The introduction of anti-VEGF molecules has dramatically reduced blindness attributable to neovascular age-related macular degeneration (nAMD). These molecules however have a relatively short intraocular half-life, which requires frequent injections on a regular basis, typically ranging from 4 to 12 weeks when a treat and extend injection protocol is utilized [1]. The frequent dosing intervals can be a significant burden for the patient and their families, and can be a cause for discontinuation of the therapy.
The anti-VEGF clinical trials have demonstrated that the visual gains attained with current anti-VEGF monotherapy are +8 to +10 ETDRS letters. However, this significant benefit does not appear to improve with increased dosage or frequency of injections, thus indicating a possible therapeutic ceiling [2]. Trials such as PrONTO and SUSTAIN tried to extend the dosing interval while retaining the efficacy and were able to reduce the number of injections to 5.6 for 12 months of study; however, vision was negatively impacted [3, 4]. HORIZON trial suggests that less frequent injections may have an incremental decline in best-corrected visual acuity (BCVA) when compared to monthly injections of ranibizumab [5].
The next generation of anti-VEGF molecules are designed to provide a better dosing regimen with a sustained duration of action. Recently, brolucizumab, an scFv molecule has been filed for biologics license application (BLA) from FDA [6]. Faricimab is a unique bispecific mAb being developed on crossmAb platform to simultaneously inhibit Ang2 and VEGF and has been shown superior to anti-VEGF monotherapy with ranibizumab [7]. The most novel of all the molecules in pipeline for intravitreal therapy is Abicipar Pegol. (Allergan Inc, Switzerland) [1].
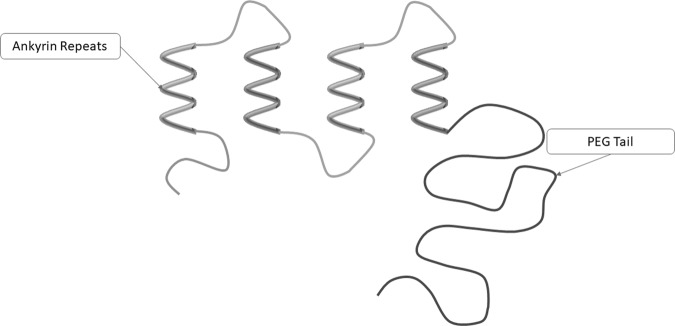
Abicipar Pegol is an anti-VEGF molecule based on the designed ankyrin repeat proteins (DARPin) therapeutics (Molecular Partners AG, Switzerland). DARPins are derived from naturally occurring ankyrin protein repeats (Fig. 1). The repeats are usually limited to four to six in numbers and thus lead to a right-handed solenoid structure with hydrophobic core and a large, grooved, solvent accessible binding surface [8]. Libraries of DARPin molecules of varying repeats numbers have been generated by a patented technology of protein engineering and recombinant DNA technology.
Fig. 1.
Molecular structure of DARPin
Early in-vivo and in-vitro trials identified few DARPin molecules that had the potential for ocular use. Intravitreal injections of DARPin in mouse models prevented retinal vascularization, while topical drops prevented corneal neovascularisation related to suture tracts in rabbits. It also reduced vascular leakage from retinal vessels in rabbits and prevented LASER induced choroidal neovascularisation in rats. It also had a favorable toxicity profile which led to the development of anti-VEGF DARPin molecule [1].
Abicipar was engineered as MP-0112 by Molecular Partners to have a longer ocular half-life with faster systemic clearance. Due to their low molecular weight and no immunoglobulin component, naive DARPins are rapidly cleared from the systemic circulation by kidneys. A poly-ethylene glycol tail was added to the repeat protein which helped the molecule to stay above renal clearance threshold [8, 9]. It was the first DARPin molecule that entered clinical development with an aim to reduce the number of intravitreal injections needed.
Initial preclinical trials showed significantly longer half-life of the molecule (6 days) following intravitreal injections in rabbits compared with aflibercept (4.7 days) and ranibizumab (2.5 days). Phase 1 dose escalation study showed the maximum tolerated dose (MTD) to be 1 mg with visual gains over the 16 weeks of study period in patients of nAMD. The trial also showed that at 2 mg dosing, the systemic circulation of drug fell below detectable level in 2 weeks, indicating a reduced systemic exposure and antidrug antibody formation. Phase 1 trial with DME patients also showed improvement in BCVA and reduction in central subfield thickness up to 0.4 mg dosing. Both trials showed high rate of inflammation attributable to the impurities in the drug formulation [10, 11].
The manufacturing process of the molecule was modified before the phase 2 trial was initiated, which was conducted in 3 phases for nAMD. The MTD for the new formulation was established at 4.2 mg in the stage 1 trial. Stage 2 established the molecule to have a similar duration of action, when duration for disease recurrence was compared with ranibizumab. Stage 3 REACH trial established the noninferiority of the molecule’s 2-monthly dosing when compared with monthly regimen of ranibizumab [12].
Another smaller cohort phase 2 study compromised of two trials, BAMBOO and CYPRESS with 25 patients each which concluded ranibizumab to demonstrate higher visual gains when compared with abicipar [13]. The visual gains in ranibizumab arm were almost double than achieved in ANCHOR and MARINA trials, and thus the results were attributed to smaller cohort and concluded that the results cannot be applied over general population.
PALM trial, a phase 2 study on patients with DME was conducted on 151 patients. The study though was not powered to show statistical significance, it showed an improvement of 7.2 letters in 2mgQ12w, 7.1 letters in 2mgQ8w, 4.9 letters in 1mgQ8w, and 9.6 letters in ranibizumab 0.5 mg monthly dosing regimen [14].
Phase 3 trials of abicipar for nAMD, dubbed as SEQUOIA and CEDAR, demonstrated similar efficacy of 2 mg abicipar in 6 and 8 injections compared to 13 injections of ranibizumab at 52 weeks primary end point in terms of visual gains. These phase 3 pivotal trials were the first to have a treatment group assigned at baseline to 12-week dosing (after a series of loading injections). The stability of visual gains in participants of q8w (94.8% in SEQUOIA, 91.7% in CEDAR) and q12w (91.3%, 91.2%) dosing of abicipar was similar to that for participants of q4w dosing of ranibizumab (96.0%, 95.5%) [15].
The rates of intraocular inflammation (IOI) in both the trials were higher in patients receiving abicipar compared to subjects treated with ranibizumab. Investigation identified impurities, potentially from fragments of E. coli, an integral part of the manufacturing process of DARPins, in the formulation. This led the sponsors of the trials to modify the manufacturing process of abicipar which was evaluated in an open label 28 weeks trial for safety. MAPLE trial demonstrated a reduced IOI rate of 8.9% with 1.6% case of severe IOI, when compared with up to 16.3% in phase 2. The phase 3 trials for DME are expected to be conducted with abicipar obtained from the modified manufacturing process [16].
Spurred by the positive outcomes of phase 3 trials in nAMD, the FDA has accepted a BLA and the European Medicines Agency has validated a marketing authorization application for abicipar pegol [17]. The promise of extended duration anti-VEGF treatment with q12 week apicipar dosing while maintaining good visual outcomes (and hopefully reduced rates of IOI with the new formulation) could usher in an a new era of decreased treatment burden to the benefit of patients and their families. Trials are summarized in Table 1.
Table 1.
Summary of clinical trials
| Phase of Trial | Nature of trial | Disease under evaluation | Number of participants | Drugs and dosage | Primary endpoint | Primary outcome | Additional remarks |
|---|---|---|---|---|---|---|---|
| Phase 1 [10] | Dose escalation study, open label | nAMD | 32 | 0.04 mg, 0.15 mg, 0.4 mg, 1 mg, 2 mg, 3.6 mg | 16 weeks | Maximum tolerated dose (MTD) | MTD was established to be 1 mg. Thirteen of the patients showed intraocular inflammation |
| Phase 1 [11] | Dose escalation study, open label | DME | 18 | 0.04 mg, 0.15 mg, 0.4 mg, 1 mg, 2 mg, 3.6 mg | 16 weeks | Maximum tolerated dose (MTD) | 0.04, 0.15, and 0.4 mg drugs were only administered. Eleven patients showed IOI |
| Phase 2 Stage 1 [12] | Efficacy and safety | nAMD | 18 |
1 mg (n = 3) 2 mg (n = 6) 3 mg (n = 6) 4.2 mg (n = 6) single intravitreal injection |
24 weeks | Change from baseline in central retinal thickness (CRT) | MTD causing reduction in CRT of the Abicipar with new modification and purification process was 4.2 mg with no adverse events reported |
| Phase 2 Stage 2 [12] | Assess the duration of action | nAMD | 183 |
Abicipar 4.2 mg (n = 67) (0, 16 weeks) Abicipar 3.0 mg (n = 58) (0, 16 weeks) Ranibizumab (n = 58) (0, 16 weeks) |
16 weeks | Duration for disease recurrence | Duration for disease recurrence was 59, 57, and 57 days in abicipar 4.2 mg, abicipar 3 mg and ranibizumab 0.5 mg cohorts respectively after 1 injection at baseline. Mean duration for recurrence increased to 85, 112, and 111 days after 2nd dose at 16 weeks |
| Phase 2 Stage 3 (REACH) [12] | Head to head comparison with ranibizumab | nAMD | 64 |
Abicipar 1 mg (n = 25) (0, 4, 8 weeks) Abicipar 2 mg (n = 23) (0, 4, 8 weeks) Ranibizumab 0.5 mg (n = 16) (0, 4, 8, 12, 16 weeks) |
16 weeks | Gain in BCVA | Study showed noninferiority of abicipar to ranibizumab with a 2-month extended durability of visual gain at primary endpoint of 16 weeks |
| Phase 2 (BAMBOO) [13] | Head to head comparison with ranibizumab | nAMD | 25 (Japan) |
Abicipar 1 mg (n = 10) (0, 4, 8 weeks) Abicipar 2 mg (n = 10) (0, 4, 8 weeks) Ranibizumab 0.5 mg (n = 5) (0, 4, 8, 12, 16 weeks) |
16 weeks | Gain in BCVA | Study showed ranibizumab had better visual gains when compared with abicipar. Significance could not be established due to smaller cohort |
| Phase 2 (CYPRESS) [13] | Head to head comparison with ranibizumab | nAMD | 25 (United States) |
Abicipar 1 mg (n = 10) (0, 4, 8 weeks) Abicipar 2 mg (n = 10) (0, 4, 8 weeks) Ranibizumab 0.5 mg (n = 5) (0, 4, 8, 12, 16 weeks) |
16 weeks | Gain in BCVA | |
| Phase 2 (PALM) [14] | Head to head comparison with ranibizumab | DME | 151 |
Abicipar 1 mg (n = 43) q8w Abicipar 2 mg (n = 42) q8w Abicipar 2 mg (n = 45) q12w Ranibizumab 0.5 mg (n = 21) q4w 3 monthly loading doses in all arms followed by dosage as applicable till 28 weeks |
28 weeks | Gain in BCVA | Study was not powered for statistical significance. Visual gains were 4.9 letters in abicipar 1mgq4w, 7.1 in 2 mgq8w and 7.2 in 2mgq12w when compared with ranibizumab |
| Phase 3 (SEQUIOA) [15] | Head to head comparison with ranibizumab | nAMD | 946 |
Abicipar 2mgq12w Abicipar 2mgq8w Ranibizumab 0.5mgq4w |
52 weeks | Percentage of patients with BCVA change from baseline ≤ 15 letters |
91.3% in abicipar 2mgq12w 94.8% in abicipar 2mgq8w 96.0% in ranibizumab 0.5mgq4w |
| Phase 3 (CEDAR) [15] | Head to head comparison with ranibizumab | nAMD | 939 |
Abicipar 2mgq12w Abicipar 2mgq8w Ranibizumab 0.5mgq4w |
52 weeks | Percentage of patients with BCVA change from baseline ≤ 15 letters |
91.2% in abicipar 2mgq12w 91.7% in abicipar 2mgq8w 95.5% in ranibizumab 0.5mgq4w |
| Phase 3 (MAPLE) [16] | Safety of abicipar from a refined manufacturing process | nAMD | 128 | 3 monthly injections of 2 mg abicipar | 28 weeks | Rate of IOI | 8.9% participants developed IOI, 1.6% were severe (1 case of iritis and uveitis each). No endophthalmitis |
Compliance with ethical standards
Conflict of interest
Ashish Sharma: Consultant—Novartis India, Allergan Global, Intas India, Bayer India. Nilesh Kumar: None. BD Kuppermann: Clinical research—Alcon, Alimera, Allegro, Allergan, Apellis, Clearside, Genentech, GSK, Ionis, jCyte, Novartis, Regeneron, ThromboGenics; Consultant—Alimera, Allegro, Allergan, Cell Care, Dose, Eyedaptic, Galimedix, Genentech, Glaukos, Interface Biologics, jCyte, Novartis, Ophthotech, Regeneron, Revana, Theravance Biopharma. Francesco Bandello: Consultant—Allergan, Bayer, Boehringer- Ingelheim, Fidia Sooft, Hofmann La Roche, Novartis, NTC Pharma, Sifi, Thrombogenics, Zeiss.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stahl A, Stumpp MT, Schlegel A, Ekawardhani S, Lehrling C, Martin G, et al. Highly potent VEGF-A-antagonistic DARPins as anti-angiogenic agents for topical and intravitreal applications. Angiogenesis. 2013;16:101–11. doi: 10.1007/s10456-012-9302-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smithwick E, Stewart MW. Designed ankyrin repeat proteins: a look at their evolving use in medicine with a focus on the treatment of chorioretinal vascular disorders. AIAAMC. 2017;16:1–1. doi: 10.2174/1871523016666170502115816. [DOI] [PubMed] [Google Scholar]
- 3.Lalwani GA, Rosenfeld PJ, Fung AE, Dubovy SR, Michels S, Feuer W, et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO study. Am J Ophthalmol. 2009;148:43–58.e1. doi: 10.1016/j.ajo.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 4.Holz FG, Amoaku W, Donate J, Guymer RH, Kellner U, Schlingemann RO, et al. Safety and efficacy of a flexible dosing regimen of ranibizumab in neovascular age-related macular degeneration: the SUSTAIN study. Ophthalmology. 2011;118:663–71. doi: 10.1016/j.ophtha.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 5.Singer MA, Awh CC, Sadda S, Freeman WR, Antoszyk AN, Wong P, et al. HORIZON: an open-label extension trial of ranibizumab for choroidal neovascularization secondary to age-related macular degeneration. Ophthalmology. 2012;119:1175–83. doi: 10.1016/j.ophtha.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 6.Novartis announces FDA filing acceptance and Priority Review of brolucizumab (RTH258) for patients with wet AMD. Novartis. 2019. https://www.novartis.com/news/media-releases/novartis-announces-fda-filing-acceptance-and-priority-review-brolucizumab-rth258-patients-wet-amd
- 7.Klein C, Schaefer W, Regula JT, Dumontet C, Brinkmann U, Bacac M, et al. Engineering therapeutic bispecific antibodies using CrossMab technology. Methods. 2019;154:21–31. doi: 10.1016/j.ymeth.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Plückthun A. Designed ankyrin repeat proteins (DARPins): binding proteins for research, diagnostics, and therapy. Annu Rev Pharmacol Toxicol. 2015;55:489–511. doi: 10.1146/annurev-pharmtox-010611-134654. [DOI] [PubMed] [Google Scholar]
- 9.Sennhauser G, Grütter MG. Chaperone-assisted crystallography with DARPins. Structure. 2008;16:1443–53. doi: 10.1016/j.str.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Souied EH, Devin F, Mauget-Faÿsse M, Kolář P, Wolf-Schnurrbusch U, Framme C, et al. Treatment of exudative age-related macular degeneration with a designed ankyrin repeat protein that binds vascular endothelial growth factor: a phase I/II study. Am J Ophthalmol. 2014;158:724–732.e2. doi: 10.1016/j.ajo.2014.05.037. [DOI] [PubMed] [Google Scholar]
- 11.Campochiaro PA, Channa R, Berger BB, Heier JS, Brown DM, Fiedler U, et al. Treatment of diabetic macular edema with a designed ankyrin repeat protein that binds vascular endothelial growth factor: a phase I/II study. Am J Ophthalmol. 2013;155:697–704.e2. doi: 10.1016/j.ajo.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 12.Callanan D, Kunimoto D, Maturi RK, Patel SS, Staurenghi G, Wolf S, et al. Double-masked, randomized, phase 2 evaluation of abicipar pegol (an Anti-VEGF DARPin Therapeutic) in neovascular age-related macular degeneration. J Ocul Pharmacol Ther. 2018;34:700–9. doi: 10.1089/jop.2018.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kunimoto D, Ohji M, Maturi RK, Sekiryu T, Wang Y, Pan G, et al. Evaluation of Abicipar Pegol (an Anti-VEGF DARPin Therapeutic) in patients with neovascular age-related macular degeneration: studies in Japan and the United States. Ophthalmic Surg Lasers Imaging Retina. 2019;50:e10–22. doi: 10.3928/23258160-20190129-13. [DOI] [PubMed] [Google Scholar]
- 14.Abicipar Pegol PALM Study Phase 2 Data in Diabetic Macular Edema (DME) Presented at 2016 AAO Annual Meeting—Molecular Partners. 2016. https://www.molecularpartners.com/abicipar-pegol-palm-study-phase-2-data-in-diabetic-macular-edema-dme-presented-at-2016-aao-annual-meeting/
- 15.Allergan and Molecular Partners Announce Two Positive Phase 3 Clinical Trials for Abicipar pegol 8 and 12-week Regimens for the Treatment in Patients with Neovascular Age-Related Macular Degeneration—Molecular Partners. 2018. https://www.molecularpartners.com/allergan-and-molecular-partners-announce-two-positive-phase-3-clinical-trials-for-abicipar-pegol-8-and-12-week-regimens-for-the-treatment-in-patients-with-neovascular-age-related-macular-degeneration/
- 16.Allergan and Molecular Partners Announce Topline Safety Results from MAPLE study of Abicipar pegol—Molecular Partners. 2019. https://www.molecularpartners.com/allergan-and-molecular-partners-announce-topline-safety-results-from-maple-study-of-abicipar-pegol/
- 17.FDA, European Medicines Agency advance abicipar pegol applications for wet AMD. 2019. https://www.healio.com/ophthalmology/retina-vitreous/news/online/%7B401f1470-71d1-4ebd-907e-5f13e0b9cd99%7D/fda-european-medicines-agency-advance-abicipar-pegol-applications-for-wet-amd