The detailed review ‘Using the CODIT model to explain secondary metabolites of xylem in defence systems of temperate trees against decay fungi’ (Morris et al., 2020) breaks new ground in understanding the biosynthesis of secondary metabolites (SMs) that resist (1) the loss of sapwood function after wounding and (2) the spread of fungal infection. The review summarizes the current state of research and provides fingerposts for the direction of new research.
COMPARTMENTALIZATION AND CODIT
For tree biology, the term ‘compartmentalization’ refers to (1) the general processes by which trees grow and defend themselves and (2) the specifics of how trees resist the spread of infection through the setting of constitutive and induced boundaries (Shigo, 1984). Individual anatomical and physiological features of compartmentalization may be in either or both categories. The CODIT model describes patterns of wood decay on the basis of conceptual walls that correspond largely to visible anatomical features such as wood rays and tree-ring boundaries (Shigo and Marx, 1977).
The compartmentalization concept and the CODIT model were originally presented as a systems approach to understand patterns of tree growth, response to injury, and the decay process in living trees. The review notes that prior to Shigo’s work, researchers generally considered the sapwood of living trees to be dead and unresponsive. Rather than as a process, decay was understood in terms of the end products: punky wood or the void remaining in the tree after wood degradation. Within the compartmentalization concept, wood decay is a process continuum from wood formation by the vascular cambium, followed by cellular differentiation, maturation (including apoptosis) and shedding. Morris et al. (2020) focus their attention primarily on the inducible elements of reaction zone formation for CODIT Wall 2 and Wall 3, with some mention of Wall 1.
When I first received a review draft of Morris et al. (2020), I was surprised by the title. Not because of the topic, which has been central to my USDA Forest Service research and development programme for more than 40 years, but because the authors based their presentation on the CODIT model. As noted in the review, CODIT was developed as a teaching tool for practitioners (e.g. arborists, foresters, wildlife managers). The lushly illustrated and simply written CODIT booklet (Shigo and Marx, 1977) effectively conveyed the message that sapwood was alive and responsive, and formed boundaries to resist the loss of function and the spread of infection. The co-author H. G. Marx was not a researcher but a publications specialist charged with keeping the narrative flow linear and to the point. The CODIT booklet summarized more than 10 years of forest pathology research published primarily in journals of professional societies (e.g. Shigo, 1965), combined with unpublished observations of the natural history of trees and associated fungi.
To me, the CODIT booklet was the ‘classic comics’ version of this body of research. Useful for educational purposes and to introduce concepts to non-specialists, but not intended to bear the weight of formal scientific criticism (Smith, 2006). Alternatively, some researchers use the term CODIT as being identical to compartmentalization itself, rather than as a conceptual summary or model. If CODIT and compartmentalization are taken to be the same thing, then criticism of the shortcomings of the comic book may replace a deeper understanding of both the assets and uncertainties of the parent research literature. For practitioners interested in improving tree care, this likely matters little. ‘Picture books’ summarizing technical work continue to be valuable for practitioners [e.g. Dujesiefken and Liese, 2015 (cited in Morris et al., 2020); Shortle and Dudzik, 2012]. CODIT is still taught in the practitioner community, but I can think of no US-based researcher on wood physiology or pathology that uses the CODIT model to focus or explain their research to other scientists.
APOPLASTIC MARKERS OF SYMPLASTIC PROCESSES
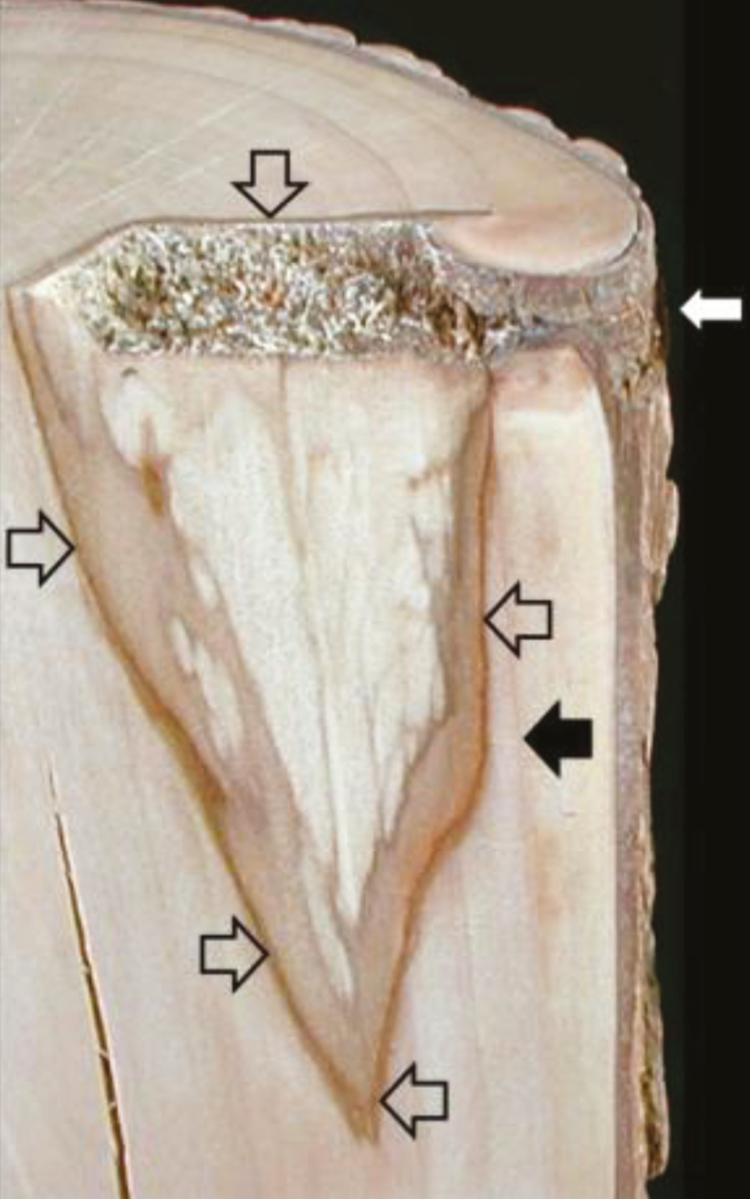
Sapwood contains two types of transport and communication systems, one based on geometric orientation of anisotropic cells (radial or axial) and one based on being within or outside of the interconnected network of cellular contents at maturity (symplast and apoplast). The walls of the CODIT model relate to anatomical markers within the apoplast, such as along the lengths of apoplastic conducting elements (Wall 1), heavily lignified latewood or bands of terminal parenchyma (Wall 2), and ray assemblages (Wall 3) (Fig. 1). However, the strength of these walls and the effectiveness of compartmentalization depend on symplastic events, from plug formation to other metabolic shifts to produce SMs, such as terpenes and phenolics. The CODIT walls are apoplastic markers of symplastic responses, usually resulting in the death of sapwood parenchyma.
Fig 1.
Compartmentalization of decay in sweetgum (Liquidambar styraciflua) associated with a 1-year-old bore hole injury (white arrow). The most recently formed reaction zone separates wound-initiated discoloration from healthy sapwood (open arrows) present at the time of injury, corresponding to CODIT Walls 1–3. The barrier zone (solid black arrow), corresponding to Wall 4, separates wood formed after injury from wood present at the time of injury. The bleached wood with zone lines indicates active decay, surrounded by wound-initiated discoloration bounded by the reaction and barrier zones. (Originally published in Shortle and Dudzik, 2012)
The review by Morris et al. (2020) properly emphasizes that SM production depends on living parenchyma with sufficient energy reserves to shift metabolism and to form compartmentalization boundaries spatially associated with the CODIT walls. The cell death in the induction of SMs limits the spread of infection but at a metabolic cost. Most obvious, perhaps, is the death of axial parenchyma associated with tyloses of vessels and other plugging processes. Less obvious, perhaps, is that successful compartmentalization results in the death of parenchyma and cessation of ordered hydraulic flow within the bounded compartment. Successful compartmentalization breaks the symplastic connections from the affected compartment to the rest of the tree. This reduces sapwood volume and cross-sectional area available to conduct xylem sap, store starch and dynamically respond to future injury.
Induced production of SMs was recognized early as a key feature of compartmentalization (e.g. Shortle, 1979; other references cited in Morris et al., 2020). Early research on SMs in reaction zones of hardwoods focused on hydrolysable tannins derived from the shikimic acid pathway. Although usually sampled along the convenient radial plane (corresponding to CODIT Wall 3; Smith, 1996), the reaction zone was considered comparable irrespective of the position of the model walls. Expanding the classes of SMs in Morris et al. (2020) is a welcome addition and may point to useful areas of future research concerning the energetics of trade-offs between growth and defence.
A minor quibble with terminology is that Morris et al. (2020) refer to reaction zones that form once and that remain effective as ‘static’ and reaction zones that form repeatedly as ‘dynamic’. I interpret the repeated formation of reaction zones as resulting from failure of ineffective, earlier-formed boundaries. Repeated formation of reaction zones indicates failure, at least at that spatial position. Particularly within Shigo’s publications, a ‘dynamic’ process is considered as being relatively more effective than a ‘static’ process. But in Morris et al. (2020) this shading of meaning seems to be reversed.
CODIT AND MICROBIAL SUCCESSION
The concept of microbial succession resulting in wood breakdown was as central to the compartmentalization concept as the physiological and anatomical boundaries (page 2 of Shigo and Marx, 1977). The mapping of microbial communities associated with dissected decay columns yielded patterns of infection by ruderal bacteria, yeasts and hyphomycetous ‘pioneer’ fungi at the edge of columns of wound-initiated discoloration and decay (e.g. Shigo and Sharon, 1970). Without using the term, Shigo interpreted these patterns as a ‘space for time substitution’ and he inferred from the spatial position along the column of wound-initiated discoloration and decay that fungi involved in decay occurred in a temporal sequence.
At least some portion of the succession of fungi colonizing wound-associated wood can be explained using classical r- and K-selection principles. Immediately or shortly after sapwood wounding, short-term opportunistic ruderal communities form that are followed by one or more additional stages before being dominated by long-term stress-tolerant specialists. Of course, primary pathogens (e.g. species of Armillaria) can directly infect and cause substantial decay in the apparent absence of succession. The above may seem beyond the scope of Morris et al. (2020), yet the review does refer to research on the soil-borne hyphomycetous genus Trichoderma as an antagonist of decay fungi. Interestingly, co-workers and I suggested that interruption or interference with the posited succession could help explain the role of Trichoderma treatments to slow the spread of decay fungi (Smith et al., 1981).
WHAT’S NEXT FOR CODIT AND COMPARTMENTALIZATION?
The original Shigo and Marx booklet (1977), long out of print but fortunately available by download (https://www.fs.usda.gov/treesearch/pubs/5292) bears another look. Despite my initial misgivings, CODIT provides a handy commonsense language for complicated processes of tree protection and defence. The CODIT language of induced metabolic shifts of the dynamic symplast associated with the apoplastic framework, oriented along the radial, tangential and axial planes, provides a shorthand for describing and locating these fundamental survival processes.
Morris et al. (2020) is thoughtful and thought-provoking. The review stands as a useful waystation in the ongoing development of these concepts of tree protection and defence. More than that, Morris et al. (2020) provides an opportunity for researchers of sapwood physiology and pathology to consider the usefulness of the CODIT model as a framework to understand fundamental research beyond the immediate needs of the practitioner community.
LITERATURE CITED
- Morris H, Hietala AM, Jansen S, et al. 2020. Using the CODIT model to explain secondary metabolites of xylem in defence systems of temperate trees against decay fungi. Annals of Botany 125: 701– 720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigo AL. 1965. The pattern of decay and discoloration in northern hardwoods. Phytopathology 55: 648–652. [Google Scholar]
- Shigo AL. 1984. Compartmentalization: a conceptual framework for understanding how trees grow and defend themselves. Annual Review of Phytopathology 22: 189–214. [Google Scholar]
- Shigo AL, Marx H. 1977. CODIT (compartmentalization of decay in trees). USDA Agricultural Information Bulletin 405. Washington, DC: U.S. Department of Agriculture, Forest Service; https://www.fs.usda.gov/treesearch/pubs/5292. [Google Scholar]
- Shigo AL, Sharon EM. 1970. Mapping columns of discolored and decayed tissues in sugar maple, Acer saccharum. Phytopathology 60: 232–237. [Google Scholar]
- Shortle WC. 1979. Compartmentalization of decay in red maple and hybrid poplar trees. Phytopathology 69: 410–413. [Google Scholar]
- Shortle WC, Dudzik KR. 2012. Wood decay in living and dead trees: a pictorial overview. General Technical Report NRS-97. Newtown Square, PA: U.S. Department of Agriculture, Forest Service, Northern Research Station.https://www.fs.usda.gov/treesearch/pubs/40899. [Google Scholar]
- Smith KT. 1996. Phenolics and compartmentalization in the sapwood of broad-leaved trees. In: Dashek WV, ed. Methods in plant biochemistry and molecular biology. Boca Raton, FL: CRC Press, 189–198. [Google Scholar]
- Smith KT. 2006. Compartmentalization today. Arboricultural Journal 29: 173–184. [Google Scholar]
- Smith KT, Blanchard RO, Shortle WC. 1981. Postulated mechanism of biological control of decay fungi in red maple wounds treated with Trichoderma harzianum. Phytopathology 71: 496–498. [Google Scholar]