Abstract
Background
Mitochondria play a diversity of physiological and metabolic roles under conditions of abiotic or biotic stress. They may be directly subjected to physico-chemical constraints, and they are also involved in integrative responses to environmental stresses through their central position in cell nutrition, respiration, energy balance and biosyntheses. In plant cells, mitochondria present various biochemical peculiarities, such as cyanide-insensitive alternative respiration, and, besides integration with ubiquitous eukaryotic compartments, their functioning must be coupled with plastid functioning. Moreover, given the sessile lifestyle of plants, their relative lack of protective barriers and present threats of climate change, the plant cell is an attractive model to understand the mechanisms of stress/organelle/cell integration in the context of environmental stress responses.
Scope
The involvement of mitochondria in this integration entails a complex network of signalling, which has not been fully elucidated, because of the great diversity of mitochondrial constituents (metabolites, reactive molecular species and structural and regulatory biomolecules) that are linked to stress signalling pathways. The present review analyses the complexity of stress signalling connexions that are related to the mitochondrial electron transport chain and oxidative phosphorylation system, and how they can be involved in stress perception and transduction, signal amplification or cell stress response modulation.
Conclusions
Plant mitochondria are endowed with a diversity of multi-directional hubs of stress signalling that lead to regulatory loops and regulatory rheostats, whose functioning can amplify and diversify some signals or, conversely, dampen and reduce other signals. Involvement in a wide range of abiotic and biotic responses also implies that mitochondrial stress signalling could result in synergistic or conflicting outcomes during acclimation to multiple and complex stresses, such as those arising from climate change.
Keywords: Mitochondria, abiotic stress, biotic stress, mitochondrial stress, mitochondria-derived signalling, retrograde signalling, interorganellar signalling, reactive oxygen species, programmed cell death
Introduction
Mitochondria are present in all plant cells, where they carry out vital functions of nutrition, metabolism and biosynthesis. Plant mitochondria present various biochemical peculiarities, such as cyanide-insensitive alternative respiration mediated by alternative oxidase (AOX) (Elthon and McIntosh, 1987), and, besides integration with ubiquitous eukaryotic compartments, their functioning is coupled with plastid functioning, whether in photosynthetic or non-photosynthetic tissues, and whether under light, shade or dark conditions. Their electron transport chain (ETC) and oxidative phosphorylation system (OXPHOS) provide the necessary energy for survival and development of the plant in spatial or temporal collaboration with photosynthetic processes (Schwarzländer and Finkemeier, 2013; Wurzinger et al., 2018). Given their strong involvement in energy homeostasis, mitochondria are key organelles for plant responses to environmental stresses (Crawford et al., 2018). Moreover, they function through an important surface of contact and strong biochemical exchanges with the cytosol, and thus, directly or indirectly, with all of the cell compartments. Environmental stresses can directly affect mitochondrial activities, thus leading to situations of mitochondrial perturbation or stress. Conversely, perturbations and stresses that affect other cell compartments can reach and modify mitochondrial functioning (Van Aken et al., 2016). The involvement of mitochondria in the integration of plant cell responses to stresses entails a complex network of signalling which has not been fully elucidated, given the great diversity of mitochondrial constituents (metabolites, reactive molecular species, structural and regulatory biomolecules) that are linked to stress and programmed cell death (PCD) signalling pathways (Møller, 2001; Mittler et al., 2004; Gupta et al., 2011; Jacoby et al., 2012; Mano, 2012; Schwarzländer and Finkemeier, 2013; Hildebrandt et al., 2015; Welchen and Gonzalez, 2016; Wang et al., 2018; Cui et al., 2019; Huang et al., 2019). The present review analyses this complexity of mitochondrial stress signalling connexions through the specific cases of ETC and OXPHOS. Drawing on different examples of abiotic and biotic stresses, we discuss how mitochondrial ETC and OXPHOS can be involved in stress perception and transduction, stress signal amplification, or cell stress response modulation
SIGNALLING DYNAMICS OF MITOCHONDRIAL ENERGY METABOLISM
Signalling energy metabolites
The ETC and OXPHOS sustain the major mitochondrial function of ATP generation, in relation to the metabolic dynamics of tricarboxylic acids (TCAs), acetyl-CoA, ADP, oxidized (NAD+) or reduced (NADH) β-nicotinamide adenine dinucleotide, oxidized (FAD) or reduced (FADH2) flavin adenine dinucleotide (Bohovych and Khalimonchuk, 2016). The NAD+/NADH and FAD/FADH2 equilibria are maintained through the TCA cycle, the ETC and OXPHOS (Bohovych and Khalimonchuk, 2016). Because of these strong connections with central metabolites, mitochondria can be key organelles for the perception of stresses harmful to the plant (Crawford et al., 2018). Environmental stresses can directly or indirectly disrupt ETC and OXPHOS activity and perception of the disturbance can be transduced to the nuclear genome with consequent regulation of metabolism- and stress-related genes (Che-Othman et al., 2017). Under conditions that lead to mitochondrial dysfunctioning or damage, decrease in ATP production can lead to depletion of ATP levels and increase in ADP and AMP (Bailey-Serres et al., 2011; Pedrotti et al., 2018). Situations where carbohydrates are limiting or eventually run out, such as the end of night during night–day cycles, or extended darkness, result in energy limitation or starvation (Baena-González and Sheen, 2008; Pedrotti et al., 2018). The resulting decrease in ATP levels can induce retrograde signals from mitochondria to the genome, resulting in the induction of alternative respiratory pathways such as amino acid and fatty acid catabolism (Schwarzländer and Finkemeier, 2013; Hildebrandt et al., 2015). Furthermore, fluctuations in the ATP level can indirectly act on other metabolites, and these metabolites can themselves mediate retrograde signalling. It has thus been established or hypothesized that acetyl-CoA, TCA cycle intermediates and NADH may act as retrograde signals from the mitochondria to the nucleus (Schwarzländer and Finkemeier, 2013; Ashrafi et al., 2018; Wagner et al., 2018). This may lead to the hypothesis that the efficiency of the stress response may depend on the quantity and diversity of mitochondrial stress signals.
Metabolic energy sensors
The plant sucrose-non-fermenting (SNF)-related kinase 1 (SnRK1) is a central metabolic sensor that belongs to a highly conserved eukaryotic protein kinase family (Crozet et al., 2014). Sensing of variations in adenylate nucleotide and sugar levels by the SnRK1 sensor provides the cell with information on energy and nutritional status and on the necessary adjustments for stress tolerance (Baena-González and Sheen, 2008). SnRK1 functions closely with TOR (target of rapamycin) kinase, another sugar sensor inducing antagonistic growth-related responses (Baena-González and Sheen, 2008). SnRK1 and TOR kinase therefore function as hubs for energy, growth and stress sensing. Interplay between soluble sugar fluctuations, SnRK1 and TOR kinase results in large-scale transcriptome reprogramming that contributes to homeostasis restoration, cell survival, autophagy regulation and adaptive responses (Baena-González and Sheen, 2008; Janse van Rensburg et al., 2019). The regulatory properties of SnRK1 imply that it can perceive variations in adenylate nucleotide levels as well as those in several other metabolites, such as sucrose, glucose, trehalose-6-P, glucose-1-P or glucose-6-P (Crozet et al., 2014). Although the links between SnRK1 and the different cellular compartments, especially mitochondria and plastids, are not fully elucidated, its cytoplasmic localization implies that it can integrate the perception of metabolic cues originating from or regulated by different sources (Crozet et al., 2014; Wurzinger et al., 2018). The regulation of SnRK1 in response to stress and metabolic changes can result in transcriptional regulation of stress-related genes or in post-translational phosphorylation regulation of key metabolic enzymes (Broeckx et al., 2016; Nukarinen et al., 2016; Wurzinger et al., 2018). Unexpectedly, phosphorylation regulation also affects some mitochondrial proteins (Wurzinger et al., 2018), thus suggesting regulatory loops between mitochondrial retrograde AMP/ADP/ATP signals, cytoplasmic SnRK1, mitochondrial functioning and mitochondrial metabolites (acetyl-CoA, TCA cycle intermediates, NADH) that are themselves candidate retrograde signals (Schwarzländer and Finkemeier, 2013; Ashrafi et al., 2018; Wagner et al., 2018). However, in contrast to the characterization of SnRK1 as a metabolic sensor of adenylate nucleotides and carbohydrates, the mechanisms of retrograde signalling by mitochondrial metabolites are not yet fully elucidated. Citrate may act through specific receptors or transporters, as is the case in bacteria (Schwarzländer and Finkemeier, 2013), or through epigenetic modifiers, in association with acetyl-CoA, as is the case in animal cells (Shaughnessy et al., 2014).
MITOCHONDRIAL REACTIVE OXYGEN AND NITROGEN SPECIES
Dynamics of electron fluxes and reactive oxygen species
The flux of electrons through the ETC and oxygen reduction can produce partially reduced reactive oxygen species (ROS), such as superoxide ion and hydrogen peroxide (Møller, 2001; Murphy, 2009). ETC complexes I, II and III (Fig. 1) are major sites of ROS production (Gleason et al., 2011; Andreyev et al., 2015). On the one hand, superoxide and hydrogen peroxide are continuously produced by natural ETC functioning. On the other hand, several metabolic and stress situations lead to a more reduced state of the ETC and increased ROS production (Møller, 2001). This occurs for instance under situations of ETC inhibition or ETC slow-down, whenever ADP availability is reduced by stress. Thus, respiratory inhibitors can inhibit ETC complexes, and induce over-reduction of various parts of the ETC, thus leading to superoxide overproduction (Li et al., 2003; Schwarzländer et al., 2009). Stress-induced alterations of mitochondrial ultrastructure, as occur for instance during salt stress, can directly affect or damage ETC and OXPHOS functioning (Garcia de la Garma et al., 2015).
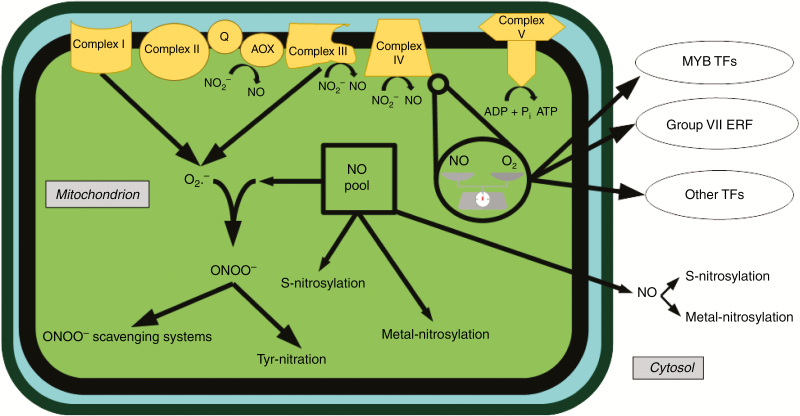
Fig. 1.
Mitochondrial dynamics and cellular effects of NO. Nitric oxide can be produced at different levels of the ETC (Planchet et al., 2005; Castello et al., 2006; Gupta and Igamberdiev, 2011; Alber et al., 2017; Vishwakarma et al., 2018). A balance between NO and O2 exists in the mitochondria (Vieira and Kroemer, 2003; Amirsadeghi et al., 2007). Nitric oxide can lead to the formation of peroxynitrite (Radi et al., 2002; Gupta et al., 2011). The balance between NO and O2 may act as a hypoxic signal involving several classes of transcription factors (Castello et al., 2006; Gibbs et al., 2015). Q, quinone pool; TF, transcription factor.
Abiotic stresses usually lead to superoxide overproduction in mitochondria (Basu et al., 2001; Dixit et al., 2002), and the action of superoxide dismutases (SODs) generates hydrogen peroxide from superoxide (Turrens, 2003). Whereas superoxide has a very short half-life, hydrogen peroxide has a longer half-life and can cross membranes through aquaporins (Bienert et al., 2007), thus potentially serving as a signal linking mitochondria to other cell compartments (Bienert et al., 2007; Bohovych and Khalimonchuk, 2016).
This ROS-based dynamics from the mitochondria has been associated with ROS signalling mechanisms (Møller and Sweetlove, 2010; Ng et al., 2014; Noctor and Foyer, 2016) that involve oxidized intermediates, Ca2+ and phosphorylation processes (Møller and Sweetlove, 2010; Ng et al., 2014) and with transcriptional and post-transcriptional alterations of cell functioning that can lead to regulation of growth and development programmes, including PCD (Gao et al., 2008; Liu et al., 2014; Wu et al., 2015; Singh et al., 2016; Cui et al., 2019). Mitochondrial ROS signalling and mitochondrial perturbation signalling are linked to developmental processes regulated by auxins (Kerchev et al., 2014; Yang et al., 2014; Wang and Auwerx, 2017). In Arabidopsis FtsH4 mutants, which are affected in a mitochondrial protein-processing protease, accumulation of mitochondrial hydrogen peroxide is associated with deregulation of auxin homeostasis, cell cycle dysregulation and impairment of meristem activity (Zhang et al., 2014; Dolzblasz et al., 2018). Mutation of the ABA-overly-sensitive-8 (ABO8) splicing factor, which is necessary for correct expression of the NAD4 component of complex I, results in increased mitochondrial ROS production, abscisic acid (ABA) hypersensitivity, reduced auxin accumulation/signalling and reduced meristem activity (Yang et al., 2014). This crosstalk between mitochondrial ROS, hormones and development shows that mitochondrial ROS signalling may be mediated, at least in part, by hormone signalling pathways.
Under conditions of biotic stress, PCD is part of the hypersensitive response (HR) that decreases the growth and development of biotrophic pathogens (Dangl and Jones, 2001). This HR is related to an increase in ROS levels (Mur et al., 2007). Besides plasma-membrane-localized NADPH oxidases (Suzuki et al., 2011), mitochondria are also an important source of ROS (Møller, 2001) for pathogen-defence oxidative bursts and HR (Allan and Fluhr, 1997; Cvetkovska and Vanlerberghe, 2012a; Cvetkovska et al., 2013). Characterization of the Arabidopsis mosaic-death-1 (mod1) mutant (Wu et al., 2015) has shown that PCD could be triggered by higher ROS production resulting from the destabilization of ETC complex I. Interestingly, in the mod1 mutant the destabilization of ETC complex I is due to chloroplastic fatty acid biosynthesis deficiency, thus emphasizing that ROS-based mitochondrial signalling could link perturbation in another type of subcellular compartment with PCD induction.
Moreover, ROS-based mitochondrial signalling appears to involve multiple mechanisms and multiple levels of regulation. Stress-induced PCD involves two phases of ROS induction. The first phase is immediately triggered after stress perception, while a second phase appears a few hours after stress perception. The initial ROS phase may be related to mitochondrial permeability transition. Mitochondrial membrane permeability is tightly regulated, but during stress-induced PCD mitochondrial membranes become permeable to bigger solutes, which modifies normal functioning and leads to ETC inhibition, ROS overproduction and PCD induction (Rostovtseva et al., 2005; Scott and Logan, 2008; Van Aken and Van Breusegem, 2015). This multi-step induction has been demonstrated by sequential hydrogen peroxide treatments (Murik et al., 2014). ETC-related increases in ROS production can reinforce mitochondrial dysfunction, which, in turn, can enhance ROS production, thus generating a self-amplifying loop of ROS dynamics in the mitochondria (Amirsadeghi et al., 2007). Under conditions of biotic stress, such amplifying loops can be triggered by plant growth regulators, such as salicylate, by pathogenic toxins, such as harpin (Krause and Durner, 2004) or victorin (Yao et al., 2002), and by virulence (Amirsadeghi et al., 2007).
Proline regulation of ROS dynamics
In plant cells, proline is a multi-usage amino acid with implications in growth and development as well as in stress responses (Szabados and Savouré, 2010). Proline is generally synthesized in the cytosol from glutamate under the control of pyrroline-5-carboxylate synthetase (P5CS) and pyrroline-5-carboxylate reductase (P5CR). Its oxidative degradation leading to glutamate production occurs in mitochondria through a two-step mechanism that involves proline dehydrogenase (ProDH) and pyrroline-5-carboxylate dehydrogenase (P5CDH). A variety of stress situations (drought, high temperature, low temperature, heavy metal, pathogen infection, anaerobiosis, nutrient deficiency, atmospheric pollution, UV irradiation) lead to proline accumulation resulting from upregulation of P5CS (proline synthesis) and downregulation of ProDH (proline catabolism) (Verbruggen and Hermans, 2008). Alternatively, other stress situations are associated with ProDH (proline catabolism) upregulation in parallel with proline accumulation (Verslues et Sharma, 2010; Garcia de la Garma et al., 2015).
Proline dehydrogenase is localized at the inner face of the mitochondrial inner membrane in association with the ETC (Cabassa-Hourton et al., 2016). As a flavoenzyme, ProDH can feed electrons to the ETC (Rasmusson and Møller, 2011; Schertl and Braun, 2014), but whenever stress-related perturbations lead to ETC over-reduction, reduced FADH2 in ProDH can directly reduce oxygen to superoxide and hydrogen peroxide, thus contributing to mitochondrial ROS production (Liang et al., 2013). The presence of proline and the activity of ProDH therefore interact with the optimal or stressed status of ETC, with ROS production and redox status as biochemical and signalling outputs (Rasmusson and Møller, 2011). This ROS production from proline/P5C conversion through ProDH activity plays an important role in PCD and HR induction under conditions of biotic stress (Monteoliva et al., 2014). Proline mitochondrial metabolism has thus been shown to contribute significantly to oxidative bursts involved in pathogen defence (Verslues and Sharma, 2010). Moreover, proline-based ROS production shows links with the functioning of plasma-membrane-localized respiratory burst NADPH oxidase homologue D (RBOHD) (Fabro et al., 2016). Under conditions of biotic stress, RBOHD provides apoplastic ROS involved in oxidative bursts and in pathogen-triggered immunity (Fabro et al., 2016). Weaker immune responses and a decrease in ROS generation by RBOHD were observed in prodh mutants, and ProDH activation or inhibition treatments showed that ProDH activity modulated RBOHD activity (Fabro et al., 2016). Given the importance of RBOHD for systemic ROS and Ca2+ wave signalling through the plant (Gilroy et al., 2014), the relationship between ProDH, mitochondrial ROS production and RBOHD shows potential links between the stress status of mitochondria and systemic signalling. Moreover, the ProDH/PC5CDH pathway is involved in the regeneration of glutamate and may thus contribute to the dynamics of the glutamate pool (Schertl and Braun, 2014). Glutamate has been associated with signalling processes involving glutamate-receptor-like channels (Forde and Roberts, 2014; Toyota et al., 2018) and with systemic Ca2+ responses to wounding and pathogens (Forde and Roberts, 2014; Hilleary and Gilroy, 2018; Toyota et al., 2018). Glutamate may thus mediate another level of interaction between mitochondrial proline dynamics and systemic signalling.
On the other hand, involvement in senescence processes (Cecchini et al., 2011; Zhang and Becker, 2015) strongly suggests that the roles of proline-based ROS production are much wider than the biotic stress context. Overexpression of heat-shock factors has thus been shown to improve heat tolerance and to increase salt sensitivity through alterations of ProDH activity and proline catabolism (Wu et al., 2018). Moreover, the regulation of ProDH gene expression under conditions of abiotic (Wu et al., 2018) or biotic (Monteoliva et al., 2014) stresses indicates that control of mitochondrial proline catabolism may be important for stress acclimation.
Dynamics of electron fluxes and nitric oxide
Nitric oxide (NO) has been shown to be involved as a reactive nitrogen species (RNS) and through its signalling properties in multiple mechanisms and physiological processes from seed germination to flowering and senescence, whether under optimal conditions or under conditions of abiotic and biotic stress (Gupta et al., 2011). Nitric oxide can reach and affect multiple targets in different subcellular compartments. At protein level, NO has three main modalities of action (Fig. 1): S-nitrosylation, metal-nitrosylation and tyrosine nitration (Besson-Bard et al., 2008). S-Nitrosylation consists in the addition of an NO group to cysteine residues. Targeted proteins are connected to a wide range of functions, such as metabolism, signalling, stress responses, redox homeostasis and cellular architecture (Astier et al., 2011). S-Nitrosylation can alter transcription factors, such as MYB or group VII ethylene response factors (ERFs) (Dubos et al., 2010; Bailey-Serres et al., 2011; Gibbs et al., 2015), thus leading to differential gene expression, which shows the importance of NO as a signalling molecule. Metal-nitrosylation affects major proteins such as haemoglobins, aconitases, lipoxygenases, catalases, cytochrome c oxidase and ascorbate peroxidases by binding to the haem structure (Besson-Bard et al., 2008). Tyrosine nitration results from the reactivity of peroxynitrite (ONOO−), which leads to the addition of a nitronium (NO2+) group to tyrosine residues and generally to loss of protein function (Besson-Bard et al., 2008; Astier et al., 2011). Through these multiple biochemical actions, NO is also connected to other secondary messengers, such as Ca2+. Elicitation of tobacco cells induces the mobilization of intracellular Ca2+ through modifications of Ca2+ channels by NO (Lamotte et al., 2004).
Mitochondria play a major role in NO synthesis and dynamics and therefore in the control of NO effects on cellular functions (Fig. 1). Whereas the existence of a mitochondrial isozyme of NO synthase is not established, mitochondria possess a wide range of NO sources (Moreau et al., 2008). Nitric oxide can be produced at different steps of the ETC (Castello et al., 2006; Gupta and Igamberdiev, 2011; Alber et al., 2017). Complex III is involved in NO production by nitrite reduction (Alber et al., 2017). Complex IV cytochrome c oxidase (COX), especially under conditions of anoxia or hypoxia, shows significant nitrite reductase activity and can thus represent one of the most important sources of NO in the cell (Castello et al., 2006). Finally, ETC inhibition studies have suggested that AOX may also carry out reduction of nitrite to NO (Planchet et al., 2005). Moreover, the work of Vishwakarma et al. (2018) indicates that such AOX-dependent NO production preferentially occurs under anoxia or hypoxia conditions. Nitric oxide plays an important role at the mitochondrial ETC level, as it can inhibit OXPHOS (Yamasaki et al., 2001) and COX (Vieira and Kroemer, 2003; Amirsadeghi et al., 2007). Inhibition of COX leads to an increase in O2 concentration in the vicinity of COX (Vieira and Kroemer, 2003; Amirsadeghi et al., 2007). A balance between NO and O2 therefore exists in the mitochondria and may act as a hypoxic signal (Fig. 1), involving several classes of transcription factors, such as MYB transcription factors and group VII ERFs (Castello et al., 2006; Bailey-Serres et al., 2011; Gibbs et al., 2015). As soon as COX produces NO, complete depletion of O2 in the mitochondria is avoided, and NO production triggers the induction of hypoxia-responsive genes. Alternatively, NO can react with other ROS, thus leading to the formation of peroxynitrite, which can trigger its own regulatory pathways (Gupta and Igamberdiev, 2011). This balance between NO, O2 and peroxynitrite plays important roles not only in abiotic stresses, such as hypoxia, but also in biotic stresses. Under conditions of biotic stress, oxidative bursts consume O2 in the vicinity of COX, which leads to NO synthesis and activation of the HR (Modolo et al., 2005) and induction of pathogenesis-related genes (Parani et al., 2004). Moreover, the balance between NO, O2 and peroxynitrite results not only from NO synthesis mechanisms, but also from NO scavenging systems, some of which are mitochondrial (Fig. 1). Complexes I and III of the mitochondrial ETC are important producers of the anion superoxide (Andreyev et al., 2015). Peroxynitrite that results from NO and superoxide reaction can be scavenged by specific mechanisms (Radi et al., 2002). A potential NO-scavenging mechanism involving superoxide formation and Ca2+-dependent external NAD(P)H dehydrogenases has also been reported (de Oliveira et al., 2008). Finally, since NO can bind to and inhibit COX, and since S-nitrosylation can be passive, mitochondrial structures are an important pool of immobilized NO. The respective activities of all of these mechanisms of synthesis, immobilization and scavenging contribute to maintain NO at a setpoint that prevents NO-dependent nitrosative damage.
Alternative oxidase as an ROS and RNS signalling rheostat
The AOX terminal oxidase of plant mitochondria (Elthon and McIntosh, 1987) exists as different isoforms encoded by a small multigene family that is differentially regulated (Ho et al., 2008; Giraud et al., 2009; Hanqing et al., 2010). Unlike the COX pathway, AOX does not pump protons and therefore does not contribute to the proton motive force (McIntosh, 1994; Finnegan et al., 2003; Millenaar and Lambers, 2003). On the other hand, by preventing over-reduction of ETC, AOX can regulate the generation of ROS (Maxwell et al., 1999), especially under stress conditions (Vanlerberghe et al., 2009). As an example, in the case of heavy metal stress, overexpression of AOX facilitates stress tolerance, whereas wild-type plants are affected by reduced respiration, increased ROS production and decreased cell viability (Liu et al., 2014).
Various signals can modulate AOX expression. Besides its inhibitory effects on mitochondrial COX (Vieira and Kroemer, 2003; Amirsadeghi et al., 2007), NO increases the expression of AOX (Huang et al., 2002; Gupta et al., 2012). Jasmonate, ethylene and salicylate induce the increase in AOX transcription (Fung et al., 2006; Wang et al., 2010; Zhang et al., 2012). Expression of AOX1 can also be induced by ABA and hydrogen peroxide in Arabidopsis (Ho et al., 2008; Giraud et al., 2009). Cvetkovska and Vanlerberghe (2012a, 2013) highlight that, according to the nature of the pathogen, Nicotiana tabacum is able to regulate differentially the expression of AOX, thus modulating the production of mitochondrial ROS, the mitochondrial oxidative burst and therefore HR. Pseudomonas syringae pv. maculicola triggers an HR reaction with no parallel induction of AOX. In contrast, P. syringae pv. phaseolica induces pathogen defences without HR in parallel with an increase in AOX and MnSOD proteins. The plant is thus able to downregulate AOX despite the presence of AOX-inducing regulators (Cvetkovska et al., 2013). Moreover, AOX seems to play different roles depending on the type of pathogen. Silencing of AOX causes increased susceptibility of Nicotiana attenuata to a piercing–sucking insect and accelerated cell death against pathogenic P. syringae pv. tomato, but this AOX regulation was not involved in responses to attacks by chewing herbivores (Zhang et al., 2012). Given its implications in ETC functioning (McIntosh, 1994; Finnegan et al., 2003; Millenaar and Lambers, 2003) and in NO production (Planchet et al., 2005), and given its stress-dependent regulation (Ho et al., 2008; Giraud et al., 2009), AOX may therefore be considered as an ETC-related rheostat for mitochondrial ROS and RNS dynamics and therefore for mitochondrial ROS and RNS signalling (Cvetkovska and Vanlerberghe, 2012b; Kumari et al., 2019). More generally, as shown by mitochondrial biogenesis mutants (Yang et al., 2014; Zhang et al., 2014; Dolzblasz et al., 2018), the developmental and hormonal context controls and determines the level of expression of mitochondrial components, such as ETC components, thus specifying a setpoint for the capacity to generate ROS and RNS.
SIGNALLING DYNAMICS OF MITOCHONDRIAL SUPRAMOLECULES
Proteome and metallome dynamics
The abundance of metal-containing proteins in the ETC implies that mitochondria possess an important metallome, including in particular Cu and Fe (Tan et al., 2010; Jacoby et al., 2012). Under conditions of direct or indirect oxidative stress, metal-containing proteins are affected in their conformation, in their electrophilicity, in protein–protein interactions and in protein–membrane interactions. Oxidative-stress-mediated damage can thus trigger the release of metals from mitochondrial proteins (Fig. 2). Metal release can directly affect biological activity and therefore ETC and mitochondrial homeostasis in relation to the ROS-amplifying loop described above (Amirsadeghi et al., 2007; Tan et al., 2010). Excess Cu or Fe has thus been shown to stimulate ROS production in mitochondria and affect mitochondrial respiration (Keunen et al., 2011). Moreover, released metals can act as the catalysts of metal-catalysed oxidation affecting oxidation-prone amino acid residues, such as arginine, lysine, proline, cysteine and histidine, in susceptible proteins (Møller et al., 2011; Jacoby et al., 2012), with potential triggering of the mitochondrial dysfunction/ROS-amplifying loop. Resulting damaged proteins tend to aggregate, which again is likely to disturb mitochondrial homeostasis (Jacoby et al., 2012). Finally, the release of metal catalysts in an oxidative environment including superoxide and hydrogen peroxide activates the Haber–Weiss reaction or the Fenton reaction, which lead to hydroxyl radical (HO.) production (Mittler et al., 2004). This highly reactive compound adds another level to ROS damage (Fig. 2) that can affect lipids and proteins and contribute to the ROS- and metal-amplifying loops described above.
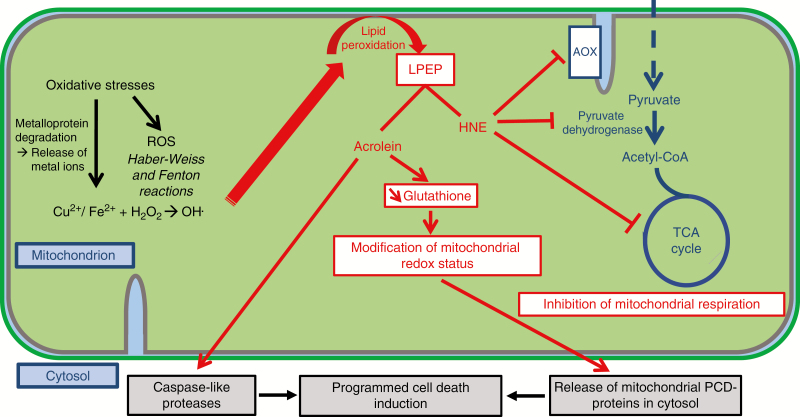
Fig. 2.
Mechanisms of mitochondrial oxidative stress sensing through metallome and lipid peroxidation dynamics. Oxidative-stress-mediated damage triggers the release of metals from mitochondrial proteins and the released metals are the catalysts of metal-catalysed oxidation (Møller et al., 2011; Jacoby et al., 2012). Cascades of reactions lead to the formation of lipid peroxidation products, such as acrolein and 4-hydroxy-2-nonenal (HNE), with wide-ranging effects on metabolism, redox status and programmed cell death (Winger et al., 2005, 2007; Mano, 2012; Biswas and Mano, 2015, 2016). LPEP, lipid peroxidation end products.
Lipid peroxides and reactive carbonyl species
The hydroxyl radical described above (Fig. 2) is a strong inducer of lipid peroxidation and therefore tightly linked to membrane dynamics. Resulting lipid peroxides or lipid peroxidation end products are reactive carbonyl species (RCS) with highly electrophilic groups that can actively react with other molecules as they are negatively charged (Møller et al., 2011; Jacoby et al., 2012). Cascades of reactions lead to the formation of derived RCS, such as acrolein and 4-hydroxy-2-nonenal (HNE) (Mano, 2012). Acrolein and HNE can form Michael adducts on thiol and amino groups of proteins (Mano, 2012); HNE can thus inhibit several enzymes involved in TCA cycle and respiration, such as malate dehydrogenase, α-ketoglutarate dehydrogenase and pyruvate dehydrogenase (Fig. 2). HNE also has an inhibitory impact on ETC proteins, including AOX (Winger et al., 2005, 2007). However, through signalling mechanisms, this inhibition can also induce the activation of AOX gene expression and AOX protein synthesis, in parallel with the induction of other stress-related genes (Winger et al., 2007). Finally, HNE can modify, through formation of Michael adducts, the interactions between glyceraldehyde-3-phosphate dehydrogenase and mitochondria (Winger et al., 2007).
Mitochondria are therefore directly involved in cascades of biochemical events that link environmental constraints acting on mitochondrial ETC functioning (Amirsadeghi et al., 2007), ROS and metal dynamics (Jacoby et al., 2012), RCS (Mano, 2012) and post-translational modifications of cell functioning, including further disruption of mitochondrial respiration (Winger et al., 2005, 2007). Such cascades of events involving RCS can lead to PCD regulation. Accumulation of acrolein can activate caspase-like proteases, which play a major role in the induction of PCD (Biswas and Mano, 2015, 2016). Acrolein increase also leads to decrease in glutathione, which modifies the redox status of mitochondria. The altered redox status and inactivation of pyruvate dehydrogenase (Fig. 2) can affect mitochondrial membranes through formation of pores through which PCD-signalling proteins can exit the mitochondrion (Biswas and Mano, 2015).
Finally, the impact of RCS such as HNE on differential gene expression indicates that RCS could be involved in ROS signalling and act as retrograde signals from the mitochondria to the nucleus (Møller and Sweetlove, 2010; Schwarzländer and Finkemeier, 2013). Further work would be required to determine whether this RCS retrograde signalling includes direct biochemical modifications of DNA.
Cardiolipin and cytochrome c
Functioning of mitochondrial ETC and OXPHOS requires correct protein–protein and protein–membrane interfaces. As a small haem protein located in the mitochondrial intermembrane space and carrying electrons between complex III and complex IV, cytochrome c plays important roles in these interfaces. Moreover, differential expression of cytochrome c-encoding genes under conditions of abiotic (cold, heat, UV-B) and biotic stress suggests that cytochrome c dynamics is involved in stress responses (Welchen et al., 2009; Welchen and Gonzalez, 2016). In particular, cytochrome c plays a major role in PCD responses, through triggering pro-apoptotic routes and inhibiting pro-survival factors (Kobylińska et al., 2017). The mechanisms of cytochrome c involvement in apoptosis have been thoroughly described in mammalian cells (Kagan et al., 2009), but the mechanisms of its implication in plant PCD remain to be fully elucidated.
Plant responses to pathogens (Krause and Durner, 2004) and to abiotic stresses, such as heat stress (Vacca et al., 2006), have been associated with release of cytochrome c from the outer face of the inner mitochondrial membrane into the cytoplasm and triggering of PCD (Fig. 3). This release occurs in two steps, consisting in initial dissociation from cardiolipin followed by formation of the permeability transition pore in the outer mitochondrial membrane. Cytochrome c interacts electrostatically with cardiolipin, which anchors it to the outer face of the mitochondrial inner membrane (Vacca et al., 2006; De Paepe et al., 2014). Arabidopsis mutants lacking cardiolipin show an increased sensitivity to PCD induction (Pineau et al., 2013; De Paepe et al., 2014). Moreover, mammalian cell studies suggest that several post-translational modifications of cytochrome c play a role in its interaction with cardiolipin. Thus, nitration can trigger cytochrome c degradation (Díaz-Moreno et al., 2011). As described above, under conditions of mitochondrial dysfunctioning or damage, decrease in ATP production can lead to depletion of ATP levels and increase in ADP and AMP (Bailey-Serres et al., 2011; Pedrotti et al., 2018). In the case of mammalian cells, it has been shown that such ATP depletion can cause cytochrome c dephosphorylation, which in turn can induce cardiolipin peroxidation and the detachment of cytochrome c from the inner mitochondrial membrane (Yu et al., 2008; Kagan et al., 2009; Sanderson et al., 2013).
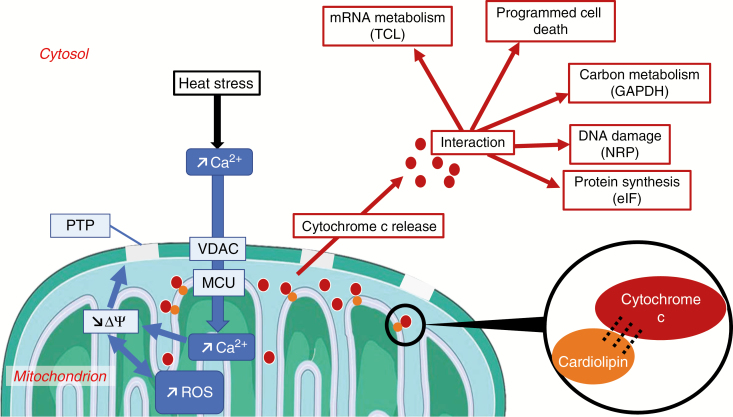
Fig. 3.
Mechanisms of cytochrome c release in mitochondria under heat stress conditions. Increase in cytosolic Ca2+ can cause Ca2+ influx into the mitochondria through the outer and inner mitochondrial membranes, with the involvement of voltage-dependent anion channels (VDAC) and of the mitochondrial calcium uniporter (MCU) (Li et al., 2013; Rikhvanov et al., 2014; Wagner et al., 2015, 2016). Ca2+ influx into the matrix can lead to dissipation of the transmembrane potential and ROS production, thus enabling the formation of permeability transition pores (PTP) and cytochrome c release (Gao et al., 2008; Vianello et al., 2012; Zancani et al., 2015). Cytochrome c release involves mechanisms of destabilization of cytochrome c–cardiolipin interactions (Vacca et al., 2006; Pineau et al., 2013; De Paepe et al., 2014). In the cytosol, cytochrome c interactions can affect nucleic acid and protein dynamics, carbon metabolism and regulation of programmed cell death (Martínez-Fábregas et al., 2013). Blue and red arrows respectively indicate mitochondrial and cytochrome c-dependent processes. Δψ, transmembrane potential; eIF, translation initiation factor; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; NRP, nucleosome assembly protein-related protein; TCL, transcriptional coactivator-like.
Heat stress responses also show a link between cytochrome c release and Ca2+ dynamics. Heat stress induces increases in cytosolic and mitochondrial Ca2+ concentrations that are mediated by influxes from apoplastic and intracellular compartments (Rikhvanov et al., 2014). Among several channels and transporters of Ca2+, voltage-dependent anion channels in the outer mitochondrial membrane and the mitochondrial calcium uniporter in the inner mitochondrial membrane are involved in Ca2+ influx into mitochondria (Li et al., 2013; Rikhvanov et al., 2014; Wagner et al., 2015, 2016). This accumulation of Ca2+ in the mitochondrial matrix as well as ROS formation can induce a decrease in the size of the transmembrane electrical potential (Gao et al., 2008), which is linked to the formation of permeability transition pores (Vianello et al., 2012; Zancani et al., 2015). Altered redox status can also affect mitochondrial membranes through formation of pores (Biswas and Mano, 2015). In other words, release of cytochrome c from the outer face of the inner mitochondrial membrane occurs in a context of permeabilization of the outer mitochondrial membrane that promotes the release of cytochrome c and other factors into the cytosol (Petrosillo et al., 2003; Vianello et al., 2012; Zancani et al., 2015).
In the cytosol, cytochrome c can interact with several partners (Martínez-Fábregas et al., 2013): a nucleosome assembly protein-related protein involved in DNA dynamics, a transcriptional coactivator-like protein involved in mRNA metabolism, a translation initiation factor, a glyoxylase involved in oxidative stress, glyceraldehyde-3-phosphate dehydrogenase, and a cysteine protease involved in PCD. Thus, sequences of events that lead to the release of cytochrome c can be initiated (Figs 1–3) by ETC dysfunction, ATP depletion and phosphorylation dynamics, oxidative processes, NO dynamics, metal ion dynamics, glutathione and redox status, lipid peroxidations or Ca2+ dynamics. Such perturbations and different combinations thereof are known to occur in response to a variety of environmental and climate change stresses, such as hypoxia (Bailey-Serres et al., 2011), carbohydrate limitation (Pedrotti et al., 2018), heat (Vacca et al., 2006; Rikhvanov et al., 2014), salinity (Biswas and Mano, 2015) or pathogenic toxins (Krause and Durner, 2004). On the other hand, given the diversity of the retrograde targets of cytochrome c (Fig. 3), it can be speculated that variable combinations of triggering events, variable intensities of cytochrome c release, and variable levels of targets, depending on cell type and developmental stage, can yield different outcomes (regulation, survival or PCD) (Vianello et al., 2012; Welchen and Gonzalez, 2016).
MITOCHONDRIAL CROSSTALK SIGNALLING AND INTERORGANELLAR STRESS INTEGRATION
Transcriptional and post-translational interorganellar stress integration
The different mechanisms of mitochondria–nucleus retrograde signalling (Schwarzländer and Finkemeier, 2013; Ashrafi et al., 2018; Wagner et al., 2018) are essential for the coordination of stress responses in eukaryotic cells (De Souza et al., 2017; Crawford et al., 2018). Moreover, in the plant cell the activities of mitochondria and plastids must be tightly coordinated in order to integrate stress-related carbon, energy and redox requirements (Crawford et al., 2018). This coordination includes the multiple sensing and signalling activities associated with mitochondria and plastids (Foyer and Noctor, 2003). The importance of mitochondria–nucleus and chloroplast–nucleus retrograde signalling emphasizes the coordinating role of nuclear transcriptional regulation (Crawford et al., 2018). However, the timescale of transcriptional responses suggests that rapid stress acclimation must also involve post-translational crosstalk between mitochondria and chloroplasts.
Gene expression regulation
Chloroplasts and mitochondria present distinct sets of retrograde signals. Chloroplast retrograde signalling is mediated by ROS and metabolites including β-cyclocitral, 2-C-methyl-d-erythritol-2,4-cyclodiphosphate (MecPP), 3′-phosphoadenosine 5′-phosphate (PAP) and intermediates of the tetrapyrrole biosynthesis pathway (Crawford et al., 2018). The retrograde signals of mitochondria include energy metabolites, cytochrome c, ROS and PAP (Møller and Sweetlove, 2010; Møller, 2016; de Souza et al., 2017; Van Aken and Pogson, 2017; Waszczak et al., 2018). Stress conditions modulate the levels of all of these retrograde signals, including ROS and PAP, which are common to chloroplasts and mitochondria (Estavillo et al., 2011; Chan et al., 2016a, b).
Stress-related perturbations of the mitochondrial ETC are associated with superoxide and hydrogen peroxide accumulation (Basu et al., 2001; Dixit et al., 2002; Bienert et al., 2007). Longer half-life allows hydrogen peroxide to move into the cytosol (Bienert et al., 2007). Hydrogen peroxide can interact with the endoplasmic reticulum and induce post-translational modifications of ARABIDOPSIS TRANSCRIPTION ACTIVATION FACTOR/CUP-SHAPED COTYLEDON (ANAC) transcription factors ANAC013 and ANAC017 (De Clercq et al., 2013; Ng et al., 2013; Meng et al., 2019). Hydrogen peroxide-induced modifications are associated with the release of transcription factors from the endoplasmic reticulum. ANAC017 has been shown to contain, in addition to its C-terminal transmembrane region, a rhomboid protease cleavage site that needs to be cleaved to induce transcription factor release from the endoplasmic reticulum (Ng et al., 2013). Action of ANAC017 and ANAC013 on the nuclear genome activates a wide range of genes. ANAC017 induces the expression of AOX1a, ANAC013, WRKY, DRE2B and RAP2 genes (Ng et al., 2013). ANAC013 interacts with mitochondrial dysfunction domain-containing promoters of mitochondrial dysfunction genes, such as AOX1a (De Clercq et al., 2013). Besides ANAC013 and ANAC017, WRKY15/WRKY40/WRKY63 (Vanderauwera et al., 2012; Van Aken et al., 2013) and ABSCISIC ACID INSENSITIVE4 (ABI4) (Giraud et al., 2009) transcription factors have been identified as additional downstream regulators of mitochondrial retrograde signalling. Moreover, ANAC017, WRKY40 and ABI4 are also involved in chloroplast retrograde signalling (Koussevitzky et al., 2007; Shang et al., 2010; Van Aken et al., 2013, 2016), thus demonstrating the convergence of retrograde signalling cascades between mitochondria and chloroplasts (Schwarzländer and Finkemeier, 2013; Wagner et al., 2018). The involvement of ANAC017 in both mitochondrial and chloroplast retrograde signalling suggests the possible convergence of mitochondria-derived (Møller, 2016) and chloroplast-derived (Maruta et al., 2012) hydrogen peroxide signals. Differential gene induction under different modalities of hydrogen peroxide production and of abiotic stresses (Maruta et al., 2012; Ng et al., 2013; Van Aken et al., 2016), however, indicates that hydrogen peroxide-dependent convergence is a complex regulatory process rather than the result of mere signalling overlapping.
3′-Phosphoadenosine 5′-phosphate originates from the cytosolic degradation of 3′-phosphoadenosine 5′-phosphosulphate (Bohrer et al., 2015), and can accumulate in mitochondria and chloroplasts. In chloroplasts, its levels are controlled by the activity of SAL1 PAP phosphatase, which catalyses its conversion into AMP and Pi (Estavillo et al., 2011). Under stress conditions, increased levels of hydrogen peroxide decrease SAL1 activity, thus resulting in higher levels of PAP, which can be exported from the chloroplast to the cytosol and the nucleus (Chan et al., 2016a, b). Besides its localization in chloroplasts, SAL1 undergoes alternative targeting to mitochondria (Estavillo et al., 2011; Van Aken and Pogson, 2017). The combination of PAP accumulation, SAL1 targeting and ROS dynamics is likely to be the basis for the involvement of PAP dynamics in mitochondria–cytosol–nucleus communication, but the export of PAP from mitochondria to the nucleus remains to be characterized. PAP is involved in the transcriptional regulation of stress-response genes (Estavillo et al., 2011). Moreover, within the nucleus, PAP inhibits the RNA-degrading activity of 5′-3′exo-ribonucleases, thus resulting in a regulation of RNA processing (Estavillo et al., 2011). Interestingly, PAP and ANAC017 are involved in overlapping retrograde signalling pathways that control similar sets of genes (Van Aken and Pogson, 2017). Moreover, PAP, ANAC013 and ANAC017 signalling pathways are all negatively regulated by the transcriptional hub protein RADICAL-INDUCED CELL DEATH1 (Waszczak et al., 2018). As is the case for hydrogen peroxide signalling, these relationships highlight the interactions between mitochondrial and chloroplastic signalling pathways.
Glutathione post-translational interactions
Mitochondria and chloroplasts are connected by biochemical links and physical contacts (Foyer et al., 2009; Pérez-Sancho et al., 2016), which may be the basis for post-translational interorganellar crosstalk, as in the case of glutathione. Glutathione is synthesized in the cytosol and chloroplasts (Noctor et al., 2012). Glutathione is then distributed between the cytosol, chloroplasts, mitochondria and peroxisomes (Zechmann et al., 2008; Noctor et al., 2012), thus reflecting the importance of glutathione production and allocation within the plant cell. The ATP requirement for glutathione synthesis (Noctor et al., 2012) may be modulated by ATP availability from mitochondria and chloroplasts, in relation to metabolic energy sensing (Baena-González and Sheen, 2008; Janse van Rensburg et al., 2019). Glutathione is mostly found in its reduced form (GSH) which is oxidized by ROS, including hydrogen peroxide, to its disulphide form (GSSG). GSH can then be regenerated from GSSG by reductive processes. These interconversions of GSH and GSSG are carried out by sets of cytosolic, chloroplastic and mitochondrial enzymes (Mittler et al., 2004). Glutathione is therefore important for the control of ROS and redox homeostasis, at the overall plant cell level (Schwarzländer et al., 2008; Noctor et al., 2012; Attacha et al., 2017) and in the mitochondria (Schwarzländer et al., 2008; Passaia et al., 2013; Attacha et al., 2017). Reciprocal changes of the GSH:GSSG ratio reflect the dynamics of ROS production, which, in the case of mitochondria, is tightly linked to ETC and OXPHOS functioning (Basu et al., 2001; Dixit et al., 2002; Turrens, 2003). The highly reduced glutathione redox potential (EGSH) that derives from the GSH:GSSG ratio (Meyer et al., 2007; Meyer, 2008; Schwarzländer et al., 2008) can therefore act as a sensitive sensor of oxidative homeostasis and stress, and of ETC and OXPHOS functioning, in the mitochondria. Global accumulation of GSSG has been shown to induce an increase in total glutathione in the cell both through gene expression regulation and through post-translational effects (Noctor et al., 2012). Decrease in GSH concentration in mitochondria and modifications of mitochondrial redox status are related to induction of PCD processes (Fig. 2). Because of interconnections of glutathione synthesis and allocation within the plant cell, it could also be hypothesized that the fluctuations of glutathione in the mitochondria could induce glutathione-related responses in the cytosolic and plastidial compartments.
Through glutathionylation, which forms a disulphide bond between GSH and cysteine residues, GSH is able to mediate post-translational modifications of proteins (Noctor et al., 2012; Zaffagnini et al., 2012). This post-translational modification prevents permanent oxidation of protein thiols by ROS and changes protein conformation, thus affecting protein dynamics and activity (Zaffagnini et al., 2012). Important targets of glutathionylation include proteins that are found both in mitochondria and in chloroplasts, including the redox regulatory proteins thioredoxins (TRX) and peroxiredoxins (PRX) (Rouhier et al., 2008; Martí et al., 2011; Liebthal et al., 2018; Thormählen et al., 2018). Thioredoxins are disulphide reducers widely involved in redox signalling and ROS protection (Geigenberger et al., 2017). Although mostly described in plastids, TRX systems are also present in mitochondria (Balmer et al., 2004; Martí et al., 2011; Yoshida et al., 2013; Daloso et al., 2015; Geigenberger et al., 2017). Thus, in pea leaves, the mitochondrial TRXo1 isoform is involved in protection from oxidative stress (Martí et al., 2011). Peroxiredoxins are thiol-peroxidases acting in antioxidant mechanisms and redox signalling (Liebthal et al., 2018). Like TRXs, PRXs are cysteine proteins that can undergo glutathionylation. Hyperoxidation of plastidial PRX leads to inactivation of peroxidase activity, thus resulting in hydrogen peroxide increase (Liebthal et al., 2018). Thioredoxins and PRXs interact with and potentially regulate myriad target proteins related to carbon, nitrogen and ATP metabolisms, including carbon assimilation, mitochondrial respiration and AOX (Geigenberger et al., 2017; Liebthal et al., 2018). This complexity of interactions and their localization in both mitochondria and plastids suggest that TRXs and PRXs play an important role at the interface between mitochondria and chloroplasts (Geigenberger et al., 2017). The crosstalk that is required for integrative functioning of TRXs and PRXs between mitochondria and chloroplasts has been ascribed to various metabolites, such as NAPDH, dihydroxyacetone phosphate, malate and glycolate (Balmer et al., 2004; Geigenberger et al., 2017). However, through its interactions with ETC functioning and through glutathionylation, glutathione may also be an important post-translational signal between mitochondria and chloroplasts.
Ascorbate post-translational interactions
Ascorbate is widely present in the plant cell, in the cytosol, in chloroplasts and in mitochondria (Smirnoff and Wheeler, 2000; Smirnoff, 2018). It is a key antioxidant molecule for detoxifying ROS produced under stress conditions (Smirnoff, 2018). Biosynthesis in plant cells involves the d-mannose/l-galactose (Smirnoff–Wheeler) pathway, where the final step consists in the oxidation of l-galactono-1,4-lactone to L-ascorbate (Smirnoff, 2018). While the initial steps occur in the cytosol, the last step is specifically carried out by l-galactono-1,4-lactone dehydrogenase (GalDH) associated with complex I of the mitochondrial ETC (Bartoli et al., 2000; Smirnoff and Wheeler, 2000; Schertl et al., 2012; Venkatesh and Park, 2014; Smirnoff, 2018). This association of ascorbate synthesis with the mitochondrial ETC is both structural and functional. GalDH forms part of subcomplexes of complex I and GalDH activity donates electrons to the ETC (Bartoli et al., 2000; Schertl et al., 2012; Smirnoff, 2018). From the site of production at the outer face of the inner mitochondrial membrane, ascorbate must then be allocated to the different cell compartments (Smirnoff, 2018).
Ascorbate is directly involved in antioxidant defence and ROS scavenging in all the different compartments where it is found (Smirnoff, 2018). Removal of hydrogen peroxide relies on ascorbate peroxidases catalysing the oxidation of ascorbate to monodehydroascorbate and reduction of hydrogen peroxide to H2O. The unstable monodehydroascorbate yields dehydroascorbate. Reduction of monodehydroascorbate and dehydroascorbate to ascorbate is carried out by monodehydroascorbate reductases and GSH-dependent dehydroascorbate reductases (Mittler et al., 2004). Oxidized glutathione (GSSG) is recycled to GSH through NADPH-dependent glutathione reductases, thus sustaining the ascorbate–glutathione cycle (Noctor and Foyer, 1998; Mittler et al., 2004).
Mitochondria are the site of active ascorbate dynamics through direct involvement in its synthesis providing the necessary pool for subcellular allocation and through utilization of the ascorbate–glutathione cycle for removal of hydrogen peroxide, whose production is tightly linked to ETC and OXPHOS functioning (Basu et al., 2001; Dixit et al., 2002; Turrens, 2003). This ascorbate dynamics is thus linked to various mitochondrial signals that have been described above, such as hydrogen peroxide and glutathione. The influence of this dynamics on allocation of ascorbate to the chloroplasts may also have an impact on chloroplast-derived signals such as hydrogen peroxide (Estavillo et al., 2011; Chan et al., 2016a, b). Moreover, ascorbate itself is thought to play a signalling role in cell division and embryo development (Gallie, 2013).
Stress-related perturbations are bound to act on mitochondrial ascorbate dynamics through their effects on mitochondrial ETC and on superoxide and hydrogen peroxide accumulation (Basu et al., 2001; Dixit et al., 2002; Bienert et al., 2007). Mitochondrial ascorbate dynamics is therefore likely to be involved in signalling interactions between stresses and plant cell acclimation. Moreover, the d-mannose/l-galactose pathway of ascorbate synthesis in the mitochondria is regulated by a number of stress-related mechanisms, involving activation by high light, ethylene and ROS treatment and inhibition by prolonged darkness (Wang et al., 2013). Such mechanisms suggest that maintenance or adjustment of the ascorbate pool size may be important for stress acclimation, whether in terms of biochemical action or in terms of signalling impact (Stevens et al., 2018).
MITOCHONDRIAL PROCESSING AND DYNAMICS OF STRESS HORMONE INFORMATION
Under conditions of abiotic or biotic stress, mitochondrial functioning occurs in a cellular context where stress regulators and hormones, such as ABA, cytokinins, ethylene, salicylate and jasmonate, accumulate (Koornneef and Pieterse, 2008; Wang et al., 2010; Verslues, 2016; Zhu, 2016), which can directly interact with mitochondrial functions. At low concentrations, salicylate acts as an uncoupling agent, but at higher concentrations, salicylate can act on complexes I and III of the mitochondrial ETC (Norman et al., 2004; Czarnocka and Karpinski, 2018). Different molecular forms of cytokinins and of cytokinin analogues can interfere with mitochondrial respiration (Miller, 1982; Alberto et al., 2017). ABA may interfere with ATP/ADP exchanges between mitochondria and cytosol transport through inhibition of mitochondrial adenine nucleotide translocators (ANTs) (Kharenko et al., 2011; Berkowitz et al., 2016). The affinity of ANTs for ATP is higher than their affinity for ABA, but under stress conditions increased ABA levels may inhibit ADP/ATP exchange (Kharenko et al., 2011), thus leading to decreases in ATP transfer to the cytosol or of ADP replenishment to the mitochondria (Fig. 4). This direct link between ABA and ATP dynamics was characterized by in vitro studies (Kharenko et al., 2011). Its relevance to in vivo cell functioning, which must depend on the relative in vivo concentrations of ATP, ADP and ABA, remains to be validated.
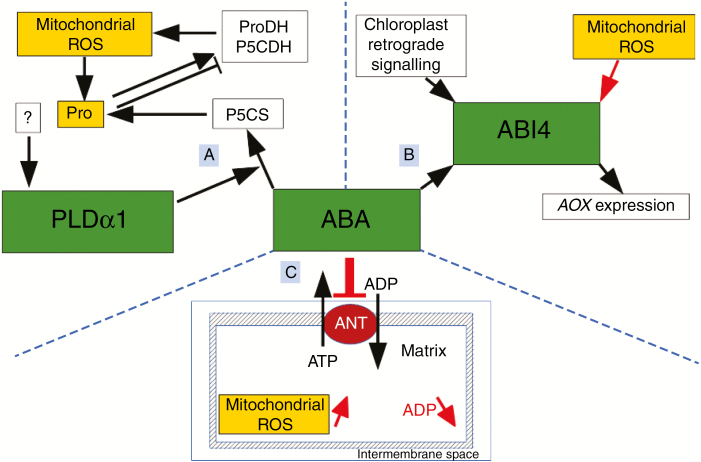
Fig. 4.
Signalling interactions between ABA and mitochondrial ROS. (A) Under salt stress conditions, proline (Pro) and mitochondrial ROS are involved in regulatory loops involving ABA pathway phospholipase Dα1 (PLDα1) (Pokotylo et al., 2012; Garcia de la Garma et al., 2015). Depending on stress conditions, proline dehydrogenase (ProDH) can be downregulated (Verbruggen and Hermans, 2008) or upregulated (Verslues et Sharma, 2010; Garcia de la Garma et al., 2015) by proline. Phospholipase Dα1 appears to be regulated by stress-related factors that remain to be identified (Garcia de la Garma et al., 2015). (B) The ABA-insensitive-4 (ABI4) transcription factor integrates mitochondrial retrograde signals, in particular ETC-derived ROS (Schwarzländer and Finkemeier, 2013; Waszczak et al., 2018), with chloroplast retrograde signals and ABA signalling (Koussevitzky et al., 2007; Giraud et al., 2009; Zhang et al., 2013). (C) According to in vitro studies, ABA may act on ATP/ADP and ROS dynamics through inhibition of mitochondrial adenine nucleotide translocator (ANT) (Kharenko et al., 2011; Berkowitz et al., 2016). Decrease in ADP availability in mitochondria is associated with the activation of ROS production (Basu et al., 2001; Møller, 2001; Dixit et al., 2002; Li et al., 2003; Turrens, 2003). P5CS, pyrroline-5-carboxylate synthetase; P5CDH, pyrroline-5-carboxylate dehydrogenase.
Decrease in ADP availability in mitochondria leads to accumulation of electrons in the ETC (Møller, 2001). All of these interactions, with mitochondrial respiration or with ATP/ADP dynamics, are therefore associated with the activation of ROS production (Basu et al., 2001; Møller, 2001; Dixit et al., 2002; Li et al., 2003; Turrens, 2003). Enhanced ROS production in the mitochondria is associated with transcriptional and post-translational regulation, with hydrogen peroxide retrograde signalling, with membrane permeability modifications and cytochrome c release (Fig. 3), and with PCD induction (Gao et al., 2008; Liu et al., 2014; Wu et al., 2015; Carraro and Bernardi, 2016; Cui et al., 2019). In parallel, modifications of ATP synthesis or of ATP/ADP exchange can be sensed by the SnRK1 metabolic sensor, with subsequent effects of transcriptional regulation of stress-related genes or post-translational phosphorylation regulation of key metabolic enzymes (Broeckx et al., 2016; Nukarinen et al., 2016; Wurzinger et al., 2018). Consequently, salicylate, cytokinins and ABA are likely to influence directly mitochondrial ROS signalling, interorganellar stress integration and regulation of PCD induction. Mitochondria can therefore be considered as organellar transducers of stress hormone signalling and, given the convergence of action on ROS and ATP/ADP ratios, as organellar integrators of multiple stress hormone signals.
Abscisic acid signalling is thought to be involved in feedback regulation of mitochondrial ROS signalling during salt stress (Pokotylo et al., 2012; Garcia de la Garma et al., 2015). Salt stress response is associated with induction of ROS production in mitochondria (Garcia de la Garma et al., 2015). In contrast with other stress situations, this salt-stress-induced ROS production in turn enhances proline accumulation and activation of mitochondrial ProDH and P5CDH activities (Garcia de la Garma et al., 2015). Proline accumulation and enhanced proline catabolism activate electron fluxes through the mitochondrial ETC, thus exacerbating mitochondrial ROS production (Liang et al., 2013; Schertl and Braun, 2014; Garcia de la Garma, 2015). Through unknown mechanisms, this stress-related regulatory loop appears to be connected to phospholipase D (PLD) α1 (Fig. 4), which is involved in activation of ABA signalling and enhancement of P5CS expression in relation to proline biosynthesis (Garcia de la Garma et al., 2015). On the one hand, this regulation of ROS amplification can lead to PCD (Figs 2 and 3). On the other hand, although its exact role remains to be elucidated, PLDα1 is related to enhanced salt tolerance (Garcia de la Garma et al., 2015). Moreover, besides transducing information from the canonical ABA signalling pathway (Zhang et al., 2013), the ABI4 transcription factor integrates mitochondrial and chloroplast retrograde signals (Koussevitzky et al., 2007; Giraud et al., 2009). This convergence (Fig. 4) affects in particular the regulation of mitochondrial AOX expression (Giraud et al., 2009), and is therefore related to the rheostat functions of AOX in mitochondrial ROS and RNS dynamics (Cvetkovska and Vanlerberghe, 2012b; Kumari et al., 2019). Several ETC-related mitochondrial stress signals therefore depend on pathways that mobilize stress hormone signalling mechanisms, especially ABA signalling. Conversely, such interactions can be seen as interferences with hormone-signalling pathways, and therefore as mechanisms of mitochondrial modulation of hormone signalling.
Conclusions
Plant mitochondria are endowed with a diversity of multi-directional hubs of stress signalling (Bohovych and Khalimonchuk, 2016; Welchen and Gonzalez, 2016). These mitochondrial stress signalling hubs are involved in the responses to a wide range of abiotic stresses (cold, drought, extended darkness, heat, heavy metals, high light, hydrogen peroxide, hypoxia, salinity, UV-B, xenobiotics), biotic stresses (piercing–sucking insects, Pseudomonas spp., toxins) and developmental, nutritional and hormonal perturbations (ABA, carbohydrate starvation, ethylene, jasmonate, nitrogen starvation, PCD, salicylate). Table 1 highlights the diversity of environmental stresses, signals and responses that are related to mitochondrial ETC and OXPHOS. Most of these stresses, with various degrees of frequency and intensity, play a role in the combination of climate change drivers affecting plant communities (Komatsu et al., 2019).
Table 1.
Examples of involvement of mitochondrial ETC- and OXPHOS-related signalling in environmental stress responses
| Environmental stress | Mitochondrial signals | Stress response | Reference |
|---|---|---|---|
| Drought | Proline | Redox regulation | Liang et al. (2013) |
| ABA | ATP/ADP | Not characterized | Kharenko et al. (2011) |
| Cold | Ca2+ | Not characterized | Li et al. (2013) |
| Heat | Ca2+, cytochrome c | PCD | Rikhvanov et al. (2014) |
| Heat, salinity | Redox potential | PCD regulation | Schwarzländer et al. (2009) |
| Salinity | Proline, ROS | ABA response | Garcia de la Garma et al. (2015) |
| Darkness | ATP/ADP | Alternative respiratory pathways | Pedrotti et al. (2018) |
| High light | Ascorbate | Heat-shock response | Stevens et al. (2018) |
| Hypoxia | NO | Ethylene response | Gibbs et al. (2015) |
| Experimental oxidative stress | RCS | PCD, redox regulation | Winger et al.(2005, 2007) |
| Lead | ROS, cytochrome c | PCD regulation | Kobylińska et al. (2017) |
| Chromium | ROS | Redox regulation | Dixit et al. (2002) |
| Xenobiotics | ATP/ADP | Alternative respiratory pathways | Alberto et al. (2017) |
| Piercing–sucking insects | AOX | Resistance | Zhang et al. (2012) |
| Pseudomonas spp. | AOX | Resistance | Zhang et al. (2012) |
| Flagellin | Proline | Immunity | Fabro et al. (2016) |
| Toxins | ROS | PCD | Yao et al. (2002) |
It can therefore be assumed that, as multi-stress signalling hubs related to energy and redox balance, mitochondria could function as integrators of multiple climate change signals and be involved in acclimation to climate change (Munné-Bosch et al., 2013). However, involvement in such a wide range of responses also implies that mitochondrial stress signalling could lead to synergistic or conflicting outcomes during acclimation to multiple and complex stresses, such as those arising from climate change.
The mechanisms of these mitochondrial stress signalling hubs involve regulatory loops and regulatory rheostats, whose functioning can amplify and diversify some signals or, conversely, dampen and reduce other signals. As emphasized in the present review, ETC and OXPHOS are centrally involved in this network of mitochondrial signals. Moreover, expression levels of ETC and OXPHOS components are controlled by the developmental and hormonal context, thus implying that ETC and OXPHOS stress signalling dynamics can function at different setpoints of respiratory activity, ATP production and ROS production. However, the involvement of other mitochondrial structures and processes, such as membrane contacts or Ca2+ dynamics, that are also connected to signalling mechanisms should be further investigated. Moreover, mitochondrial signalling hubs may involve not only functioning and perturbations of functioning, but also biogenetic processes such as protein import and supramolecular assembly. Thus, ETC and OXPHOS homeostasis is likely to act not only on respiration and energy functioning, but also on the correct assembly of ETC and OXPHOS complexes. Finally, further research should be carried out on the potential links between the mitochondrial signalling hubs, nuclear epigenetic regulation (Shaughnessy et al., 2014) and mitochondrial epigenetics (van der Wijst and Rots, 2015). Understanding these links should bring new insights into the involvement of mitochondria in the processes of stress memory and priming (Hilker and Schmülling, 2019).
The regulation of mitochondrial respiration and of mitochondria/cell crosstalk has been recognized to be a major determinant of plant vigour and crop productivity (Amthor et al., 2019). The objective of decreasing respiratory carbon loss for improvement of crop productivity will depend on the identification of relevant genetic and physiological targets (Amthor et al., 2019). However, the search for such targets should not be confined to metabolic and biosynthetic processes, and should take into account signalling interactions that are likely to sustain integrative and adaptive responses, and thus especially mitochondria-related signalling.
FUNDING
This work was supported by recurrent funding from the University of Rennes 1 (France) and the Centre National de la Recherche Scientifique (CNRS, France). Research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
ACKNOWLEDGEMENTS
We thank Professor Jean-Pierre Carde (University of Bordeaux, France), Dr Gwenola Gouesbet (University of Rennes 1/CNRS, France), Dr Antoine Gravot (University of Rennes 1/INRA, France) and Dr Cécile Sulmon (University of Rennes 1/CNRS, France) for fruitful discussions and comments. We also thank the reviewers for their thorough analysis and their insightful comments and suggestions.
LITERATURE CITED
- Alber NA, Sivanesan H, Vanlerberghe GC. 2017. The occurrence and control of nitric oxide generation by the plant mitochondrial electron transport chain. Plant, Cell & Environment 40: 1074–1085. [DOI] [PubMed] [Google Scholar]
- Alberto D, Couée I, Sulmon C, Gouesbet G. 2017. Root-level exposure reveals multiple physiological toxicity of triazine xenobiotics in Arabidopsis thaliana. Journal of Plant Physiology 212: 105–114. [DOI] [PubMed] [Google Scholar]
- Allan A, Fluhr R. 1997. Two distinct sources of elicited reactive oxygen species in tobacco epidermal cells. Plant Cell 9: 1559–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amirsadeghi S, Robson C, Vanlerberghe G. 2007. The role of the mitochondrion in plant responses to biotic stress. Physiologia Plantarum 129: 253–266. [Google Scholar]
- Amthor JS, Bar-Even A, Hanson A, et al. 2019. Engineering strategies to boost crop productivity by cutting respiratory carbon loss. Plant Cell 31: 297–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreyev AY, Kushnareva YE, Murphy AN, Starkov AA. 2015. Mitochondrial ROS metabolism: 10 years later. Biochemistry (Moscow) 80: 517–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafi M, Azimi-Moqadam M-R, Moradi P, Mohseni-Fard E, Shekari F, Kompany-Zareh M. 2018. Effect of drought stress on metabolite adjustments in drought tolerant and sensitive thyme. Plant Physiology and Biochemistry 132: 391–399. [DOI] [PubMed] [Google Scholar]
- Astier J, Rasul S, Koen E et al. 2011. S-nitrosylation: an emerging post-translational protein modification in plants. Plant Science 181:527–533. [DOI] [PubMed] [Google Scholar]
- Attacha S, Solbach D, Bela K, et al. 2017. Glutathione peroxidase-like enzymes cover five distinct cell compartments and membrane surfaces in Arabidopsis thaliana. Plant Cell & Environment 40: 1281–1295. [DOI] [PubMed] [Google Scholar]
- Baena-González E, Sheen J. 2008. Convergent energy and stress signaling. Trends in Plant Science 13: 474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Serres J, Fukao T, Gibbs DJ, et al. 2011. Making sense of low oxygen sensing. Trends in Plant Science 17: 129–138. [DOI] [PubMed] [Google Scholar]
- Balmer Y, Vensel WH, Tanaka CK, et al. 2004. Thioredoxin links redox to the regulation of fundamental processes of plant mitochondria. Proceedings of the National Academy of Sciences of the USA 101: 2642–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli CG, Pastori GM, Foyer CH. 2000. Ascorbate biosynthesis in mitochondria is linked to the electron transport chain between complexes III and IV. Plant Physiology 23: 335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu U, Good AG, Taylor GJ. 2001. Transgenic Brassica napus plants overexpressing aluminium-induced mitochondrial manganese superoxide dismutase cDNA are resistant to aluminium. Plant Cell & Environment 24: 1269–1278. [Google Scholar]
- Berkowitz O, De Clercq I, Van Breusegem F, Whelan J. 2016. Interaction between hormonal and mitochondrial signalling during growth, development and in plant defence responses. Plant, Cell & Environment 39: 1127–1139. [DOI] [PubMed] [Google Scholar]
- Besson-Bard A, Pugin A, Wendehenne D. 2008. New insights into nitric oxide signaling in plants. Annual Review of Plant Biology 59: 21–39. [DOI] [PubMed] [Google Scholar]
- Bienert GP, Møller ALB, Kristiansen KA, et al. 2007. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. Journal of Biological Chemistry 282: 1183–1192. [DOI] [PubMed] [Google Scholar]
- Biswas M, Mano J. 2015. Lipid peroxide-derived short-chain carbonyls mediate hydrogen peroxide-induced and salt-induced programmed cell death in plants. Plant Physiology 168: 885–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas M, Mano J. 2016. Reactive carbonyl species activate caspase-3-like protease to initiate programmed cell death in plants. Plant Cell Physiology 57: 1432–1442. [DOI] [PubMed] [Google Scholar]
- Bohovych I, Khalimonchuk O. 2016. Sending out an SOS: mitochondria as a signaling hub. Frontiers in Cell and Developmental Biology 4: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohrer A-S, Kopriva S, Takahashi H. 2015. Plastid-cytosol partitioning and integration of metabolic pathways for APS/PAPS biosynthesis in Arabidopsis thaliana. Frontiers in Plant Science 5: 751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broeckx T, Hulsmans S, Rolland F. 2016. The plant energy sensor: evolutionary conservation and divergence of SnRK1 structure, regulation and function. Journal of Experimental Botany 67: 6215–6252. [DOI] [PubMed] [Google Scholar]
- Cabassa-Hourton C, Schertl P, Bordenave-Jacquemin M, et al. 2016. Proteomic and functional analysis of proline dehydrogenase 1 link proline catabolism to mitochondrial electron transport in Arabidopsis thaliana. Biochemical Journal 473: 2623–2634. [DOI] [PubMed] [Google Scholar]
- Carraro M, Bernardi P. 2016. Calcium and reactive oxygen species in regulation of the mitochondrial permeability transition and of programmed cell death in yeast. Cell Calcium 60: 102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castello PR, David PS, McClure T, Crook Z, Poyton RO. 2006. Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: implications for oxygen sensing and hypoxic signaling in eukaryotes. Cell Metabolism 3: 277–287. [DOI] [PubMed] [Google Scholar]
- Cecchini NM, Monteoliva MI, Alvarez ME. 2011. Proline dehydrogenase is a positive regulator of cell death in different kingdoms. Plant Signaling & Behavior 6: 1195–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KX, Mabbitt PD, Phua SY, et al. 2016. a Sensing and signaling of oxidative stress in chloroplasts by inactivation of the SAL1 phosphoadenosine phosphatase. Proceedings of the National Academy of Sciences of the USA 113: E4567–E4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KX, Phua SY, Crisp P, McQuinn R, Pogson BJ. 2016b Learning the languages of the chloroplast: retrograde signaling and beyond. Annual Review of Plant Biology 67: 25–53. [DOI] [PubMed] [Google Scholar]
- Che-Othman MH, Millar AH, Taylor NL. 2017. Connecting salt stress signalling pathways with salinity-induced changes in mitochondrial metabolic processes in C3 plants. Plant, Cell & Environment 40: 2875–2905. [DOI] [PubMed] [Google Scholar]
- Crawford T, Lehotai N, Strand Å. 2018. The role of retrograde signals during plant stress responses. Journal of Experimental Botany 69: 2783–2795. [DOI] [PubMed] [Google Scholar]
- Crozet P, Margalha L, Confraria A, et al. 2014. Mechanisms of regulation of SNF1/AMPK/SnRK1 protein kinases. Frontiers in Plant Science 5: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui F, Brosché M, Shapiguzov A, et al. 2019. Interaction of methyl viologen-induced chloroplast and mitochondrial signalling in Arabidopsis. Free Radical Biology and Medicine 134: 555–566. [DOI] [PubMed] [Google Scholar]
- Cvetkovska M, Vanlerberghe GC. 2012a Coordination of a mitochondrial superoxide burst during the hypersensitive response to bacterial pathogen in Nicotiana tabacum. Plant, Cell & Environment 35: 1121–36 [DOI] [PubMed] [Google Scholar]
- Cvetkovska M, Vanlerberghe GC. 2012b Alternative oxidase modulates leaf mitochondrial concentrations of superoxide and nitric oxide. New Phytologist 195: 32–39. [DOI] [PubMed] [Google Scholar]
- Cvetkovska M, Vanlerberghe GC. 2013. Alternative oxidase impacts the plant response to biotic stress by influencing the mitochondrial generation of reactive oxygen species: alternative oxidase and the biotic stress response. Plant, Cell & Environment 36: 721–732. [DOI] [PubMed] [Google Scholar]
- Cvetkovska M, Alber N, Vanlerberghe G. 2013. The signaling role of a mitochondrial superoxide burst during stress. Plant Signaling & Behavior 8:e22749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnocka W, Karpiński S. 2018. Friend or foe? Reactive oxygen species production, scavenging and signaling in plant response to environmental stresses. Free Radical Biology and Medicine 122: 4–20. [DOI] [PubMed] [Google Scholar]
- Daloso DM, Müller K, Obata T, et al. 2015. Thioredoxin, a master regulator of the tricarboxylic acid cycle in plant mitochondria. Proceedings of the National Academy of Sciences of the USA 112: E1392–E1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangl J, Jones J. 2001. Plant pathogens and integrated defence responses to infection. Nature 411: 826–833. [DOI] [PubMed] [Google Scholar]
- De Clercq I, Vermeirssen V, Van Aken O, et al. 2013. The membrane-bound NAC transcription factor ANAC013 functions in mitochondrial retrograde regulation of the oxidative stress response in Arabidopsis. Plant Cell 25: 3472–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Paepe R, Lemaire SD, Danon A. 2014. Cardiolipin at the heart of stress response across kingdoms. Plant Signaling & Behavior 9: e29228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Moreno I, García-Heredia JM, Díaz-Quintana A, Teixeira M, De la Rosa MA. 2011. Nitration of tyrosines 46 and 48 induces the specific degradation of cytochrome c upon change of the heme iron state to high-spin. Biochimica et Biophysica Acta 1807: 1616–1623. [DOI] [PubMed] [Google Scholar]
- Dixit V, Pandey V, Shyam R. 2002. Chromium ions inactivate electron transport and enhance superoxide generation in vivo in pea (Pisum sativum L. cv. Azad) root mitochondria. Plant, Cell & Environment 25: 687–693. [Google Scholar]
- Dolzblasz A, Gola EM, Sokolowska K, Smakowska-Luzan E, Twardawska A, Janska H. 2018. Impairment of meristem proliferation in plants lacking the mitochondrial protease AtFTSH4. International Journal of Molecular Sciences 19: e853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, Lepiniec L. 2010. MYB transcription factors in Arabidopsis. Trends in Plant Science 15: 573–581. [DOI] [PubMed] [Google Scholar]
- Elthon T, McIntosh L. 1987. Identification of the alternative terminal oxidase of higher plant mitochondria. Proceedings of the National Academy of Sciences of the USA 84: 8399–8403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estavillo GM, Crisp PA, Pornsiriwong W, et al. 2011. Evidence for a SAL1-PAP chloroplast retrograde pathway that functions in drought and high light signaling in Arabidopsis. Plant Cell 23: 3992–4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabro G, Rizzi YS, Alvarez ME. 2016. Arabidopsis proline dehydrogenase contributes to flagellin-mediated PAMP-triggered immunity by affecting RBOHD. Molecular Plant-Microbe Interactions 29: 620–628. [DOI] [PubMed] [Google Scholar]
- Finnegan P, Umbach A, Wilce J. 2003. Prokaryotic origins for the mitochondrial alternative oxidase and plastid terminal oxidase nuclear genes. FEBS Letters 555: 425–430. [DOI] [PubMed] [Google Scholar]
- Forde BG, Roberts MR. 2014. Glutamate receptor-like channels in plants: a role as amino acid sensors in plant defence? F1000Prime Reports 6: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. 2003. Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiologia Plantarum 119: 355–364. [Google Scholar]
- Foyer CH, Bloom AJ, Queval G, Noctor G. 2009. Photorespiratory metabolism: genes, mutants, energetics, redox signaling. Annual Review of Plant Biology 60: 455–484. [DOI] [PubMed] [Google Scholar]
- Fung RW, Wang CY, Smith DL, Gross KC, Tao Y, Tian M. 2006. Characterization of alternative oxidase (AOX) gene expression in response to methyl salicylate and methyl jasmonate pre-treatment and low temperature in tomatoes. Journal of Plant Physiology 163: 1049–1060. [DOI] [PubMed] [Google Scholar]
- Gallie DR. 2013. l-Ascorbic acid: a multifunctional molecule supporting plant growth and development. Scientifica (Cairo) 2013: 795964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Xing D, Li L, Zhang L. 2008. Implication of reactive oxygen species and mitochondrial dysfunction in the early stages of plant programmed cell death induced by ultraviolet-C overexposure. Planta 227: 755–767. [DOI] [PubMed] [Google Scholar]
- Garcia de la Garma J, Fernandez-Garcia N, Bardisi E, et al. 2015. New insights into plant salt acclimation: the roles of vesicle trafficking and reactive oxygen species signalling in mitochondria and the endomembrane system. New Phytologist 205: 216–239. [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Thormählen I, Daloso DM, Fernie AR. 2017. The unprecedented versatility of the plant thioredoxin system. Trends in Plant Science 22: 249–262. [DOI] [PubMed] [Google Scholar]
- Gibbs DJ, Conde JV, Berckhan S, Prasad G, Mendiondo GM, Holdsworth MJ. 2015. Group VII ethylene response factors coordinate oxygen and nitric oxide signal transduction and stress responses in plants. Plant Physiology 169: 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy S, Suzuki N, Miller G, et al. 2014. A tidal wave of signals: calcium and ROS at the forefront of rapid systemic signaling. Trends in Plant Science 19: 623–630. [DOI] [PubMed] [Google Scholar]
- Giraud E, Van Aken O, Ho LHM, Whelan J. 2009. The transcription factor ABI4 is a regulator of mitochondrial retrograde expression of ALTERNATIVE OXIDASE1a. Plant Physiology 150: 1286–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason C, Huang S, Thatcher LF, et al. 2011. Mitochondrial complex II has a key role in mitochondrial-derived reactive oxygen species influence on plant stress gene regulation and defense. Proceedings of the National Academy of Sciences of the USA 108: 10768–10773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta KJ, Igamberdiev AU. 2011. The anoxic plant mitochondrion as a nitrite: NO reductase. Mitochondrion 11: 537–543. [DOI] [PubMed] [Google Scholar]
- Gupta KJ, Igamberdiev AU, Manjunatha G, et al. 2011. The emerging roles of nitric oxide (NO) in plant mitochondria. Plant Science 181: 520–526. [DOI] [PubMed] [Google Scholar]
- Gupta KJ, Shah JK, Brotman Y, et al. 2012. Inhibition of aconitase by nitric oxide leads to induction of the alternative oxidase and to a shift of metabolism towards biosynthesis of amino acids. Journal of Experimental Botany 63: 1773–1784. [DOI] [PubMed] [Google Scholar]
- Hanqing F, Kun S, Mingquan L, et al. 2010. The expression, function and regulation of mitochondrial alternative oxidase under biotic stresses. Molecular Plant Pathology 11: 429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt TM, Nunes Nesi A, Araújo WL, Braun HP. 2015. Amino acid catabolism in plants. Molecular Plant 8: 1563–1579. [DOI] [PubMed] [Google Scholar]
- Hilker M, Schmülling T. 2019. Stress priming, memory, and signalling in plants. Physiologia Plantarum 42: 753–761. [DOI] [PubMed] [Google Scholar]
- Hilleary R, Gilroy S. 2018. Systemic signaling in response to wounding and pathogens. Current Opinion in Plant Biology 43: 57–62. [DOI] [PubMed] [Google Scholar]
- Ho LHM, Giraud E, Uggalla V, et al. 2008. Identification of regulatory pathways controlling gene expression of stress-responsive mitochondrial proteins in Arabidopsis. Plant Physiology 147: 1858–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Braun HP, Gawryluk RMR, Millar AH. 2019. Mitochondrial complex II of plants: subunit composition, assembly, and function in respiration and signaling. Plant Journal 98: 405–417. [DOI] [PubMed] [Google Scholar]
- Huang X, Von-Rad U, Durner J. 2002. Nitric oxide induces transcriptional activation of the nitric oxide-tolerant alternative oxidase in Arabidopsis suspension cell. Planta 215: 914–923. [DOI] [PubMed] [Google Scholar]
- Jacoby R, Li L, Huang S, Lee C, Millar A, Taylor N. 2012. Mitochondrial composition, function and stress response in plants. Journal of Integrative Plant Biology 54: 887–906. [DOI] [PubMed] [Google Scholar]
- Janse van Rensburg HC, Van den Ende W, Signorelli S. 2019. Autophagy in plants: both a puppet and a puppet master of sugars. Frontiers in Plant Science 10: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan VE, Bayir HA, Belikova NA, et al. 2009. Cytochrome c/cardiolipin relations in mitochondria: a kiss of death. Free Radical Biology and Medicine 46: 1439–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerchev PI, De Clercq I, Denecker J, et al. 2014. Mitochondrial perturbation negatively affects auxin signaling. Molecular Plant 7: 1138–1150. [DOI] [PubMed] [Google Scholar]
- Keunen E, Remans T, Bohler S, Vangronsveld J, Cuypers A. 2011. Metal-induced oxidative stress and plant mitochondria. International Journal of Molecular Sciences 12: 6894–6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharenko OA, Boyd J, Nelson KM, Abrams SR, Loewen MC. 2011. Identification and characterization of interactions between abscisic acid and mitochondrial adenine nucleotide translocators. Biochemical Journal 437: 117–123. [DOI] [PubMed] [Google Scholar]
- Kobylińska A, Reiter RJ, Posmyk MM. 2017. Melatonin protects cultured tobacco cells against lead-induced cell death via inhibition of cytochrome c translocation. Frontiers in Plant Science 8: 1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu KJ, Avolio ML, Lemoine NP, et al. 2019. Global change effects on plant communities are magnified by time and the number of global change factors imposed. Proceedings of the National Academy of Sciences of the USA 116: 17867–17873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef A, Pieterse C. 2008. Cross talk in defense signaling. Plant Physiology 146: 839–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koussevitzky S, Nott A, Mockler TC, et al. 2007. Signals from chloroplasts converge to regulate nuclear gene expression. Science 316: 715–719. [PubMed] [Google Scholar]
- Krause M, Durner J. 2004. Harpin inactivates mitochondria in Arabidopsis suspension cells. Molecular Plant-Microbe Interactions 17: 131–139. [DOI] [PubMed] [Google Scholar]
- Kumari A, Pathak PK, Bulle M, Igamberdiev AU, Gupta KJ. 2019. Alternative oxidase is an important player in the regulation of nitric oxide levels under normoxic and hypoxic conditions in plants. Journal of Experimental Botany 70: 4345–4354. [DOI] [PubMed] [Google Scholar]
- Lamotte O, Gould K, Lecourieux D, et al. 2004. Analysis of nitric oxide signaling functions in tobacco cells challenged by the elicitor cryptogein. Plant Physiology 135: 516–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Ragheb K, Lawler G, et al. 2003. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. Journal of Biological Chemistry 278: 8516–8525. [DOI] [PubMed] [Google Scholar]
- Li Z-Y, Xu Z-S, He G-Y, Yang G-X, Chen M, Li L-C, Ma Y. 2013. The voltage-dependent anion channel 1 (AtVDAC1) negatively regulates plant cold responses during germination and seedling development in Arabidopsis and interacts with calcium sensor CBL1. International Journal of Molecular Sciences 14: 701–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Zhang L, Natarajan SK, Becker DF. 2013. Proline mechanisms of stress survival. Antioxidants & Redox Signaling 19: 998–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebthal M, Maynard D, Dietz K-J. 2018. Peroxiredoxins and redox signaling in plants. Antioxidants & Redox Signaling 28: 609–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Li Z, Wang Y, Xing D. 2014. Overexpression of ALTERNATIVE OXIDASE1a alleviates mitochondria-dependent programmed cell death induced by aluminium phytotoxicity in Arabidopsis. Journal of Experimental Botany 65: 4465–4478. [DOI] [PubMed] [Google Scholar]
- Mano J. 2012. Reactive carbonyl species: their production from lipid peroxides, action in environmental stress, and the detoxification mechanism. Plant Physiology and Biochemistry 59: 90–97. [DOI] [PubMed] [Google Scholar]
- Martí MC, Florez-Sarasa I, Camejo D, et al. 2011. Response of mitochondrial thioredoxin PsTrxo1, antioxidant enzymes, and respiration to salinity in pea (Pisum sativum L.) leaves. Journal of Experimental Botany 62: 3863–3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Fábregas J, Díaz-Moreno I, González-Arzola K, et al. 2013. New Arabidopsis thaliana cytochrome c partners: a look into the elusive role of cytochrome c in programmed cell death in plants. Molecular and Cellular Proteomics 12: 3666–3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruta T, Noshi M, Tanouchi A, et al. 2012. H2O2-triggered retrograde signaling from chloroplasts to nucleus plays specific role in response to stress. Journal of Biological Chemistry 287: 11717–11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell D, Wang Y, McIntosh L. 1999. The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proceedings of the National Academy of Sciences of the USA 96: 8271–8276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh L. 1994. Molecular biology of the alternative oxidase. Plant Physiology 105: 781–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Li L, De Clercq I, et al. 2019. ANAC017 coordinates organellar functions and stress responses by reprogramming retrograde signaling. Plant Physiology 180: 634–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AJ. 2008. The integration of glutathione homeostasis and redox signaling. Journal of Plant Physiology 165: 1390–1403. [DOI] [PubMed] [Google Scholar]
- Meyer AJ, Brach T, Marty L, et al. 2007. Redox-sensitive GFP in Arabidopsis thaliana is a quantitative biosensor for the redox potential of the cellular glutathione redox buffer. Plant Journal 52: 973–986. [DOI] [PubMed] [Google Scholar]
- Millenaar F, Lambers H. 2003. The alternative oxidase: in vivo regulation and function. Plant Biology 5: 2–15. [Google Scholar]
- Miller CO. 1982. Cytokinin modification of mitochondrial function. Plant Physiology 69: 1274–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. 2004. Reactive oxygen gene network of plants. Trends in Plant Science 9: 1360–1385. [DOI] [PubMed] [Google Scholar]
- Modolo LV, Augusto O, Almeida IMG, Magalhaes JR, Salgado I. 2005. Nitrite as the major source of nitric oxide production by Arabidopsis thaliana in response to pseudomonas syringae. FEBS Letters 579: 3814–3820. [DOI] [PubMed] [Google Scholar]
- Møller IM. 2001. Plant mitochondria and oxidative stress: electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annual Review of Plant Biology 52: 561–591. [DOI] [PubMed] [Google Scholar]
- Møller IM. 2016. What is hot in plant mitochondria? Physiologia Plantarum 157: 256–263. [DOI] [PubMed] [Google Scholar]
- Møller IM, Sweetlove LJ. 2010. ROS signalling—specificity is required. Trends in Plant Science 15: 370–374. [DOI] [PubMed] [Google Scholar]
- Møller IM, Rogowska-Wrzesinska A, Rao RS. 2011. Protein carbonylation and metal-catalyzed protein oxidation in a cellular perspective. Journal of Proteomics 74: 2228–2242. [DOI] [PubMed] [Google Scholar]
- Monteoliva MI, Rizzi YS, Cecchini NM, Hajirezaei M-R, Alvarez ME. 2014. Context of action of proline dehydrogenase (ProDH) in the hypersensitive response of Arabidopsis. BMC Plant Biology 14: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau M, Lee GI, Wang Y, Crane BR, Klessig DF. 2008. AtNOS/AtNOA1 is a functional Arabidopsis thaliana cGTPase and not a nitric-oxide synthase. Journal of Biological Chemistry 283: 32957–32967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munné-Bosch S, Queval G, Foyer CH. 2013. The impact of global change factors on redox signaling underpinning stress tolerance. Plant Physiology 161: 5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mur L, Kenton P, Lloyd A, Ougham H, Prats E. 2007. The hypersensitive response; the centenary is upon us but how much do we know? Journal of Experimental Botany 59: 501–520. [DOI] [PubMed] [Google Scholar]
- Murik O, Elboher A, Kaplan A. 2014. Dehydroascorbate: a possible surveillance molecule of oxidative stress and programmed cell death in the green alga Chlamydomonas reinhardtii. New Phytologist 202: 471–484. [DOI] [PubMed] [Google Scholar]
- Murphy MP. 2009. How mitochondria produce reactive oxygen species. Biochemical Journal 417: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng S, De Clercq I, Van Aken O et al. 2014. Anterograde and retrograde regulation of nuclear genes encoding mitochondrial proteins during growth, development, and stress. Molecular Plant 7: 1075–1093. [DOI] [PubMed] [Google Scholar]
- Ng S, Ivanova A, Duncan O, et al. 2013. A membrane-bound NAC transcription factor, ANAC017, mediates mitochondrial retrograde signaling in Arabidopsis. Plant Cell 25: 3450–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, Foyer CH. 1998. Ascorbate and glutathione: keeping active oxygen under control. Annual Review of Plant Physiology and Plant Molecular Biology 49: 249–279. [DOI] [PubMed] [Google Scholar]
- Noctor G, Foyer CH. 2016. Intracellular redox compartmentation and ROS-related communication in regulation and signaling. Plant Physiology 171: 1581–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, Mhamdi A, Chaouch S, Han Y, et al. 2012. Glutathione in plants: an integrated overview. Plant, Cell & Environment 35: 454–484. [DOI] [PubMed] [Google Scholar]
- Norman C, Howell KA, Millar AH, Whelan JM, Day DA. 2004. Salicylic acid is an uncoupler and inhibitor of mitochondrial electron transport. Plant Physiology 134: 492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nukarinen E, Nägele T, Pedrotti L, et al. 2016. Quantitative phosphoproteomics reveals the role of the AMPK plant ortholog SnRK1 as a metabolic master regulator under energy deprivation. Scientific Reports 6: 31697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parani M, Rudrabhatla S, Myers R, et al. 2004. Microarray analysis of nitric oxide responsive transcripts in Arabidopsis. Plant Biotechnology Journal 2: 359–366. [DOI] [PubMed] [Google Scholar]
- de Oliveira HC, Wulff A, Saviani EE, Salgado I. 2008. Nitric oxide degradation by potato tuber mitochondria: evidence for the involvement of external NAD(P)H dehydrogenases. Biochimica et Biophysica Acta 1777: 470–476. [DOI] [PubMed] [Google Scholar]
- Passaia G, Fonini LS, Caverzan A, et al. 2013. The mitochondrial glutathione peroxidase GPX3 is essential for H2O2 homeostasis and root and shoot development in rice. Plant Science 208: 93–101. [DOI] [PubMed] [Google Scholar]
- Pedrotti L, Weiste C, Nägele T, et al. 2018. Snf1-RELATED KINASE1-controlled C/S1-bZIP signaling activates alternative mitochondrial metabolic pathways to ensure plant survival in extended darkness. Plant Cell 30: 495–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Sancho J, Tilsner J, Samuels AL, Botella MA, Bayer EM, Rosado A. 2016. Stitching organelles: organization and function of specialized membrane contact sites in plants. Trends in Cell Biology 26: 705–717. [DOI] [PubMed] [Google Scholar]
- Petrosillo G, Ruggiero FM, Paradies G. 2003. Role of reactive oxygen species and cardiolipin in the release of cytochrome c from mitochondria. FASEB Journal 17: 2202–2208. [DOI] [PubMed] [Google Scholar]
- Pineau B, Bourge M, Marion J, et al. 2013. The importance of cardiolipin synthase for mitochondrial ultrastructure, respiratory function, plant development, and stress responses in Arabidopsis. Plant Cell 25: 4195–4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planchet E, Gupta KJ, Sonoda M, Kaiser WM. 2005. Nitric oxide emission from tobacco leaves and cell suspensions: rate limiting factors and evidence for the involvement of mitochondrial electron transport. Plant Journal 41: 732–743. [DOI] [PubMed] [Google Scholar]
- Pokotylo IV, Kretinin SV, Kravets VS. 2012. Role of phospholipase D in metabolic reactions of transgenic tobacco cax1 cells under the influence of salt stress. Cytology and Genetics 46: 131–135. [PubMed] [Google Scholar]
- Radi R, Cassina A, Hodara R, Quijano C, Castro L. 2002. Peroxynitrite reactions and formation in mitochondria. Free Radical Biology and Medicine 33: 1451–1464. [DOI] [PubMed] [Google Scholar]
- Rasmusson AG, Møller IM. 2011. Mitochondrial electron transport and plant stress. In: F Kempken, ed. Plant mitochondria. >New York Dordrecht Heidelberg; London: Springer, 357–381. [Google Scholar]
- Rikhvanov EG, Fedoseeva IV, Pyatrikas DV, Borovskii GB, Voinikov VK. 2014. Role of mitochondria in the operation of calcium signaling system in heat-stressed plants. Russian Journal of Plant Physiology 61: 141–153. [Google Scholar]
- Rostovtseva TK, Tan W, Colombini M. 2005. On the role of VDAC in apoptosis: fact and fiction. Journal of Bioenergetics and Biomembranes 37: 129–142. [DOI] [PubMed] [Google Scholar]
- Rouhier N, Lemaire SD, Jacquot J-P. 2008. The role of glutathione in photosynthetic organisms: emerging functions for glutaredoxins and glutathionylation. Annual Review of Plant Biology 59: 143–166. [DOI] [PubMed] [Google Scholar]
- Sanderson TH, Mahapatra G, Pecina P, et al. 2013. Cytochrome c is tyrosine 97 phosphorylated by neuroprotective insulin treatment. PLoS ONE 8: e78627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schertl P, Braun HP. 2014. Respiratory electron transfer pathways in plant mitochondria. Frontiers in Plant Science 5: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schertl P, Sunderhaus S, Klodmann J, Grozeff GE, Bartoli CG, Braun HP. 2012. l-Galactono-1,4-lactone dehydrogenase (GLDH) forms part of three subcomplexes of mitochondrial complex I in Arabidopsis thaliana. Journal of Biological Chemistry 287: 14412–14419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzländer M, Finkemeier I. 2013. Mitochondrial energy and redox signaling in plants. Antioxidants & Redox Signaling 18: 2122–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzländer M, Fricker MD, Müller C, et al. 2008. Confocal imaging of glutathione redox potential in living plant cells. Journal of Microscopy 231: 299–316. [DOI] [PubMed] [Google Scholar]
- Schwarzländer M, Fricker MD, Sweetlove LJ. 2009. Monitoring the in vivo redox state of plant mitochondria: effect of respiratory inhibitors, abiotic stress and assessment of recovery from oxidative challenge. Biochimica et Biophysica Acta 1787: 468–475. [DOI] [PubMed] [Google Scholar]
- Scott I, Logan DC. 2008. Mitochondrial morphology transition is an early indicator of subsequent cell death in Arabidopsis. New Phytologist 177: 90–101. [DOI] [PubMed] [Google Scholar]
- Shang Y, Yan L, Liu ZQ, et al. 2010. The Mg-chelatase H subunit of Arabidopsis antagonizes a group of WRKY transcription repressors to relieve ABA-responsive genes of inhibition. Plant Cell 22: 1909–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaughnessy DT, McAllister K, Worth L, et al. 2014. Mitochondria, energetics, epigenetics, and cellular responses to stress. Environmental Health Perspectives 122: 1271–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Singh S, Parihar P, et al. 2016. Reactive oxygen species (ROS): beneficial companions of plants’ developmental processes. Frontiers in Plant Science 7: 1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff N. 2018. Ascorbic acid metabolism and functions: a comparison of plants and mammals. Free Radical Biology and Medicine 122: 116–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff N, Wheeler GL. 2000. Ascorbic acid in plants: biosynthesis and function. Critical Reviews in Biochemistry and Molecular Biology 35: 291–314. [DOI] [PubMed] [Google Scholar]
- de Souza A, Wang J-Z, Dehesh K. 2017. Retrograde signals: integrators of interorganellar communication and orchestrators of plant development. Annual Review of Plant Biology 68: 85–108. [DOI] [PubMed] [Google Scholar]
- Stevens RG, Baldet P, Bouchet JP, et al. 2018. A systems biology study in tomato fruit reveals correlations between the ascorbate pool and genes involved in ribosome biogenesis, translation, and the heat-shock response. Frontiers in Plant Science 9: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Miller G, Morales J, Shulaev V, Torres M, Mittler R. 2011. Respiratory burst oxidases: the engines of ROS signaling. Current Opinion in Plant Biology 14: 691–699. [DOI] [PubMed] [Google Scholar]
- Szabados L, Savouré A. 2010. Proline: a multifunctional amino acid. Trends in Plant Science 15: 89–97. [DOI] [PubMed] [Google Scholar]
- Tan Y, O’Toole N, Taylor N, Millar A. 2010. Divalent metal ions in plant mitochondria and their role in interactions with proteins and oxidative stress-induced damage to respiratory function. Plant Physiology 152: 747–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thormählen I, Naranjo B, Trujillo-Hernandez JA, Reichheld J-P, Cejudo FJ, Geigenberger P. 2018. On the elaborate network of thioredoxins in higher plants. Progress in Botany 80: 1–23. [Google Scholar]
- Toyota M, Spencer D, Sawai-Toyota S, et al. 2018. Glutamate triggers long-distance, calcium-based plant defense signaling. Science 361: 1112–1115. [DOI] [PubMed] [Google Scholar]
- Turrens JF. 2003. Mitochondrial formation of reactive oxygen species. Journal of Physiology 552: 335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacca RA, Valenti D, Bobba A, Merafina RS, Passarella S, Marra E. 2006. Cytochrome c is released in a reactive oxygen species-dependent manner and is degraded via caspase-like proteases in tobacco Bright-Yellow 2 cells en route to heat shock-induced cell death. Plant Physiology 141: 208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Aken O, De Clercq I, Ivanova A, et al. 2016. Mitochondrial and chloroplast stress responses are modulated in distinct touch and chemical inhibition phases. Plant Physiology 171: 2150–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Aken O, Pogson BJ. 2017. Convergence of mitochondrial and chloroplastic ANAC017/PAP-dependent retrograde signalling pathways and suppression of programmed cell death. Cell Death and Differentiation 24: 955–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Aken O, Van Breusegem F. 2015. Licensed to kill: mitochondria, chloroplasts, and cell death. Trends in Plant Science 20: 754–766. [DOI] [PubMed] [Google Scholar]
- Van Aken O, Zhang B, Law S, Narsai R, Whelan J. 2013. AtWRKY40 and AtWRKY63 modulate the expression of stress-responsive nuclear genes encoding mitochondrial and chloroplast proteins. Plant Physiology 162: 254–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Wijst MG, Rots MG. 2015. Mitochondrial epigenetics: an overlooked layer of regulation? Trends in Genetics 31: 353–356. [DOI] [PubMed] [Google Scholar]
- Vanlerberghe G, Cvetkovska M, Wang J. 2009. Is the maintenance of homeostatic mitochondrial signaling during stress a physiological role for alternative oxidase? Physiologia Plantarum 137: 392–406. [DOI] [PubMed] [Google Scholar]
- Vanderauwera S, Vandenbroucke K, Inzé A, et al. 2012. AtWRKY15 perturbation abolishes the mitochondrial stress response that steers osmotic stress tolerance in Arabidopsis. Proceedings of the National Academy of Sciences of the USA 109: 20113–20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh J, Park S. 2014. Role of L-ascorbate in alleviating abiotic stresses in crop plants. Botanical Studies 55: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen N, Hermans C. 2008. Proline accumulation in plants: a review. Amino Acids 35: 753–759. [DOI] [PubMed] [Google Scholar]
- Verslues PE. 2016. ABA and cytokinins: challenge and opportunity for plant stress research. Plant Molecular Biology 91: 629–640. [DOI] [PubMed] [Google Scholar]
- Verslues PE, Sharma S. 2010. Proline metabolism and its implications for plant-environment interaction. Arabidopsis Book 8: e0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianello A, Casolo V, Petrussa E, et al. 2012. The mitochondrial permeability transition pore (PTP) – an example of multiple molecular exaptation? Biochimica et Biophysica Acta 1817: 2072–2086. [DOI] [PubMed] [Google Scholar]
- Vieira H, Kroemer G. 2003. Mitochondria as targets of apoptosis regulation by nitric oxide. IUBMB Life 55: 613–616. [DOI] [PubMed] [Google Scholar]
- Vishwakarma A, Kumari A, Mur LAJ, Gupta KJ. 2018. A discrete role for alternative oxidase under hypoxia to increase nitric oxide and drive energy production. Free Radical Biology and Medicine 122: 40–51. [DOI] [PubMed] [Google Scholar]
- Wagner S, Behera S, De Bortoli S, et al. 2015. The EF-hand Ca2+ binding protein MICU choreographs mitochondrial Ca2+ dynamics in arabidopsis. Plant Cell 27: 3190–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S, De Bortoli S, Schwarzländer M, Szabò I. 2016. Regulation of mitochondrial calcium in plants versus animals. Journal of Experimental Botany 67: 3809–3829. [DOI] [PubMed] [Google Scholar]
- Wagner S, Van Aken O, Elsässer M, Schwarzländer M. 2018. Mitochondrial energy signaling and its role in the low-oxygen stress response of plants. Plant Physiology 176: 1156–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Liang X, Huang J, Zhang D, Lu H, Liu Z, Bi Y. 2010. Involvement of ethylene and hydrogen peroxide in induction of alternative respiratory pathway in salt-treated Arabidopsis calluses. Plant Cell Physiology 51: 1754–1765. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang Z, Huang R. 2013. Regulation of ascorbic acid synthesis in plants. Plant Signaling & Behavior 8: e24536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Auwerx J. 2017. Systems phytohormone responses to mitochondrial proteotoxic stress. Molecular Cell 68: 540–551. [DOI] [PubMed] [Google Scholar]
- Wang Y, Berkowitz O, Selinski J, Xu Y, Hartmann A, Whelan J. 2018. Stress responsive mitochondrial proteins in Arabidopsis thaliana. Free Radical Biology and Medicine 122: 28–39. [DOI] [PubMed] [Google Scholar]
- Waszczak C, Carmody M, Kangasjärvi J. 2018. Reactive oxygen species in plant signaling . Annual Review of Plant Biology 69: 209–236. [DOI] [PubMed] [Google Scholar]
- Welchen E, Gonzalez DH. 2016. Cytochrome c, a hub linking energy, redox, stress and signaling pathways in mitochondria and other cell compartments. Physiologia Plantarum 157: 310–321. [DOI] [PubMed] [Google Scholar]
- Welchen E, Viola IL, Kim HJ, et al. 2009. A segment containing a G-box and an ACGT motif confers differential expression characteristics and responses to the Arabidopsis Cytc-2 gene, encoding an isoform of cytochrome c. Journal of Experimental Botany 60: 829–845. [DOI] [PubMed] [Google Scholar]
- Winger AM, Millar AH, Day DA. 2005. Sensitivity of plant mitochondrial terminal oxidases to the lipid peroxidation product 4-hydroxy-2-nonenal (HNE). Biochemical Journal 387: 865–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winger AM, Taylor NL, Heazlewood JL, Day DA, Millar AH. 2007. The cytotoxic lipid peroxidation product 4-hydroxy-2-nonenal covalently modifies a selective range of proteins linked to respiratory function in plant mitochondria. Journal of Biological Chemistry 282: 37436–37447 [DOI] [PubMed] [Google Scholar]
- Wu J, Sun Y, Zhao Y, et al. 2015. Deficient plastidic fatty acid synthesis triggers cell death by modulating mitochondrial reactive oxygen species. Cell Research 25: 621–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Liang J, Wang C, et al. 2018. Overexpression of lily HsfA3s in Arabidopsis confers increased thermotolerance and salt sensitivity via alterations in proline catabolism. Journal of Experimental Botany 69: 2005–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurzinger B, Nukarinen E, Nägele T, Weckwerthe W, Teige M. 2018. The SnRK1 kinase as central mediator of energy signaling between different organelles. Plant Physiology 176: 1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H, Shimoji H, Ohshiro Y, Sakihama Y. 2001. Inhibitory effects of nitric oxide on oxidative phosphorylation in plant mitochondria. Nitric Oxide 5: 261–270. [DOI] [PubMed] [Google Scholar]
- Yang L, Zhang J, He J, et al. 2014. ABA-mediated ROS in mitochondria regulate root meristem activity by controlling PLETHORA expression in arabidopsis. PLoS Genetics 10: e1004791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao N, Tada Y, Sakamoto M, et al. 2002. Mitochondrial oxidative burst involved in apoptotic response in oats. Plant Journal 30: 567–579. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Noguchi K, Motohashi K, Hisabori T. 2013. Systematic exploration of thioredoxin target proteins in plant mitochondria. Plant Cell Physiology 54: 875–892. [DOI] [PubMed] [Google Scholar]
- Yu H, Lee I, Salome AR, Yu K, Hüttemann M. 2008. Mammalian liver cytochrome c is tyrosine-48 phosphorylated in vivo, inhibiting mitochondrial respiration. Biochimica et Biophysica Acta 1777: 1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaffagnini M, Bedhomme M, Marchand CH, et al. 2012. Redox regulation in photosynthetic organisms: focus on glutathionylation. Antioxidants & Redox Signaling 16: 567–586. [DOI] [PubMed] [Google Scholar]
- Zancani M, Casolo V, Petrussa E, et al. 2015. The permeability transition in plant mitochondria: the missing link. Frontiers in Plant Science 6: 1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechmann B, Mauch F, Sticher L, Müller M. 2008. Subcellular immunocytochemical analysis detects the highest concentrations of glutathione in mitochondria and not in plastids. Journal of Experimental Botany 59: 4017–4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Becker DF. 2015. Connecting proline metabolism and signaling pathways in plant senescence. Frontiers in Plant Science 6: 552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Oh Y, Li H, Baldwin IT, Galis I. 2012. Alternative oxidase in resistance to biotic stresses: Nicotiana attenuata AOX contributes to resistance to a pathogen and a piercing-sucking insect, but not Manduca sexta larvae. Plant Physiology 160: 1453–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zhang D, Yang C. 2014. AtFtsH4 perturbs the mitochondrial respiratory chain complexes and auxin homeostasis in Arabidopsis. Plant Signaling & Behavior 9: e29709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZW, Feng LY, Cheng J, et al. 2013. The roles of two transcription factors, ABI4 and CBFA, in ABA and plastid signalling and stress responses. Plant Molecular Biology 83: 445–448. [DOI] [PubMed] [Google Scholar]
- Zhu JK. 2016. Abiotic stress signaling and responses in plants. Cell 167: 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]