Abstract
Background and Aims
Global warming has large effects on the performance and spatial distribution of plants, and increasingly facilitates the spread of invasive species. Particularly vulnerable is the vegetation of cold environments where indigenous plants selected for cold tolerance can have reduced phenotypic plasticity and associated lower capacity to respond to warming temperatures. In contrast, invasive species can be phenotypically plastic and respond positively to climate change, but at the expense of stress tolerance.
Methods
We investigate this trade-off in traits, measuring the photosynthetic response to warming, chilling tolerance and specific leaf area (SLA) of Pooid grasses. We compare this between invasive and non-invasive grasses and correlate this to their range expansions on a cold sub-Antarctic island that has warmed significantly in the past five decades. We determined whether these responses remained consistent after temperature acclimation.
Key Results
Invasive species responded strongly to warming, increasing photosynthetic rates by up to 2-fold, while non-invasive species did not respond. The response was associated with increased stomatal conductance, but not with modified photosynthetic metabolism. Electrolyte leakage and SLA were higher in invasive than in non-invasive species. Acclimation altered the photosynthetic response and invasive species responded to warm temperatures irrespective of acclimation, while non-invasive species responded only after acclimation to warm temperature.
Conclusions
Traits scaled linearly with rates of range expansion and demonstrate that under sub-Antarctic conditions, anthropogenic warming over the last 50 years may have favoured species with greater capacity to respond photosynthetically to warming to the detriment of species that cannot, and negated the advantage that chilling tolerance would have conferred on endemic species in the past. This suggests that species of cold ecosystems could be particularly vulnerable to warming as selection for stress tolerance has limited their responsiveness to environmental change, while introduced invasive species may have no such limitations. We show mechanistic evidence of the physiology that underpins an apparent trade-off between warming and chilling tolerance traits.
Keywords: Climate change, invasive grasses, temperature response, chilling sensitivity, temperature acclimation, Pooiid grasses
INTRODUCTION
Climate change has large effects on the performance and spatial distribution of plants (Doxford and Freckleton, 2012), including through direct response to changes in temperature, atmospheric CO2 concentrations and rainfall patterns. In cold environments, invasions by introduced species are the primary causes of ecosystem and biodiversity change (Kennedy, 1995; Chown et al., 2012a). Warming, about which future predictions can be made with high levels of certainty (IPCC, 2014), is thought to be facilitating these invasions (reviewed by Walther et al., 2010), but the relative responses of native and invasive species, and the underlying physiological mechanisms, are poorly understood.
Non-native invasive plants are likely to be well suited to warming conditions as they often have traits useful in coping with environmental change that have allowed them to colonize new habitats (Walther et al., 2010; Lembrechts et al., 2016). Traits include those related to strong dispersal and increased reproductive output, tolerance of a wide range of environmental conditions and those associated with high productivity (Rejmánek and Richardson, 1996; Kolar and Lodge 2001; van Kleunen et al. 2010). Of particular importance is phenotypic plasticity that enables a high growth rate, which is positively associated with invasive species (van Kleunen et al., 2010; Vila et al., 2011) and can facilitate establishment and spread under conditions of increased resource availability and disturbance (Chown et al., 2012b). Native species do not necessarily respond less positively to warming than do introduced species (Sorte et al., 2013), and high growth rates may be better associated with invasive than with non-native species, regardless of their origin.
Traits such as fast growth and high productivity are associated with traits such as high photosynthetic rates, increased stomatal conductance and the production of leaves with low carbon costs [high specific leaf area (SLA)]. Such traits can maximize carbon assimilation but can come at the cost of traits that confer tolerance to disturbance and environmental extremes. For example, fast-growing species that require a high availability of resources tend to have short-lived leaves, while slow-growing species invest resources in durable thick leaves (Reich et al., 1997; Wright et al., 2004, 2005). Such trade-offs also exist across the transition from warm to cold environments, and a productivity–freezing tolerance trade-off has been demonstrated in species of oak that span a tropical–temperate gradient (Koehler et al., 2012). The basis for this is likely to be physiological, for example the production of stress proteins that prevent ice nucleation, and altered carbohydrate metabolism and membrane stability are all associated with cold adaptation (Zuniga et al., 1994; Yamashita et al., 2002; Sadve et al., 2008; Janmohammadi et al., 2015), but come at the expense of lowered photosynthetic rates (Smallwood and Bowles, 2002; Zhen and Ungerer, 2008; Zhen et al., 2011). Plants adapted to cold environments show decreased photosynthetic rates under warm conditions (Tieszen et al., 1981; Xiong et al., 1999). Cold environments that are warming create conditions where traits that enable positive responses to warmer temperatures confer an advantage over those that confer cold tolerance (Smallwood and Bowles, 2002; Zhen and Ungerer, 2008; Zhen et al., 2011; Koehler et al., 2012).
In order to explore the trade-off between traits associated with a positive response to warming temperatures against those that confer chilling tolerance, we compared introduced invasive and native grasses on sub-Antarctic Marion Island in the Southern Ocean (46°52'34''S, 37°51'32''E). Over the last 50 years, Marion Island has experienced a substantial increase in annual and monthly minimum and maximum temperatures, such that the climate has become warmer and drier with an increase in the hours of sunshine (Smith, 2002).
The species selected were phylogenetically constrained within the Pooideae subfamily, included six of the nine grass species present on Marion Island, and were distinguished as invasive or non-invasive based on the rates at which they have spread on the island. We tested the hypothesis that the photosynthetic rates of invasive grasses respond positively to warming but at the expense of cold tolerance, whereas non-invasive grasses are less able to respond to warming but have higher cold tolerance. We correlated the reciprocal pattern in these traits to the rate at which grasses have invaded Marion Island and determined how the photosynthetic response was modified by acclimation to warm or cold temperatures.
MATERIALS AND METHODS
Weather data
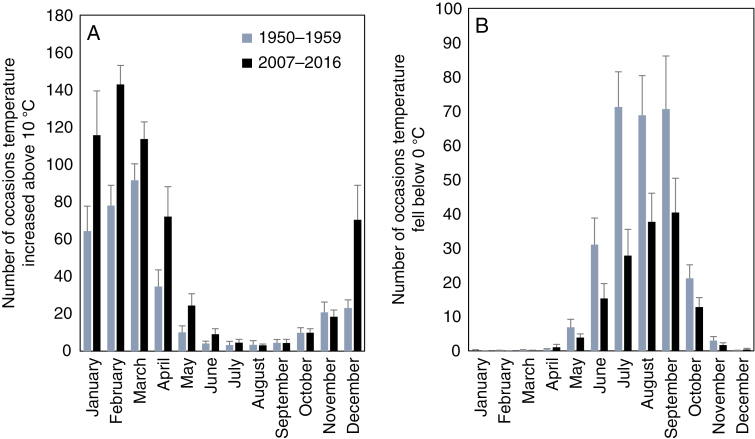
Weather data were obtained from the South African Weather Service and were recorded on the hour as an instantaneous measurement and at heights above the ground according to international standards. The meteorological station is on the eastern side of Marion Island (46°52'34''S, 37°51'32''E, 24 m) on the coastal plain where all plants used in this study were collected. Previous analyses of these data have shown that in the 1950s average daily minimum and maximum temperatures for Marion Island ranged between 2.8 and 7.6 °C. Over the last subsequent 50 years, average minimum and maximum temperatures have increased by 0.28 and 0.24 °C per decade, respectively. To investigate how this has altered the frequency and duration of both cold and warm events, we analysed the number of occasions and successive numbers of hours within each month, averaged across the period 1950–1959, or 2007–2016, that temperatures increased above 10 °C or fell below 0 °C. These thresholds were chosen as our photosynthetic measurements determined responses to temperatures increased from 10 to 20° C, while chilling sensitivity analyses used sub-zero temperatures.
Grass characteristics and collection
Six Pooiid grass species, Poa cookii (Hook.f.), Poa pratensis (L.), Poa annua (L.), Agrostis magellanica (Lam.), Agrostis stolonifera (L.) and Elymus repens (L.) Gould 1947, were collected in the vicinity of Marion Base (46°52'34''S, 37°51'32''E), at low altitude and within 1 km from the sea. These species inhabit mires, or borders of mires, and can be easily excavated to maintain intact root systems.
Two of the species (P. cookii and A. magellanica) were present on the island in the earliest vegetation survey (Huntley, 1967), have not subsequently spread and are considered native and non-invasive (Gremmen, 1997). They are found on all the cold sub-Antarctic islands, but the distribution of A. magellanica also extends to New Zealand South Island and Tierra del Fuego in South America (Gremmen and Smith, 2004). Elymus repens is thought to have been introduced by sealers in the 1950s, but has not spread beyond a single 250 m2 site and has been classified as non-invasive by Gremmen (1997). It originates from temperate climates in the northern hemisphere, and is found to some extent in cool climates at higher altitudes within warmer regions (CABI, 2017). Poa annua was first recorded in 1948 and has subsequently spread at a rate of 1.48 km2 year–1 (le Roux et al., 2013). It originates from temperate Eurasia but has spread all over the world, including into tropical and warm temperate regions. Poa pratensis and A. stolonifera were first recorded on Marion Island in 1965 and have spread at rates of 0.29 and 0.56 km2 year–1, respectively (le Roux et al., 2013). Both are from warm temperate climates and native to Eurasia and North Africa (CABI, 2017). Poa annua, P. pratensis and A. stolonifera are classified as invasive species (Gremmen, 1997). Hence, the grasses that have not spread on Marion Island since the 1960s originate from cold relatively homogenous environments, while those that have spread originate from warmer and heterogeneous climates.
Intact sods (20–30 cm in diameter), excavated during the April/May period of 2015 (late summer), were put into plastic bags that enclosed the soil and roots and were well watered. Plants were stored for a maximum of 1 d in laboratory incubators at 15 °C and a photosynthetically active photon flux density (PPFD) of 300 μmol m–2 s–1. This intensity was not dissimilar to the average daytime solar radiation of 340 μmol m–2 s–1 measured during the growing season. Fresh plants were routinely collected, and plants used for gas exchange measurements were never older than 1 d.
Gas exchange
Photosynthetic light responses (A:PPFD) were constructed at three temperatures in order to determine the response of light-saturated photosynthetic rates and light compensation points to warming. Fully expanded, single or duplicate leaves from separate tillers were used for measuring the photosynthetic light responses for each of the six grass species using a Licor 6400 photosynthesis system with simultaneous measures of chlorophyll fluorescence (Lincoln, NE, USA). Light intensities were sequentially decreased from a value of 500 μmol m–2 s–1, and at each light intensity gas exchange parameters were recorded after they had stabilized and a minimum period of 1 min was exceeded. The CO2 concentration of sample air was controlled at 40 Pa, and vapour pressure deficit (VPD) was maintained at <1 kPa. Light responses were repeated on between three and five replicate plants per species at leaf temperatures of 10, 15 and 20 °C.
Individual replicate responses of A to PPFD (average responses given in Supplementary data Fig. S3 and summary statistics in Table S1) were fitted with the equation A = Asat × [1 – exp(b – c × PPFD)] (Causton and Dale, 1990); where Asat is the light-saturated photosynthetic rate [μmol (CO2) m–2 s–1]. Values of Asat, b and c were determined using the Solver function in Microsoft Excel 2010, using a routine to minimize the sum of squares of the errors (SSE). On termination of experiments, the areas of leaves from each species were measured using calibrated digital photographs (Image-J), and dry masses were recorded for the calculation of SLA.
Relative electron transport rates (ETRs) were calculated as: ETR = ΦPSII × incident PPFD × 0.5 × 0.84 (Genty et al., 1989). Here, ΦPSII is the quantum yield of photosystem II photochemistry, 0.84 accounts for the absorption of incident light and 0.5 (α) accounts for the fact that absorbed light is used by both PSI and PSII. In order for ETR to be accurately estimated, it is necessary to measure leaf absorption and α, we did not do this and assume α values of 0.84 and 0.5, respectively, and hence our ETR values are approximations. Responses of ETR to PPFD (Supplementary data Fig. S4; Table S1) were fitted with the equation: ETR = ETRsat × [1 – exp(–b × PPFD)]; where ETRsat (μmol electrons m–2 s–1) and b are fitted parameters.
The response of photosynthesis to increasing CO2 (A:Ci) at two temperatures was measured to determine if the photosynthetic response to warming was underpinned by altered metabolic or stomatal mechanisms. A:Ci responses were constructed according to Long and Bernacchi (2003), selecting leaves and using conditions as before, at a saturating PPFD of 500 μmol m–2 s–1, and were repeated at 10 and 20 °C. As the number of individuals of E. repens on Marion Island is very limited, this species was not included in A:Ci analyses. CO2 response curves (Supplementary data Fig. S5) were fitted using the fitting utility of Sharkey et al. (2007), according to the equations of Farquhar et al. (1980) and von Caemmerer (2000), which give estimates of dark respiration (RD), Vcmax and Jmax in units of μmol (CO2) m–2 s–1. Summary statistics associated with A:Ci fits are given in Supplementary data Table S2. Stomatal limitation (SL) was calculated from A:Ci responses as: SL = [(X – A)/A] × 100. Where X is the photosynthetic rate at an intercellular CO2 concentration (Ci) of 40 Pa, and A is photosynthetic rate measured with an atmospheric CO2 concentration of 40 Pa. Rates of photosynthesis (A) and corresponding stomatal conductance to CO2 (gST), measured at ambient CO2 concentrations of 40 Pa, were obtained during the construction of A:Ci curves. These represented the first point measured on each leaf at the start of A:Ci analyses.
Acclimation
In order to determine if photosynthetic responses acclimate to prevailing conditions, three replicates of five of the grass species (P. cookii, P. pratensis, P. anua, A. magellanica and A. stolonifera) were maintained for two weeks in growth cabinets (MIR-254, Sanyo Electric Co., Ltd Japan) at either 1 or 20 °C. These temperatures represent the extreme that plants would be exposed to without freezing and the maximum daily temperatures that plants would be exposed to in the field. As before, plants were excavated as intact sods with roots and soil, and were enclosed in plastic bags. These plants were kept well watered and were illumined at 300 μmol m–2 s–1 on a 10:14 h day:night cycle. After 2 weeks, the photosynthetic rates of two tillers from each plant were measured at a leaf temperatures of 20 °C, with leaf chamber conditions as before. Leaves were selected as the second or third leaf from the apical leaf and had expanded, but probably not developed, under the acclimation conditions.
Electrolyte leakage
Electrolyte leakage is a measure of chilling sensitivity and was measured to determine if it showed a reverse pattern to the photosynthetic warming response. Leaves from four or five plants of each species were freshly harvested from the field and cut into 5 mm lengths, and a weighed sample (approx. 40 mg) of leaf material was sealed in Eppendorf vials with 3 mL of distilled water. Paired samples were then incubated for 14 h at 20 or –8 °C. Following this period, the leakage of electrolytes from the leaves was measured using a calibrated conductivity meter (Cyberscan 600, Eutech Instruments). Electrolyte leakage (μS cm–1 g–1) was calculated as the conductivity measured for the –8 °C solution minus that of the solution maintained at 20 °C, normalized by the measured leaf weights (Pérez-Harguindeguy et al., 2013).
Statistics
Temperature count data were compared between the periods 1950–1959 and 2007–2016 using a linear model with period and months as fixed effects, using a Poisson distribution. Plant response variables (RD, ΦCO2, Vcmax, Jmax, Asat, ETRsat, electrolyte leakage and SLA) were regressed against the rates that species have spread on Marion Island (spread data extracted from le Roux et al., 2013) using restricted maximum likelihood (REML) mixed effects models with rate of spread as a fixed effect and species as a random factor. The significance of the rate of spread as an explanatory variable was determined using Wald χ2 test Type II using the R-package car. Marginal R2 values associated with the fixed effect (rate of spread) were calculated according to Nakagawa and Schielzeth (2013), using the R package MuMIn.
Mixed models were fitted separately for each experimental temperature. Regression lines were fitted using the data from replicate plants for response variables despite each species only having a single rate of spread. The same approach was used to relate photosynthesis to stomatal conductance. All statistical analyses were performed in R version 3.3.1 (R Core Team, 2014).
RESULTS
Temperature changes on Marion Island
The increase in average temperatures (0.26 °C per decade) on Marion Island (le Roux and McGeoch, 2008) has been accompanied by an increase in the number of occasions that temperatures exceeded 10 °C, as was apparent when records from 1950–1959 were compared with data from 2007–2016 (Fig. 1A; F2, 237= 12.1, P < 0.0001). This increase was evident in all months of the year and was accompanied by a decrease in the number of occasions when temperatures declined below 0 °C (Fig. 1b; F2, 237= 58.7, P < 0.0001).
Fig. 1.
The monthly average number of times that temperature exceeded 10 °C (A) or declined below 0 °C (B) compared between the periods 1950–1959 and 2007–2016. Vertical bars indicate the s.e.
Temperature acclimation requires days to weeks of altered temperature (Atkin and Tjoelker, 2003; Atkin et al., 2006; Sage and Kubien, 2007; Armstrong et al., 2008; Smith and Dukes, 2013), but the temporal variation in temperature on Marion Island is such that this does not occur despite recent warming. The average number of consecutive hours that temperatures rose above 10 °C was highest in the summer months and increased in the 2007–2016 period relative to the 1950–1959 (F1, 239= 13.6, P < 0.0003; Supplementary data Fig. S1a, b). However, consecutive hours amounted to only a little over a third of a day in the 1950s and this was only doubled in the more recent period. The length of the periods of consecutive mild hourly temperatures increased exponentially as the threshold temperature was decreased but, even at a threshold of 6 °C, the longest average period amounted to <5 d. The maximum consecutive periods of hours where temperature exceeded 8 °C amounted to little more than a single day over the 20 years of data analysed (Supplementary data Fig. S2).
Photosynthetic response to increasing temperature
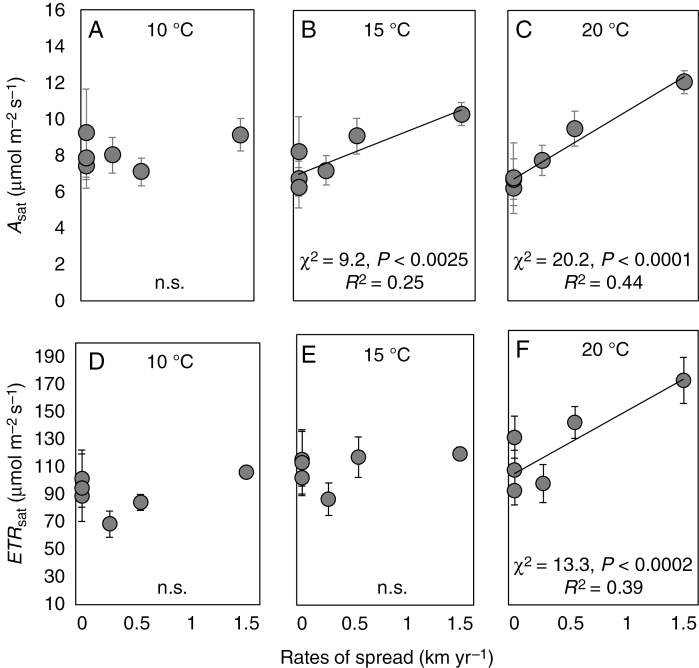
The light-saturated photosynthetic rate (Asat), estimated from curve fitting, ranged from around 6 μmol m–2 s–1 to as high as 14 μmol m–2 s–1 (Supplementary data Fig. S3) and at warmer temperatures correlated positively to the rate at which species have spread on Marion Island (Fig. 2A–C). The correlations were not significant at 10 °C, but both the regression coefficients and significance of the linear fits increased at 15 and 20 °C. The relationship at 20 °C did not depend only on the data from the species that has spread most rapidly on the island (Poa annua), and remained significant if these data were omitted from the analysis (χ2 = 5.3, P < 0.022, R2 = 0.20). Like Asat, ETRsat was only different between species at higher temperatures (Supplementary data Fig. S4) and was correlated to rates of spread, but only when the temperature was increased to 20 °C (Fig. 2E–G).
Fig. 2.
Light-saturated photosynthetic rate (Asat; A–C) and electron transport rate (ETRsat; D–F) measured at the three indicated temperatures correlated to the rate at which grass species have spread on Marion island. Points are means ± s.e. and lines were fitted to individual replicate data.
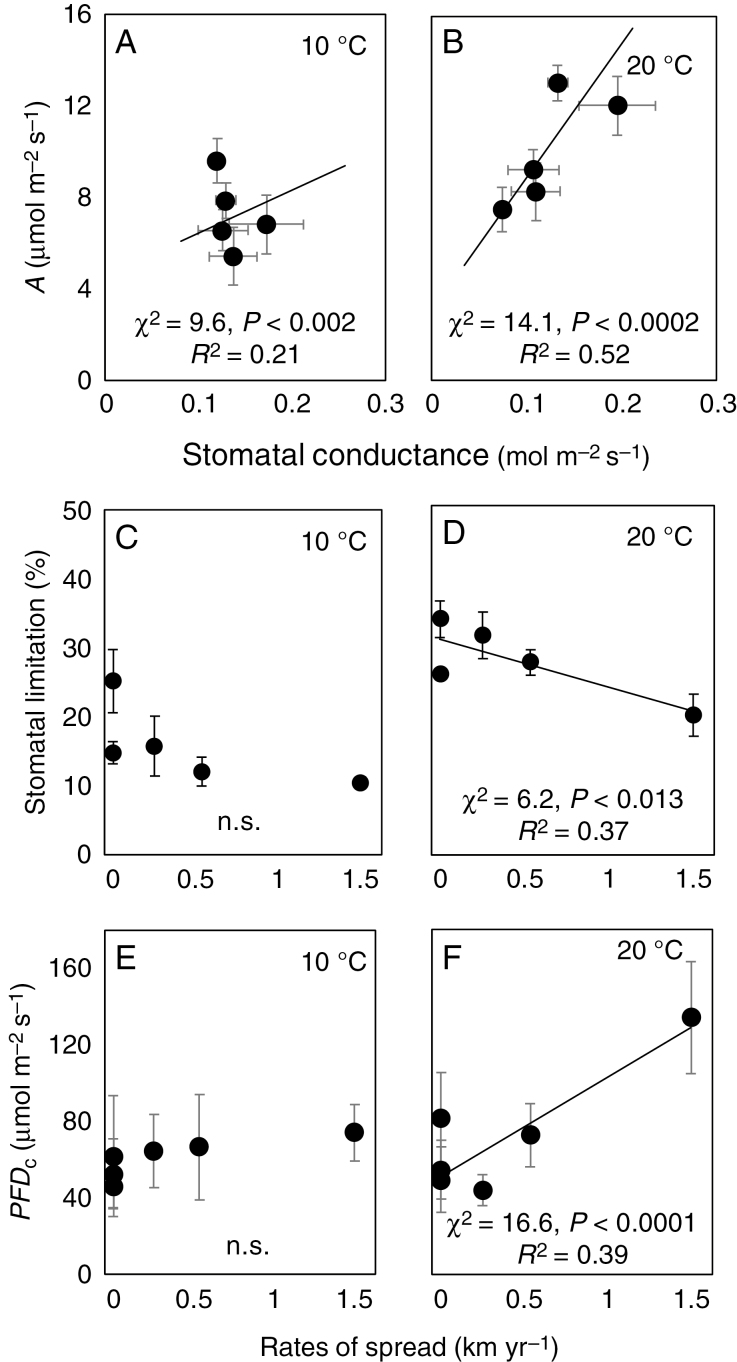
Photosynthetic rates (A) extracted from CO2 response curves (Supplementary data Fig. S5) showed the same temperature pattern as Asat, were higher in invasive than in non-invasive species and were positively correlated to rates of spread (data not shown; χ2 = 18.7, P < 0.0001, R2 = 0.52). Higher A was correlated with higher stomatal conductance (gST) at a both 10 and 20 °C, but the increase in the R2 value at 20 °C shows the increased dependence of A on gST at the higher temperature (Fig. 3A, B). This relationship meant that the stomatal limitation (SL) was negatively correlated to the rate at which species have spread and that the photosynthesis of fast spreading species was less limited by stomatal conductance than was that of the species which have not spread (Fig. 3C, D). This SL correlation was significant at 20 °C (χ2 = 6.2, P < 0.013, R2 = 0.37) but not at 10 °C.
Fig. 3.
Photosynthetic rate (A) correlated to stomatal conductance (A and B). Stomatal limitation (C and D) and photosynthetic light compensation points (PFDc; E and F) correlated to rates of spread. Correlations are compared between measurement temperatures of 10 and 20 °C. Net photosynthetic rates and stomatal conductance were extracted from A:Ci responses at an ambient CO2 concentration of 40 Pa and PFDc values were determined from A:PPFD responses. Filled circles are means (n ≥ 3) ± s.e. Lines were fitted to replicate data.
The light compensation point (PFDc) extracted for A:PPFD curves, which is an indication of rates of respiration, was higher for species showing the greatest rates of spread, but only when measurements were made at 15 °C (data not shown; χ2 = 13.5, P < 0.0002, R2 = 0.33) or 20 °C, and not at 10 °C (Fig. 3E, F).
Species’ abilities to spread were not related to differences in temperature responsiveness of non-stomatal photosynthetic metabolism. Rates of spread were not correlated to quantum efficiency of CO2 assimilation (ΦCO2), maximum rate of Rubisco carboxylation (Vcmax) or maximum rate of photosynthetic electron transport (Jmax) at either 10 or 20 °C (data not shown). However, an analysis that included temperature as an interaction showed that both Vcmax and Jmax were significantly higher at 20 °C than at 10°C (Vcmax: χ2 = 75.6, P < 0.0001; Jmax: χ2 = 5.0, P < 0.024), while ΦCO2 did not respond to temperature.
Chilling sensitivity and specific leaf area
Electrolyte leakage ranged from <1000 to almost 8200 μS cm–1 g–1 between species, and was positively correlated with the rate of spread (Fig. 4A). Like the response Asat, this analysis remained significant if the data for P. annua were omitted (χ2 = 10.8, P < 0.001, R2 = 0.49). This meant that species with high rates of spread had high electrolyte leakage. Hence, the species with the greatest photosynthetic temperature responsiveness were also the species that showed the greatest increase in chilling sensitivity, and had the greatest rates of spread.
Fig. 4.
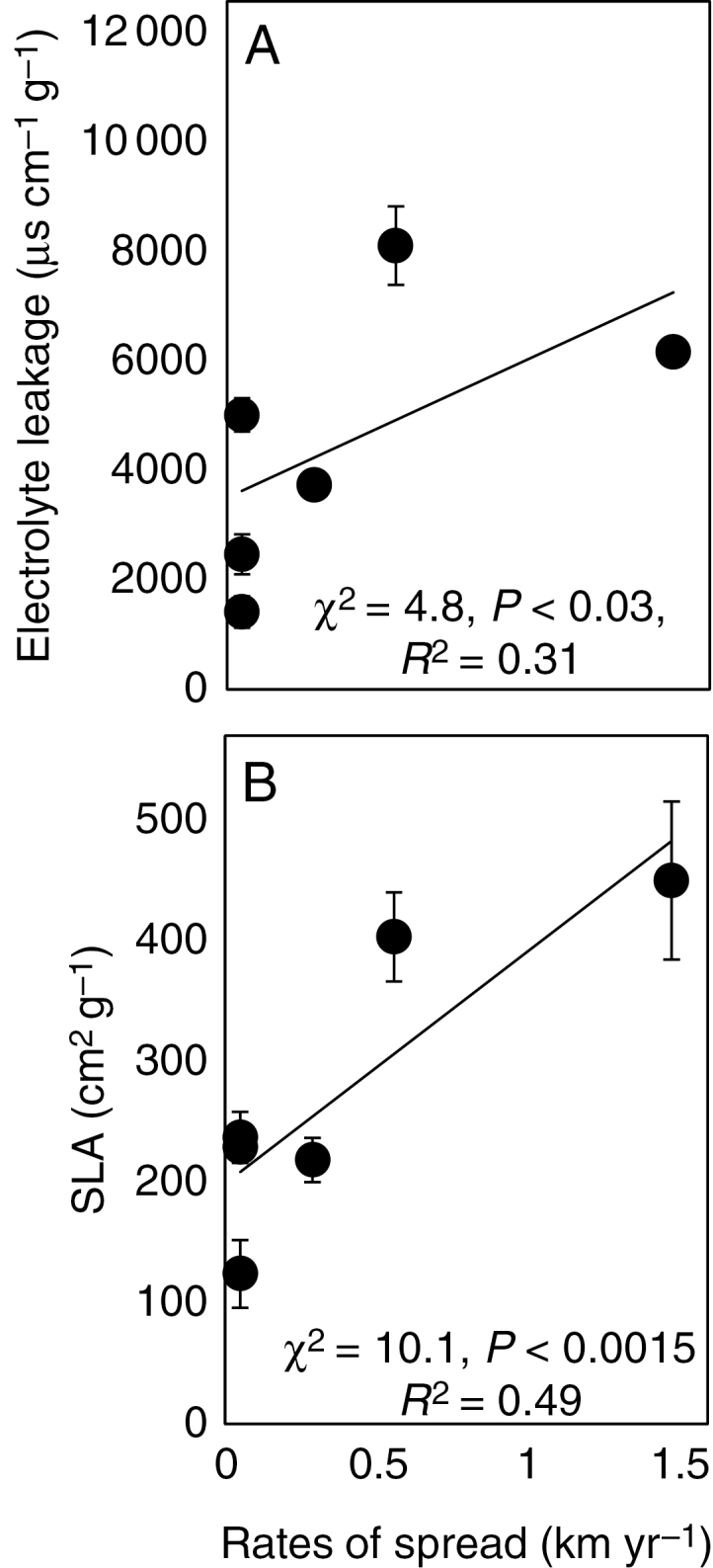
Electrolyte leakage (A) and SLA (B) in response to rates of spread. Filled circles are means (n ≥ 4) ± s.e. Lines were fitted to replicate data.
Like electrolyte leakage, SLA was positively correlated with the rate of spread (Fig. 4B). This meant that the species with the greatest rates of spread produced leaf area for the lowest biomass cost and had the most responsive photosynthetic increase to warming, but were most chilling sensitive.
Acclimation
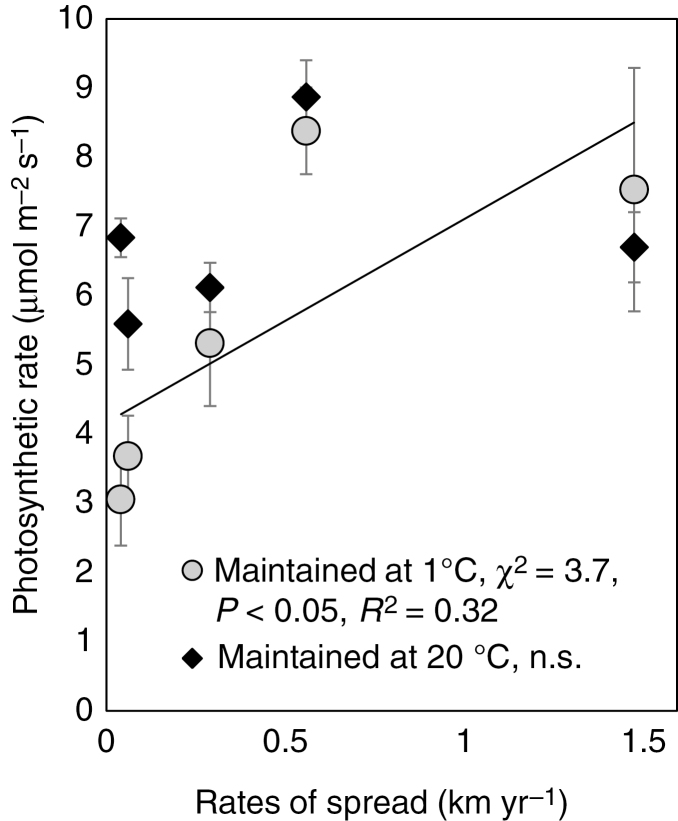
When pre-treated at 20 °C for 2 weeks, all species, irrespective of the rates at which they have spread on the island, had similar photosynthetic rates (Fig. 5). In contrast, when maintained at 1 °C, photosynthetic rates correlated to rates of spread. Photosynthetic rates of invasive species were unaffected by pre-treatment, with no significant difference between plants pre-treated at 1 or 20 °C.
Fig. 5.
Photosynthetic rates of grasses measured at 20 °C after a 2 week pre-treatment in growth chambers at either 1 or 20 °C. Filled circless are means (n ≥ 3) ± s.e. Lines were fitted to replicate data and the values have been staggered on the x-axis to improve visual appearance.
DISCUSSION
Surviving in cold terrestrial environments such as those of the sub-Antarctic requires a suite of traits, morphological and physiological, that confer stress tolerance. The selection of stress tolerance traits can limit phenotypic plasticity and the ability to survive and compete under conditions of changing climate (Valladares et al., 2007; Gratani, 2014). In contrast, phenotypic plasticity has been positively associated with biological invasions (Daehler, 2003; Pysek and Richardson, 2007) and with the ability to respond to changing conditions (Fordyce, 2006; Nicotra et al., 2010). Hence, in a historically thermally stable and cold environment that has recently experienced unprecedented rates of warming, the trade-off of these traits (chilling tolerance vs. warming responsiveness) is likely to differentiate between species, changing competitive hierarchies and their ability to spread across the environment. We demonstrate such a trade-off for grasses on Marion Island. Species that have not spread in the past 50 years were chilling tolerant, photosynthetically unresponsive to warming and had low SLA. In contrast, species that have been invading the island showed marked increases in photosynthesis with temperature, were chilling sensitive and had higher SLA.
The invasive species responded to warm temperatures irrespective of acclimation, while the non-spreading species did not. The ability to respond immediately to warm temperatures suggests a greater phenotypic plasticity, which would be advantageous under climatic conditions where warm episodes are sporadic and not sustained over long periods (several days). Under these conditions, a requirement for acclimation would be disadvantageous. Although we did not determine the minimum required length of acclimation, it generally takes days and may not be completed until new leaves develop (Talts et al., 2004; Atkin et al., 2006; citations in Vårhammar et al., 2014; Slot and Kitajima, 2015). Differences in plasticity may arise from evolutionary adaptation to fluctuations in temperatures found in the species’ native distribution range (Gratani, 2014). Greater plasticity may be a characteristic of fast-growing invasive species in comparison with slow-growing non-invasive counterparts (Atkin et al., 2006; Pysek and Richardson, 2007). Other studies on sub-Antarctic grasses have reached similar conclusions about phenotypic plasticity, which was higher in invasive than in native species (Molina-Montenegro et al., 2013). Invasions at high elevation, and hence low temperatures, are also associated with generalist species with broad environmental tolerances (Steyn et al., 2017).
Photosynthetic responsiveness was associated with increased stomatal conductance at warm temperatures and not with altered rates of Rubisco carboxylation or ribulose bisphosphate (RuBP) regeneration. The differential temperature responsiveness between species meant that at warm temperatures, the most invasive species had photosynthetic rates double those of the non-invasive species. Presumably, and as has been shown for other invasive species, high photosynthetic productivity underpins fast growth (Pattison et al., 1998), increased fitness (Molina-Montenegro et al., 2013) and, in combination with other functional traits, an increased ability to expand distributional range (Zinnert et al., 2013). Higher rates of respiration in the invasive species, as indicated by the values of PFDc, would support the suggestion of faster growth, as the two are frequently correlated (Amthor, 1989; Poorter and Bongers, 2006).
Associated with higher photosynthetic rates, SLA was higher in invasive than in non-invasive species, increasing carbon gain for biomass investment with positive effects on relative growth rates (Poorter and Remkes, 1990; Baruch and Goldstein, 1999; Rejmanek et al., 2005; Leishman et al., 2007). In contrast, decreased SLA has been linked to cold adaptation (He et al., 2006; Ma et al., 2010), chilling tolerance and lower photosynthetic capacity (Reich et al., 1997; Westoby et al., 2002; Wright et al., 2004). All these were evident for the non-invasive grass species that have inhabited Marion Island for the longest periods of time and established when the climate was cooler and chilling events more frequent. The recent warming with increased daily maxima and minima (le Roux and McGeoch, 2008), and the concomitant decrease in the frequency of chilling events, appears to have decreased the need for chilling tolerance while giving an advantage to invasive species that are chilling sensitive, but have higher productivity and are able to respond to warming without acclimation.
Our conclusions depend on the validity of assessments of rates of spread, and le Roux et al. (2013) paid particular attention to the possibility that historic surveys of Marion Island may not have detected all plants of a particular species. They defined their data as representing ‘a minimum assessment of area occupancy’, and used mapping procedures with a 30 s resolution which would have minimized potential error. We also measured electrolyte leakage at only two temperatures and hence cannot demonstrate a linear response between leakage and chilling within a species, and so our conclusions are limited to cell membrane damage across a 20 °C to –8 °C temperature drop.
All measured traits scaled linearly with the observed rates of range expansion among the Pooid grass species considered in this study, and key responses remained significant even when the data for species with the greatest range expansion were omitted. Similar differences in responsiveness may explain the highly species-specific range expansions that have been observed for 14 of the 22 vascular plant species found on Marion Island (le Roux et al., 2008b). Our findings demonstrate that species introduced from warmer environments may become invasive in cold environments under conditions of climate change, since they are phenotypically plastic and can respond to warming to the detriment of native species selected to tolerate stress. Understanding species-level trait trade-offs and their relationship with potential invasiveness advances our understanding of responses at community, ecosystem and functional group scales.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following.
Figure S1: number of consecutive days for months of the year that temperatures equalled or exceeded the indicated temperatures. Figure S2: maximum number of consecutive days within the indicated 10 year periods that temperatures equalled or exceeded values indicated on the x-axis. Figure S3: average A:PPFD responses for the indicated grass species. Figure S4: average ETR:PPFD responses for the indicated grass species. Figure S5: average A:Ci responses for the indicated grass species. Table S1: summary statistics of fits of photosynthetic rate and relative electron transport rate (ETR) to photosynthetically active photon flux density. Table S2: summary statistics of fits of photosynthetic rate to intercellular CO2 concentration.
FUNDING
We thank the South African National Research Foundation for funding under the South African National Antarctic Programme (SNGR grant SNA14072580160).
ACKNOWLEDGEMENTS
We thank the Department of Environmental Affairs for logistical support during field work. Field collections were carried out under permits cleared by the Prince Edward Islands Advisory Committee (PEIAC). B.R., V.S. and G.M. conceived the idea; B.R. designed the methods; B.R., A.E. and M.R. collected the data; and B.R. and G.M. wrote the manuscript, with contributions from the other authors. The authors have no competing interests to declare.
LITERATURE CITED
- Amthor JS. 1989. Respiration and crop productivity. New York: Springer Verlag. [Google Scholar]
- Atkin OK, Scheurwater I, Pons TL. 2006. High thermal acclimation potential of both photosynthesis and respiration in two lowland Plantago species in contrast to an alpine congeneric. Global Change Biology 12: 500–515. [Google Scholar]
- Atkin OK, Tjoelker MG. 2003. Thermal acclimation and the dynamic response of plant respiration to temperature. Trends in Plant Science 8: 343–351. [DOI] [PubMed] [Google Scholar]
- Armstrong AF, Badger MR, Day DA, et al. . 2008. Dynamic changes in the mitochondrial electron transport chain underpinning cold acclimation of leaf respiration. Plant, Cell & Environment 31: 1156–1169. [DOI] [PubMed] [Google Scholar]
- Atkin OK, Tjoelker MG. 2003Thermal acclimation and the dynamic response of plant respiration to temperature. Trends in Plant Science 8: 343–351. [DOI] [PubMed] [Google Scholar]
- Baruch Z, Goldstein G. 1999. Leaf construction cost, nutrient concentration, and net CO2 assimilation of native and invasive species in Hawaii. Oecologia 121: 183–192. [DOI] [PubMed] [Google Scholar]
- CABI 2017. Invasive species compendium. Wallingford, UK: CAB International; www.cabi.org/isc. [Google Scholar]
- von Caemmerer S. 2000. Biochemical models of leaf photosynthesis. Victoria, Australia: CSIRO Publishing. [Google Scholar]
- Causton DR, Dale MP. 1990. The monomolecular and rectangular hyperbola as empirical models of the response of photosynthetic rate to photon flux density, with application to three Veronica species. Annals of Botany 65: 389–394. [Google Scholar]
- Chown SL, Huiskes AHL, Gremmen NJM, et al. . 2012. a Continent-wide risk assessment for the establishment of nonindigenous species in Antarctica. Proceedings of the National Academy of Sciences, USA 109: 4938–4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chown SL, Lee JE, Hughes KA, et al. . 2012. b Challenges to the future conservation of the Antarctic. Science 337: 158–159. [DOI] [PubMed] [Google Scholar]
- Daehler CC. 2003. Performance comparisons of co-occurring native and alien invasive plants: implications for conservation and restoration. Annual Review of Ecology, Evolution, and Systematics 34: 183–211. [Google Scholar]
- Doxford SW, Freckleton RP. 2012. Changes in the large-scale distribution of plants: extinction, colonisation and the effects of climate. Journal of Ecology 100: 519–529. [Google Scholar]
- Farquhar GD, von Caemmerer S, Berry JA. 1980. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149: 78–90. [DOI] [PubMed] [Google Scholar]
- Fordyce JA. 2006. The evolutionary consequences of ecological interactions mediated through phenotypic plasticity. Journal of Experimental Biology 209: 2377–2383. [DOI] [PubMed] [Google Scholar]
- Genty B, Briantais JM, Baker NR. 1989. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta 990: 87–92. [Google Scholar]
- Gratani L. 2014. Plant phenotypic plasticity in response to environmental factors. Advances in Botany 2014: 1–17. [Google Scholar]
- Gremmen NJM. 1997. Changes in the vegetation of sub-Antarctic Marion Island resulting from introduced vascular plants. In: Battaglia B, Valencia J, Walton DWH, eds. Antarctic communities: species, structure and survival. Cambridge: Cambridge University Press, 417–423. [Google Scholar]
- Gremmen NJM, Smith VR. 2004. The flora of Marion and Prince Edward Islands CD-ROM. The Netherlands: Data Analyse Ecologie. [Google Scholar]
- He JS, Wang Z, Wang X, et al. . 2006. A test of the generality of leaf trait relationships on the Tibetan Plateau. New Phytologist 170: 835–848. [DOI] [PubMed] [Google Scholar]
- Huntley BJ. 1967. A preliminary account of the vegetation of Marion and Prince Edward Island. South African Journal of Science 63: 235–241. [Google Scholar]
- IPCC 2014. Climate change 2014: impacts, adaptation, and vulnerability. Part A: global and sectoral aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change Field CB, Barros VR, Dokken DJ, et al. eds. Cambridge, UK and New York, USA: Cambridge University Press, 1–32. [Google Scholar]
- Janmohammadi M, Zolla L, Rinalducci S. 2015. Low temperature tolerance in plants: changes at the protein level. Phytochemistry 117: 76–89. [DOI] [PubMed] [Google Scholar]
- Kennedy AD. 1995. Antarctic terrestrial ecosystem response to global environmental change. Annual Review of Ecology and Systematics 26: 683–704. [Google Scholar]
- Koehler K, Center A, Cavender-Bares J. 2012. Evidence for a freezing tolerance–growth rate trade-off in the live oaks (Quercus series Virentes) across the tropical–temperate divide. New Phytologist 193: 730–744. [DOI] [PubMed] [Google Scholar]
- Kolar CS, Lodge DM. 2001. Progress in invasion biology: predicting invaders. Trends in Ecology & Evolution 16: 199–204. [DOI] [PubMed] [Google Scholar]
- Leishman MR, Haslehurst T, Ares A, Baruch Z. 2007. Leaf trait relationships of native and invasive plants: community- and global-scale comparisons. New Phytologist 176: 635–643. [DOI] [PubMed] [Google Scholar]
- Lembrechts JJ, Pauchard A, Lenoir J, et al. . 2016. Disturbance is the key to plant invasions in cold environments. Proceedings of the National Academy of Sciences, USA 113: 14061–14066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SP, Bernacchi CJ. 2003. Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. Journal of Experimental Botany 54: 2393–2401. [DOI] [PubMed] [Google Scholar]
- Ma W, Shi P, Li W, He YT, Zhang XZ, Shen ZX, Chai SY. 2010. Changes in individual plant traits and biomass allocation in alpine meadow with elevation variation on the Qinghai–Tibetan Plateau. Science China: Life Sciences 53: 1142–1151. [DOI] [PubMed] [Google Scholar]
- Molina-Montenegro MA, Salgado-Luarte C, Oses R, Torres-Díaz C. 2013. Is physiological performance a good predictor for fitness? Insights from an invasive plant species. PLoS One 8: e76432. doi: 10.1371/journal.pone.0076432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Schielzeth H. 2013. A general and simplemethod for obtaining R2 from generalized linear mixed-effects models. Methods in Ecology and Evolution 4: 133–142. [Google Scholar]
- Nicotra AB, Atkin OK, Bonser SP, et al. . 2010. Plant phenotypic plasticity in a changing climate. Trends in Plant Science 15: 1360–1385. [DOI] [PubMed] [Google Scholar]
- Pattison RR, Goldstein G, Ares A. 1998. Growth, biomass allocation and photosynthesis of invasiveand native Hawaiian rainforest species. Oecologia 117: 449–459. [DOI] [PubMed] [Google Scholar]
- Pérez-Harguindeguy N, Díaz S, Garnier E, et al. . 2013. New handbook for standardised measurement of plant functional traits worldwide. Australian Journal of Botany 61: 167–234. [Google Scholar]
- Poorter H, Remkes C. 1990. Leaf area ratio and net assimilation rate of 24 wild species differing in relative growth rate. Oecologia 83: 553–559. [DOI] [PubMed] [Google Scholar]
- Poorter L, Bongers F. 2006. Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology 87: 1733–1743. [DOI] [PubMed] [Google Scholar]
- Pyšek P, Richardson DM. 2007. Traits associated with invasiveness in alien plants: where do we stand? In: Nentwig W, ed. Biological invasions. Berlin Heidelberg: Springer-Verlag,97–125. [Google Scholar]
- R Core Team 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Reich PB, Walters MB, Ellsworth DS. 1997. From tropics to tundra: global convergence in plant functioning. Proceedings of the National Academy of Sciences 94: 13730–13734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejmánek M, Richardson DM. 1996. What attributes make some plant species more invasive? Ecology 77: 1655–1661. [Google Scholar]
- Rejmánek M, Richardson DM, Higgins SI, Pitcairn MJ, Grotkopp E. 2005. Ecology of invasive plants: state of the art. In: Mooney HA, Mack RN, McNeely JA, Neville LE, Schei PJ, Waage JK, eds. Invasive alien species: a new synthesis. Washington, DC:Island Press, 104–161. [Google Scholar]
- le Roux PC, McGeoch MA. 2008. Changes in climate extremes, variability and signature on sub-Antarctic Marion Island. Climatic Change 86: 309–329. [Google Scholar]
- le Roux PC, Ramaswiela T, Kalwij JM, et al. . 2013. Human activities, propagule pressure and alien plants in the sub-Antarctic: tests of generalities and evidence in support of management. Biological Conservation 161: 18–27. [Google Scholar]
- Sadve SR, Rudi H, Asp T, Rognli OA. 2008. Tracking the evolution of a cold stress associated gene family in cold tolerant grasses. BMC Evolutionary Biology 8: 245. doi: 10.1186/1471-2148-8-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF, Kubien DS. 2007. The temperature response of C3 and C4 photosynthesis. Plant, Cell & Environment 30: 1086–1106. [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Bernacchi CJ, Farquhar GD, Singsass EL. 2007. Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant & Cell Physiology 30: 1035–1040. [DOI] [PubMed] [Google Scholar]
- Slot M, Kitajima K. 2015. General patterns of acclimation of leaf respiration to elevated temperatures across biomes and plant types. Oecologia 117: 885–900. [DOI] [PubMed] [Google Scholar]
- Smallwood M, Bowles DJ. 2002. Plants in a cold climate. Philosophical Transactions of the Royal Society B: Biological Sciences 357: 831–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NG, Dukes JS. 2013Plant respiration and photosynthesis in global-scale models: incorporating acclimation to temperature and CO2. Global Change Biology 19: 45–63. [DOI] [PubMed] [Google Scholar]
- Smith VR. 2002. Climate change in the sub-Antarctic: an illustration from Marion Island. Climate Change 52: 345–357. [Google Scholar]
- Sorte CJB, Ibáñez I, Blumenthal DM, et al. . 2013. Poised to prosper? A cross-system comparison of climate change effects on native and non-native species performance. Ecological Letters 16: 261–270. [DOI] [PubMed] [Google Scholar]
- Steyn C, Greve M, Robertson MP, Kalwij JM, le Roux PC. 2017. Alien plant species that invade high elevations are generalists: support for the directional ecological filtering hypothesis. Journal of vegetation science 28: 337–346. [Google Scholar]
- Talts P, Pärnik T, Gardeström P, Keerberg O. 2004. Respiratory acclimation in Arabidopsis thaliana leaves at low temperature. Journal of Plant Physiology 161: 573–579. [DOI] [PubMed] [Google Scholar]
- Tieszen LL, Lewis MC, Miller PC, Mayo J, Chapin FS, Oechel W. 1981. An analysis of processes of primary production in tundra growth forms. In: Bliss LC, Heal OW, Moore JJ, eds. Tundra ecology: a comparative analysis. Cambridge: Cambridge University Press, 285–356. [Google Scholar]
- Valladares F, Gianoli E, Gómez JM. 2007. Ecological limits to plant phenotypic plasticity. New Phytologist 176: 749–763. [DOI] [PubMed] [Google Scholar]
- van Kleunen M, Weber E, Fischer M. 2010. A meta-analysis of trait differences between invasive and non-invasive plant species. Ecological Letters 13: 235–245. [DOI] [PubMed] [Google Scholar]
- Vårhammar A, Wallin G, McLean CM, et al. . 2014. Photosynthetic temperature responses of tree species in Rwanda: evidence of pronounced negative effects of high temperature in montane rainforest climax species. New Phytologist 206: 1000–1012. [DOI] [PubMed] [Google Scholar]
- Vilà M, Espinar JL, Hejda M, et al. . 2011. Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecological Letters 14: 702–708. [DOI] [PubMed] [Google Scholar]
- Walther R-G. 2010. Community and ecosystem responses to recent climate change. Philosophical Transactions of the Royal Society B: Biological Sciences 365: 2019–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westoby M, Falster DS, Moles AT, Vesk PA, Wright IJ. 2002Plant ecological strategies: some leading dimensions of variation between species. Annual Review of Ecology and Systematics 33: 125–159. [Google Scholar]
- Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F. 2004. The worldwide leaf economics spectrum. Nature 428: 821–827. [DOI] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Cornelissen JHC, et al. . 2005. Modulation of leaf economic traits and trait relationships by climate. Global Ecology & Biogeography 14: 411–421. [Google Scholar]
- Xiong FS, Ruhland CT, Day TA. 1999. Photosynthetic temperature response of the Antartic vascular plants Colobanthus quitensis and Deschampsia antartica. Physiologia Plantarum 106: 276–286. [Google Scholar]
- Yamashita Y, Nakamura N, Omiya K, et al. . 2002. Identification of an antifreeze lipoprotein from Moraxella sp. of Antarctic origin. Bioscience, Biotechnology, and Biochemistry 66: 239–247. [DOI] [PubMed] [Google Scholar]
- Zhen Y, Ungerer MC. 2008. Clinal variation in freezing tolerance among natural accessions of Arabidopsis thaliana. New Phytologist 177: 419–427. [DOI] [PubMed] [Google Scholar]
- Zhen Y, Dhakal P, Ungerer MC. 2011. Fitness benefits and costs of cold acclimation in Arabidopsis thaliana. American Naturalist 178: 44–52. [DOI] [PubMed] [Google Scholar]
- Zinnert JC, Shiflett SA, Vick JK, Young DR. 2013. Plant functional traits of a shrub invader relative to sympatric native shrubs. Ecosphere 4: 1–119. [Google Scholar]
- Zúñiga GE, Alberdi M, Fernández J, Montiel P, Corcuera LJ. 1994. Lipid content in leaves of Deschampsia antarctica Desv. from Maritime Antarctic. Phytochemistry 37: 669–672. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.