Abstract
Background and Aims
There are a number of disparate models predicting variation in plant chemical defences between species, and within a single species over space and time. These can give conflicting predictions. Here we review a number of these theories, before assessing their power to predict the spatial–temporal variation of thapsigargins between and within populations of the deadly carrot (Thapsia garganica). By utilizing multiple models simultaneously (optimum defence theory, growth rate hypothesis, growth–differentiation balance hypothesis, intra–specific framework and resource exchange model of plant defence), we will highlight gaps in their predictions and evaluate the performance of each.
Methods
Thapsigargins are potent anti-herbivore compounds that occur in limited richness across the different plant tissues of T. garganica, and therefore represent an ideal system for exploring these models. Thapsia garganica plants were collected from six locations on the island of Ibiza, Spain, and the thapsigargins quantified within reproductive, vegetative and below-ground tissues. The effects of sampling time, location, mammalian herbivory, soil nutrition and changing root-associated fungal communities on the concentrations of thapsigargins within these in situ observations were analysed, and the results were compared with our model predictions.
Key Results
The models performed well in predicting the general defence strategy of T. garganica and the above-ground distribution of thapsigargins, but failed to predict the considerable proportion of defences found below ground. Models predicting variation over environmental gradients gave conflicting and less specific predictions, with intraspecific variation remaining less understood.
Conclusion
Here we found that multiple models predicting the general defence strategy of plant species could likely be integrated into a single model, while also finding a clear need to better incorporate below-ground defences into models of plant chemical defences. We found that constitutive and induced thapsigargins differed in their regulation, and suggest that models predicting intraspecific defences should consider them separately. Finally, we suggest that in situ studies be supplemented with experiments in controlled environments to identify specific environmental parameters that regulate variation in defences within species.
Keywords: Thapsia garganica, Apiaceae, chemical defence, herbivory, soil, root-associated fungi, fungal inhibition, plant defence model, optimal defence theory, growth rate hypothesis, growth–differentiation balance hypothesis, resource exchange model of plant defence
INTRODUCTION
How plants optimize and utilize their energy supply under stress has long been of interest to ecologists and evolutionary biologists alike (Thornley, 1972). In this regard, the process of herbivory has been a major focus for the majority of studies, where a plant balances the fitness cost of a loss of photosynthetic capacity against the cost of defence (Tuomi et al., 1988). There is a plethora of specialized metabolites used in chemical defence against herbivores, with compounds making tissue unpalatable due to toxicity, but they all require photosynthates for their production (Gershenzon, 2017). However, when not required, the production of specialized anti-herbivore compounds has repeatedly been shown to negatively impact growth rates, and consequently the level of chemical defences is tightly regulated (Sampedro et al., 2011). Defence compounds can be constitutively produced, but will vary substantially in quantity both within different tissues of an individual plant (Gershenzon, 2017) and between individuals of the same species (Maldonado et al., 2017). Alternatively, chemical defences can be induced when the plant is antagonized by specific stimuli (Moreira et al., 2014). Consequently, a number of different models have been developed to predict the resource allocation of plants to specialized anti-herbivore metabolites. However, different models often give conflicting predictions, with many exceptions observed (Le Bot et al., 2009; Gutbrodt et al., 2012; Kleczewski et al., 2012) and no clear optimum approach established. A better understanding of the resource allocation for plant chemical defence is needed to help guide ecological studies, conservation efforts and sustainable production of medicinal and other useful natural products.
Evolutionary theories of plant defence investment
The optimal defence theory (ODT) was one of the earliest models to consider defence through an evolutionary perspective (McKey, 1974; Rhoades, 1979). It predicts that with increasing herbivory the relative cost of chemical defence is reduced, and this can be over evolutionary time frames or within a single life cycle. While the ODT suggests that there are competing functions for resources, so that defence has a cost in terms of growth and reproductive success, other defensive theories have more explicit expectations in this regard and are preferred (Stamp, 2003). However, it remains the only major model to address in planta variation, predicting that tissues of higher value to plant fitness will be better defended. It has performed well in predicting the relative defence levels of flowers (Kessler and Halitschke, 2009) and fruits (Zangerl and Rutledge, 1996), but performs less well for tissues with lower fitness values, like leaves. However, as a general rule, vegetative tissue is suggested to have a lower fitness value than reproductive tissue, and is therefore less protected (Zangerl and Bazzaz, 1992; Hamilton et al., 2001).
The growth-rate hypothesis (GRH), which is sometimes referred to as the resource availability hypothesis (RAH; Coley et al., 1985), expands upon the ODT by suggesting that plant defences and growth rates vary around their ability to regrow after a herbivory event. Plants that have evolved within low-resource or stressful environments have slower growth rates and allocate a higher proportion of photosynthates to defences instead of growth (Karban and Baldwin, 1997; Grime, 2006). Consequently, they are more likely to have evolved a suite of resistance traits, including higher levels of constitutive chemical defences and fewer induced defences than their faster-growing counterparts (Coley, 1988; Grime, 1977, 2006). Similarly, the growth–differentiation balance hypothesis (GDBH) model also predicts a plant’s level of chemical defence in response to resource availability. Under the GDBH, two processes regulate the plant’s entire photosynthate use. Growth refers to any process requiring substantial cell division and elongation, such as the production of roots, stems and leaves, whilst differentiation is essentially everything else, including herbivore defence (Loomis, 1932, 1953). Shortages of nutrients and water are thought to slow growth more than photosynthesis. Hence, as carbohydrates accumulate within the plant, the fitness reduction for differentiation processes is lower. Growth and differentiation are hypothesized to be mutually exclusive. Therefore, plants invest in growth-based strategies in resource-rich environments (high competition) and in differentiation-based strategies in low-resource environments (low competition).
Theories of defence investment in response to environmental variation
Both the GRH and GDBH can also be applied to predict the level of defence within a single species, where differences are more likely derived from environmental rather than evolutionary processes. Unlike the GRH, the GDBH hypothesizes that maximum chemical defence occurs when enough nutrients are present to allow specialized metabolites to be readily synthesized, but before nutrient levels drive growth-based life strategies (as opposed to increasing nutrients leading to decreasing defences under the GRH). This was developed in part due to multiple studies finding that defence compounds were in the highest concentrations at intermediary levels of nutrition (Mihaliak and Lincoln, 1985; Wilkens et al., 1996; Glynn et al., 2007; Scogings, 2018). This suggests that plants form a growth–differentiation continuum over environmental gradients (Herms and Mattson, 1992).
Ecological processes vary over differing geographical scales (Laughlin and Messier, 2015). The macro- and micro-evolutionary processes that shape patterns in chemical defences differently may also vary at different scales in much the same way. For example, well-defended plants were found to reduce chemical defences with increasing soil nutrients (Nerg et al., 1994; Wainhouse and Ashburner, 1996; Woods et al., 2012; Pratt and Mooney, 2013; Couture et al., 2015; Agrawal et al., 2015; Stevens et al., 2016), whilst faster-growing but less defended species showed no variation in chemical defences associated with changing soil nutrients (Hahn and Maron, 2016). Upon noting these likely differences between inter- and intraspecific variation in chemical defences, Hahn and Maron (2016) theorized that broad resource environments select for growth–defence strategies across species (i.e. high- or low-resource environments) as stated in the GRH and GDBH, but extend this to hypothesize that intraspecific growth–resistance relations are selected by smaller-scale environmental factors, such as herbivore community composition (Abdala-Roberts et al., 2015), climatic variation (Woods et al., 2012; Pratt and Mooney, 2013; Anstett et al., 2015) and growing season length (Lehndal and Ågren, 2015), alongside more fine-scale fluctuations in nutrients. They go on to construct a framework for predicting intraspecific patterns of plant growth and defence (intraspecific framework, ISF) within environmental gradients, predicting that increasing resource availability across an environmental gradient will select for faster growth rates within species. They further suggest that constitutive and induced defences are differentially regulated, with constitutive defences indirectly positively correlated with resources within species, while induced defences are favoured in lower-resource environments.
Theories of defence investment in response to changing plant-associated microbes
The vast majority of plants form mutualistic associations with microbes (van der Heijden et al., 2015), potentially changing their nutrient profiles (Barrow and Osuna, 2002; Porras-Soriano et al., 2009). Nonetheless, they were not incorporated into models of plant defence until relatively recently (Vannette and Hunter, 2011). The resource exchange model of plant defence (REMPD) looks to integrate nutrient exchange dynamics between plants and soil mutualists in order to predict plant defences within a species, but is the least experimentally validated model (Román et al., 2011; Vannette and Hunter, 2011, 2013; Barber et al., 2013; Xiao et al., 2013; Meier and Hunter, 2018). Finding links between specific plant-associated fungi and plant chemical defence is complex due to the enormous array of fungi, bacteria and other soil microorganisms that can simultaneously interact with a single plant (van der Heijden et al., 2015). This makes the identification of groups or individuals associated with changing plant chemistry extraordinarily challenging. As a result, support for the REMPD comes predominantly from in vitro studies. However, in the last decade high-throughput sequencing techniques have revolutionized the process of characterizing complex microbial communities. This can in turn be used to attempt to understand microbial functioning in processes (Barnes et al., 2018), such as in situ plant chemical defence.
Predicting and validating variation of thapsigargins in Thapsia garganica
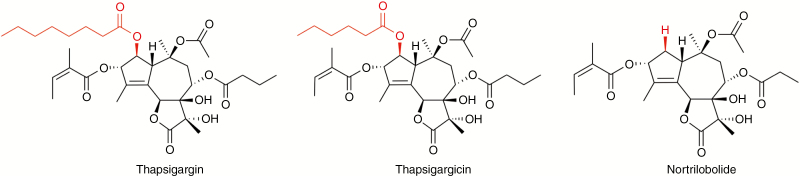
Thapsia (Apiaceae) species are commonly referred to as deadly carrots, since reports of their poisonous qualities have emerged (Gerard et al., 1597). Their toxicity has been known for centuries, with citations dating back to Theophrastus (372–287 BC). It is a small genus of herbaceous perennials (Weitzel et al., 2014), restricted to the Mediterranean from Portugal and Morocco to Turkey and Greece (Smitt et al., 1996), with Thapsia garganica being the most widely distributed species (Pujadas-Salvà and Rosselló, 2003). Thapsia garganica is reported to contain three major anti-herbivore compounds (thapsigargins): thapsigargin (Rasmussen et al., 1978; Andersen et al., 2015), thapsigargicin (Christensen et al., 1982) and nortrilobolide (Smitt and Christensen, 1991) (Fig. 1). They have been found to be potent inhibitors of the sarco-endoplasmic reticulum Ca2+-ATPase (SERCA) in mammalian cells, leading to cell apoptosis (Thastrup et al., 1990), thereby explaining the remarkable method by which T. garganica keeps mammalian herbivores at bay. Accordingly, T. garganica is very easy to spot in fields, as its toxicity prevents it from being grazed and it remains tall compared with the surrounding vegetation (Fig. 2A, B, D). The concentration of thapsigargin varies greatly between individuals, with T. garganica containing 0.2–1.2 and 0.7–1.5 % of thapsigargin within the roots and the ripe fruits, respectively (Smitt et al., 1995).
Fig. 1.
Chemical structures of thapsigargin, thapsigargicin and nortrilobolide used in this study, with differences in structure highlighted in red.
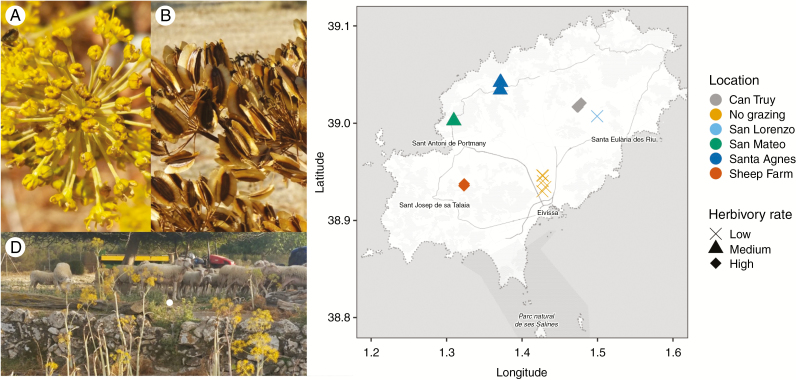
Fig. 2.
Thapsia garganica (A) flowers and (B) fruits. (C) Map of sampling locations collected across the island of Ibiza. (D) T. garganica populations growing under intense herbivory pressure at the sheep farm site.
Investigations into plant chemical defences are simplified when a small array of compounds are expressed throughout a plant (Zangerl and Rutledge, 1996). Therefore, with its small number of variable thapsigargins present across tissue types, T. garganica represents an ideal system for exploring models of plant chemical defence. In this work, we evaluate the predictive power of the aforementioned five models (ODT, GRH, GDBH, ISF and REMPD) by using them to predict the distribution and variation of the three major thapsigargins within T. garganica (Table 1). We subsequently quantified thapsigargins from naturally occurring specimens of T. garganica, with populations spanning a mammalian herbivory gradient within its native range on the island of Ibiza. Plants were partitioned into reproductive tissue (flowers, fruits, bracts), vegetative tissue (leaves, senesced leaves, stems) and below-ground tissue (root periderm and inner root). Temporal variation was assessed by repeat sampling once every 3 weeks over a 6-week period (three sampling points). Soil nutrition was recorded for each plant, alongside DNA metabarcoding of the root-associated fungal communities. Using these extensive collections and metadata, predictions from each model were evaluated for their ability to predict the in situ variation of thapsigargins between and within individuals of T. garganica, and the factors that regulate them.
Table 1.
Predictions produced from models of plant defences, predicting within- and between-plant variations in thapsigargins within T. garganica
| (A) Predicting the general defence strategy in T. garganica | |
| Theory | Prediction |
| Optimal defence theory (ODT) | A1 T. garganica is highly apparent, and is therefore highly defended |
| Growth rate hypothesis (GRH) | A2 T. garganica is adapted to low resources, and therefore has high constitutive defences and low induced defences |
| Growth–differentiation balance hypothesis (GDBH) | A3 Herbivory is a far greater threat than competition; therefore T. garganica is highly defended |
| (B) Predicting in planta variation of thapsigargins within T. garganica | |
| Theory | Prediction |
| ODT | B1 Thapsigargins vary in concentration between tissue types, and are highest in reproductive tissue |
| (C) Predicting intraspecific variation of thapsigargins within T. garganica | |
| Theory | Prediction |
| ODT | C1a Thapsigargins will increase with increasing herbivory rates |
| C1b Thapsigargins will vary in concentration temporally | |
| GRH | C2 Increasing nutrients decrease thapsigargin concentrations |
| GDBH | C3 Increasing nutrients increase thapsigargin concentrations to a maximum, before declining with further nutrients |
| Resource exchange model of plant defence (REMPD) | C4 Changing fungal communities are associated with changing thapsigargin concentrations |
| Intraspecific framework (ISF) | C5a There is a positive correlation between resources and constitutive defences |
| C5b Decreasing resources will increase induced defences |
METHODS
Sample collection
Sampling of Thapsia garganica was performed from early May to the end of June 2015 on Ibiza, Spain, during three sampling trips separated by 3-week intervals. Six sampling locations were chosen (Table 2 and Fig. 2C) that were of varying land use and mammalian herbivory pressure. Herbivory pressure was determined from the description of land use given by the landowners as low (fenced fields to keep grazing cattle out), medium (occasionally grazed over the year, but lacking fencing) and high (daily presence of cattle) (see land use descriptions in Table 2).
Table 2.
Collection sites chosen in Ibiza, Spain, along with sampling location names
| Sampling location | GPS coordinates | Land use | Herbivory level | C:N ratio | Phosphorus (mg kg−1 ) | Potassium (mg kg−1 ) | pH |
|---|---|---|---|---|---|---|---|
| No grazing | 38°56′47″ N, 1°25′36″ S | Disused farm land, fenced | Low | 38.69 ± 10.27 | 24.16 ± 7.96 | 10 233.5 ± 3876.0 | 8.4 ± 0.2 |
| San Lorenzo | 39°0′26″ N, 1°29′58″ S | Disused almond grove, grass overgrown, not grazed, partially fenced | Low | 8.68 ± 1.34 | 33.68 ± 1.38 | 5499.1 ± 235.6 | 8.3 ± 0.1 |
| Santa Agnes | 39°0′10″ N, 1°18′34″ S | Almond grove, ground cleared, infrequently grazed | Medium | 35.50 ± 10.09 | 13.72 ± 6.04 | 5529.0 ± 417.5 | 8.3 ± 0.3 |
| San Mateo | 39°2′31″ N, 1°22′16″ S | Olive and almond grove, ground ploughed | Medium | 20.04 ± 7.109 | 23.64 ± 2.34 | 5096.5 ± 411.2 | 8.4 ± 0.1 |
| Can Truy | 39°1′00″ N, 1°28′27″ S | Grazing ground for sheep, grazed frequently but not daily | High | 27.08 ± 10.01 | 19.68 ± 8.73 | 4615.7 ± 534.7 | 8.4 ± 0.1 |
| Sheep farm | 38°56′10″ N, 1°19′24″ S | Sheep enclosure, cattle present daily | High | 33.83 ± 3.78 | 15.92 ± 5.77 | 18 285.1 ± 6274.7 | 8.4 ± 0.2 |
Sampling was targeted to be approximately before fruiting, when leaves are active, but also during and after fruiting, when leaves have senesced. For every individual we sampled roots and, when possible, stems, leaves (fresh or senesced), bracts, flowers and fruits, but as a result of the above-ground biomass of T. garganica senescing over the sampling period, sampling numbers were limited during the second and third sampling times (Table 3). Plant chemical defences can be stored in specialized structures to avoid autotoxicity (Rosenthal and Berenbaum, 1992) and MALDI imaging of the roots has shown thapsigargins to be concentrated near the root periderm of T. garganica (Andersen et al., 2017). We therefore partitioned root samples into the outer periderm and inner tissue, sampling 10–20 cm below ground. At each site, five plants were destructively sampled, with a voucher specimen deposited at the herbarium of the Natural History Museum of Denmark (C). Plant tissue samples for HPLC analysis were collected, dried in silica gel and stored in darkness. Fresh samples for DNA analysis were kept in RNAlater™ (Invitrogen, USA) at 4 °C during the field stay (maximum 3 d) and stored at −20 °C upon return. Additionally, ~200 g of soil was collected from the base of each plant from the upper 20 cm of the soil profile, loosely covered and air-dried.
Table 3.
Full list of samples that underwent HPLC analysis to quantify thapsigargins, over the 13-week sampling period
| Area | Leaves | Flowers | Fruit | Root | Periderm | Bract | Stem | Time point |
|---|---|---|---|---|---|---|---|---|
| Sheep farm | 4 | 5 | 2 | 5 | 5 | Week 1 | ||
| CanTruy | 5 | 5 | 5 | 5 | 2 | |||
| SanMateo | 6 | 7 | 5 | 5 | ||||
| SantAgnes | 5 | 10 | 5 | 5 | ||||
| SanLorenzo | 3 | 5 | 1 | 5 | 5 | 1 | ||
| NoGrazing | 3 | 6 | 5 | 5 | ||||
| Sheep farm | 2 | 1 | 5 | 4 | 4 | Week 7 | ||
| CanTruy | 5 | 3 | 5 | 3 | 3 | |||
| SanMateo | 2 | 2 | 2 | 3 | 3 | |||
| SantAgnes | 1 | 1 | 1 | |||||
| SanLorenzo | ||||||||
| NoGrazing | 3 | 3 | 6 | 5 | ||||
| Sheep farm | 3 | 2 | 1 | 1 | 3 | 3 | Week 13 | |
| CanTruy | 2 | 3 | 3 | |||||
| SanMateo | 2 | 2 | 2 | 3 | 4 | |||
| SantAgnes | ||||||||
| SanLorenzo | ||||||||
| NoGrazing | 1 | 1 | 1 | 2 | 2 |
HPLC analyses
To identify thapsigargin, thapsigargicin and nortrilobolide, standards previously extracted from T. garganica fruits were used (López et al., 2018). For each compound, standard solutions diluted in 80 % methanol were prepared in triplicate (dilution concentrations: 5, 25, 50, 200, 400, 500, 600, 800 and 1000 µg mL−1). To quantify the analytes of interest, calibration curves were generated by running the standard dilutions in triplicate on an HPLC. Peak areas were plotted against the concentrations of the standard dilutions; the calibration curve for each standard had a correlation coefficient of 0.999.
Silica-dried plant material was homogenized in liquid nitrogen by mortar and pestle followed by 5 min at 30 Hz in a Tissue Lyser II (Qiagen, Germany) with 2-mm glass beads in 2-mL Eppendorf tubes, before being analysed by HPLC as previously outlined by Mohamed Ibrahim et al. (2018) (Supplementary Data Method S1).
Characterizing the abiotic environment
To determine the edaphic properties of the soil, samples were sieved to <2 mm and homogenized by mortar and pestle. The pH of soil samples was determined by mixing 10 mL of soil with 25 mL of Milli-Q water, vigorously mixing for 15 min on a mixing tray at room temperature before immediately measuring the pH with a meter (Mettler Toledo, Denmark). Both the total carbon and nitrogen available to the plant in the soil (CNS analysis) was calculated by mixing 50 mg of soil with 50 mg of tungsten and compressing into a ball in inert foil. Samples underwent a dry combustion procedure on a Vario Macro Cube Elementar analyser (Elementar Analysen Systeme, Germany) using sulphanilamide and acetanilide as standard compounds for the calibration of the results. Phosphorus (P) and potassium (K) were simultaneously measured, with soil digested in a mixture of 2.5 mL of 70 % HNO3 (nitric acid in distilled water) in a pressurized microwave oven (25 min, 154 bars, 250 °C, 1500 W; Ultrawave, Milestone, USA). Following digestion, samples were diluted in 50 mL of distilled water and underwent a multi-elemental analysis using ICP-OES inductively coupled plasma optical emission spectrometry (Model 5100, Agilent) coupled with a Meinhard nebulizer and cyclonic spray chamber (flow injection volume 50 µL, flow rate 0.2 mL min−1, mobile phase 3.5 % HNO3) following Olsen et al. (2016). The instrument was run in collision mode with helium gas. Data were acquired and processed using the MassHunter 4.1 chromatographic software package. Elements were calculated as previously described (Olsen et al., 2016) and validated using external calibration standards corresponding to elemental ratios typically found in plants, with data quality ensured using NIST 1515 Apple Leaf certified reference material (National Institute of Standards and Technology, USA). Data that exceeded the limit of quantification were accepted, with accuracy >95 % and the coefficient of variation between five replicates <3 %.
Fungal metabarcoding and toxicity screening
DNA from the periderm and inner root was extracted using a PowerSoil DNA isolation kit (MP Biomedicals, USA) according to the manufacturer’s guidelines, with lysis performed by two rounds of mechanical lysis at 30 Hz for 30 s in a TissueLyser II (Qiagen, Germany). DNA extracts underwent DNA metabarcoding as described by Barnes et al. (2016), using the universal fungal internal transcribed spacer (ITS) primers glITS7 (Ihrmark et al., 2012) and ITS4 (Gardes and Bruns, 1993) (Supplementary Data Method S2).
From a total of 1 946 964 reads produced via metabarcoding, a total of just 6121 were assigned to fungal species (0.3 %), with reads derived from both the root periderm and inner root samples primarily consisted of reads from the plant host (0.5 and 0.3 % respectively). Given the lack of fungal reads in the inner roots and periderms of T. garganica, the toxicities of thapsigargin, thapsigargicin and notrilobolide were tested in vitro. Petri dishes were established by adding 100 µL (1000 mg L−1 stock) of one of the three thapsigargins to 20 mL of potato dextrose agar (PDA) giving a final concentration of 5 mg L−1. Additionally, plates containing 100 µL of pooled root extracts (that had been quantified by HPLC) in 20 mL of PDA were also established, giving final concentrations of 3.7 µg L−1 thapsigargin, 2.1 µg L−1 thapsigargicin and 0.8 µg L−1 nortrilobolide. Untreated PDA plates served as controls. Common plant-associated fungi from the two most abundant phyla [a Fusarium sp. (Ascomycota) and a Clitopilus sp. (Basidiomycota)] were initaily grown in untreated PDA and incubated until a homogeneous fungal mycelium had formed. Single 4-mm diameter plugs of each fungus were transplanted into each of the four treatment plates and the untreated PDA controls (n = 15 for each fungus). For each plate, the diameter of fungal colonies was measured in two places in a cross formation over time, and repeated until the control colonies were spread over three-quarters of the Petri dish.
Statistical analyses
All statistical analyses were performed in statistical computing environment R. Due to sampling time 1 being the most complete, and to avoid the confounding effects of temporal variation, correlations of expression of thapsigargins with locality and mammalian herbivory and between plant parts were investigated from samples collected during this time point only. Correlations between compounds were assessed using Pearson’s correlation coefficients. Mixed linear modelling was performed to assess differences in individual thapsigargins between tissue types, sites and mammalian herbivory levels, with the plant of origin serving as the random effect. Significance was calculated for each by likelihood ratio testing (χ2) performed using the drop1 function in the lme4 package (Bates et al., 2014). Meanwhile, as it was only possible to sample above-ground tissue in some of the sampling times, statistical analysis of temporal variation was limited to the below-ground tissue of time points 1 and 2 only. Significant temporal variation of the three thapsigargins was assessed in the root periderm and inner root independently, using Mann–Whitney tests within R’s native statistics package.
As the below-ground tissue from sampling time 1 was ubiquitously available, the effects of environmental variation on the concentration of thapsigargins was investigated using partitioned root periderm and inner root tissue independently. Initially, the co-expression of (log10 normalized) thapsigargins in the root periderm and inner roots was investigated with paired t-tests. Individual thapsigargins (log10 normalized) from either the root periderm or inner roots underwent mixed linear modelling to test for environmental variation in each of the three major defence compounds (with soil pH, C:N ratio, P, K and mammalian herbivory serving as fixed effects and plant part serving as the random effect). As before, likelihood ratio tests (χ2) were performed using the drop1 function to identify significantly correlated variables.
In assessing fungal toxicity, two-way ANOVAs were performed for each of the Fusarium sp. and Clitopilus sp. fungi, where treatment (controls, thapsigargin, thapsigargicin, nortrilobolide and plant extracts) and time (in hours after inoculation) were analysed against the rank of diameter (due to lack of normality).
RESULTS
Predicting the general defence strategy of T. garganica
All three thapsigargins were detectable in some of the sample types, but varied substantially in concentration both between and within tissue types. Thapsigargin itself ranged from being undetectable to 5.76 mg kg−1, and was the most abundant defence compound with a mean concentration of 0.70 ± 0.07 mg kg−1. Thapsigargicin was also extremely variable and the second most abundant of the thapsigargins, which ranged from being undetectable to 8.05 mg kg−1, with a mean of 0.39 ± 0.07 mg kg−1. Thapsigargin and thapsigargicin strongly correlated with each other (rs = 0.780, P > 0.001) and were both detectable in the majority of samples analysed (94.6 %). Nortrilobolide had a mean concentration of just 0.12 ± 0.02 mg kg−1 and was undetectable in 64.3 % of samples. While nortrilobolide was weakly correlated with thapsigargin (rs = 0.200, P = 0.041), it was not correlated with thapsigargicin (rs = 0.186, P = 0.060).
Predicting in planta variation of thapsigargins in T. garganica
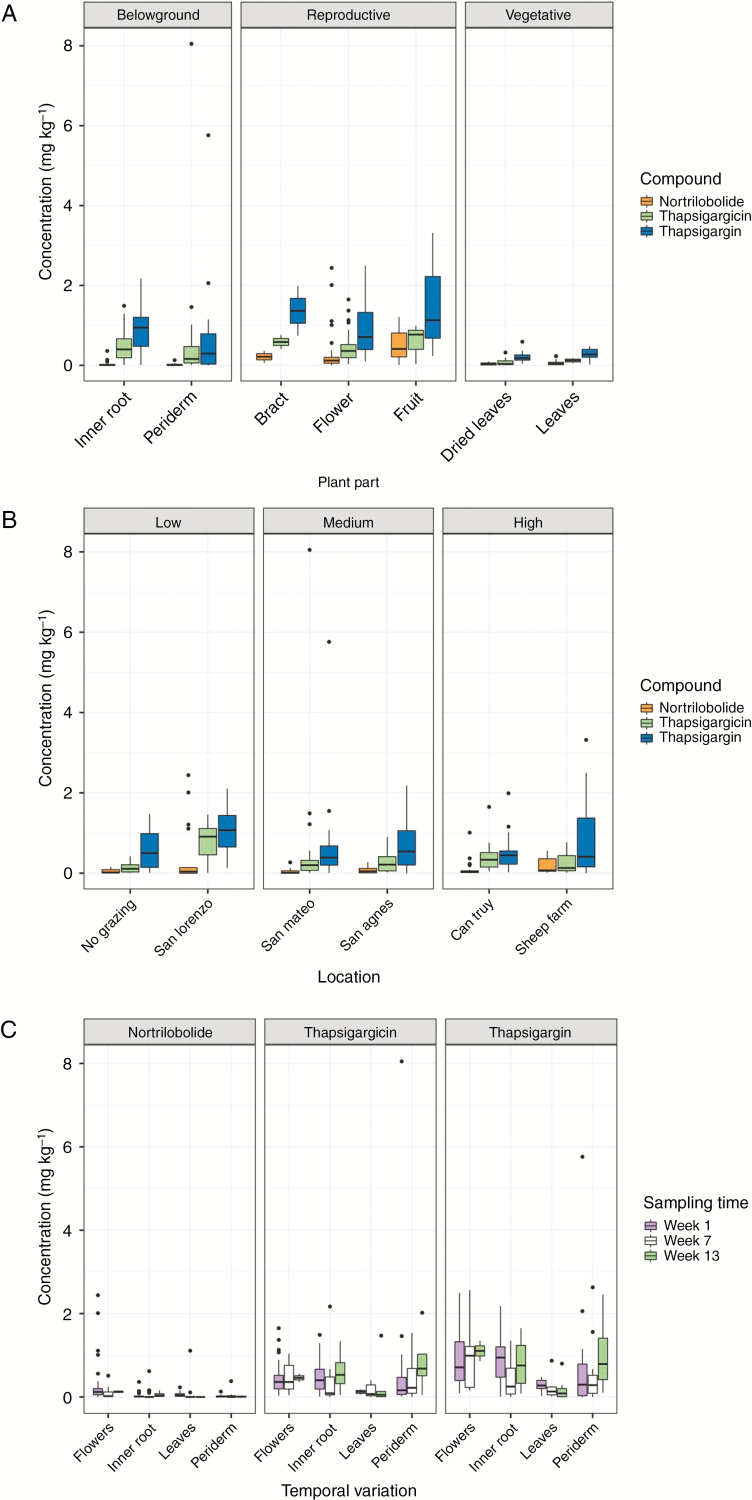
To avoid the confounding effects of temporal variation between sites, the tissue parts were investigated using the samples from the first time point only. The likelihood ratio test (LRT) of mixed linear models revealed significant variation between sites for thapsigargicin (LRT = 22.3, P < 0.001) and nortrilobolide (LRT = 19, P = 0.002) but not for thapsigargin (LRT = 8.908, P = 0.113). Thapsigargin and thapsigargicin were both at highest concentrations in the San Lorenzo site (low herbivory), averaging 1.00 and 0.75 mg kg−1, respectively, whilst thapsigargin was lowest in the Can Truy site (high herbivory; 0.50 mg kg−1) and thapsigargicin in the no-grazing site (low herbivory; 0.14 mg kg−1). Despite significant variation between sites in thapsigargin and nortrilobolide, when sites were analysed as low, medium or high herbivory, herbivory did not significantly influence any of the thapsigargin (LRT = 0.76, P = 0.684), thapsigargicin (LRT = 2.22, P = 0.330) or nortrilobolide (LRT = 1.00, P = 0.605) levels (Fig. 3A).
Fig. 3.
Relative distribution of thapsigargin, thapsigargicin and nortrilobolide from Thapsia garganica (A) within the different plant tissues, (B) between different sampling locations that had differing intensities of mammalian herbivory, and (C) over time. There was significant variation in all three thapsigargins between plant parts, while significant variation between sites was limited to thapsigargicin and nortrilobolide. Due to sampling limitation, only variation in the inner roots and root periderms from time points 1 and 2 underwent statistical analyses for temporal variation, with only thapsigargin in the inner roots varying significantly temporally.
Due to the inconsistent availability of above-ground biomass parts, the analysis of temporal variation in thapsigargins was limited to the below-ground parts (root periderm and inner root tissue; Fig. 3B) from time points 1 and 2. Within the inner roots, thapsigargin significantly decreased in abundance between sampling times 1 and 2, from a mean of 0.88 ± 0.12 mg kg−1 to 0.46 ± 0.10 mg kg−1. However, neither thapsigargicin (W = 163.0, P = 0.104) nor nortrilobolide (W = 146.5, P = 0.476) varied significantly during this period. There were also no significant differences in all three thapsigargins over time in the root periderm [thapsigargin (W = 218.5, P = 0.903), thapsigargicin (W = 230.5, P = 0.725) and nortrilobolide (W = 123, P = 0.280)].
Variation in thapsigargins was investigated from the samples collected in time point 1. Mixed linear modelling revealed that thapsigargin (LRT = 34.5, P < 0.001), thapsigargicin (LRT = 52.5, P < 0.001) and nortrilobolide (LRT = 41.5, P < 0.001) all varied significantly between plant parts (Fig. 3C). Thapsigargin and thapsigargicin were highest in the reproductive tissue (mean: fruits, 1.56 ± 0.92 and 0.60 ± 0.29 mg kg−1, respectively; flowers, 0.88 ± 0.10 and 0.43 ± 0.29 mg kg−1; bracts, 1.37 ± 0.63 and 0.59 ± 0.18 mg kg−1) followed by the below-ground tissue (inner roots, 0.84 ± 0.10 and 0.45 ± 0.08 mg kg−1; root periderm, 0.59 ± 0.20 and 0.51 ± 0.29 mg kg−1), and then vegetative tissue (fresh, 0.28 ± 0.06 and 0.12 ± 0.02 mg kg−1; senesced, 0.20 ± 0.03 and 0.07 ± 0.02 mg kg−1). Nortrilobolide was also highest in reproductive tissue (fruits, 0.54 ± 0.35; flowers, 0.29 ± 0.08; bracts, 0.22 ± 0.16 mg kg−1), but was in significantly higher abundance in vegetative tissues (senesced leaves, 0.03 ± 0.01; fresh leaves, 0.07 ± 0.03 mg kg−1) than in below-ground tissue (inner root, <0.01 ± 0.02; root periderm, 0.01 ± 0.01 mg kg−1).
Predicting intraspecific variation of thapsigargins within T. garganica: abiotic regulation
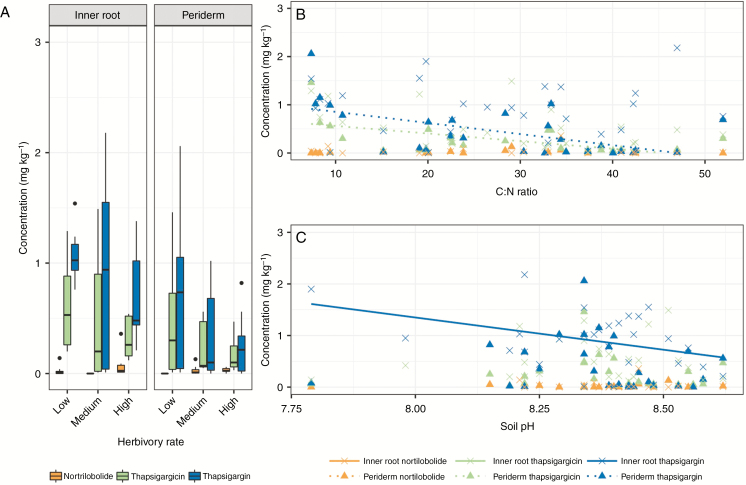
As below-ground tissue was present for all samples of time point 1, the effects of environmental variation on thapsigargin concentrations on the inner roots and root periderm were analysed. As before, thapsigargin and thapsigargicin correlated positively in the inner roots (rs = 0.904, P > 0.001) and root periderm (rs = 0.470, P = 0.013), but nortrilobolide did not significantly correlate with either thapsigargin (rs < 0.01, P = 0.967 and rs < 0.01, P = 0.980, respectively) or thapsigargicin (rs <−0.01, P = 0.967 and rs = −0.13; P = 0.602) (Fig. 4A). Surprisingly, whilst thapsigargin concentration correlated significantly between the inner root and the root periderm (d.f. = 25, t = 2.412, P = 0.024) of the same plants, neither thapsigargicin nor nortrilobolide concentrations correlated.
Fig. 4.
Relative distribution of thapsigargin, thapsigargicin and nortrilobolide in inner roots and root periderms of Thapsia garganica (A) across the herbivory gradient, (B) with changing C:N ratio and (C) with changing soil pH. Lines of best fit are drawn with significant interactions. The C:N ratio was significantly correlated with thapsigargin and thapsigargicin in the root periderm, and soil pH was significantly correlated with thapsigargin in the inner root. Note that for Fig. 4 only, a single sample has been excluded as it contained exceptionally high thapsigargin concentrations (5.8 mg kg−1 of thapsigargin and 8.0 mg kg−1 of thapsigargicin).
Given the lack of co-correlation between the different tissue types, the effects of environmental regulation (C:N ratio, P, K, soil pH and mammalian herbivory level) were analysed in the inner root and root periderm independently for each individual thapsigargin. Both thapsigargin (LRT = 5.488, P = 0.019) and thapsigargicin (LRT = 5.560, P = 0.018) correlated with C:N ratio in the root periderm (Fig. 4B). Soil pH (LRT = 5.869, P = 0.015) correlated with thapsigargin in the root periderm (Fig. 4C). Nortrilobolide did not correlate with any explanatory factors in the root periderm, but correlated with K (LRT = 14.663, P < 0.001), soil pH (LRT = 6.659, P = 0.012) and herbivory (LRT = 11.815, P = 0.003) in the inner roots.
Predicting intraspecific variation of thapsigargins within T. garganica: root-associated fungi
The root periderm and inner root tissues from sampling time point 1 also underwent DNA metabarcoding to characterize the associated below-ground fungal communities. Despite there being a deliberate poor match between host DNA and the fungal targeting primers, >99.7 % of reads produced by metabarcoding were assigned to the ITS region for roots of all 30 sampled plants of T. garganica, leaving nominal reads assigned to the fungal community. The plant PCR products were ~100 bp shorter than predicted amplicon length for fungi, whilst the PCR products of the surrounding soil were of the expected length. We therefore hypothesized that T. garganica could inhibit fungal associations, potentially via thapsigargin, thapsigargicin and nortrilobolide.
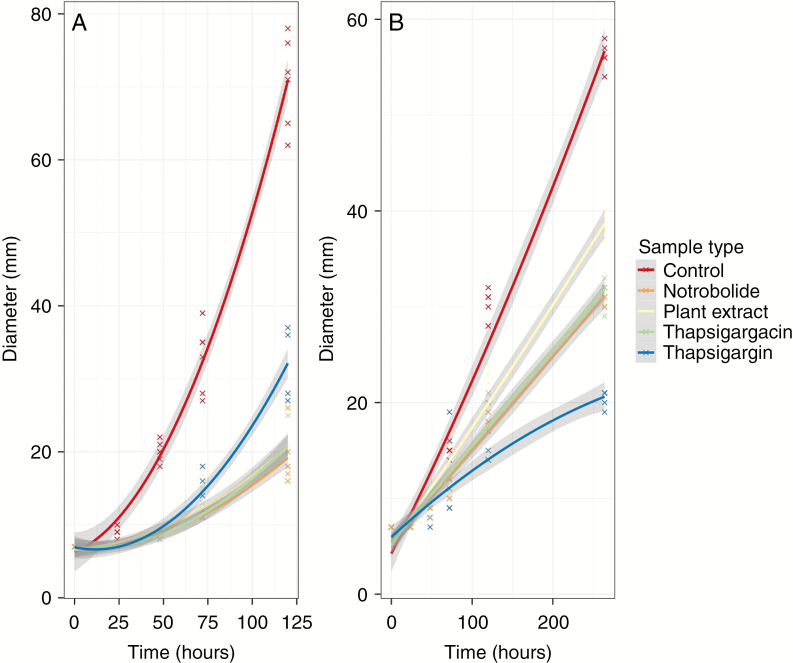
The toxicity assay tested the inhibition of growth by T. garganica-derived compounds on common plant-associated fungi, a Fusarium sp. and a Clitopilus sp. Whilst the Fusarium sp. grew quicker than the Clitopilus sp., the growth of both fungi was significantly reduced by all three compounds and by the raw plant extracts that were previously quantified by HPLC (Fusarium sp.: d.f. = 4, F = 352.7, P < 0.001; Clitopilus sp.: d.f. = 4, F = 415.4, P < 0.001). Fungal colonies at least halved in size during the incubation period (Fig. 5). Tukey tests revealed that the ascomycete fungi were affected most by the plant extract, then by nortrilobolide and thapsigargicin equally, whilst thapsigargin also significantly slowed fungal growth, but least (Fig. 5A). Conversely, Clitopilus sp. fungal growth was also significantly slowed, but least by the plant extract, followed by thapsigargicin and nortrilobolide equally (Fig. 5B). Thapsigargin was the most effective in inhibiting Clitopilus sp. growth.
Fig. 5.
(A) Fusarium sp. and (B) Clitopilus sp. fungi were grown on PDA. The medium was untreated in the control or had 100 µL of 1000 p.p.m. standards added to 25 mL of liquid medium before setting. Additionally, 100 µL of methanol plant extract (from roots) was added to 25 mL of medium before setting to screen for alternative fungal inhibitors. Both fungi had their growth significantly reduced by all treatments.
DISCUSSION
In our analyses, we show that the three major thapsigargins within wild populations of T. garganica vary considerably between different tissue types and spatially, and modestly over time. Here we evaluate how these observations compare with predictions made with theories of plant investment (Table 1).
Predicting the general defence strategy of T. garganica
All models predicted that T. garganica would be highly defended (Table 1, predictions A). Given that substantial quantities of the potent thapsigargins were observed in nearly all tissue types in most plants, results here suggest the models performed well.
The ODT predicted that T. garganica would be well defended due to being highly apparent within the ecosystem (prediction A1; Fig. 2D). However, as a herbaceous plant, T. garganica is predicted to invest fewer resources in defence than woody species (prediction A1). These defences are predicted to be in low concentrations and effective against generalist herbivores rather than specialists (qualitative defences rather than quantitative; Smilanich et al., 2006). Thapsigargins are terpenes that are qualitative defence compounds toxic to a wide variety of organisms (Fox, 1981). However, they were also present in relatively high abundance across the different tissues of T. garganica, and therefore the ODT performed well in predicting the species’ general defensive strategy. The GDBH also predicted high defences (prediction A3), but as a result of herbivory being a far greater threat than competition within the relatively sparsely populated, nutrient-poor Ibizan soils. The GRH similarly predicted considerable chemical defences within T. garganica that are produced constitutively, as it is adapted to a low-resource environment (prediction A2), and therefore adopts a defence-based life strategy rather than a growth-based strategy to overcome its decreased ability to compensate for tissues lost to herbivory. Logically, these concepts are likely interlinked in many ecosystems, with increasing soil nutrients increasing the biomass an ecosystem can support (de Graaff et al., 2006), which in turn increases competition and reduces how apparent individual plants are.
Predicting in planta variation of thapsigargins in T. garganica
Unlike the other theories, the ODT can also be used to predict how defences are distributed within plants, stating that the most valuable tissues, or those at greatest risk of herbivory, are better defended (prediction B1; Rhoades, 1979). This led to the general statement that vegetative parts are easier to replace than reproductive parts (Zangerl and Bazzaz, 1992; Hamilton et al., 2001). Here we find that fruits and flowers are particularly well protected, fitting well with this prediction. Furthermore, T. garganica has a very low germination rate, only 15 % in some instances (Makunga et al., 2003); low reproductive success might therefore explain the high investment in protecting reproductive tissue. Meanwhile, the low concentration of thapsigargins found in the leaves suggests they are of lower fitness value to the plant. This too, seems logical, given that T. garganica is distributed only in areas of high solar radiation without competition for light. Meanwhile, the below-ground biomass had greater concentrations of thapsigargins than leaves, but less than the reproductive tissue, and represents a considerable sink of photosynthates. Below-ground plant chemical defences can play integral roles in ecosystems, shaping soil microbial communities (van der Putten, 2003), yet their variation between and within species remains poorly characterized and they are generally not well integrated within current chemical defence theories (Tsunoda et al., 2017). Further studies comparing the plant’s above- and below-ground tissue value and vulnerability, coupled with chemical defences, are required to evaluate whether the ODT is applicable to below-ground plant biomass (Tsunoda and van Dam, 2017).
Predicting intraspecific variation of thapsigargins within T. garganica: abiotic regulation
Predicting intraspecific variation of thapsigargins within T. garganica was more challenging than predicting the general defensive strategy of T. garganica. Models sometimes gave conflicting and generally fewer specific predictions about the within-species regulation of thapsigargins when compared with the inter-specific models, and the observed variation of thapsigargins was compatible with multiple conflicting models.
The ODT predicts that increasing herbivory will increase chemical defences (Rhoades, 1979; prediction C1a). In this study, we observed variation between sites, but limited support for this was derived from mammalian herbivory, with effects limited to nortrilobolide in the inner root. According to the land use strategies given (Table 2), the herbivory pressure of the different sites has been maintained for many years (D. Marban, ThapsIbiza S.L., Spain, pers. comm.), which could explain the lack of correlation between mammalian herbivory and the concentrations of thapsigargins. Furthermore, whilst herbivory pressure is the basis for its chemical defence mechanisms, in fact local cattle do not eat T. garganica and it is usually found in large numbers and intact, demonstrating an acquired knowledge of the post-ingestive effects of the species. Despite the toxicity of thapsigargins to a wide range of eukaryotes (Thastrup et al., 1990; Vaca et al., 1994; Fiandra et al., 2006; Johnson et al., 2009), other types of herbivory cannot be ruled out. Literature on insect herbivory within T. garganica is scarce, but evidence of insect damage, observed as damaged leaves and bored fruits and stems, was observed in the field. In addition, the larvae of the Mediterranean moth Agonopterix thapsiella have been reported to be specialists in feeding on the young leaves and developing flower buds of T. garganica, and those of Depressaria veneficella on the stems (Stainton, 1869). The ODT additionally states that plants will be most protected mid-season (Zangerl and Rutledge, 1996; prediction C1b), as the cost of herbivory is greatest when compared with the early and late seasons; however, we found limited temporal variation in thapsigargins. It should be noted that there was temporal variation in the presence of above-ground biomass (i.e. stems, leaves, bracts, flowers and fruits), which were all only intermittently present during our sampling, and therefore total thapsigargins within a plant may still vary considerably over time. Given the toxicity of these compounds, the plant may also simply be inedible throughout the sampling period, leading to limited differences in chemical defences associated with neither the herbivory gradient nor time.
Unlike the ODT, the GRH and GDBH make explicit expectations about nutrient gradients affecting plant defences. The GRH predicts that increasing nutrients, increases growth and reduces defences (prediction C2; Grime, 2006), whilst the GDBH predicts there is an optimal level of nutrients where defences peak, before a growth strategy is preferred and defences decrease (prediction C3 not compatible with the GRH). Optimum chemical defences have been observed with intermediate nutrient levels within single species (Mihaliak and Lincoln, 1985; Wilkens et al., 1996; Glynn et al., 2007; Scogings, 2018) and the GDBH is viewed as the most advanced model of plant investment in defence. Results here further support the idea that the GDBH should subsume the GRH in explaining intraspecific variation in chemical defences (Stamp, 2003), despite the notable exceptions to the GDBH that have been observed (Le Bot et al., 2009; Kleczewski et al., 2012; Gutbrodt et al., 2012).
The ISF model predicted a positive correlation between constitutive defences and resources (prediction C5a) and a negative correlation for induced defences (prediction C5b). Results here further support previous findings that thapsigargin and thapsigargacin are constitutively biosynthesized in Thapsia plants (Christensen et al., 1997; Drew et al., 2009; Pickel et al., 2012; Andersen et al., 2017) while suggesting that nortrilobolide is induced by environmental factors. Given the observed differences in factors regulating these ‘constitutive’ and ‘induced’ defences, partitioning defences, as only the ISF model does, may improve future predictions. Furthermore, the ‘constitutive’ defences (thapsigargin and thapsigargicin) fit well with this model, increasing with a decreasing C:N ratio (increasing soil N). The key difference between predictions from the GDBH and the ISF theory is that the GDBH predicts an eventual decline in chemical defences with increasing nutrients whereas the ISF model does not, and sampling over a wider nutrient gradient is required to disentangle these two predictions. In contrast, the ‘induced’ defences (nortrilobolide) did not correlate with soil properties in either the root periderm or inner root and is therefore either less sensitive to abiotic environmental variation, or even not effected by them, and this is in contrast to what the ISF predicts. Alternatively, nortrilobolide may have been induced by one of the many environmental stimuli not tested here and, given the higher quantities observed in the above-ground biomass, insect herbivory would be of particular interest in further experiments.
Whilst soil pH is not a nutrient, it was found to significantly influence thapsigargin and thapsigargicin in the root periderm in a non-linear fashion, as predicted by the GDBH. Yet its impact on plant chemical defences is much less explored than soil nutrients. Experimental manipulations need to be performed to validate whether pH does regulate thapsigargins, but results here highlight the potential of in situ studies to find novel mechanisms of regulation that can be explored within more controlled environments.
Predicting intraspecific variation of thapsigargins in T. garganica: root-associated fungi
Here, we found that the non-specific binding of primers to T. garganica DNA led to nominal numbers of reads being fungal-derived through metabarcoding. Our subsequent fungal toxicity assay found that all three defence compounds, as well as the crude plant extracts, significantly inhibited plant-associated fungal growth. Other plant defence compounds have been shown to inhibit fungal colonization, most notably in the glucosinolates found within the Brassicaceae (Pedras and Hossain, 2011; Hu et al., 2015). In doing so, these plants inhibit potential beneficial symbioses with mycorrhizal fungi and dark septate endophytes that can enhance nutrient uptake (Barrow and Osuna, 2002; Porras-Soriano et al., 2009). However, mycorrhizal fungi function parasitically on their hosts under certain circumstances (Johnson et al., 2009). Pathogenic fungi are one of the greatest threats to a plant’s vitality (Dean et al., 2012), and these associations were also inhibited. Soil microorganisms interact with the outermost surfaces of roots, and many fungi can penetrate into or between root cells themselves (Vierheilig et al., 2001). MALDI-TOF imaging revealed large quantities of thapsigargins near the outermost surface of the root of T. garganica, and results here suggest this would likely strongly inhibit fungal interactions (Andersen et al., 2017). Furthermore, glucosinolates have also been shown to inhibit parasitic nematodes in Brassicaceae (Ntalli and Caboni, 2017) and thapsigargins might be employed as part of the below-ground defences against a wide range of below-ground biotic interactions. Although it cannot be ruled out that thapsigargins are moved to the root periderm as part of a storage strategy to avoid autotoxicity, in both scenarios there seems to be no fitness cost incurred by not having below-ground fungal associations, and this could even increase fitness. Whilst the REMPD was developed to characterize chemical defences in response to changing numbers of mutualists within a species, understanding the evolutionary and ecological determinants of plants that strongly inhibit root fungal associations could provide further insight into cost-benefits of root-associated fungal associations, and in turn give a better understanding of their influence on plant chemical defences.
Future perspectives
Here we assemble a framework to highlight available models and their practical application in predicting chemical defences at the in planta, intraspecific and interspecific levels (Table 4). Additionally, for future studies we have a few main suggestions. Initially, we propose that multiple models of chemical defence be assessed simultaneously, as each has a different utility and this will ultimately assist in the development of an integrated model able to predict chemical defences across levels. We also believe that significant improvements could be achieved by supplementing targeted analyses with untargeted metabolomic screenings, as more complex responses, including responses of non-target compounds, may be overlooked in traditional approaches (Zangerl and Berenbaum, 1997; Stamp, 2003, 2004; Gutbrodt et al., 2012). We further suggest that in situ studies could supplement experiments in controlled environments (such as reciprocal transplant studies and common garden studies), so that relevant specific environmental regulators of chemical defence can be identified before hypothesis testing, echoing Hahn and Maron (2016). Most importantly, we recommend the establishment of a gold standard of best practice for testing intraspecific variation of chemical defences in plants, as differing experimental designs and methodologies might in part be responsible for conflicting results (Scogings, 2018), and therefore hinder the development of an all-encompassing theory of plant chemical defences.
Table 4.
A framework briefly outlining the available models, the experimental setup required and the future improvements needed to predict and validate in planta, intraspecific and interspecific variation in plant chemical defences
| In planta variation | Intraspecific variation | |
|---|---|---|
| Types of experimental setup |
○ Sampling of different tissue types from the same individuals. ○ Repeated sampling of a population over time. |
○ Sampling of the same species between different populations. ○ Sampling single species over environmental gradients. |
| Relevant theories | ○ Optimal defence theory (ODT). |
○ Growth rate hypothesis (GRH). ○ Growth–differentiation balance hypothesis (GDBH). ○ Resource exchange model of plant defence (REMPD). ○ Intraspecific framework for variation in plant defences (ISF). |
| Current status |
○ The ODT is the only major theory to predict in planta variation. ○ The level of chemical defences is hypothesized to vary with the value of the tissue to the plant, as well as with the level of herbivory. ○ This ‘value’ is not well defined, but generally reproductive tissue is thought to be of higher value than leaves and is therefore better defended. ○ Its applicability to below-ground defences has not been tested thoroughly. |
○ The ISF is the most advanced theory in predicting chemical defences at the intraspecific level, expanding upon the GRH by partitioning induced and constitutively active defences. ○ However, the ISF suggests a linear relationship between defences and resources, whilst the GDBH suggests there is an optimum. ○ The specific environmental factors that regulate chemical defences remain poorly defined. |
| Future improvement |
○ More studies are needed to determine the relative value of root tissue to plants. ○ Further quantification of below-ground herbivory rates are required. ○ At the intraspecific level, other theories are considered superior to the ODT, therefore the in planta aspects of the ODT should be amalgamated with these more sophisticated models of intraspecific variation. |
○ Further experimental observations are required to determine whether partitioning-induced and constitutive defences are valid. ○ Experimental in situ observations should be performed to identify putative environmental determinants of chemical defences, before validation within glasshouse studies. |
Conclusions
In this work we show that T. garganica was constitutively defended by potent anti-herbivore and anti-fungal compounds, thapsigargins, which were highly variable both within and between plants. We found 10-fold differences in thapsigargin concentrations associated with tissue type. We also found 2-fold differences associated with spatial variation, which were linked to soil properties, whilst there was limited temporal variation in thapsigargins. We found that the theories predicting inter-specific variation in plant defences effectively predicted the growth strategy and level of defences within T. garganica (Koricheva, 2002). We also found that one theory, the ODT, effectively predicted the distribution of thapsigargins in the above-ground but not below-ground biomass. Models performed least well in predicting intraspecific variation in chemical defences, but results here further suggest that the GRH should be subsumed by the GDBH. We could not differentiate between the ISF model and the GDBH in performance; however, sampling over a wider nutrient gradient would allow this to be resolved. Ultimately, we are some way from a ‘grand unifying theory’ in predicting intraspecific variation in plant chemical defences, but with the increasing availability of high-throughput analyses of DNA (both of plants and their microbial symbionts) and chemistry, and databases such as WorldClim and the Global Biodiversity Information Facility (GBIF), we can inch ever closer.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Method S1: HPLC analysis. Method S2: fungal sequencing and analysis.
FUNDING
This work received funding from the People Programme (Marie Curie Actions) of the European Union’s Seventh Framework Programme FP7/2007–2013/ under REA grant agreement (606895), The Danish Council for Independent Research (4005-00158B) and from the Aage V. Jensen Foundation, Denmark (112172).
ACKNOWLEDGEMENTS
The HPLC standards were extracted and purified under the direction of Søren Brøgger Christensen and Huizhen Liu at the Department of Drug Design and Pharmacology, University of Copenhagen, Denmark. Special thanks to Dorian Marban of ThapsIbiza S.L., Spain, for his invaluable advice on T. garganica growing conditions, and to Georgios I. Zervakis (Agricultural University of Athens, Greece) for insight on the Pleurotus fungus associated with T. garganica. The authors declare that they have no conflicts of interest.
LITERATURE CITED
- Abdala-Roberts L, Mooney KA, Quijano-Medina T, Campos-Navarrete MJ, González-Moreno A, Parra-Tabla V. 2015. Comparison of tree genotypic diversity and species diversity effects on different guilds of insect herbivores. Oikos 124: 1527–1535. [Google Scholar]
- Agrawal AA, Hastings AP, Bradburd GS, et al. 2015. Evolution of plant growth and defense in a continental introduction. American Naturalist 186: E1–E15. [DOI] [PubMed] [Google Scholar]
- Andersen TB, López CQ, Manczak T, Martinez K, Simonsen HT. 2015. Thapsigargin—from Thapsia L. to mipsagargin. Molecules 20: 6113–6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen TB, Martinez-Swatson KA, Rasmussen SA, et al. 2017. Localization and in-vivo characterization of Thapsia garganica CYP76AE2 indicates a role in thapsigargin biosynthesis. Plant Physiology 174: 56–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstett DN, Ahern JR, Glinos J, Nawar N, Salminen J-P, Johnson MTJ. 2015. Can genetically based clines in plant defence explain greater herbivory at higher latitudes? Ecology Letters 18: 1376–1386. [DOI] [PubMed] [Google Scholar]
- Barber NA, Kiers ET, Theis N, Hazzard RV, Adler LS. 2013. Linking agricultural practices, mycorrhizal fungi, and traits mediating plant–insect interactions. Ecological Applications 23: 1519–1530. [DOI] [PubMed] [Google Scholar]
- Barnes CJ, Maldonado C, Frøslev TG, Antonelli A, Rønsted N. 2016. Unexpectedly high beta-diversity of root-associated fungal communities in the Bolivian Andes. Frontiers in Microbiology 7: 1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CJ, van der Gast CJ, McNamara NP, Rowe R, Bending GD. 2018. Extreme rainfall affects assembly of the root-associated fungal community. New Phytologist 220: 1172–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow JR, Osuna P. 2002. Phosphorus solubilization and uptake by dark septate fungi in fourwing saltbush, Atriplex canescens (Pursh) Nutt. Journal of Arid Environments 51: 449–459. [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S. 2014. Fitting linear mixed-effects models using lme4. arXiv preprint arXiv:1406.5823.
- Le Bot J, Bénard C, Robin C, Bourgaud F, Adamowicz S. 2009. The “trade-off” between synthesis of primary and secondary compounds in young tomato leaves is altered by nitrate nutrition: experimental evidence and model consistency. Journal of Experimental Botany 60: 4301–4314. [DOI] [PubMed] [Google Scholar]
- Christensen SB, Andersen A, Smitt UW. 1997. Sesquiterpenoids from Thapsia species and medicinal chemistry of the thapsigargins. Fortschritte Der Chemie Organischer Naturstoffe 71: 129–167. [DOI] [PubMed] [Google Scholar]
- Christensen SB, Kjoeller Larsen I, Rasmussen U, Christophersen C. 1982. Thapsigargin and thapsigargicin, two histamine liberating sesquiterpene lactones from Thapsia garganica. X-ray analysis of the 7,11-epoxide of thapsigargin. Journal of Organic Chemistry 47: 649–652. [Google Scholar]
- Coley PD. 1988. Effects of plant growth rate and leaf lifetime on the amount and type of anti-herbivore defense. Oecologia 74: 531–536. [DOI] [PubMed] [Google Scholar]
- Coley PD, Bryant JP, Chapin FS. 1985. Resource availability and plant antiherbivore defense. Science 230: 895–899. [DOI] [PubMed] [Google Scholar]
- Couture JJ, Serbin SP, Townsend PA. 2015. Elevated temperature and periodic water stress alter growth and quality of common milkweed (Asclepias syriaca) and monarch (Danaus plexippus) larval performance. Arthropod-Plant Interactions 9: 149–161. [Google Scholar]
- Dean R, Kan JA, Pretorius ZA, et al. 2012. The top 10 fungal pathogens in molecular plant pathology. Molecular Plant Pathology 13: 414–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew DP, Krichau N, Reichwald K, Simonsen HT. 2009. Guaianolides in Apiaceae: perspectives on pharmacology and biosynthesis. Phytochemistry Reviews 8: 581–599. [Google Scholar]
- Fiandra L, Casartelli M, Giordana B. 2006. The paracellular pathway in the lepidopteran larval midgut: modulation by intracellular mediators. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 144: 464–473. [DOI] [PubMed] [Google Scholar]
- Fox LR. 1981. Defense and dynamics in plant-herbivore systems. American Zoologist 21: 853–864. [Google Scholar]
- Gardes M, Bruns TD. 1993. ITS primers with enhanced specificity for basidiomycetes – application to the identification of mycorrhizae and rusts. Molecular Ecology 2: 113–118. [DOI] [PubMed] [Google Scholar]
- Gerard J, Dodoens R, Priest R, Norton J. 1597. The herball, or, generall historie of plantes. Gathered by John Gerarde of London, master in chirurgerie. London: John Norton. [Google Scholar]
- Gershenzon J. 2017. The cost of plant chemical defense against herbivory: a biochemical perspective. In: Bernays EA, ed. Insect-plant interactions (1993). Abington, UK: CRC Press, 121–190. [Google Scholar]
- Glynn C, Herms DA, Orians CM, Hansen RC, Larsson S. 2007. Testing the growth–differentiation balance hypothesis: dynamic responses of willows to nutrient availability. New Phytologist 176: 623–634. [DOI] [PubMed] [Google Scholar]
- De Graaff MA, van Groenigen KJ, Hungate SJ, van Kessel C. 2006. Interactions between plant growth and soil nutrient cycling under elevated CO2: a meta‐analysis. Global Change Biology 12: 2077–2091. [Google Scholar]
- Grime JP. 1977. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. American Naturalist 111: 1169–1194. [Google Scholar]
- Grime JP. 2006. Plant strategies, vegetation processes, and ecosystem properties. Chichester: John Wiley & Sons. [Google Scholar]
- Gutbrodt B, Dorn S, Mody K. 2012. Drought stress affects constitutive but not induced herbivore resistance in apple plants. Arthropod-Plant Interactions 6: 171–179. [Google Scholar]
- Hahn PG, Maron JL. 2016. A framework for predicting intraspecific variation in plant defense. Trends in Ecology & Evolution 31: 646–656. [DOI] [PubMed] [Google Scholar]
- Hamilton JG, Zangerl AR, DeLucia EH, Berenbaum MR. 2001. The carbon–nutrient balance hypothesis: its rise and fall. Ecology Letters 4: 86–95. [Google Scholar]
- van der Heijden MGA, Martin FM, Selosse M-A, Sanders IR. 2015. Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytologist 205: 1406–1423. [DOI] [PubMed] [Google Scholar]
- Herms DA, Mattson WJ. 1992. The dilemma of plants: to grow or defend. Quarterly Review of Biology 67: 283–335. [Google Scholar]
- Hu P, Hollister EB, Somenahally AC, Hons FM, Gentry TJ. 2015. Soil bacterial and fungal communities respond differently to various isothiocyanates added for biofumigation. Frontiers in Microbiology 5: 729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihrmark K, Bödeker ITM, Cruz-Martinez K, et al. 2012. New primers to amplify the fungal ITS2 region – evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiology Ecology 82: 666–677. [DOI] [PubMed] [Google Scholar]
- Johnson NA, Liu F, Weeks PD, et al. 2009. A tomato ER-type Ca2+-ATPase, LCA1, has a low thapsigargin-sensitivity and can transport manganese. Archives of Biochemistry and Biophysics 481: 157–168. [DOI] [PubMed] [Google Scholar]
- Karban R, Baldwin IT. 1997. Induced responses to herbivory. Chicago: University of Chicago Press. [Google Scholar]
- Kessler A, Halitschke R. 2009. Testing the potential for conflicting selection on floral chemical traits by pollinators and herbivores: predictions and case study. Functional Ecology 23: 901–912. [Google Scholar]
- Kleczewski NM, Herms DA, Bonello P. 2012. Nutrient and water availability alter belowground patterns of biomass allocation, carbon partitioning, and ectomycorrhizal abundance in Betula nigra. Trees 26: 525–533. [Google Scholar]
- Koricheva J. 2002. Meta-analysis of sources of variation in fitness costs of plant antiherbivore defenses. Ecology 83: 176–190. [Google Scholar]
- Laughlin DC, Messier J. 2015. Fitness of multidimensional phenotypes in dynamic adaptive landscapes. Trends in Ecology & Evolution 30: 487–496. [DOI] [PubMed] [Google Scholar]
- Lehndal L, Ågren J. 2015. Latitudinal variation in resistance and tolerance to herbivory in the perennial herb Lythrum salicaria is related to intensity of herbivory and plant phenology. Journal of Evolutionary Biology 28: 576–589. [DOI] [PubMed] [Google Scholar]
- Loomis W. 1932. Growth-differentiation balance vs. carbohydrate-nitrogen ratio. Proceedings of the American Society of Horticultural Science 29: 240–245. [Google Scholar]
- Loomis W. 1953. Growth and differentiation – an introduction and summary. In: Matyssek R, Schnyder H, Osswald W, Ernst D, Munch JC, Pretsch H, eds. Growth and differentiation in plants. Ames: Iowa State College Press, 1–17. [Google Scholar]
- López CQ, Corral P, Lorrain-Lorrette B, Martinez-Swatson K, Michoux F, Simonsen HT. 2018. Use of a temporary immersion bioreactor system for the sustainable production of thapsigargin in shoot cultures of Thapsia garganica. Plant Methods 14: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makunga NP, Jäger AK, van Staden J. 2003. Micropropagation of Thapsia garganica—a medicinal plant. Plant Cell Reports 21: 967–973. [DOI] [PubMed] [Google Scholar]
- Maldonado C, Barnes CJ, Cornett C, et al. 2017. Phylogeny predicts the quantity of antimalarial alkaloids within the iconic yellow Cinchona bark (Rubiaceae: Cinchona calisaya). Frontiers in Plant Science 8: 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKey D. 1974. Adaptive patterns in alkaloid physiology. American Naturalist 108: 305–320. [Google Scholar]
- Meier AR, Hunter MD. 2018. Arbuscular mycorrhizal fungi mediate herbivore-induction of plant defenses differently above and belowground. Oikos 127: 1759–1775. [Google Scholar]
- Mihaliak CA, Lincoln DE. 1985. Growth pattern and carbon allocation to volatile leaf terpenes under nitrogen-limiting conditions in Heterotheca subaxillaris (Asteraceae). Oecologia 66: 423–426. [DOI] [PubMed] [Google Scholar]
- Mohamed Ibrahim AM, Martinez-Swatson KA, Benkaci-Ali F, Cozzi F, Zoulikha F, Simonsen HT. 2018. Effects of gamma irradiation and comparison of different extraction methods on sesquiterpene lactone yields from the medicinal plant Thapsia garganica L. (Apiaceae). Journal of Applied Research on Medicinal and Aromatic Plants 8: 26–32. [Google Scholar]
- Moreira X, Mooney KA, Rasmann S, et al. 2014. Trade-offs between constitutive and induced defences drive geographical and climatic clines in pine chemical defences. Ecology Letters 17: 537–546. [DOI] [PubMed] [Google Scholar]
- Nerg A, Kainulainen P, Vuorinen M, Hanso M, Holopainen JK, Kurkela T. 1994. Seasonal and geographical variation of terpenes, resin acids and total phenolics in nursery grown seedlings of Scots pine (Pinus sylvestris L.). New Phytologist 128: 703–713. [Google Scholar]
- Ntalli N, Caboni P. 2017. A review of isothiocyanates biofumigation activity on plant parasitic nematodes. Phytochemistry Reviews 16: 827–834. [Google Scholar]
- Olsen LI, Hansen TH, Larue C, et al. 2016. Mother-plant-mediated pumping of zinc into the developing seed. Nature Plants 2: 16036. [DOI] [PubMed] [Google Scholar]
- Pedras MSC, Hossain S. 2011. Interaction of cruciferous phytoanticipins with plant fungal pathogens: indole glucosinolates are not metabolized but the corresponding desulfo-derivatives and nitriles are. Phytochemistry 72: 2308–2316. [DOI] [PubMed] [Google Scholar]
- Pickel B, Drew DP, Manczak T, Weitzel C, Simonsen HT, Ro D-K. 2012. Identification and characterization of a kunzeaol synthase from Thapsia garganica: implications for the biosynthesis of the pharmaceutical thapsigargin. Biochemical Journal 448: 261–271. [DOI] [PubMed] [Google Scholar]
- Porras-Soriano A, Soriano-Martín ML, Porras-Piedra A, Azcón R. 2009. Arbuscular mycorrhizal fungi increased growth, nutrient uptake and tolerance to salinity in olive trees under nursery conditions. Journal of Plant Physiology 166: 1350–1359. [DOI] [PubMed] [Google Scholar]
- Pratt JD, Mooney KA. 2013. Clinal adaptation and adaptive plasticity in Artemisia californica: implications for the response of a foundation species to predicted climate change. Global Change Biology 19: 2454–2466. [DOI] [PubMed] [Google Scholar]
- Pujadas-Salvà AJ, Rosselló J. 2003. Thapsia L. In: Castroviejo S, Aedo C, Cirujano S. et al. , eds. Flora Ibérica. Vol. X. Araliaceae-Umbelliferae. Madrid: Real Jardín Botánico, C.S.I.C, 401–410. [Google Scholar]
- van der Putten WH. 2003. Plant defense belowground and spatiotemporal processes in natural vegetation. Ecology 84: 2269–2280. [Google Scholar]
- Rasmussen U, Brøgger Christensen S, Sandberg F. 1978. Thapsigargine and thapsigargicine, two new histamine liberators from Thapsia garganica L. Acta Pharmaceutica Suecica 15: 133–140. [PubMed] [Google Scholar]
- Rhoades DF. 1979. Evolution of plant chemical defense against herbivores. In: Rosenthal GA, Janzen DH, eds. Herbivores: their interaction with secondary plant metabolites. New York: Academic Press, 3–54. [Google Scholar]
- de Román M, Fernández I, Wyatt T, Sahrawy M, Heil M, Pozo MJ. 2011. Elicitation of foliar resistance mechanisms transiently impairs root association with arbuscular mycorrhizal fungi. Journal of Ecology 99: 36–45. [Google Scholar]
- Rosenthal G, Berenbaum M. 1992. Herbivores: their interactions with secondary metabolites: evolutionary and ecological processes. San Diego: Academic Press. [Google Scholar]
- Sampedro L, Moreira X, Zas R. 2011. Costs of constitutive and herbivore-induced chemical defences in pine trees emerge only under low nutrient availability. Journal of Ecology 99: 818–827. [Google Scholar]
- Scogings PF. 2018. Foliar flavonol concentration in Sclerocarya birrea saplings responds to nutrient fertilisation according to growth-differentiation balance hypothesis. Phytochemistry Letters 23: 180–184. [Google Scholar]
- Smilanich AM, Fincher RM, Dyer LA. 2006. Does plant apparency matter? Thirty years of data provide limited support but reveal clear patterns of the effects of plant chemistry on herbivores. New Phytologist 210: 1044–1057. [DOI] [PubMed] [Google Scholar]
- Smitt UW, Christensen SB. 1991. Nortrilobolide, a new potent guaianolide secretagogue from Thapsia garganica. Planta Medica 57: 196–197. [DOI] [PubMed] [Google Scholar]
- Smitt UW, Jäger AK, Adsersen A, Gudiksen L. 1995. Comparative studies in phytochemistry and fruit anatomy of Thapsia garganica and T. transtagana, Apiaceae (Umbelliferae). Botanical Journal of the Linnean Society 117: 281–292. [Google Scholar]
- Smitt UW, Jäger AK, Nyman U. 1996. Thapsia garganica L.: in vitro culture, somatic embryogenesis, and the production of thapsigargins. In: Bajaj PDYPS, ed. Biotechnology in Agriculture and Forestry. Medicinal and Aromatic Plants IX. Berlin: Springer, 402–409. [Google Scholar]
- Stainton HT. 1869. The Tineina of Southern Europe. London: John Van Voorst. [Google Scholar]
- Stamp N. 2003. Theory of plant defensive level: example of process and pitfalls in development of ecological theory. Oikos 102: 672–678. [Google Scholar]
- Stamp N. 2004. Can the growth–differentiation balance hypothesis be tested rigorously? Oikos 107: 439–448. [Google Scholar]
- Stevens MT, Brown SC, Bothwell HM, Bryant JP. 2016. Biogeography of Alaska paper birch (Betula neoalaskana): latitudinal patterns in chemical defense and plant architecture. Ecology 97: 494–502. [DOI] [PubMed] [Google Scholar]
- Thastrup O, Cullen PJ, Drøbak BK, Hanley MR, Dawson AP. 1990. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proceedings of the National Academy of Sciences of the USA 87: 2466–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornley JHM. 1972. A model to describe the partitioning of photosynthate during vegetative plant growth. Annals of Botany 36: 419–430. [Google Scholar]
- Tsunoda T, van Dam NM. 2017. Root chemical traits and their roles in belowground biotic interactions. Pedobiologia 65: 58–67. [Google Scholar]
- Tsunoda T, Krosse S, van Dam NM. 2017. Root and shoot glucosinolate allocation patterns follow optimal defence allocation theory. Journal of Ecology 105: 1256–1266. [Google Scholar]
- Tuomi J, Niemelä P, Stuart Chapin F, Bryant JP, Sirén S. 1988. Defensive responses of trees in relation to their carbon/nutrient balance. In: Mattson WJ, Levieux J, Bernard-Dagan C, eds. Mechanisms of woody plant defenses against insects. New York: Springer, 57–72. [Google Scholar]
- Vaca L, Sinkins WG, Hu Y, Kunze DL, Schilling WP. 1994. Activation of recombinant trp by thapsigargin in Sf9 insect cells. American Journal of Physiology 267: C1501–C1505. [DOI] [PubMed] [Google Scholar]
- Vannette RL, Hunter MD. 2011. Plant defence theory re-examined: nonlinear expectations based on the costs and benefits of resource mutualisms. Journal of Ecology 99: 66–76. [Google Scholar]
- Vannette RL, Hunter MD. 2013. Mycorrhizal abundance affects the expression of plant resistance traits and herbivore performance. Journal of Ecology 101: 1019–1029. [Google Scholar]
- Vierheilig H, Knoblauch M, Juergensen K, van Bel AJ, Grundler FM, Piché Y. 2001. Imaging arbuscular mycorrhizal structures in living roots of Nicotiana tabacum by light, epifluorescence, and confocal laser scanning microscopy. Canadian Journal of Botany 79: 231–237. [Google Scholar]
- Wainhouse D, Ashburner R. 1996. The influence of genetic and environmental factors on a quantitative defensive trait in spruce. Functional Ecology 10: 137–143. [Google Scholar]
- Weitzel C, Rønsted N, Spalik K, Simonsen HT. 2014. Resurrecting deadly carrots: towards a revision of Thapsia (Apiaceae) based on phylogenetic analysis of nrITS sequences and chemical profiles. Botanical Journal of the Linnean Society 174: 620–636. [Google Scholar]
- Wilkens RT, Spoerke JM, Stamp NE. 1996. Differential responses of growth and two soluble phenolics of tomato to resource availability. Ecology 77: 247–258. [Google Scholar]
- Woods EC, Hastings AP, Turley NE, Heard SB, Agrawal AA. 2012. Adaptive geographical clines in the growth and defense of a native plant. Ecological Monographs 82: 149–168. [Google Scholar]
- Xiao M, Zhang Ye, Chen X, et al. 2013. Transcriptome analysis based on next-generation sequencing of non-model plants producing specialized metabolites of biotechnological interest. Journal of Biotechnology 166: 122–134. [DOI] [PubMed] [Google Scholar]
- Zangerl AR, Bazzaz FA. 1992. Theory and pattern in plant defense allocation. In: Fritz RS, Simms EL, eds. Plant resistance to herbivores and pathogens, ecology, evolution, and genetics. Chicago: University of Chicago Press, 363–392. [Google Scholar]
- Zangerl AR, Berenbaum MR. 1997. Cost of chemically defending seeds: furanocoumarins and Pastinaca sativa. American Naturalist 150: 491–504. [DOI] [PubMed] [Google Scholar]
- Zangerl AR, Rutledge CE. 1996. The probability of attack and patterns of constitutive and induced defense: a test of optimal defense theory. American Naturalist 147: 599–608. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.