Abstract
Background and Aims
Signal grass (Urochloa decumbens) is a widely used pasture grass in tropical and sub-tropical areas due to its high aluminiun (Al) resistance. However, the underlying mechanisms conferring this resistance are not clearly understood.
Methods
The Al concentrations of bulk root tissues and the intracellular compartment were examined, including the impact of a metabolic inhibitor, carbonyl cyanide m-chlorophenyl hydrazone (CCCP). Next, we examined changes in the properties of signal grass root tissues following exposure to toxic levels of Al, including the cell wall cation exchange capacity (CEC), degree of methylation and concentrations of cell wall fractions.
Key Results
Although signal grass was highly resistant to Al, there was a delay of 24–48 h before the expression of this resistance. We found that this delay in the expression of Al resistance was not related to the total Al concentration in the bulk apical root tissues, nor was it related to changes in the Al bound to the cell wall. We also examined changes in other properties of the cell wall, including the CEC, degree of methylation and changes in the concentration of pectin, hemicellulose and cellulose. We noted that concentrations of intracellular Al decreased by approx. 50 % at the same time that the root elongation rate improved after 24–48 h. Using CCCP as a metabolic inhibitor, we found that the intracellular Al concentration increased approx. 14-fold and that the CCCP prevented the subsequent decrease in intracellular Al.
Conclusions
Our results indicate that the delayed expression of Al resistance was not associated with the Al concentration in the bulk apical root tissues or bound to the cell wall, nor was it associated with changes in other properties of the cell wall. Rather, signal grass has an energy-dependent Al exclusion mechanism, and this mechanism requires 24–48 h to exclude Al from the intracellular compartment.
Keywords: Aluminium toxicity, cation exchange capacity (CEC), degree of methylation, intracellular aluminium, pectin, signal grass
INTRODUCTION
Signal grass is a perennial pasture grass that is widely grown due to its adaptation to tropical conditions. It is used as a pasture for livestock production but also for revegetation of disturbed sites, and is planted in Australia, tropical America and south-eastern Asia (Wenzl et al., 2001). Many soils in these tropical and sub-tropical areas are acid (Von Uexküll and Mutert, 1995), with elevated concentration of soluble aluminium (Al) which is known to be highly toxic to plant roots.
Plants differ in their resistance to toxic concentrations of Al (Wenzl et al., 2001; Kopittke et al., 2016), indicating that a variety of resistance mechanisms exist (Choudhary et al., 2011). The root cell wall is the first site to come into contact with Al during plant uptake, with the properties of the root surface regulating the processes of water and elemental uptake (Szatanik-Kloc et al., 2017). Of particular interest in the present study is that is known that most Al that accumulates within plant roots is bound strongly to the cell walls. For example, Taylor et al. (2000) directly measured the Al uptake and distribution in Chara corallina and found that the cell wall accounted for > 99.5 % of the total Al. The carboxyl groups of pectin are known to make the largest contribution to the negative charge of the cell walls. Indeed, a key factor during Al binding and accumulation is the magnitude of the negative charge of the pectin, with this determined by the degree of methylation. Importantly, a range of studies have shown the physiological importance of this Al binding to the cell wall (Horst et al., 2010), and it has recently been shown that this strong and rapid binding of Al to the cell wall is the primary lesion of Al toxicity in roots of soybean (Glycine max) (Kopittke et al., 2015).
It has been reported that signal grass is hyper-resistant to Al and it is able to grow at Al concentrations considerably higher than most other plant species (Wenzl et al., 2002b). However, it remains unclear what mechanisms are used by signal grass to provide this resistance. For example, it has been found that its resistance is not related to Al-induced organic acid secretion (Wenzl et al., 2001), with Wenzl et al. (2002a) finding that although internal detoxification of Al by organic acids may contribute somewhat, ‘internal detoxification of Al by organic acids does not appear to be the principal mechanism responsible for the superior resistance of Brachiaria [Urochloa] decumbens’. It is possible, however, that there is a metabolically driven exclusion mechanism in signal grass. Importantly, it has been found that full resistance expression in signal grass requires a lag time of about 72–96 h, with this being preceded by a sensitive phase (Arroyave et al., 2017).
Given that the cell wall has been shown to have an important role in the expression of Al toxicity, the present study aimed to determine whether the high level of Al resistance observed in signal grass is related to changes in cell wall properties, or whether it is associated with an internal detoxification mechanism. Using nutrient solutions containing a toxic level of Al, root growth and morphology were examined first. Then we examined the Al concentration in apical root tissues and in the cell wall, with active exclusion of Al investigated using the metabolic inhibitor CCCP. In general, CCCP causes an uncoupling of the proton gradient that is established during the normal activity of electron carriers in the electron transport chain. This chemical which reduces the ability of ATP synthase to function optimally is suitable as a metabolic inhibitor for plants. In addition, its effect had been reported previously on Chara coralline (Taylor et al., 2000). The changed properties of the cell wall including CEC, degree of cell wall methylation and concentration of four cell wall fractions were also examined. The present study will provide new information when considering mechanisms of Al resistance in signal grass.
MATERIALS AND METHODS
Plant materials and general growth conditions
All experiments were conducted at The University of Queensland (St Lucia, Australia) in controlled environmental conditions. Seeds of signal grass (‘Basilisk’) were scarified by placing in concentrated sulfuric acid for 10 min before being washed with deionized water. Seeds were then soaked in aerated deionized water for 6 h before being germinated in a rolled paper towel soaked with tap water for 2 d (Li et al., 2018). Uniform seedlings were selected and then held by Perspex strips above continuously aerated basal nutrient solutions containing 1 mM CaCl2 and 5 μM H3BO3 (pH 4.2) in 650 mL beakers for 18 h before Al treatment (see below). Each Perspex strip held seven seedlings, forming one experimental unit. During the entire experimental period, seedlings were growing in a controlled environment at a temperature of 25 °C during both the light and dark periods, and with high-pressure sodium lamps providing 12 h per day of photosynthetically active radiation at 1500 µmol m–2 s–1.
Experiment 1: dose response curves, root morphology and thermodynamic modelling
Dose response curves were determined in order to examine the effect of Al on the elongation rate of signal grass roots. After 18 h growth, seedlings were transferred from the basal solutions (see above) to Al-containing treatment solutions. In total, ten treatments were investigated, i.e. 0, 25, 50, 100, 150, 200, 250, 300, 600 and 1000 µm Al. The treatment solutions were prepared in 600 mL beakers filled to the brim (650 mL) with the appropriate solution, with the Al added using a 65 mm stock solution of AlCl3·6H2O. The Al-containing treatment solutions also contained 1 mm CaCl2 and 5 µm H3BO3. Solutions were adjusted to pH 4.2 using 0.1 m HCl and were continuously aerated. Each of the ten treatments was replicated three times, yielding a total of 30 experimental units. Seedlings were grown in these Al-containing solutions for 5 d. Solution pH was checked every 24 h and, if necessary, adjusted using 0.1 m HCl.
To determine root length, seedlings were photographed using a digital camera at the time of transfer to the Al-containing solutions (0 h) and every 12 h thereafter across the 120 h experimental period. Using these images, root lengths were calculated using ImageJ v1.45s (available at: http//imagej.nih.gov/ij/), with changes in root length used to calculate the root elongation rate (RER). Root morphology was observed using light microscopy after 24, 48 and 96 h Al exposure. Samples of the nutrient solutions (10 mL) were collected after 0 h and 120 h, filtered using a 0.22 µm membrane filter (Millipore) and acidified with 20 µL of concentrated HCl prior to measurement by ICP-MS (inductively coupled plasma mass spectrometry) for Al. Thermodynamic modelling was used to predict the speciation of the nutrient solutions, with Visual MINTEQ 3.1 (available at https://vminteq.lwr.kth.se/) used to calculate the Al3+ and Ca2+ activities. The log K values (Supplementary data Table S1) are those recommended by Nordstrom and May (1996).
Experiment 2: Al concentrations in bulk apical root tissues, cell wall and intracellular space
This experiment aimed to determine concentrations of Al in the bulk root apical tissues, the Al in the cell wall and the Al in the intracellular space. We also examined the impact of the metabolic inhibitor CCCP on these Al concentrations.
A total of 16 treatments were examined, consisting of four periods of exposure (0, 24, 48 and 96 h) with 0 μm Al and 0 µm CCCP, three periods of exposure (24, 48 and 96 h) with 200 μm Al and 0 μm CCCP, three periods of exposure (24, 48 and 96 h) with 200 μm Al and 10 μm CCCP, three periods of exposure (24, 48 and 96 h) with 1000 μm Al and 0 μm CCCP and three periods of exposure (24, 48 and 96 h) with 1000 μm Al and 10 μm CCCP. These Al concentrations were selected based upon the results from Experiment 1 which showed that both 200 and 1000 µm Al were sufficient to cause a reduction in the RER (see the Results). The four time periods (0, 24, 48 and 96 h) were selected as they included the sensitive phase (24–48 h) which preceded a partial recovery in the RER associated with the delayed development of Al resistance (96 h) (see the Results). The concentration of CCCP was based upon a previous study, where it was shown not to influence cell vitality (Reid et al., 1996). There were two replicates for each of the total of 16 treatments, with each replicate containing 300–400 seedlings. The nutrient solutions were adjusted to pH 4.2 using 0.1 m HCl. The metabolic inhibitor CCCP was dissolved in 95 % (v/v) ethanol, and thus aliquots of ethanol were added to all treatment solutions to achieve a final concentration of 0.95 % (v/v).
After the required periods of exposure (0, 24, 48 and 96 h), the apical root tissues (0–10 mm) were harvested, rinsed with deionized water and divided into two portions. The first portion (bulk tissue) was weighed, dried at 65 °C and then digested in 5 mL of a 1:5 mixture of nitric acid and perchloric acid. The digests were analysed using ICP-MS to determine the Al concentration. The second portion was prepared in order to analyse the Al concentration of the cell wall. The 0–10 mm apical root tissues were placed in 96 % ethanol and homogenized using a mortar and pestle placed on ice. The collected homogenized root tissue was washed with ethanol (96 %), gently shaken and centrifuged at 4000 g for 10 min with the supernatant discarded; this process was repeated three times (Schmohl and Horst, 2000). After centrifugation, the pellet was dried at 65 °C. The cell wall materials were digested as described above and the Al concentrations determined using ICP-MS. The intracellular Al concentration was calculated as the difference between bulk apical tissue and apical cell wall Al concentrations. For the treatment samples, seeds were germinated using a rolled paper towel as described above. After germination, >1000 uniform seedlings were selected and held using shade cloth suspended above a basal nutrient solution in a 22 L container for another 24 h growth, to achieve a suitable root length for the experiment.
Experiment 3: measurement of root tissue cation exchange capacity
The CEC of the root tissues was determined by lanthanum (La) sorption using the centrifugation method described by Wehr et al. (2010b). Seeds were germinated in a rolled paper towel and seedlings were grown as described above. A total of ten treatments were used as outlined in Experiment 2, each with two replicates. Each replicate contained 300–400 apical root tips to provide enough tissues to determine the La concentration. These treatments consisted of three Al concentrations (0, 200 and 1000 μm Al) and four periods of Al exposure (0, 24, 48 and 96 h). The treatment solutions were adjusted to pH 4.2 using 0.1 m HCl.
After growth for the required period of time, the 0–10 mm apical root tissues were harvested, rinsed with deionized water and homogenized using a mortar and pestle placed on ice. Next, the homogenized root tissue was collected and mixed with 10 mL of 0.01 m citric acid and then centrifuged. The supernatant was discarded and the pellet washed with another 10 mL of 0.01 m citric acid. After centrifugation, 5 mL of 5 mm LaCl3 was added to the remaining pellet and stirred for 5 min before placing on ice for 0.5 h. This step was repeated twice to allow the pellet to fully resuspend. After centrifugation, the pellet was washed with deionized water three times and dried at 65 °C. The dry plant tissues were weighed and digested as described in Experiment 2. The digests were analysed using ICP-OES (inductively coupled plasma optical emission spectroscopy) to determine the La concentration.
Experiment 4: measurement of the degree of cell wall methylation
Seedlings were grown as described in Experiment 2, with initial growth in basal solution before being transferred to Al-containing nutrient solutions in 22 L containers. A total of ten treatments were investigated, with 0 μm Al concentrations for 0, 24, 48 and 96 h, and 200 and 1000 μm Al for 24, 48 and 96 h, with each treatment having two replicates containing 300–400 seedlings each. At the required time, apical root tissues (0–10 mm) were prepared as described in Experiment 2. Titrimetric analysis was used to determine the degree of methylation of the cell wall. Using 5 mL of the cell wall material, samples were titrated to between pH 7 and 8 using 0.02 m NaOH, with the volume recorded as the initial titre. Next, 1 mL of 0.5 m NaOH was added to each sample while purging with N2 gas. The solutions were then titrated to neutral after the addition of 1 mL of 0.5 m HCl. This was recorded as the saponification titre, which represents the degree of methylation (Walter, 1991). The degree of methylation was calculated as:
where VS is the saponification titre (mL) and VT is the total titre (initial titre plus saponification titre, mL).
Experiment 5: changes in root cell wall fractions within Al exposure
Experiment 5 aimed to examine concentrations of four cell wall fractions over time, i.e. pectin, hemicellulose 1, hemicellulose 2 and cellulose. The treatments consisted of three Al concentrations (0, 200 and 1000 µm Al) and four periods of exposure (0, 24, 48 and 96 h), with each treatment containing two replicates. Each replicate contained 300–400 seedlings. Seedlings were grown in 22 L containers as described in Experiment 2. At the appropriate times, apical root tissues (0–10 mm) were rinsed, harvested and the cell wall materials were prepared as described in Experiment 2. The dried and weighed cell wall material was then sequentially extracted. The pectin was extracted using 0.5 % ammonium oxalate in a boiling water bath for 1 h, with this process repeated twice and the supernatants collected. The pellets were subsequently extracted with 4 % KOH at room temperature for assessment of the hemicellulose 1 fraction and with 24 % KOH for assessment of the hemicellulose 2 fraction. The pellets were then dried and weighed, and considered as the cellulose fraction (Zhong and Lauchli, 1993).
The cell wall fractions were quantified using a spectrophotometer according to the colorimetric method described by Blumenkrantz and Asboe-Hansen (1973). It is important to note that the method used in the present experiment is only suitable for measuring those cell wall fractions with carboxylic functional groups. Although there are likely to be polysaccharides without carboxylic groups, the present study aims to examine the charged cell wall fractions, as these are responsible for Al binding. Generally, 2 mL of chilled (4 °C) concentrated sulfuric acid was added to 0.2 mL of each fraction in reaction vials kept in an ice–water bath. Next, 1 mL of deionized water was added and incubated for 4 h at room temperature (25 °C). Then, 1.2 mL of a sulfuric acid/tetraborate reagent was added to each reaction vial in an ice–water bath to ensure that the samples remained cool. Samples were shaken in a vortex mixer and heated in a water bath at 100 °C for 5 min. After cooling again in an ice–water bath, 20 µL of a 3-phenylphenol solution was added and the samples were gently shaken. After 5 min, absorbance measurements were conducted at 520 nm using the spectrophotometer. Both galacturonic acid and glucose were used as calibration standards. The pectin, hemicellulose 1 and hemicellulose 2 were expressed as galacturonic acid equivalents (GaEs), while cellulose was expressed as glucose (µg) equivalents (Schmohl and Horst, 2000).
RESULTS
Experiment 1: dose response curves, root morphology and thermodynamic modelling
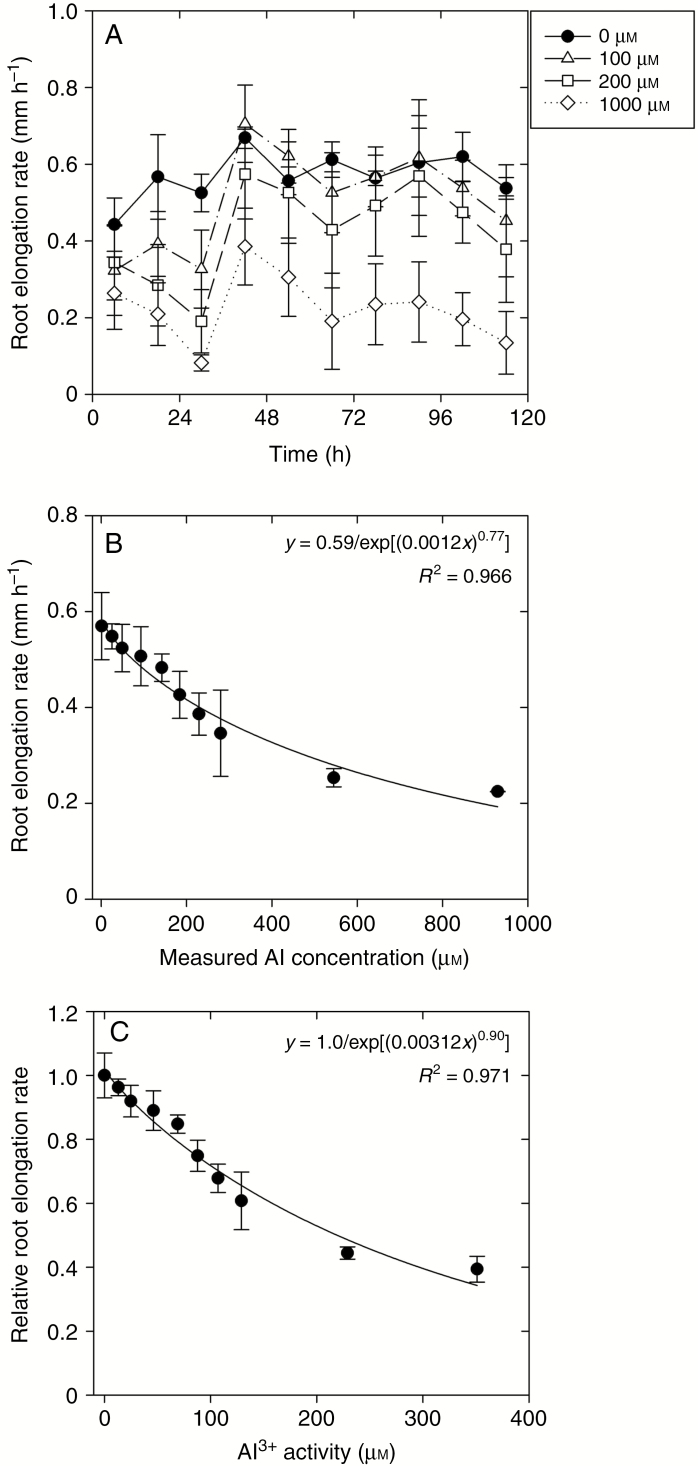
For Experiment 1, the RER in control solutions (0 µm Al) was approx. 0.6 mm h–1 across the 120 h experimental period (Fig. 1A, B). This value for the RER in the Al-free solutions is similar to that reported previously for this plant species, with Arroyave et al. (2013) reporting a value of about 0.65 mm h–1. However, when averaged across the entire experimental period, addition of Al decreased the RER, with a 25 % reduction in the RER predicted to occur at an Al concentration of 184 µm and a 50 % reduction at 460 µm (Fig. 1B, C).
Fig. 1.
Effect of increasing Al concentrations on the signal grass root elongation rate (RER) during 120 h in basal solution (Experiment 1). (A) The RER in basal solution over time when exposed to 0, 100, 200 and 1000 µm Al (nominal concentrations). (B) The RER in basal solution after 120 h growth in a control or in solutions containing one of nine measured Al concentrations. (C) Effect of Al3+ activity on the relative RER. Values are the arithmetic mean of two replicates, with each replicate containing seven seedlings. The vertical bars correspond to the s.d.
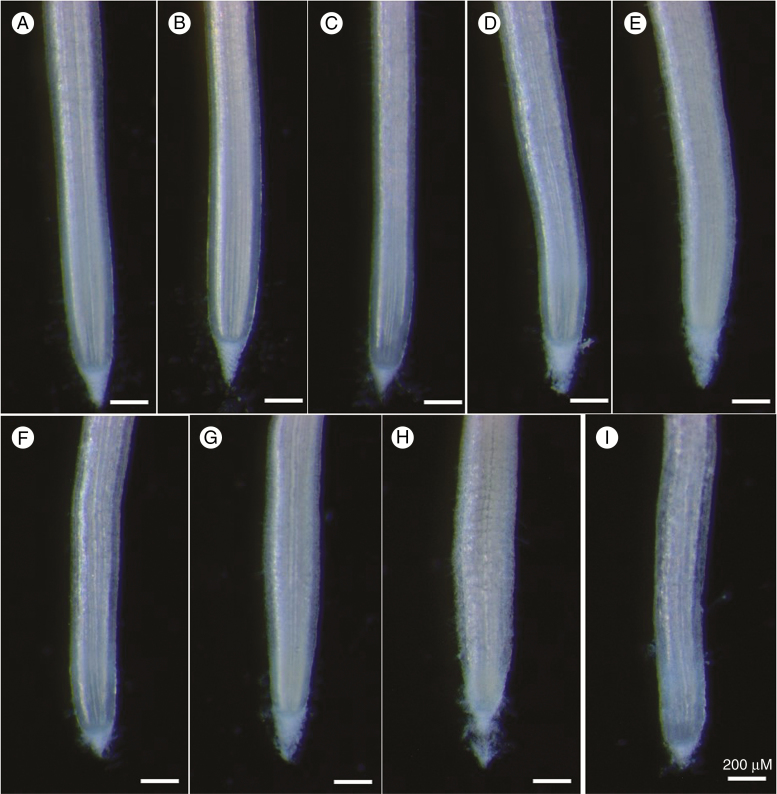
Interestingly, examination of the change in the RER over time (Fig. 1A) showed that although exposure to Al caused an initial decrease in the RER (0–24 h), it subsequently increased in all Al-containing solutions after 24–48 h. For example, for the 100 µm Al treatment, the RER decreased to 0.33 mm h–1 after 30 h, but increased again to 0.69 mm h–1 after 48 h, with this latter value being similar to that in the control (0.67 mm h–1 after 48 h) (Fig. 1A). Indeed, for 100 µm Al, although the RER decreased substantially during the initial 24 h period, it was similar to the control across the remainder of the experimental period (Fig. 1A). Similar trends were observed at higher levels of Al, although the RER did not necessarily recover to values similar to those observed in Al-free solutions. Light microscopy was used to examine changes in root morphology associated with this exposure to Al. It was observed that increases in Al concentration were associated with the disintegration of the root surface tissues (rhizodermis and outer cortex) and the associated formation of root ruptures (Fig. 2). Moreover, formation of root ruptures started after 24 h exposure, with obvious disintegration apparent after 48 h. The morphological symptoms of the roots did not further develop during the 48–96 h period as no new root ruptures occurred (Fig. 2).
Fig. 2.
Light micrographs of signal grass root apices (Experiment 1). Roots were exposed to 0 µm Al (A, B, C), 200 µm Al (D, E, F) and 1000 µm Al (G, H, I) (nominal concentrations) for 24 h (A, D, G), 48 h (B, E, H) and 96 h (C, F, I). The scale bar in (I) applies to all images.
We used thermodynamic modelling to determine if changes in the RER could be due to differences in Al concentrations or activities over time. However, the solution concentrations of Al measured after 0 and 120 h were similar to the nominal values, and concentrations did not change over the duration of the experiments (Table 1). Specifically for treatments containing nominal Al concentrations of 25–1000 µm Al, measured Al values ranged from 25.6 to 929 µm, with the corresponding values calculated for Al3+ activity being 13.1–351 µm (Table 1). The Ca2+ activity, calculated from nominal Ca concentrations, decreased slightly with increasing Al, ranging from 675 to 782 µm (Table 1).
Table 1.
Predicted activities of Ca2+ and Al3+ in basal solution from the measured Al concentrations used in Experiment 1 (measured values are the average after 0 and 120 h)
| Nominal Al (µm) | Measured Al (µm) | Ca2+ activity (µm) | Al3+ activity (µm) |
|---|---|---|---|
| 0 | 1.02 | 782 | 0.57 |
| 25 | 25.6 | 778 | 13.1 |
| 50 | 49.4 | 775 | 24.9 |
| 100 | 93.1 | 768 | 46.1 |
| 150 | 142 | 760 | 69.0 |
| 200 | 184 | 754 | 87.8 |
| 250 | 229 | 748 | 107 |
| 300 | 280 | 741 | 129 |
| 600 | 545 | 710 | 229 |
| 1000 | 929 | 675 | 351 |
The Al3+ activity was calculated from measured Al concentrations, and the Ca2+ activity was calculated from nominal Ca concentrations.
Total concentration of Ca: 1000 µm.
Experiment 2: Al concentrations in bulk apical root tissues, apical cell wall and intracellular space
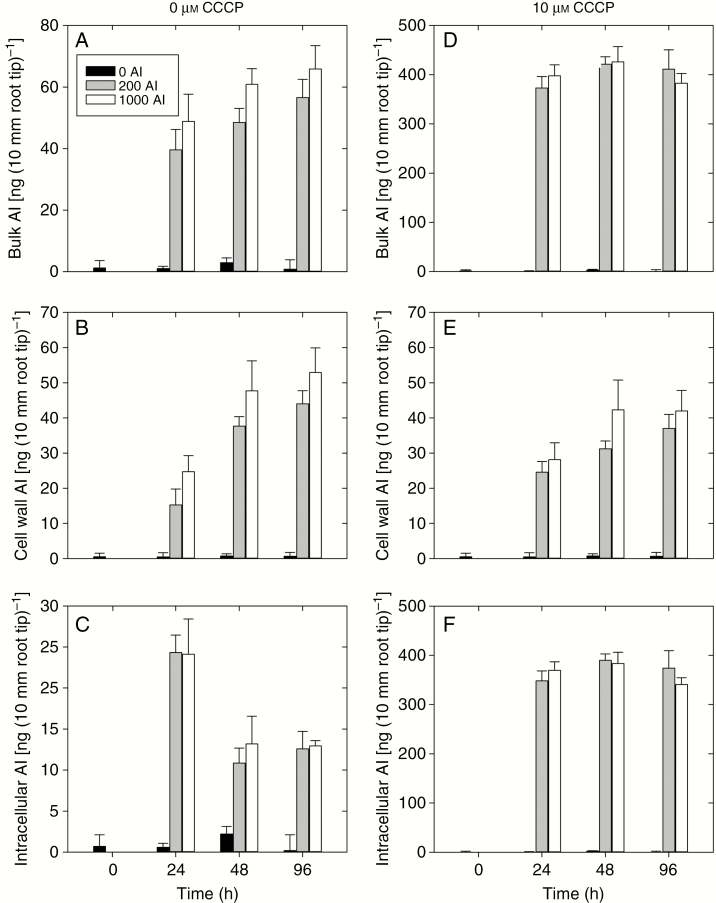
In the bulk apical root tissues (0–10 mm) in the absence of the metabolic inhibitor CCCP, the Al concentration increased rapidly upon exposure to Al, with the concentration after 24 h ranging from 39 ng (10 mm root tip)–1 at 200 µm Al to 49 ng (10 mm root tip)–1 at 1000 µm Al (Fig. 3A). The tissue Al concentrations continued to increase over time, reaching values after 96 h of 57 ng (10 mm root tip)–1 at 200 µm Al and 66 ng (10 mm root tip)–1 at 1000 µm Al (Fig. 3A). We then examined the concentration of Al that accumulated specifically within the apical cell wall materials (Fig. 3B). After 24 h, the Al concentration in the cell wall materials was 15 ng (10 mm root tip)–1 at 200 µm Al and 25 ng (10 mm root tip)–1 at 1000 µm Al, increasing after 96 h to 44 ng (10 mm root tip)–1 at 200 µm Al and to 53 ng (10 mm root tip)–1 at 1000 µm Al (Fig. 3B). For the intracellular tissues, the Al concentrations were similar in both the 200 and 1000 µm Al treatments across all time points (Fig. 3C). Interestingly, however, intracellular Al concentrations reached their maximum value after 24 h [approx. 25 ng (10 mm root tip)–1] before decreasing to approx. 13 ng (10 mm root tip)–1 after 96 h (Fig. 3 C).
Fig. 3.
Concentrations of Al in signal grass bulk apical tissues, apical cell wall and intracellular Al after 0, 24, 48 and, 96 h treatment in 0, 200 and 1000 µm Al basal solutions with 0 µm CCCP (left) and with 10 µm CCCP (right) (Experiment 2). The Al concentrations are expressed on a per 10 mm root tip basis. Values are the arithmetic mean (with the s.d.) of two replicates. It should be noted that the scales of the y-axis are different.
When exposed to 10 µm CCCP, the Al concentrations in the bulk apical tissues were approx. 8-fold higher than in the corresponding treatments without CCCP (Fig. 3D). This increase in the bulk tissue Al concentration in the presence of CCCP was not associated with an increase in the cell wall Al concentration (Fig. 3E), but rather with a substantial increase in the intracellular Al. Indeed, intracellular Al concentrations increased from approx. 10–25 ng (10 mm root tip)–1 to approx. 350–400 ng (10 mm root tip)–1 (Fig. 3F). Furthermore, it was noted that not only was the intracellular Al concentration higher upon addition of CCCP, but there was no subsequent reduction in concentration after 48 h, as was observed in the absence of CCCP (Fig. 3F).
Experiment 3: measurement of root tissue cation exchange capacity
The CEC values for the root apical tissues were determined using La sorption to examine if these values changed over time in accordance with the observed changes in the RER. For the control, CEC values generally remained constant across the entire experimental period, i.e. 14–15 µmol g–1 (Table 2). However, exposure to Al resulted in an increase in the CEC values of the apical root tissues. Specifically, the CEC values were observed to increase over time, but also increase as the solution Al concentration increased. For example, at 200 µm Al, the apical root tissue CEC values increased from 15.6 µmol g–1 after 0 h to 57.6 µmol g–1 after 96 h, with the magnitude of this increase being greater in the initial period of the experiment (Table 2). A similar trend in CEC was also found for the 1000 µm Al treatment, although the increase in CEC was greater than observed for 200 µm Al. For example, after 96 h exposure, the CEC was measured to be 57.6 µmol g–1 at 200 µm Al but 77.4 µmol g–1 at 1000 µm Al (Table 2).
Table 2.
Effect of Al exposure on cell wall cation exchange capacity (CEC)
| Treatment time (h) | Cell wall CEC (µmol g–1) | ||
|---|---|---|---|
| 0 µm Al | 200 µm Al | 1000 µm Al | |
| 0 | 15.6 (±2.1) | 15.6 (±2.1) | 15.6 (±2.1) |
| 24 | 14.7 (±4.2) | 34.6 (±4.6) | 48.5 (±4.4) |
| 48 | 14.1 (±1.1) | 49.7 (±2.4) | 62.1 (±3.6) |
| 96 | 14.3 (±3.6) | 57.6 (±6.3) | 77.4 (±5.8) |
The La sorption by the apical signal grass root tissues was analysed after 0, 24, 48 and 96 h.
Experiment 4: measurement of the degree of cell wall methylation
The degree of methylation for the cell wall was examined using titrimetric analysis (Table 3). Again, concentrations of 0, 200 and 1000 µm Al were used, with the RER initially decreasing at 200 and 1000 µm (0–24 h) before subsequently increasing again (24–48 h, Fig. 1). In the Al-free nutrient solution, the degree of methylation remained relatively constant over time, ranging between 88 and 92 % over the entire 96 h experimental period (Table 3). At solution Al concentrations of 200 and 1000 µm, the degree of methylation first decreased slightly after 24 h before increasing again to levels similar to that in the control after 48 h (Table 3). For example, for the 1000 µm Al treatment, the degree of methylation decreased from 92 % after 0 h to 77 % after 24 h, before subsequently returning to 93 % after 48 h (Table 3).
Table 3.
Effect of Al exposure on the degree of cell wall methylation after 0, 24, 48 and 96 h
| Treatment time (h) | Degree of cell wall methylation (%) | ||
|---|---|---|---|
| 0 µm Al | 200 µm Al | 1000 µm Al | |
| 0 | 92 (±0.5) | 92 (±0.5) | 92 (±0.5) |
| 24 | 88 (±1.2) | 83 (±1.3) | 77 (±2.1) |
| 48 | 87 (±1.1) | 96 (±2.4) | 93 (±1.9) |
| 96 | 90 (±1.0) | 94 (±2.2) | 97 (±4.3) |
Values are the arithmetic mean (with the s.d.) of two experimental replicates (each replicate contains 300–400 apical root tips) and are expressed on a dry mass basis.
Experiment 5: changes in root cell wall fractions within Al exposure
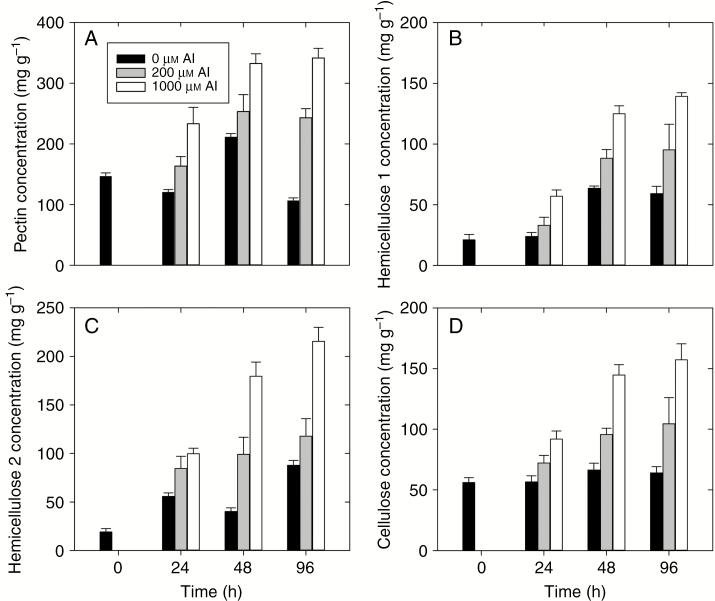
Changes in four different cell wall fractions (pectin, hemicellulose 1, hemicellulose 2 and cellulose) were examined over time following exposure to Al (Fig. 4). For pectin, in the Al-free treatment (control), the pectin concentration varied between 110 and 200 mg g–1 during the 96 h experimental period (Fig. 4A). Addition of Al (either 200 or 1000 µm) resulted in an increase in pectin concentration, with the magnitude of this increasing with Al (Fig. 4A). For example, the pectin concentration increased from 140 mg g–1 after 0 h to 250 mg g–1 (200 µm Al) and 330 mg g–1 (1000 µm Al) after 48 h.
Fig. 4.
Cell wall fractions extracted from the apical root tissues (0–10 mm) of signal grass exposed to 0, 200 and 1000 µm Al for 0, 24, 48 or 96 h. Concentrations are expressed on a dry mass basis per gram of cell wall. Values are the arithmetic mean (with the s.d.) of two replicates which contain 300–400 apical root tips each.
For hemicellulose 1, concentrations generally increased over time for all three Al treatments (0, 200 and 1000 µm Al), although the magnitude of this increase was greater at higher Al. For example, the hemicellulose 1 concentration increased from 21 to 63 mg g–1 after 96 h for the control, but from 21 to 139 mg g–1 at 1000 µm Al (Fig. 4B). Changes in the hemicellulose 2 fraction were similar to those described for hemicellulose 1. Specifically, the hemicellulose concentration increased over time for all three treatments, but the magnitude of this increase was greater at higher Al concentrations (Fig. 4C).
Finally, consideration was given to the cellulose concentration. For cellulose, the concentration in Al-free treatment (control) was generally constant over time, being about 60 mg g–1 (Fig. 4D). However, an increase of the Al concentration resulted in increases in cellulose. At 200 µm Al, the cellulose concentration increased from 56 to 104 mg g–1 after 96 h, while at 1000 µm Al, cellulose increased from 56 to 157 mg g–1 after 96 h (Fig. 4D).
DISCUSSION
Signal grass is a widely used pasture which is known to have a delayed Al resistance. In the present study, using plant growth experiments, we found that this delayed resistance was not associated with a change in properties of the cell wall, with this being confirmed by measuring CEC, the degree of methylation as well as concentrations of pectin, hemicellulose 1, hemicellulose 2 and cellulose. However, we have shown that the improvement in the RER that occurs after approx. 48 h corresponds to a marked decrease in the intracellular Al concentration, with this being confirmed by the addition of the metabolic inhibitor CCCP, which prevents the decrease in intracellular Al that occurs after 48 h. These results indicate that the delayed resistance of signal grass to Al is not due to changes in cell wall properties or the binding of Al to the cell wall, but rather that it is likely to be associated with a delayed ability of signal grass to exclude Al from the intracellular compartment.
Signal grass has a high Al resistance
As has been reported previously, we have found that signal grass is highly Al resistant. In the present study, in the absence of Al, the RER of signal grass in basal nutrient solution was 0.6 mm h–1, which is similar to that reported previously by Arroyave et al. (2013) for this species. However, the addition of Al reduced the RER, with the effective concentration of Al that reduced root elongation by 50 % (EC50) calculated to be 460 µm (Fig. 1). Importantly, this value for the EC50 is considerably higher than the EC50 of other plants, including 5 µm for wheat (Triticum aestivum, ‘Scout 66’), 15–20 µm for maize (Zea mays) and 75–200 µm for rice (Oryza sativa, ‘Nipponbare’) (Kopittke et al., 2016). The EC50 value of 460 µm corresponds well to previous reports for this plant species (Wenzl et al., 2001). Although Brachiaria is highly tolerant of Al, this resistance comes at a cost for the plant in that the RER decreases with Al concentrations.
We also observed that Al toxicity in signal grass was associated with the development of ruptures on the root surface, with these being due to the disintegration of root tissues (Fig. 2). This morphological symptom occurred from 24 h and did not further develop after 48 h, as no obvious new root ruptures were observed at 96 h. Not surprisingly, similar morphological symptoms have been observed previously in a wide range of plant species including soybean, cowpea (Vigna unguiculata), maize and pea (Pisum sativum) (Blancaflor et al., 1998; Yamamoto et al., 2001; Kopittke et al., 2008, 2015). The formation of ruptures is the most commonly reported morphological symptom when exposed to Al and is due to the slower elongation of the outer tissues where Al is binding strongly compared with the inner tissues where Al concentrations are markedly lower (Kopittke et al., 2016).
Expression of Al resistance is delayed
Although signal grass was found to have a high Al resistance, this was preceded by a sensitive phase for the first 24–48 h (Fig. 1). This observation of an initial sensitive phase is also in agreement with previous studies (Arroyave et al., 2011, 2017). However, the mechanism conferring this Al resistance in signal grass remains unknown. Indeed, it has been reported previously that it is not associated with organic acid secretion, or with the alkalinization of the apical rhizosphere (Wenzl et al., 2001).
We first examined whether the delay in the expression of Al resistance could be explained by changes related to the cell wall. This is because the cell wall has increasingly been shown to play a critical role in the expression of Al toxicity in a range of plant species (Horst et al., 2010; Kopittke et al., 2015). We examined whether the delay in Al resistance in signal grass was associated with a change in cell wall properties.
However, changes in the Al bound to the cell wall did not appear to be related to the improvement in the RER after the initial sensitive phase (Fig. 3). In addition, we also measured changes in CEC, degree of cell wall methylation and polysaccharide concentrations, but again found that none of these parameters could explain the improvement in the RER observed after the initial sensitive phase (Tables 1–3). This is despite previous findings in a range of plant species that changes in cell wall properties can influence the plant response to Al. For example, Eticha et al. (2005) reported a different degree of methylation in Al-sensitive and Al-resistant genotypes. Similarly, it has been reported previously that the binding of Al to the cell wall is positively correlated to Al-induced pectin formation (Horst et al., 2010), with similar results observed for hemicellulose in wheat, and cellulose in tobacco (Nicotiana tabacum) and squash (Cucurbita maxima) (Van et al., 1994; Chang et al., 1999; Tabuchi and Matsumoto, 2001). However, in the present study, changes in these cell wall fractions were not associated with delayed expression of Al resistance (Fig. 1). The increase of pectin and hemicellulose concentrations is due to Al-inhibited degradation of turnover (Wehr et al., 2010a). This makes cell walls stiffer and causes ruptures. An increase in the CEC corresponds to an increase in cell wall polysaccharides (mainly hemicellulose) but not a change in the degree of cell wall methylation. In grasses, hemicellulose (xylans and glucuronoarabinoxylans) is more important than pectin in contributing to the CEC (grasses have less pectin in cell wall).
Interestingly, however, it was observed that the initiation of the Al resistance phase after approx. 24–48 h corresponded to a substantial decrease in the Al concentration in the intracellular compartment (Fig. 3). Indeed, the Al concentration in the intracellular compartment decreased substantially, from approx. 25 ng (10 mm root tip)–1 after 24 h to 13 ng (10 mm root tip)–1 after 48 h. This was then examined through the addition of CCCP, which is known to inhibit the flux across the plasma membrane. Upon the addition of CCCP, we observed that the concentration of Al in the intracellular compartment increased approx. 14-fold. Furthermore, we no longer observed a decrease in the intracellular Al concentration at 48 h. These results suggest that the delay of Al resistance is likely to be associated with Al concentration in the intracellular compartment in root tissues, and that the high Al resistance is the result of an energy-dependent Al exclusion mechanism.
Signal grass is highly Al resistant, but there is an initial delay in the expression of this resistance. In the present study, we found that Al decreases upon exposure to Al before improving again after 24–48 h. We initially hypothesized that this delayed expression of Al resistance was due to a change in cell wall properties which altered the binding of Al to the cell wall. However, the concentration of Al binding to the cell wall materials was not related to changes in the RER, nor were changes in cell wall properties such as the CEC or the degree of cell wall methylation. However, we found that the Al concentration in the intracellular compartment decreased by approx. 50 % after 24 h, with this decrease in intracellular Al corresponding to delayed expression of Al resistance. Upon the addition of the metabolic inhibitor CCCP, the concentration of Al in the intracellular compartment increased approx. 14-fold compared with the control. Moreover, the concentration of intracellular Al did not decrease after 48 h, as observed in the Al-treated roots in the absence of CCCP. Our results support the hypothesis that part of the mechanism of Al resistance is based on the energy-dependent Al exclusion metabolism.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of Table S1: values of log K used for thermodynamic modelling with MINTEQ 3.1.
ACKNOWLEDGEMENTS
Z.L. is a recipient of a scholarship from the China Scholarship Council (201506300048) and of a University of Queensland International Scholarship (UQI). P.M.K. is the recipient of an Australian Research Council (ARC) Future Fellowship (FT120100277), and P.W. is the recipient of an ARC Discovery Early Career Researcher Award (DECRA, DE130100943).
LITERATURE CITED
- Arroyave C, Barceló J, Poschenrieder C, Tolrà R. 2011. Aluminium-induced changes in root epidermal cell patterning, a distinctive feature of hyperresistance to Al in Brachiaria decumbens. Journal of Inorganic Biochemistry 105: 1477–1483. [DOI] [PubMed] [Google Scholar]
- Arroyave C, Tolrà R, Thuy T, Barceló J, Poschenrieder C. 2013. Differential aluminum resistance in Brachiaria species. Environmental and Experimental Botany 89: 11–18. [Google Scholar]
- Arroyave C, Tolrà R, Chaves L, de Souza MC, Barceló J, Poschenrieder C. 2017. A proteomic approach to the mechanisms underlying activation of aluminium resistance in roots of Urochloa decumbens. Journal of Inorganic Biochemistry 181: 145–151. [DOI] [PubMed] [Google Scholar]
- Blancaflor EB, Jones DL, Gilroy S. 1998. Alterations in the cytoskeleton accompany aluminum-induced growth inhibition and morphological changes in primary roots of maize. Plant Physiology 118: 159–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenkrantz N, Asboe-Hansen G. 1973. New method for quantitative determination of uronic acids. Analytical Biochemistry 54: 484–489. [DOI] [PubMed] [Google Scholar]
- Chang YC, Yamamoto Y, Matsumoto H. 1999. Accumulation of aluminium in the cell wall pectin in cultured tobacco (Nicotiana tabacum L.) cells treated with a combination of aluminium and iron. Plant, Cell & Environment 22: 1009–1017. [Google Scholar]
- Choudhary AK, Singh D, Kumar J. 2011. A comparative study of screening methods for tolerance to aluminum toxicity in pigeonpea [Cajanus cajan (L.) Millspaugh]. Australian Journal of Crop Science 5: 1419–1426. [Google Scholar]
- Eticha D, Stass A, Horst WJ. 2005. Cell-wall pectin and its degree of methylation in the maize root-apex: significance for genotypic differences in aluminium resistance. Plant, Cell & Environment, 28: 1410–1420. [Google Scholar]
- Horst WJ, Wang Y, Eticha D. 2010. The role of the root apoplast in aluminium-induced inhibition of root elongation and in aluminium resistance of plants: a review. Annals of Botany, 106: 185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopittke PM, Blamey FPC, Menzies NW. 2008. Toxicities of soluble Al, Cu, and La include ruptures to rhizodermal and root cortical cells of cowpea. Plant and Soil 303: 217–227. [Google Scholar]
- Kopittke PM, Moore KL, Lombi E, et al. 2015. Identification of the primary lesion of toxic aluminum in plant roots. Plant Physiology 167: 1402–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopittke PM, Menzies NW, Wang P, Blamey FPC. 2016. Kinetics and nature of aluminium rhizotoxic effects: a review. Journal of Experimental Botany 67: 4451–4467. [DOI] [PubMed] [Google Scholar]
- Li Z, Wang P, Menzies NW, Kopittke PM. 2018. Defining appropriate methods for studying toxicities of trace metals in nutrient solutions. Ecotoxicology and Environmental Safety 147: 872–880. [DOI] [PubMed] [Google Scholar]
- Nordstrom DK, May HM. 1996. Aqueous equilibrium data for mononuclear aluminum species. Boca Raton, FL: CRC/Lewis Publishers. [Google Scholar]
- Reid RJ, Rengel Z, Smith FA. 1996. Membrane fluxes and comparative toxicities of aluminium, scandium and gallium. Journal of Experimental Botany 47: 1881–1888. [Google Scholar]
- Schmohl N, Horst WJ. 2000. Cell wall pectin content modulates aluminium sensitivity of Zea mays (L.) cells grown in suspension culture. Plant, Cell & Environment, 23: 735–742. [Google Scholar]
- Szatanik-Kloc A, Szerement J, Józefaciuk G. 2017. The role of cell walls and pectins in cation exchange and surface area of plant roots. Journal of Plant Physiology 215: 85–90. [DOI] [PubMed] [Google Scholar]
- Tabuchi A, Matsumoto H. 2001. Changes in cell-wall properties of wheat (Triticum aestivum) roots during aluminum-induced growth inhibition. Physiologia Plantarum 112: 353–358. [DOI] [PubMed] [Google Scholar]
- Taylor GJ, McDonald-Stephens JL, Hunter DB, Bertsch PM, Elmore D, Rengel Z, Reid RJ. 2000. Direct measurement of aluminum uptake and distribution in single cells of Chara corallina. Plant Physiology 123: 987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van HL, Kuraishi S, Sakurai N. 1994. Aluminum-induced rapid root inhibition and changes in cell-wall components of squash seedlings. Plant Physiology 106: 971–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Uexküll H, Mutert E. 1995. Global extent, development and economic impact of acid soils. Plant and Soil 171: 1–15. [Google Scholar]
- Walter R. 1991. Analytical and graphical methods for pectin. San Diago, CA: Academic Press. [Google Scholar]
- Wehr JB, Blamey FPC, Kopittke PM, Menzies NW. 2010a Comparative hydrolysis and sorption of Al and La onto plant cell wall material and pectic materials. Plant and Soil 332: 319–330. [Google Scholar]
- Wehr JB, Blamey FPC, Menzies NW. 2010b Comparison between methods using copper, lanthanum, and colorimetry for the determination of the cation exchange capacity of plant cell walls. Journal of Agricultural and Food Chemistry 58: 4554–4559. [DOI] [PubMed] [Google Scholar]
- Wenzl P, Patino GM, Chaves AL, Mayer JE, Rao IM. 2001. The high level of aluminum resistance in signalgrass is not associated with known mechanisms of external aluminum detoxification in root apices. Plant Physiology 125: 1473–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzl P, Chaves AL, Patiño GM, Mayer JE, Rao IM. 2002a Aluminum stress stimulates the accumulation of organic acids in root apices of Brachiaria species. Journal of Plant Nutrition and Soil Science 165: 582–588. [Google Scholar]
- Wenzl P, Mayer JE, Rao IM. 2002b Aluminum stress inhibits accumulation of phosphorus in root apices of aluminum-sensitive but not aluminum-resistant Brachiaria cultivar. Journal of Plant Nutrition 25: 1821–1828. [Google Scholar]
- Yamamoto Y, Kobayashi Y, Matsumoto H. 2001. Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiology 125: 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Lauchli A. 1993. Changes of cell wall composition and polymer size in primary roots of cotton seedlings under high salinity. Journal of Experimental Botany 44: 773–778. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.