Abstract
Background
In trees, secondary metabolites (SMs) are essential for determining the effectiveness of defence systems against fungi and why defences are sometimes breached. Using the CODIT model (Compartmentalization of Damage/Dysfunction in Trees), we explain defence processes at the cellular level. CODIT is a highly compartmented defence system that relies on the signalling, synthesis and transport of defence compounds through a three-dimensional lattice of parenchyma against the spread of decay fungi in xylem.
Scope
The model conceptualizes ‘walls’ that are pre-formed, formed during and formed after wounding events. For sapwood, SMs range in molecular size, which directly affects performance and the response times in which they can be produced. When triggered, high-molecular weight SMs such as suberin and lignin are synthesized slowly (phytoalexins), but can also be in place at the time of wounding (phytoanticipins). In contrast, low-molecular weight phenolic compounds such as flavonoids can be manufactured de novo (phytoalexins) rapidly in response to fungal colonization. De novo production of SMs can be regulated in response to fungal pathogenicity levels. The protective nature of heartwood is partly based on the level of accumulated antimicrobial SMs (phytoanticipins) during the transitionary stage into a normally dead substance. Effectiveness against fungal colonization in heartwood is largely determined by the genetics of the host.
Conclusion
Here we review recent advances in our understanding of the role of SMs in trees in the context of CODIT, with emphasis on the relationship between defence, carbohydrate availability and the hydraulic system.We also raise the limitations of the CODIT model and suggest its modification, encompassing other defence theory concepts. We envisage the development of a new defence system that is modular based and incorporates all components (and organs) of the tree from micro- to macro-scales.
Keywords: CODIT, decay fungi, plant defence, parenchyma, plant–fungi interactions, heartwood, induced systemic resistance, non-structural carbohydrate (NSC), sapwood, secondary metabolites (SMs), secondary xylem, systemic induced resistance
INTRODUCTION
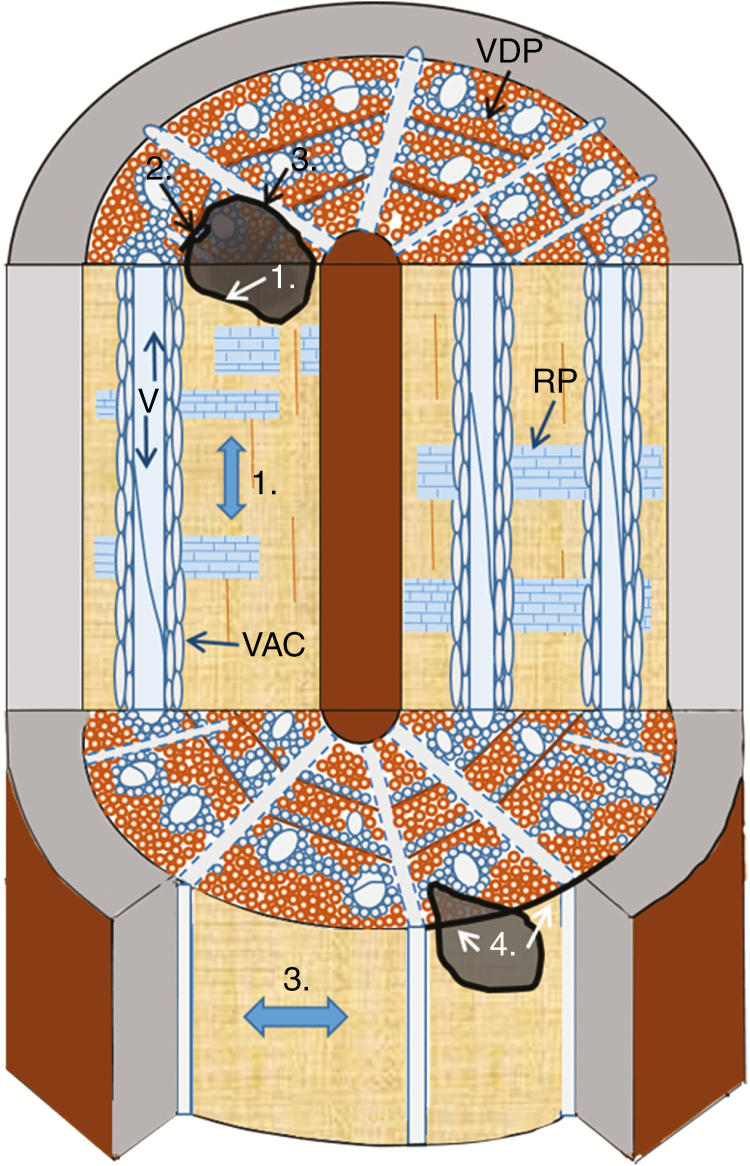
In terms of longevity, size and ecological dominance, trees are among the most successful life forms to have evolved. The latter characteristics are largely a consequence of a unique ability to form highly specialized tissues, where, like other organisms, trees are exposed to a wide variety of potential pathogens throughout their often very long life span. To defend themselves successfully, trees combine constitutive defences with short- and long-term inducible defences. The ultimate function of these defences is to protect the nutrient- and energy-rich phloem, the vascular cambium and the transpiration stream in the sapwood. Defence compartments in trees can be observed at macro- and microscopic scales. This review focuses on the molecular scale of the secondary xylem tissue in response to wood decay fungi where an expanded version of the CODIT model (Compartmentalization of Damage/Dysfunction in Trees) is used to explain defence processes (Fig. 1).
Fig. 1.
Conceptual drawing illustrating the three-dimensionality of xylem and the four walls of the CODIT mode in a typical temperate angiosperm tree (A). Numbers 1–4 with arrows represent the CODIT walls from weakest to strongest: wall 1, prevents decay spread in axial directions by plugging vessels with tyloses and gels from vessel-associated cells (VAC); wall 2, the annual rings restrict decay in radial directions through lignified fibres and polyphenolic SM production from ray and marginal axial parenchyma (MAP); wall 3, ray parenchyma (RP) plugs vessels from VAC and is critical in reaction zone development; and wall 4 or the barrier zone is a lignified and suberized parenchymatous zone of phenolic compounds initiated by the cambium after wounding that prevents decay spreading outwardly (and inwardly) into new sapwood. VDP, vessel-distant parenchyma connecting vessel to vessel (V) and ray to ray, forming a continuous 3-D lattice in axial, radial and lateral directions. Darkened regions represent decay columns.
Secondary or ‘special’ metabolites (SMs) are normally defined – with the exception of lignin and suberin – as low-molecular weight secondary products of plants and fungi derived from primary metabolites, and are not considered active in growth processes (Fig. 2; Scharf et al., 2014; Pusztahelyi et al., 2015). SMs are a crucial part of the tree defence system, which also includes the lignification of secondary walls (Miedes et al., 2014), the secretion of peptides called defensins (Terras et al., 1995; Lacerda et al., 2014) and reactive oxygen species (Inupakutika et al., 2016). The latter are involved in programmed cell death in plants during reaction zone formation (a key component of CODIT) against pathogenic fungi in xylem tissue (Inupakutika et al., 2016). Trees possess a huge diversity of SMs, where different chemical classes are constitutively present or synthesized de novo depending on the threat and the type of the secondary xylem tissue; for example, whether it is sapwood (active defence) or heartwood (constitutive defence). Xylem is composed of four main cell types, namely tracheids, vessels, fibres (the last two only found in angiosperms) and parenchyma. Parenchyma and living fibres are the only living components of functional xylem (sapwood). The key functions of xylem are water conduction (i.e. vessels and tracheids), storage of non-structural carbohydrates (NSCs) and water (i.e. parenchyma and living fibres), and mechanical support (i.e. tracheids and fibres). Symplast is formed by plasma continuum through cell–cell connections via plasmodesmata within mainly simple pits and is interwoven among conductive tissues and fibres forming two kinds, ray and axial parenchyma. Together, ray and axial parenchyma form a 3-D lattice in radial and axial directions and provide a functional link between phloem and the water transport system. Since the vast majority of xylem is composed of dead cells, inducible defences rely on the living parenchyma and the 3-D network for signalling, synthesis and transport of SMs (Figs 1 and 2A, B, E). It is important to mention that there is remarkable variation among and between angiosperms and gymnosperms in the organization of xylem and in the presence and frequency of cell types, and this should be considered when applying the CODIT model (Fig. 2).
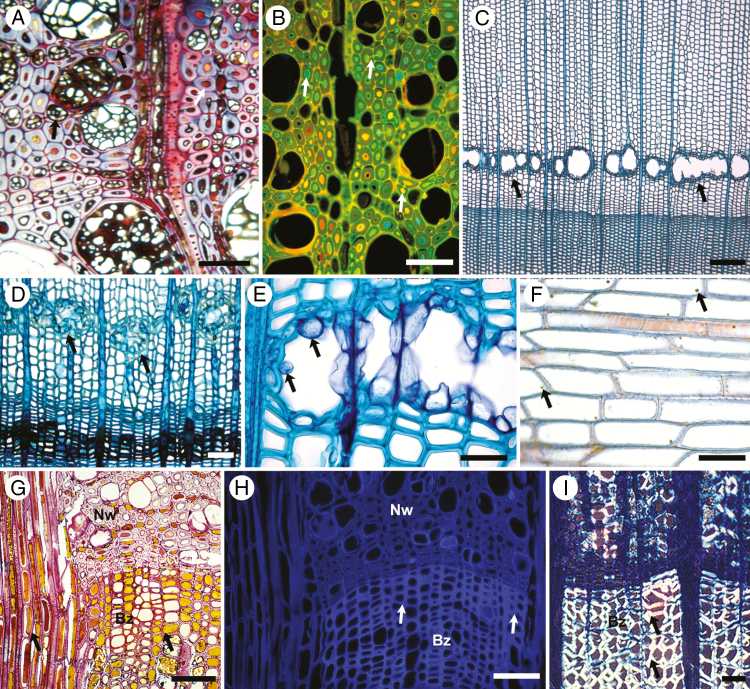
Fig. 2.
Micrographs of a range of angiosperm and conifer tree species showing reaction zone (A–F) and barrier zone (G–I) development as part of the CODIT tree defence system. (A) Fagus sylvatica (Fagaceae): pseudosclerotial plates or dark strands of melanized mycelia can be seen in the vessels and comprise zone lines formed by the ascomycete Kretzschmaria deusta; polyphenolic SMs occlude fibre tracheids (white arrow), which are synthesized in vessel-associated cells (black arrows); scale bar = 50 μm. (B) F. sylvatica: reaction zone stained with acridine orange under a fluorescence microscope showing polyphenols in fibre tracheid lumina and in adjoining pits (white arrows); scale bar = 50 μm. (c) Picea abies (Pinaceae): a ring of uninduced constitutive resin canals tangentially oriented along the early wood (black arrows); scale bar = 200 μm. (D) P. abies: resin-induced traumatic resin canals forming a distinct tangentially oriented impervious barrier that operates to prevent axial and radial spread of fungi in wall 1 and wall 2 of CODIT, respectively. Epithelial cells produce tylosoids that protrude into the canals, completely closing them (black arrows); scale bar = 50 μm. (E) P. abies: close-up of tylosoid development from epithelial cells lining the resin canals; scale bar = 30 μm. (F) Robinia pseudoacacia (Fabaceae): a single chlamydospore deposit (arrows) in each ray cell from fungal hyphae belonging to the basidiomycete Laetiporus sulphureus; scale bar = 50 μm. (G) Platanus × acerifolia (Platanaceae): deposits of low-molecular weight polyphenolic SMs accumulate in axial parenchyma of the barrier zone prior to suberization and lignification of cell walls (Bz), an anatomically distinct region that differs from normal wood (Nw); scale bar = 50 μm. (H) P. × acerifolia: fluorescence microscopy of image (G) shows suberin and lignification of axial parenchyma cells walls, two high-molecular weight SMs that are part of inducible defences in barrier zone formation, (I) Tilia platyphyllos (Malvaceae): heavily suberized axial parenchyma with polyphenolic SM deposits in the barrier zone in response to treatments from a range of decay fungi (black arrows); scale bar = 50 μm. Images (A), (B), (G), (H) and (I) are the copyright of Francis W. M. R. Schwarze.
The physiological mechanisms leading to tree mortality are a complex interaction between abiotic and biotic stresses (Cailleret et al., 2017), and tree defence and resistance capabilities (Boddy and Rayner, 1983; Shigo, 1984; Pearce, 1996; Morris et al., 2016a). Only living parenchyma cells within plant tissues are responsive to stimuli through forming the active component of tree defence systems in roots, leaves and stems. Dead parenchyma, whose extractives (released during cell death) are responsible for the dark coloration in heartwood, together with heavily lignified fibres or tracheids (forming annual rings in temperate tree species), occluded vessels from tyloses (outgrowths from the protoplast of vessel-associated parenchyma cells) and suberized periderm, form the physical and biologically inactive defence.
In wood, both active and physical/pre-formed defences can be explained by the CODIT model, with ‘D’ originally standing for ‘Decay’ but superseded by ‘Damage or Dysfunction’ in light of later findings in our understanding of tree defence processes (Shigo, 1970, 1984; Shortle, 1979; Liese and Dujesiefken, 1996). CODIT is a concept showing how trees are highly compartmented perennial organisms formed of discrete 3-D units that when under threat from any abiotic or biotic stress can trigger the tree into walling-off or compartmentalizing damaged or decayed regions. The tree compartmentalizes by the formation of reaction zones together with the conceptual ‘walls’ (barriers) already present at the time of attack (Morris et al., 2016a). The original intention of Alex Shigo, the creator of the CODIT model in the 1970s, was to make the science of tree compartmentalization accessible to practitioners within forestry and arboriculture, giving them a sound scientific foundation prior to carrying out work on trees (Shigo and Marx, 1977). In the 1970s, wood in living trees was considered a dead substance by practitioners and even by many wood scientists (Schenk, 2018). Therefore, the distribution of an early CODIT booklet aimed at the layman was a breakthrough in forestry and arboriculture in terms of changing our understanding of wood in living trees and our treatment of them. However, due to the model’s simplicity, it was open to enormous scrutiny by the scientific community, irrespective of its original purpose. The scrutiny mostly hinged on what caused the tree to compartmentalize, whether that be decay fungi, as the original acronym suggested, or any impairment of the tree defence system that allows the entrance of air to impede the hydraulic system (Boddy and Rayner, 1983). Making a strong case for the CODIT model and the concept’s usefulness in explaining defence systems in trees in relation to fungi is subject to the premise that fungi can pose a threat to tree function and that the threat is met with an appropriate response. Our modern understanding of CODIT is that it encompasses the trees’ response to any desiccation-inducing threat, whether biotic or abiotic. However, a shortcoming in our understanding of CODIT lies in the uncertainty of whether the tree can recognize a biological threat from a non-biological threat and recognize different fungal classes and discriminate between different fungal classes, which is a focus in this review.
CODIT still holds huge relevance in practical tree applications as demonstrated in relatively recent texts (Gilman, 2011; Dujesiefken and Liese, 2015). Moreover, the model can also be used by researchers to advance our understanding of tree pathology, as was intended by Shigo in later works (Shigo, 1984). Another key point is that traditionally the CODIT model only explained defence processes from a macroscopic viewpoint, with little attention given to cellular and molecular levels (Shigo and Tippett, 1981). Using a chainsaw, Shigo and co-workers made approx. 15 000 dissections of trees in the forest before developing the CODIT model from observations of large cross-sections and longitudinal slices of wood (Shigo, 1991). However, since the introduction of the CODIT model, our knowledge of tree–fungal interactions has widened, and we now have a far greater understanding of defence processes, especially at the cellular level. Moreover, the CODIT model was traditionally used to explain tree defence processes in relation to decay fungi generally confined to secondary xylem, a projection of previous studies into decay and boundary-setting concepts in trees (Hartig, 1878; Küster, 1913; Hepting, 1935). Although the concept was developed for the xylem alone, the model could be used effectively to explain all stress-related interferences within and beyond xylem (Boddy and Rayner, 1983; Liese and Dujesiefken, 1989, 1996; Morris et al., 2016a). Compartmentalization has been studied extensively in bark (Biggs, 1992), but not linked to xylem to help us understand CODIT as an integrated system, when considering that both phloem and xylem are connected through ray parenchyma.
This interdisciplinary review has a wood structure–function premise that focuses on our current knowledge of SMs in wood defence using the CODIT model (Figs 1 and 2). We integrate our current understanding of tissue and cellular processes with the concept and reality of compartmentalization, one of the foundational concepts derived from forest pathology in the 20th century (Manion, 2003). How trees’ cells and tissues communicate through chemical signalling during defence is still largely unknown compared with model herbaceous annuals and perennials such as Arabidopsis thaliana. The latter is mostly due to the complex structure of long-lived and highly compartmented woody perennials, the difficulty in carrying out experiments and the general lack of clonal trees for reliable and consistent experiments. We discuss both the explicit and implicit properties of the CODIT model and its applicability to the level of microscopy in light of current biological knowledge. In the context of CODIT, we examine the trade-offs between constitutive and inducible defences against decay fungi, alongside the strategies of fungi to overcome host defences. Also, for the purpose of this review, SMs are everything outside of what are strictly classified as primary metabolites, with the latter including nucleic acids, proteins and polysaccharides.
XYLEM STRUCTURE–FUNCTION AND CONSTITUTIVE VS. INDUCED DEFENCES
Xylem and the CODIT model
According to the CODIT model, sapwood is made up of four protective boundary-setting conceptual walls, which range from weak to strong in ascending order. Walls 1–3 form discrete anatomical units that are constitutively formed, while wall 4, the ‘so-called’ barrier zone, is different in that it is produced after wounding, is usually enforced by suberin and is formed by the cambium or cambial derivatives (Fig. 1). Suberin is a complex of suberin polyphenolics (SPPs) and suberin polyaliphatics (SPAs) that are cross-linked by glycerol, embedded with soluble waxes and laminated to the interior side of the plant cell walls (Bernards, 2002). Although suberin probably evolved primarily as a gas- and water-tight layer between cells, it is also highly resistant to decay fungi, with only a few fungal species able to degrade the substance, e.g. Heterobasidion annosum and Armillaria mellea (Swift, 1965; Biggs, 1987; Boddy and Rayner, 1983). Wall 1 is formed of tracheary elements (conductive cells of either angiosperms or conifers) and the adjacent living axial parenchyma contact cells that can plug vessels by tyloses or production of, for example, polyphenols to impede bi-directional fungal spread along the stem above and below the wound (Morris et al., 2018a), and via stem–branch attachments. Wall 2 comprises the latewood of annual rings of temperate tree species, and ray parenchyma present intermittently along a growth ring. For example, for wall 2 in Tilia platyphyllos, highly lignified fibres, often accompanied by marginal bands of axial parenchyma formed between ray units, are only separated by intermittent ray parenchyma, which prevents radial spread of fungi (both inward and outward). Wall 3 describes ray parenchyma that inhibits lateral fungal spread, with ray units ranging in height and width from short, uniseriate to high, multiseriate rays (>10-seriate in some species, and >1 mm in height; IAWA Committee, 1989). Finally, wall 4 or the barrier zone is a region composed of usually multiple layers of enlarged ray and axial parenchyma cells (up to 30 cell layers wide; Pearce, 1996) that prevents fungal decay spread into new xylem laid down to the barrier zone’s exterior.
All these conceptual walls are designed to be chemically bolstered by phytoalexins from ray and axial parenchyma in response to wounds to greater or lesser degrees, with NSC reserves providing the source for their production (Swarbrick, 1926; Wargo, 1977a; Kemp and Burden, 1986). The synthesis of phytoalexins in response to pathogenic fungi probably depends on: (1) the structural integration and strength of the walls (reaction zones and barrier zone); (2) the fitness of the tree at the time of infection (water status and sufficient NSC reserves; Thalmann and Santelia, 2017); (3) the stage of cambial activity during the year; (4) the level of connectivity between axial and ray parenchyma within sapwood and their link to the secondary phloem; and (5) the pathogenic potential of the fungus. All factors related to tree fitness depend on the tree’s genetic constitution (CODIT walls and phytoanticipins) and its relative plasticity (phytoalexins) when under attack, with tree species having contrasting defence strategies depending on survival priorities. For instance, research has shown trade-offs between bark and xylem, with having strong constitutive and induced defences in both tissues highly unlikely (Biggs, 1986; Romero and Bolker, 2008). Also in xylem, the lack of suberin in the reaction zone but its abundant presence in the barrier zone, as observed in T. platyphyllos, implies a trade-off between walls 1–3 and wall 4 (Fig. 2I; Baum and Schwarze, 2002). In secondary xylem, response to wounding almost always comes at a severe cost, namely the death of wood parenchyma and the cessation of water transport in conducting conduits during reaction zone formation (Shigo and Marx, 1977; Schwarze et al., 2000a; Oliva et al., 2012). However, in bark, living cells are not always sacrificed following wounding, as observed in some conifer species, where localized accumulation of methyl jasmonate-induced ethylene resulted in polyphenol production in the polyphenolic parenchyma cells along with enhanced sclereid lignification, but apparently without cell death (Hudgins and Franceschi, 2004). Trade-offs in carbon allocation between different components of the tree are likely to be the case across species, with further research necessary to confirm this.
The tree’s immune system
There are two known branches forming the plant immune system: the first branch is non-specific and applies to a range of fungi, herbivores and mechanical wounds, while the second branch discriminates between general threats and virulent pathogens (Jones and Dangl, 2006). Through different response strategies depending on the level of threat, plant energy costs are balanced by administering appropriate SM doses, deploying the second level of defence (pathogen-specific response) only as a last resort. However, regarding the latter, our current knowledge of pathogen-specific responses in trees is limited to only a few studies, making it an important avenue for research.
In general, a process known as pattern-triggered immunity (PTI; Bigeard et al., 2015; Nasir et al., 2018) involves the activation of jasmonic and salicylic acid pathways, which communicate to target pathogenic fungi. While SMs from the jasmonic acid-mediated pathway are effective against fungi that kill parenchyma directly (necrotrophs), salicylic acid, on the other hand, triggers the accumulation of hydrogen peroxide (H2O2), resulting in controlled programmed cell death in the immediate vicinity of the pathogen (biotrophs). The hypersensitive response forms a defence against biotrophs (fungi with haustoria) by retarding fungal growth and spread to other parts of the plant (Glazebrook, 2005). All decay fungi, the subject of this review, lie in a spectrum between saprotrophic and necrotrophic.
Sapwood as a 3-D structure in relation to defence
An important factor of parenchyma cells is their arrangement as a 3-D network (Spicer, 2014; Morris et al., 2018a, b) with symplastic continuity throughout secondary xylem and living bark being intermeshed in an apoplastic skeleton (Carlquist, 2018). We still do not know how the level of symplastic interconnectivity plays a role in CODIT, signalling, synthesis and transport of SMs from a systems biology standpoint. It is reasonable to suggest that the close alignment of the living cells running vertically (axial parenchyma; CODIT walls 1 and 2) and radially (ray parenchyma; CODIT wall 3) and their intertwinement allows for the easy transfer of phytohormone signals from the immediate contact region of the pathogen. The former shows that reaction zones also form in advance of decay spread in some species, which is referred to as a dynamic reaction zone in conifers (Shain, 1995) and the temperate angiosperms species T. platyphyllos and Eucalyptus nitens (Barry et al., 2002; Baum and Schwarze, 2002). However, the signalling that leads to reaction zone development at distance from the wound or decay colony in secondary xylem is unknown (Eyles et al., 2010; Germain and Séguin, 2011). Also, a dynamic reaction zone may not form in response to the detection of fungal hyphae, but in response to the ingress of air that seeps through upon breakdown of the previous reaction zone. Communication between living cells of ray and axial parenchyma through small densely packed connecting pores called plasmodesmata (Morris et al., 2016b) could be augmented through combinations of the phytohormones jasmonic acid, salicylic acid (SMs regulated by COI1 and EIN2 genes), ethylene and abscisic acid, allowing trees to respond in advance of decay/disease spread. Another important factor to consider is the speed at which host defence signalling macromolecules can travel, which is likely to be extremely limited in parenchyma alone; to keep pace with advancing decay or pathogen-related mycotoxins would demand a faster apoplastic route. Utilizing vessels for long-distance signalling transport could meet defence demands, but this requires a depositary system from vessel-associated cells (parenchyma in contact with vessels) into vessels and possibly the retrieval of defence proteins along route, which involves apoplastic to symplastic transport as suggested by Słupianek et al. (2019). The latter workers provide visual evidence of clathrin-mediated and clathrin-independent endocytosis in three temperate angiosperm tree species through loading experiments into the dead vessels using various fluorescent tracers, while also measuring a heightened metabolic activity in vessel-associated cells. Direct evidence of endocytosis helps explain long-distance transport defence signalling in plant–fungi interactions (Mbengue et al., 2016).
Conifers, owing to an early derived anatomical and functional make-up, have a symplastic system that is less densely connected three-dimensionally. Less integration of symplast in conifers is a likely trade-off with other functions, i.e. tracheids are narrow and have a torus–margo pit membrane mechanism, making them less prone to embolism (Choat et al., 2012; Morris et al., 2016b), therefore not requiring assistance from abundant contact parenchyma cells, especially axial parenchyma (Morris et al., 2018b), while resin canals surrounded by epithelial cells are specialized in defence against pathogens and general damage, and are a likely substitute for ‘typical’ axial parenchyma (Evert, 2006). Conifers are mostly composed of unicellular ray parenchyma (rarely bicellular), where ‘typical’ axial parenchyma fractions are generally low and completely absent in some genera (e.g. Taxus spp.; Morris et al., 2016b). The low parenchyma fraction and the subsequent arrangement means that all ray parenchyma and in a few cases axial parenchyma is in contact with water-conducting tracheids (Evert, 2006). Conifers use a different strategy that is both constitutive and inducible, but pre-formed. Oleoresin, a terpenoid SM present in resin canals, provides various conifers with both mechanical and chemical defence (Smith et al., 2016). The oleoresin terpenoids act as deterrents or are directly toxic to insects and pathogens, while the volatile components of oleoresin also act as airborne signalling molecules in recognition of the host by the bark beetles (Zulak and Bohlmann, 2010).
Constitutive resin canals (or intercellular canals; IAWA Committee, 2004) in Picea spp., for example, are arranged in defensive bands across xylem, as seen in cross-section, and are surrounded by epithelial cells that synthesize resin, once triggered (Evert, 2006). The banded arrangement of the canals, similar to marginal axial parenchyma in a range of angiosperm trees (Fig. 2C), probably form the constitutive element (wall 1 and 2 of CODIT), and are induced once living cells abutting the ducts are triggered into filling them with terpenoid SMs. However, marginal ducts in conifers appear to serve only in defence (Ferrenberg et al., 2014), while marginal axial parenchyma in angiosperms is likely to be multifunctional (Carlquist, 2018). For example, in angiosperm trees, prior to defence activation, marginal axial parenchyma serves to connect ray to ray, and the ray cells are connected to the phloem via the cambium for the transport of water and assimilate, which function in the osmoregulation of the water transport system (Morris et al., 2018a, b). Resin canals, when closed, can form a reaction zone (wall 1 and 2 of CODIT) that completely circumvents the sapwood in tangential and axial directions, which is similar in construct and alignment to the barrier zone (wall 4), the only key difference being that the former develops during wounding and the latter after wounding (Figs 1 and 2C, D, G, H). Resin canals are positioned radially among ray parenchyma (called fusiform rays) in some conifers (Evert, 2006), forming wall 3 of CODIT and helping prevent lateral fungal spread. The differences in anatomy and function in the context of three-dimensionality between conifers and angiosperms are clear, where defence and hydraulic trade-offs in each wood type allow for adaptations that suit their geographical distributions and site conditions.
REACTION ZONES AND SECONDARY METABOLITES
The formation of reaction zones
Reaction zones form through programmed cell death at the interface between sound and decayed wood. Macroscopically, reaction zones mostly appear as dark brown necrotic regions that should not be confused with zone lines (Fig. 2A). Zone lines are black on account of melanin accumulation and are produced by a number of fungal species in response to antagonism from another fungus, or from another strain from the same fungal species and also in response to living cells along the reaction zone (Pearce, 1991). Reaction zones are rows of dead parenchyma filled with antimicrobial polyphenolic compounds (phytoalexins) and are often structurally reinforced with lignin and suberin (phytoalexins) prior to programmed cell death (Fig. 2A, B, D). Our knowledge of reaction zone formation in response to decay fungi remains poorly understood, as is our classification of the mode of nutrition of wood decay fungi along the gradient saprotroph–(hemi)biotroph–necrotroph. Moreover, programmed cell death in trees (reaction zones 1–3) occurs against any desiccation-inducing threat, but with no specificity to a causative agent, as observed in a range of non-woody crop and model plants (Eyles et al., 2010; Germain and Séquin, 2011). In the latter studies, clear evidence of salicylic acid pathways activated in response to biotrophs was observed in crop plants, but not in trees. While there is plenty of evidence to support the role of jasmonic acid pathways for PTI in trees (Hudgins et al., 2004), evidence is scant for the role of the salicylic acid pathway in secondary xylem (Ollerstam and Larsson, 2003; Germain and Séquin, 2011). However, it is likely that salicylic acid does occur in secondary xylem and that there is molecular cross-talk between the two phytohormones.
Also, we cannot rule out involvement of salicylic acid pathways in reaction zone formation (CODIT 1–3). Germain and Séquin, (2011) conclude that a defence model comparable to that found in short-lived herbaceous species (Durrant and Dong, 2004; Nasir et al., 2018) may be transferrable to the genetic model tree species Populus trichocarpa. However, reservations for this assumption are based on the longevity of trees vs. the short generation time of herbaceous plants. Moreover, there is the question of plant height in relation to systemic acquired resistance and the role of salicylic acid in biotrophic and hemibiotrophic fungal interactions and whether or not sufficient doses of salicylic acid can be modulated in trees due to a plant size restriction. On this account, it is argued that salicylic acid pathways may not exist at all in trees regarding PTI. Although evidence of systemic induced resistance against necrotrophs (a form of PTI that is not to be confused with induced systemic resistance triggered by beneficial microbes; see Table 1) is lacking in angiosperm trees, there are studies showing that it occurs in the xylem of some conifer species, Pinus nigra and Picea abies (Krekling et al., 2004; Luchi et al., 2005; Blodgett et al., 2007).
Table 1.
Overview of key terms used in tree defence systems
| Term | Meaning and references |
|---|---|
| Biotroph | A specialized pathogenic fungus that feeds off living parenchyma without killing them and usually causes disease. Possesses appressoria or haustoria |
| Brown rot fungi | Decay fungi specialized in breaking down cellulose in plant secondary cells walls using specialized digestive enzymes, such as cellulase (Schwarze et al., 2000a) |
| Dynamic reaction zone | A migrating reaction zone that keeps pace with the advancing decay by developing into previously functional sapwood and preceded by drying wood. Typically found in conifers and some angiosperms (Shain, 1995; Barry et al., 2002; Baum and Schwarze, 2002) |
| Induced systemic resistance | A resistance mechanism in plants triggered by beneficial microbes, e.g. Trichoderma harzianum or Mycorrhiza spp. Priming of whole plant body is through activation of jasmonate and ethylene and by salicylic acid signalling pathways (Pieterse et al., 2014) |
| Jasmonic pathway | Leads to localized synthesis of jasmonic acid, an SM plant hormone essential for the immune response against necrotrophic pathogens (Pieterse et al., 2014) |
| Necrotroph | Pathogenic fungi that actively kill host cells as a source of nourishment |
| Pathogen | A pathogen is a disease-causing organism. A pathogen is interpreted here as the ability to kill or cause parenchyma death or pass reaction zone defences in sapwood. Pathogenicity ranges from low to high along a saprotroph–necrotroph gradient for decay fungi |
| Pattern-triggered immunity | An innate immune plant recognition system triggered by activating the plasma membrane in response to pathogenic fungi. Part of a two-tiered defence system alongside effector-triggered immunity (Jones and Dangl, 2006) |
| Phytoalexin | Secondary metabolites produced by parenchyma in response to a plant pathogen or saprotroph |
| Phytoanticipin | Pre-existing or constitutively produced SMs that interact and inhibit fungal colonization, e.g. in heartwood |
| Salicylic pathway | Leads to an accumulation of the phenolic SM salicylic acid around the fungal infection or wound site in response to effector-triggered immunity (Betsuyaku et al., 2018) |
| Saprotroph | A decay fungus that solely lives on dead tissue, especially regarding heart rot decay. Has low pathogenicity (e.g. Fistulina hepatica; Schwarze et al., 2000c) |
| Soft rot fungi | Similar to a brown rot, these fungi selectively degrade lignin, but the fungal hyphae grow in the cell wall and follow the direction of the microfibrils (Schwarze et al., 2000b) |
| Static reaction zone | A non-migrating reaction zone formed between sound and discoloured wood in response to fungi or any desiccation-inducing threat. Typically found in angiosperms (Shain, 1995) |
| Systemic induced resistance | Infection by a pathogen which results in an induced or greater resistance to further challenges by the same pathogen (Bonello et al., 2001) |
| Vessel-associated cells | Parenchyma in direct association with the vessels that have specialized functions compared with vessel-distant cells (Morris et al., 2018b) |
| White rot fungi | Decay fungi specialized in breaking down lignin using specialized digestive enzymes, e.g. laccase. A simultaneous white rot can degrade cellulose and lignin at equal rates, whereas a selective delignification white rot primarily involves lignin digestion (Schwarze et al., 2000a) |
Several studies demonstrate how conifers respond specifically to decay fungi through SM production and reaction zone formation, and at distances from the initial site of infection (Hudgins and Franceschi, 2004; Krekling et al., 2004). Upon wounding and subsequent inoculation of Norway spruce by the pathogenic white rot fungus Heterobasidion annosum (necrotroph–saprotrophic lifestyle; Raffaello and Asiegbu, 2017), traumatic resin canals were triggered by a signal, propagating in an axial direction (wall 1 and 2 of CODIT) at about 2.5 cm d–1 and forming up to 30 cm above single inoculation points (Krekling et al., 2004). The size and number of traumatic ducts appeared to reduce with distance from the inoculation site, indicating a gradual decline in the signal that led to their formation (Krekling et al. 2004). Jasmonate-induced ethylene production was shown to be responsible for reprogramming of the vascular cambial zone for the formation of traumatic resin ducts in conifer stems (Hudgins and Franceschi, 2004). Concerning the composition of constitutive and induced resin, the latter is more monoterpene enriched (Martin et al., 2002) and thus less viscous, which facilitates resin flow in xylem. Reaction zone formation occurring at distances from the initial infection site is in line with microbe-associated molecular pattern (MAMP), microbe-triggered immunity (MTI) and effector-triggered immunity (ETI) described for herbaceous plants (Jones and Dangl, 2006; Dodds and Rathjen, 2010). There are conflicting reports on the toxicity of resin components towards wood decay fungi, with some reports for example indicating that volatile terpenes are more toxic than resin acids to white rot fungi (Cobb, 1968; Yamada, 1992). The inconsistencies in resin toxicity between different studies could partly be due to the fact that constitutive and induced resins may differ in composition and that certain trees such as pines and spruce species produce two kinds of resin, a supersaturated solution of resin acids produced by vertical ducts and fatty acid-enriched resin produced by ray-associated canals (Sjöström 1993).
So, although reaction zones are formed in response to wounding in general, there is a heightened response to fungal pathogens by some conifers, an important modification for our understanding of the CODIT model. Based on the premise that fungi follow wounding, a less robust response during the early stages of compartmentalization is likely to be a form of priming and related to the protection of the hydraulic system, thereby maintaining NSC reserves in the event of a fungal attack.
Dynamic vs. static reaction zones
Two kinds of reaction zone are produced in trees, dynamic and static reaction zones. Dynamic reaction zones are successive reaction zones formed at increasing distances from the wound into formerly healthy sapwood, which are probably related to a poor host response and characterized by a lack of polyphenolic compounds (e.g. in sapwood of a range of conifers; Shain, 1995). Dynamic reaction zones may occur more frequently in species that lack defence specialization and investment in axial parenchyma; however, some conifers invest heavily in a network of radial and axial resin canals (P. abies; Fig. 2C–E). A static reaction zone, assumed in most angiosperm tree species, is formed with a higher quality and quantity of SMs and usually prevents any further progress of fungal spread in the region, as was shown in F. sylvatica when compared with T. platyphyllos (Baum and Schwarze, 2002). Furthermore, a second static reaction zone can be formed upon the failure of the first, but only if NSC reserves for conversion into SMs are in plentiful supply. Reaction zones, unlike the barrier zone (wall 4 of CODIT), are not continuous, where gaps between axial and ray parenchyma connections can provide entrance points for fungi to spread across xylem tissue. The level of symplastic connectivity between living cells varies greatly between angiosperm tree species and directly affects the ability of a host to compartmentalize. For instance, the effectiveness of de novo suberin synthesis against decay spread in parenchyma cell walls, vessel linings and in tyloses of many tree species is limited by the degree of discontinuity between ray and axial parenchyma, and living fibres (Pearce, 1990; Yamada, 2011).
Through the chemical and physical nature of a reaction zone that involves metabolism of NSC into antimicrobial materials (e.g. polyphenolic compounds, suberin, etc.), the boundary is particularly important against ingress of air and decay fungi. However, seasonality plays an important role regarding how effective reaction zones are against a fungal threat, which directly concerns the tree’s ability to synthesize SMs (Schwarze and Baum, 2000; Dujesiefken et al., 2005). During the dormant season, Schwarze and Fink (1997) observed no phenolic SMs in front and behind the reaction zone after colonization by the white rot fungus Inonotus hispidus in Platanus × acerifolia, allowing the fungus to successfully breach wall 3 (ray parenchyma) of the tree’s defence system. The inability to produce phenolic SMs during certain times of the year highlights the shortcomings of the defence system where pre-formed boundaries (i.e. the parenchyma cell interconnectivity of wall 1–3) are presumably only partially effective against fungal pathogens when phytoalexins are not induced. While heartwood and the dead outer bark can have solely a constitutive role in defence, the design of sapwood (and secondary phloem) is based around having an integrated system of pre-formed and induced defences. The primary role of the 3-D interconnected symplastic network is in providing a transport link between phloem and xylem, and ultimately in preserving the integrity of the hydraulic system and protecting the cambium (Tyree and Zimmermann, 2002). However, the bidirectional channel for transport is sacrificed for defence purposes, culminating in the death of parenchyma and the blocking of tracheary elements. This changeover represents a critical trade-off between the conservation of xylem (and phloem) hydraulic and storage space, and successful compartmentalization of the decayed/diseased region.
SECONDARY METABOLITES IN SECONDARY XYLEM
Taxonomy and definition of secondary metabolites in microbe–plant interactions
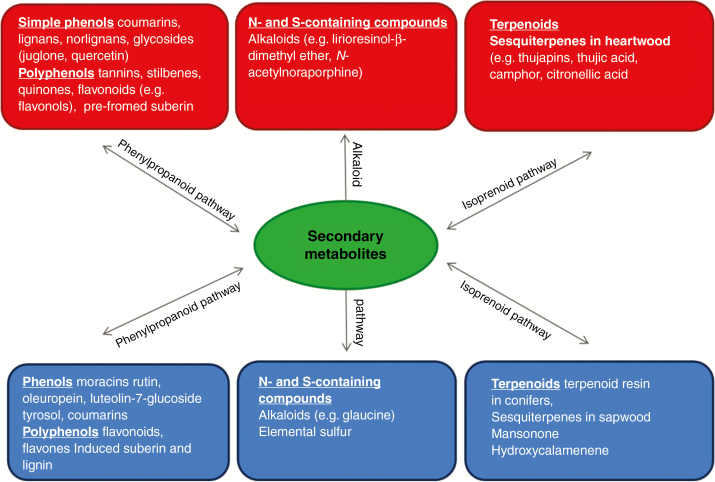
Phytoalexins are a heterogeneous group of antibiotics, which fall into a number of classes including terpenoids, alkaloids, lignans and glycosteroids (Smith, 1996). Phytoanticipins are constitutive by nature or a pre-formed component of defence, such as those found in heartwood (e.g. polyphenols, quinones, saponins, terpenoids, stilbenes snd tannins; refer to Figs 3 and 4, and Supplementary data Table S1 for a breakdown of phytoalexins and phytoanticipins and the SM classes to which they belong.
Fig. 3.
Diagram of major chemical groups and the main chemical pathways found in the secondary xylem of trees. Phytoanticipins (in red) are pre-formed secondary metabolites (SMs), while phytoalexins (in blue) are induced as part of a host response to decay fungi.
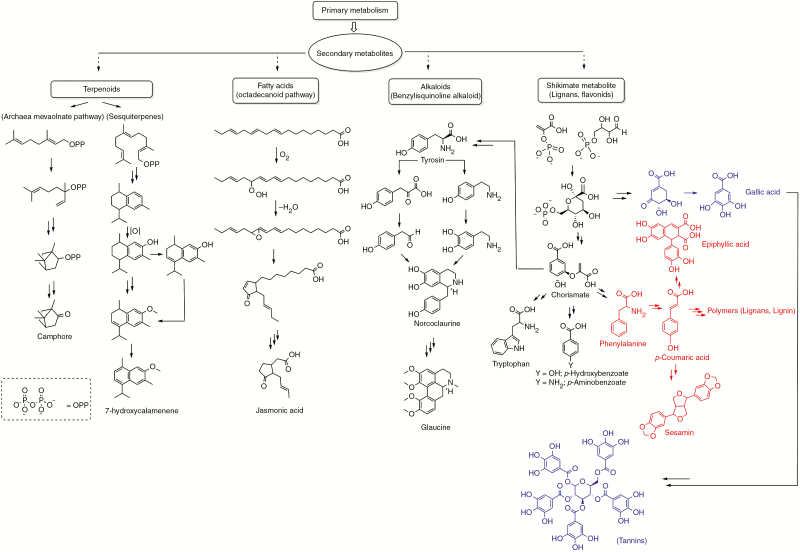
Fig. 4.
The biosynthesis of the major chemical defence groups in secondary xylem tissue: terpenoids, fatty acids, alkaloids and simple to complex phenols. We include all compounds outside of primary metabolism under the framework of secondary metabolites, including complex molecular structures such as lignin. The latter is produced through the phenylpropanoid pathway.
Secondary metabolites of heartwood and sapwood
In the absence of infection, heartwood has a range of characteristics that distinguish it from sapwood. Heartwood is a dead substance, except for rare circumstances where parenchyma activity has been detected (i.e. sandalwood; Celedon and Bohlmann, 2018). Also, heartwood is low in free oxygen, has comparatively low moisture and high CO2 content (Hietala et al., 2015), and generally has an increased durability, with some species having a much higher resistance to rot than others (Metsä-Kortelainen and Viitanen, 2009), making the environment hostile to fungi. At the transition zone during heightened metabolic activity just prior to programmed cell death, ray parenchyma releases antimicrobial SMs, phytoanticipins that accumulate in heartwood (Chattaway, 1949; Magel et al., 2001). In Cryptomeria japonica (Japanese cedar), ferruginol, a diterpenoid SM, was found to accumulate in both ray and axial parenchyma at the transition zone (Imai et al., 2005). Similarly, axial parenchyma is thought to play a role in heartwood formation of Juglans nigra (black walnut), with contrasting luminance values between inclusions in ray and axial parenchyma suggesting qualitative differences between synthesized phenolic SMs (Phelps and McGinnes, 1983). However, the key functional differences may lie between vessel-associated ray and axial parenchyma and their vessel-distant counterparts, where the former develop gels and tyloses to fill vessel or tracheid lumina at the transition zone, while the latter release mostly SMs synthesized via phenylpropanoid and isoprenoid pathways (Figs 3 and 4). Raman spectroscopy used together with image analysis software might provide us with a way to detect different tones of coloration in heartwood related to the parenchymal source from where SMs are derived, along with the area (fraction %) the shaded regions occupy (see Felhofer et al., 2018).
Three heartwood models have been described, which follow an order based on SM production at the sapwood–heartwood interface and its subsequent accumulation within heartwood: the Robinia type I, the Juglans type II (Magel et al., 1994; Kampe and Magel, 2013) and the newly proposed Santalum type III, with the latter based on the retention of living parenchyma in heartwood (Celedon and Bohlmann, 2018). There is no appreciable production of SMs at the transition zone in the Robinia type I model from ray parenchyma, with significant increases of SMs from ray parenchyma at the transition zone in the Juglans type II model. The darker the coloration of heartwood, the greater the resistance to microbial rot in general, as characterized by a higher quantity and quality of phenolic- and terpenoid-based SMs (Shigo and Hillis, 1973; Hillis, 1987). When heartwood forms, the active defence associated with living parenchyma is replaced with a constitutive or passive defence (Shain, 1995). Aside from phenolic SMs filling the lumina of fibres and vessels (tracheids in conifers), aspirated and encrusted pits together with suberin and lignin deposits in cell walls form physical barriers. Some species invest heavily in heartwood defence (phytoanticipins), predominantly owing to their genetic make-up (Hillis, 1987; Hazenburg and Yang, 1991), but also from environmental influences such as growth rate and photosynthate production (Wilkins and Stamp, 1990), nutrient availability (Brix and Mitchell, 1983) and site conditions (Climent et al., 2002). Also, the parenchyma fraction of the sapwood was found to be related to heartwood extractive richness in Pseudotsuga menziesii (Hemingway and Hillis, 1970) and heartwood diameter in Pinus canariensis, which influences the ratio between sapwood and heartwood (Climent et al., 1998). The SMs released from ray and axial parenchyma at the transition zone include the following broad classifications, which all play a role in heartwood durabilty: (1) flavonoids (Harborne and Williams, 2000); (2) quinones (Lukmandaru and Takahashi, 2009); (3) stilbenes (Felhofer et al., 2018); and (4) tannins (Haslam, 1989).
In a few studies, SMs extracted from sapwood were shown to be analogous to phytoanticipins extracted from heartwood (Burden and Kemp, 1984; Shain, 1995). One of these SMs is mansonone, a sesquiterpenoid found in Ulmus spp. Mansonone is constitutively synthesized in heartwood and actively produced in sapwood in response to Dutch elm disease, a vascular wilt disease. In Sorbus aucuparia, the antifungal compound aucuparin, a biphenyl derivative, is normally associated with heartwood, but has been found in the sapwood as a phytoalexin (Kokubun and Harborne, 1995). Compounds occurring in both heartwood and sapwood (along with bark) are probably quite common across species, but research is still limited in the quantification of SMs in trees, particularly regarding phytoalexin accumulation. Carbon investment into heartwood probably trades off against inducible defences in sapwood (and bark), where a balance between constitutive defences and induced defences may well depend on the nature of the threat and the frequency of attacks, as well as the ecological strategy (life history) of the tree species.
Two other tree defence-focused models that could help explain investment in heartwood (constitutive defence), and possible trade-offs with sapwood (constitutive and induced defence) in relation to heartwood–sapwood defence allocation, include the extended growth/differentiaton model (GDBe; Herms and Mattson, 1992) and the protein competition model (PCM; Jones and Hartley, 1999). Both conceptual models address partitioning to SMs by the phenylpropanoid biosynthetic pathway but predict opposite trends in response to environmental constraints, e.g. increased levels of CO2. The protein competition model predicts an inverse relationship between protein and phenolic synthesis at the cellular, tissue and whole-plant level. In the latter model, both proteins and phenolics use the same phenylalanine precursor. However, a biochemical trade-off occurs in response to phenylalanine ammonia-lyase (PAL) activity, where PAL activation leads to the synthesis of phenolics, while its inhibition results in proteins; this is on account of environmental pressure dictating demands for growth and development (proteins), or structure, protection and defence (phenolics; Figs 3 and 4). Stressful environmental conditions and subsequent slow growth should cause the activation of PAL and phenolic synthesis, while conditions that promote growth should result in protein synthesis at the expense of defence investment. However, tests using the GDBe model showed a rise in constitutive SMs (i.e. tannins and lignins) in Betula papyrifera and Populus tremuloides wood in response to CO2 enrichment and increased biomass (Mattson et al., 2005), but did not discriminate between heartwood and sapwood. More research is required here, with a heartwood–sapwood focus on a range of conifers and angiosperm trees to see if CO2 and growth affect the proportion of SMs being allocated into constitutive vs. induced defences. Although the environment certainly plays a role in partitioning of SMs, heartwood SM accumulation for many species of conifer, and diffuse and ring porous angiosperm trees is under strong genetic control (Zobel and Jett, 1995).
SECONDARY METABOLITES AND THE REACTION ZONES OF CODIT
Reaction zones and phytoalexins
Although walls 1–3 of the CODIT concept are in place at the time of wounding, it is important to note that they are only constitutive in terms of their spatial positioning and close alignment (Morris et al., 2016a, 2018b), and the degree of lignification of fibres at the growth ring boundary of temperate trees (wall 2; Fig. 1). Parenchyma becomes activated into phytoalexin synthesis on recognition of a threat, which is triggered in the cell wall before sending signals to the plasma membrane (Hammerschmidt, 1999). Phytoalexin SM compounds in reaction zones are most commonly derived via the phenylpropanoid pathway. However, compounds derived through other pathways have been extracted from reaction zones in response to fungi, including alkaloids (Chen et al., 1976) and terpene-based phytoalexins in both angiosperms (Burden and Kemp, 1984; Melcher et al., 2003) and conifers (Flodin, 1979; Schuck, 1982; Keeling and Bohlmann, 2006).
Other than histochemical confirmation by microscopy, there are still relatively few accounts characterizing polyphenolic SMs (phytoalexins) in reaction zones (and the barrier zone). Of polyphenolic compounds, flavonoids have been successfully extracted from reaction zones in a range of temperate tree species (Feucht and Treutter, 1999; Baum and Schwarze, 2002). Through high-performance liquid chromatography with chemical reaction detection (HPLC-CRD) and spectrophotometric analysis, flavanols (a class of flavonoid) were found to differ significantly in quantity and quality between the reaction zones of Fagus sylvatica (common beech) and T. platyphyllos (large-leaved lime). Catechin, a monomeric flavanol with antifungal properties against decay fungi (Ardi et al., 1998), is found in high amounts in F. sylvatica compared with T. platyphyllos (Baum and Schwarze, 2002), whereas the fungitoxic epicatechin is found only in T. platyphyllos. Of interest is that the highest amounts of phenolic SMs were found on the healthy side of the reaction zone, while the reaction zone margin facing the decay column showed a build-up of cell wall-bound proanthocyanidins (Baum and Schwarze, 2002). In another study, Prunus cerasus (sour cherry) and Prunus avium (sweet cherry or gean) also showed great differences in flavanol content (Feucht and Treutter, 1999), demonstrating that even congeneric trees (Prunus in this case) can show great variation in SM concentration and chemical composition, which probably plays a role in their ability to compartmentalize successfully after wounding.
During the formation of reaction zones in sapwood, phytoalexin SMs are generally thought to be synthesized in large doses only in response to fungi and bacteria, while being found to accumulate to a lesser degree against abiotic stresses (Yang et al., 1989); however, abiotic wounding vs. wounding associated with fungi and the level of response in host cells has long been debated in tree defence studies (Shigo and Tippett, 1981; Boddy and Rayner, 1983). Although there is evidence of a heightened response to the Dutch elm wilt fungus Ophiostoma nova-ulmi in Ulmus americana, where mansonone accumulation is correlated with resistance to the pathogen (Jeng et al., 1983; Duchesne et al., 1985), there is no work to our knowledge demonstrating a similar result for decay fungi. However, a study by Shortle and Cowling (1978) demonstrated how early non-decay colonizers failed to induce discoloration (reaction zone formation) from living parenchyma, while decay fungi prompted strong discoloration (defence response) in excised live sapwood tissue. The latter work shows that trees can differentiate harmful from non-harmful fungi and respond accordingly, demonstrating a level of recognition at the molecular level. One must bear in mind that the success of fungitoxic SMs during reaction zone formation is largely dependent on the inoculum potential and pathogenicity of the fungus; if pathogenicity exceeds SM toxicity, reaction zones can be breached (Boddy and Rayner, 1983).
Reaction zones, wood anatomy and its modification
Reaction zones comprise numerous cell types, including vessel-distant ray and axial parenchyma, libriform fibres, tracheids (conifers and angiosperms) and vessels. Vessel-associated cells often form an airtight sleeve around the conduits (Braun, 1984; Morris et al., 2018a, b). In the reaction zone, de novo synthesized polyphenolic SMs from ray and axial parenchyma leak into neighbouring libriform fibres, vascular tracheids and vessels, thus strengthening the region (Schwarze and Fink, 1997). Through exocytosis along the plasma membrane of parenchyma cells, polyphenols can be secreted from parenchyma cells through pit membranes into adjacent vessels or fibres as precursor molecules with subsequent polymerization within vessel and fibre lumina. However, we do not know precisely how polyphenols cross the plasmalemma, amorphous layer and pit membrane of cell walls at the parenchyma–vessel or parenchyma–tracheid pit membranes. Moreover, SM compounds were often involved in the modification of fibre and vessel cell wall structures by forming a narrow black-stained dense layer along the inner wall surface, perhaps through cross-linking with cell wall molecules (Melcher et al., 2003; Schmitt and Koch, 2009). The de novo enhancement of pre-existing lignin along with the synthesis of suberin in parenchyma in the reaction zones are also reported for a range of tree species (Biggs, 1987; Geiger et al., 1989). Localized lignification of xylem cell walls was observed in Hevea brasiliensis (rubber tree) in response to the decay fungus Rigidoporus lignosus, resulting in a 30 % increase in lignin content (Geiger et al., 1989). Also, in a large range of temperate angiosperm and coniferous tree species, lamellar suberin was frequently found to form along the inner cell wall structure of ray parenchyma (wall 3 of CODIT; Fig. 1), but less often in marginal axial parenchyma (wall 2 of CODIT; Fig.1) (Biggs, 1987). The latter finding reveals differences in the defence functions between ray and axial parenchyma, and is among the key evidence for wall 3 being the strongest of the walls in place at the time of wounding. Suberin initiation in parenchyma cell walls of Ulmus minor and subsequent increased resistance to the wilt pathogen O. nova-ulmi were found to be induced by the application of exogenous phenolic compounds (Martín et al., 2008). Although the latter study concerns tree phenols originating from external application, it could be suggested that phenol accumulation in parenchyma triggers suberin and lignin deposition in response to pathogenic decay fungi; the amount of suberin and lignin synthesized de novo may be related to the composition of the simple phenols produced in the cytoplasm. Simple phenols could form phenolic moieties that are used in lignin and suberin biosynthesis.
Vessel-associated cells in reaction zones are an anatomically and chemically distinct cell type compared with vessel-distant parenchyma and react differently to wounding due to their association with conduits (Morris et al., 2018b). Regarding defence in angiosperm trees, the core function of the vessel-associated cells is their involvement in clogging the vessels to prevent longitudinal spread of fungal pathogens (wall 1 of CODIT). Along with preventing fungal spread, the spread of air is restricted following desiccation of wound-associated tissue. However, vessel-associated cells are also defensively involved along ray margins in contact with vessels and laterally where axial parenchyma contact vessels (walls 2 and 3 of CODIT). In contrast to vessel-distant parenchyma, vessel-associated cells are smaller with few amyloplasts (starch-storing organelles), few plastids and small vacuoles, but have dense cytoplasm, more plentiful mitochondria, large nuclei and many ribosomes (Morris et al., 2018a). The combination of smaller vacuoles that house polyphenolic SMs such as tannins and fewer amyloplasts for storage of NSC suggests that vessel-associated cells are limited in their capacity to manufacture polyphenolic SMs compared with vessel-distant parenchyma, where water transport-related functions (and tylosis) take precedence in the former (Holbrook and Zwieniecki, 1999, Goldstein et al., 1998; Morris et al., 2018a).
Reaction zones, tyloses and gels
When tyloses/gels (formed of cell wall content, lignin, suberin, phenolic compounds and pectins; De Micco et al., 2016) are triggered into clogging the vessels either indirectly following embolism formation or directly in response to vascular pathogens from the vessel side, vessel-associated cells die (Fig. 2A). Tylosis development results in the death of vessel-associated cells together with the cessation of translocation in the affected vessels. There are exceptions, however, where vessel-associated cells remain alive and have functions post-vessel translocation cessation (Spicer, 2014), and where localized lignification results in thickening of the secondary walls of parenchyma–vessel pit membrane of rice and aubergine, preventing bacterial and fungal spores from entering parenchyma (Benhamou, 1995; Hilaire et al., 2001). For the latter, vessel-associated cells remain alive and continue a normal but perhaps reduced role in hydraulic upkeep. Further studies are required here with a tree focus to provide evidence for the occurrence of localized lignification in response to decay fungi.
The mechanism triggering tyloses or gels (also known as gums) together with the composition of the substance probably depends on whether it is trauma or embolism induced. Trauma-induced gels have a high abundance of antimicrobial SMs from different chemical pathways including catechol, flavonoids and coumarins (Rioux et al., 1998; Del Rio et al., 2001; Baum and Schwarze, 2002), indicating that a different secondary metabolic pathway is possibly triggered in vessel-associated cells in response to pathogenesis (Wallis and Truter, 1978). A recent study by Leśniewska et al. (2017) revealed that down-regulation of Pectin Methlyesterase1 (PtxtPME1) via jasmonates (i.e. jasmonic acid and methyl jasmonate), when working in synergy with 1-aminocyclopropane-1-carboxylic acid (ACC), trigger gel and tylosis development in aspen plantlets. Jasmonates and ACC were found to depend on ethylene signalling, while treatments with salicylic acid had no effect. The latter finding demonstrates the key role SMs play in tylosis and gel production in vessel-associated cells, but it is not clear if greater jasmonic acid concentrations occur in response to pathogenesis when compared with normal wounding. Jasmonic acid concentrations may vary depending on the extent of the wounding and on the size of the interface between responsive host cells and the fungus.
Trauma-induced gels (i.e. polyphenols, pectin and hemicelluloses), compared with tyloses, can be secreted through smaller pit apertures than parenchyma–vessel pit membranes, and may be complementary to tyloses in the wider development of reaction zones, where the gels can fill both fibres and vascular tracheids together with vessels (Fig. 2A, B). Studies of two temperate genera, Betula and Tilia, show that in response to wounds, vessel-associated cells produce and secrete gels into neighbouring imperforate tracheary elements and vessels through interconnecting pit membranes, which involves modification of both the protective layer located on the parenchyma side of the pit membrane and the pit membrane itself, which allow antimicrobial substances through (Schmitt and Liese, 1990, 1992). Deposits of polyphenols in fibre tracheids originating from axial parenchyma in response to I. hispidus (a white rot fungus) were also found in Platanus × acerifolia (London plane tree) (Schwarze and Fink, 1997). The effectiveness of the constitutive and induced barrier (wall 1 of CODIT; Fig. 1) is seasonal, with the most intense defensive responses occurring between May and November (Schmitt and Liese, 1992), which is linked to carbohydrate mobilization (Tixier et al., 2019). However, the exact period during the growing season in which the response is most effective against fungal pathogens would be likely to depend on NSC availability (Essiamah and Eschrich, 1985; Sauter and Wisniewski, 1996; Hoch et al., 2003).
In extant conifers, ray contact cells are not known to produce tyloses and clog tracheids. However, the epithelial cells that surround resin canals appear to completely clog intercellular resin canals in response to fungi by a similar process to tylosis, but intrusions in the case of conifers are referred as tylosoids (Evert, 2006). Tylosoids have been defined by the IAWA Committee (1964) as differing from tyloses in that they do not pass through a pit cavity as found in parenchyma–vessel pit membranes in angiosperms (Fig. 2E). However, from a functional perspective, the latter difference should be regarded as superficial; similar to the function of vessel-associated cells in angiosperms, the blocking of resin canals in reaction zone formation prevents axial and radial spread of decay (wall 1 and wall 2 of CODIT; Fig. 2D, E).
Secondary metabolites and the barrier zone
The parenchyma of the barrier zone is filled with NSCs in the form of starch, which becomes mobilized and converted into polyphenolic SMs, while in many species lignin and suberin are induced in the cell walls, making the barrier zone both a chemical and an anatomical construct (Tippett and Shigo, 1981; Shigo, 1984; Rioux et al., 1995). The strength of the barrier zone varies between species and is largely genetically controlled, with successful compartmentalization of the fungus dependent on the effectiveness of wall 4 of CODIT and the tree’s ability to synthesize phenolic-based SMs (Figs 1 and 2G–I). For example, Schwarze et al. (2007) speculated that the higher resistance of the barrier zone in Platanus × acerifolia compared with Fraxinus excelsior when colonized by I. hispidus was due to the impregnation of the cell walls with suberin in the former and the complete absence of suberin in the latter. Suberin is especially difficult to degrade by fungi, which may explain why the barrier zone is regarded as the strongest wall of the CODIT model (Pearce and Rutherford, 1981; Pearce, 1996; Schwarze et al., 2000a). White rot fungi in the genus Armillaria represent some of the few fungi that are capable of degrading suberin (Swift, 1965), but suberin degradation has only been reported in periderm. The timing of SM synthesis is also a critical factor determining the effectiveness of the barrier zone, which depends on the genetic propensity of the species, the fitness of the individual at the time of infection, the type of SM being produced and the time of the year. For instance, polyphenolic SMs can be produced more rapidly than lignin and suberin, which is related to the molecular complexity of the compounds, the pathways involved and the degree of carbon investment (Fig. 2G, I). In general, the accumulation of polyphenolic SMs in the large vacuoles of temperate tree species was followed by lignin synthesis and deposition of suberin (Rioux and Ouellett, 1991a). The deposition of lignin was found to be higher in barrier zones than in cells of uncolonized xylem (Fig. 2H), demonstrating its crucial importance in structural defence (Rioux and Ouellette, 1991a; Pearce, 1996). Interestingly, determining when parenchyma dies after suberin deposition is difficult, but the process towards death is thought to be irreversible once suberization is initiated (Rioux and Ouellette, 1991b).
SECONDARY METABOLITES AND TREE–FUNGI INTERACTIONS
Secondary metabolites and wood decay fungi
There are three main types of fungal wood decay, namely brown rot, soft rot and white rot, with the latter divided into sub-types, simultaneous white rots and selective delignification (Table 1). The decay mode and the genetic potential of the fungus to interact with tree defences can influence whether constitutive and/or induced plant defences are used. For instance, brown rot fungi are generally confined to heartwood (phytoanticipins) and are weakly pathogenic, whereas white rot and soft rot fungi are more likely to be associated with sapwood (phytoalexins and phytoanticipins) and have a higher level of pathogenicity (Supplementary data Table S2). Brown rot fungi have high stress tolerance and use a heart rot strategy to colonize heartwood, a hostile substance to most fungi, being low in O2, high in CO2 and high in antifungal SMs (Rayner and Boddy, 1988). The release of small molecules of H2O2 and hydroxyl radicals by brown rot fungi allows them to degrade cellulose and hemicellulose in cell walls, but leave lignin largely unmodified due to an inability to produce lignin peroxidase, manganese peroxidase and laccase (Hatakka, 1994; Goodell, 2003). Heart rot specialists generally have low ability to detoxify de novo synthesized fungitoxic or antifeedant SMs associated with reaction zones and rarely establish in sapwood. Some white rot fungi such as Phellinus igniarus are also heart rot specialists and are usually delimited to the heartwood (Seehann, 1979), while Ganoderma lipsiense, a weakly pathogenic white rot involved in selective delignification of cell walls, can slowly migrate into sapwood; however, mycelia are usually upheld by strong reaction and barrier zones (Terho et al., 2007). In contrast, Ganoderma adspersum is moderately to highly pathogenic, with an ability to degrade polyphenols and suberin along reaction zones (Schwarze and Ferner, 2003).
The brown rot fungal basidiomycetes Fistulina hepatica and Laetiporus sulphureus use different strategies when decaying the durable heartwood of Quercus robur, Q. petraea and Castanea sativa (all Fagaceae family). Unable to degrade cell walls until a late stage, F. hepatica readily breaks down tannins (polyphenolic SMs), giving the heartwood a red-brown coloration, while L. sulphureus degrades the cellulose and hemicellulose in the cell wall, but cannot break down tannins (Schwarze et al., 2000b, 2003). Fistulina hepatica deploys the phenoloxidase, laccase and the hydrolytic enzyme tanninase to degrade tannins into simple sugars for consumption, whereas L. sulphureus lacks tanninases and the ability to metabolize parenchyma cell walls (Schwarze et al., 2000a).
Fungal pathogenicity should not be viewed as being in conflict with their ecological significance, especially regarding nutrient recycling. The degree of pathogenicity between different decay fungi just highlights the huge diversity in strategies used by them in the context of CODIT. The strategies vary depending on the key fungal lifestyles that range in a gradient from necrotrophy to saprotrophy. No decay fungi are completely necrotrophic or saprotrophic, but fall somewhere along a gradient between them. Pathogenic decay fungi have what could be considered stage-specific necrotrophy upon sapwood colonization, suggesting trade-offs between wood decay and parasitism (Olson et al., 2012). For instance, in a range of deciduous broadleaved trees, the decay fungi Chondrostereum purpureum (silver-leaf disease fungus) and Amylostereum laevigatum combine necrotrophic and saprotrophic strategies to kill trees rapidly through hydraulic dysfunction from reaction zone formation and direct toxicity. Moreover, the infected trees are relatively small in diameter, so formation of reaction zones probably comes with significant sacrifice. The biphenyl phytoalexin aucuparin has been isolated in the reaction zones of Malus pumila presumably in response to C. purpureum (Kemp and Burden, 1986), which may form successful walls 2 and 3 of CODIT; Fig. 1). However, CODIT wall 1 is easily bypassed through excretion directly into the transpiration stream of fungal sesquiterpene SM toxins, causing foliar silvering followed by tree death (Strunz et al., 1997). Such fungi that use multiple strategies take advantage of wall 1, the weakest defence construct of the CODIT system (Fig. 1). Amylostereum laevigatum and L. sulphureus both cause decay in the European yew, Taxus baccata. However, the differences in decay strategies that the latter conifer has compared with deciduous broadleaf trees against these fungi is undocumented.
Secondary metabolites, beneficial endophytes and wood decay fungi
Micro-organisms are known to critically affect host physiology and performance where evolution and ecology of plants and animals might be better understood in the context of a holobiont, a concept explaining the relationship between host and all associated microbes (Agler et al., 2016). Many endophytes that have developed a co-evolutionary relationship with trees were found to be beneficial to tree health by suppressing decay-causing fungi, while other endophytes that may lack a close evolutionary coupling with the host become pathogenic under the right conditions. The endophytic fungus Taxomyces andreanae, considered specific to Pacific yew (Taxus brevifolia), produces the SM paclitaxel (taxol), an antifungal diterpenoid that can be produced de novo in response to decay-causing fungi (Zhou et al., 2010; Jia et al., 2016). Paclitaxel is present in all Taxus species as well as in Ginkgo biloba and Wollemia nobilis, two extremely long-lived gymnosperm trees. Several host-specific endophytes can produce paclitaxel, including Paraconiothyrium sp., associated with the European yew T. baccata (Talbot, 2015). Through in vitro experiments with Taxus plantlets and fungal culture tests, the pathogenic wood decay fungi Heterobasidion annosum, Phaeolus schweinitzii and Perenniporia subacida were found to be suppressed by paclitaxel produced from the endophyte Paraconiothyrium (Soliman et al., 2015). The authors showed how the endophyte, on recognition of the threat, migrates to pathogen entry points (cracks in the bark) where paclitazel is synthesized in extracellular hydrophobic bodies, only to be released by exocytosis upon contact with fungi. Here, the released antifungal compound from the extracellular bodies coalesces to form a continuous barrier blocking the entrance way to the decay fungi. The latter demonstrates remarkably how endophytes can form a crucial part of tree defence systems that may hold the key to their host’s longevity.
Another important genus of ‘mostly’ symptomless (absence of disease expression) endophytes are Trichoderma spp., root symbionts that behave as mycoparasites while inducing systemic plant defences to protect the plant through the secretion of various SMs and proteins (Harman et al., 2004; Mukherjee et al., 2013). Trichoderma are even known to suppress and kill a range of pathogenic decay fungi through several mechanisms (Grosclaude et al., 1973; Harman et al., 2004; Schubert et al., 2008; Ribera et al., 2017). Trichoderma harzianum was successful against Phellinus noxius (brown root rot disease) in a wood block test (Schwarze et al., 2012; Ribera et al., 2017), while T. atroviride showed a high antagonistic potential against the moderately pathogenic Ganoderma adspersum and I. hispidus, and the ascomycete Kretzschmaria deusta after fresh pruning wounds were inoculated with the decay fungi, but only had a weak effect against the basidiomycete Polyporus squamosus (Schubert et al., 2008). Trichoderma uses SM compounds, volatile organic compounds, fungal toxic cell wall-degrading enzymes (chitinases and glucanases) and appressoria (clamp-like structures) to parasitize other fungi. For instance, Trichoderma spp. are regarded as the richest source of peptaibiotics (Zeilinger et al., 2016), where this class of SMs act in concert with hydrolytic enzymes to antagonize pathogenic fungi (Schirmböck et al., 1994). Other SMs associated with the antagonistic behaviour in Trichoderma include: terpenes, peptides, gliovirn, gliotoxin, pyrenes, siderophores and isocyane metabolites (Zeilinger et al., 2016).
Plant systemic resistance works through the manipulation of the SM hormones, jasmonic acid, salicylic acid and ethylene, by asymptomless endophytes (e.g. Trichoderma spp. and mycorrhizae). The latter fungi can induce host phytoalexin production (priming) against a specific target, a process referred to as induced systemic resistance (Harman et al., 2004; Pieterse et al., 2014). The induction of resistance in the whole body of herbaceous plants is clear (Vinale et al., 2008; Eyles et al., 2010; Tytgat et al., 2013; Martínez-Medina et al., 2017), but how Trichoderma (or similar endophytes) activates the defence system in trees remains poorly understood. There is certainly no known evidence to support salicylic acid signalling pathways in secondary xylem (Eyles et al., 2010; Germain and Séquin, 2011). Only a few studies on conifers provide evidence for induced systemic resistance in trees, but only in response to necrotrophs (Bonello et al., 2001). The production of phenolic phytoalexins is considered to be terminal for the wood parenchyma involved (reaction zones, walls 1–3), so perhaps signalling is only conducted between roots and leaves to alleviate symptoms from foliar pathogens, with secondary xylem only used to transport the defence molecules; however, overcoming the long-distance signalling pathway in trees challenges this hypothesis (but see Kollist et al., 2018).
Tree defence systems and non-beneficial endophytes
Relatively few endophytes are host specific, and have undergone co-evolutionary adaptation with their host plant as in the case of Taxus spp. (Sieber, 2007). Symbiosis of most tree endophytes appears to sway more towards commensalism, where it is not clear what health benefits are gained by the host other than a fast decomposition rate at the ecological level. Such endophytes rest as chlamydospores in the xylem of healthy trees until conditions become suitable, such as the onset of drought, changes in nutrient availability or changes in oxygen levels (Rayner, 1986). In a range of Chilean tree species, the chlamydospores of important decay-causing fungi were found in living xylem ray parenchyma, including the basidiomycetes Bjerkandera adusta and Inonotus sp., as well as the ascomycete Xylaria sp. (Oses et al., 2008), while the defence system remained apparently inactivated. Fomes fomentarius, a white rot decay-causing basidiomycete not previously considered an endophyte, was also found in the healthy wood of F. sylvatica (Baum et al., 2003). For the latter fungus, the chlamydospore stage was found in the rays of F. sylvatica and more rarely in the vessels of F. sylvatica and Quercus robur (Schwarze et al., 2000a). The spores of decay-causing endophytes are thought to enter wood passively through wounds and possibly lenticels, allowing the fungus to eventually spread throughout xylem before switching from latent to active colonization (Schwarze et al., 2000a; Baum et al., 2003). It remains unknown how spores enter xylem parenchyma, as there is a size restriction to spore entry. The pore size of intervessel pit membranes in fresh (i.e. never-dried) samples for a number of trees species were found to range between 5 and 20 nm after using colloidal gold particles (Choat et al., 2003), and vessel–parenchyma pits are presumably of a similar size. Aside from sapwood endophytes, the brown rot fungus Laetiporus sulphureus provides an example of a heartwood endophyte whose chlamydospores have been observed in the ray cells and fibres of Robinia pseudoacacia (false locust tree) heartwood (Fig. 2F). We suspect they enter the ray system of heartwood from the vascular system in functional sapwood and probably remain there in a spore state until xylem dysfunction allows for hyphal development. Once germinated, the hyphae spread centripetally (from sapwood to heartwood) by degrading cell wall structures using chitinase and H2O2 to access fibres and ray parenchyma, or they await the transition from sapwood to heartwood. Remarkably, there was no visual evidence in cross-section of the presence of hyphae in the ray cells where the chlamydospores rest (Fig. 2F). We speculate that an individual hypha of L. sulphureus burrows through the vessel–parenchyma pit membrane, releasing a single chlamydospore in each ray cell (Fig. 2F), before undergoing self-parasitism. However, although self-parasitism is an important part of nitrogen recycling in many fungi at a higher ecological level (Gruber and Seidl-Seiboth, 2012), there is no direct evidence for it in this species.
The strategy used by the decay-causing ‘endophytes’ appears to be a ‘chink in the armour’ of the tree’s CODIT system, where fungal spores gain access to xylem tissue without activating parenchyma into reaction zone formation until at a late stage when the in situ latent fungi become infectious. Regarding the deposition of chlamydospores in living parenchyma from a range of white rot fungi, the question of how fungal spores avoid activating the synthesis of phytoalexins is unknown; they most probably degrade the vessel–parenchyma pit membrane structure (or tracheid–parenchyma pits in non-vessel-bearing plants) from the vessel side, which normally activate either lignification of the cell wall to decrease the aperture size to prevent spore entry (common in the case of vascular wilts) or trigger tylosis (Wallis and Truter, 1978). We suggest that there is perhaps a specialized gelatinous layer surrounding the spore that makes it undetectable to parenchyma or that enzymes manipulate the defence signalling pathways (CODIT), rendering the defence system ineffective. Moreover, spores during transit in the water-conducting system fail to germinate, which might be related to low oxygen levels, or the interaction with various compounds in xylem sap. This is an important avenue of research that requires exploration.
DROUGHT STRESS, NON-STRUCTURAL CARBOHYDRATE RESERVES AND SECONDARY METABOLITES
Considering current concerns about climate change and shifts in rainfall patterns at many places on this planet, the tree defence system may become more vulnerable to various pathogens. Serious pathogens during times of drought include opportunistic decay fungi such as the white rot complex Armillaria mellea, which in turn pave the way for secondary decay fungi (more saprotrophic). When the tree’s immune system is low, the CODIT system can be overwhelmed and easily breached, which can result in complete failure and tree death. A key to successful tree defence upon compromise of constitutive tiers lies in the availability of NSC (Wargo, 1972; Kemp and Burden, 1986; Hartmann and Trumore, 2016). Starch reserves within the parenchyma of the secondary xylem are critical to offset the effects of short-term drought, as shown by O’Brien et al. (2015) in temperate tree species. Moreover, total parenchyma fractions (ray and axial parenchyma combined) provide the uppermost limit for the amount of NSC that can amass and in turn be immobilized in sapwood of temperate trees (Plavcová et al., 2016). During drought, NSCs act as a buffer through osmotic adjustments (Sala et al., 2012), but are also available for conversion to SMs for defence, as drought brings with it an increased risk of attack from pathogens. As SMs are converted from NSCs, the amount of starch available for conversion may have serious implications in relation to tree defence capabilities during drought stress (Wargo, 1977b; Shigo and Tippett, 1981; Rolshausen et al., 2010). Therefore, temperate tree species with more parenchyma and a greater capacity for NSC storage, particularly in storage cells of ray parenchyma (Chattaway, 1951), should have greater defence potential owing to subsequent abundant chemical availability. Research into grapevines by Rolshausen et al. (2010) shows that concentrations of polyphenolic compounds vary between cultivars, with higher concentrations proving more resilient against the fungal canker- and decay-causing Eutypa lata, a disease often coupled with Phomopsis viticola, and a serious concern for wine growers.
Much recent research has focused on SM synthesis during drought stress; however, the focus appears to concentrate on water deficits in leaf xylem tissue rather than in wood (McKeirnan et al., 2014, 2016; Niinemets, 2016). The latter works describe how NSC is converted into SMs in response to drought stress irrespective of the presence of pathogens. With the exception of SMs involved in tylosis, which are mostly suberized, the production of SMs solely in response to drought is, as far as we know, undocumented for woody xylem tissue. Moreover, it is unclear and perhaps unlikely that leaf defence strategies in trees can be extrapolated to woody parts. Different plant organs probably have different strategies in response to drought. Here, we postulate that there is a trade-off between leaves and secondary xylem in the approach to drought stress. Leaves, like bark, are on the frontline of defence in trees and, being disposable organs and the site of photosynthesis, can afford to invest in a more costly strategy.
In leaves, the onset of drought signals stress-related phytohormones and enzymes from the phenolic pathway to activate secondary metabolism from primary pathways, where fresh reserves of NSC are available to be converted to SMs (McDowell, 2011; McKiernan et al., 2016). In addition, the accumulation of organic alkaloids such as quercitol, presumably arising from vascular parenchyma, has been found to function as osmotica in the leaves of Eucalyptus (Merchant et al., 2006; Arndt et al., 2008), but there is no evidence of this for stems. For leaves, this is outlined as a trade-off between growth, leaf hydraulic function and defence (Herms and Mattson, 1992; Caretto et al., 2015), and falls under the relatively recent discipline termed metabolomics (Trethewey, 2004). Also, in chlorenchyma of leaves (and bark of many species), the chloroplasts are specialist organelles involved in active defence against pathogens: as the first organelles to receive stress signals before shutting down photosynthesis, chloroplasts liaise with the cell membrane, endoplastic reticulum and nuclei to form an interorganellular immune response that is highly effective against pathogens (Serrano et al., 2016). While generally absent in wood parenchyma, chloroplasts may be a key distinction between leaves and stem defence capabilities, providing an explanation for greater defence plasticity in frontline defence along with more rapid plant immune responses.
In sapwood, post-drought hydraulic recovery is largely dependent on the upkeep of vessel-associated cells and vessel or tracheid translocation. However, in temperate angiosperms, a reason for the cessation of translocation in vessels in compartmented regions of sapwood on account of embolism might be speculated to preserve the integrity of the stem hydraulic system as a whole by preventing rapid spread of emboli and, indirectly, fungal spread. The latter scenario is explained by various models similar in principle to CODIT, including: hydraulic segmentation (Tyree and Zimmermann, 2002), hydraulic sectoriality (Orians et al., 2002; Zanne et al., 2006) and hydraulic redundancy (Schenk et al., 2008). Tree hydraulics is often viewed separately from tree defence, but the two functions are intertwined; when the hydraulic system falters, the defence system is activated. The integrity of the water conductive system against the ingress of air (embolism formation) is foremost to safeguard the tree, and all tree responses to fungal pathogens revolve around maintenance of the hydraulic system (Rayner and Boddy, 1988; Schenk et al., 2008). Safeguarding the tree requires careful readjustment of carbon imbalances (Sala et al., 2012; Oliva et al., 2014). Overcompensation in response to fungi (an overactive CODIT) can result in depletion of resources and can also disrupt hydraulic activity through excessive tylosis, a tipping point often resulting in premature death. Overcompensation is a known occurrence for Ulmus spp. (elm) in response to Dutch elm disease and for Pinus spp. in response to Heterobasidion (Rishbeth, 1951; Kirisits, 2007). An overactive CODIT is especially critical when fungal activity is coupled with abiotic stresses such as drought. Also, excessive compartmentalization reduces xylem space otherwise used for NSC storage and transport, placing greater stress on the tree at the whole-plant level (Shigo, 1982; Oliva et al., 2012; Smith, 2015; Hartmann and Trumbore, 2016). In light of this, the CODIT system, which is confined to xylem (largely sapwood), needs modification to integrate more distal organs for a whole-tree approach.
CONCLUSION AND OUTLOOK
In this review we have extended the applicability of the CODIT model to help us further our understanding of tree defence processes at micro-scales in relation to decay fungi. Research at the micro-scale now clarifies that trees recognize differences between threats, and can even identify levels of threat within fungi, new findings which should be factored into the CODIT model. Moreover, the CODIT model and the four walls are mostly limited to the xylem (particularly sapwood), which fails to recognize that the tree is an interconnected modular-based system, where trade-offs exist between the hydraulic system and defence in a continuum across leaves, bark, roots and stem. To better understand bark, for instance, detailed histology and experiments on a range of species with different bark thicknesses is required to interpret trade-offs within bark (between the phloem and periderm) and between bark and xylem. The co-ordination between modules should form an all-encompassing system based on a new CODIT concept design that should make the model applicable to all tree defects, insects, diseases and necrotrophic–saprotrophic decay fungi. Also, the CODIT model should not only belong under the remit of forestry, but also should become available to our understanding of compartmentalization in plants from genetic to micro–macro scales across all plant-based disciplines. The concept’s focus on wood decay has prevented its usefulness beyond forestry.
Increased parenchyma and vessel interconnectivity in angiosperm trees should allow for more rapid signalling, synthesis and transport of SMs. However, greater vessel connectivity, although hydraulically more efficient, can result in an increase in embolism and fungal spread, the idea behind hydraulic-focused models, such as hydraulic sectoriality (Orians et al., 2002; Zanne et al., 2006) and hydraulic redundancy (Schenk et al., 2008). CODIT should envelop the latter models to embrace the important trade-offs between defence and hydraulics, such as how reaction zones form at the expense of water transport, resulting in often regionalized hydraulic cessation upon successful compartmentalization of the decayed or damaged region. Conifers lack a highly integrated 3-D system and are anatomically and functionally distinct from angiosperms, deserving a different approach when applying CODIT. Another important aspect of 3-D connectivity is related to signalling and the long-distance transport of jasmonic and salicylic acid from root-based symbionts such as Trichoderma spp. and mycorrhizae. Large-scale field studies are required to investigate if induced systemic resistance from the latter endophytic fungi can occur in trees, as has been shown in model crop plants. Specifically, we need to understand if a heightened immunity against pathogenic fungi can be triggered across the whole plant body without activating cell death and reaction zone formation in sapwood, when considering the complexity, size and longevity of trees.
We also need to investigate SM production speed and SM composition in reaction zones and the barrier zone in both conifers and angiosperm trees using control tests with just mechanical damage vs. mechanical damage together with a range of pathogenic decay fungi. The latter tests might universally demonstrate that heightened response occurs to pathogenic fungi, with noticeable differences between pathogens, depending on toxicity levels, compared with only mechanical damage and air ingress. An understanding surrounding the allocation of constitutive SMs during ontogeny (genetic control) and inducible SMs during growth and development also highlights shortcomings in our knowledge of xylem and beyond xylem tree defence, which need to be addressed. The CODIT system is a pragmatic approach to explaining and understanding defence processes in trees. With more research alongside the expansion of the current model, we can make it a more widely used framework to better understand genetic influence, microbial–plant interactions and cost–benefit trade-offs in trees.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following.
Table S1: low-molecular weight and complex secondary metabolites found in the reaction zones and barrier zone of CODIT in a range of tree species.
Table S2: overview of the major fungi associated with xylem tissue in trees.
FUNDING
This work was supported by the Innosuisse - Schweizerische Agentur für Innovationsförderung [Project no. 26083.1 PFIW-IW 8].
ACKNOWLEDGEMENTS
The authors are grateful to Kevin Smith and the two anonymous reviewers for critical and valuable comments on the manuscript. H.M conceived, initiated and managed the project. All authors contributed equally to the writing and ideas within the final manuscript.
LITERATURE CITED
- Agler MT, Ruhe J, Kroll S, et al. . 2016. Microbial hub taxa link host and abiotic factors to plant microbiome variation. PLoS Biology 14: e1002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardi R, Kobiler I, Jacoby B, Keen NT, Prusky D. 1998. Involvement of epicatechin biosynthesis in the activation of the mechanism of resistance of avocado fruits to Colletotrichum gloesporioides. Physiological Molecular Plant Pathology 53: 269–285. [Google Scholar]
- Arndt SK, Livesley S, Merchant A, Bleby T, Grierson P. 2008. Quercitol and osmotic adaptation of field grown Eucalyptus under seasonal drought stress. Plant, Cell & Environment 31: 915–924. [DOI] [PubMed] [Google Scholar]
- Barry KM, Davies NW, Mohammed CM. 2002. Effect of season and different fungi on phenolics in response to xylem wounding and inoculation in Eucalyptus nitens. Forest Pathology 32: 163–178. [Google Scholar]
- Baum S, Schwarze FWMR. 2002. Large-leaved lime (Tilia platyphyllos) has a low ability to compartmentalize decay fungi via reaction zone formation. New Phytologist 154: 481–490. [DOI] [PubMed] [Google Scholar]
- Baum S, Sieber TN, Schwarze FWMR, Fink S. 2003. Latent infections of Fomes fomentarius in the xylem of European beech (Fagus sylvatica). Mycological Progress 2: 141–148. [Google Scholar]
- Benhamou N. 1995. Ultrastructural and cytochemical aspects of the response of eggplant parenchyma cells in direct contact with Verticillium-infected xylem vessels. Physiological and Molecular Plant Pathology 46: 321–338. [Google Scholar]
- Bernards MA. 2002. Demystifying suberin. Canadian Journal of Botany 80: 227–240. [Google Scholar]
- Betsuyaku S, Katou S, Takebayashi Y, Sakakibara H, Nomura N, Fukuda H. 2018. Salicylic acid and jasmonic acid pathways are activated in spatially different domains around the infection site during effector-triggered immunity in Arabidopsis thaliana. Plant and Cell Physiology 59: 439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigeard J, Colcombet J, Hirt H. 2015. Signaling mechanisms in pattern-triggered immunity (PTI). Molecular Plant 8: 521–539. [DOI] [PubMed] [Google Scholar]
- Biggs AR. 1986. Comparative anatomy of host response of two peach cultivars inoculated with Leucostoma cincta and L. personii. Phytopathology 76: 905–912. [Google Scholar]
- Biggs AR. 1987. Occurrence and location of suberin in wound reaction zones in xylem of 17 tree species. Phytopathology 77: 718–725. [Google Scholar]
- Biggs AR. 1992. Anatomical and physiological responses of bark tissues to mechanical injury. In: Blanchette RA, Biggs AR, eds. Defense mechanisms of woody plants against fungi. Berlin: Springer-Verlag, 13–40. [Google Scholar]
- Blodgett JT, Eyles A, Bonello P. 2007. Organ-dependent induction of systemic resistance and systemic susceptibility in Pinus nigra inoculated with Sphaeropsis sapinea and Diplodia scrobiculata. Tree Physiology 27: 373–382. [DOI] [PubMed] [Google Scholar]
- Boddy L, Rayner ADM. 1983. Origins of decay in living deciduous trees: the role of moisture content and a re-appraisal of the expanded concept of tree decay. New Phytologist 94: 623–641. [Google Scholar]
- Bonello P, Gordon TR, Storer AJ. 2001. Systemic induced resistance in Monterey pine. Forest Pathology 31: 99–106. [Google Scholar]
- Braun HJ. 1984. The significance of the accessory tissues of the hydrosystem for osmotic water shifting as the second principle of water ascent, with some thoughts concerning the evolution of trees. International Association of Wood Anatomists Bulletin 5: 275–294. [Google Scholar]
- Brix H, Mitchell AK. 1983. Thinning and nitrogen fertilization effects on sapwood development and relationships of foliage quantity to sapwood area and basal area in Douglas-fir. Canadian Journal of Forest Research 13: 384–389. [Google Scholar]
- Burden RS, Kemp MS. 1984. Sesquiterpene phytoalexins from Ulmus glabra. Phytochemistry 23: 383–385. [Google Scholar]
- Cailleret M, Jansen S, Robert EMR, et al. . 2017. A synthesis of radial growth patterns preceding tree mortality. Global Change Biology 23: 1675–1690. [DOI] [PubMed] [Google Scholar]
- Carlquist S. 2018. Living cells in wood 3. Overview; functional anatomy of the parenchyma network. The Botanical Review 84: 242–294. [Google Scholar]
- Caretto S, Linsalata V, Colella G, Mita G, Lattanzio V. 2015. Carbon fluxes between primary metabolism and phenolic pathway in plant tissues under stress. International Journal of Molecular Science 16: 26378–26394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celedon JM, Bohlmann J. 2018. An extended model of heartwood secondary metabolism informed by functional genomics. Tree Physiology 38: 311–319. [DOI] [PubMed] [Google Scholar]
- Chattaway MM. 1949. The development of tyloses and secretion of gum in heartwood formation. Australian Journal of Biological Sciences 2: 227–240. [Google Scholar]
- Chattaway MM. 1951. Morphological and functional variations in the rays of pored timbers. Australian Journal of Biological Sciences 4: 12–27. [PubMed] [Google Scholar]
- Chen C, Chang H, Cowling EB, Hsu CH, Gates RP. 1976. Aporphine alkaloids and lignans formed in response to injury of sapwood in Liriodendron tulipifera. Phytochemistry 15: 1161–1167. [Google Scholar]
- Choat B, Ball M, Luly L, Holtum J. 2003. Pit membrane porosity and waterstress-induced cavitation in four co-existing dry rainforest tree species. Plant Physiology 131: 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choat B, Jansen S, Brodribb TJ, et al. . 2012. Global convergence in the vulnerability of forests to drought. Nature 491: 752–755. [DOI] [PubMed] [Google Scholar]
- Climent J, Gil L, Pardos JA. 1998. Xylem anatomical traits related to resinous heartwood formation in Pinus canariensis Sm. Trees 12: 139–145. [Google Scholar]
- Climent J, Chambel MR, Pérez E, Gil L, Pardos J. 2002. Relationship between heartwood radius and early radial growth rate, tree age, and climate in Pinus canariensis. Canadian Journal of Forest Research 32: 103–111. [Google Scholar]
- Cobb FW, Krstic M, Zavarin E, Barber HW. 1968. Inhibitory effects of volatile oleoresin components on Fomes annosus and four Ceratocystis spp. Phytopathology 58: 1327–1335. [Google Scholar]
- Del Rio JA, Gonzales A, Fuster MD, et al. . 2001. Tylose formation and changes in phenolic compounds of grape roots infected with Phaemoniella chlamydospora and Phaeoacremonium species. Phytopathologia Mediterranea 40: 394–399. [Google Scholar]
- De Micco V, Balzano A, Wheeler EA, Baas P. 2016. Tyloses and gums: a review of structure, function and occurrence of vessel occlusions. International Association of Wood Anatomists 37: 186–295. [Google Scholar]
- Dodds PN, Rathjen JP. 2010. Plant immunity: towards an integrated view of plant–pathogen interactions. Nature Review Genetics 11: 539–548. [DOI] [PubMed] [Google Scholar]
- Duchesne LC, Jeng RS, Hubbes M. 1985. Accumulation of phytoalexins in Ulmus americana in response to infection by a nonaggressive and an aggressive strain of Ophiostoma ulmi. Canadian Journal of Botany 63: 678–680. [Google Scholar]
- Dujesiefken D, Liese W. 2015. The CODIT principle – implications for best practice. Champaign, IL: International Society of Arboriculture. [Google Scholar]
- Dujesiefken D, Liese W, Shortle W, Minocha R. 2005. Response of beech and oaks to wounds made at different times of the year. European Journal of Forest Research 124: 113–117. [Google Scholar]
- Durrant WE, Dong X. 2004. Systemic acquired resistance. Annual Review of Phytopathology 42: 185–209. [DOI] [PubMed] [Google Scholar]
- Essiamah S, Eschrich W. 1985. Changes of starch content in the storage tissues of deciduous trees during winter and spring. International Association of Wood Anatomists Bulletin New Series 6: 97–106. [Google Scholar]
- Evert RF. 2006. Esau’s plant anatomy: meristems, cells, and tissues of the plant body: their structure, function, and development, 3rd edn. Hoboken, NJ: John Wiley and Sons Inc. [Google Scholar]
- Eyles A, Bonello P, Ganley R, Mohammed C. 2010. Induced resistance to pests and pathogens in trees. New Phytologist 185: 893–908. [DOI] [PubMed] [Google Scholar]
- Felhofer M, Prats-Mateu B, Bock P, Gierlinger N. 2018. Antifungal stilbene impregnation: transport and distribution on the micron-level. Tree Physiology 38: 1526–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrenberg S, Kane JM, Mitton JB. 2014. Resin duct characteristics associated with tree resistance to bark beetles across lodgepole and limber pines. Oecologia 174: 1283–1292. [DOI] [PubMed] [Google Scholar]
- Feucht W, Treutter D. 1999. The role of flavan-3-ol and proanthocyanidins in plant defense. In: Inderjit KMM, ed. Principles and practices in chemical ecology. Boca Raton, FL: CRC Press, 307–338. [Google Scholar]
- Flodin K. 1979. Effects of monoterpenes on Fomes annosus (Fr,) Cooke and its phenol oxidase activity. European Journal of Forest Pathology 9: 1–6. [Google Scholar]
- Geiger JP, Rio B, Nicole M, Nandris D. 1989. Peroxidase production in tissues of the rubber tree following infection by root rot fungi. Physiological and Molecular Plant Pathology 34: 241–246. [Google Scholar]
- Germain H, Séguin A. 2011. Innate immunity: has poplar made its BED? New Phytologist 189: 678–687. [DOI] [PubMed] [Google Scholar]
- Gilman E. 2011. An illustrated guide to pruning, 3rd edn. Albany, NY: Delmar Publishers. [Google Scholar]
- Glazebrook J. 2005. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annual Review of Phytopathology 43: 205–227. [DOI] [PubMed] [Google Scholar]
- Goldstein G, Andrade JL, Meinzer FC, et al. . 1998. Stem water storage and diurnal patterns of water use in tropical forest canopy trees. Plant, Cell & Environment 21: 397–406. [Google Scholar]
- Goodell B. 2003. Brown-rot fungal degradation of wood: our evolving view. In: Barry G, Nicholas DD, Schultz TP, eds. Wood deterioration and preservation. Washington, DC: American Chemical Society, 97–118. [Google Scholar]
- Grosclaude C, Ricard JL, Dubos B. 1973. Inoculation of Trichoderma viride spores via pruning shears for biological control of Stereum purpureum on plum tree wounds. Plant Disease Reporter 57: 25–28. [Google Scholar]
- Gruber S, Seidl-Seiboth V. 2012. Self versus non-self: fungal cell wall degradation in Trichoderma. Microbiology 158: 26–34. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt R. 1999. Induced disease resistance: how do induced plants stop pathogens? Physiological and Molecular Plant Pathology 55: 77–84. [Google Scholar]
- Harborne JB, Williams CA. 2000. Advances in flavonoid research since 1992. Phytochemistry 55: 481–504. [DOI] [PubMed] [Google Scholar]
- Harman GE, Howell CR, Viterbo A, Chet I, Lorito M. 2004. Trichoderma species – opportunistic, avirulent plant symbionts. Nature Reviews Microbiology 2: 43–56. [DOI] [PubMed] [Google Scholar]
- Hartig R. 1878. Die Zersetzungserscheinungen des Holzes der Nadelholzbaume und der Eiche in Forstlicher, Botanischer und Chemischer Richtung. Berlin: Springer-Verlag. [Google Scholar]
- Hartmann H, Trumbore S. 2016. Understanding the roles of non-structural carbohydrates in forest trees – from what we can measure to what we want to know. New Phytologist 211: 386–403. [DOI] [PubMed] [Google Scholar]
- Haslam E. 1989. Gallic acid derivatives and hydrolyzable tannins. In: Rowe JW, eds. Natural products of woody plants: chemicals extraneous to the lignocellulosic cell wall. Berlin: Springer-Verlag, 399–438. [Google Scholar]
- Hatakka A. 1994. Lignin‐modifying enzymes from selected white‐rot fungi: production and role from in lignin degradation. FEMS Microbiology Reviews 13: 125–135. [Google Scholar]
- Hazenberg G, Yang KC. 1991. The relationship of tree age with sapwood and heartwood width in black spruce, Picea mariana (Mill.) B.S.P. Holzforschung 45: 417–320. [Google Scholar]
- Hemingway RW, Hillis WE. 1970. Heartwood formation in living stumps of Douglas-fir. Wood Science and Technology 4: 246–254. [Google Scholar]
- Hepting GH. 1935. Decay following fire in young Mississippi Delta hardwoods. USDA Bulletin 409: 1–32. [Google Scholar]
- Herms DA, Mattson WJ. 1992. The dilemma of plants: to grow or defend. Quarterly Review of Biology 67: 282–335. [Google Scholar]
- Hietala AM, Dörsch P, Kvaalen H, Solheim H. 2015. Carbon dioxide and methane concentrations in Norway spruce stems infected by white-rot fungi. Forests 6: 3304–3325. [Google Scholar]
- Hilaire E, Young SA, Willard LH, et al. . 2001. Vascular defense responses in rice: peroxidase accumulation in xylem parenchyma cells and xylem wall thickening. Molecular Plant Microbe Interactions 14: 1411–1419. [DOI] [PubMed] [Google Scholar]
- Hillis WE. 1987. Heartwood and tree exudates. New York: Springer-Verlag. [Google Scholar]
- Hoch G, Richter A, Korner C. 2003. Non-structural carbon compounds in temperate forest trees. Plant, Cell & Environment 26: 1067–1081. [Google Scholar]
- Holbrook NM, Zwieniecki MA. 1999. Embolism repair and xylem tension: do we need a miracle? Plant Physiology 120: 7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudgins JW, Franceschi VR. 2004. Methyl jasmonate-induced ethylene production is responsible for conifer phloem defense responses and reprogramming of stem cambial zone for traumatic resin duct formation. Plant Physiology 135: 2134–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IAWA Committee. 1989. IAWA list of microscopic features for hardwood identification. International Association of Wood Anatomists Bulletin 10: 219–332. [Google Scholar]
- IAWA Committee. 2004. IAWA list of microscopic features for softwood identification. International Association of Wood Anatomists 25: 1–70. [Google Scholar]
- IAWA Committee on Nomenclature. 1964. Multilingual glossary of terms used in wood anatomy. Verlagsanstalt Buchdruckerei Konkordia: Switzerland. [Google Scholar]
- Imai T, Tanabe K, Kato T, Fukushima K. 2005. Localization of ferruginol, a diterpene phenol, in Cryptomeria japonica heartwood by time-of-flight secondary ion mass spectrometry. Planta 221: 549–556. [DOI] [PubMed] [Google Scholar]
- Inupakutika MA, Sengupta S, Devireddy AR, Azad RK, Mittler R. 2016. The evolution of reactive oxygen species metabolism. Journal of Experimental Botany 67: 5933–5943. [DOI] [PubMed] [Google Scholar]
- Jeng RS, Alfenas AC, Hubbes M, Dumas M. 1983. Presence and accumulation of fungitoxic substances against Ceratocystis ulmi in Ulmus americana: possible relation to induced resistance. European Journal of Forest Patholology 13: 239–244. [Google Scholar]
- Jia M, Chen L, Xin HL, et al. . 2016. A friendly relationship between endophytic fungi and medicinal plants: a systematic review. Frontiers in Microbiology 7: 906. doi: 10.3389/fmicb.2016.00906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Dangl JL. 2006. The plant immune system. Nature 444: 323–329. [DOI] [PubMed] [Google Scholar]
- Jones CG, Hartley SE. 1999. A protein competition model of phenolic allocation. Oikos 86: 27–44. [Google Scholar]
- Kampe A, Magel E. 2013. New insights into heartwood and heartwood formation. In: Fromm J, ed. Cellular aspects of wood formation. Berlin: Springer, 71–95. [Google Scholar]
- Keeling CI, Bohlmann J. 2006. Genes, enzymes and chemicals of terpenoid diversity in the constitutive and induced defence of conifers against insects and pathogens. New Phytologist 170: 657–675. [DOI] [PubMed] [Google Scholar]
- Kemp MS, Burden RS. 1986. Phytoalexins and stress metabolites in the sapwood of trees. Phytochemistry 25: 1261–1269. [Google Scholar]
- Kirisits T. 2007. Fungal associates of European bark beetles with special emphasis on the ophiostomatoid fungi. In: Lieutier F, Day KR, Battisti A, Grégoire JC, Evans H, eds. Bark and wood boring insects in living trees in Europe, a synthesis. Dordrecht: Kluwer, 185–223. [Google Scholar]
- Kokubun T, Harborne JB. 1995. Antifungal biphenyl compounds are the phytoalexins of the sapwood of Sorbus aucuparia. Phytochemistry 40: 57–59. [Google Scholar]
- Kollist H, Zandalinas SI, Sengupta S, Nuhkat M, Kangasjärvi J, Mittler R. 2018. Rapid responses to abiotic stress: priming the landscape for the signal transduction network. Trends in Plant Science 24: 25–37. [DOI] [PubMed] [Google Scholar]
- Krekling T, Franceschi VR, Krokene P, Solheim H. 2004. Differential anatomical response of Norway spruce stem tissues to sterile and fungus infected inoculations. Trees 18: 1–9. [Google Scholar]
- Küster E. 1913. Pathological plant anatomy. Madison, WI: University of Wisconsin Press. [Google Scholar]
- Lacerda AF, Vasconcelos EA, Pelegrini PB, de Sa MF. 2014. Defensins and their role in plant defense. Frontiers in Microbiology 5: 116. doi: 10.3389/fmicb.2014.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leśniewska J, Ohman D, Krzeslowska M, et al. . 2017. Defense responses in aspen with altered pectinmethylesterase activity reveal the hormonal inducers of tyloses. Plant Physiology 173: 1409–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liese W, Dujesiefken D. 1996. Wound reactions of trees. In: Raychaudhuri SP, Maramorosch K, eds. Forest trees and palms: diseases and control. Lebanon, NH: Science Publishers, 21–35. [Google Scholar]
- Luchi N, Ma R, Capretti P, Bonello P. 2005. Systemic induction of traumatic resin ducts and resin flow in Austrian pine by wounding and inoculation with Sphaeropsis sapinea and Diplodia scrobiculata. Planta 221: 75–84. [DOI] [PubMed] [Google Scholar]
- Lukmandaru G, Takahashi K. 2009. Radial distribution of quinone in plantation teak (Tectona grandis L.f.). Annals of Forest Science 66: 605. [Google Scholar]
- Magel E, Jay-Allemand C, Ziegler H. 1994. Formation of heartwood substances in the stemwood of Robinia pseudoacacia L. II. Distribution of nonstructural carbohydrates and wood extractives across the trunk. Trees 8: 165–171. [Google Scholar]
- Magel E, Abdel-Latif A, Hampp R. 2001. Non-structural carbohydrates and catalytic activities of sucrose metabolizing enzymes in trunks of two Juglans species and their role in heartwood formation. Holzforschung 55: 135–145. [Google Scholar]
- Manion PD. 2003. Evolution of concepts in forest pathology. Phytopathology 93: 1052–1055. [DOI] [PubMed] [Google Scholar]
- Martin D, Tholl D, Bohlmann J. 2002. Methyl Jasmonate induces traumatic resin ducts, terpenoid resin biosynthesis, and terpenoid accumulation in developing xylem of Norway spruce stems. Plant Physiology 129: 1003–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín JA, Solla A, Domingues MR, Coimbra MA, Gil L. 2008. Exogenous phenol increase resistance of Ulmus minor to Dutch elm disease through formation of suberin-like compounds on xylem tissues. Environmental and Experimental Botany 64: 97–104. [Google Scholar]
- Martínez-Medina A, Appels FV, Van Wees SC. 2017. Impact of salicylic acid- and jasmonic acid-regulated defences on root colonization by Trichoderma harzianum T-78. Plant Signaling & Behavior 12: e1345404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson WJ, Julkunen-Tiitto R, Herms DA. 2005. CO2 enrichment and carbon partitioning to phenolics: do plant responses accord better with the protein competition or the growth–differentiation balance models? Oikos 111: 337–347. [Google Scholar]
- Mbengue M, Bourdais G, Gervasi F, et al. . 2016. Clathrin-dependent endocytosis is required for immunity mediated by pattern recognition receptor kinases. Proceedings of the National Academy of Sciences, USA 113: 11034–11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell NG. 2011. Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiology 155: 1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan AB, Hovenden MJ, Brodribb TJ, Potts BM, Davies NW, O’Reilly-Wapstra JM. 2014. Effect of limited water availability on foliar plant secondary metabolites of two Eucalyptus species. Environmental and Experimental Botany 105: 55–64. [Google Scholar]
- McKiernan AB, Potts BM, Brodribb TJ, et al. . 2016. Responses to mild water deficit and rewatering differ among secondary metabolites but are similar among provenances within Eucalyptus species. Tree Physiology 36: 133–147. [DOI] [PubMed] [Google Scholar]
- Melcher E, Jungel P, Mollendorf B, Schmitt U. 2003. Identification of a hydroxy substituted calamenene – a sesquiterpene associated with wound reactions in non-infected xylem of Tilia spp. Phytochemistry 62: 707–713. [DOI] [PubMed] [Google Scholar]
- Merchant A, Tausz M, Arndt SK, Adams MA. 2006. Cyclitols and carbohydrates in leaves and roots of 13 Eucalyptus species suggest contrasting physiological responses to water deficit. Plant, Cell & Environment 29: 2017–2029. [DOI] [PubMed] [Google Scholar]
- Metsä-Kortelainen S, Viitanen H. 2009. Decay resistance of sapwood and heartwood of untreated and thermally modified Scots pine and Norway spruce compared with some other wood species. Wood Material Science and Engineering 3: 105–114. [Google Scholar]
- Miedes E, Vanholme R, Boerjan W, Molina A. 2014. The role of the secondary cell wall in plant resistance to pathogens. Frontiers in Plant Science 5: 358. doi: 10.3389/fpls.2014.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris H, Brodersen CR, Schwarze FWMR, Jansen S. 2016a The parenchyma of secondary xylem and its critical role in tree defense against fungal decay in relation to the CODIT model. Frontiers in Plant Science 7: 1665. doi: 10.3389/fpls.2016.01665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris H, Plavcová L, Cvecko P, et al. . 2016. b. A global analysis of parenchyma tissue fractions in secondary xylem of seed plants. New Phytologist 209: 1553–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris H, Plavcová L, Gorai M, et al. . 2018. a Vessel‐associated cells in angiosperm xylem: highly specialized living cells at the symplast–apoplast boundary. American Journal of Botany 105: 151–160. [DOI] [PubMed] [Google Scholar]
- Morris H, Gillingham MAF, Plavcová L, et al. . 2018. b. The amount and spatial arrangement of axial parenchyma is related to vessel diameter in woody angiosperms. Plant, Cell & Environment 44: 245–260. [DOI] [PubMed] [Google Scholar]
- Mukherjee PK, Horwitz BA, Singh US, Mukherjee M, Schmoll M. 2013. Trichoderma in agriculture, industry and medicine: an overview. In: Prasun K, Mukherjee PK, Horwitz BA, Singh US, Schmoll M, eds. Trichoderma: biology and applications. Wallingford, Oxon: CABI Publishers, 1–10. [Google Scholar]
- Nasir F, Tian L, Chang C, Li X, Gao Y, Tran PL, Tian C. 2018. Current understanding of pattern-triggered immunity and hormone-mediated defense in rice (Oryza sativa) in response to Magnaporthe oryzae infection. Seminars in Cell and Developmental Biology 83: 95–105. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü. 2016. Uncovering the hidden facets of drought stress: secondary metabolites make the difference. Tree Physiology 36: 129–132. [DOI] [PubMed] [Google Scholar]
- O’Brien MJ, Burslem DFRP, Caduff A, Tay J, Hector A. 2015. Contrasting non-structural carbohydrate dynamics of tropical tree seedlings under water deficit and variability. New Phytologist 205: 1083–1094. [DOI] [PubMed] [Google Scholar]
- Ollerstam O, Larsson S. 2003. Salicylic acid mediates resistance in the willow Salix viminalis against the gall midge Dasineura marginemtorquens. Journal of Chemical Ecology 29: 163–74. [DOI] [PubMed] [Google Scholar]
- Oliva J, Camarero JJ, Stenlid J. 2012. Understanding the role of sapwood loss and reaction zone formation on radial growth of Norway spruce (Picea abies) trees decayed by Heterobasidion annosum s.l. Forest Ecology and Management 274: 201–209. [Google Scholar]
- Oliva J, Stenlid J, Martínez-Vilalta J. 2014. The effect of fungal pathogens on the water and carbon economy of trees: implications for drought-induced mortality. New Phytologist 203: 1028–1035. [DOI] [PubMed] [Google Scholar]
- Olson A, Aerts A, Asiegbu F, et al. . 2012. Insight into trade-off between wood decay and parasitism from the genome of a fungal forest pathogen. New Phytologist 194: 1001–1013. [DOI] [PubMed] [Google Scholar]
- Orians CM, Ardon M, Mohammad BA. 2002. Vascular architecture and patchy nutrient availability generate within-plant heterogeneity in plant traits important to herbivores. American Journal of Botany 89: 270–278. [DOI] [PubMed] [Google Scholar]
- Oses R, Valenzuela S, Freer J, Sanfuentes E, Rodríguez J. 2008. Fungal endophytes in xylem of healthy Chilean trees and their possible role in early wood decay. Fungal Diversity 33: 77–86. [Google Scholar]
- Pearce RB. 1990. Occurrence of decay-associated xylem suberization in a range of woody species. European Journal of Forest Pathology 20: 275–289. [Google Scholar]
- Pearce RB. 1991. Reaction zone relics and the dynamics of fungal spread in the xylem of woody angiosperms. Physiological and Molecular Plant Pathology 39: 41–55. [Google Scholar]
- Pearce RB. 1996. Antimicrobial defences in the wood of the living trees. New Phytologist 132: 203–233. [Google Scholar]
- Pearce RB, Rutherford J. 1981. A wound-associated suberization barrier to the spread of decay in the sapwood of oak (Quercus robur). Physiological Plant Pathology 19: 359–369. [Google Scholar]
- Phelps JE, McGinnes EA Jr. 1983. Growth-quality evaluation of black walnut wood. Part III – an anatomical study of color characteristics of black walnut veneer. Wood Fiber Science 15: 212–218. [Google Scholar]
- Pieterse CM, Zamioudis C, Berendsen RL, Weller DM, Van Wees SC, Bakker PA. 2014. Induced systemic resistance by beneficial microbes. Annual Review of Phytopathology 52: 347–375. [DOI] [PubMed] [Google Scholar]
- Plavcová L, Hoch G, Morris H, Ghiasi S, Jansen S. 2016. The amount of parenchyma and living fibers affects storage of nonstructural carbohydrates in young stems and roots of temperate trees. American Journal of Botany 113: 603–612. [DOI] [PubMed] [Google Scholar]
- Pusztahelyi T, Holb IJ, Pócsi I. 2015. Secondary metabolites in fungus–plant interactions. Frontiers in Plant Science 6: 573. doi: 10.3389/fpls.2015.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaello T, Asiegbu FO. 2017. Small secreted proteins from the necrotrophic conifer pathogen Heterobasidion annosum s.l. (HaSSPs) induce cell death in Nicotiana benthamiana. Scientific Reports 7: 8000. doi: 10.1038/s41598-017-08010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner ADM. 1986. Water and the origin of decay in trees. In: Ayres PG, Boddy L, eds. Water, fungi and plants. Cambridge: Cambridge University Press, 321–341. [Google Scholar]
- Rayner ADM, Boddy L. 1988. Fungal decomposition of wood: its biology and ecology. Chichester: John Wiley International. [Google Scholar]
- Ribera J, Fink S, Bas MC, Schwarze FWMR. 2017. Integrated control of wood destroying basidiomycetes combining Cu-based wood preservatives and Trichoderma spp. PLoS One 12: e0174335. doi: 10.1371/journal.pone.0174335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioux D, Ouellette GB. 1991a Barrier zone formation in host and nonhost trees inoculated with Ophiostoma ulmi. I. Anatomy and histochemistry. Canadian Journal of Botany 69: 2055–2073. [Google Scholar]
- Rioux D, Ouellette GB. 1991. b. Barrier zone formation in host and nonhost trees inoculated with Ophiostoma ulmi. II. Ultrastructure. Canadian Journal of Botany 69: 2074–2083. [Google Scholar]
- Rioux D, Chamberland H, Simard M, Ouellette GB. 1995. Suberized tyloses in trees: an ultrastructural and cytochemical study. Planta 196: 125–140. [Google Scholar]
- Rioux D, Nicole M, Simard M, Ouellette GB. 1998. Immunocytochemical evidence that secretion of pectin occurs during gel (gum) and tylosis formation in trees. Phytopathology 88: 494–505. [DOI] [PubMed] [Google Scholar]
- Rishbeth J. 1951. Observations on the biology of Fomes annosus, with particular reference to East Anglian pine plantations. III. Natural and experimental infection of pines, and some factors affecting severity of the disease. Annals of Botany 15: 221–246. [Google Scholar]
- Rolshausen PE, Urbez-Torres JR, Rooney-Latham S, Eskalen A, Smith RJ, Gubler WD. 2010. Evaluation of pruning wound susceptibility and protection against fungi associated with grapevine trunk diseases. American Journal of Enology and Viticulture 61: 113–119. [Google Scholar]
- Romero C, Bolker BM. 2008. Effects of stem anatomical and structural traits on responses to stem damage: an experimental study in the Bolivian Amazon. Canadian Journal of Forest Research 38: 611–618. [Google Scholar]
- Sala A, Woodruff DR, Meinzer FC. 2012. Carbon dynamics in trees: feast or famine. Tree Physiology 32: 764–775. [DOI] [PubMed] [Google Scholar]
- Sauter JJ, Wisniewski M, Witt W. 1996. Interrelationships between ultrastructure, sugar levels, and frost hardiness of ray parenchyma cells during frost acclimation and deacclimation in poplar (Populus × canadensis Moench <robusta>) wood. Journal of Plant Physiology 149: 451–461. [Google Scholar]
- Scharf DH, Heinekamp T, Brakhage AA. 2014. Human and plant fungal pathogens: the role of secondary metabolites. PLoS Pathogens 10: e1003859. doi: 10.1371/journal.ppat.1003859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk HJ. 2018. Wood: biology of a living tissue. American Journal of Botany 105: 139–141. [DOI] [PubMed] [Google Scholar]
- Schenk HJ, Espino S, Goedhart CM, Nordenstahl M, Martinez-Cabrera HI, Jones CS. 2008. Hydraulic integration and shrub growth form linked across continental aridity gradients. Proceedings of the National Academy of Sciences, USA 105: 11248–11253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmböck M, Lorito M, Wang YL, et al. . 1994. Parallel formation and synergism of hydrolytic enzymes and peptaibol antibiotics, molecular mechanisms involved in the antagonistic action of Trichoderma harzianum against phytopathogenic fungi. Applied and Environmental Microbiology 60: 4364–4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt U, Koch G. 2009. Characterisation of wound reaction compounds in the xylem of Tilia Americana L. by electron microscopy and cellular UV-microspectophotometry. New Zealand Journal of Forest Science 39: 233–241. [Google Scholar]
- Schmitt U, Liese W. 1990. Wound reaction of the parenchyma in Betula. International Association of Wood Anatomists Bulletin New Series 11: 413–420. [Google Scholar]
- Schmitt U, Liese W. 1992. Seasonal influences on early wound reactions in Betula and Tilia. Wood and Fiber Science 26: 405–412. [Google Scholar]
- Schubert M, Fink S, Schwarze WMRF. 2008. Evaluation of Trichoderma spp. as a biocontrol agent against wood decay fungi in urban trees. Biological Control 45: 111–123. [Google Scholar]
- Schuck HJ. 1982. Monoterpenes and resistance of conifers to fungi. In: Heybioek HM, Stephan BR, von Weissenberg K, eds. Resistance to diseases and pests in forest trees. Wageningen: Pudoc, 169–175. [Google Scholar]
- Schwarze FWMR. 2007. Wood decay under the microscope. Fungal Biology Reviews 21: 133–170. [Google Scholar]
- Schwarze FWMR, Baum S. 2000. Mechanisms of reaction zone penetration by decay fungi in wood of beech (Fagus sylvatica). New Phytologist 146: 129–140. [Google Scholar]
- Schwarze FWMR, Ferner D. 2003. Ganoderma on trees – differentiation of species and studies of invasiveness. Arboricultural Journal 27: 59–77. [Google Scholar]
- Schwarze FWMR, Fink S. 1997. Reaction zone penetration and prolonged persistence of xylem rays in London plane wood degraded by the Basidiomycete Inonotus hispidus. Mycological Research 101: 1207–1214. [Google Scholar]
- Schwarze FWMR, Mattheck C, Engels J. 2000a Fungal strategies of wood decay in trees. Heidelberg: Springer. [Google Scholar]
- Schwarze FWMR, Baum S, Fink S. 2000. b. Duak modes of degradation by Fistulina hepatica in xylem cell walls of Quercus robur. Mycological Research 104: 846–852. [Google Scholar]
- Schwarze FWMR, Fink S, Deflorio G. 2003. Resistance of parenchyma cells in wood to degradation by brown rot fungi. Mycological Progress 2: 267–274. [Google Scholar]
- Schwarze FWMR, Jauß F, Spencer C, Hallam C, Schubert M. 2012. Evaluation of an antagonistic Trichoderma strain for reducing the rate of wood decomposition by the white rot fungus Phellinus noxius. Biological Control 61: 160–168. [Google Scholar]
- Seehann G. 1979. Holzzerstörende Pilze an Straßen- und Parkbäumen in Hamburg. Mitteilungen der Deutschen Denrologischen Gesellschaft 71: 193–221. [Google Scholar]
- Serrano I, Audran C, Rivas S. 2016. Chloroplasts at work during plant innate immunity. Journal of Experimental Botany 67: 3845–3854. [DOI] [PubMed] [Google Scholar]
- Shain L. 1995. Stem defense against pathogens. In: Gartner BL, ed. Plant stems: physiology and functional morphology. San Diego, CA: Academic Press, 383–406. [Google Scholar]
- Shigo AL. 1970. An expanded concept of decay in living trees. In: Shigo AL, ed. Interactions of organisms in the process of decay of forest trees. Quebec: Laval University Bulletin, 7–9. [Google Scholar]
- Shigo AL. 1982. Trees resistant to spread of decay associated with wounds. In: Heybroek HM, Stephen BR, von Weissenberg K, eds. Resistance in forest trees to diseases and pests. Wageningen: Pudoc, 103–109. [Google Scholar]
- Shigo AL. 1984. Compartmentalization: a conceptual framework for understanding how trees grow and defend themselves. Annual Review of Phytopathology 22: 189–214. [Google Scholar]
- Shigo AL. 1991. Modern arboriculture: a systems approach to the care of trees and their associates. Durham, NH: Shigo and Trees. [Google Scholar]
- Shigo AL, Hillis WE. 1973. Heartwood, discolored wood and microorganisms in living trees. Annual Review of Phytopathology 11: 197–222. [Google Scholar]
- Shigo AL, Marx H. 1977. CODIT (Compartmentalization of decay in trees). Bulletin of the U.S. Department of Agriculture 405: 1–73. [Google Scholar]
- Shigo AL, Tippett JT. 1981. Compartmentalization of American elm tissues infected by Ceratocystis ulmi. Plant Disease 65: 715–718. [Google Scholar]
- Shortle WC. 1979. Mechanisms of compartmentalization of decay in living trees. Phytopathology 69: 1147–1151. [Google Scholar]
- Shortle WC, Cowling EB. 1978. Interaction of live sapwood and fungi commonly found in discolored and decayed wood. Phytopathology 68: 617–623. [Google Scholar]
- Sieber TN. 2007. Endophytic fungi in forest trees: are they mutualists? Fungal Biology Reviews 21: 75–89. [Google Scholar]
- Sjöström E. 1993. Wood chemistry: fundamentals and applications. 2nd edn. San Diego, CA: Academic Press. [Google Scholar]
- Słupianek A, Kasprowicz-Maluśki A, Myśkow E, Turzańska M, Sokołowska K. 2019. Endocytosis acts as transport pathway in wood. New Phytologist 222: 1846–1861. [DOI] [PubMed] [Google Scholar]
- Smith CJ. 1996. Accumulation of phytoalexins: defence mechanism and stimulus response system. New Phytologist 132: 1–45. [DOI] [PubMed] [Google Scholar]
- Smith KT. 2015. Compartmentalization, resource allocation, and wood quality. Current Forestry Reports 1: 8–15. [Google Scholar]
- Smith KT, Arbellay E, Falk DA, Sutherland EK. 2016. Macroanatomy and compartmentalization of recent fire scars in three North American conifers. Canadian Journal of Forest Research 46: 535–542. [Google Scholar]
- Soliman SS, Greenwood JS, Bombarely A, et al. . 2015. An endophyte constructs fungicide-containing extracellular barriers for its host plant. Current Biology 25: 2570–2576. [DOI] [PubMed] [Google Scholar]
- Spicer R. 2014. Symplastic networks in secondary vascular tissues: parenchyma distribution and activity supporting long-distance transport. Journal of Experimental Botany 65: 1829–1848. [DOI] [PubMed] [Google Scholar]
- Strunz GM, Bethel R, Dumas MT, Boyonoski N. 1997. On a new synthesis of sterpurenes and the bioactivity of some related Chondrostereum purpureum sesquiterpene metabolites. Canadian Journal of Chemistry 75: 742–753. [Google Scholar]
- Swarbrick T. 1926. The healing of wounds in woody stems. Journal of Pomology and Horticultural Science 5: 526–534. [Google Scholar]
- Swift MJ. 1965. Loss of suberin from bark tissue rotted by Armillaria mellea. Nature 207: 436–437. [Google Scholar]
- Talbot NJ. 2015. Plant immunity: a little help from fungal friends. Current Biology 25: 1074–1076. [DOI] [PubMed] [Google Scholar]
- Terho M, Hantula J, Hallaaksela AM. 2007. Occurrence and decay patterns of common wood-decay fungi in hazardous trees felled in the Helsinki City. Forest Pathology 37: 420–432. [Google Scholar]
- Terras FRG, Eggermont K, Kovaleva V, et al. . 1995. Small cysteine-rich antifungal proteins from radish: their role in host defense. The Plant Cell 7: 573–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thalmann M, Santelia D. 2017. Starch as a determinant of plant fitness under abiotic stress. New Phytologist 214: 943–951. [DOI] [PubMed] [Google Scholar]
- Tippett JT, Shigo AL. 1981. Barrier zone formation: a mechanism of tree defense against vascular pathogens. International Association of Wood Anatomists Bulletin 2: 163–168. [Google Scholar]
- Tixier A, Gambetta GA, Godfrey J, Orozco J, Zwieniecki A. 2019. Non-structural carbohydrates in dormant woody perennials; the tale of winter survival and spring arrival. Frontiers for Global Change 2: 18. doi: 10.3389/ffgc.2019.00018. [Google Scholar]
- Trethewey RN. 2004. Metabolite profiling as an aid to metabolic engineering in plants. Current Opinion in Plant Biology 7: 196–201. [DOI] [PubMed] [Google Scholar]
- Tyree MT, Zimmermann MH. 2002. Xylem structure and the ascent of sap. Berlin: Springer. [Google Scholar]
- Tytgat TOG, Verhoeven KJF, Jansen JJ, et al. . 2013. Plants know where it hurts: root and shoot jasmonic acid induction elicit differential responses in Brassica oleracea. PLoS One 8: e65502. doi: 10.1371/journal.pone.0065502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinale F, Sivasithamparam K, Ghisalberti EL, Marra R, Woo SL, Lorito M. 2008. Trichoderma–plant–pathogen interactions. Soil Biology and Biochemistry 40: 1–10. [Google Scholar]
- Wallis FM, Truter SJ. 1978. Histopathology of tomato plants infected with Pseudomonas solanacearum with emphasis on ultrastructure. Physiological Plant Pathology 13: 307–318. [Google Scholar]
- Wargo PM. 1972. Defoliation-induced chemical changes in sugar maple roots stimulate growth of Armillaria mellea. Phytopathology 62: 1278–1283. [Google Scholar]
- Wargo MP. 1977a Wound closure in sugar maples: adverse effects of defoliation. Canadian Journal of Forest Research 7: 410–414. [Google Scholar]
- Wargo PM. 1977. b. Armillariella mellea and Agrilus bilineatus and mortality of defoliated oak trees. Forest Science 23: 485–492. [Google Scholar]
- Wilkins AP, Stamp CM. 1990. Relationship between wood colour, silvicultural treatment and rate of growth of Eucalyptus grandis (Hill) Maiden. Wood Science and Technology 24: 297–304. [Google Scholar]
- Yamada T. 1992. Biochemistry of gymnosperm xylem responses to fungal invasion. In: Blanchette RA, Biggs AR, eds. Defense mechanisms of woody plants against fungi. Berlin: Springer-Verlag, 13–40. [Google Scholar]
- Yamada Y, Awano T, Fujita M, Takabe K. 2011. Living wood fibers act as large-capacity ‘single-use’ starch storage in black locust (Robinia pseudoacacia). Trees - Structure and Function 25: 607–616. [Google Scholar]
- Yang D, Jeng R, Hubbes M. 1989. Mansonone accumulation in elm callus induced by elicitors of Ophiostornn ulmi, and general properties of elicitors. Canadian Journal of Botany 67: 3490–3497. [Google Scholar]
- Zanne AE, Sweeney K, Sharma M, Orians CM. 2006. Patterns and consequences of differential vascular sectoriality in 18 temperate tree and shrub species. Functional Ecology 20: 200–206. [Google Scholar]
- Zeilinger S, Gruber S, Bansal R, Mukherjee PK. 2016. Secondary metabolism in Trichoderma – chemistry meets genomics. Fungal Biology Reviews 30: 74–90. [Google Scholar]
- Zhou X, Zhu H, Liu L, Lin J, Tang K. 2010. A review: recent advances and future prospects of taxol-producing endophytic fungi. Applied Microbiology and Biotechnology 86: 1707–1717. [DOI] [PubMed] [Google Scholar]
- Zobel BJ, Jett JB. 1995. Genetics of wood production. Berlin: Springer-Verlag. [Google Scholar]
- Zulak KG, Bohlmann J. 2010. Terpene biosynthesis and specialized vascular cells of conifer defense. Journal of Integrative Plant Biology 52: 86–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.