Abstract
Background and Aims
We have recently shown that an Arabidopsis thaliana double mutant of type III phosphatidylinositol-4-kinases (PI4Ks), pi4kβ1β2, constitutively accumulated a high level of salicylic acid (SA). By crossing this pi4kβ1β2 double mutant with mutants impaired in SA synthesis (such as sid2 impaired in isochorismate synthase) or transduction, we demonstrated that the high SA level was responsible for the dwarfism phenotype of the double mutant. Here we aimed to distinguish between the SA-dependent and SA-independent effects triggered by the deficiency in PI4Kβ1 and PI4Kβ2.
Methods
To achieve this we used the sid2pi4kβ1β2 triple mutant. High-throughput analyses of phytohormones were performed on this mutant together with pi4kβ1β2 and sid2 mutants and wild-type plants. Responses to pathogens, namely Hyaloperonospora arabidopsidis, Pseudomonas syringae and Botrytis cinerea, and also to the non-host fungus Blumeria graminis, were also determined. Callose accumulation was monitored in response to flagellin.
Key Results
We show here the prominent role of high SA levels in influencing the concentration of many other tested phytohormones, including abscisic acid and its derivatives, the aspartate-conjugated form of indole-3-acetic acid and some cytokinins such as cis-zeatin. We show that the increased resistance of pi4kβ1β2 plants to the host pathogens H. arabidopsidis, P. syringae pv. tomato DC3000 and Bothrytis cinerea is dependent on accumulation of high SA levels. In contrast, accumulation of callose in pi4kβ1β2 after flagellin treatment was independent of SA. Concerning the response to Blumeria graminis, both callose accumulation and fungal penetration were enhanced in the pi4kβ1β2 double mutant compared to wild-type plants. Both of these processes occurred in an SA-independent manner.
Conclusions
Our data extensively illustrate the influence of SA on other phytohormone levels. The sid2pi4kβ1β2 triple mutant revealed the role of PI4Kβ1/β2 per se, thus showing the importance of these enzymes in plant defence responses.
Keywords: pi4kβ1β2/PI4Ks, callose, salicylic acid, phytohormones, isochorismate synthase 1, biotic stress, pathogens, Arabidopsis thaliana
INTRODUCTION
Salicylic acid (SA) is a phytohormone that has a role in many plant physiological processes, although this has mainly been documented in plant responses to biotic stress when SA accumulates within tissues, both at the site of attack and in a systemic manner (Vlot et al., 2009; Janda and Ruelland, 2015). In plants, SA is biosynthesized via two pathways. One is dependent on phenylalanine ammonia-lyase (PAL; EC 4.3.1.24), which catalyses the conversion of phenylalanine to trans-cinnamic acid. In the other pathway, the key enzyme is isochorismate synthase (ICS; EC 5.4.4.2), which catalyses the isomerization of chorismate to isochorismate (Dempsey et al., 2011). The ICS-dependent pathway was shown to be responsible for most SA accumulation upon pathogen attack. In Arabidopsis thaliana, two ICS isoforms exist, but the major role in SA biosynthesis is played by ICS1. The ICS1 mutant is known as sid2 for salicylic acid induction deficient 2 (Wildermuth et al., 2001; Wagner et al., 2013; Cui et al., 2017). When SA levels increase, downstream signalling events are triggered, and the best described molecular pathway is dependent on NONEXPRESSOR OF PATHOGENESIS RELATED 1 (NPR1). Upon SA action, homo-oligomeric NPR1 protein undergoes dissociation by reduction and the resulting monomers move into the nucleus where they interact with TGA-transcription factors to induce the expression of SA responsive genes. An NPR1-independent pathway also exists in response to SA (Janda and Ruelland, 2015). The activation of SA signalling pathways leads to robust changes in the plant transcriptome, including defence-related genes (Seyfferth and Tsuda, 2014). Among the immune responses affected by changes in SA levels or by SA treatments is the accumulation of callose (Kohler et al., 2002; Dong et al., 2008; Antignani et al., 2015), a (1,3)-β-glucan occurring in plant cell walls.
The signalling pathways triggered by SA remain the subject of current research. We have shown that phosphoinositides, the phosphorylated derivatives of phosphatidylinositol (PI), are involved in SA transduction. Indeed, PI can be phosphorylated at the D4 position of the inositol ring by phosphatidylinositol-4-kinases (PI4Ks) thus leading to phosphatidylinositol 4-phosphate (PI4P), which can be phosphorylated further to phosphatidylinositol-4,5-bisphosphate (PI-4,5-P2). There are two types of PI4Ks according to their primary sequences and pharmacological sensitivities. Type II PI4Ks are inhibited by adenosine while type III PI4Ks are inhibited by micromolar concentrations of wortmannin, a steroid metabolite produced by the fungus Penicillium funiculosum (Nakanishi et al., 1995; Balla, 2007; Krinke et al., 2007). From the A. thaliana genome, 12 putative PI4K isoforms have been identified. Eight belong to type II (AtPI4Kγ1–8) and four belong to type III (AtPI4Kα1 and α2, AtPI4Kβ1 and β2) (Delage et al., 2012; Janda et al., 2014). We have shown that type III PI4Ks are activated when A. thaliana suspension cells respond to SA, thus leading to an increase in PI4P and PI-4,5-P2 (Krinke et al., 2007). PI-4,5-P2 can act as a cofactor to some phospholipase Ds (Pappan et al., 1998). Interestingly, there is overlap between SA-responsive genes controlled by PI4Ks and those controlled by phospholipase Ds, leading to the working model that in response to SA, PI4P and PI-4,5-P2 are produced with PI-4,5-P2 acting as a cofactor for a phospholipase D, whose product, PA, will trigger a signalling cascade (Krinke et al., 2009; Kalachova et al., 2016).
To better characterize the role of PI4Ks in the response to SA, we have used A. thaliana mutants altered in type III PI4Ks. We previously worked on a double mutant defective in the two PI4Kβ genes. Surprisingly, pi4kβ1β2 exhibited a constitutively high SA level that resulted in constitutive high transcription of SA-responsive genes such as PR-1 (PATHOGENESIS RELATED 1). Therefore, PI4Ks are not only involved in SA transduction but they can also impact SA concentration. Furthermore, this double mutant exhibited dwarfism and was more resistant to the bacterial pathogen Pseudomonas syringae pv. maculicola ES4326 (Sasek et al., 2014). The pi4kβ1β2 plant was crossed with mutants impaired in components of SA synthesis (sid2, impaired in ICS1 expression; eds1), SA transduction (npr1) or a mutant expressing bacterial SA-hydroxylase (NahG) that degrades SA to catechol. The resulting triple mutants allowed us to conclude that the dwarf phenotype of pi4kβ1β2 plants was dependent on SA accumulation and its transduction via the NPR1 pathway (Janda et al., 2014; Sasek et al., 2014).
In the present study, our aim was to identify amongst the cellular and physiological processes affected by the deficiency of PI4Kβ1β2 those that were either SA-dependent or SA-independent. To achieve this, we used the sid2pi4kβ1β2 triple mutant that does not accumulate SA and exhibits wild-type-sized rosettes (Sasek et al., 2014). In this mutant, the effects of PI4Kβ1 and PI4Kβ2 mutations would not be masked by high SA levels. We showed that hormonal levels and pathogen resistance were mainly dependent on SA. However, we could show that sid2pi4kβ1β2 plants accumulated higher amounts of callose in response to flg22 and wounding. Interestingly, this SA-independent callose accumulation was also observed during early stages of interactions with Blumeria graminis when penetration was observed. Our data suggest that PI4Ks are involved in plant immune responses not only through SA accumulation but also via SA-independent processes.
MATERIALS AND METHODS
Plant material, growth conditions
In this study, we used the following genotypes of A. thaliana: Columbia-0 (WT), sid2-3 (Gross et al., 2006), npr1-1 (Cao et al., 1997), NahG (Delaney et al., 1994), pi4kβ1β2 (SALK_040479/SALK_09069; Preuss et al., 2006), sid2pi4kβ1β2, NahGpi4kβ1β2 and npr1pi4kβ1β2 mutants previously described (Sasek et al., 2014).
All plants were cultivated in Jiffy 7 peat pellets at 22 °C with 70 % relative humidity. All plants were watered without additional fertilizers. Plants were routinely cultivated in daily cycles of 10 h light (100–130 μE m−2 s−1) and 14 h dark. Plants that would be used for hormonal analysis or transcription analysis were cultivated under 16 h light (130–150 μE m−2 s−1) and 8 h dark.
Pathogen inoculation
Two-week-old plants grown at high density in one pot were sprayed with Hyaloperonospora arabidopsidis NoCo2 spores (~100 spores μL–1). The infected plants were cultivated in closed transparent plastic boxes at high humidity for 6 d under 16 h light/8 h dark (100–130 μE m−2 s−1) at 19 °C. For analysis, leaves collected from one pot were considered as one sample (for each genotype, 11 samples were analysed). Spores were counted under a microscope using a Bürker chamber and expressed as relative spore number (%), where relative spore number for a given control genotype (WT or sid2) was set to 100 %. The spores were counted as spores per milligram of tissue fresh weight. The experiments (WT vs. pi4kβ1β2 and sid2 vs. sid2pi4kβ1β2) were conducted independently.
Inoculation with P. syringae was performed according to Katagiri et al. (2002) with modifications. Bacteria were cultivated overnight on King’s B medium plates containing rifampicin (50 µg µL–1). P. syringae pv. tomato DC3000 (Pst DC3000) and P. syringae pv. tomato DC3000 AvrRpt2 (Pst DC3000 AvrRpt2) were taken from the respective plate and resuspended in 10 mm MgCl2 to give an OD600 of 0.001. Four-week-old plants were infiltrated with this suspension.
One disc (6 mm) from one leaf, three leaves at a similar developmental stage from one plant and three plants were collected as one sample of one genotype at 0 days post-inoculation (dpi) and 3 dpi (3 dpi only for Pst DC3000; 2 dpi for Pst DC3000 AvrRpt2). Leaf discs were ground in 10 mm MgCl2 and decimal dilutions were made. Colony forming units were counted.
Four-week-old A. thaliana plants were treated with 6 µL drops containing Botrytis cinerea BMM spores (5 × 104 spores mL–1) by applying a single drop to each leaf, with three leaves at a similar developmental stage inoculated for each plant. Treated plants were placed into closed plastic boxes and kept in low light (16 h light/8 h dark, 21 °C; 10–μE m−2 s−1) for 56 h post-inoculation (hpi).
Blumeria graminis f. sp. hordei (Bgh) was cultivated continuously on fresh barley (‘Golden promise’) grown under short day conditions (19 °C, 10/14 h, 50 % humidity, at a light intensity of 70 μmol m−2 s−1). Plants, ~4 weeks old, were inoculated by spreading spores from infected barley onto the adaxial side of their leaves (from leaf to leaf). The 5th–6th leaves were cut off at selected times hours post-inoculation (hpi) and cleared with 96 % ethanol or chloral hydrate. For penetration rate, fungal structures were stained with 250 mg mL–1 trypan blue in a lactophenol/ethanol solution (Vogel and Somerville, 2000). Stained leaves were observed by classical epifluorescence microscopy or bright-field microscopy using a Zeiss AxioImager ApoTome2 (objective 100×).
Callose deposition
Four-week-old A. thaliana plants were treated for 24 h with 100 nm flg22 or infiltrated with Pst DC3000. Distilled water infiltration was used as a control (mock) treatment. Infiltrated leaves were decoloured in ethanol/glacial acetic acid (3:1, v/v). The leaves were then rehydrated in successive baths of 70 % ethanol (at least 1 h), 50 % ethanol (at least 1 h), 30 % ethanol (at least 1 h) and water (at least 2 h). Leaves were stained for 4 h with 0.01 % aniline blue in 150 mm K2HPO4, pH 9.5. Callose deposition was observed by fluorescence microscopy using a Zeiss AxioImager ApoTome2 (objective 10×). In Bgh infection analysis, we calculated only callose spots using the high circularity function of the measurement settings at an interval of 0.5–1 which allowed us to distinguish only the cells with the size exclusion limit for spots corresponding to either encased haustoria or enormous papilla. Images were processed with ImageJ software. At least four leaves from three independent plants were analysed for each variant.
RNA extraction and qPCR analysis
Plant tissues were homogenized in 2-mL screw-cap tubes containing 1 g of 1.3-mm-diameter silica beads using a FastPrep-24 instrument (MP Biomedicals, USA). Total RNA was isolated using a Spectrum Plant Total RNA kit (Sigma-Aldrich, USA) and treated with a DNA-free kit (Ambion, USA). Subsequently, 1 µg of RNA was converted to cDNA with M-MLV RNase H–Point Mutant reverse transcriptase (Promega Corp., USA) and an anchored oligo dT21 primer (Metabion, Germany). Gene transcription was quantified by qPCR using a LightCycler 480 SYBR Green I Master kit and a LightCycler 480 (Roche, Switzerland). The PCR conditions employed were 95 °C for 10 min followed by 45 cycles of 95 °C for 10 s, 55 °C for 20 s and 72 °C for 20 s. Melting curve analyses were then carried out. Relative transcription was normalized to the housekeeping genes SAND or TIP41 (Czechowski et al., 2005). Primers were designed using PerlPrimer v1.1.21 (Marshall, 2004). The primers used were CalS1_FP, AAGAGCGGAGGGTCACTTTG; CalS1_RP, GGCGACACGAATAGACGGAT; CalS12_FP, TTCACTCCGTTTTCCCGAGG; and CalS12_RP, GGAGAGAGACGCATCTGAGC.
Analysis of plant hormones
Plant hormones were extracted from 100 mg of frozen tissues and their concentrations were determined as previously described (Dobrev and Vankova, 2012; Dobrev and Kaminek, 2002) after the addition of appropriate internal standards. Hormone analysis was carried out on four samples, each taken from three plants. Briefly, samples were homogenized in tubes with 1.3-mm silica beads using a FastPrep-24 instrument (MP Biomedicals). Samples were then extracted with a methanol/H2O/formic acid (15:4:1, by vol.) mixture, which was supplemented with stable isotope-labelled phytohormone internal standards (10 pmol per sample) in order to check recovery during purification and to validate the quantification. The clarified supernatants were subjected to solid phase extraction using Oasis MCX cartridges (Waters Co., USA). The eluates were evaporated to dryness and the generated solids were dissolved in 30 µL of 15 % (v/v) acetonitrile in water. Hormones were separated and quantified by Ultimate 3000 high-performance liquid chromatography (Dionex, USA) coupled to a 3200 Q TRAP hybrid triple quadrupole/linear ion trap mass spectrometer (Applied Biosystems, USA) as described by Dobrev et al. (2017). Metabolite levels were expressed in pmol/g fresh weight (f. wt).
Statistical analysis
At least three independent biological replicates were performed for all experiments. Statistical analysis was conducted by paired t-test or ANOVA with Tukey honestly significant difference (HSD) multiple mean comparison post hoc test. The number of analysed samples was specified for each condition. The correlation matrix for hormonal levels was prepared using R-software Hmisc and corrplot packages based on the Pearson correlation (R Core Team, 2014).
RESULTS
The pi4kβ1β2 double mutant has altered phytohormonal levels
Our goal was to identify SA-dependent and SA-independent processes triggered by the double pi4kβ1β2 mutation. To do so, we used a sid2pi4kβ1β2 triple mutant. If a process triggered by the double pi4kβ1β2 mutation is SA-dependent, then it should disappear in the sid2pi4kβ1β2 triple mutant. On the other hand, if a process triggered by the double pi4kβ1β2 mutation is SA-independent, then it should still be observed in the sid2pi4kβ1β2 triple mutant.
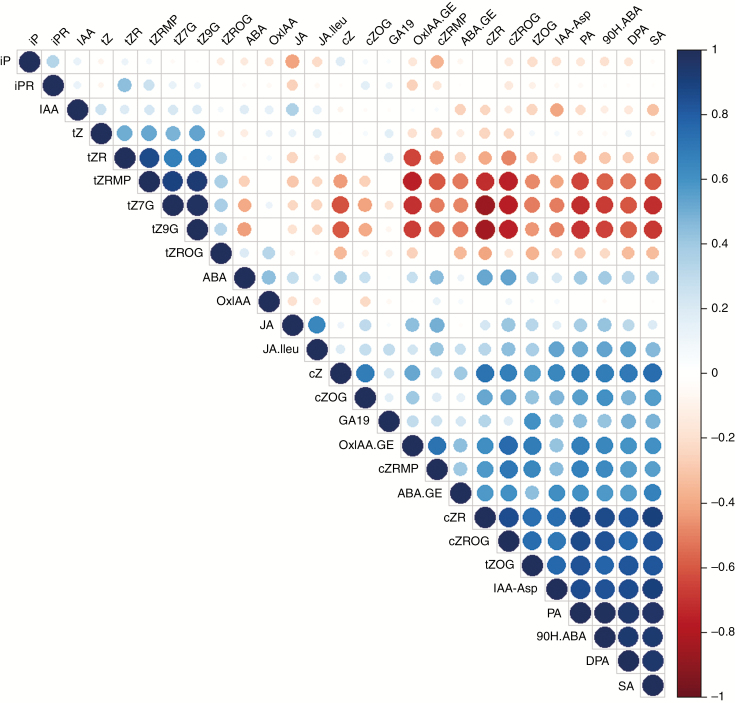
Because the main effect of the double pi4kβ1β2 mutation was on the level of SA, we decided to quantify a broad spectrum of phytohormones in the sid2pi4kβ1β2 triple mutant. The hormone levels obtained were compared to those of pi4k β1β2, sid2 and WT plants. A first look allowed us to establish that the pi4k β1β2 double mutation does not impact only SA levels. Many hormone-related metabolites showed significantly different levels in pi4k β1β2 plants when compared to WT plants while most of them remained at WT levels in sid2pi4kβ1β2 (Supplementary Data Table S1). In our previous study, we created an additional triple mutant by crossing pi4kβ1β2 with an NahG mutant impaired in SA accumulation: NahGpi4kβ1β2 (Sasek et al., 2014). To confirm and to strengthen the results obtained with sid2pi4kβ1β2, we also quantified phytohormones in NahGpi4kβ1β2, together with their corresponding single mutants (Table S1). From these data, we built a correlation matrix (Fig. 1). Many hormone levels were correlated to higher SA levels (Pearson correlation >0.7) as seen for the abscisic acid (ABA) derivatives such as 9-hydroxy-ABA (9OH-ABA), phaseic acid (PA) and dihydrophaseic acid (DPA). However, the high levels of DPA, PA and 9OH-ABA observed in pi4kβ1β2 vs. WT were no longer seen in the triple mutants with low SA; therefore, these metabolite levels were controlled by SA in the pi4kβ1β2 double mutant (Fig. S1A).
Fig. 1.
Correlation matrix between phytohormone levels. The matrix was built using the Pearson correlation of 27 hormone-related metabolites from 24 independent samples corresponding to six genotypes (WT, sid2, NahG, pi4kβ1β2, sid2pi4kβ1β2 and NahGpi4kβ1β2; four plants per genotype). Positive correlations are displayed in blue and negative correlations in red. Colour intensity and the size of the circles are proportional to the correlation coefficients. Red rectangles highlight the correlation between SA and other hormones.
Because the main genetic pathway of the SA response is controlled by NPR1, we investigated whether the hormonal control of SA was NPR1-dependent. This was achieved using a previously generated npr1pi4kβ1β2 triple mutant where the SA level was 30-fold that of the WT and 4-fold that of pi4kβ1β2. Interestingly, levels of DPA, PA and 9OH-ABA were still high in npr1pi4kβ1β2, showing that the effect of SA on these metabolites was only partially NPR1-dependent. Note that ABA levels did not correlate with SA (Pearson correlation 0.3), indicating that the action of SA on ABA derivatives is probably not directly connected to the biosynthesis of ABA, but with its metabolism.
Other hormones with high Pearson correlations to SA were the Asp conjugated form of indole-3-acetic acid (Asp-IAA) and some cytokinins. The increased level of Asp-IAA observed in pi4kβ1β2 was no longer observed in the triple mutants with low SA. However, it was still visible in the npr1pi4kβ1β2 triple mutant, suggesting that this metabolite is controlled by SA but it is only partially NPR1-independent (Supplementary Data Fig. S1B). The pattern of IAA was different from that of Asp-IAA, suggesting that SA control of Asp-IAA is on aspartate conjugation. As for cytokinins, the increase in cis-zeatin (cZ), cis-zeatin-riboside (cZR), cis-zeatin-7-N-glucoside (cZ7G), cis-zeatin-riboside-O-glucoside (cZROG) and trans-zeatin-O-glucoside (tZOG) and the decrease in trans-zeatin-7-N-glucoside (tZ7G) and trans-zeatin-9-N-glucoside (tZ9G) in the pi4kβ1β2 double mutant were SA-driven. In contrast to the other hormones tested, SA’s action on tZROG appeared to be partially independent of NPR1 (Fig. S1C).
We identified one hormone for which its level was altered in pi4kβ1β2 plants independently of SA: the increase in oxIAA-GE observed in pi4kβ1β2 was still visible in the triple mutants with low SA. Note that oxIAA did not follow the same pattern (Supplementary Data Fig. S2).
Pathogen resistance, of pi4kβ1β2 plants is SA-dependent
At the hormonal level, our data show that the major change induced by the pi4kβ1β2 double mutation was an increase in SA, with this change determining the levels of many other hormones. Because a major role of SA is related to biotic stress responses, we reasoned that processes related to biotic stress, dependent or not on SA, might also be altered in the double mutant.
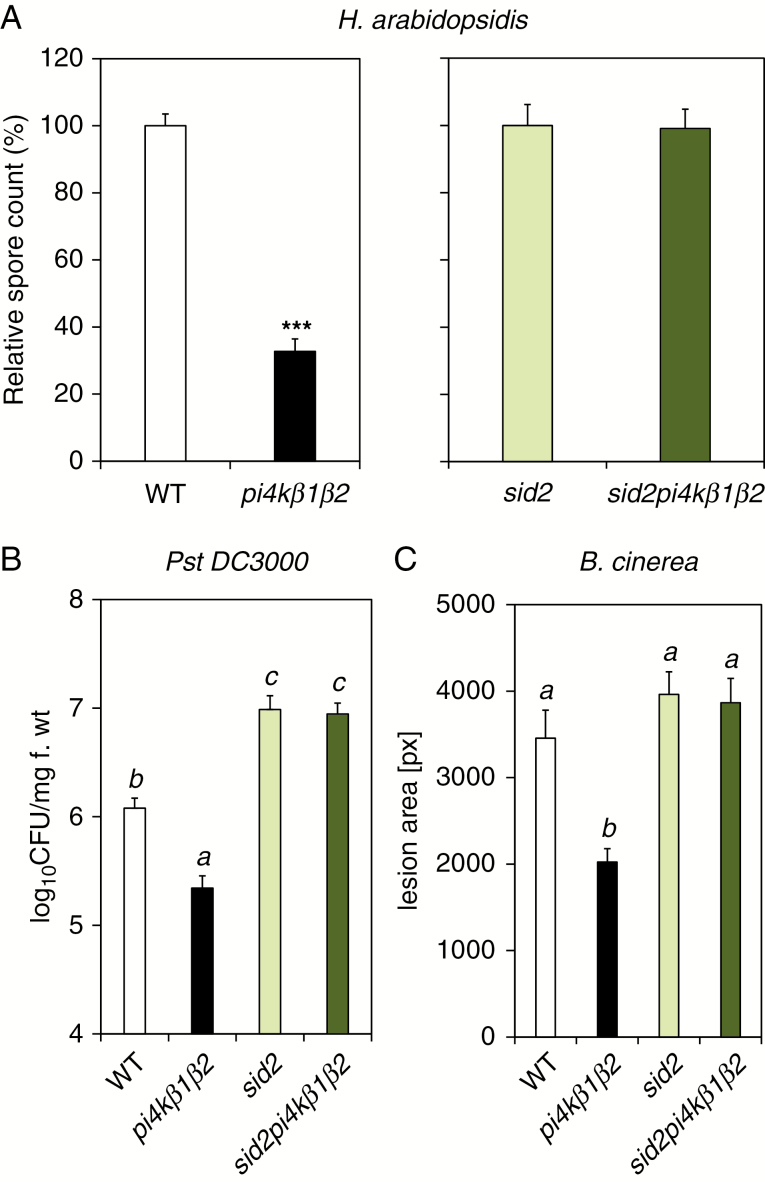
Whether the PI4K double mutation per se was accompanied by an enhanced resistance to pathogens was investigated. Comparing resistance in sid2pi4k β1β2 plants to that in pi4k β1β2 or sid2 would allow us to distinguish between the effectiveness of SA-dependent and SA-independent responses. Therefore, different pathogens with different lifestyles (biotrophs, hemibiotrophs and necrotrophs) were tested (Glazebrook, 2005). The pi4kβ1β2 double mutant plants were more resistant to the biotroph H. arabidopsidis NoCo2 compared to WT. However, sid2pi4kβ1β2 resistance was similar to that of sid2 plants (Fig. 2A). We then studied resistance to the hemibiotroph P. syringae pv. tomato DC3000 in its wild type (Pst DC3000) or AvrRpt2-expressing form (Pst DC3000 AvrRpt2). Pst DC3000 AvrRpt2 leads to a strong effector-triggered immunity (ETI) response compared to Pst DC3000. With both forms, pathogen development was reduced in pi4k β1β2 plants compared to the WT while sid2pi4kβ1β2 resistance was comparable to that of sid2 and lower than WT plants (Fig. 2B, Supplementary Data Fig. S3). Unexpectedly, the double mutant also showed an increased resistance to the necrotroph Botrytis cinerea which was also SA-dependent as sid2pi4kβ1β2 resistance was similar to that of sid2 and WT plants (Fig. 2C). For each pathogenic assay, triple mutant resistance was similar to sid2, indicating that SA-dependent pathways were dominant in the immune response. Putative mechanisms regulated by PI4K activity alone were not sufficient to establish pathogen resistance.
Fig. 2.
Resistance to biotic stresses of pi4kβ1β2 and sid2pi4kβ1β2 mutants. (A) Resistance to the biotroph Hyaloperonospora arabidopsidis. (B) Resistance to the hemibiotroph Pseudomonas syringae pv. syringae DC3000. Infiltration treatment of 4-week-old-plants, with six independent samples. (C) Resistance to the necrotroph Botrytis cinerea. Four- to 5-week-old A. thaliana were inoculated with a 6-µL drop containing spores of Botrytis cinerea (50 000 spores mL–1), placed into a plastic box and kept in the dark for 56 h. Statistical differences between the genotypes for B and C were assessed using ANOVA, with a Tukey honestly significant difference (HSD) multiple mean comparison post hoc test. Different letters indicate a significant difference, Tukey HSD, P < 0.05, n = 10 for A, n = 7 for B and n = 27 for C. ***Indicate difference from WT, t-test, P < 0.001.
The enhanced level of basal callose deposition in pi4kβ1β2 is mainly SA-dependent, while stress-induced callose accumulation is not
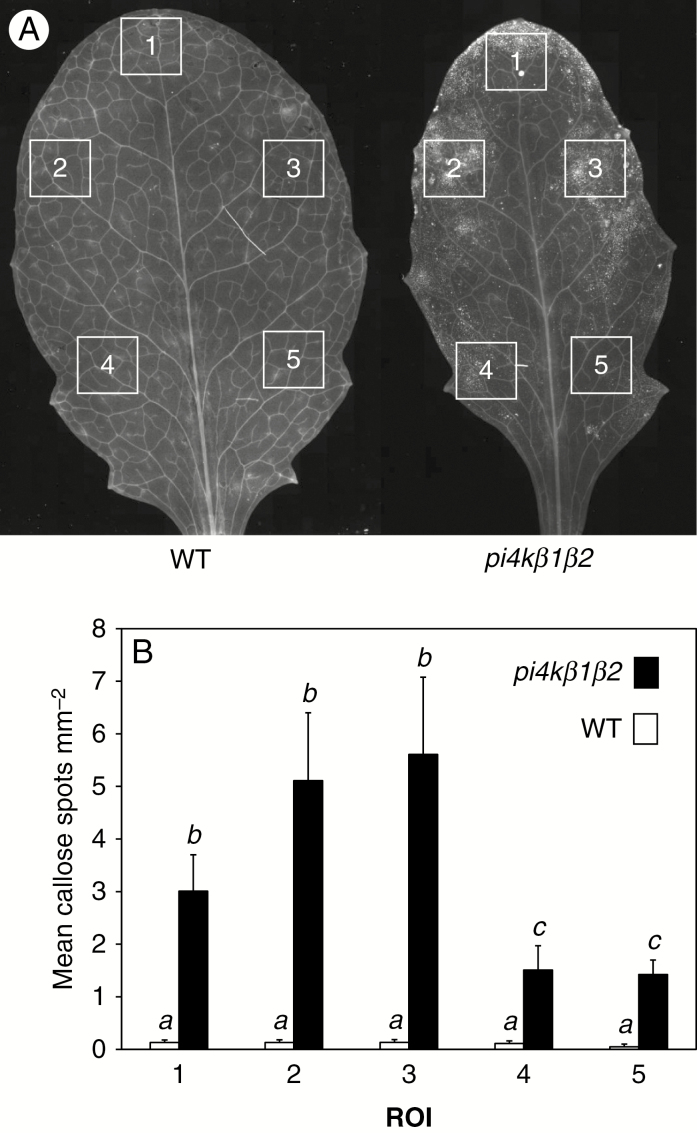
SA levels modulate numerous processes associated with immune responses, including the strengthening of leaf tissues and particularly cell walls around the infection site by lignification and callose accumulation (Voigt, 2014). Interestingly, SA pretreatment also has a positive effect on flagellin-induced callose accumulation (Yi et al., 2014). We therefore studied callose levels, accumulated in leaf tissues in response to treatment with the flagellin epitope (flg22). We first studied callose accumulation in the absence of flg22 treatment (control; Fig. 3A). The pi4kβ1β2 double mutant exhibited a constitutively high level of callose deposition, as previously shown (Antignani et al., 2015). We were able to show that a spatial pattern in callose accumulation existed, as the examination of different regions of interest (ROI) indicated a higher accumulation in the upper part of the leaf edges (Fig. 3B).
Fig. 3.
Pattern of callose accumulation in pi4kβ1β2 leaves. (A) Aniline blue staining, and fluorescence microscopy. Scale bar = 500 µm. (B) Callose particles accumulated in different ROI. The squares represent the ROI. Data are presented as means ±s.e.m. Statistical differences were assessed using a two-way ANOVA, with a Tukey honestly significant difference (HSD) multiple mean comparison post hoc test. Different letters indicate a significant difference, Tukey HSD, P < 0.05. n = 11.
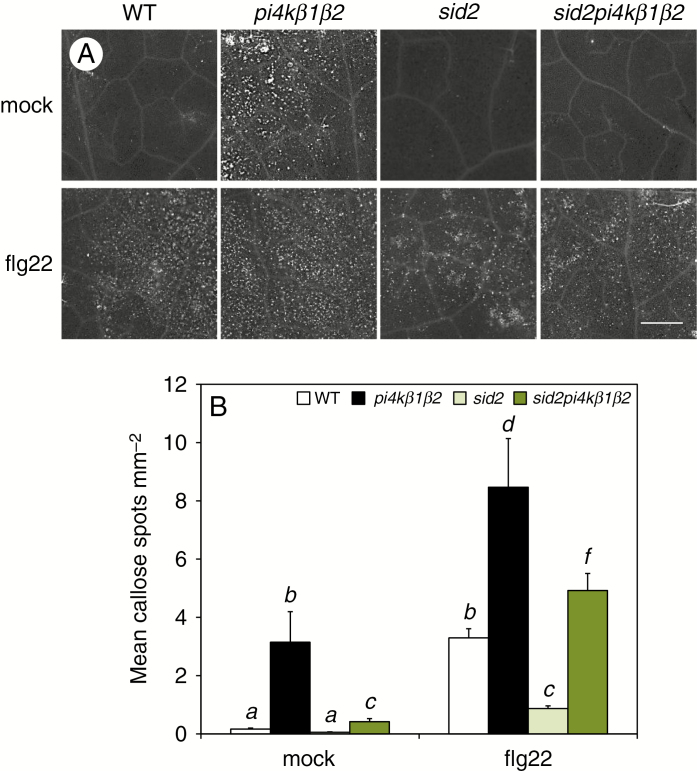
Callose accumulation was then assessed in either mock-infiltrated or flg22-treated plants (Fig. 4). In this case, callose accumulation was much higher in pi4kβ1β2 when compared to WT plants (Fig. 4). The sid2pi4kβ1β2 triple mutant was used to investigate whether high callose deposition in the double mutant depended on its high SA level. For mock treatments, callose deposition was much lower in sid2pi4kβ1β2 compared to pi4kβ1β2 plants. This provides arguments for an SA-dependent higher basal callose deposition. This was confirmed by the response to flg22. In WT plants, flg22 induced 20 times more callose compared to control mock infiltrations. Again, the callose level in pi4kβ1β2 was higher (two-fold) compared to WT plants. This increase was reduced when the sid2 mutation was introduced into the pi4kβ1β2 double mutant as the level in the triple mutant was about two-fold lower than in the double mutant and in the same range as the WT level. This indicated that SA was a major inducer of callose accumulation in the pi4kβ1β2 genotype context. Yet, the sid2pi4kβ1β2 triple mutant exhibited a higher callose deposition than sid2 plants. Therefore, the pi4kβ1β2 double mutation per se had a role in the high callose accumulation observed in the pi4kβ1β2 double mutant.
Fig. 4.
Callose deposition in response to flagellin. (A) Representative images of callose accumulated in leaves of 4-week-old A. thaliana plants by aniline blue staining, 24 h after infiltration with 0.1 µm flg22 or mock infiltration. Scale bar = 1cm. (B) Quantification of callose particles. Values represent an average of five ROI. Data are presented as means +s.d. For each treatment, statistical differences between the genotypes were assessed using a one-way ANOVA, with a Tukey honestly significant difference (HSD) multiple mean comparison post hoc test. Different letters indicate a significant difference, Tukey HSD, P < 0.01, n = 4.
We also studied callose accumulation in leaf tissues in response to mechanical wounding (Supplementary Data Fig. S4). Again, in response to wounding, the PI4K double mutation enhanced callose accumulation via an SA-dependent pathway.
Whether callose overaccumulation correlated with the transcription of callose synthases (CalSs) was then investigated. Among 12 callose synthases, CalS1 and CalS12 have been shown to be related to SA and/or biotic stresses (Dong et al., 2008). The transcript levels of CalS1 and CalS12 were tested by qPCR in WT, pi4kβ1β2, sid2 and sid2pi4kβ1β2 plants treated or not with flg22. No correlation was observed between CalS1 and CalS12 transcript levels and callose accumulation (Supplementary Data Fig. S5).
pi4kβ1β2 has altered non-host resistance that is SA-independent
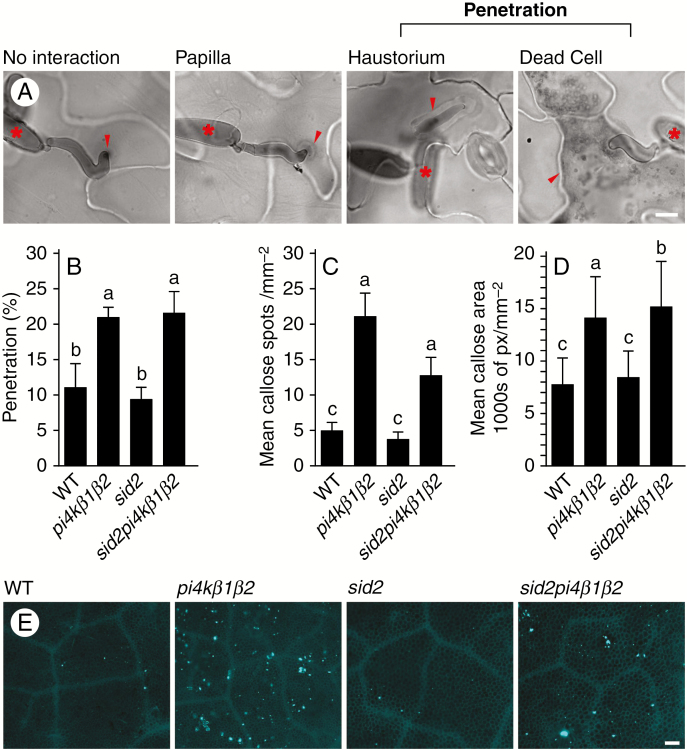
The establishment of non-host resistance is based on different mechanisms, involving vesicular secretion as well as callose accumulation (Collins et al., 2003; Assaad et al., 2004; Takemoto et al., 2006; Böhlenius et al., 2010; Lee et al., 2017). To study the role of PI4K in such responses, we tested penetration success and callose production in response to the non-host pathogen Blumeria graminis f. sp. hordei (Bgh). In Bgh/A. thaliana interactions, callose accumulates in defensive papillae and haustorial encasements or around dead cells (Jacobs et al., 2003; Assaad et al., 2004; Ellinger et al., 2014). The enhanced number of plant cells with developed haustoria or dead cells reflects the penetration success of fungal hyphae (Fig. 5A). In our experiments a higher penetration correlated with greater callose accumulation in the plant tissue. In particular, the pi4kβ1β2 double mutant showed an enhanced successful penetration of Bgh 24 hpi, as seen by the enhanced number of haustoria and dead cells. A similar defect in penetration resistance was seen in sid2/pi4kβ1β2, indicating the SA-independent character of this phenomenon (Fig. 5B). Both pi4kβ1β2 and sid2/pi4kβ1β2 accumulated more callose (Fig. 5C) and over larger areas compared to WT plants (Fig. 5C, D). Thus, the lower penetration resistance accompanied by callose accumulation in pi4kβ1β2 was independent of the SA pathway.
Fig. 5.
Resistance of pi4kβ1β2 to penetration by the non-host pathogen Blumeria graminis f. sp. hordei (Bgh). (A) Four types of interactions counted in the penetration success analysis after trypan blue staining. Scale bar = 5 µm. (B) Data showing penetration success of Bgh 24 hpi in each genotype: the mean number of cells with either haustoria or dead cells, respectively. (C) Data showing mean area of a callose spot per mm2 at 24 hpi after interaction with Bgh spores. (D) Data showing mean area of a callose spot per mm2 at 24 hpi after interaction with Bgh spores. Data for B, C and D were processed by ANOVA, with a Tukey honestly significant difference (HSD) multiple mean comparison post hoc test, the data represent one independent experiment, and the experiment was repeated four times. Letters indicate a significant difference, Tukey HSD, P < 0.01. (E) Pictures demonstrating callose staining with aniline blue 24 hpi with Bgh. Scale bar = 100 µm.
DISCUSSION
The aim of this study was to investigate SA-dependent and SA-independent processes caused by the pi4k β1β2 double mutation. As previously shown (Sasek et al., 2014), this mutant accumulates a constitutively high level of SA. In pi4k β1β2, SA biosynthesis is dependent on ICS1, as demonstrated by an absence of SA accumulation in the sid2pi4kβ1β2 triple mutant with impaired ICS1 transcription (Sasek et al., 2014). This was confirmed with the hormone analysis described in the present study. The sid2pi4kβ1β2 triple mutant lacking high SA was used as a tool to distinguish between SA-dependent and SA-independent effects caused by the double mutation in pi4kβ1β2.
We first measured phytohormonal levels in fully developed leaves of 4-week-old A. thaliana WT, pi4kβ1β2, sid2 and sid2pi4kβ1β2 plants. To our knowledge, this is the broadest phytohormonal study carried out with an SA over-accumulating mutant (in our case pi4kβ1β2) and its comparison with a plant having the same background but with an impaired SA pathway (sid2pi4kβ1β2). The level of 15 hormone derivatives (excluding SA) was altered in pi4k β1β2 compared to WT leaves. For 13 of these, this was SA-dependent. We identified two metabolites, cis-zeatin-riboside-5’-monophosphate and glucosylesters of oxindole-3-acetic acid, for which the pi4k β1β2 double mutation effect was SA-independent. The same conclusions were also reached with another triple mutant, NahGpi4kβ1β2. Amongst the hormones controlled by SA were ABA derivatives such as DPA, PA and 9OH-ABA. As ABA levels did not correlate with SA, the action of SA on ABA derivatives did not appear to act on ABA biosynthesis but on its metabolism. Such an effect of SA on ABA catabolism is poorly described. A slight induction of ABA 8′-hydroxylase expression was observed after 24 h of SA treatment of rice seedlings (Mega et al., 2015). In an A. thaliana cpr22 mutant (constitutive expressor of PR genes 22), the increase of SA and ABA levels due to a high to low humidity shift was also followed by the SA-dependent expression of genes encoding ABA-metabolizing enzymes (Mosher et al., 2010).
Other hormones displaying a high correlation to SA were Asp-IAA and some cytokinins (SA positively controlled cZROG and tZOG and negatively controlled tZ7G and tZ9G). The pattern of IAA was different from that of Asp-IAA, suggesting that SA control of Asp-IAA was on aspartate conjugation. Aspartate conjugation is catalysed by GH3.2-GH3.6 (Normanly, 2010), with our transcriptome data obtained with in vitro grown pi4kβ1β2 seedlings (Sasek et al., 2014) indicating that GH3.3 (At2g23170) was overexpressed in the double mutant. It would be interesting to investigate whether it was responsible for the Asp-IAA /SA correlation in our double mutant.
Our results demonstrate the major role of hormonal cross-talk between SA and other hormones but only a minor role of impairment of PI4Kβ1/β2 per se. Because a major role of SA is related to responses to biotic stresses, we reasoned that other processes related to biotic stress, whether SA-dependent or not, could also be altered in the double mutant. We tested the resistance of WT, pi4kβ1β2, sid2 and sid2pi4kβ1β2 plants to representative biotrophic (oomycete H. arabidopsidis NoCo2), hemibiotrophic (bacteria Pst DC3000) and necrotrophic (fungus Botrytis cinerea) pathogens. The results clearly showed that resistance to these pathogens was dependent on a high SA content. Resistance to H. arabidopsidis NoCo2 and Pst DC3000 was perhaps not surprising as resistance to such pathogens is generally associated with SA signalling (Glazebrook, 2005). On the other hand, the role of SA in regulating resistance to necrotrophs is rather uncommon. Indeed, plant defence against necrotrophs is commonly associated with jasmonic acid signalling (Ferrari et al., 2003; Glazebrook, 2005). However, Ferrari et al. (2003) showed that resistance to B. cinerea could be dependent on high SA levels, in accordance with our observations. A similar finding was reported for defence response to the necrotroph Sclerotinia sclerotiorum (Novakova et al., 2014). Moreover, we tested A. thaliana ETI by using a bacterial strain highly expressing the AvrRpt2 effector (Pst DC3000 AvrRpt2). An ETI response can induce the expression of genes commonly associated with SA, such as PR-1, in an SA-independent manner in A. thaliana (Tsuda et al., 2013). Yet, we found that the higher resistance of pi4kβ1β2 plants to Pst DC3000 AvrRpt2 was SA-dependent. In conclusion, the higher resistance of the pi4kβ1β2 mutant to all the host pathogens assayed was strongly SA-dependent.
Non-host resistance, efficient against non-adapted pathogens, does not rely fully on the SA pathway. Here we show that pi4kβ1β2 exhibited an SA-independent defective resistance towards penetration of the non-host pathogen Bgh.
Callose is a linear polysaccharide (1,3-β-glucan) occurring in plant cells where it is important for many plant physiological processes such as cytokinesis (Chen and Kim, 2009). Callose accumulation is triggered in response to pathogens and is used as a common test of pathogen-triggered immunity upon treatment with typical pathogen-associated molecular patterns such as flg22, the epitope of flagellin (Luna et al., 2011). In mock inoculated pi4k β1β2, callose deposition was greater than in WT leaves, thus confirming the findings of Antignani et al. (2015). Interestingly, this was also true for the sid2pi4kβ1β2 triple mutant when compared to sid2 plants, thus indicating an SA-independent phenomenon. Following inoculation with flg22, an increase in callose deposition was observed in all genotypes tested, but this was still higher in pi4kβ1β2 compared to WT leaves, and callose deposition was higher in sid2pi4kβ1β2 with respect to sid2 plants. Therefore, it seems that the pi4kβ1β2 double mutation per se enabled higher callose deposition under biotic stress conditions.
The biosynthesis of callose occurs outside of the cell (Ellinger and Voigt, 2014). Accumulation can be regulated at different levels: transcriptional, translational, or during enzyme transport to the plasma membrane and out of the cell via vesicular trafficking. Phosphorylation and direct translocation of callose synthase is crucial in the regulation of biosynthesis, whereas transcriptional control might have only a minor role (Ellinger and Voigt, 2014). Our data on the transcription levels of CALS1 and CALS12 indicate that in pi4kβ1β2 a transcriptional effect is not involved in the observed over-accumulation of callose. So how is it possible to explain the action on callose of the pi4kβ1β2 double mutation per se? A number of reports indicate that PI4Ks can impact trafficking. In A. thaliana, PI4Kβ1 was shown to be recruited by the GTP-bound Rab4b GTPAse. Both RabA4b and PI4Kβ1 localize to budding secretory vesicles in the trans-Golgi network (TGN) and to secretory vesicles en route to the cell surface. A pi4kβ1β2 double mutant produces secretory vesicles of highly variable sizes (Preuss et al., 2006; Kang et al., 2011; Antignani et al., 2015). The product of PI4K activity, PI4P massively accumulates at the plasma membrane and at early endosomes/TGN and Golgi (Platre and Jaillais, 2016; Noack and Jaillais, 2017). Therefore, PI4Kβ1/β2 are important in vesicle trafficking. Interestingly, inhibiting PI4K with phenylarsine oxide (PAO) suppressed the salt-induced endocytosis of plasma membrane intrinsic protein 2;1 (Ueda et al., 2016). Similarly, inhibiting PI4K led to the internalization of CELLULOSE SYNTHASE3 from the plasma membrane (Fujimoto et al., 2015). Can the impact of PI4K betas on trafficking explain the increased callose accumulation? Callose biosynthesis and accumulation have been shown to be affected by vesicle trafficking (Ellinger and Voigt, 2014). PI4Ks have been shown to play an important role in cytokinesis (Lin et al., 2019), especially in the correct organization of the vesicles at the cell division pla β1β2ne and further formation of a cell plate. During phragmoplast formation, PI4Kβ1 probably interacts with MPK4, a member of the MAP65 protein family that regulates microtubule organization (Lin et al.,, 2019). Callose is also essential for cytokinesis (Thiele et al., 2009). We can therefore only speculate whether the effects of PI4Ks on callose, cytokinesis and trafficking are interconnected. Interestingly, the role of SA in these processes has not been tested. Because PI4Kβ1/β2 can impact the secretory pathway, they could also impact the translocation of callose synthases. Furthermore, PMR4 (CALS12) binds to small RabA4c GTPase at the TGN and PI4Kβ1 binds to RabA4b GTPase, the most similar small GTPase to RabA4c, at the TGN (Böhlenius et al., 2010). Note that the impact of PI4K betas on trafficking could also explain our non-host resistance data. The syp121 mutant altered in a SNARE protein involved in trafficking has been reported to accumulate SA and also display defective non-host resistance (Collins et al.,, 2003).
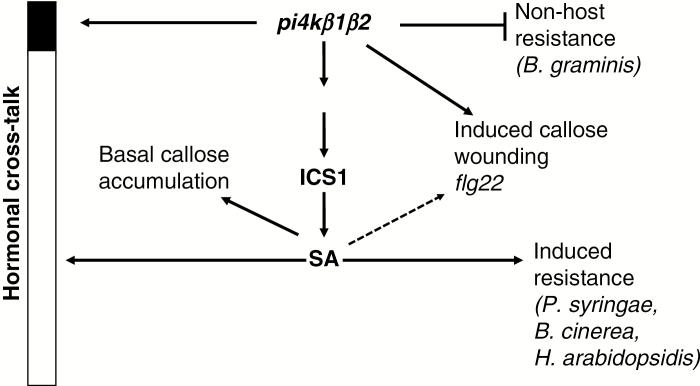
In conclusion (Fig. 6), the pi4kβ1β2 double mutant constitutively accumulated a high SA level via ICS1/SID2 and this had considerable impact on other hormone levels and was associated with an increased resistance to several plant pathogens (P. syringae, H. arabidopsidis, Botrytis cinerea). The pi4kβ1β2 double mutation also affected pathogen-related processes in a high SA-independent manner as seen by differences in callose accumulation in response to flg22, to Bgh infection, to wounding, and the higher penetration success of Bgh. The identification of such processes directly affected by the mutation on PI4Ks will now allow us to better investigate the role of these enzymes, in relation to signalling or trafficking events.
Fig. 6.
Schematic representation of the effects of the pi4kβ1β2 double mutation on Arabidopsis plants.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: Phytohormone levels in 4-week-old plants. Fig. S1: Levels of hormones controlled by SA in 4-week-old mutant and WT plants. Fig. S2; Levels of hormones not controlled by SA in 4-week-old mutant and WT plants. Fig. S3: Resistance to P. syringae pv. tomato DC3000 AvrRpt2. Fig. S4: Callose deposition in response to wounding. Fig. S5: Relative transcription of some CalS genes in untreated rosette leaves or 24 h after infiltration with 0.1 µm flg22.
FUNDING
This work was supported by the Czech Science Foundation [grant number 17-05151S]. Collaboration between the Ukrainian and French teams was partially supported by a ‘Projet international de coopération scientifique’ from the ‘Centre National de la Recherche Scientifique’ (2015–2018). T.K. received the Visegrad scholarship (2016–2017) [grant number 51600349]; she also benefited from the Program of Postdoctoral Fellowships of the Czech Academy of Sciences [grant number TK 919220]. This study was also supported by a European Regional Development Fund-Project ‘Centre for Experimental Plant Biology’ [grant number CZ.02.1.01/0.0/0.0/16_019/0000738].
ACKNOWLEDGEMENTS
We thank Lucie Lamparová and Romana Pospíchalová for their technical support and Michael Hodges for language editing.
LITERATURE CITED
- Antignani V, Klocko AL, Bak G, Chandrasekaran SD, Dunivin T, Nielsen E. 2015. Recruitment of PLANT U-BOX13 and the PI4Kbeta1/beta2 phosphatidylinositol-4 kinases by the small GTPase RabA4B plays important roles during salicylic acid-mediated plant defense signaling in Arabidopsis. Plant Cell 27: 243–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaad FF, Qiu JL, Youngs H, et al. . 2004. The PEN1 syntaxin defines a novel cellular compartment upon fungal attack and is required for the timely assembly of papillae. Molecular Biology of the Cell 15: 5118–5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balla T. 2007. Imaging and manipulating phosphoinositides in living cells. Journal of Physiology 582: 927–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhlenius H, Mørch SM, Godfrey D, Nielsen ME, Thordal-Christensen H. 2010. The multivesicular body-localized GTPase ARFA1b/1c is important for callose deposition and ROR2 syntaxin-dependent preinvasive basal defense in barley. The Plant Cell 22: 3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Glazebrook J, Clarke JD, Volko S, Dong X. 1997. The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 88: 57–63. [DOI] [PubMed] [Google Scholar]
- Collins NC, Thordal-Christensen H, Lipka V, et al. . 2003. SNARE-protein-mediated disease resistance at the plant cell wall. Nature 425: 973–977. [DOI] [PubMed] [Google Scholar]
- Cui H, Gobbato E, Kracher B, Qiu J, Bautor J, Parker JE. 2017. A core function of EDS1 with PAD4 is to protect the salicylic acid defense sector in Arabidopsis immunity. New Phytologist 213: 1802–1817. [DOI] [PubMed] [Google Scholar]
- Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR. 2005. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiology 139: 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delage E, Ruelland E, Guillas I, Zachowski A, Puyaubert J. 2012. Arabidopsis type-III phosphatidylinositol 4-kinases beta1 and beta2 are upstream of the phospholipase C pathway triggered by cold exposure. Plant and Cell Physiology 53: 565–576. [DOI] [PubMed] [Google Scholar]
- Delaney TP, Uknes S, Vernooij B, et al. . 1994. A central role of salicylic Acid in plant disease resistance. Science 266: 1247–1250. [DOI] [PubMed] [Google Scholar]
- Dempsey DMA, Vlot AC, Wildermuth MC, Klessig DF. 2011. Salicylic Acid biosynthesis and metabolism. Arabidopsis Book 9: e0156–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrev PI, Hoyerova K, Petrasek J. 2017. Analytical determination of auxins and cytokinins. Methods in Molecular Biology 1569: 31–39. [DOI] [PubMed] [Google Scholar]
- Dobrev PI, Kaminek M. 2002. Fast and efficient separation of cytokinins from auxin and abscisic acid and their purification using mixed-mode solid-phase extraction. Journal of Chromatography A 950: 21–29. [DOI] [PubMed] [Google Scholar]
- Dobrev PI, Vankova R. 2012. Quantification of abscisic acid, cytokinin, and auxin content in salt-stressed plant tissues. Methods in Molecular Biology 913: 251–261. [DOI] [PubMed] [Google Scholar]
- Dong X, Hong Z, Chatterjee J, Kim S, Verma DP. 2008. Expression of callose synthase genes and its connection with Npr1 signaling pathway during pathogen infection. Planta 229: 87–98. [DOI] [PubMed] [Google Scholar]
- Ellinger D, Glockner A, Koch J, et al. . 2014. Interaction of the Arabidopsis GTPase RabA4c with its effector PMR4 results in complete penetration resistance to powdery mildew. Plant Cell 26: 3185–3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinger D, Voigt CA. 2014. Callose biosynthesis in Arabidopsis with a focus on pathogen response: what we have learned within the last decade. Annals of Botany 114: 1349–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S, Plotnikova JM, De Lorenzo G, Ausubel FM. 2003. Arabidopsis local resistance to Botrytis cinerea involves salicylic acid and camalexin and requires EDS4 and PAD2, but not SID2, EDS5 or PAD4. Plant Journal 35: 193–205. [DOI] [PubMed] [Google Scholar]
- Fujimoto M, Suda Y, Vernhettes S, Nakano A, Ueda T. 2015. Phosphatidylinositol 3-kinase and 4-kinase have distinct roles in intracellular trafficking of cellulose synthase complexes in Arabidopsis thaliana. Plant and Cell Physiology 56: 287–298. [DOI] [PubMed] [Google Scholar]
- Glazebrook J. 2005. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annual Review of Phytopathology 43: 205–227. [DOI] [PubMed] [Google Scholar]
- Gross J, Cho WK, Lezhneva L, et al. . 2006. A plant locus essential for phylloquinone (vitamin K1) biosynthesis originated from a fusion of four eubacterial genes. Journal of Biological Chemistry 281: 17189–17196. [DOI] [PubMed] [Google Scholar]
- Chen XY, Kim JY. 2009. Callose synthesis in higher plants. Plant Signaling and Behaviour 4: 489–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs AK, Lipka V, Burton RA, et al. . 2003. An Arabidopsis callose synthase, GSL5, is required for wound and papillary callose formation. Plant Physiology 15: 2503–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda M, Sasek V, Ruelland E. 2014. The Arabidopsis pi4kIIIbeta1beta2 double mutant is salicylic acid-overaccumulating: a new example of salicylic acid influence on plant stature. Plant Signaling and Behaviour 9: e977210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda M, Ruelland E. 2015. Magical mystery tour: salicylic acid signalling. Environmental and Experimental Botany 114: 117–128. [Google Scholar]
- Kalachova T, Puga-Freitas R, Kravets V, et al. . 2016. The inhibition of basal phosphoinositide-dependent phospholipase C activity in Arabidopsis suspension cells by abscisic or salicylic acid acts as a signalling hub accounting for an important overlap in transcriptome remodelling induced by these hormones. Environmental and Experimental Botany 123: 37–49. [Google Scholar]
- Kang BH, Nielsen E, Preuss ML, Mastronarde D, Staehelin LA. 2011. Electron tomography of RabA4b- and PI-4Kbeta1-labeled trans Golgi network compartments in Arabidopsis. Traffic 12: 313–329. [DOI] [PubMed] [Google Scholar]
- Katagiri F, Thilmony R, He SY. 2002. The Arabidopsis thaliana-pseudomonas syringae interaction. Arabidopsis Book 1: e0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler A, Schwindling S, Conrath U. 2002. Benzothiadiazole-induced priming for potentiated responses to pathogen infection, wounding, and infiltration of water into leaves requires the NPR1/NIM1 gene in Arabidopsis. Plant Physiology 128: 1046–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krinke O, Ruelland E, Valentová O, et al. . 2007. Phosphatidylinositol 4-Kinase activation is an early response to salicylic acid in Arabidopsis suspension cells. Plant Physiology 144: 1347–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krinke O, Flemr M, Vergnolle C, et al. . 2009. Phospholipase D activation is an early component of the salicylic acid signaling pathway in Arabidopsis cell suspensions. Plant Physiology 150: 424–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H-A, Lee H-Y, Seo E, et al. . 2017. Current understandings of plant Nonhost Resistance. Molecular Plant–Microbe Interactions 30: 5–15. [DOI] [PubMed] [Google Scholar]
- Lin F, Krishnamoorthy P, Schubert V, Hause G, Heilmann M, Heilmann I. 2019. A dual role for cell plate-associated PI4Kβ in endocytosis and phragmoplast dynamics during plant somatic cytokinesis. The EMBO Journal 38: e100303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna E, Pastor V, Robert J, Flors V, Mauch-Mani B, Ton J. 2011. Callose deposition: a multifaceted plant defense response. Molecular Plant–Microbe Interactions 24: 183–193. [DOI] [PubMed] [Google Scholar]
- Marshall OJ. 2004. PerlPrimer: cross-platform, graphical primer design for standard, bisulphite and real-time PCR. Bioinformatics 20: 2471–2472. [DOI] [PubMed] [Google Scholar]
- Mega R, Meguro-Maoka A, Endo A, et al. . 2015. Sustained low abscisic acid levels increase seedling vigor under cold stress in rice (Oryza sativa L.). Scientific Reports 5: 13819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher S, Moeder W, Nishimura N, et al. . 2010. The lesion-mimic mutant cpr22 shows alterations in abscisic acid signaling and abscisic acid insensitivity in a salicylic acid-dependent manner. Plant Physiology 152: 1901–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi S, Catt KJ, Balla T. 1995. A wortmannin-sensitive phosphatidylinositol 4-kinase that regulates hormone-sensitive pools of inositolphospholipids. Proceedings of the National Academy of Sciences of the USA 92: 5317–5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noack LC, Jaillais Y. 2017. Precision targeting by phosphoinositides: how PIs direct endomembrane trafficking in plants. Current Opinion in Plant Biology 40: 22–33. [DOI] [PubMed] [Google Scholar]
- Normanly J. 2010. Approaching cellular and molecular resolution of auxin biosynthesis and metabolism. Cold Spring Harbor Perspectives in Biology 2: a001594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novakova M, Sasek V, Dobrev PI, Valentova O, Burketova L. 2014. Plant hormones in defense response of Brassica napus to Sclerotinia sclerotiorum - reassessing the role of salicylic acid in the interaction with a necrotroph. Plant Physiology and Biochemistry 80: 308–317. [DOI] [PubMed] [Google Scholar]
- Pappan K, Austin-Brown S, Chapman KD, Wang X. 1998. Substrate selectivities and lipid modulation of plant phospholipase D alpha, -beta, and -gamma. Archives of Biochemistry and Biophysics 353: 131–140. [DOI] [PubMed] [Google Scholar]
- Platre MP, Jaillais Y. 2016. Guidelines for the use of protein domains in acidic phospholipid imaging. Methods in Molecular Biology 1376: 175–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss ML, Schmitz AJ, Thole JM, Bonner HK, Otegui MS, Nielsen E. 2006. A role for the RabA4b effector protein PI-4Kbeta1 in polarized expansion of root hair cells in Arabidopsis thaliana. Journal of Cell Biology 172: 991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team 2014. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Sasek V, Janda M, Delage E, et al. . 2014. Constitutive salicylic acid accumulation in pi4kIIIbeta1beta2 Arabidopsis plants stunts rosette but not root growth. New Phytologist 203: 805–816. [DOI] [PubMed] [Google Scholar]
- Seyfferth C, Tsuda K. 2014. Salicylic acid signal transduction: the initiation of biosynthesis, perception and transcriptional reprogramming. Frontiers in Plant Science 5: 697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto D, Jones DA, Hardham AR. 2006. Re‐organization of the cytoskeleton and endoplasmic reticulum in the Arabidopsis pen1‐1 mutant inoculated with the non‐adapted powdery mildew pathogen, Blumeria graminis f. sp. hordei. Molecular Plant Pathology 7: 553–563. [DOI] [PubMed] [Google Scholar]
- Thiele K, Wanner G, Kindzierski V, et al. . 2009. The timely deposition of callose is essential for cytokinesis in Arabidopsis. The Plant Journal 58: 13–26. [DOI] [PubMed] [Google Scholar]
- Tsuda K, Mine A, Bethke G, et al. . 2013. Dual regulation of gene expression mediated by extended MAPK activation and salicylic acid contributes to robust innate immunity in Arabidopsis thaliana. PLoS Genetics 9: e1004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda M, Tsutsumi N, Fujimoto M. 2016. Salt stress induces internalization of plasma membrane aquaporin into the vacuole in Arabidopsis thaliana. Biochemical and Biophysical Research Communications 474: 742–746. [DOI] [PubMed] [Google Scholar]
- Vlot AC, Dempsey DA, Klessig DF. 2009. Salicylic acid, a multifaceted hormone to combat disease. Annual Review of Phytopathology 47: 177–206. [DOI] [PubMed] [Google Scholar]
- Vogel J, Somerville S. 2000. Isolation and characterization of powdery mildew-resistant Arabidopsis mutants. Proceedings of the National Acadademy of Sciences USA 97: 1897–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt CA. 2014. Callose-mediated resistance to pathogenic intruders in plant defense-related papillae. Frontiers in Plant Science 5: 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S, Stuttmann J, Rietz S, et al. . 2013. Structural basis for signaling by exclusive EDS1 heteromeric complexes with SAG101 or PAD4 in plant innate immunity. Cell Host & Microbe 14: 619–630. [DOI] [PubMed] [Google Scholar]
- Wildermuth MC, Dewdney J, Wu G, Ausubel FM. 2001. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414: 562–565. [DOI] [PubMed] [Google Scholar]
- Yi SY, Shirasu K, Moon JS, Lee SG, Kwon SY. 2014. The activated SA and JA signaling pathways have an influence on flg22-triggered oxidative burst and callose deposition. PLoS One 9: e88951. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.