Abstract
The Gremlin-2 (GREM2) plays crucial roles in modulating bone homeostasis through the bone morphogenetic protein-2 pathway. However, GREM2 gene variants in osteoporosis were less frequent in a Chinese population. Therefore, the present study recruited 310 patients with osteoporosis and 339 healthy postmenopausal women to assess the correlation of GREM2 gene polymorphisms with the risk of osteoporosis. Polymerase chain reaction (PCR) and Sanger sequencing were utilized to genotype samples. The results showed that GREM2 gene rs4454537, not rs11588607, polymorphism was significantly associated with an increased risk of osteoporosis in postmenopausal women. Moreover, stratified analyses indicated a significant association between rs4454537 polymorphisms and body mass index of <25 kg/m2. Additionally, the association between GREM2 rs4454537 polymorphism and clinical characteristics was assessed, which showed that this locus decreased the bone mineral density (BMD) in postmenopausal osteoporotic individuals. Furthermore, individuals with CC genotype appeared to have a higher GREM2 expression compared with those bearing the TT genotype of rs4454537 polymorphism. However, the genotype distribution of rs4454537 polymorphism showed no statistical difference between osteoporotic patients as a function of fracture status. In summary, GREM2 rs4454537 polymorphism decreases BMD and increases osteoporotic risk in postmenopausal women.
Keywords: Case–control study, GREM2, osteoporosis, postmenopausal women
Introduction
Osteoporosis is a systemic condition wherein patients exhibit low bone mass and microstructural degeneration of osseous tissue, resulting in increased bone fragility and fracture susceptibility [1]. The mean prevalence of osteoporosis in older adults was about 15.7% and it is reported to be 202.43 million people in China [2]. This condition is most common among women, who account for >80% of cases, with incidence being particularly high in women after menopause [3,4]. Many factors, including loss of bone mineral density (BMD), vitamin D deficiencies, estrogen deficiency, environmental factors, and several genetic factors can drive postmenopausal osteoporosis [5]. Cheung et al. found that Gremlin-2 (GREM2) regulated osteoblasts differentiation and osteogenesis [6,7] and its polymorphism was related to low BMD [8].
GREM2 is a key bone morphogenetic protein (BMP) antagonist [9]. GREM2, as a BMP antagonist, competes with the BMP receptor to regulate the activity of BMP ligands [10,11]. BMP activity changes within fully developed bone are linked to osteoporosis, osteoarthritis, and decreased bone fracture healing capacity [12]. Additionally, previous studies have shown that GREM2 is regulated by the Wnt/β-catenin signaling pathway; it participates in osteoblast differentiation and is involved in osteogenesis [6,7]. Thus, it is believed that GREM2 might be a prominent candidate gene for susceptibility to osteoporosis.
GREM2 gene variants have been studied in various diseases [13,14], but rarely in osteoporosis. Kaminski et al. reported no significant association between GREM2 rs4454537 polymorphism and osteoporosis risk in Polish postmenopausal women [15]. This finding has not been confirmed in a Chinese population. Furthermore, GREM2 rs11588607 and rs4454537 polymorphisms were related to a low femoral neck BMD and whole hip BMD, respectively, and rs4454537 was successfully replicated in an independent cohort [8]. The accelerated loss of hip BMD could contribute to osteoporotic fracture [16]. Therefore, this case–control study was designed as a means of exploring how GREM2 gene polymorphisms impact osteoporosis susceptibility, BMD levels, and fracture incidence.
Materials and methods
Subjects
Totally, we recruited 649 unrelated Chinese postmenopausal women from the First Affiliated Hospital of Soochow University, including 310 osteoporosis patients and 339 healthy postmenopausal women. Osteoporosis was diagnosed based upon World Health Organization criteria. The inclusion criteria consisted of an age range of 40–90 years, reaching the menopause at least one year ago, no hormone replacement therapy, or drugs affecting bone mass. Patients with an unbalanced thyroidal condition, kidney failure, malabsorption syndrome, and active neoplastic disease were not included in the present study. During a year of follow-up, 181 of 310 osteoporosis patients were found to have fractured. Among the 181 fracture cases, 54 (29.8%) were lumbar spine compression fractures, 36 (19.9%) were intertrochanteric fractures, 36 (19.9%) were distal radius fractures, 22 (12.2%) were thoracic compression fractures and 33 (18.2%) were ankle fractures. The control group consisted of 339 postmenopausal women with the same age range in the same region. The subjects were interviewed using a standard questionnaire to collect data on demographics, BMI, age at menopause, and medical history. All the subjects submitted informed consent forms before enrollment. The study protocol was confirmed by the Ethics Committee of the First Affiliated Hospital of Soochow University and performed according to the guidelines of the Helsinki Declaration.
BMD measurement
Two radiologists assessed total hip BMD and BMD at the lumbar spine (L2–L4), with these radiologists being blinded to the other medical data. A dual-energy X-ray absorptiometer (Lunar Co., WI, U.S.A.) was used for these measurements. The coefficients of variation of the BMD values were 0.2%. BMDs were recorded as g/cm2 and a percentage of peak bone mass in the controls (T-score). Based on these measurements, the subjects were classified into the following categories: osteopenic (Y score between -2.5 and -1), osteoporotic (T-score under -2.5), and healthy groups (T-score over -1).
Blood sampling and genotyping
A 2-ml peripheral blood sample was collected from all the participants, and genomic DNA was extracted from the whole blood with a TIANamp blood DNA kit (Tiangen Biotech, Beijing, China) based on provided directions, followed by storage at −20°C for further tests. Genotyping of GREM2 rs11588607 and rs4454537 polymorphisms was conducted via polymerase chain reaction (PCR) and Sanger sequencing techniques. The specific primers were rs4454537: 5′-TCTGTATTGGGCTGTTGT-3′ (F) and 5′-CTGCTTAATTTGGTGGGT-3′ (R); rs11588607: 5′-CTTTAGGTTTGGGTTGAT-3′ (F) and 5′-TAGCCTTGCTTTACTTCT-3′ (R). The PCR products were sent to Genscript. Inc (Nanjing, Jiangsu, China) for Sanger sequencing procedure. Approximately 10% of the samples of all the subjects underwent repeated genotyping, and the genotypes were 100% concordant.
Real-time PCR analysis
Total RNA was extracted from whole blood using the Trizol reagent (Invitrogen). Real-time PCR was performed after reverse transcription with a Roche LC 480 system with a QuantiTect SYBR Green RT-PCR kit (Qiagen, Hilden, Germany). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was included as the internal control. Data were analyzed by Ct (2−ΔΔCt) method and expressed as the fold change compared with GAPDH. Primers were used for RT-PCR were as follows: GREM2, 5′-AGAGTGACTGGTGCAAGACG-3′ (forward) and 5′-TGATTCGGAAAGGTGGGTCG-3′ (reverse); and GAPDH, 5′-GTTCCAATATGATTCCACCC-3′ (forward) and 5′- AGGGATGATGTTCTGGAGAG-3′(reverse).
Statistical analyses
All the data were tested on Statistical Product and Service Solutions (SPSS) 22.0 (IBM, Chicago, U.S.A.). The Student’s t-test or chi-squared (χ2) test were used for comparing demographic and clinical differences between the cases and control groups. A χ2 analysis was used to evaluate whether the genotype distribution of healthy controls conformed to the Hardy–Weinberg equilibrium (HWE). Stratified analyses were conducted in terms of drinking, smoking, BMI, and age. The correlation between GREM2 gene polymorphisms and the risk of osteoporosis was assessed via logistic regression by calculating the odds ratios (ORs) and 95% confidence intervals (CI). P < 0.05 indicated a significant difference.
Results
Characteristics of the study population
The details of all the subjects are summarized in Table 1. The patients and controls were 62.77 and 62.79 years of age on average, respectively. No significant inter-group differences were identified in age, BMI, and serum phosphorus levels. In addition, the distributions of smokers and drinkers in the case group were comparable to those in the control group, whereas serum calcium, L2-L4 BMD, and T-scores were significantly lower in the osteoporotic patients versus controls (P<0.05).
Table 1. The general characteristics of study participants.
| Variable | Cases (n=310) | Controls (n=339) | P |
|---|---|---|---|
| Age (years) | 62.77 ± 8.42 | 62.79 ± 8.30 | 0.980 |
| BMI (kg/m2) | 24.40 ± 1.44 | 24.26 ± 1.52 | 0.230 |
| Serum calcium (mg/dl) | 9.31 ± 0.65 | 9.55 ± 0.57 | <0.001 |
| Serum phosphorus (mg/dl) | 4.55 ± 2.76 | 4.32 ± 3.01 | 0.327 |
| Smoking (%) | 25 (8.1%) | 23 (6.8%) | 0.702 |
| Drinking (%) | 61 (19.7%) | 58 (17.1%) | 0.406 |
| Fracture (%) | 181 (58.4%) | ||
| BMD L2-L4 (g/cm3) | 0.73 ± 0.06 | 1.01 ± 0.06 | <0.001 |
| T-score | −3.19 ± 0.39 | 0.12 ± 0.10 | <0.001 |
Abbreviations: BMD, bone mineral density; BMI, body mass index.
The analysis of GREM2 gene variants
Table 2 presents the genotypes and allele distributions of GREM2 rs11588607 and rs4454537 polymorphisms. The HWE testing revealed no obvious deviation in genotypic frequency in the controls, indicating that these subjects were representatives of the local population. GREM2 rs11588607 polymorphism exhibited negative correlation with the risk of postmenopausal osteoporosis under five models. Logistic regression analyses showed that CC genotype of rs4454537 polymorphism markedly elevated the risk of osteoporosis (CC vs. TT: OR = 1.85, 95% CI: 1.01–3.38, P=0.047) (Table 2). Furthermore, the C allele of rs4454537 polymorphism was correlated with a higher risk of osteoporosis (C vs. T: OR = 1.29, 95% CI: 1.01–1.65, P=0.041) (Table 2). Subgroup analyses were conducted in terms of age, BMI, smoking, and drinking status. No significant association was observed with the risk of osteoporosis in any subgroup for rs11588607. However, there was a significantly higher risk of osteoporosis in BMI < 25 kg/m2 (Table 3).
Table 2. The association of genotype and allele of GREM2 rs11588607/rs4454537 polymorphism with osteoporosis risk.
| Genotype | Genotypes and alleles | Frequencies, N(%) | OR (95% CI) | P | |
|---|---|---|---|---|---|
| Cases (n=310) | Controls (n=339) | ||||
| Rs11588607 | |||||
| CC | 145 (46.8%) | 169 (49.9%) | 1.0 | ||
| CT | 133 (42.9%) | 143 (42.2%) | 1.08 (0.78,1.50) | 0.626 | |
| TT | 32 (10.3%) | 27 (8.0%) | 1.38 (0.79,2.41) | 0.257 | |
| TT+CT | 165 (53.2%) | 170 (50.1%) | 1.13 (0.83,1.54) | 0.433 | |
| CC+TT | 278 (89.7%) | 312 (92.0%) | 1.0 | ||
| TT | 32 (10.3%) | 27 (8.0%) | 1.33 (0.78,2.28) | 0.298 | |
| C allele | 423 (68.2%) | 481 (70.9%) | 1.0 | ||
| T allele | 197 (31.8%) | 197 (29.1%) | 1.14 (0.90,1.44) | 0.288 | |
| Rs4454537 | |||||
| TT | 156 (50.3%) | 192 (56.6%) | 1.0 | ||
| TC | 124 (40.0%) | 127 (37.5%) | 1.20 (0.87,1.66) | 0.268 | |
| CC | 30 (9.7%) | 20 (5.9%) | 1.85 (1.01,3.38) | 0.047 | |
| CC+TC | 154 (49.7%) | 147 (43.4%) | 1.29 (0.95,1.76) | 0.107 | |
| TC+TT | 280 (90.3%) | 319 (94.1%) | 1.0 | ||
| CC | 30 (9.7%) | 20 (5.9%) | 1.71 (0.95,3.08) | 0.074 | |
| T allele | 436 (70.3%) | 511 (75.4%) | 1.0 | ||
| C allele | 184 (29.7%) | 167 (24.6%) | 1.29 (1.01,1.65) | 0.041 | |
Bold values are statistically significant (P<0.05).
Table 3. Stratified analyses between rs11588607/rs4454537 polymorphisms and the risk of osteoporosis.
| Variable | Case/Control | Heterozygous | Homozygous | Recessive | Dominant | ||
|---|---|---|---|---|---|---|---|
| CC | CT | TT | CT vs. CC | TT vs. CC | TT vs. CT+CC | TT+CT vs. CC | |
| rs11588607 | |||||||
| Age | |||||||
| <60 years | 55/65 | 49/60 | 13/10 | 0.94 (0.55,1.60); 0.815 | 1.40 (0.57,3.44); 0.470 | 1.44 (0.61,3.42); 0.412 | 1.01 (0.61,1.67); 0.976 |
| ≥60 years | 90/104 | 84/83 | 19/17 | 1.18 (0.78,1.78); 0.428 | 1.37 (0.67,2.78); 0.390 | 1.27 (0.64,2.51); 0.500 | 1.21 (0.82,1.79); 0.337 |
| BMI | |||||||
| <25 kg/m2 | 96/117 | 87/98 | 24/20 | 1.08 (0.73,1.61); 0.696 | 1.46 (0.76,2.81); 0.253 | 1.41 (0.75,2.64); 0.282 | 1.15 (0.79,1.67); 0.474 |
| ≥25 kg/m2 | 49/52 | 46/45 | 8/7 | 1.09 (0.62,1.91); 0.778 | 1.21 (0.41,3.60); 0.728 | 1.17 (0.41,3.34); 0.774 | 1.10 (0.64,1.90); 0.727 |
| Smoking | |||||||
| No | 131/155 | 124/137 | 30/24 | 1.07 (0.77,1.50); 0.690 | 1.48 (0.82,2.65); 0.190 | 1.43 (0.82,2.51); 0.212 | 1.13 (0.82,1.56); 0.450 |
| Yes | 14/14 | 9/6 | 2/3 | 1.50 (0.42,5.35); 0.532 | 0.67 (0.10,4.62); 0.682 | 0.58 (0.09,3.83); 0.571 | 1.22 (0.39,3.86); 0.733 |
| Drinking | |||||||
| No | 117/143 | 105/115 | 27/23 | 1.12 (0.78,1.60); 0.551 | 1.44 (0.78,2.63); 0.244 | 1.36 (0.76,2.45); 0.298 | 1.17 (0.83,1.65); 0.370 |
| Yes | 28/26 | 28/28 | 5/4 | 0.93 (0.44,1.96); 0.846 | 1.16 (0.28,4.80); 0.837 | 1.21 (0.31,4.73); 0.789 | 0.96 (0.47,1.97); 0.906 |
| Variable | Case/Control | Heterozygous | Homozygous | Recessive | Dominant | ||
|---|---|---|---|---|---|---|---|
| TT | TC | CC | TC vs. TT | CC vs. TT | CC vs. TC+TT | CC+TC vs. TT | |
| rs4454537 | |||||||
| Age | |||||||
| <60 years | 55/77 | 50/51 | 10/7 | 1.39 (0.82,2.36); 0.221 | 2.13 (0.73,6.22); 0.165 | 1.85 (0.65,5.27); 0.247 | 1.48 (0.89,2.46); 0.132 |
| ≥60 years | 99/115 | 74/76 | 20/13 | 1.10 (0.73,1.66); 0.657 | 1.72 (0.83,3.58); 0.148 | 1.65 (0.81,3.37); 0.168 | 1.19 (0.81,1.76); 0.384 |
| BMI | |||||||
| <25 kg/m2 | 99/135 | 89/86 | 19/14 | 1.41 (0.95,2.09); 0.087 | 1.85 (0.89,3.87); 0.102 | 1.60 (0.78,3.27); 0.202 | 1.47 (1.01,2.15); 0.044 |
| ≥25 kg/m2 | 57/57 | 35/41 | 11/6 | 0.85 (0.48,1.53); 0.594 | 1.83 (0.64,5.29); 0.263 | 1.95 (0.69,5.50); 0.205 | 0.98 (0.57,1.69); 0.939 |
| Smoking | |||||||
| No | 145/182 | 113/115 | 27/19 | 1.23 (0.88,1.73); 0.226 | 1.78 (0.95,3.34); 0.070 | 1.64 (0.89,3.01); 0.114 | 1.31 (0.95,1.81); 0.099 |
| Yes | 11/10 | 11/12 | 3/1 | 0.83 (0.26,2.72); 0.763 | 2.73 (0.24,30.66); 0.416 | 3.00 (0.29,31.11); 0.357 | 0.98 (0.31,3.07); 0.971 |
| Drinking | |||||||
| No | 129/160 | 100/108 | 20/13 | 1.15 (0.80,1.64); 0.448 | 1.91 (0.91,3.98); 0.085 | 1.80 (0.88,3.70); 0.110 | 1.23 (0.87,1.73); 0.237 |
| Yes | 27/32 | 24/19 | 10/7 | 1.50 (0.68,3.30); 0.317 | 1.69 (0.57,5.05); 0.345 | 1.43 (0.50,4.05); 0.502 | 1.55 (0.75,3.19); 0.235 |
BMI, body mass index
Bold values are statistically significant (P<0.05).
Next, the clinical and biochemical characteristics of GREM2 rs4454537 polymorphism were compared between the osteoporotic patients and the healthy controls (Table 4). There were no significant differences between the three genotypes of rs4454537 polymorphism in terms of age, BMI, serum calcium and phosphorus levels, and T-scores. However, for osteoporosis, the L2-L4 BMD of the CC genotype was significantly lower than the TT genotype, indicating that GREM2 rs4454537 polymorphism showed a significant correlation with L2-L4 BMD.
Table 4. The clinical and biochemical characteristics of GREM2 rs4454537 polymorphism among two groups.
| Variables | TT | CT | CC | P |
|---|---|---|---|---|
| Osteoporosis | ||||
| Age (years) | 63.09 ± 8.55 | 62.18 ± 8.07 | 63.57 ± 9.30 | 0.576 |
| BMI (kg/m2) | 24.54 ± 1.44 | 24.20 ± 1.35 | 24.50 ± 1.78 | 0.127 |
| Serum calcium (mg/dL) | 9.32 ± 0.66 | 9.30 ± 0.65 | 9.29 ± 0.56 | 0.954 |
| Serum phosphorus (mg/dL) | 4.73 ± 3.42 | 4.44 ± 1.96 | 4.06 ± 1.51 | 0.407 |
| BMD L2-L4 (g/cm2) | 0.74 ± 0.067 | 0.72 ± 0.063 | 0.72 ± 0.055 | 0.012 |
| T-score | -3.14 ± 0.37 | -3.25 ± 0.39 | -3.17 ± 0.43 | 0.051 |
| Control | ||||
| Age (years) | 62.81 ± 8.58 | 62.42 ± 7.86 | 64.90 ± 8.33 | 0.462 |
| BMI (kg/m2) | 24.22 ± 1.51 | 24.29 ± 1.54 | 24.42 ± 1.57 | 0.806 |
| Serum calcium (mg/dL) | 9.58 ± 0.56 | 9.53 ± 0.59 | 9.41 ± 0.44 | 0.383 |
| Serum phosphorus (mg/dL) | 4.56 ± 3.29 | 3.99 ± 1.98 | 4.18 ± 5.05 | 0.254 |
| BMD L2-L4 (g/cm2) | 1.01 ± 0.055 | 1.01 ± 0.055 | 1.00 ± 0.069 | 0.566 |
| T-score | 0.10 ± 0.01 | 0.10 ± 0.10 | 0.09 ± 0.01 | 0.688 |
Bold values are statistically significant (P<0.05).
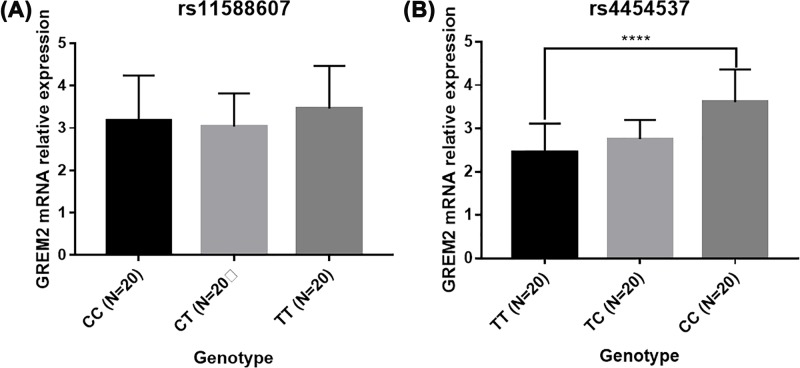
GREM2 mRNA expression in different genotype
Furthermore, we evaluated the effect of GREM2 gene polymorphisms on the GREM2 expression. For rs11588607, there is no significant difference among three genotypes with regard to the GREM2 levels (Figure 1A). However, our results indicated that the up-regulation of GREM2 were observed in CC genotype than those in TT genotype of rs4454537 polymorphism (Figure 1B).
Figure 1. GREM2 mRNA expression in different genotype.
(A) rs11588607; (B) rs4454537. ****P<0.05.
Association of GREM2 rs4454537 polymorphism with fracture risk
The present study also investigated whether GREM2 rs4454537 polymorphism was linked to the risk of osteoporotic fracture since this polymorphism conferred susceptibility to osteoporosis (Table 5). The genotype distribution of rs4454537 polymorphism was statistically comparable between osteoporotic patients with and without fracture, indicating no statistical correlation with fracture.
Table 5. The genotype and allele frequency distribution of rs4454537 polymorphism in postmenopausal osteoporosis patients with and without fracture.
| Genotypes and alleles | Frequencies, N (%) | OR (95% CI) | P | |
|---|---|---|---|---|
| Without fracture (n=129) | With fracture (n=181) | |||
| TT | 68 (52.7%) | 88 (48.6%) | 1.0 | |
| TC | 45 (34.9%) | 79 (43.6%) | 1.36 (0.84,2.20) | 0.216 |
| CC | 16 (12.4%) | 14 (7.7%) | 0.68 (0.31,1.48) | 0.326 |
| CC+TC | 61 (47.3%) | 93 (51.4%) | 1.18 (0.75,1.85) | 0.477 |
| TC+TT | 113 (87.6%) | 167 (92.3%) | 1.0 | |
| CC | 16 (12.4%) | 14 (7.7%) | 0.59 (0.28,1.26) | 0.171 |
| T allele | 181 (70.2%) | 255 (70.4%) | 1.0 | |
| C allele | 77 (29.8%) | 107 (29.6%) | 0.99 (0.70,1.40) | 0.939 |
Discussion
Our findings revealed that GREM2 rs4454537, not rs11588607, polymorphism increased osteoporosis risk in Chinese postmenopausal women, especially in subjects with BMI < 25 kg/m2. In addition, this polymorphism was linked to reduced BMD in those with osteoporosis. In contrast, the rs4454537 polymorphism allele and genotype distributions were comparable between cases and controls.
The BMP-2 pathway plays a vital role in positively modulating bone homeostasis. The suppression of BMP antagonist GREM2 increased the BMP-2-induced osteogenesis of human bone marrow-derived mesenchymal stem cells (BMSCs) [17]. GREM2 gene variants might influence the expression and function of GREM2. Several recent studies have addressed the relationship between GREM2 gene polymorphism and the risk of osteoporosis [8,15]. Cheung et al. were the first researchers to investigate the relationship between GREM2 rs11588607 and rs4454537 polymorphisms and areal BMD in a southern Chinese population with 417 cases and 359 controls [8]. They observed that GREM2 rs11588607 and rs4454537 were linked to a low BMD at the femoral neck, spine, and total hip [8].
Furthermore, the positive result of rs4454537 was repeated in an additional 454 cases and 401 controls [8]. Later, a Polish cohort with 333 osteoporosis patients and 233 healthy women exhibiting the genotype distribution of the GREM2 gene showed no significant difference between groups [15]. Herein, GREM2 rs4454537 polymorphism increased osteoporotic risk in the Chinese postmenopausal women evaluated, with 310 cases and 339 controls; however, this strong correlation did not apply to rs11588607 polymorphism. This significant association also appeared to be strong in people with BMI < 25 kg/m2. Furthermore, the mutant genotype of rs4454537 polymorphism showed a significant correlation with a decreased BMD, while it did not confer susceptibility to fracture.
To sum up, a significant association was found between GREM2 rs45454537 polymorphism and the risk of osteoporosis, which is different from the results of a study by Kaminski et al. Many factors might account for the contradictory results. First, different races and clinical heterogeneity might cause different genotype frequencies of rs4454537 polymorphism in the Polish [15] and Chinese populations. Second, different sample sizes should also be taken into consideration. Additionally, GREM2 rs4454537 polymorphism was found to be associated with a decreased BMD in Hong Kong [8] and mainland China (the present study) populations. In addition, the role of this polymorphism in the fracture risk was investigated, which is different from the results of a study by Cheung [8]. Although GREM2 rs4454537 polymorphism decreased BMD, it was not associated with the risk of fracture. We could not rule out the possibility of false negative results due to the limited sample size.
The present study has certain limitations. First, the study was hospital-based; therefore, selection bias was inevitable. Second, the gene–environment interactions were not investigated in the present study, including gene–diet and gene–physical activity variables. Three, the medium sample size might make the study underpowered. Fourth, the GREM2 expression levels in the three genotypes of rs4454537 polymorphism were not measured, which was a serious limitation.
It was concluded that GREM2 rs4454537 polymorphism decreases the BMD and increases osteoporotic risk among postmenopausal women. Studies of other Chinese populations are urgently needed to explore the relationship between this polymorphism and postmenopausal susceptibility to osteoporosis.
Abbreviations
- BMD
bone mineral density
- CI
confidence interval
- GREM2
Gremlin-2
- OR
odds ratio
- PCR
polymerase chain reaction
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported in part by grants from the National Natural Science Foundation of China [grant number 81702190 (to Feng Cai)]; the Natural Science Foundation of Jiangsu Province [grant number BK20170370 (to Feng Cai)]; the Graduate Student Scientific Research Innovation Projects of Jiangsu Province [grant number KYCX18_0176 (to Lei Zhu)].
Author Contribution
Conceived and designed the experiments: Feng Cai and Liang Chen. Performed the experiments: Yu Feng, Lei Zhu, Ling-Jun Wang and Bing-Jie Niu. Analyzed the data: Yu Feng and Yong Gu. Contributed reagents/materials/analysis tools: Bing-Jie Niu. Wrote the paper: Yu Feng and Lei Zhu.
References
- 1.(1993) Consensus Development Conference on Osteoporosis. Hong Kong, April 1-2, 1993. Am. J. Med. 95, 1S–78S [PubMed] [Google Scholar]
- 2.Lin X. et al. (2015) Epidemiology and management of osteoporosis in the People's Republic of China: current perspectives. Clin. Interv. Aging. 10, 1017–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Daghri N.M. et al. (2014) Inflammatory and bone turnover markers in relation to PTH and vitamin D status among Saudi postmenopausal women with and without osteoporosis. Int. J. Clin. Exp. Med. 7, 2812–2819 [PMC free article] [PubMed] [Google Scholar]
- 4.Oishi Y. et al. (2012) The IL6 gene polymorphism -634C>G and IL17F gene polymorphism 7488T>C influence bone mineral density in young and elderly Japanese women. Gene 504, 75–83 [DOI] [PubMed] [Google Scholar]
- 5.Musumeci M. et al. (2009) Genetic and environmental factors in human osteoporosis from Sub-Saharan to Mediterranean areas. J. Bone Miner. Metab. 27, 424–434 10.1007/s00774-009-0041-2 [DOI] [PubMed] [Google Scholar]
- 6.Suzuki D. et al. (2012) BMP2 differentially regulates the expression of Gremlin1 and Gremlin2, the negative regulators of BMP function, during osteoblast differentiation. Calcif. Tissue Int. 91, 88–96 10.1007/s00223-012-9614-5 [DOI] [PubMed] [Google Scholar]
- 7.Ideno H. et al. (2009) Protein related to DAN and cerberus (PRDC) inhibits osteoblastic differentiation and its suppression promotes osteogenesis in vitro. Exp. Cell Res. 315, 474–484 10.1016/j.yexcr.2008.11.019 [DOI] [PubMed] [Google Scholar]
- 8.Cheung C.L. et al. (2013) Genetic variants in GREM2 are associated with bone mineral density in a southern Chinese population. J. Clin. Endocrinol. Metab. 98, E1557–E1561 10.1210/jc.2013-1983 [DOI] [PubMed] [Google Scholar]
- 9.Kantaputra P.N. et al. (2015) GREMLIN 2 Mutations and Dental Anomalies. J. Dent. Res. 94, 1646–1652 10.1177/0022034515608168 [DOI] [PubMed] [Google Scholar]
- 10.Mulloy B. and Rider C.C. (2015) The Bone Morphogenetic Proteins and Their Antagonists. Vitam. Horm. 99, 63–90 10.1016/bs.vh.2015.06.004 [DOI] [PubMed] [Google Scholar]
- 11.Gustafson B. et al. (2015) BMP4 and BMP Antagonists Regulate Human White and Beige Adipogenesis. Diabetes 64, 1670–1681 10.2337/db14-1127 [DOI] [PubMed] [Google Scholar]
- 12.Rosen V. (2009) BMP2 signaling in bone development and repair. Cytokine Growth Factor Rev. 20, 475–480 10.1016/j.cytogfr.2009.10.018 [DOI] [PubMed] [Google Scholar]
- 13.Velez J.I. et al. (2013) Pooling/bootstrap-based GWAS (pbGWAS) identifies new loci modifying the age of onset in PSEN1 p.Glu280Ala Alzheimer's disease. Mol. Psychiatry 18, 568–575 10.1038/mp.2012.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pei Y.F. et al. (2016) Genome-wide association meta-analyses identified 1q43 and 2q32.2 for hip Ward's triangle areal bone mineral density. Bone 91, 1–10 10.1016/j.bone.2016.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaminski A. et al. (2018) Association betweenGREM2 gene polymorphism with osteoporosis and osteopenia in postmenopausal women. Eur. J. Obstet. Gynecol. Reprod. Biol. 228, 238–242 10.1016/j.ejogrb.2018.07.009 [DOI] [PubMed] [Google Scholar]
- 16.Christiansen B.A. et al. (2018) Incident fracture is associated with a period of accelerated loss of hip BMD: the Study of Osteoporotic Fractures. Osteoporos. Int. 29, 2201–2209 10.1007/s00198-018-4606-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang C.L. et al. (2017) Gremlin2 Suppression Increases the BMP-2-Induced Osteogenesis of Human Bone Marrow-Derived Mesenchymal Stem Cells Via the BMP-2/Smad/Runx2 Signaling Pathway. J. Cell. Biochem. 118, 286–297 10.1002/jcb.25635 [DOI] [PubMed] [Google Scholar]