Abstract
Objectives
Vitamin B6 deficiency is associated with a wide spectrum of clinical syndromes. While vitamin B6 status is primarily assessed by measuring the biologically active form of the vitamin, pyridoxal 5-phosphate (PLP), concurrent measurement of the final metabolite 4-pyridoxic acid (PA) can provide additional information regarding supplement intake and hypophosphatasia. The aim of this study is to develop a simple method traceable to the NIST standard reference material 3950 for simultaneous detection of PLP and PA.
Design & methods
A one-step reverse phase HPLC method with fluorescence detection was developed by evaluating different derivatization conditions, the use of an internal standard and different calibration strategies. The assay analytical performance was evaluated.
Results
Pre-column derivatization with semicarbazide showed the best overall performance in terms of signal to noise ratio, retention time and peak shape when compared to pre- or post-column derivatization with chlorite, pre-column or in-mobile phase derivatization using sodium bisulfite. The final method provided an analytical measurement range from 7.8 to 350 nmol/L for PLP and 3.3–339 nmol/L for PA, total imprecision <15% and <5% for PLP and PA respectively. Calibration against the NIST standard produced measured values within 3% of NIST assigned PLP values. The use of 4-deoxypyridoxine as internal standard did not improve precision or accuracy when compared to calibration using 5-level external standards.
Conclusions
This method combines derivatization and protein precipitation in one step and is traceable to NIST standard reference material 3950. It is simple and reliable for routine evaluation of vitamin B6 nutrition status.
Keywords: Vitamin B6, Pyridoxal 5-phosphate, 4-Pyridoxic acid, Liquid chromatography, NIST traceable
1. Introduction
According to CDC’s second national report on biochemical indicators of diet and nutrition in the U.S. population, vitamin B6 is the nutrient with the highest prevalence of deficiency in the United States. It is estimated that 11% of the population aged 1 year and older is at risk for vitamin B6 deficiency (<20 nmol/L) [1,2]. Symptoms of vitamin B6 deficiency can include dermatitis, glossitis, depression, confusion, convulsions, and anemia. Inborn errors affecting vitamin B6 metabolism have been associated with vitamin B6-dependent epilepsy [3]. Additionally, vitamin B6 deficiency can lead to homocysteinemia due to the crucial roles of vitamin B6-dependent enzymes in converting homocysteine to cysteine. An inverse relationship between vitamin B6 levels and oxidative stress, inflammation, cardiovascular disease and cancer has also been reported, although these results remain debatable [4].
Vitamin B6 generically refers to a collective of six interconvertible 3-hydroxy-2-methylpyridine compounds: pyridoxine (PN), pyridoxamine (PM), and pyridoxal (PL) and their phosphorylated derivatives pyridoxine 5′-phosphate (PNP), pyridoxamine 5′-phosphate (PMP), and pyridoxal 5′-phosphate (PLP) [5]. Among these vitamers, PLP is the bioactive form and serves as cofactor in more than 160 different catalytic functions. Since most of the PLP-dependent enzymes are involved in amino acid biosynthesis and degradation, vitamin B6 plays an important role in the metabolism of neurotransmitters, such as dopamine, serotonin, glycine, glutamate, and γ-aminobutyric acid (GABA) [5]. In addition to its function as a cofactor, PLP has also been suggested as a scavenger of reactive oxygen species.
While serum or plasma PLP is considered the best single indicator of vitamin B6 status, 4-pyridoxic acid (the end product of vitamin B6 catabolism) can be useful for assessing recent intake. Vitamin B6 is usually measured in clinical laboratories by either high performance liquid chromatography (HPLC) with fluorometric detection or by LC-MS/MS [6]. Several inter-laboratory comparison studies have revealed a wide dispersion of results between methods and/or laboratories [6], suggesting that standardization would be helpful in promoting analytical accuracy. To address this, we have developed a simple HPLC method combining protein precipitation and derivatization into a single step to reduce intra-assay variation. Furthermore, we calibrated the assay standard curve using the vitamin B6 reference standard to make the assay traceable to the National Institute of Standards and Technology (NIST) standard reference material 3950 (NIST SRM 3950; certified concentrations for serum PLP and PA by the CDC HPLC method and LC-MS/MS method). Inter-method harmony is expected to be improved when more laboratories adopt a NIST SRM 3950 traceable calibration.
2. Materials and methods
2.1. Chemicals and reagents
Pyridoxal 5′-phosphate monohydrate, 4-Pyridoxic acid, semicarbazide hydrochloride and glycine were purchased from Sigma-Aldrich (St Louis, MO, USA). NIST SRM 3950 (vitamin B6 in frozen human serum) was purchased from NIST (Gaithersburg, MD). Perchloric acid (70% solution in water), sodium phosphate dibasic anhydrous, methanol (HPLC grade), sodium hydroxide solution (50%, HPLC grade) and phosphoric acid (85%, HPLC grade) were purchased from Fisher Scientific (Hampton NH). Solutions were filtered through a 0.22-μm filter before use. Charcoal-stripped plasma was purchased from SeraCare Life Sciences (Milford, MA). Water was generated using a Millipore water system.
2.2. Preparation of calibrators and controls
Primary stock solutions of 1 mmol/L PLP and 1 mmol/L PA were prepared in pure water. These stock solutions were stored at −70 °C and stable for 1 year. The exact concentration of the 1 mmol/L PLP solution was determined using a spectrophotometer with molar absorption coefficient of 6550 L/mol per cm at 388 nm in 0.1 M NaOH. A combined PLP and 4-PA stock solution was then prepared by diluting the 1 mmol/L stocks with 0.1 M HCL to a final concentration of 30 μmol/L. This solution was then further diluted using charcoal-stripped plasma to prepare 5 levels of calibrators ranging from 10 nmol/L to 400 nmol/L of PLP and PA. Calibrator stability was evaluated by testing aliquots of the working solution stored at −70C at various time points and comparing peak area of each calibrator to the initial values obtained when the calibrators were freshly prepared. Based on this criterion, calibrators are stable for 3 months at −70 °C.
2.3. Assign value for the initial lot of the calibrators using NIST SRM 3950
One unit of SRM3950 consists of two vials of frozen human serum representing two different concentration levels. The certified concentration values for each level are based on the agreement of results from the isotope dilution LC-MS/MS method at NIST and the HPLC/fluorescence detection method at the Centers for Disease Control and Prevention (CDC). To assign a value for the initial lot of the assay calibrators, the two levels of NIST SRM 3950 were processed together with the in-house calibrators. A two-point linear calibration curve was constructed using the peak areas of the two NIST reference standards and the assigned concentrations for PLP and PA. The concentration of the highest calibrator was obtained utilizing the curve. The concentration of the other four levels of calibrators were calculated using the values of the highest calibrator and dilution factor. The PLP and PA concentration in each new lot of the diluent for calibrator preparation (charcoal stripped plasma) was also determined using the NIST two-point calibration curve descripted above. The sample treatment procedure of the charcoal stripped plasma is the same as plasma samples.
Two level of plasma-based quality controls were also prepared for use in the study. The low control was prepared by spiking 12 nmol/L PLP and PA into charcoal-stripped plasma. The high control was prepared by pooling residual patient plasma (Lithium heparin) with normal or high concentration of PLP.
2.4. Sample preparation and analysis
A reaction mixture stock solution (in water) was prepared with 125 mg/mL semicarbazide hydrochloride and 125 mg/mL glycine. The solution was stable at room temperature for up to one week. A working B6 reaction mixture was prepared fresh on each day of use by slowly adding 70% perchloric acid to the semicarbazide/glycine stock solution at 1 to 1 ratio. When assaying, 500 μL of plasma samples, controls or calibrators were added into a 96-well plate containing 80 μL of B6 reaction mixture in each well. The plate was then vortexed at 1000 rpm for 5 min in dark at room temperature and then incubated in a refrigerator (2–8 °C) for 20 min. At the end of the incubation, the plate was centrifuged at 3,220 g (about 4000 rpm; 4 °C) for 20 min and then the generated sample supernatant was filtered using a filter plate (Agilent Captiva ND Plate 0.2 μm). Finally, 100 μL of filtrate was transferred into a 96-well glass-coated microplate containing 5 μL of 25% NaOH in each well. The plate was then loaded into the auto-sampler for injection.
2.5. Chromatographic conditions
A Thermal Vanquish UHPLC system equipped with fluorescence detector (Thermo Fisher Scientific) was used in the analysis. Separation was achieved at 25 °C using an Agilent Poroshell 120 EC-C18 column (3.0 × 50 mm, 2.7 μm; Part Number 699975-302). Mobile phase A was 25 mmol/L dibasic sodium phosphate (pH 7.0) and mobile phase B was methanol. Gradient steps were as follows: −2.5 to 0 min: equilibration with 95% A (5%B). 1.5 min: 7% B. 1.6 min: 10% B. 3.6 min: 15% B. 4.0 min: 80%B, 5.5 min: 80%B and then return to equilibration. Injection volume was 20 μL and flow rate was 0.6 mL/min. The fluorescence detector was set at an excitation wavelength of 367 nm and emission wavelength of 478 nm for PLP detection and then switched to 325/425 at 2.6 min for PA detection. Peak area was used for quantitation.
2.6. Evaluation of assay performance
Assay precision was evaluated using the two levels of QC samples. These two samples were assayed in duplicate with one replicate immediately follow the calibrators at the beginning of the run and the other replicate by the end of the run for a total of 15 days. Linearity was evaluated by diluting a plasma specimen spiked with high concentration of target analytes to at least 5 concentration levels spanning 3.3–350 nmol/L for PLP and 1.4–350 nmol/L for PA. All linearity samples were assayed in triplicate. Spike recovery at each concentration level was calculated; %Recovery = (observed concentration/spiked concentration) x100%. The lower limit of quantification (LLOQ) was defined as the lowest level where bias and imprecision were both below 15%. An inter-method comparison for PLP was performed against a reference laboratory using an unpublished HPLC method. The method comparison was performed using a total of 26 residual patient samples submitted for vitamin B6 testing. Specimen storage stability at room temperature, refrigerated and frozen (−20 °C) was evaluated using pooled patient samples with low and high concentration of vitamin B6. Stability of post-derivatized specimens was tested using both pretreated calibrators and patient specimens when stored in the autosampler at 6 °C. For all the stability studies, peak area was used for evaluation and % change of less than 15% than the original value was considered stable.
2.7. Statistical methods
Precision, method comparison, linear range and detection limits were calculated using EP Evaluator software (Release 10). Comparison between two groups was determined using t-test and P values < 0.05 were regarded as statistically significant.
3. Results and discussion
3.1. Selection of derivatization method
Although PA has a natural fluorescence which can be readily detected using fluorescence detector of a HPLC system, PLP almost always needs a chemical derivatization step to enhance fluorescence to detectable level (Rybak 2004). To develop an assay capable of detecting both PLP and PA in a single reaction, we evaluated the impact of a variety of derivatization chemicals (chlorite, bisulfite and semicarbazide) and methods (pre-column, online in mobile phase, or post-column) on PLP derivatization. We compared the performance of various derivatization methods using a high calibrator containing 360 nmol/L PLP, along with the Poroshell 120 EC-C18 column for analysis. As shown in Table 1, pre-column derivatization with sodium bisulfite give the highest signal-to-noise ratio. However, the retention time of the PLP-derivative formed with bisulfite was very short and difficult to distinguish from the diluent front, and adjustments to mobile phase and HPLC conditions were not able to improve the retention time for this approach. In contrast, pre-column derivatization with semicarbazide also generated a high signal-to-noise ratio with a more acceptable retention time on the column (1.8 min). The remaining derivatization approaches had either poorer S/N ratios or difficult retention time (Table 1). Based on these data we chose semicarbazide derivatization as the starting point for further assay optimization. It should be noted that the PLP S/N ratio from the derivatization methods mentioned above were based on the 25 mmol/L dibasic sodium phosphate (pH 7.0) mobile and Poroshell 120 EC-C18 column. Those values might vary depending on column or mobile phase used.
Table 1.
Comparison of different derivatization methods for PLP.
| Derivatization method | Excitation/emission wavelength (nm) | Signal to noise ratio | Retention time (minute) |
|---|---|---|---|
| Post-column with chlorite | 325/425 | 14.2 | 5.1 |
| Pre-column with chlorite | 325/425 | 112.6 | 3.5 |
| Pre-column with semicarbazide | 380/450 | 608.2 | 1.8 |
| In mobile phase with sodium bisulfite | 300/400 | 458.7 | 0.6 |
| Pre-column with sodium bisulfite | 300/400 | 1937.6 | 0.5 |
Different derivatization method for PLP (366 nmol/L) was compared using an Agilent Poroshell 120 EC-C18 column with 25 mmol/L dibasic sodium phosphate (pH 7.0) and methanol as mobile phase.
3.2. Selection of matrix for calibrator solution
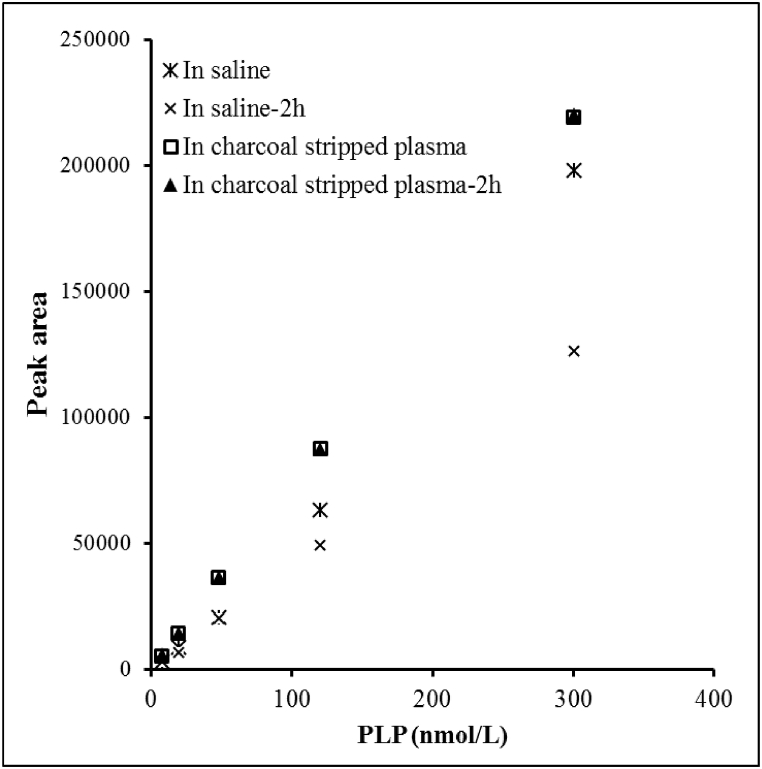
Finding a suitable matrix for calibrator preparation was challenging because pooled plasma or serum samples usually contain detectable amounts of vitamin B6. We evaluated 3% BSA solution, saline, and charcoal-stripped plasma as candidate matrices for preparing calibrators. Surprisingly, PLP was detectable at a fairly high level (22.5 nmol/L) in a 3% BSA solution prepared using commercially available BSA. Although saline did not contain PLP, the PLP fluorescent derivative was unstable in saline and decreased 16% over 2 h (Fig. 1). Charcoal-stripped plasma contained very low level of PLP ranging from 2.8 to 5.3 nmol/L in the two lots we tested. It did not contain any detectable amount of PA. In addition, PLP remained stable in charcoal-stripped plasma with no significant change in peak area observed within the 2-h period. Based on these characteristics, charcoal-stripped plasma was selected as the matrix for preparing calibrators. PA was not detectable in BSA solution and remained stable in either saline or stripped plasma.
Fig. 1.
Matrix choice for vitamin B6 calibrator solution. PLP derivatized fluorescence product is not stable in saline but remains stable in charcoal stripped plasma.
3.3. Evaluation of assay performance characteristics
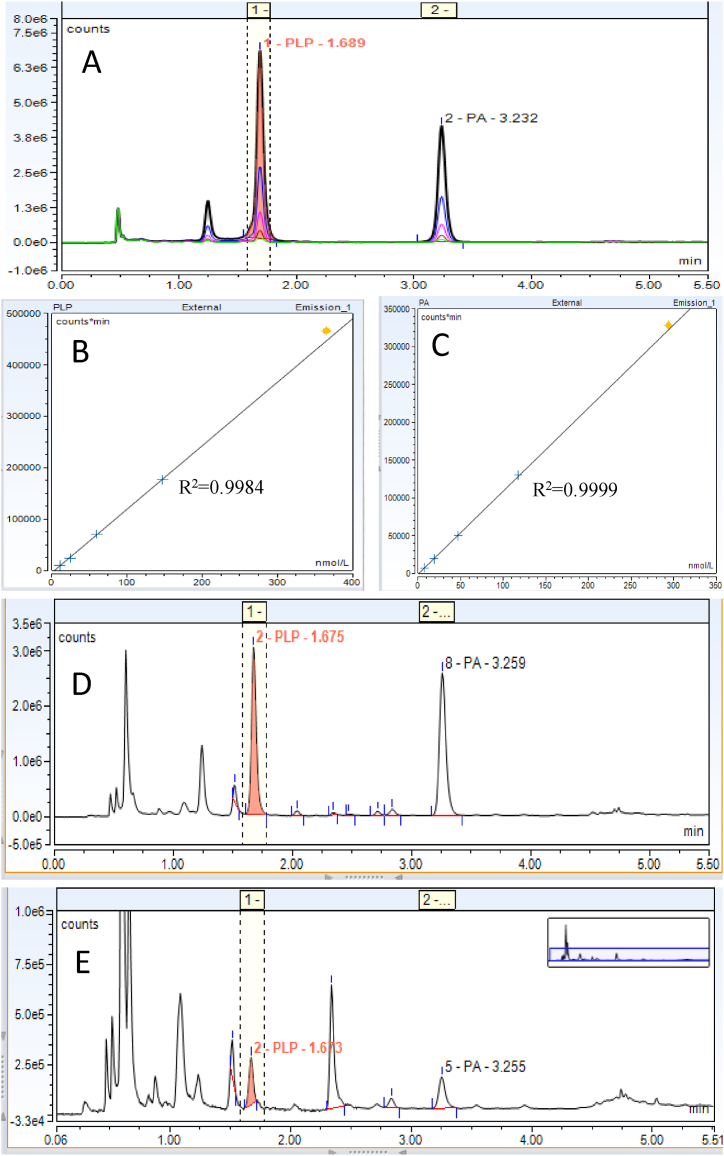
Typical assay chromatograms and standard curves were shown in Fig. 2. The analytical performance characteristics of the assay are summarized in Table 2. The total CV from the 15-day precision study was less than 10% for PLP and less than 5% for PA which is well within the total allowable error of 26% specified in the Westgard website (https://www.westgard.com/biodatabase1.htm). AMR of the assay spanned the biologically-relevant target levels for both PLP and PA. The AMR of PLP and PA quantitation covers the 2.5th to 97.5th percentile (11.3–302 nmol/L) and 2.5th to 95th percentile (8.7–194 nmol/L) of PLP and PA concentration respectively in U.S populations based upon the values reported in the 2nd National Report on Biochemical Indicators of Diet and Nutrition in the U.S. population [1].
Fig. 2.
Representative chromatograms of vitamin B6 and standard curves. A: Overlay chromatograms PLP and PA from five levels of calibrators. B: A typical standard curve of PLP. C: A typical standard curve of PA. D: Chromatogram of a patient sample with normal level of PLP (130.5 nmol/L) and normal concentration of PA (154.5 nmol/L). E. Chromatograms of a patient sample with vitamin B6 deficiency (PLP 11.3 nmol/L, PA 10.0 nmol/L). The yellow diamond indicates the highest calibrator. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Table 2.
Assay performance characteristics.
| Assay Precision | ||||||
|---|---|---|---|---|---|---|
| Analytes | Low Control |
High Control |
||||
| Mean (nmol/L) | CV% Intra-assay |
CV% Inter-assay |
Mean (nmol/L) | CV% Intra-assay |
CV% Inter-assay |
|
| PLP (n = 30) | 21.3 | 8.3 | 6.2 | 304.3 | 1.2 | 5.6 |
| PA (n = 30) | 11.9 | 1.4 | 0.7 | 304.0 | 0.7 | 1.8 |
| Accuracy Evaluation using NIST Standard Reference Material 3950 | ||||||
|---|---|---|---|---|---|---|
| Analytes | NIST level 1 (n = 3) |
NIST Level 2 (n = 3) |
||||
| Assigned value | Measured Value | % Difference | Assigned Value | Measured Value | % Difference | |
| PLP (nmol/L) | 18.6 ± 0.6 | 18.2 ± 1.6 | −10.8–5.9% | 36.4 ± 1.2 | 35.8 ± 2.1 | −5.0 to 5.0% |
| PA (nmol/L) | 121 | 121.8 ± 0.7 | −0.7 to 0.7% | 202 | 201.0 ± 0.6 | −1.7 to 0.5% |
| Analytical sensitivity and Analytical Measurement Range | |||
|---|---|---|---|
| Analytes | LLOD | LLOQ | Analytical Measurement Range |
| PLP | 4.5 nmol/L | 7.8 nmol/L | 7.8–350.1 nmol/L |
| PA | 3.3 nmol/L | 3.3 nmol/L | 3.3–339.3 nmol/L |
| Sample and Reagent Storage Stability | |||
|---|---|---|---|
| Ambient | Refrigerated | Frozen (−70 °C) | |
| Plasma | 4 h | 24 h | 1 year |
| Post-derivatization specimen | 3 days | ||
| Calibrators | NA | NA | 2 months |
3.4. Evaluation of potential internal standard
To determine if the inclusion of an internal standard would improve the analytical performance of the assay, we evaluated 17 chemicals for use as an IS (Supplementary Table 1). The criteria for an optimal IS included: 1) a chemical structure similar to PLP; 2) ability to produce fluorescence which is stable in acidic conditions; 3) a peak which can reach baseline separation with other peaks in patient samples. 4) appropriate retention time on C18 column. Among the 17 chemicals we tested, only six compounds retained fluorescence and showed acceptable retention time after the sample preparation/derivatization procedure (Supplementary Table 1). Out of these 6 candidates, five were either found to be present in pooled patient samples or overlapped with a large peak in pooled patient samples. 4-deoxypyridoxine hydrochloride was the only candidate that successfully fulfilled our criteria and it has been used as an IS in vitamin B6 analysis of food sample, animal tissue or cell culture. Using 4-deoxypridoxin HCL as an internal standard, a 3-day precision study was performed. The use of the IS actually led to an increased in CV for both PLP (11.5%–17.3%) and PA (4.2%–7.3%). This loss of precision appeared to be primarily due to variation of the IS peak area. Additionally, the wavelength required to detect 4-deoxypyridoxine hydrochloride was Excitation 310 nm/Emission 380 nm, which requires a switch to 367 nm/478 nm for PLP detection within 0.3 min. Any delay of the timely switch in excitation and emission wavelength between the closely eluting 4-deoxypyridoxine and PLP peaks could result in failure of PLP peak detection. Because of these limitations, the use of an IS did not appear to provide any improvements in precision for this assay. However, the baseline precision using external standards appears to be acceptable for clinical use.
3.5. Analytical accuracy and inter-method comparison
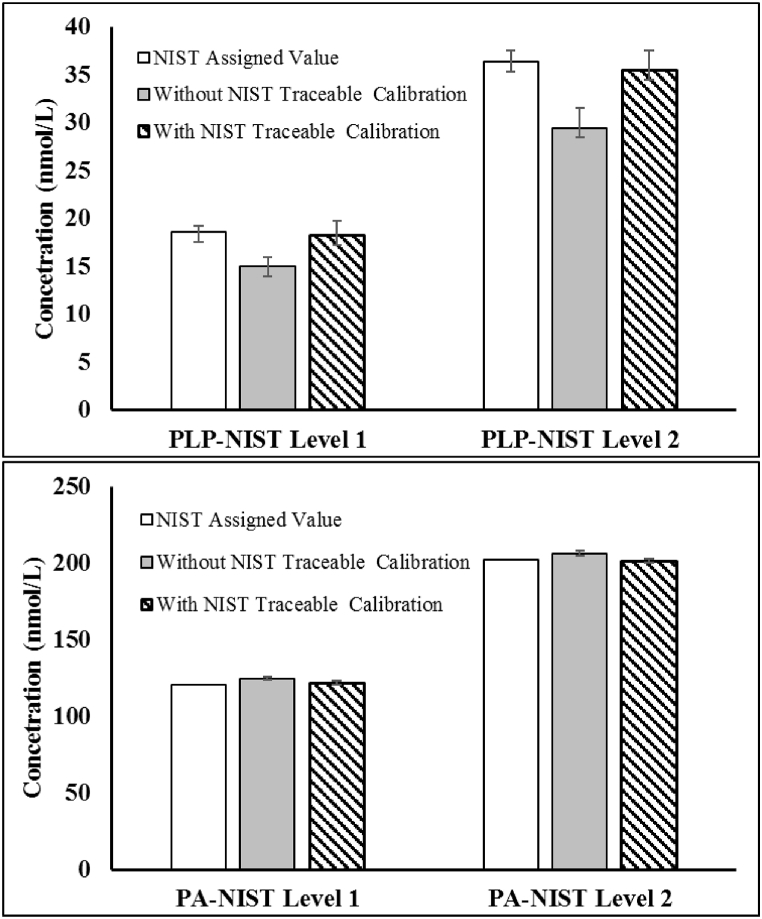
The mean spike recovery of PLP across the AMR was 100.3% ranging from 92.0 to 116.6%. The average recovery of PA was 105.4% ranging from 95.2 to 117.5%. Assay accuracy was next evaluated by assaying the NIST SRM 3950 as unknowns (Fig. 3). Certified values of NIST SRM 3950 are based on the agreement of results from isotope dilution liquid chromatography/tandem mass spectrometry at NIST and liquid chromatography/fluorescence detection at the CDC [7,8]. Using the spectrophotometry-based values for calibration, the % difference between the measured values and the assigned value of PLP was −19.5% and −18.9% for the level 1 and level 2 standard material respectively. After changing the calibrator value based on NIST traceable calibration, the accuracy was greatly improved and the % difference reduced to −2.1% and −1.7% respectively. The difference of PA measurement with or without NIST traceable calibration was insignificant. Both the routine weight-based calibration and the NIST calibration generated accurate measurement of PA with % difference less than ±5% from the assigned value.
Fig. 3.
Accuracy of the in-house vitamin B6 assay in measuring the NIST standard reference material 3950. The top panel shows that measured results of PLP were about 19% lower than the NIST assigned value using the in-house assay with calibrator values assigned by absorbance measurement. After the calibrators were calibrated against the NIST SRM 3950, measured results of PLP matched the NIST assigned value with difference less than 5%. The Bottom panel shows that measured results of PA matched the NIST assigned value with or without the calibrators calibrating against the NIST reference standards.
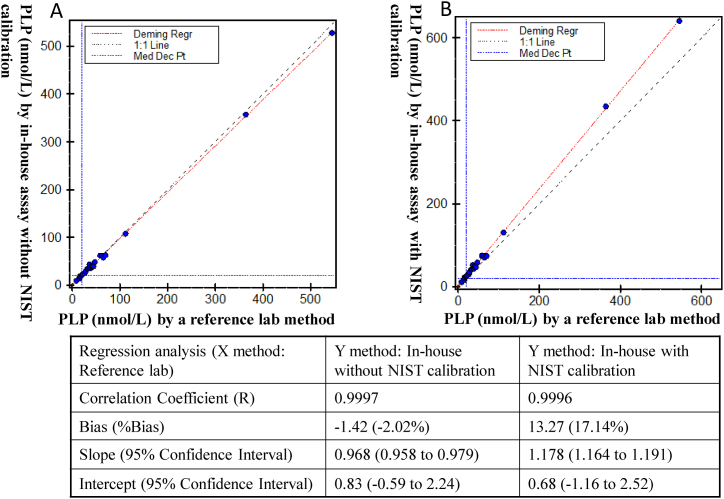
Additionally, we compared the in-house method with a commercially-available Vitamin B6 assay (measuring PLP only) performed by a large reference laboratory. As shown in Fig. 4, without the NIST calibration (using the spectrophotometry-based calibration), the two methods showed equivalent performance for PLP quantitation. However, after the in-house assay was calibrated to the NIST materials, a 17% bias was observed between the two methods. These results suggest that the reference lab method was not traceable to the NIST SRM 3950 and it would generate about 17% difference if used to measurement the NIST SRM 3950.
Fig. 4.
Method comparison between the in-house vitamin B6 assay with/without NIST calibration and a reference laboratory method (n = 26). Left panel: A. Comparison of the in-house HPLC method with a reference lab HPLC method showed equivariant measurement of PLP in plasma specimens using absorbance assigned calibrator values. B. Bias was observed between the in-house HPLC method and the reference lab method after the in-house calibrators were calibrated against the NIST standard reference material 3950. The black dashed line in panel A and B indicate the 1:1 line.
3.6. Clinical relevance and reference interval
The CDC’s National Second Nutrition Report used 20 nmol/L as cutoff in identifying vitamin B6 deficiency. This cutoff value is derived from the Recommended Dietary Allowance and has been selected by the Institute of Medicine as the basis for the Estimated Average Requirement [2]. A plasma or serum PLP concentration of 20 nmol/L is usually not associated with immediate health risk, but it allows a moderate safety margin to prevent potential harm of deficiency. It should be noted that the analytical method for vitamin B6 quantitation at the CDC is one of the two methods contributed to the certified values of the NIST SRM 3950. Even though many clinical laboratories also adopted the same cutoff (<20 nmol/L) for diagnosis of vitamin B6 deficiency, the wide dispersion of results, especially at lower PLP concentration, among different methods as reported in literature [6,9] could lead to discrepancy in diagnosis. Here, we provided a practical approach to harmonize the vitamin B6 measurement method and thus standardize vitamin B6 deficiency evaluation. Vitamin B6 deficiency has been associated with a wide spectrum of symptoms. With this simple and accurate method, it can either identify patient with vitamin B6 deficiency and correct the condition simply by taking multivitamin supplement or exclude vitamin B6 deficiency to focus on other possible causes.
4. Conclusion
Vitamin B6 deficiency is the most prevalent nutrient deficiency with prevalence greater than iron and vitamin D deficiency according to the CDC second Nutrition Report. We developed a simple one-step HPLC method for evaluation of vitamin B6 status by measuring the bioactive PLP and final metabolite PA simultaneously. The assay procedure was simplified into a one-step approach by combining the protein precipitation and derivatization procedure without compromising analytical performance. The method is fast, precise and sensitive enough to detect vitamin B6 concentrations below 2.5th percentile among the general U.S. population. More importantly, the method has been calibrated against the NIST SRM 3950 to facilitate inter-method harmonization. In summary, this article provides a simple and practical approach to promote inter-laboratory/inter-method consistency and standardization in evaluation of B6 nutrition status for clinical laboratories.
CRediT authorship contribution statement
Xiaochun Zhang: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing - original draft, Writing - review & editing. Xiaoying Tang: Methodology, Validation, Formal analysis, Investigation. Thomas M. Daly: Writing - original draft, Writing - review & editing.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.plabm.2020.e00160.
Appendix A. Supplementary data
References
- 1.CDC . 2012. Second National Report on Biochemical Indicators of Diet and Nutrition in the U.S. Population.https://www.cdc.gov/nutritionreport/pdf/Nutrition_Book_complete508_final.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfeiffer C.M., Sternberg M.R., Schleicher R.L., Haynes B.M., Rybak M.E., Pirkle J.L. The CDC’s Second National Report on Biochemical Indicators of Diet and Nutrition in the U.S. Population is a valuable tool for researchers and policy makers. J. Nutr. 2013;143(6) doi: 10.3945/jn.112.172858. 938S-47S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akiyama T., Hayashi Y., Hanaoka Y., Shibata T., Akiyama M., Tsuchiya H., Yamaguchi T., Kobayashi K. Pyridoxal 5’-phosphate, pyridoxal, and 4-pyridoxic acid in the paired serum and cerebrospinal fluid of children. Clin. Chim. Acta. 2017;472:118–122. doi: 10.1016/j.cca.2017.07.032. [DOI] [PubMed] [Google Scholar]
- 4.Albersen M., Bosma M., Luykx J.J., Jans J.J., Bakker S.C., Strengman E., Borgdorff P.J., Keijzers P.J., van Dongen E.P., Bruins P., de Sain-van der Velden M.G., Visser G., Knoers N.V., Ophoff R.A., Verhoeven-Duif N.M. Vitamin B-6 vitamers in human plasma and cerebrospinal fluid. Am. J. Clin. Nutr. 2014;100(2):587–592. doi: 10.3945/ajcn.113.082008. [DOI] [PubMed] [Google Scholar]
- 5.Ueland P.M., Ulvik A., Rios-Avila L., Midttun O., Gregory J.F. Direct and functional biomarkers of vitamin B6 status. Annu. Rev. Nutr. 2015;35:33–70. doi: 10.1146/annurev-nutr-071714-034330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoad K.E., Johnson L.A., Woollard G.A., Walmsley T.A., Briscoe S., Jolly L.M., Gill J.P., Greaves R.F. Vitamin B1 and B6 method harmonization: comparison of performance between laboratories enrolled in the RCPA Quality Assurance Program. Clin. Biochem. 2013;46(9):772–776. doi: 10.1016/j.clinbiochem.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 7.Midttun O., Hustad S., Solheim E., Schneede J., Ueland P.M. Multianalyte quantification of vitamin B6 and B2 species in the nanomolar range in human plasma by liquid chromatography-tandem mass spectrometry. Clin. Chem. 2005;51(7):1206–1216. doi: 10.1373/clinchem.2005.051169. [DOI] [PubMed] [Google Scholar]
- 8.Rybak M.E., Pfeiffer C.M. Clinical analysis of vitamin B(6): determination of pyridoxal 5’-phosphate and 4-pyridoxic acid in human serum by reversed-phase high-performance liquid chromatography with chlorite postcolumn derivatization. Anal. Biochem. 2004;333(2):336–344. doi: 10.1016/j.ab.2004.06.036. [DOI] [PubMed] [Google Scholar]
- 9.Rybak M.E., Jain R.B., Pfeiffer C.M. Clinical vitamin B6 analysis: an interlaboratory comparison of pyridoxal 5’-phosphate measurements in serum. Clin. Chem. 2005;51(7):1223–1231. doi: 10.1373/clinchem.2005.050278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.