Abstract
Introduction
Research on cigarette smokers suggests cognitive and behavioral impairments. However, much remains unclear how the functional neurobiology of smokers is influenced by nicotine state. Therefore, we sought to determine which state, be it acute nicotine abstinence or satiety, would yield the most robust differences compared with nonsmokers when assessing neurobiological markers of nicotine dependence.
Methods
Smokers (N = 15) and sociodemographically matched nonsmokers (N = 15) were scanned twice using a repeated-measures design. Smokers were scanned after a 24-hour nicotine abstinence and immediately after smoking their usual brand cigarette. The neuroimaging battery included a stop-signal task of response inhibition and pseudocontinuous arterial spin labeling to measure cerebral blood flow (CBF). Whole-brain voxel-wise analyses of covariance were carried out on stop success and stop fail Stop-Signal Task contrasts and CBF maps to assess differences among nonsmokers, abstinent smokers, and satiated smokers. Cluster correction was performed using AFNI’s 3dClustSim to achieve a significance of p < .05.
Results
Smokers exhibited higher brain activation in bilateral inferior frontal gyrus, a brain region known to be involved in inhibitory control, during successful response inhibitions relative to nonsmokers. This effect was significantly higher during nicotine abstinence relative to satiety. Smokers also exhibited lower CBF in the bilateral inferior frontal gyrus than nonsmokers. These hypoperfusions were not different between abstinence and satiety.
Conclusions
These findings converge on alterations in smokers in prefrontal circuits known to be critical for inhibitory control. These effects are present, even when smokers are satiated, but the neural activity required to achieve performance equal to controls is increased when smokers are in acute abstinence.
Implications
Our multimodal neuroimaging study gives neurobiological insights into the cognitive demands of maintaining abstinence and suggests targets for assessing the efficacy of therapeutic interventions.
Introduction
Tobacco smoking is the major cause of preventable death in the United States and other developed countries. According to the US Centers for Disease Control and Prevention (www.cdc.gov), smoking causes nearly 6 million deaths per year globally; current trends predict this will reach 8 million by 2030.1 Using the United States as an example, more than 5 million Americans younger than 18 years today are expected to die from a smoking-related illness, which is higher than the number of deaths caused by HIV, illicit drug misuse, suicide, murder, and motor vehicle injuries, combined. Each year approximately 30% of smokers try to quit, with the vast majority of attempts (~90%) ending in relapse. Tobacco remains the most widely abused substance, and evidence suggests that abuse is linked to the nicotine content of tobacco.2 Thus, assessing the neural correlates of cigarette smoking has been of great interest to researchers.
Structural neuroimaging studies using voxel-based morphometry have found that cigarette smokers, compared with never-smokers, have smaller gray matter volume mainly in the prefrontal cortex, medial frontal cortex, thalamus, and cingulate cortex.3,4 Moreover, diffusion tensor imaging studies have shown that cigarette smoking is associated with reduced microstructural integrity of white matter5 and higher fractional anisotropy in the prefrontal tracts, cingulum, corpus callosum,6 and the frontoparietal tracts.7 Consequently, it has commonly been assumed that these nicotinic associations with structural differences in several key regions in the brain that play an important role in attention, working memory, and other cognitive domains could lead to the functional impairments observed in chronic smokers in behavioral tasks and functional magnetic resonance imaging (fMRI) studies.8,9 In addition to associations between long-term nicotine use and brain structure, fMRI studies in cigarette smokers have found increased neural activation in the frontal gyrus, insula, caudate, and amygdala after nicotine administration10, as well as a link between nicotine administration and brain circuitry that mediates attentional processing and withdrawal symptoms.11 In addition, other resting-state and event-related fMRI studies found that nicotine administration induced deactivation in the default mode network and improved cognitive performance and activity in the network related to executive control and attention.12
In humans, the effects of nicotine and smoking on cerebral perfusion and energy metabolism are poorly documented. In one recent study using positron emission tomography, global gray matter cerebral blood flow (CBF) was reduced relative to nonsmokers at the end of an overnight abstinence and increased (relative to abstinent smokers) after smoking resumption, suggesting an acute restoration of the hypoperfusion by resumption of smoking.13 In a more recent study using arterial spin labeling perfusion fMRI, 4 hours of abstinence were enough to show significant reductions in resting CBF in multiple nodes of the brain’s mesocorticolimbic network, disrupting neural processing.14 Earlier studies provided evidence suggesting increases in global CBF and oxygen consumption after smoking cigarettes, indicating coupled stimulation of perfusion and energy metabolism,15 although an earlier study based on the radioactive Xenon method showed no changes in cerebral metabolic rate for oxygen after smoking.16 Reports of regional CBF revealed mixed effects where acute nicotine administration evoked relative increases in CBF in visual cortex, thalamus, and cerebellum17 and decreases in the anterior cingulate cortex and the ventral striatum. Autoradiographic studies with [14C] deoxyglucose showed that acute nicotine stimulates the cerebral metabolic rate of glucose by approximately 50% in specific brain regions, notably in the thalamus and in parts of the rat cortex.18 In another study, intravenous nicotine evoked a global 10% decrease in cerebral metabolic rate of glucose in healthy male smokers,19 whereas other studies showed increased [18F]fluorodeoxyglucose uptake in left thalamus.20
Response inhibition, an important form of behavioral impulse control, is the ability to inhibit behavioral responses to salient approach cues. The Stop-Signal Task (SST) and the GO/NO-GO task are common measures of response inhibition and have highlighted inhibitory control deficits in people with substance use disorders and other addictions.21,22 A number of studies assessing smoking effects on response inhibition have shown performance and neural activity differences between smokers, former smokers, and never- smokers.21,22 Using a GO/NO-GO task, Nestor et al. examined neural correlates of response inhibition and error monitoring in current smokers, former smokers who had at least 1 year of abstinence prior to study participation (average 7 years of abstinence), and never-smokers. Only current smokers demonstrated behavioral deficits in response inhibition relative to never-smokers. The neuroimaging results showed that while current and former smokers demonstrated decreased activation of the inferior frontal gyrus (IFG) compared with never-smokers, former smokers demonstrated increased activation of the left anterior cingulate cortex for successful response inhibition trials compared with current smokers.22 In a similar study examining how response inhibition and error monitoring processes differ as a function of smoking status, Weywadt et al. reported that never-smokers, current smokers, and former smokers performed similarly on the task and similarly engaged the inferior frontal gyrus and dorsal anterior cingulate cortex.23 However, in contrast to Nestor et al., they reported higher activation levels for successful inhibition in the cerebellum for current and former smokers compared with never-smokers.
Studying the neurobiological markers of nicotine dependence such as prefrontal differences in perfusion and neurocognitive processes such as impaired impulse control could greatly aid in understanding the challenges faced by smokers attempting abstinence. As reviewed, findings from previous studies investigating inhibitory control impairment and CBF changes in smokers relative to nonsmokers are scarce with some inconsistencies in findings. In addition, multimodal imaging that assesses both CBF and impulse control in the same participants permits testing their relationship related to nicotine state that may provide a more comprehensive understanding of the brain mechanisms underlying addiction and efforts to quit smoking. It is unclear whether the functional changes in smokers reported in the literature are present both when acutely abstinent or satiated and therefore reflect trait- or state-like characteristics of smokers. Here, we use fMRI to study inhibitory control using the SST and resting-state CBF in nonsmokers and smokers, who were assessed both when abstinent and when satiated. First, we performed whole-brain analyses to locate gray matter regions showing task activation and CBF differences between smokers and nonsmokers. Then, we performed region of interest (ROI)-based analyses on the resulting regions to determine the impact of current smoking status on differences compared with nonsmokers. We hypothesize, given the few neuroimaging studies comparing smokers versus nonsmokers and showing inconsistent results using the SST or the GO/NO-GO task,21–23 that brain regions rich in nicotinic acetylcholine receptors such as the IFG, ventral striatum, and the thalamus would be expected to exhibit changes in BOLD activation during nicotine abstinence or satiety. For CBF, given the existing evidence illustrating the detrimental effects of smoking on blood circulation and neuroimaging studies in heavy smokers,13,23 we hypothesize that lower global CBF would be observed in smokers relative to nonsmokers.
Methods
Participants
Smokers and nonsmokers were recruited from the local community. At their baseline visit, participants were informed about the course of the study and provided written consent. A total of N = 17 current smokers without plans to quit in the next 6 months and N = 16 nonsmokers (never-smokers or those who only smoked a few cigarettes during their lifetime) matched on age, sex, handedness, and years of education were recruited. All participants were free from MRI contraindications. Participants were compensated at the end of each visit. The study was approved by the University of Vermont Institutional Review Board.
Baseline Visit
Smokers were instructed to smoke ad libitum prior to attending their baseline visit. After providing written consent, all participants completed questionnaires related to their demographics, nicotine dependence, and other substance use and psychiatric symptoms. Nicotine dependence was assessed using the Fagerström Test for Nicotine Dependence (FTND).24 The FTND scores range from 0 to 10 where higher scores indicate greater nicotine dependence. Participants were also tested for depression and anxiety using the Beck Depression Inventory (BDI)25 and the Overall Anxiety Severity and Impairment Scale (OASIS)26 questionnaires, respectively. In addition, participants completed the European School Survey Project on Alcohol and Drugs (ESPAD) questionnaire where they reported the number of occasions during which they used Marijuana or drank alcohol. The ESPAD category scores are as follows: Score (lifetime occurrences): 0 (0), 1 (1–2), 2 (3–5), 3 (6–9), 4 (10–19), 5 (20–39), and 6 (>40). Participants reporting current psychiatric and/or neurological disorders, history of trauma, or injury causing loss of consciousness were excluded from the study. Urine drug tests were administered to all participants. Participants testing positive for cocaine, amphetamine, methamphetamine, tetrahydrocannabinol, methylenedioxymethamphetamine (commonly known as ecstasy), methadone, buprenorphine, opiates, oxycodone, phencyclidine, barbiturates, or benzodiazepine were excluded. Expired breath carbon monoxide (CO) was measured at the conclusion of the visit for all participants. Participants also practised the fMRI task to be completed on a later date inside the scanner.
Scanning Visits
Smokers and nonsmokers were asked to complete two identical neuroimaging visits, separated by at least 1 week. At one of the scans, smokers were instructed to commit to a 24-hour smoking abstinence verified by breath CO content prior to scanning. Participants were considered abstinent if their CO value at the abstinent scan was less than half of their CO value at the baseline visit. For the other scan, smokers were asked to smoke one of their usual brand cigarettes immediately prior to entering the MRI facility. CO was measured again prior to the scan. The order of the abstinent or satiated scan was randomized across study participants. For satiated smokers, the approximate time between smoking their last cigarette and the start time of scanning was 45 minutes.
One participant who tested positive for drug use and two others who dropped out after the first scan were excluded from the study. The final sample consisted of N = 15 nonsmokers and N = 15 smokers who met the inclusion criteria and completed both neuroimaging sessions.
MRI Acquisition
The neuroimaging battery included fMRI during which participants performed a stop-signal task of response inhibition and pseudocontinuous arterial spin labeling to measure brain perfusion. All data were acquired using a Philips 3T Achieva dStream MRI scanner using a 32-channel head coil. BOLD functional MRI data were acquired using a 2D gradient-echo echo-planar imaging sequence with echo time (TE)/repetition time (TR) = 35/2000 ms, 240 × 240 mm field of view, acquisition matrix 64 × 64, and SENSE acceleration factor of 2. Whole-brain coverage included 33 slices, 4 mm thick, with 0.4-mm interslice gap, interleaved odd–even acquisition. High-resolution T1-weighted images were acquired using a turbo field echo technique, with 256 × 256 × 256 mm field of view, acquisition matrix 320 × 320 × 320 to give 0.8-mm isotropic resolution, SENSE factors of 2.3/2.6 AP/RL, and TE/TR/inversion time (TI) = 4/5/9.8/950 ms.
The Stop Signal Task
Brain activation maps of inhibitory control were generated from the SST fMRI paradigm. Participants were instructed to respond to regularly presented visual go stimuli (arrows pointing left or right) but to withhold their motor response when the go stimulus was followed unpredictably by a stop signal (an arrow pointing upwards). A block contained 400 go trials and 80 variable delay stop trials with between 3 and 7 go trials between 2 stop trials. Stopping difficulty was manipulated across trials by varying the delay between the onset of the go arrow and the stop arrow (stop-signal delay [SSD]) using a tracking algorithm in which a participant’s performance dictates the SSD of the subsequent stop trial to reach an SSD that elicits 50% stopping accuracy. Stimulus duration in go trials was 1000 ms and in stop trials varied (0–900 ms in 50-ms steps) in accordance with the tracking algorithm (initial SSD = 250 ms).
In the tracking algorithm, successful stopping results in a longer SSD in the following stop trial, which reduces the probability of successful inhibition, and unsuccessful stopping results in a shorter SSD in the following stop trial, which increases the probability of successful inhibition. This algorithm allows for simple calculation of the Stop Signal Reaction Time (SSRT), or the time required to inhibit an already initiated motor response.27 The SSRT is an indirect measure of inhibitory ability and commonly used as the main outcome variable of the SST.28 In this study, the SSRT was calculated using the integration method that involves subtracting the mean SSD from the nth response time in the response time distribution, with n equals to the percentage of successful inhibitions on stop trials.29
fMRI Processing
Image preprocessing was completed using the AFNI tools (https://afni.nimh.nih.gov).30 Six-parameter rigid-body motion correction was executed using a Fourier interpolation to align all images to the first volume of the run. The functional images were spatially coregistered to the skull-stripped anatomical image. The latter was normalized into MNI coordinate space using a 12-parameter affine transformation, and the resulting transformation matrix was then applied to the functional images. All functional images were then spatially smoothed using a 6-mm Gaussian kernel. The signal intensity at each voxel was then scaled to a mean of 100.
Next, SPM 12 was used to generate, from the SST data, contrast images for successful inhibitions (“stop success”) and unsuccessful inhibitions (“stop fail”), both versus an implicit baseline. CBF parametric maps were generated from pseudocontinuous arterial spin labeling data using a Matlab script based on the method of Aslan et al.,31 which consists of a coregistration followed by a subtraction of the control and label images, with appropriate scaling based on estimated parameters for T1 of brain tissue, arterial blood, and the thalamus.
Whole-Brain Analyses
Whole-brain voxel-wise analyses of covariance (ANCOVAs) were carried out on the two SST contrasts (stop success and stop fail) and CBF maps using the general linear model, with the VBM toolbox of SPM12, to assess differences between nonsmokers versus abstinent smokers, nonsmokers versus satiated smokers, and abstinent versus satiated smokers. Control scans were picked, so they had an equal number of first and second scans as the smoker condition. Age, sex, years of education, and marijuana use were included as nuisance covariates in the design matrix. The resulting set of voxel values constituted a Statistical Parametric Map of the t statistic (SPM{t}). We used 3dClustSim, a cluster correction procedure available in AFNI to estimate the probability of false-positive clusters using a noise volume simulation assuming the autocorrelation function is given by a mixed-model of the form a*exp(−r*r/[2*b*b]) + (1 − a)*exp(−r/c), to determine a minimum cluster size so as to achieve a corrected significance of p less than .05 with an initial cluster-forming voxel-wise threshold of p less than .001. Clusters bigger than 409 and 421 voxels were considered significant for the SST and CBF analyses, respectively. The demographics of the participants included in the between-group analyses are detailed in Table 1.
Table 1.
The Demographics and Performance Measures of Smokers and Nonsmoker Controls
| Measure (M ± SD) | Group/condition | |||
|---|---|---|---|---|
| Nonsmokers | Smokers | Nonsmokers vs. smokers, p | ||
| Age | 22.6 ± 1.5 | 24.8 ± 5.8 | .328 | |
| Sex (males %) | 63 | 78 | .490 | |
| Years of education | 16 ± 1 | 14 ± 1 | .221 | |
| Baseline CO ppm | 2.1 ± 0.4 | 12.2 ± 4.7 | .000 | |
| Cigarettes/d | — | 10.4 ± 3.6 | — | |
| Fagerström | 0 | 2.37 ± 1.82 | .000 | |
| BDI | 2.5 ± 4.39 | 4.87 ± 3.28 | .373 | |
| OASIS | 2.73 ± 2.6 | 2.93 ± 1.5 | .517 | |
| Lifetime marijuana use | 4.2 ± 2.93 | 6.71 ± 0.61 | .006 | |
| Marijuana use in the past 12 mo | 3.33 ± 2.76 | 5.92 ± 1.32 | .002 | |
| Marijuana use in the past week | 0.93 ± 1.5 | 1.57 ± 1.80 | .301 | |
| Lifetime alcohol use | 5.93 ± 2.82 | 7 ± 0.00 | .091 | |
| Alcohol use in the past 12 mo | 5.4 ± 2.13 | 6.28 ± 1.32 | .105 | |
| Alcohol use in the past week | 1.26 ± 0.79 | 1.64 ± 0.92 | .254 | |
| SST performance | Nonsmokers | Abstinent smokers | Satiated smokers | Pairwise comparisons, p |
| SSRT (ms) | 201 ± 29 | 209 ± 37 | 205 ± 34 | >.41 |
| GO response time (ms) | 391 ± 64 | 408 ± 72 | 402 ± 65 | >.39 |
| Correct stop trials (%) | 52.1 ± 2.5 | 50.4 ± 2 | 51.7 ± 2 | >.7 |
Marijuana and alcohol use were assessed using the ESPAD and are coded as follows: Score (lifetime occurrences): 0 (0), 1 (1–2), 2 (3–5), 3 (6–9), 4 (10–19), 5 (20–39), 6 (>40). BDI = Beck Depression Inventory; CO = carbon monoxide; OASIS = Overall Anxiety Severity and Impairment Scale; SSRT = Stop Signal Reaction Time; SST = Stop-Signal Task.
ROI-Level Analyses
To test for corresponding effects and correlations across modalities, mean values were extracted for all participants from the significant clusters obtained from the voxel-wise SST and CBF comparisons. Next, between-group (smokers vs. nonsmokers) and within-group (abstinent vs. satiated smokers) comparisons were carried out using ANCOVA and repeated-measures ANCOVA models, respectively. In addition, the correlation between SST and CBF was assessed using Pearson’s test. Analyses were performed with SPSS 17, and age, sex, years of education, and marijuana use were included as nuisance covariates.
Results
Response inhibition activity and CBF measures were analyzed in the final sample of 15 nonsmokers and 15 smokers. There were no differences in age, sex distribution, years of education, alcohol use, or depression and anxiety measures between nonsmokers and smokers (p > .51) (Table 1). However, marijuana use was significantly higher in smokers (p = .002) and was included as a nuisance covariate in all analyses.
SST behavioral performance for each scan for each participant underwent quality control prior to inclusion in the study to check whether they meet the Congdon et al.’s32 reliability criteria of response inhibition. All participants had correct Stop trials of at least 47%, correct GO trials of at least 81% and SSRT values of at least 173 ms; thus, they were all included in the study. There were no differences (p > .39) in inhibitory control performance among the three conditions when comparing SSRT, GO reaction time, or correct stop trial percentage with a series of ANCOVAs between nonsmokers and abstinent smokers, nonsmokers and satiated smokers, and abstinent and satiated smokers (Table 1). In addition, participants did not show significant practice effects from their first to second scans (p > .3).
Whole-Brain SST Analyses
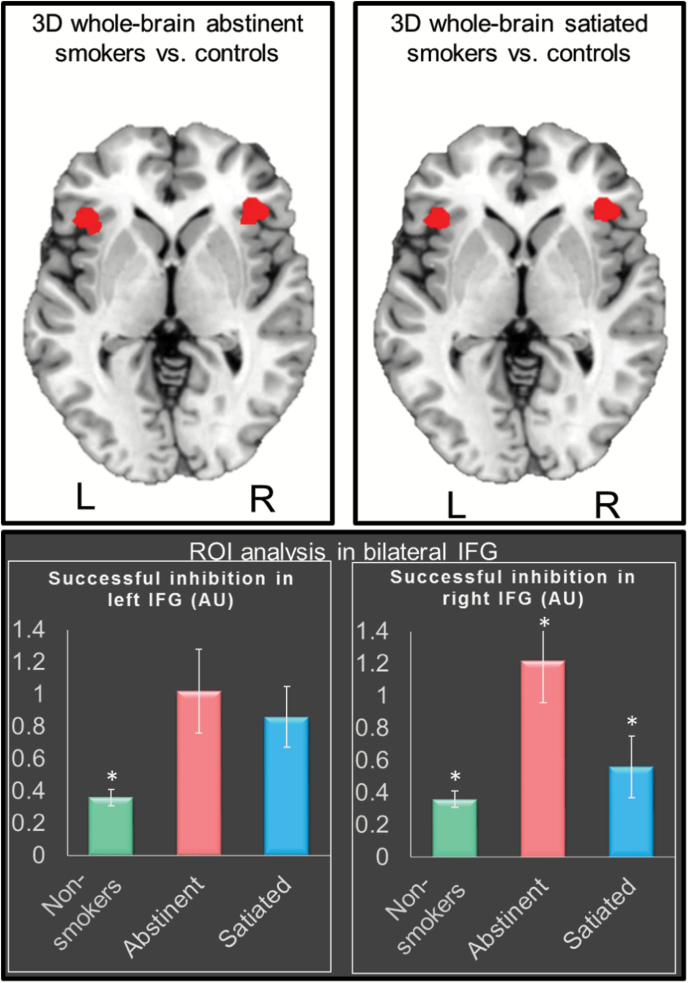
After cluster-wise correction for multiple comparisons at p < .05, whole-brain between-group comparisons indicated significantly higher neural activity for the stop success contrast in abstinent smokers compared with nonsmokers, in the bilateral IFG (Figure 1; Table 2). Similarly, significantly higher activity for stop success was observed in satiated smokers compared with nonsmokers, also in the bilateral inferior frontal IFG (Figure 1; Table 2). There was an 81% overlap between the two contrasts (abstinent vs. nonsmokers and satiated vs. nonsmokers) (Figure 1). Within-subject whole-brain comparisons did not show clusters surviving correction for multiple comparisons in the abstinent versus satiated stop success contrast. No significant clusters were observed in any of the stop fail between- or within-group comparisons.
Figure 1.
Top row: Whole-brain analyses showing greater successful inhibition activity (t maps cluster-corrected at p < .05, cluster size ≥ 421) in abstinent smokers and satiated smokers relative to controls in bilateral inferior frontal gyrus (IFG), with 81% overlap across the two comparisons. Bottom row: Region of interest (ROI)-level analyses in the bilateral IFG showing higher successful inhibition activity in abstinent smokers relative to satiated and nonsmokers. The ROIs were defined as the overlapping areas across the two whole-brain comparisons. *Significantly different from the two other groups with p < .001.
Table 2.
MNI Peak Coordinates of Clusters Showing Significant Response Inhibition or CBF Changes Between Smokers and Nonsmokers
| Whole-brain analysis | ROI | MNI peak coordinates | |||
|---|---|---|---|---|---|
| x | y | z | |||
| Response inhibition | Abstinent vs. nonsmokers | Left IFG | −55 | 27 | 8 |
| Right IFG | 57 | 30 | 3 | ||
| Satiated vs. nonsmokers | Left IFG | −49 | 23 | 4 | |
| Right IFG | 51 | 25 | 1 | ||
| CBF | Abstinent vs. nonsmokers | Left IFG | −47 | 29 | 10 |
| Right IFG | 49 | 24 | 4 | ||
| Occipital cortex | 14 | −99 | 3 | ||
| Satiated vs. nonsmokers | Left IFG | −51 | 26 | 2 | |
| Right IFG | 51 | 26 | 6 | ||
| Occipital cortex | 18 | −101 | 1 | ||
CBF = cerebral blood flow; IFG = inferior frontal gyrus; ROI = region of interest.
Whole-Brain Perfusion Analyses
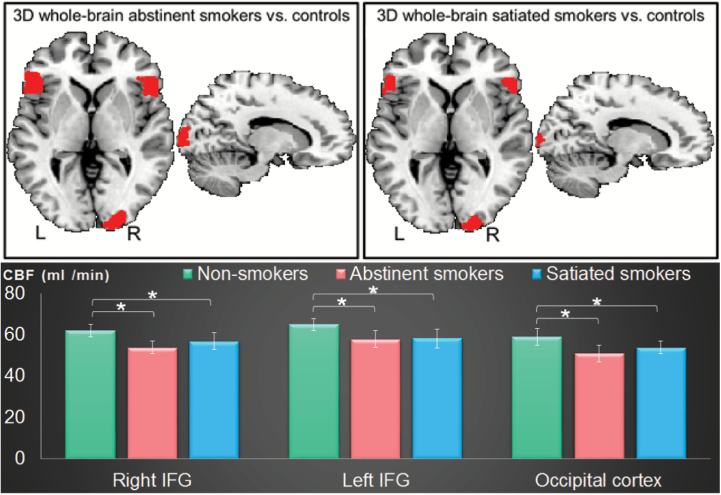
After cluster-wise correction for multiple comparisons at p < .05, significantly lower CBF was observed in abstinent smokers compared to nonsmokers, in the bilateral IFG and the occipital gyrus (Figure 2; Table 2). Similarly, significantly lower CBF was observed in satiated smokers compared with nonsmokers, in the same bilateral IFG and occipital gyrus regions, with a 35% overlap in the observed clusters across the two contrasts (abstinent vs. nonsmokers and satiated vs. nonsmokers) (Figure 2; Table 2). No regions showed higher CBF in the smoker conditions compared with nonsmokers. No clusters survived correction for multiple comparisons in the abstinent versus satiated CBF comparisons.
Figure 2.
Top row: Whole-brain analyses showing lower cerebral blood flow (CBF) (t maps cluster-corrected at p < .05, cluster size ≥ 433) in abstinent smokers and satiated smokers relative to controls in bilateral inferior frontal gyrus (IFG), occipital cortex, and insula with 35% overlap between the two contrasts. Bottom row: Region of interest–level analyses in bilateral IFG and occipital cortex showing lower CBF in abstinent smokers relative to satiated smokers and non-smokers.*Significantly different from the two other groups with p < .001.
ROI-Level Analyses
Given the high overlap in the clusters showing fMRI activation differences between nonsmokers and the abstinent and satiated smokers, we created bilateral IFG ROIs that included just the overlapping voxels. A repeated-measures analysis of variance indicated significantly higher activity in abstinent smokers compared with satiated smokers in the right IFG (p = .005) (Figure 1).
Similarly, we overlapped the clusters showing significant differences in CBF between abstinent smokers and nonsmokers and between satiated smokers and nonsmokers. A repeated-measures analysis of variance indicated no differences between abstinent and satiated smokers (p = .15) (Figure 2).
Finally, to assess the correlation between successful inhibition activation and CBF values between smokers and nonsmokers, we created bilateral IFG ROIs that included just the overlapping voxels across the two BOLD and CBF modalities (two bilateral ROIs for nonsmokers and abstinent smokers and two others for nonsmokers and satiated smokers). Pearson’s correlation test revealed no significant correlations for the nonsmokers and abstinent smokers or for the nonsmokers and satiated smokers, in either of the two corresponding IFG ROIs, with r less than 0.09 and p greater than .16. However, FTND scores were significantly negatively correlated with CBF in abstinent smokers (r = 0.38, p = .03) and satiated smokers (r = 0.4, p = .027).
Discussion
In this study investigating brain differences between smokers and nonsmokers, whole-brain analyses showed that abstinent and satiated smokers had higher inhibitory control-related activity in bilateral IFG and lower CBF in the bilateral IFG and in the occipital cortex, compared with nonsmoker controls. Subsequent ROI-level analyses in these regions indicated that the task activation effects were more pronounced in abstinent relative to satiated smokers. These alterations may directly contribute to nicotine dependence and increase the difficulties that many smokers experience when attempting to quit.
Smoker Versus Nonsmoker Differences
Our whole-brain analysis of successful response inhibition revealed differences between smokers and nonsmokers in the IFG. The IFG is a key region involved in inhibitory control33 and has been shown in functional imaging studies to be associated with response inhibition in paradigms such as the SST34 and the GO/NO-GO task.35 In addition, genetic studies suggest that brain regions rich in nicotinic acetylcholine receptors (nAChRs) (eg, IFG, ventral striatum, thalamus) would be expected to exhibit changes in activation during nicotine abstinence or satiety.36 To explain, the stimulation of nAChRs is intimately linked with effects of smoking as shown, for example, in Brody.11(p2)Nicotine binds to α4β2 nAChRs on dopaminergic neurons in the ventral tegmental area,11(p2) which project to the nucleus accumbens, amygdala, and the prefrontal cortex.37 In chronic smokers, a single cigarette produces nearly complete saturation and desensitization of α4β2 nAChRs.11(p2) In contrast to nicotine delivery, abstinence from nicotine increases the availability of unbound α4β2 nAChRs, which in turn, contributes to smoking urges.36
It has been shown that those with substance use disorders in general have altered function in extensive areas of the cortex (especially in the frontal and temporal cortex), during cognitive memory tasks, inhibition, and decision-making.38 In the rare studies investigating the neural correlates of motor response inhibition in smokers using the GO/NO-GO task, Weywadt et al. recently observed, in accordance with our findings, increased activation in 35 current smokers compared with 35 nonsmokers in regions of the brain implicated in successful response inhibition including parts of the parietal lobe and the cerebellum. Moreover, this was observed when behavioral performance was similar between the groups.23 However, they did not observe activation differences in the IFG, which might be related to age differences between our young sample (mean age ± SD = 23 ± 4) compared with theirs (mean age ± SD = 59 ± 10). In fact, aging has been linked to changes in processes that support cognitive control,39 suggesting these processes might engage different regions in younger than older individuals. In addition, Galvan et al.40 reported that activation of the IFG correlated positively with the severity of nicotine dependence in a younger sample of adolescents who ranged between 15 and 21 years old (although it did not distinguish between smokers and nonsmokers), suggesting this region might be more relevant for younger samples. In contrast, Nestor et al. reported, in a smaller sample, performance deficits and reduced successful inhibition activation in 13 smokers compared with 13 never-smokers in prefrontal cortical regions including bilateral IFG.22 It is important to note that, in comparison to the work of Nestor et al., the SST used in the present study applies an adaptive algorithm that may have reduced any potential group differences in behavioral performance that would be observed with the GO/NO-GO task that they used. Future work might benefit from using multiple measures of inhibition to better understand potential task differences in nicotine-dependent individuals.
The lower perfusion in smokers than nonsmokers that we observed is consistent with the literature reporting a hypoperfusion in smokers in the frontal gyrus, anterior cingulate cortex, insula, right temporal cortex, and dorsolateral prefrontal cortex in adolescents and adults.13,41 Similar effects were observed in elderly smokers as well.42 Moreover, the nasal administration of nicotine17,19 or cigarette smoking43 during scanning resulted in decreased global cerebral perfusion compared with nonsmokers. Likewise, smokers who smoked ad lib prior to single-photon emission computed tomography scanning (including the morning of the scan) had decreased global brain activity compared with former smokers and nonsmokers.11 These findings are supported by studies using transcranial Doppler ultrasound or inhalation methods to measure responses to smoking, showing diminished CBF in the occipital cortex.11,17 This is also in line with studies reporting that smokers are at higher risk for cardiovascular disease and atherosclerosis, which hardens the blood vessels, narrows arteries, and slows blood flow.23
In the present study, no significant correlations were observed between the response inhibition BOLD activations and CBF measures in bilateral IFG. This is consistent with recent findings studying the mechanisms of vascular regulation that underlie resting-state BOLD fluctuations,44,45 which are still incompletely understood. These findings suggested that the degree of BOLD–CBF coupling is not homogeneous in the brain and is significantly decreased as the ratio of large vessels to tissue volume increases. For instance, BOLD fluctuations at the sites of medium-to-small vessels (more proximal to local neuronal activity) present near the default mode network regions (such as the medial prefrontal cortex, the posterior cingulate cortex and the inferior parietal lobule) are more closely regulated by dynamic regulations in CBF, whereas CBF regulation decreases closer to large veins (near the bilateral IFG), which are more distal to neuronal activity and thus are more affected by non-neuronal physiological noise.44,45 On the other hand, the increased response inhibition activity coupled with decreased CBF in smokers in the same IFG regions points toward a compensation mechanism allowing them to perform equally on cognitive tasks such as the SST.
Abstinent Smokers Versus Satiated Smokers
Our ROI-level analyses show that the increased activation of smokers associated with response inhibition is enhanced when abstinent. Careful separation of the cognitive domains affected by nicotinic stimulation has pointed out that cigarette smoking and nicotine administration enhance neurotransmission through prefrontal and paralimbic cortico-basal ganglia-thalamic circuits,46 regardless of smoking status in one study.47 This suggests that nicotine’s effects on attentional performance are probably mediated by nicotinic receptor activation. These acute beneficial effects of nicotine may explain why IFG activation in smokers is less when satiated than abstinent.
In the only study, we are aware of assessing CBF differences after overnight abstinence (using positron emission tomography), CBF was reduced by 17% relative to nonsmokers in frontal and parietal regions at the end of abstinence and increased by 8% at 60minutes after smoking resumption, suggesting an acute restoration by resumption of smoking.13 The rapid reversal is consistent with the stimulant effect of nicotine or other components of tobacco smoke on flow-metabolism coupling in the brain, as also indicated by the reversible effects of smoking on cardiovascular fitness.13 This study also suggested that brain energy metabolism is distinctly compromised in abstaining smokers, which may have implications for their cognitive function. In addition, in the studies examining regional activity responses to nicotine or smoking, the most common findings are relative increases in activity in the prefrontal cortex (including the dorsolateral prefrontal cortex, and inferior frontal, medial frontal, and orbitofrontal gyri), thalamus, and the occipital cortex.17 The neurobiological mechanisms by which nicotine or smoking change CBF are not thoroughly understood. The presynaptic nicotinic acetylcholine receptors may mediate nicotine-induced increases in CBF, as shown in rat cerebral cortex.48 In addition, [14C]deoxyglucose autoradiographic studies suggest that the α4β2 nAChRs presumably drive local cerebrometabolic effects of nicotine.13
Limitations
In our study, nicotine withdrawal was not measured. According to behavioral and psychological studies, valid withdrawal symptoms such as anger, anxiety, depression, difficulty concentrating, impatience, insomnia, and restlessness peak within the first week and last for 2–4 weeks.49 Although we anticipate that all our regular smokers were experiencing some level of acute withdrawal arising from their overnight abstinence, information on the magnitude of withdrawal effects may have explained some portion of the interindividual variation in BOLD activation and perfusion effects.14,50 A second limitation of this article is the relatively small sample size with just 20 subjects per group. However, since we found very similar effects in task activation and in blood perfusion in two different between-group, whole-brain analyses (abstinent vs. nonsmokers and satiated vs. nonsmokers), we believe that this is strong evidence that the observed effects are reliable. Another limitation of this study is the low nicotine exposure in our smokers opening up the possibility that our findings may not generalize to heavier smokers. However, it is interesting to note that similar neuroimaging effects to what we found in our sample have been observed in heavier smoking groups, as detailed earlier. It is also worth noting that finding these effects with relatively low nicotine smoking exposure in a young sample may well suggest even greater effects in heavier users.
In summary, we show here that abstinent and satiated smokers are characterized by higher fMRI brain activation during successful response inhibitions and lower CBF in bilateral IFG relative to nonsmokers. These clear response inhibition and CBF differences compared with nonsmokers, regardless of smoking state, suggest a possible trait-like difference between smokers and nonsmokers or an effect of chronic smoking that is discernible across abstinent or satiated states. These effects in key brain regions involved in impulse control may contribute to the enormous difficulties that so many smokers experience when attempting to quit and suggest targets for assessing the efficacy of therapeutic interventions.
Funding
This study was funded by Tobacco Center of Regulatory Science award P50DA036114 from the National Institute on Drug Abuse and Food and Drug Administration (FDA) and by Centers of Biomedical Research Excellence award P20GM103644 from the National Institute of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration.
Declaration of Interests
None declared.
Acknowledgment
BC and PAS contributed equally to this study.
References
- 1. U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General; 2014. SurgeonGeneral.gov; https://www.surgeongeneral.gov/library/reports/50-years-of-progress/index.html. Accessed November 14, 2017. [Google Scholar]
- 2. Higgins ST, Heil SH, Sigmon SC, et al. Addiction potential of cigarettes with reduced nicotine content in populations with psychiatric disorders and other vulnerabilities to tobacco addiction. JAMA Psychiatry. 2017;74(10):1056–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pan P, Shi H, Zhong J, et al. Chronic smoking and brain gray matter changes: evidence from meta-analysis of voxel-based morphometry studies. Neurol Sci. 2013;34(6):813–817. [DOI] [PubMed] [Google Scholar]
- 4. Gallinat J, Meisenzahl E, Jacobsen LK, et al. Smoking and structural brain deficits: a volumetric MR investigation. Eur J Neurosci. 2006;24(6):1744–1750. [DOI] [PubMed] [Google Scholar]
- 5. Gons RAR, van Norden AGW, de Laat KF, et al. Cigarette smoking is associated with reduced microstructural integrity of cerebral white matter. Brain. 2011;134(7):2116–2124. [DOI] [PubMed] [Google Scholar]
- 6. Hudkins M, O’Neill J, Tobias MC, Bartzokis G, London ED. Cigarette smoking and white matter microstructure. Psychopharmacology (Berl). 2012;221(2):285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liao Y, Tang J, Deng Q, et al. Bilateral fronto-parietal integrity in young chronic cigarette smokers: a diffusion tensor imaging study. PLoS One. 2011;6(11):e26460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Azizian A, Monterosso JR, Brody AL, Simon SL, London ED. Severity of nicotine dependence moderates performance on perceptual-motor tests of attention. Nicotine Tob Res. 2008;10(4):599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xu J, Mendrek A, Cohen MS, et al. Effect of cigarette smoking on prefrontal cortical function in nondeprived smokers performing the Stroop Task. Neuropsychopharmacology. 2007;32(6):1421–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lawrence NS, Ross TJ, Stein EA. Cognitive mechanisms of nicotine on visual attention. Neuron. 2002;36(3):539–548. [DOI] [PubMed] [Google Scholar]
- 11. Brody AL. Functional brain imaging of tobacco use and dependence. J Psychiatr Res. 2006;40(5):404–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tanabe J, Nyberg E, Martin LF, et al. Nicotine effects on default mode network during resting state. Psychopharmacology (Berl). 2011;216(2):287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vafaee MS, Gjedde A, Imamirad N, et al. Smoking normalizes cerebral blood flow and oxygen consumption after 12-hour abstention. J Cereb Blood Flow Metab. 2015;35(4):699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Franklin TR, Jagannathan K, Hager N, et al. Brain substrates of early (4h) cigarette abstinence: identification of treatment targets. Drug Alcohol Depend. 2018;182:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wennmalm A. Effect of cigarette smoking on basal and carbon dioxide stimulated cerebral blood flow in man. Clin Physiol. 1982;2(6):529–535. [DOI] [PubMed] [Google Scholar]
- 16. Skinhoj E, Olesen J, Paulson OB. Influence of smoking and nicotine on cerebral blood flow and metabolic rate of oxygen in man. J Appl Physiol. 1973;35(6):820–822. [DOI] [PubMed] [Google Scholar]
- 17. Domino EF, Minoshima S, Guthrie SK, et al. Effects of nicotine on regional cerebral glucose metabolism in awake resting tobacco smokers. Neuroscience. 2000;101(2):277–282. [DOI] [PubMed] [Google Scholar]
- 18. Marenco T, Bernstein S, Cumming P, Clarke PB. Effects of nicotine and chlorisondamine on cerebral glucose utilization in immobilized and freely-moving rats. Br J Pharmacol. 2000;129(1):147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stapleton JM, Gilson SF, Wong DF, et al. Intravenous nicotine reduces cerebral glucose metabolism: a preliminary study. Neuropsychopharmacology. 2003;28(4):765–772. [DOI] [PubMed] [Google Scholar]
- 20. Gehricke JG, Potkin SG, Leslie FM, et al. Nicotine-induced brain metabolism associated with anger provocation. Behav Brain Funct. 2009;5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xin Z, Ting LX, Yi ZX, Li D, Bao ZA. Response inhibition of cigarette-related cues in male light smokers: behavioral evidence using a two-choice oddball paradigm. Front Psychol. 2015;6:1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nestor L, McCabe E, Jones J, Clancy L, Garavan H. Differences in “bottom-up” and “top-down” neural activity in current and former cigarette smokers: evidence for neural substrates which may promote nicotine abstinence through increased cognitive control. Neuroimage. 2011;56(4):2258–2275. [DOI] [PubMed] [Google Scholar]
- 23. Weywadt CR, Kiehl KA, Claus ED. Neural correlates of response inhibition in current and former smokers. Behav Brain Res. 2017;319:207–218. [DOI] [PubMed] [Google Scholar]
- 24. Huang CL, Lin HH, Wang HH. The psychometric properties of the Chinese version of the Fagerström Test for Nicotine Dependence. Addict Behav. 2006;31(12):2324–2327. [DOI] [PubMed] [Google Scholar]
- 25. Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev. 1988;8(1):77–100. [Google Scholar]
- 26. Norman SB, Cissell SH, Means-Christensen AJ, Stein MB. Development and validation of an Overall Anxiety Severity And Impairment Scale (OASIS). Depress Anxiety. 2006;23(4):245–249. [DOI] [PubMed] [Google Scholar]
- 27. Logan GD, Schachar RJ, Tannock R. Impulsivity and inhibitory control. Psychol Sci. 1997;8(1):60–64. [Google Scholar]
- 28. Logan GD, Cowan WB. On the ability to inhibit thought and action: a theory of an act of control. Psychol Rev. 1984;91(3):295. [DOI] [PubMed] [Google Scholar]
- 29. Band GP, van der Molen MW, Logan GD. Horse-race model simulations of the stop-signal procedure. Acta Psychol (Amst). 2003;112(2):105–142. [DOI] [PubMed] [Google Scholar]
- 30. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. [DOI] [PubMed] [Google Scholar]
- 31. Aslan S, Xu F, Wang PL, et al. Estimation of labeling efficiency in pseudocontinuous arterial spin labeling. Magn Reson Med. 2010;63(3):765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Congdon E, Mumford JA, Cohen JR, Galvan A, Canli T, Poldrack RA. Measurement and reliability of response inhibition. Front Psychol. 2012;3:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci. 2014;18(4):177–185. [DOI] [PubMed] [Google Scholar]
- 34. Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6(2):115–116. [DOI] [PubMed] [Google Scholar]
- 35. Steele VR, Aharoni E, Munro GE, et al. A large scale (N = 102) functional neuroimaging study of response inhibition in a Go/NoGo task. Behav Brain Res. 2013;256:529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Staley JK, Krishnan-Sarin S, Cosgrove KP, et al. Human tobacco smokers in early abstinence have higher levels of beta2* nicotinic acetylcholine receptors than nonsmokers. J Neurosci. 2006;26(34):8707–8714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162(8):1403–1413. [DOI] [PubMed] [Google Scholar]
- 38. Verdejo-Garcia A, Benbrook A, Funderburk F, David P, Cadet J-L, Bolla KI. The differential relationship between cocaine use and marijuana use on decision-making performance over repeat testing with the Iowa Gambling Task. Drug Alcohol Depend. 2007;90(1):2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Braver TS, Barch DM. A theory of cognitive control, aging cognition, and neuromodulation. Neurosci Biobehav Rev. 2002;26(7):809–817. [DOI] [PubMed] [Google Scholar]
- 40. Galván A, Poldrack RA, Baker CM, McGlennen KM, London ED. Neural correlates of response inhibition and cigarette smoking in late adolescence. Neuropsychopharmacology. 2011;36(5):970–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang Z, Suh J, Duan D, et al. A hypo-status in drug-dependent brain revealed by multi-modal MRI. Addict Biol. 2017;22(6):1622–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Siennicki-Lantz A, Reinprecht F, Wollmer P, Elmståhl S. Smoking-related changes in cerebral perfusion in a population of elderly men. Neuroepidemiology. 2008;30(2):84–92. [DOI] [PubMed] [Google Scholar]
- 43. Yamamoto Y, Nishiyama Y, Monden T, Satoh K, Ohkawa M. A study of the acute effect of smoking on cerebral blood flow using 99mTc-ECD SPET. Eur J Nucl Med Mol Imaging. 2003;30(4):612–614. [DOI] [PubMed] [Google Scholar]
- 44. Polimeni JR, Fischl B, Greve DN, Wald LL. Laminar analysis of 7T BOLD using an imposed spatial activation pattern in human V1. Neuroimage. 2010;52(4):1334–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tak S, Wang DJ, Polimeni JR, Yan L, Chen JJ. Dynamic and static contributions of the cerebrovasculature to the resting-state BOLD signal. Neuroimage. 2014;84:672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Newhouse PA, Potter A, Singh A. Effects of nicotinic stimulation on cognitive performance. Curr Opin Pharmacol. 2004;4(1):36–46. [DOI] [PubMed] [Google Scholar]
- 47. Ernst M, Heishman SJ, Spurgeon L, London ED. Smoking history and nicotine effects on cognitive performance. Neuropsychopharmacology. 2001;25(3):313–319. [DOI] [PubMed] [Google Scholar]
- 48. Uchida S, Kawashima K, Lee TJ. Nicotine-induced NO-mediated increase in cortical cerebral blood flow is blocked by beta2-adrenoceptor antagonists in the anesthetized rats. Auton Neurosci. 2002;96(2): 126–130. [DOI] [PubMed] [Google Scholar]
- 49. Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res. 2007;9(3):315–327. [DOI] [PubMed] [Google Scholar]
- 50. Ashare RL, Falcone M, Lerman C. Cognitive function during nicotine withdrawal: implications for nicotine dependence treatment. Neuropharmacology. 2014;76(Pt B):581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]