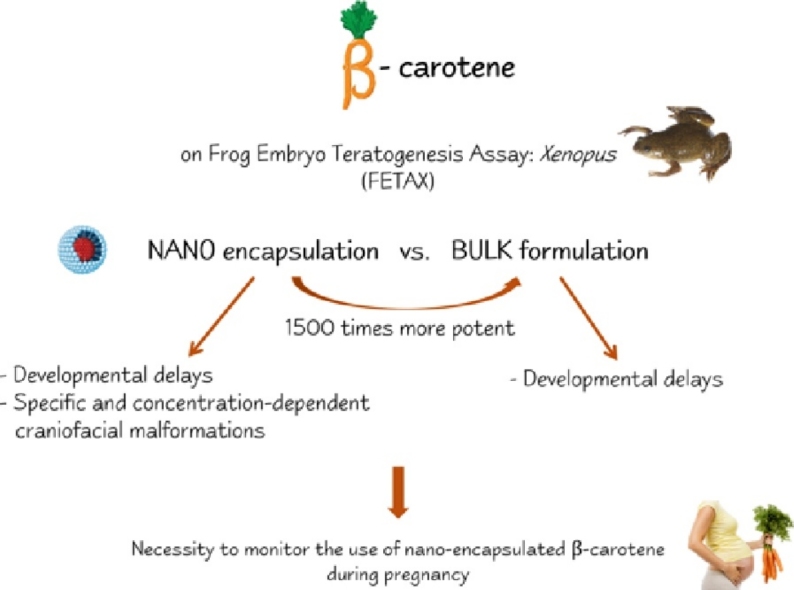
Graphical abstract
Keywords: Functional food, β-carotene, FETAX, Alternative model
Highlights
-
•
Effects of bulk and nanoencapsulated βcarotene on Xenopus laevis embryo development.
-
•
NANO βcarotene induced specific malformations at craniofacial and eye level.
-
•
Necessity to monitor the use of nanoencapsulated βcarotene during pregnancy.
-
•
FETAX: a rapid screening test for micronutrient evaluation after nanoencapsulation.
Abstract
Vitamin A plays a vital role during embryo development as most precursor of embryonic retinoic acid, a key morphogen during embryogenesis. Carotenoids, including β-carotene, are important vegetal source of Vitamin A and in contrast to teratogenic potential of animal-derived retinoids, β‐carotene is usually considered freed from embryotoxic effects and supplements in pregnancy with β-carotene are suggested. The aim of the present work is to evaluate the effect of bulk and nano-encapsulated β-carotene on embryo development, by using the animal model Frog Embryo Teratogenesis Assay: Xenopus- FETAX. Xenopus laevis embryos were exposed from late gastrula till pharyngula (the phylotypic stage for vertebrates) to the concentrations of BULK β-carotene 150-3000 ng/mL and NANO β-carotene 0.75-30 ng/mL. At pharyngula stage, some embryos were processed for whole mount neural crest cell immunostaining, while others embryos were allowed to develop till tadpole for morphological and histological evaluation of neural crest cells-derived structures. In this model, the nano-encapsulated β-carotene induced specific malformations at craniofacial and eye level, while the bulk formulation only induced developmental delays. Finally, the applied alternative animal model resulted a rapid and sensitive screening method able to re-evaluate the teratogenic profile of nano-encapsulated micronutrients.
1. Introduction
Vitamin A plays a key role during embryo development because it’s the main precursor of embryonic retinoic acid (RA), a crucial morphogen during embryogenesis [1]. In mammalian species, RA is essential during the developmental and reproductive phases for directing the growth and differentiation of cells, tissues and organs [2,3]. In vertebrates, RA is also considered the main molecule involved in craniofacial morphogenesis in vertebrates, and in activating the expression of a cascade of growth factors and genes controlling craniofacial development [4].
The deficiency, as well as the excess of embryonic RA, is associated with malformations in many districts, including cranio-facial defects in humans and animals [1,3,[5], [6], [7], [8], [9]]. Therefore, the regulation of RA amount that is available to the embryo at specific times and to a given site is of critical importance.
In rats, congenital malformations caused by the administration of vitamin A excess during pregnancy were first described by Cohaln (1954) [10], and later confirmed with overlapping defects patterns in different animal species [11].
The similarities of the teratological effects of vitamin A deficiency and excess indicates common targets and an important role for vitamin A in the embryo development [12].
These teratogenic effects, produced by vitamin A over-intake or the clinical use of synthetic retinoids, are classified as FRS (Fetal Retinoid Syndrome). Among characteristics of FRS, cranio-facial defects and encephalic, cardio-vascular and thymic abnormalities are reported [13]. Vitamin A or retinoids supplementation should be, by consequence, contraindicated during pregnancy.
Carotenoids, including β-carotene, are an important vegetal source of vitamin A; in mammals they are obtained from the mother, and in egg-laying species they are stored in the eggs as provitamin A. Thanks to their molecular structure, carotenoids show anti-inflammatory and antioxidant activities such as binding and eliminating several free radicals, ROS included and quenching singlet oxygen, thus suppressing lipid peroxidation and preventing oxidative damage [14]. For this reason, a diet rich in fresh vegetables with a high vitamins content (especially vitamins C and E) and other substances such as carotenoids has long been suggested, in mammals, including humans, as a protection against cardiovascular diseases, tumors and inflammatory states [14].
In human nutrition β-carotene contributes more than 30 % to vitamin A intake in Western countries, and in many populations it is the exclusive source of vitamin A [15]. It has been established that the recommended daily dose of vitamin A (retinol) is 600 μg/day in adult women, 700 μg/day in pregnant women and 1000 μg/day during lactation, which corresponds to approximately 3.6, 4.2 and 6 mg/day of β-carotene, respectively [16]. The maximum tolerable dose of vitamin A is 3000 μg/day, corresponding to 18 mg/day of β carotene [16].
In contrast to teratogenic potential of animal-derived retinoids, β‐carotene is usually considered free of embryotoxic effects [17,18] and supplements before, during and after pregnancy with β-carotene have been suggested. In fact, it has been proved that β-carotene could be useful to achieve the necessary quantity of vitamin A for the correct embryonic development, improving fetal development and growth, and reducing the incidence of preterm births, birth defects, risk of infections and postpartum mortality [15].
β-carotene is photosensitive and because of its strong antioxidant action it can oxidize very quickly causing alterations of the product to which it is supplemented [19]. In order to improve the stability and the bioavailability of this compounds, β-carotene is also used for supplementation in nano-encapsulated form, despite the toxico-kinetic and toxico-dynamic profile obtained for the bulk form is probably different after nano-encapsulation [20].
In recent years, nano-encapsulation technology greatly increased in food industry thanks to its advantages. Nano-encapsulation is generally used to deliver different nutraceutical products and bioactive molecules like vitamins and antioxidants, allowing production of functional foods with enhanced functionality and stability by protecting the encapsulated materials from environmental, enzymatic and chemical changes, and by ameliorating their organoleptic properties [21]. Lipid-based nano-encapsulation systems enhance, for example, the performance of lipophilic molecules by improving their solubility. In these systems, the lipophilic substance (core) is surrounded by an amphiphilic coat (shell) made of surface-active material that enhances solubility in aqueous media [22]. Nano-encapsulation provides significant savings to formulators, because it reduces the amount of active ingredients, increasing their bioavailability and shelf-life [23]. Nevertheless, nano-encapsulation raised some questions about the potential toxic effects of nano-encapsulated nutrients in food and beverages on human health and environment. This technology can, infact, modify absorption, distribution, metabolism, and excretion processes [24,25]. For these reasons, the European Food Safety Agency (EFSA) and the Food and Drug Administration (FDA) promoted the search for valid alternative methods able to identify and evaluate the risks deriving from nanotechnologies [26].
Simple culture systems, like rat and Xenopus laevis embryos in vitro development, have already been proposed as animal models to assess the developmental toxicity of nano-encapsulated molecules and the effects of nutrient excess or deficiency on embryos [[27], [28], [29], [30], [31], [32], [33]] thanks to the interclass's similarities at both morphological and molecular level at the phylotypic stages (pharyngula, as named by Ballard [34], corresponding in vertebrates to the neurulation and branchial arch organization stages). However, because of physiological differences in provitamin A carotenoid absorption between rodents and humans, rodents are not suitable animal models for studying human carotenoid absorption [35,16].
Among the non-mammalian models, the standardized Frog Embryo Teratogenesis Assay: Xenopus (FETAX) test represents a high sensitive tool to evaluate potential embryotoxicity effects [[36], [37], [38], [39]]. Based on the amphibian Xenopus laevis, FETAX protocol is a well known and powerful bioassay used not only to screen the teratogenic potential of single compounds (developmental toxicants, such as pesticides or some metal oxide nanoparticle), but also to evaluate their possible joint action [31,[40], [41], [42], [43], [44], [45]]. Due to its rapid aquatic development, numerous and large size eggs and easily manipulating embryos, X. laevis is one of the most used model system for vertebrate experimental embryology [36]. These characteristics led to the development of assay conditions to systematically test the teratogenicity of any chemical compound of interest and to determine and categorize the induced developmental malformations [37]. In recent years, thanks to its ability to predict human teratogens with a 75 % accuracy [30], the use of FETAX has been extended to the establishment of models for human diseases in addition to its classical use as ecotoxicological test [45,46]. Last but not least, FETAX is considered an alternative animal-free model, and as such is not subject to mandatory regulative rules [32].
Moreover, it must be considered that nano-encapsulation of Vitamin A was demonstrated to be able to enhance embryotoxicity in different developmental models, X. laevis included [47].
The aim of the work is to evaluate and compare the effect of bulk and nano-encapsulated β-carotene on X. laevis embryo development by using a modified version [47] of the alternative animal-free model FETAX, with the final goal to eventually reconsider vitamin nano-encapsulation toxicological evaluation.
2. Material and method
2.1. Chemicals
All analytical grade reagents, β-carotene (BULK β-carotene), 3-amino-benzoic acid ethylester (MS222) and salts for FETAX solution were purchased from Sigma-Aldrich S.r.l., Italy. The traded nano-formulations with (NANO β-carotene) or without (SHELL) encapsulated β-carotene were kindly provided by Aquanova® (Novasol® GmbH, Germany). All suspensions and solutions were prepared in FETAX whose composition in mg/L was: 625 NaCl, 96 NaHCO3, 30 KCl, 15 CaCl2, 60 CaSO4 •2H2 O, and 70 MgSO4). For every experiment, solutions were freshly prepared and maintained in the dark. Nano micelles (with or without β-carotene) have been supplied as a 1% emulsion in vegetable oil of nanoliposomes, formed by a shell of lipids from soya lecithin and a core of β-carotene (NANO β-carotene) or lecithin itself (SHELL). The product micelle is stable with respect to pH and temperature and has a diameter of approximately 30 nm.
2.2. Experimental design
2.2.1. FETAX methodology
Each assay was performed in triplicate. Adults of X. laevis (Harlan Italia, Bresso, Italy) were maintained in controlled conditions (T = 20+2 °C; pH = 7.5+0.5; Conductivity = 1000 ± 100 μS; 12 h light/dark cycle) in an automatic breeding system (TecnoPlus, Techniplast, Italia) and fed a semisynthetic diet two times per week (XE40 by Mucedola; Settimo Milanese, Italy). Embryos were obtained from natural mating of couples. After mating, embryos transferred in Petri dishes and processed according to Battistoni et al. [47].
Embryos were exposed from NF stage 13 (early neurula stage) to NF stage 26 (phylotypic stage) [36] to BULK β-carotene (150–300 – 750–1500 ng/mL dissolved in DMSO) or NANO β-carotene (0.75–1.5 – 3–7.5 – 15–30 ng/mL dissolved in FETAX). BULK and NANO β-carotene concentrations were selected on the basis of preliminary range finding tests, considering the maternal plasma concentrations reported in Goldberg as reference to set concentrations for the bulk form [48]. A control group exposed to the shell alone (SHELL) and a control group containing the solvent used for the bulk form (1 μL DMSO /mL FETAX, CON + DMSO) were also prepared. At NF stage 26, 5 embryos/group/replicate were processed for immunostaining, while the remaining were removed from treatment solution, rinsed and incubated in FETAX solution. Morphology was evaluated when NF 37 and 46 were reached in controls. At NF stage 37 and 46, 5 embryos/group/replicate were also processed for histological evaluation.
2.2.2. Immunostaining for CRABPI
Immunostaining of CRABPI was used to visualize the migrating neural crest cells [49]. The whole mount immunostaining procedures on X. laevis embryos have been previously described respectively in Menegola et al. [50] and Di Renzo et al. [41]. The monoclonal antibody was the anti-CRABPI (cellular retinoic acid binding protein), ABR, Italy, dilution 1:500. The anti-mouse-Ig-peroxidase (Fab fragment Boehringer, Italy) was diluted 1:40. Immunoreactivity was visualized with the substrate 4-Cl-1-naphtol (Sigma, Italy) and 0.006 % H2O2. Stained cells appeared dark brown at light microscope. Negative control was performed without primary antibody incubation.
2.2.3. Histological analysis
For the histological analysis, control and exposed larvae at NF stage 37 and 46 were overdosed with anesthetic MS-222 and fixed overnight in Bouin’s fluid. After fixation, larvae were rinsed in tap and distilled water, dehydrated in an ascending ethanol series and embedded in Bio-plast tissue embedding medium (Bio-optica, Italy). Seven micrometers frontal serial sections of the entire larvae were obtained by a Reichert rotative microtome and stained with Hematoxylin-Eosin. All larvae organs and tissues were considered.
2.2.4. Acridine orange
In order to obtain a detailed and three-dimensional view of the morphological structures, control and exposed larvae at NF stages 37 and 46 were overdosed with anesthetic MS-222, fixed in 10 % buffered formalin overnight at 4 °C, rinsed and stained with acridine orange (Sigma, Italy; 5 mg/mL PBS), according to the method described by Zucker et al. [51] and, after washing in PBS, viewed under a fluorescence microscope.
2.3. Statistical analysis
Data on abnormalities, expressed in percentage of malformed samples, were analyzed by Chi-square test. The level of significance was set at p < 0.05.
2.4. Benchmark-dose analysis
Benchmark-dose (BMD) method was applied on abnormalities. Data were modeled by using PROAST 67 software in order to characterize the single dose response curves on total abnormal larvae and to find the relative potency factor (RPF) of BULK versus NANO β-carotene. Additionally, dose-response evaluation of specific effect categories (classified as malformations, severe malformations and extreme malformations) and their BMDs for Benchmark-response (BMR) at 50 % of NANO β-carotene was performed.
3. Results
3.1. Stage 46 larvae, morphological evaluation
Controls and X. laevis larvae exposed from NF stage 13 to NF stage 26 to the BULK and NANO β-carotene at the end of the test reached NF stage 46. The totality of unexposed larvae developed normally, displaying well distinguishable craniofacial portions, well-expanded gill basket, linear brain parallel to the body axis and normally developed eye. No malformations were recorded in larvae exposed to BULK β-carotene at any tested concentration. Anyway, developmental delays were recorded starting from dose 750 ng/mL with consequent decrease of larvae displaying normal phenotype with a significant linear trend (Fig. 1 A-A’; Table 1).
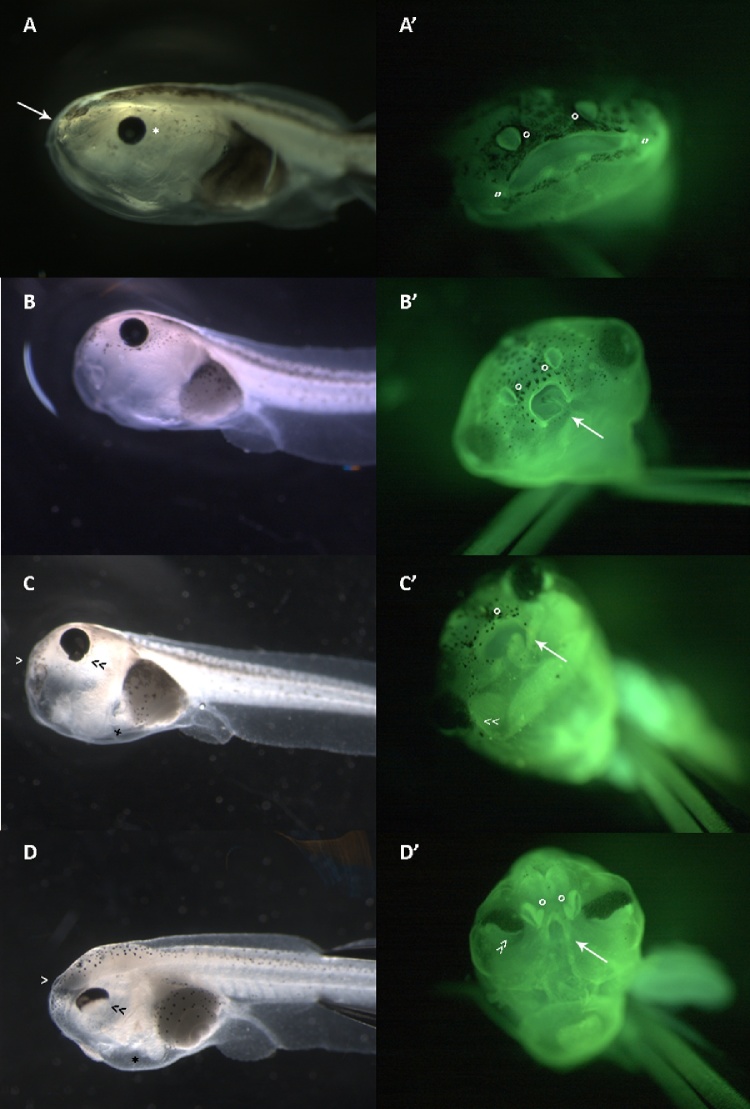
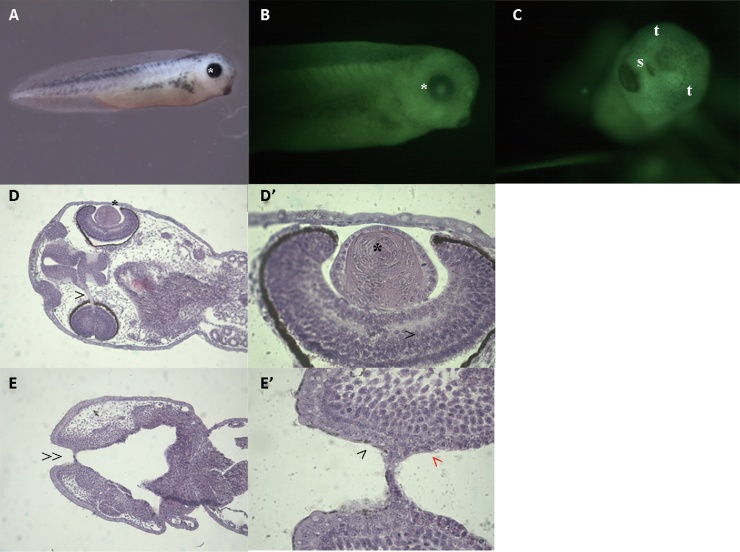
Fig. 1.
Morphological appearance of NF stage 46 larvae before (A-B-C-D) and after fixation and acridine orange staining according to Zucker et al. [51] (A’-B’-C’-D’). Magnification 20X (A-B-C-D); magnification 32X (A’-B’-C’-D’).
A: lateral view of a control larva with normal phenotype. Note the dorsal neural tube (NT), the well-expanded gill basket (GB), the normal eye (*), the intestine (I) and the oral opening (arrow).
A': frontal view of a larva with normal phenotype stained with acridine orange. The naris (o) are well distinguishable, as well as the well-expanded oral cavity (‘’).
B: lateral view of a NANO β-carotene 3 ng/mL larva with malformed phenotype with round-shape head (NT).
B': frontal view of a malformed larva with round-shape head stained with acridine orange. The nasal pits (o) are well formed but in a different inclination due to the round-shape head. The oral cavity present marked reduction accompanied by oedema (arrow).
C: lateral view of a larva exposed to NANO β-carotene 7,5 ng/mL with severely malformed phenotype with round-shape and coerced head. Note the extremely reduced oral cavity (>), the abnormal shape of the eye (<<) and the presence of oedema in the cardiac region (*).
C': frontal view of a severely malformed larva with round-shape and coerced head stained with acridine orange. Note the nasal pits barely visible (o), the abnormal eyes (<<) and the altered oral cavity (arrow).
D: lateral view of a larva exposed to NANO β-carotene 30 ng/mL with extremely malformed phenotype with funnel shaped oral cavity (>). Note the not linear and swollen neural tube (NT), the abnormal eye with half-moon shape (<<) and the oedema in the cardiac region (*).
D': frontal view of an extremely malformed larva with funnel shaped oral cavity stained with acridine orange. Note the irregular nasal pits (o), the half-moon shape eye (>>) and the oral cavity, almost a small split (arrow).
Table 1.
Abnormalities observed in X. laevis larvae after BULK and NANO β-carotene exposure (%).
| CONT + DMSO | BULK 300 | BULK 750 | BULK 1500 | BULK 3000 | |
|---|---|---|---|---|---|
| Total examined | 37 | 10 | 29 | 29 | 58 |
| Normal** | 94.59 | 100.00 | 68.97 | 82.76 | 58.62 |
| Developmental delay** | 5.41 | 0.00 | 31.03 | 17.24 | 41.38 |
| Abnormal | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| malformed | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| severly malformed | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| extremely malformed | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| SHELL | NANO 075 | NANO 1,5 | NANO 3 | NANO 7,5 | NANO 15 | NANO 30 | |
|---|---|---|---|---|---|---|---|
| Total examined | 13 | 24 | 44 | 44 | 44 | 10 | 27 |
| Normal** | 100.00 | 95.83 | 63.64 | 31.82 | 0.00 | 0.00 | 0.00 |
| Developmental delay** | 0.00 | 4.17 | 29.55 | 40.91 | 4.55 | 0.00 | 0.00 |
| Abnormal** | 0.00 | 0.00 | 6.82 | 27.27 | 95.45 | 100.00 | 100.00 |
| malformed** | 0.00 | 0.00 | 2.27 | 27.27 | 31.82 | 20.00 | 0.00 |
| severly malformed** | 0.00 | 0.00 | 2.27 | 0.00 | 29.55 | 20.00 | 7.41 |
| extremely malformed** | 0.00 | 0.00 | 2.27 | 0.00 | 34.09 | 60.00 | 92.59 |
p < 0.001, Chi-squared test for trend.
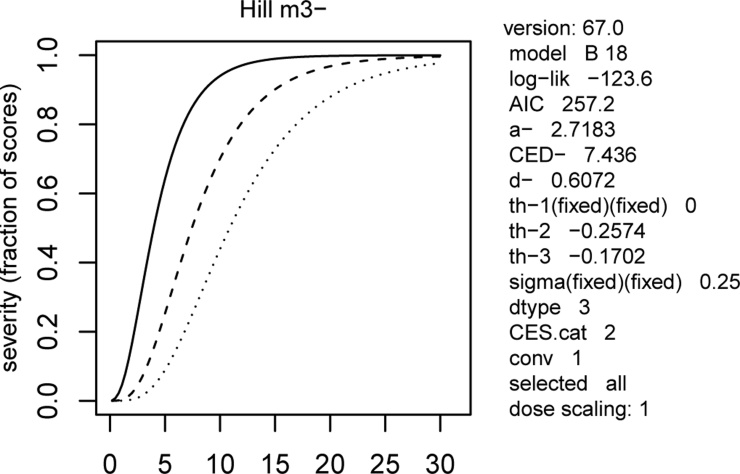
On the contrary, the exposure to NANO β-carotene resulted effective in inducing developmental delays and abnormal development of the head in a concentration-dependent manner, with a significant linear trend (p < 0.01). Malformed larvae were recorded starting from dose 1.5 ng/mL and at dose 7.5 they appeared with different grade of severity (Fig. 1; Table 1). Dose 15 ng/mL resulted 100 % teratogenic and the 92.6 % of larvae exposed to 30 ng/mL showed the most severe phenotype (Table 1). The BMDs for BMR at 50 % resulted nearly 4, 7 and 10 nM respectively for malformations, severe malformations and extreme malformations (Fig. 2).
Fig. 2.
Evaluation of the benchmark doses (BMDs) for Benchmark-response (BMR) at 50 % of the different categories (malformations, severe malformations and extreme malformations) observed in larvae exposed to NANO β-carotene. Malformations: continuous dose-response curve; Severe malformations: dashed dose-response curve; Extreme malformations: dotted dose-response curve.
In detail, sever reduction of the anterior dorsal part of the head accompanied by edema at the level of oral cavity and of the craniofacial and cardiac regions, a marked reduction of the oral opening, abnormal eye and abnormal position of the adhesive organ and of the gills were recorded. Digestive tract and the tail were normally shaped (Fig. 1 B-C-D). The acridine orange staining highlighted the abnormalities at the eyes and at the oral cavity (Fig. 1 B’-C’-D’).
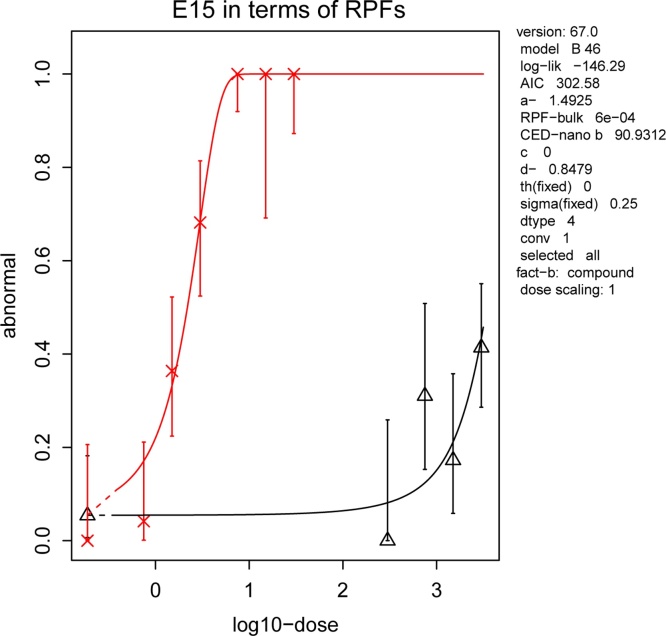
The BMD approach indicated a RPF for the BULK versus NANO β-carotene equal to 6*10−4 (C.I. (Fig. 3).
Fig. 3.
Evaluation of the relative potency factor (RPF) of the NANO formulation (dose-response curve with crosses) in respect to the BULK form (dose-response curve with triangles) of β-carotene. RPF was calculated at the effective concentration at 50 % (EC50). RPF of the nano-encapsulated β-carotene was 6*10−4 (BMD modelling by using PROAST software).
3.2. Histological evaluation
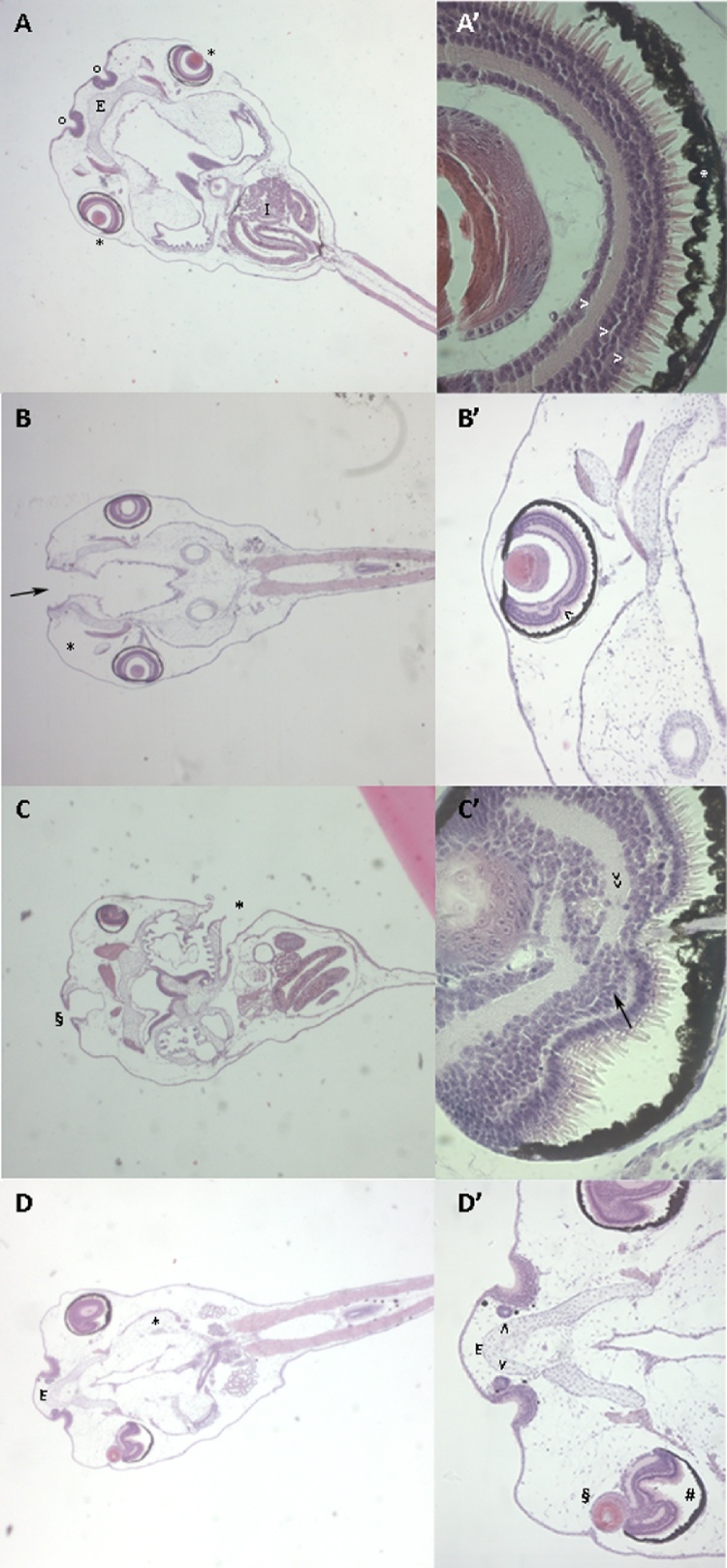
At the histological examination, cartilaginous head elements appeared abnormally shaped and fused in abnormal NANO β-carotene exposed larvae (Fig. 4). The presence of schisis at the anterior portion of the head was observed, with the impossibility to structure the naris. A progressive reduction of the cartilaginous elements of the ethmoid and of the maxillary arch was also associated with the schisis, as well as the presence of oedema in the mesenchyme of the oral cavity. The evaluation of the histological slides also revealed important abnormalities at the eye level, with progressive folding of the epithelium based on the grade of severity of the malformations registered at the external evaluation. Nevertheless, the differentiation of the different eye cell populations was maintained in all malformed larvae (Fig. 4 B-D’).
Fig. 4.
Frontal histological sections of NF stage 46 control and exposed larvae.
A: Dorsal view of a control larva with normal phenotype. Note the well-expanded ethmoid cartilage (E) in the anterior portion of the head and laterally to the olfactory mucosa (o). The eyes (*) and the well-developed intestine (I) are visible. Magnification 25 × .
A': Detail of the eye: note the pigmented layer (*) and the photoreceptor, bipolar cell and multipolar layers of the retina (>) Magnification 400 × .
B: Dorsal view of a larva exposed to NANO β-carotene 3 ng/mL with malformed phenotype. Note the anterior schisis (black arrow) and the moderate oedema in the mesenchyme of the oral cavity (*). Magnification 25 × .
B': Detail of the eye: note the slight folding at the level of the layers of the retina (<). Magnification 100 × .
C: Dorsal view of a larva exposed to NANO β-carotene 7,5 ng/mL with severely malformed phenotype. Note the anterior schisis (§) and the opened branchial basket (*). Magnification 25 × .
C': Detail of the eye: note the prominent folding of different layers of the retina (>>) and the abnormal shape of the crystalline (arrow). Magnification 100 × .
D: Dorsal view of a larva exposed to NANO β-carotene 7,5 ng/mL with extremely malformed phenotype. Note the ethmoid cartilage with elongated and flattened shape (E) and the oedematous mesenchyme (*). Magnification 25 × .
D': Detail of the anterior portion: note the disorganized tissues at the level of the nasal pits (>) and at the eye level with no centrally positioned crystalline (§) and very folded retinal layer separated by the pigmented layer (#). Magnification 100 × .
3.3. Morphological evaluation of stage 37 embryos
In order to evaluate the morphogenetic events at the base of the observed malformation, embryos at stage 37 were morphologically evaluated. Control embryo showed well pigmented eyes and well-formed adhesive organ. At the histological examination, the eye had differentiated retina with crystalline of acceptable dimensions and in a central position with respect to the optical cup. Stomodeal and pharyngeal cavity were well introflexed and ready to originate the oral cavity (Fig. 5).
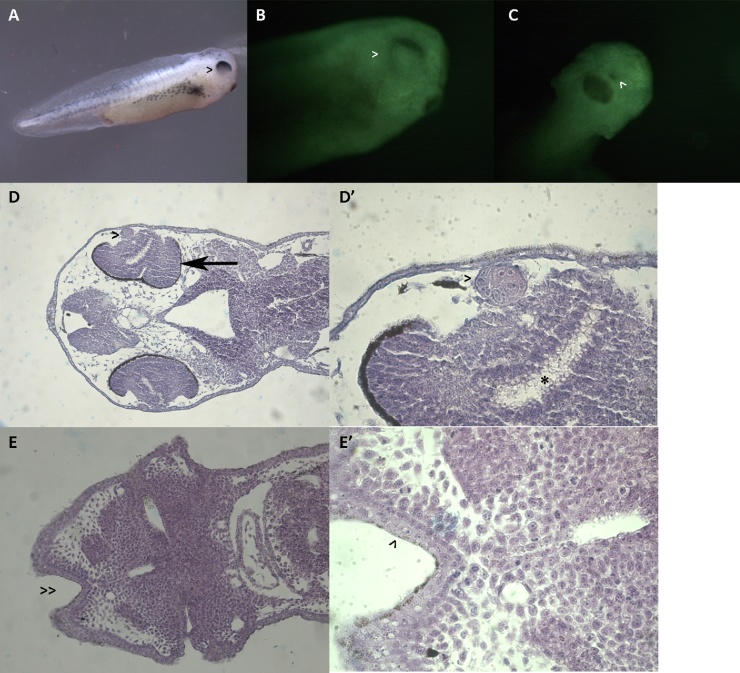
Fig. 5.
Morphological appearance of a NF stage 37 control larva before (A) and after fixation and acridine orange staining according to Zucker et al. [51] (B, C), with the corresponding histological sections (D, D’, E, E’).
A: Lateral view of a control larva with normal phenotype. Note the well-developed eye (*). Magnification 10 × .
B: Lateral view of a control larva with normal phenotype stained with acridine orange. Note the eye pigment circularly positioned and the centrally positioned crystalline (*).Magnification 40 × .
C: Frontal view of a control larva with normal phenotype stained with acridine orange. Note the round-opened stomodeum (s) and the forebrain bulges (t). Magnification 40 × .
D: Dorsal section of a control larva. Note the optical nerve (>) and the eye structure with centrally positioned crystalline and retinal layer already differentiated (*). Magnification 100 × .
D': Detail of the eye. Note the crystalline with different layers (*) and the regularly organized retinal layers (>). Magnification 400 × .
E: Ventral section of a control larva. Note the well-introflexed stomodeum (>>). Magnification 100 × .
E': Detail of E. Note the stomodeal ectoderm (black >) and the pharyngeal endoderm (red >) which are about to merge. Magnification 400 × .
On the contrary, the morphological evaluation of embryos exposed to NANO β-carotene showed abnormalities at the eye level with altered pigment distribution only visible in the upper area of the eye (Fig. 6 A).
Fig. 6.
Morphological appearance of NF stage 37 larvae exposed to NANO β-carotene before (A) and after fixation and acridine orange staining according to Zucker et al. [51] (B, C), with the corresponding histological sections (D, D’, E, E’).
A: Lateral view of a larva exposed to NANO β-carotene 15 ng/mL with alteration at the cephalic portion. Note the half-shape of the eye (>). Magnification 10 × .
B: Lateral view of a larva with alteration at the cephalic portion stained with acridine orange. Note the pigmented layers in the upper side of the eye (>). It is not possible to note the crystalline. Magnification 40 × .
C: Frontal view of a larva with alteration at the cephalic portion stained with acridine orange. Note the narrowed stomodeum (<). It is not possible to note the forebrain bulges. Magnification 40 × .
D: Dorsal section of a larva exposed to NANO β-carotene 15 ng/mL. Note the compromised eye structure with reduced crystalline (>) and with over folded retinal layers (arrow). Magnification 100 × .
D': Detail of the eye. Note the reduced crystalline (>) and the disorganized layers of the retina (*). Magnification 400 × .
E: Ventral section of a larva exposed to NANO β-carotene 30 ng/mL. Note the reduced stomodeum (>>). Magnification 100 × .
E': Detail of the reduced stomodeum (>). Magnification 400 × .
The acridine orange staining revealed an alteration in the development of crystalline and the absence of the telencephalic vesicles. In addition, stomodeum appeared reduced (Fig. 6 B–C).
At the histological examination the eye structure appeared extremely compromised in larvae exposed to NANO β-carotene 15 ng/mL: the epithelium of the optical cup was folded several times on itself and the modest-size crystalline was segregated in lateral position (Fig. 6 d-D'). Finally, in larvae exposed to NANO β-carotene 30 ng/mL the reduced funnel-shaped stomodeum did not reached the epithelium of the anterior intestine (Fig. 6 E-E’).
3.4. Whole mount CRABPI immunostaining stage 26
After CRABPI immunostaining, control X. laevis embryos at stage 26 showed immunostained tissues distributed around the optic and otic vesicles, at the level of the frontal region and at the level of the branchial arches. The distinct migratory flows of migrating cells were well distinguishable from the rhomboencephalon to the branchial arches, where the ectomesenchyme appeared condensed (Fig. 7 A-A’). Embryos exposed to NANO β-carotene show different degree of disorganization of CRABPI positive tissues: embryos exposed to dose 3 ng/mL showed partially altered migration streams with interconnection areas between frontonasal and branchial portions (data not shown). Embryos exposed to dose 30 ng/mL showed partially fused migration streams and continuous immunostained mass into the branchial region (Fig. 7 B-B’).
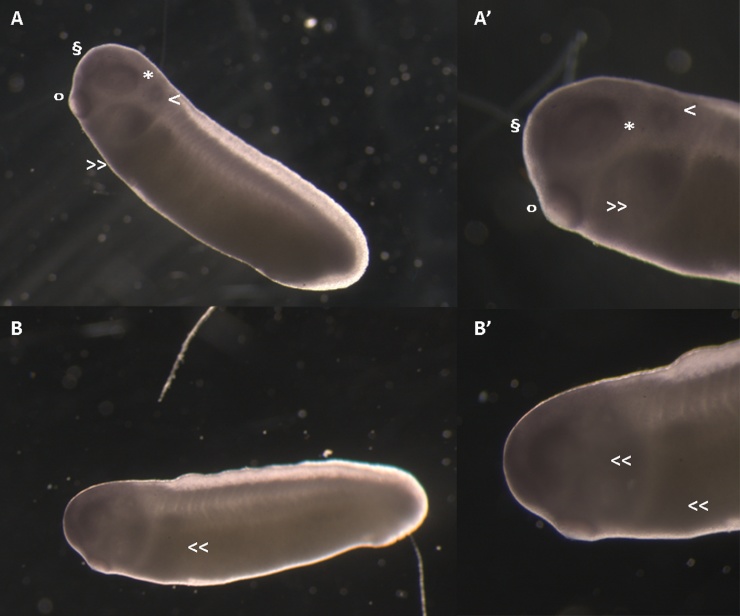
Fig. 7.
X. laevis embryos at NF stage 26 immunostained with antibody anti-CRABP (C-D'). Magnification: 25X, 40 × .
A, A’: X. laevis control embryo. Note the immunostained areas at the level of the oral cavity (o), of the frontal region (§), of the otic vesicle (>), of the optic vesicle (*), and at the level of the branchial arches with separate neural crest migratory flows (>>).
B, B': X. laevis embryos exposed to NANO-β carotene 30 ng/mL. Note the altered migration of the neural crest cells: the migration streams appear fused forming a ventrally indistinct immunostained mass with fusion regions (<<).
4. Discussion
β-carotene is a substance of plant-derived origin widely used both in the food industry and in the chemical-pharmaceutical industry as source of vitamin A [15]. The absorption of β-carotene appears to be highly variable in the population (5–65 %), depending on food- and diet-related factors, genetic characteristics and the health status of the subject [16].
As form of provitamin A, β-carotene is often recommended as a dietary supplement (also in nano-encapsulated form) for pregnant women, in order to prevent the effects of hypovitaminosis A (vitamin A is essential for embryonic development) [52,53]. In fact, low or excessive doses of vitamin A have been shown to be teratogenic, while toxicity studies in animals have shown that β-carotene in his bulk form is not embryotoxic or teratogenic and does not cause hypervitaminosis A [54].
Anyway, strong evidence suggests that the fetus has limited capacity to store β-carotene and convert most of it to RA [48]. By consequence, an increase in fetal β-carotene exposure could lead to an increase of fetal RA levels.
Nano-encapsulation raised some questions about the potential toxic effects of nano-encapsulated nutrients in food and beverages on human health and environment because it may modify absorption, distribution, metabolism, and excretion processes [24,25]. Previously published data on two different alternative animal models (rat whole embryo culture and FETAX) showed that nano-encapsulation significantly increases vitamin A embryotoxicity, probably enhancing vitamin A bioavailability [48].
The aim of this work was then to compare the effect of nano-encapsulated β-carotene to the bulk form (for which available toxicological data allow to classify β-carotene as safe during gestation). We used the alternative animal-free models Frog Embryo Teratogenesis Assay: Xenopus- FETAX. The selected concentrations for the bulk form were in the range of maternal plasma levels [49].
Our results indicated that in X. laevis embryos, during the morphogenetic phylotypic stages, the tested BULK β-carotene concentrations induced only developmental delays, starting from dose 750 ng/mL. On the contrary, the exposure to NANO β-carotene revealed that all tested concentrations were effective in inducing specific malformations at cranio-facial and eye level, although they were one hundred time lower than the BULK form. Only NANO β-carotene 0.75 ng/mL resulted ineffective in inducing malformations but was able to induce developmental delays.
The reported malformations, however, were not comparable to the alterations described for hypervitaminosis A, but seemed to be related to severe hypovitaminosis A obtained experimentally by inhibiting the retinoic acid receptors (RAR) or the RALDH2 enzyme as reported in Kennedy [55]. In their study, they showed that RA is required for the formation of the upper lip and primary palate in Xenopus laevis and a loss of RA signalling, by RALDH2 or RAR inhibition, resulted in a median facial cleft. Median facial hypoplasia, or median cleft lip and primary palate, are common malformations in humans and are often attributed to deficiency or agenesis in midface development caused by RA signalling disruption [56].
The eye malformations we observed in X. laevis larvae seem as well to correlate to situations of hypovitaminosis A. In particular, the histological analysis revealed that both the crystalline lens (in the most severe situations) and the retina were affected. These malformations were also visible in X. laevis larvae in which RA production or the binding with RAR was inhibited [55] or in RARα-γ mutant mice [57]. In fact, RA is an important morphogen also for the correct formation of the eye: the signal from RA would lead to the differentiation and correct growth of portions of the eye originating from different embryonic cell populations [58]. The presence of alternate areas of synthesis and degradation of RA between the retina and the optical calyx, allows RA to coordinate the eye formation by regulating biological activities of a family of non-steroid hormone receptors, RARα/β/γ, and RXRα/β/γ. Alterations in this RA distribution, by excess or deficiency of RA, result in specific developmental abnormalities [59]. In addition to what is stated in Goldberg [48], from recent studies it seems that β-carotene could be involved in RA metabolism by close competing at nanomolar level for the binding sites of nuclear receptors RARs, with consequent alteration of RA signal [60,61].
It was reported that three apocarotenes produced by eccentric cleavage of β carotene (β-14′-carotenal, β-14′-carotenoic acid and β-apo-13-carotenone) act as antagonists for the three isoforms of RAR, α, β, and γ. The binding affinity of these substances for the receptors is within a nanomolar range and therefore they are close competitors of RA itself. Although they can directly interact with the retinoid receptor binding site, none of these apocarotenes is revealed able to significantly activate the signal after binding with RARs receptors [61]. This aspect could result in an anti-vitamin action of β-carotene, aggravated by nano-encapsulation. It has been proposed, in fact, that the larger surface area of the small lipid-based nano-capsule, could facilitate the crossing of biological barriers, allowing the encapsulated lipophilic substances to quickly and efficiently reach the biological targets and, by consequence, enhance their bioavailability [62,63]. In addition, our results obtained on neural crest cell migration (CRABPI immunostaining) support the hypothesis that the observed alterations could be likely related to a disruption of the endogenous RA levels. In fact, neural crest cell specification and migration from hindbrain to the branchial apparatus is driven by RA and RA deficiency could induce similar migration defects in X. laevis models [64].
5. Conclusions
In this study, nano-encapsulated β-carotene was active in inducing specific different malformation patterns, contrary to the bulk form that was only active in inducing developmental delays. The results showed that nano-encapsulation could change the toxicological profile of β-carotene, suggesting that the encapsulation could increase the pro-vitamin bioavailability at target structure or the bioavailability of β-carotene-derived molecules able to inhibit retinoids signal. It can also be supposed that nano-encapsulation could promote the formations of these molecules and further evaluation are needed.
Although supplements during pregnancy with β-carotene are suggested, results from this work prompt the necessity to evaluate the use of nano-encapsulated β-carotene as it can cause teratogenic effects even at low doses, which are considered safe for the bulk form, due to its increased bioavailability.
Finally, due to its external development and accessibility throughout all development and to its easily manipulating embryo, the alternative animal models Frog Embryo Teratogenesis Assay: Xenopus- FETAX resulted a rapid screening test applicable to study, and eventually to reconsider, micronutrient hazard evaluation after nano-encapsulation.
Funding
This research did not receive any specific grant from funding in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Maria Battistoni: Methodology, Validation, Formal analysis, Investigation, Data curation, Writing - original draft, Visualization. Renato Bacchetta: Methodology, Investigation. Francesca Di Renzo: Conceptualization, Validation, Investigation, Resources, Writing - review & editing. Francesca Metruccio: Software, Formal analysis, Visualization. Elena Menegola: Conceptualization, Validation, Investigation, Writing - review & editing, Supervision, Project administration.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
References
- 1.Morriss-Kay G. Retinoic acid and development. Pathobiology. 1992;60:264–270. doi: 10.1159/000163733. [DOI] [PubMed] [Google Scholar]
- 2.Clagett-Dame M., Knutson D. Vitamin a in reproduction and development. Nutrients. 2011;3:385–428. doi: 10.3390/nu3040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piersma A.H., Hessel E.V., Staal Y.C. Retinoic acid in developmental toxicology: teratogen, morphogen and biomarker. Reprod. Toxicol. 2017;72:53–61. doi: 10.1016/j.reprotox.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki T., Oohara I., Kurokawa T. Retinoic acid given at late embryonic stage depresses sonic hedgehog and Hoxd-4 expression in the pharyngeal area and induces skeletal malformation in flounder (Paralichthys olivaceus) embryos. Dev. Growth Differ. 1999;41:143–152. doi: 10.1046/j.1440-169x.1999.00420.x. [DOI] [PubMed] [Google Scholar]
- 5.Warkany J., Schraffenberger E. Congenital malformations of the eyes induced in rats by maternal vitamin a deficiency. Exp. Biol. Med. 1944;57:49–52. doi: 10.3181/00379727-57-14695P. [DOI] [PubMed] [Google Scholar]
- 6.Lammer E.J., Chen D.T., Hoar R.M., Agnish N.D., Benke P.J., Braun J.T., Curry C.J., Fernhoff P.M., Grix A.W., Lott I.T., Richard J.M., Sun S.C. Retinoic acid embryopathy. N. Engl. J. Med. 1985;313:837–841. doi: 10.1056/NEJM198510033131401. [DOI] [PubMed] [Google Scholar]
- 7.Hathcock J.N., Hattan D.G., Jenkins M.Y., McDonald J.T., Sundaresan P.R., Wilkening V.L. Evaluation of vitamin A toxicity. Am. J. Clin. Nutr. 1990;52:183–202. doi: 10.1093/ajcn/52.2.183. [DOI] [PubMed] [Google Scholar]
- 8.Kochhar D.M. Teratogenic activity of retinoic acid. Acta Pathol. Microbiol. Scand. 2009;70:398–404. doi: 10.1111/j.1699-0463.1967.tb01308.x. [DOI] [PubMed] [Google Scholar]
- 9.Browne H., Mason G., Tang T. Retinoids and pregnancy: an update. Obstet. Gynaecol. 2014;16:7–11. doi: 10.1111/tog.12075. [DOI] [Google Scholar]
- 10.Cohlan S.Q. Excessive intake of vitamin A as a cause of congenital anomalies in the rat. Science. 1953;117:535–536. doi: 10.1126/science.117.3046.535. [DOI] [PubMed] [Google Scholar]
- 11.Rhinn M., Dolle P. Retinoic acid signalling during development. Development. 2012;139:843–858. doi: 10.1242/dev.065938. [DOI] [PubMed] [Google Scholar]
- 12.Zile M.H. Vitamin A. And embryonic development: an overview. J. Nutr. 1998;128:455S–458S. doi: 10.1093/jn/128.2.455S. [DOI] [PubMed] [Google Scholar]
- 13.Browne H., Mason G., Tang T. Retinoids and pregnancy: an update. Obstet. Gynaecol. 2014;16:7–11. doi: 10.1111/tog.12075. [DOI] [Google Scholar]
- 14.El-Baz F.K., Aly H.F., Abd-Alla H.I. The ameliorating effect of carotenoid rich fraction extracted from Dunaliella salina microalga against inflammation- associated cardiac dysfunction in obese rats. Toxicol. Rep. 2020;7:118–124. doi: 10.1016/j.toxrep.2019.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grune T., Lietz G., Palou A., Ross A.C., Stahl W., Tang G., Thurnham D., Yin S., Biesalski H.K. Β-carotene is an important vitamin a source for humans. J. Nutr. 2010;140:2268S–2285S. doi: 10.3945/jn.109.119024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.EFSA Scientific opinion on dietary reference values for vitamin a: dietary reference values for vitamin a. EFSA J. 2015;13:4028. doi: 10.2903/j.efsa.2015.4028. [DOI] [Google Scholar]
- 17.Cothran R.D., Gervasi S.S., Murray C., French B.J., Bradley P.W., Urbina J., Blaustein A.R., Relyea R.A. Carotenoids and amphibians: effects on life history and susceptibility to the infectious pathogen, Batrachochytrium dendrobatidis. Conserv. Physiol. 2015;3 doi: 10.1093/conphys/cov005. cov005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green A.S., Fascetti A.J. Meeting the vitamin a requirement: the efficacy and importance of β-Carotene in animal species. Transfus. Apher. Sci. 2016;2016:1–22. doi: 10.1155/2016/7393620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bradley D.G., Min D.B. Singlet oxygen oxidation of foods. Crit. Rev. Food Sci. Nutr. 1992;31:211–236. doi: 10.1080/10408399209527570. [DOI] [PubMed] [Google Scholar]
- 20.Gutiérrez F.J., Albillos S.M., Casas-Sanz E., Cruz Z., García-Estrada C., García-Guerra A., García-Reverter J., García-Suárez M., Gatón P., González-Ferrero C., Olabarrieta I., Olasagasti M., Rainieri S., Rivera-Patiño D., Rojo R., Romo-Hualde A., Sáiz-Abajo M.-J., Mussons M.-L. Methods for the nanoencapsulation of β-carotene in the food sector. Trends Food Sci. Technol. 2013;32:73–83. doi: 10.1016/j.tifs.2013.05.007. [DOI] [Google Scholar]
- 21.Mozafari M., Johnson C., Hatziantoniou S., Demetzos C. Nanoliposomes and Their Applications in Food Nanotechnology. J. Liposome Res. 2008;18:309–327. doi: 10.1080/08982100802465941. [DOI] [PubMed] [Google Scholar]
- 22.Wilson N., Shah N.P. Microencapsulation of vitamins. ASEAN Food J. 2007;14:1–14. [Google Scholar]
- 23.Astete C.E., Sabliov C.M., Watanabe F., Biris A. Ca(2+) cross-linked alginic acid nanoparticles for solubilization of lipophilic natural colorants. J. Agric. Food Chem. 2009;57:7505–7512. doi: 10.1021/jf900563a. [DOI] [PubMed] [Google Scholar]
- 24.Chaudhry Q., Scotter M., Blackburn J., Ross B., Boxall A., Castle L., Aitken R., Watkins R. Applications and implications of nanotechnologies for the food sector. Food Addit. Contam. Part A Chem. 2008;25:241–258. doi: 10.1080/02652030701744538. [DOI] [PubMed] [Google Scholar]
- 25.Bouwmeester H., Dekkers S., Noordam M.Y., Hagens W.I., Bulder A.S., de Heer C., ten Voorde S.E.C.G., Wijnhoven S.W.P., Marvin H.J.P., Sips A.J.A.M. Review of health safety aspects of nanotechnologies in food production. Regul. Toxicol. Pharmacol. 2009;53:52–62. doi: 10.1016/j.yrtph.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 26.EFSA Guidance on the risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain: guidance for risk assessment of engineered nanomaterials. EFSA J. 2011;9:2140. doi: 10.2903/j.efsa.2011.2140. [DOI] [Google Scholar]
- 27.Morriss G.M., Steele C.E. The effect of excess vitamin A on the development of rat embryos in culture. J. Embryol. Exp. Morphol. 1974;32:505–514. [PubMed] [Google Scholar]
- 28.Morriss G.M., Steele C.E. Comparison of the effects of retinol and retinoic acid on postimplantation rat embryos in vitro. Teratology. 1977;15:109–119. doi: 10.1002/tera.1420150115. [DOI] [PubMed] [Google Scholar]
- 29.Klug S., Lewandowski C., Wildi L., Neubert D. All-trans retinoic acid and 13-cis-retinoic acid in the rat whole-embryo culture: abnormal development due to the all-trans isomer. Arch. Toxicol. 1989;63:440–444. doi: 10.1007/BF00316445. [DOI] [PubMed] [Google Scholar]
- 30.Fort D.J., Paul R.R. Enhancing the predictive validity of frog embryo teratogenesis Assay?XEnopus (FETAX) J. Appl. Toxicol. 2002;22:185–191. doi: 10.1002/jat.848. [DOI] [PubMed] [Google Scholar]
- 31.Bonfanti P., Colombo A., Orsi F., Nizzetto I., Andrioletti M., Bacchetta R., Mantecca P., Fascio U., Vailati G., Vismara C. Comparative teratogenicity of Chlorpyrifos and Malathion on Xenopus laevis development. Aquat. Toxicol. 2004;70:189–200. doi: 10.1016/j.aquatox.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Mouche I., Malesic L., Gillardeaux O. FETAX assay for evaluation of developmental toxicity. In: Gautier J.-C., editor. Drug Saf. Eval. Humana Press; Totowa, NJ: 2011. pp. 257–269. [DOI] [Google Scholar]
- 33.Bacchetta R., Santo N., Fascio U., Moschini E., Freddi S., Chirico G., Camatini M., Mantecca P. Nano-sized CuO, TiO(2+) and ZnO affect Xenopus laevis development. Nanotoxicology. 2012;6:381–398. doi: 10.3109/17435390.2011.579634. [DOI] [PubMed] [Google Scholar]
- 34.Ballard W.W. Morphogenetic movements and fate maps of vertebrates. Am. Zool. 1981;21:391–399. doi: 10.1093/icb/21.2.391. [DOI] [Google Scholar]
- 35.Huang H.S., Goodman D.S. Vitamin A. And carotenoids. I. Intestinal absorption and metabolism of 14C-LABELLED vitamin A. Alcohol and BETA-CAROTENE in the rat. J. Biol. Chem. 1965;240:2839–2844. [PubMed] [Google Scholar]
- 36.Nieuwkoop P.D., Faber J., editors. Normal Table of Xenopus laevis (Daudin) Publishing Co.; Amsterdam: North Holland: 1956. [Google Scholar]
- 37.Fainsod A., Kot-Leibovich H. Xenopus embryos to study fetal alcohol syndrome, a model for environmental teratogenesis. Biochem. Cell Biol. 2018;96:77–87. doi: 10.1139/bcb-2017-0219. [DOI] [PubMed] [Google Scholar]
- 38.Dumont J.N., Schultz T.W., Buchanan M.V., Kao G.L. Frog embryo teratogenesis assay: xenopus (FETAX) — a short-term assay applicable to complex environmental mixtures. In: Waters M.D., Sandhu S.S., Lewtas J., Claxton L., Chernoff N., Nesnow S., editors. Short-Term Bioassays Anal. Complex Environ. Mix. III, Springer US; Boston, MA: 1983. pp. 393–405. [DOI] [Google Scholar]
- 39.Bantle J., Dawson D. Uninduced rat liver microsomes as a metabolic activation system for the frog embryo teratogenesis assay—. In: Adams W., Chapman G., Landis W., editors. Aquat. Toxicol. Hazard Assess. 10th Vol., ASTM International, 100 Barr Harbor Drive; PO Box C700, West Conshohocken, PA 19428-2959; 1988. pp. 316-316–11. [DOI] [Google Scholar]
- 40.Colombo A., Orsi F., Bonfanti P. Exposure to the organophosphorus pesticide chlorpyrifos inhibits acetylcholinesterase activity and affects muscular integrity in Xenopus laevis larvae. Chemosphere. 2005;61:1665–1671. doi: 10.1016/j.chemosphere.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 41.Di Renzo F., Bacchetta R., Sangiorgio L., Bizzo A., Menegola E. The agrochemical fungicide triadimefon induces abnormalities in Xenopus laevis embryos. Reprod. Toxicol. 2011;31:486–493. doi: 10.1016/j.reprotox.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Nations S., Wages M., Cañas J.E., Maul J., Theodorakis C., Cobb G.P. Acute effects of Fe2O3, TiO2, ZnO and CuO nanomaterials on Xenopus laevis. Chemosphere. 2011;83:1053–1061. doi: 10.1016/j.chemosphere.2011.01.061. [DOI] [PubMed] [Google Scholar]
- 43.Bacchetta R., Tremolada P., Di Benedetto C., Santo N., Fascio U., Chirico G., Colombo A., Camatini M., Mantecca P. Does carbon nanopowder threaten amphibian development? Carbon. 2012;50:4607–4618. doi: 10.1016/j.carbon.2012.05.047. [DOI] [Google Scholar]
- 44.Dawson D.A. Additive incidence of developmental malformation forXenopus embryos exposed to a mixture of ten aliphatic carboxylic acids. Teratology. 1991;44:531–546. doi: 10.1002/tera.1420440507. [DOI] [PubMed] [Google Scholar]
- 45.Sater A.K., Moody S.A. Using Xenopus to understand human disease and developmental disorders: sater and Moody. Genesis. 2017;55 doi: 10.1002/dvg.22997. [DOI] [PubMed] [Google Scholar]
- 46.Leconte I., Mouche I. Frog Embryo Teratogenesis Assay on Xenopus and Predictivity Compared with In Vivo Mammalian Studies. In: Barrow P.C., editor. Teratog. Test. Humana Press; Totowa, NJ: 2013. pp. 403–421. [DOI] [PubMed] [Google Scholar]
- 47.Battistoni M., Bacchetta R., Di Renzo F., Metruccio F., Pennati R., Menegola E. Teratogenic potential of traditionally formulated and nano-encapsulated vitamin a in two vertebrate models, Rattus norvegicus and Xenopus laevis. Int. J. Food Nutr. Sci. 2019;6:43–51. doi: 10.15436/2377-0619.19.2493. [DOI] [Google Scholar]
- 48.Goldberg J.S. Monitoring maternal Beta carotene and retinol consumption may decrease the incidence of neurodevelopmental disorders in offspring. Clin. Med. Insights Reprod. Health. 2012;6 doi: 10.4137/CMRH.S8372. CMRH.S8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanzler B., Foreman R.K., Labosky P.A., Mallo M. BMP signaling is essential for development of skeletogenic and neurogenic cranial neural crest. Dev. Suppl. 2000;127:1095–1104. doi: 10.1242/dev.127.5.1095. [DOI] [PubMed] [Google Scholar]
- 50.Menegola M.L., Broccia F., Di Renzo, Massa V., Giavini E. Relationship between hindbrain segmentation, neural crest cell migration and branchial arch abnormalities in rat embryos exposed to fluconazole and retinoic acid in vitro. Reprod. Toxicol. 2004;18:121–130. doi: 10.1016/j.reprotox.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 51.Zucker R.M., Elstein K.H., Shuey D.L., Ebron-Mccoy M., Rogers J.M. Utility of fluorescence microscopy in embryonic/fetal topographical analysis. Teratology. 1995;51:430–434. doi: 10.1002/tera.1420510608. [DOI] [PubMed] [Google Scholar]
- 52.Ross A.C., Ambalavanan N., Zolfaghari R., Li N. Vitamin A combined with retinoic acid increases retinol uptake and lung retinyl ester formation in a synergistic manner in neonatal rats. J. Lipid Res. 2006;47:1844–1851. doi: 10.1194/jlr.M600061-JLR200. [DOI] [PubMed] [Google Scholar]
- 53.Bailey R.L., Fulgoni V.L., Keast D.R., Dwyer J.T. Dietary supplement use is associated with higher intakes of minerals from food sources. Am. J. Clin. Nutr. 2011;94:1376–1381. doi: 10.3945/ajcn.111.020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bendich A. The safety of β‐carotene. Nutr. Cancer. 1988;11:207–214. doi: 10.1080/01635588809513989. [DOI] [PubMed] [Google Scholar]
- 55.Kennedy A.E., Dickinson A.J.G. Median facial clefts in Xenopus laevis: roles of retinoic acid signaling and homeobox genes. Dev. Biol. 2012;365:229–240. doi: 10.1016/j.ydbio.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 56.Allam K.A., Wan D.C., Kawamoto H.K., Bradley J.P., Sedano H.O., Saied S. The Spectrum of median craniofacial dysplasia. Plast. Reconstr. Surg. 2011;127:812–821. doi: 10.1097/PRS.0b013e318200aa08. [DOI] [PubMed] [Google Scholar]
- 57.Lohnes D., Mark M., Mendelsohn C., Dollé P., Dierich A., Gorry P., Gansmuller A., Chambon P. Function of the retinoic acid receptors (RARs) during development (I). Craniofacial and skeletal abnormalities in RAR double mutants. Dev. Suppl. 1994;120:2723–2748. doi: 10.1242/dev.120.10.2723. [DOI] [PubMed] [Google Scholar]
- 58.Matt N. Retinoic acid-dependent eye morphogenesis is orchestrated by neural crest cells. Development. 2005;132:4789–4800. doi: 10.1242/dev.02031. [DOI] [PubMed] [Google Scholar]
- 59.Cvekl A., Wang W.-L. Retinoic acid signaling in mammalian eye development. Exp. Eye Res. 2009;89:280–291. doi: 10.1016/j.exer.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Russell R.M. The enigma of β-carotene in carcinogenesis: what can be learned from animal studies. J. Nutr. 2004;134:262S–268S. doi: 10.1093/jn/134.1.262S. [DOI] [PubMed] [Google Scholar]
- 61.Eroglu A., Hruszkewycz D.P., dela Sena C., Narayanasamy S., Riedl K.M., Kopec R.E., Schwartz S.J., Curley R.W., Harrison E.H. Naturally occurring eccentric cleavage products of provitamin a β-Carotene function as antagonists of retinoic acid receptors. J. Biol. Chem. 2012;287:15886–15895. doi: 10.1074/jbc.M111.325142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Acosta E. Bioavailability of nanoparticles in nutrient and nutraceutical delivery. Curr. Opin. Colloid Interface Sci. 2009;14:3–15. doi: 10.1016/j.cocis.2008.01.002. [DOI] [Google Scholar]
- 63.McClements D.J., Rao J. Food-grade nanoemulsions: formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit. Rev. Food Sci. Nutr. 2011;51:285–330. doi: 10.1080/10408398.2011.559558. [DOI] [PubMed] [Google Scholar]
- 64.Vieux-Rochas M., Bouhali K., Baudry S., Fontaine A., Coen L., Levi G. Irreversible effects of retinoic acid pulse on Xenopus jaw morphogenesis: new insight into cranial neural crest specificationb. Birth Defects Res. B. Dev. Reprod. Toxicol. 2010;89:493–503. doi: 10.1002/bdrb.20269. [DOI] [PubMed] [Google Scholar]