Abstract
Background
Oral health problems may have numerous effects on general health, including physical fitness and performance. In this review, we aimed to systematically review the available evidence to assess the effect of oral health on general physical fitness.
Methods
We systematically performed a literature search in two different databases (PUBMED and EMBASE) without restriction to the year of publication. Articles were included if the subjects were humans and the primary aim was to assess the effects of dental and/or oral health problems on physical activity using either objective physical measurements or physical performance tests. The quality of the studies was then assessed using a Joanna Briggs Institute (JBI) Critical Appraisal tool.
Results
A total of 2651 articles were initially retrieved from the systematic search of the literature. Of these, a final total of 11 articles following the inclusion criteria were included in the review. All of the 11 articles included in the review had good methodological quality. Of the 11 articles, ten articles suggested a correlation between dental and oral condition toward physical fitness, body balance, cardiorespiratory function, and also cognitive function. Only one article found contradictory results, which showed that periodontal conditions did not correlate with the cardiorespiratory function. Malocclusion, including the number of remaining teeth, was reported in five studies (45.4%), periodontal disease was evaluated in six studies (54.5%), along with slight evaluation of periapical inflammation.
Conclusion
This review suggests that there is a negative effect of poor dental and/or oral health on physical fitness and performance.
Keywords: Health sciences, Public health, Physical activity, Physiology, Dentistry, Oral disease, Dental disease, Physical fitness, Physical performance
Health sciences; Public health; Physical activity; Physiology; Dentistry; Oral disease; Dental disease; Physical fitness; Physical performance
1. Introduction
Oral health problems may cause various adverse effects on well-being and reduce the quality of life. Local inflammation caused by poor oral health can induce a systemic inflammatory response [1, 2, 3] and affect physical fitness [4]. The systemic changes affected by either dental or oral health problems, for example, changes in serum levels of inflammatory biomarkers, such as C-Reactive Protein (CRP) and interleukin (IL), also appear in muscle injury [4] and, thus, may influence physical fitness, specifically the muscle mass, muscle strength, and muscle function [5]. Furthermore, a previous observational study found lower pro-inflammatory biomarker concentrations among individuals who engaged in more frequent and intense physical activities than those who engaged in less frequent and intense physical activities [5].
Physical fitness is defined as a set of attributes related to the ability to perform a physical activity [6]. The theoretical construct of physical fitness comprises various dimensions, including body composition and muscle performance [4]. Damage to the muscles, which may lead to decreased physical fitness, induces a systemic inflammatory response involving leukocytes and increased serum levels of pro-inflammatory cytokines, such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α [7, 8]. This inflammatory response in the muscle may lead to secondary damage to the healthy muscle structures, thereby lengthening the muscle repair process, increasing muscle soreness, and making the individual more reluctant to make his or her skeletal muscles contract [9, 10].
Since well-maintained physical fitness positively affect various biological functions [11], it is crucial to put into consideration the effect of oral health on physical fitness. There have been many studies investigating the effect of oral health on physical fitness. However, those studies were mostly limited to self-report rather than direct measures for physical fitness. As far as we are concerned, studies that assessed the relationship between oral health and physical performance using objective physical tests are scarce. Therefore, we aimed to systematically review the available studies that used objective physical measurements or running a physical performance test to assess the effect of oral health on general physical fitness.
2. Results
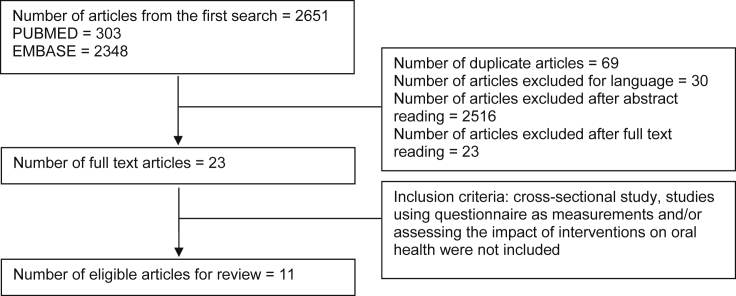
We initially retrieved a total of 303 articles from the systematic literature search in PUBMED and 2651 articles from EMBASE. After removing 69 duplicate articles and excluding 30 articles because of using a language other than English, we screened the remaining articles through titles and abstracts. From title and abstract screening, we excluded 2516 articles, which were not relevant with the review question. Subsequently, we read full-text of the remaining 23 articles. Studies, which measured physical fitness using self-reported questionnaire were excluded as the focus of this review is on objective physical measurements. A final total of 11 articles were included in the review (Figure 1). The detailed information of the acquired articles is summarized in Table 1.
Figure 1.
Flow charts of literature searching.
Table 1.
The summary of selected articles.
| No. | Title | Author | Study Design | Subject | Oral Condition | Number of Participants | Study factor/exposure | Outcome | Test Performed | Results | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Teeth and physical fitness in a community-dwelling 40 to 79-year-old Japanese population (Clinical Intervention in Aging, 29 June 2016) | Akira Inui, Ippei Takahashi, Kaori Sawada, Akimoto Naoki, Toshirou Oyama, Yoshihiro Tamura, et al | Cross sectional. Observational study |
Elderly age 40–79 years old | Occlusal condition | n = 522 (198 males and females) | Number of teeth, Occlusal condition (Eichner Index) | Physical Fitness | Timed 10 m walk test, Hand grip strength, SMM of the whole body (kg) | Number of teeth was shown to be an independent risk factor for the timed 10 m walk test in female (P value = 0.007) Number of teeth and SMM in male (P value = 0.031), Eichner index correlated with the Timed 10m walk test |
Prevention of teeth loss is important for maintaining muscle strength and its function in people aged 40–79, especially for walking ability. This cross-sectional study on a Japanese community-dwelling population revealed relationships between partial oral conditions and the muscle mass and its function. |
| 2 | Influence of dental occlusion on the athetlic performance of young elite rowers: a pilot study (CLINICS, 4 July 2018) | Eric Leroux, Stephanie Leroux, Frederic Maton, Xavier Ravalec, Olivier Sorel | Cross sectional | Members of the ‘‘Pô le France Aviron’’ (age range of 15–17 years) | Dental occlusion | N = 7 | Artificial occlusal disturbance | Athletic performance | Body balance (stabilometric test) symmetry of the muscular contraction (posturographic tests) Muscular power (Aerobic test) |
None of the three body balance parameters was significantly influenced by the artificial occlusal disturbance. The interposition of the silicone splint resulting in a 4 mm ateral deflection of the mandible increased the proportion of asymmetric muscular contractions from 14.3% to 85.7% of the participants (p = 0.025). The interposition of the silicone splint resulting in a 4 mm lateral deflection of the mandible induced a significant 17.7% reduction in the athletes' muscular power (p = 0.030). |
In this pilot study, artificial mandibular laterodeviation induced a significant alteration in the muscular power of the rowers. Such temporomandibular disorders constitute a major public health problem [37]. Based on our findings, dental occlusion examination should be regularly undertaken for young elite rowers. Moreover, for cases in which dental malocclusions are detected, a suitable treatment plan based on prosthetic, surgical and/or orthodontic care can improve athletes' performances. |
| 3 | Periodontal Disease as a Risk Indicator for Poor Physical Fitness: A Cross-Sectional Observational Study (J Periodontol, Januariy 2015 Vol 86 No. 1) | Joao Augusto P. Oliveira, Carolina B. Hoppe, Maximiliano S. Gomes, Fabiana S. Grecca, and Alex N. Haas | Cross sectional. Observational Study |
Male police officers (aged 20–56 years; mean age: 34.8 years). | Periodontal Health | N = 111 | Periodontal Disease | Physical Fitness | Physical Fitness Test (PFT): 1. Push-up exercises 2. Pull-up exercises 3. Sit-up exercises 4. Running exercise |
Individuals who reached the highest PFT score had significantly better periodontal conditions compared with those with PFT scores below the maximum. Individuals who did not reach the highest PFT score presented significantly higher mean PD (P = 0.03), mean AL (P = 0.01), BOP (P = 0.04), and number of teeth with AL ‡4 mm (P = 0.04). | Periodontal disease may be considered a risk indicator for poor physical fitness in males. If periodontal health and physical fitness are truly connected, then the prevention and treatment of periodontal diseases, with aims to ensure physical fitness, should be considered at the population level. On an individual level, maintaining periodontal health may be an important strategy for improving physical fitness related to the performance of athletes. |
| 4 | Cross sectional association between physical strength, obesity, periodontitis and number of teeth in a general population. (J Clin Periodontol 2016; 43: 401–407) |
Michael Eremenko, Christiane Pink, Reiner Biffar, Carsten O. Schmidt, Till Ittermann, Thomas Kocher and Peter Meisel | Cross sectional. Observational Study |
Participants of the Study of Health in Pomerania (SHIP-2) | Periodontal Health | N = 2089 | Clinical attachment loss, number of teeth, C-reactive protein and glycated haemoglobin | Physical strength | Handgrip strength (GS), anthropometric measures, | In multiple regression adjusted for age, body mass index (BMI) and waist-to-hip ratio (WHR) each mm of diminished periodontal attachment was associated with reduction in GS by 1.47 kg (95% CI -2.29 to -0.65) and 0.38 kg (-0.89 to 0.14) in men and women respectively. Correspondingly, each additional remaining tooth was significantly associated with higher GS. | Periodontitis is associated with GS modified mainly by anthropometric measures related to adiposity and inflammation. Putative mechanisms encompass interactions of factors declining with increasing age. |
| 5 | Association between chronic oral inflammatory burden and physial fitness in males: a cross sectional observational study | CB Hoppe, JAP Oliveira, FS Grecca, AN Haas, MS Gomes | cross sectional observational study | male police officer in Military Police of Rio Grande do Sul, Porto Alegre, Brazil | Periodontal health | N = 112 | Periodontal disease was assessed by probing depth (PD) and clinical attachment loss (AL). For radiographic analysis, both apical periodontitis (AP) and root canal treatment (RCT) variables were analysed. Endodontic Burden (EB) was calculated merging the total number of teeth with AP and/or RCT per individual. OIB was calculated combining EB and AL. |
Physical Fitness | PFT Score (a combination of physical strength and cardiorespiratory fitness) | There was no significant association between AP, RCT and EB with physical fitness. Whereas, PD, AL and OIB were significantly associated with low physical fitness (p < 0.05). Multivariate regression analysis revealed that individuals with OIB = EB ≥ 3 & AL≥4mm had a 81% lower chance of reaching the highest PFT score (OR = 0.19, 95%CI = 0.04–0.87, p = 0.03) compared to individuals with EB < 3 and & no AL≥4mm. Individuals with unfavourable periodontal parameters but with low EB (OIB = EB < 3 & AL≥4mm) showed no significant differences on the chance to reach the highest PFT score compared to participants with favourable periodontal status and low EB (OIB = EB < 3 & no AL≥4mm). |
The OIB - higher levels of EB in periodontal patients - was independently associated with poor physical fitness in males. |
| 6 | Moderate and severe periodontitis are independent risk factors associated with low cardiorespiratory fitness in sedentary non-smoking men aged between 45 and 65 years | Eberhard J, Stiesch M, Kerling A, Bara C, Eulert C, Hilfiker-Kleiner D, Hilfiker A, Budde E, Bauersachs J, Kück M, Haverich A, Melk A, Tegtbur U. | cross sectional observational study | Non-smoking healthy male aged 45–65 years | Periodontal disease | N = 72 | Periodontal disease (probing depth and clinical attachment loss) | Cardiorespiratory fitness | Analysis of oxygen consumption, questionnaire of physical activity, blood pressure, routine blood test (lipid levels, glucose concentration) | Differences between VO2peak levels in subjects with no or mild, moderate or severe periodontitis were statistically significant (p = 0.026). Individuals with low VO2peak values showed high BMI scores, high concentrations of high-sensitive C-reactive protein, low levels of high-density lipoprotein-cholesterol, and used more glucocorticoids compared to individuals with high VO2peak levels. Multivariate regression analysis showed that high age (p = 0.090), high BMI scores (p < 0.001), low levels of physical activity (p = 0.031) and moderate (p = 0.087), respectively, severe periodontitis (p = 0.033) were significantly associated with low VO2peak levels. | moderate and severe periodontitis were independently associated with low levels of CRF in sedentary men aged between 45 and 65 years. |
| 7 | Oral Condition and Health Status of Elderly 8020 Archieves in Aichi Prefecture | Masamori H., Katsumi Y., Tsukasa S., Akira O., Tooru T., Shinsuke H., Takeshi S., Toshihide N. | cross sectional observational study | Elderly | Oral condition | N = 217 | Total number of teeth, CPITN index, Salivary blood test, masticatory activity | Bone mineral density, Grip strength, balance test, BMI | X-ray absorptiometry, Handgrip strength, balance test | The percentages of CPITN code 0, 1 and 2 were 68% in the 8020 male elderly and 72% in the 8020 female elderly. The positive percentage in the salivary blood test in the 8020 male elderly was lower than that in the non-8020 elderly. Masticatory ability was 1.55g in the 8020 male elderly and 1.53g in the 8020 female elderly. Relative masticatory ability in the 8020 female elderly was 20% higher than that in the non-8020 female elderly. BMD in the 8020 female elderly was significantly higher than that in the non-8020 female elderly. Grip strength in the 8020 elderly was also significantly higher than that in the non-8020 elderly. The duration of balance test in the 8020 male elderly was 2.2 times longer than that in the non-8020 male elderly. | The 8020 elderly showed good oral condition and health status was found to be better in the 8020 elderly than that in the non-8020 elderly |
| 8 | Relationship Between Obesity and Physical Fitness and Periodontitis | Yoshihiro S., Yuko E, Takeshi M., George K., Sumio A., Sumie J., Yoshihisa Y | cross sectional observational study | Participants of health promotion program who received dental and medical examination | Periodontal health | N = 1160 | Obesity and physical fitness | Periodontal health status | Community Periodontal Index (CPI), BMI | The lowest quintile in BMI and the highest quintile in VO2max were inversely associated with severe periodontitis, ingly, in multivariate logistic regression analyses. Subjects with the combined lowest quintile in BMI and the highest quintile in VO2max had a significantly lower risk of severe periodontitis compared to subjects with other combined quintiles in BMI and in VO2max (odds ratio: 0.17; 95% confidence interval: 0.05 to 0.55). | obesity and physical fitness may have some interactive effect on periodontal health status. |
| 9 | Periodontal Infection and Cardiorespiratory Fitness in Younger Adults: Results from Continuous National Health and Nutrition Examination Survey 1999–2004 | Ashley Thai., Panos N. Papapanou, David R. Jacobs Jr, Moı¨se Desvarieux, Ryan T. Demmer | cross sectional observational study | participants were enrolled in NHANES 1999–2004 aged 20–49 years old | Periodontal infection | N = 2863 | Probing depth, clinical attachment loss | Cardiorespiratory fitness | Maximal oxygen uptake, BMI, treadmill, blood pressure test | After multivariable adjustment, mean eVO2 max levels6SE across quartiles of attachment loss were 39.7260.37, 39.6460.34, 39.5960.36, and 39.8560.39 (P = 0.99). Mean eVO2 max6SE across quartiles of probing depth were 39.5760.32, 39.7860.38, 39.1960.25, and 40.3760.53 (P = 0.28). Similarly, multivariable adjusted mean eVO2 max values were similar between healthy participants vs. those with moderate/severe periodontitis: 39.7060.21 vs. 39.7060.90 (P = 1.00). The odds ratio (OR) for low eVO2 max comparing highest vs. lowest quartile of attachment loss = 0.89 [95% CI 0.64–1.24]. The OR for comparing highest vs. lowest probing depth quartile = 0.77 [95% CI 0.51–1.15]. | Clinical measures of periodontal infection were not related to cardiorespiratory fitness in a sample of generally healthy younger adults. |
| 10 | Tooth Loss Associated with Physical and Cognitive Decline in Older Adults | G. Tsakos, RG. Watt, PL. Rouxel, C de Oliveira, P. Demakakos | Cross sectional. Observational study |
Elderly aged 60 and older | Tooth loss | N = 3166 | Number of remaining teeth | Physical and cognitive function | 10-word recall test, gait speed assessment | Edentulous participants recalled 0.88 fewer words and were 0.09 m/s slower than dentate participants after adjusting for time and demographics. Only the latter association remained significant after full adjustment, with edentulous participants being 0.02 m/s slower than dentate participants. In age-stratified analyses, baseline edentulousness was associated with both outcomes in fully adjusted models in participants aged 60 to 74 but not in those aged 75 and older. Supplementary analysis indicated significant associations between baseline edentulousness and 4-year change in gait speed and memory in participants aged 60 to 74 | Total tooth loss was independently associated with physical and cognitive decline in older adults in England. Tooth loss is a potential early marker of decline in older age. |
| 11 | The Effect of Tooth Loss on Body Balance Control Among Community-Dwelling Elderly Persons | M Yoshida, T Kikutani, G Okada, T Kawamura, M Kimura, Y Akagawa | Cross sectional. Observational study |
Participants of the 2006 Kyoto Health Seminar | Tooth loss | N = 35 (12 male, 23 female) | Occlusal condition | Physical fitness | Hand grip and leg extensor power reflected muscle strength, body balance test | The test and control groups both included 12 male and 23 female subjects. Body balance ability, measured by time spent standing on one leg with eyes open (P = .013) and functional reach (P = .037), was significantly less in the test group when compared to the control, as shown by analysis done using the Mann-Whitney U test. The stabilometer examination also indicated that sway area (an accurate indicator of postural balance) and body sway (evidence of energy consumption for postural control) while standing with eyes closed were both significantly higher in the test group (P = .035 and .048, respectively; Wilcoxon signed ranks test) than the control. |
tooth loss is a risk factor for postural instability. This further suggests that proprioceptive sensation from the periodontal ligament receptor may play a role in body balance control. |
2.1. Characteristics of the studies
The studies included in this review were from three different continents; America, Europe, and Asia in which the largest proportion were from the first two continents with a proportion of 40% each. Of the 11 studies included in this review, 4 were from Japan, 5 were from Brazil, and 2 were from Germany. The year of publication ranged from 2006 to 2018. The average number of subjects per study was 568 with a minimum of 7 and a maximum of 2089.
2.2. Methodological quality
Overall, the methodological quality of the included studies was high. All studies clearly defined the inclusion criteria of the subjects and described them as well as the study settings in detail. The measurement of oral health conditions as the exposure was reliably performed by trained and calibrated examiners. The assessment of periodontal conditions including probing depth (PD), clinical attachment level (CAL), and bleeding on probing (BOP) was carried out objectively, showing valid and reliable measurements. In addition, the outcomes were measured using a broad range of standardized physical fitness tests, such as stabilometric test to measure body balance, posturographic tests, aerobic test, physical fitness test (PFT) (consisting of push-up, pull-up, sit-up, and running exercise), and handgrip strength test.
Most of the studies had also been concerned about the potential confounding factors that might affect the result of the studies. These confounding factors included age, body mass index (BMI), frequency of exercise, serum albumin concentration, and smoking. The statistical result of these studies had been adjusted for confounding factors and some of them also stratified the results based on gender.
2.3. The effect of oral health conditions on physical fitness
Three oral health conditions were reported to have an influence on physical fitness: malocclusion, periodontitis, and periapical inflammation.
-
1.
Malocclusion
Malocclusion was reported in five studies (45.4%), with one study presented a comparison to the control population. The first study showed that dental occlusion and the number of teeth was significantly correlated with the walking ability of the elderly that measured with the timed 10m walk test [12]. Another study showed that the lateral deflection of the mandible significantly increased the proportion of asymmetric muscular contractions from 14.3% to 85.7% of the participants (p = 0.025). Mandible lateral deflection also induced a significant 17.7% reduction in the athletes’ muscular power (p = 0.030) [13]. Another study observed the Bone Mineral Density (BMD), grip strength, balance test and BMI of elderly aged 80 years and over divided into 20 or more teeth group (8020) and less than 20 teeth (non-8020) group. The study showed that the 8020 group had higher masticatory ability, which was correlated to a significantly higher BMD, and stronger handgrip strength. Besides, the 8020 group was also able to stand 1.9 times longer in the balance test. Concerning obesity, this study revealed that the elderly with well-maintained oral health had normal BMI, 22.2 in males and 22.9 in females [14]. This result was also confirmed by another study, stating that the denture wearer had a remarkably lower body balance ability, measured by time spent standing on one leg with the eyes open (P = 0.013) and functional reach (P = 0.037), compared to those with natural occlusion. Edentulism was reported to be a marker of subsequent diminished function in the elderly, both physical and cognitive function [15].
-
2.
Periodontitis
Periodontal disease was evaluated in six studies (54.5%) by a diverse group of measures including the probing depth (PD), clinical attachment loss (AL), and bleeding on probing (BOP).
Individuals who reached the highest PFT score had significantly better periodontal conditions compared with those with PFT scores below the maximum. Individuals who did not reach the highest PFT score presented significantly higher mean PD (P = 0.03), mean AL (P = 0.01), mean BOP (P = 0.04), and the number of teeth with AL ‡4 mm (P = 0.04) [16].
In multiple regression adjusted for age, body mass index (BMI) and waist-to-hip ratio (WHR), each mm of diminished periodontal attachment was associated with a reduction in handgrip strength (GS 0by 1.47 kg (95% CI -2.29 to -0.65) and 0.38 kg (-0.89 to 0.14) in men and women respectively. Correspondingly, each additional remaining tooth was significantly associated with higher GS [17].
There was a significant difference in cardiorespiratory fitness through the measurement ofVO2peak (p = 0.026) between subjects with no, mild, moderate and severe periodontitis. Subsequent measurement revealed that individuals with low VO2peak had significantly higher weight (p < 0.001), BMI scores (p < 0.001), lower level of high-density lipoprotein (HDL) (p = 0.036), higher serum level of high-sensitive CRP (hsCRP) (p = 0.045), and more glucocorticoids (p = 0.027). Further analysis with univariate regression revealed that age, BMI and no or mild periodontitis had a remarkable association with VO2peak [18]. The reversed correlation was also found in another study, showing that low BMI and high VO2max were inversely associated with severe periodontitis in the multivariate logistic regression analysis (OR: 0.17; 95% CI: 0.05 to 0.55) [19]. Interestingly, the observation on healthy young adults revealed that clinical measures of periodontal infection, such as attachment loss (OR = 0.89; 95% CI 0.64–1.24) and probing depth (OR = 0.77; 95% CI 0.51–1.15) were not related to cardiorespiratory fitness [20].
-
3.
Periapical Inflammation
One of the studies evaluated the oral inflammatory burden as the combination of periodontal and endodontic disease load. Using the radiographic analysis, both apical periodontitis (AP) and root canal treatment (RCT) variables were analyzed. The Endodontic Burden (EB) was calculated by adding the total number of teeth with AP and/or RCT per individual. Oral inflammatory burden (OIB) was calculated by combining the endodontic burden (EB) and AL. The results showed that there was no significant association between AP, RCT, and EB with physical fitness. However, PD, AL, and OIB were significantly associated with low physical fitness (p < 0.05). The results of multivariate regression analysis revealed that individuals with OIB = EB ≥ 3 & AL≥4mm had an 81% lower chance of reaching the highest PFT score (OR = 0.19, 95%CI = 0.04–0.87, p = 0.03) compared to individuals with EB < 3 and & no AL≥4mm. Individuals with unfavorable periodontal parameters but with low EB (OIB = EB < 3 & AL≥4mm) showed no significant differences in the chance to reach the highest PFT score compared to individuals with favorable periodontal status and low EB (OIB = EB < 3 & no AL≥4mm) [21].
3. Discussion
3.1. Key findings
This systematic review reveals that there is a negative effect of poor oral health on physical fitness. The oral conditions that strongly affect the physical strength were malocclusion and periodontal disease, whereas endodontic disease alone was reported not associated with poor physical performance. However, the negative effect of endodontic burden (number of teeth with apical periodontitis and/or root canal treatment) on physical fitness became more obvious when both endodontic and periodontal diseases were found in a patient. This suggests that the higher level of endodontic burden in the respondents were independently associated with poor physical fitness [21]. In addition to the current condition, endodontic burden indicates the past history of pulp and periapical disease burden. Therefore, the OIB variable arising from merging AL and EB may provide information on the individual's experience of both periodontal and endodontic diseases load [21].
The majority of the studies used periodontal health as their study factor. The severity of periodontal disease, which was assessed using some clinical parameters such as probing depth (PD), clinical attachment loss (CAL), and bleeding on probing (BOP), was related to physical strength. For instance, the increasing CAL is significantly associated with decreased handgrip strength and was reported to reduce the chance to reach the highest PFT score [16, 17, 21]. Not only CAL increment, the increasing mean of PD also reduces the chance of achieving the highest PFT score [16]. Another study found a correlation of clinical parameters of periodontal disease toward cardiorespiratory fitness and vice versa [18, 19]. While the other study revealed no correlation between measures of periodontal disease and cardiorespiratory fitness [20]. Thus, periodontal disease was considered to be a risk indicator of poor physical fitness.
Five studies concerned about the number of teeth remained in the oral cavity. One study showed that a higher number of remaining natural teeth was associated with higher handgrip strength [17]. This finding was relevant with the result of another study showing that the reduced number of teeth was independently associated with lower walking speed and muscle mass, potentially leading to lower quality of life [12]. Besides, low number of remaining teeth in the elderly, despite the use of full denture, denotes a risk factor of declining body balance control [22]. Interestingly, one study found that tooth loss not only affected physical fitness, but also associated with the decline of cognitive function [15]. The number of remaining teeth and tooth loss could be used as indicators of periodontal conditions, dental occlusion conditions, and mastication ability. These findings were confirmed by a study showing that the well-maintained mastication ability denoted an essential factor in sustaining good daily activities and social participation, since it might affect the handgrip strength, BMD, and balance test [14]. In addition to the above-mentioned findings, another study reported the negative effect of occlusal disturbance on the body posture and athletic performance [13]. The presence of occlusal disturbance could lower the muscular power of athletes [13]. Thus, the presence of dental malocclusion was also reported to have an association with poor physical fitness.
A study also analyzed several confounding factors that possibly affected the decline in physical fitness. This study revealed that those factors, such as age and gender, contributed to the rate of decline only, without disrupting the causal relationship between poor oral health and physical fitness and performance, which was actually; due to the differences in physiological process [17].
3.2. Strengths and limitations of the review
As mentioned before, the high-methodological-quality of the papers could become the main strength of the included studies. However, some limitations can still be found. Firstly, three out of five studies might arise selection bias as they used convenience samples, such as athlete group and military officer group, rather than using random samples from the general population [13, 16, 21]. These specific groups of people might have different characteristics with the population in general. In addition, selection bias could also be found in another study recruiting volunteers as their participants. The volunteers might show interest in their own health and have been healthier than other local residents [12]. Physical fitness denotes a multidimensional system, including skill- and health-related components, specifically cardiorespiratory fitness, and muscular fitness. Those can be assessed by measuring body composition, cardiorespiratory endurance, muscular fitness, and musculoskeletal flexibility. However, those modalities, of course, cannot be used to assess the physical fitness of all individuals due to personal limitations. Therefore, information from an individual's health and medical records should be considered to be suited with the testing modalities [23, 24].
Moreover, in the context of the assessment of exposure, a traditional method of clinical assessment was used for periodontal measurement. It might underestimate the periodontal status of the participants. Although remains unchanged, the traditional methods of clinical assessment of periodontitis do not provide information on whether active tissue destruction is occurring. However, a periodontal diagnostic tool provides the disease characteristics such as pocket depths, bleeding on probing, clinical attachment levels, plaque index, and alveolar bone level, which can be confirmed with radiographic imaging. Those are appropriate for differential diagnosis, disease location, and severity of infection [25]. Besides, cross-sectional observation is not able to give more detail information about the causal relationship between poor oral health and physical fitness [12]. As explained by Leroux et al (2018), that disturbance in occlusion may affect body balance after a long period of neural integration only [13]. Other factors that may also impair body balance, are cerebrovascular diseases, motor neuron diseases, or otologic symptoms, and therefore, those individuals were excluded from the study.
3.3. Significance of findings and possible mechanisms
The findings of this review suggest that there was a negative impact of poor oral health on physical fitness. Individuals with poor oral conditions were likely to have lower physical strength and performance than those with good oral conditions. This finding was interesting in particular areas, such as athletics and sports. The sports committee might often put aside their athletes' oral health and more focus on their athletes' general health. This indicated that oral health is often overlooked concerning athletes’ overall health [26], however, based on this review, the oral health conditions have a significant effect on physical performance of athletes.
Physical fitness and oral health conditions had a bidirectional relationship. Both physical fitness and oral health conditions related to one another. Oral health problems might lead to low physical performance and vice versa. For example, individuals with periodontal diseases might have a poor masticatory performance, which would affect their skeletal muscle, and thus impact on their physical strength [17, 18, 27]. Furthermore, the efficiency of masseter muscles was related to physical fitness in the elderly [28]. On the contrary, the physical function and muscle mass, including masticatory muscles, might decrease among individuals with slower ambulatory speed. This would cause poor oral hygiene and tooth loss [12]. The chance of getting periodontal disease might decrease with good physical strength through a regular exercise [29]. In addition to physical strength, the severity of periodontal disease might affect the function of the respiratory and cardiovascular system [18].
The possible explanations of the associations between the two variables had been revealed. First, studies showed that there was an association between physical activity and inflammation. Individuals reporting more frequent and more intense physical activities showed lower inflammatory biomarker concentrations, which might also repress the effect of periodontal disease [5, 18, 30, 31]. Additionally, muscle strength and periodontitis shared common risk factors, which were associated with inflammation, such as obesity, diabetes, and chronic inflammation conditions. It indicated that such factors mediated the relationship [21, 29]. One of the elements to explain this causal correlation is CRP. Its serum concentration is not only positively correlated to periodontal disease, hbut also is determined by the frequency and intensity of physical activity. CRP denotes the marker of systemic inflammation, and therefore, it may also predict the risk of myocardial infarction and stroke [18, 19, 20]. This may explain the correlation of periodontal health to physical activity and performances, obesity, and cardiorespiratory function.
The lower prevalence of periodontal disease compared to those who are not-physically-active, is correlated to the production and modulation of cytokine. In particular, routine exercise may improve periodontal condition owing to the stimulus by pro-inflammatory cytokine that will be released after exercise [32]. Conversely, the iincreased serum levels of pro-inflammatory cytokines observed in periodontal and endodontic diseases might modify the metabolism in muscle locally and lead to poorer physical fitness [16, 21]. It is plausible as the accumulation of in situ neutrophils, macrophages, and pro-inflammatory cytokines such as IL-1b, IL-6, and TNF-α were observed in muscle injury [9, 33]. In addition, the anatomy of periodontal tissue has close anatomy proximity with the bloodstream. The occurrence of periodontal disease may influence physical performance through metastatic pathways, in a similar way to the biological mechanisms linking the chronic oral diseases and other chronic systemic diseases [21, 34, 35, 36].
Another explanation of the relationship between periodontal disease and physical fitness might come from the fatigue sensations during exercise. The workload may create an intense sensation that could reduce or stop the exercise. These sensations work physiologically to protect the body from damage and to maintain homeostasis [37, 38]. However, this mechanism could be magnified due to the increasing levels of cytokines originating from periodontal disease [16].
A study that used the general population as a sample found that the correlation between periodontitis and grip strength was mainly affected by anthropometric measures, which is related to adiposity and inflammation. The presumed mechanism is the interaction between the declining factors as increasing age [17]. While similar study on younger adults found no meaningful association between periodontal infection and cardiorespiratory fitness [20]. These results are corresponding since Eremenko et al. stated that the underlying mechanism might be related to the aging process. Although the other confounding factors such as nutritional intake and awareness of exercise were not analyzed, it is unlikely that lacking these data would meaningfully bias the result. As nutritional intake and exercise are likely to be strongly correlated with several factors that were included in the analysis (i.e., body composition, blood pressure, educational level, and pulse), they are likely to have been indirectly accounted [17, 20].
Dental malocclusion is another oral health problem that may affect physical fitness. Besides dental caries, periodontal disease also contributes to tooth loss, especially in the elderly [14]. The masticatory activity may decrease due to the limited number of teeth. This may lead to reduced stimulation of the central nerve through proprioceptive sensation from periodontal tissue which causes a reduction in other physical functions [22]. The declining number of teeth and occlusal support region had been reported to be correlated with the lower speed in walking performance and body balance [12, 22]. In normal individuals, in an upright position, various afferent sensory were presented by proprioceptive, tactile, vestibular, and visual receptors. The masticatory system, specifically the masticatory muscles and periodontal ligaments providing the proprioception also contributes in body balance. Edentulism may affect the maxillomandibular position, which may also disrupt the symmetrical sternocleidomastoid muscle contraction pattern, and therefore, affect the stability of the head posture and the body balance [22]. Tooth loss, which means the reduction in periodontal ligament proprioceptive input, may also lessen the sensory input to the brain leading to the declining cognitive function [15, 39]. The other possible mechanism is the nutritional pathway. Impaired mastication is associated with poor nutritional intake in adults, and therefore, linked to the chronic deterioration of physical and cognitive function [15]. This importance of sensory input from the periodontal ligament may also explain the reason why the natural dentition is better than prostheses. This hypothesis was supported by other evidence suggesting that occlusal function may affect the function of remote muscles through cortical activation [12, 22, 39, 40].
Some of the studies considered other factors, such as stress, age, and environmental factors. Most of the articles stated the relationship between oral health and physical fitness, also with cognitive function was stronger in older individuals [15, 20, 22]. The total tooth loss may simply be a potential early marker of higher risk of frailty in later life [20]. Most of the selected studies were conducted in developed countries, and the rest took place in developing countries. Health inequity is evident in many countries. Those disparities might occur as varying social structures, including socioeconomic status, politics, ethnics, culture, and gender. However, the underlying factors influencing health disparities in developed countries might be different from developing countries, and thus, further research is required to analyze the strength of the correlation between oral health and physical fitness according to the level of development of the country [41].
3.4. Limitations of this review
We decided to include observational studies only in this review as our study focuses on the epidemiology of oral disease and its effect. This focus prevents to draw causal inferences between oral health problems and physical fitness. It also results in some studies being excluded and only eleven articles included in this review. In addition, we decided to include any methods of measurement of physical fitness, resulting in various methods were reviewed and it was quite challenging to conclude. Finally, the included studies have different sample size with a huge gap between the smallest and the largest number, affecting the quality of data analysis.
4. Future research
Finally, considering that there are still limited number of studies to understand the correlation between poor oral health toward physical fitness and performance, we suggest to conduct an epidemiological study using the general population, with additional analysis involving the possible confounding factors, such as age, gender, socio-economic background, and habitual daily physical activity. According to Hariyani et al, the duration of follow up denotes a factor affecting a disease incidence [42]. Therefore, a further longitudinal observation on representative samples to understand both short-term and long-term effects of the degree of oral disease burden on physical fitness and performance is also necessary. Considering that human body constitutes a complex entity with its ability to adapt by means of a physiological process, a longitudinal study is required to give a better explanation about the negative impact of poor oral health toward physical fitness and performance [43].
5. Conclusion
Within the limitations of this review, we conclude that there was a negative effect of poor dental health toward physical fitness and performance, and also the cognitive function. In addition to athletes, the impacts could also be more distinctive in the elderly. The primary outcome of this review could be a persuasive argument to encourage both the athletes, the elderly and the authorities to be more attentive toward the oral health conditions of athletes and of course, other related groups to improve their quality of life.
6. Methods
We conducted a systematic review of the available literature to answer the focused question—What is the effect of oral health on physical fitness?
The following eligibility criteria were used when considering studies for this review:
-
⁃
Observational study design;
-
⁃
Language restriction: English only
-
⁃
Research subjects are humans without any age restrictions
-
⁃
Study factor/exposure: All types of dental and oral health problems (dental caries, periodontitis, edentulous, occlusal disturbance, etc). Any measures of oral health (eg, Decayed Missing Filled Teeth (DMFT))
-
⁃
Outcome of study: Physical fitness, which was objectively assessed by using physical fitness tests regardless of the types of the test. Physical fitness which was assessed by using a questionnaire was excluded
-
⁃
Any impact of oral health on physical fitness/performance.
We conducted a serial group discussions prior to the data accumulation, to adjust the perception regarding the operational definition of all variables, also standardize the data extraction. The problems faced during the data extraction would be solved with a further group discussion.
6.1. Search strategy
We searched PUBMED and EMBASE as the sources for studies, with no date restrictions were applied. We decided to focus on PUBMED and EMBASE since they are the largest pharmaceutical and biomedical database. Moreover, PUBMED is considered as the gold standard for biomedical database searching. Therefore, we believed that focusing on those two would be unlikely to lessen the number of articles we got [42, 44, 45]. We chose these two databases as they were considered as major biomedical databases. We anticipated a wide range of terms for possibly relevant studies and therefore designed a sensitive electronic search strategy. We used unique subject headings to each database (MeSH for PubMed and Emtree for Embase).
We developed a subject-specific search strategy using the following terms: stomatognathic diseases, mouth diseases, periodontal diseases, tooth diseases, periodontitis, athletic performance, physical fitness, physical fitness test, and exercise test.
6.2. Study selection
Articles, which were considered to meet the inclusion criteria were selected based on the title and abstract by two authors. The data selection was subsequently performed by two authors separately, then the results were compared. Any disagreements or disambiguates were then resolved through a discussion with all the authors. Data were extracted, tabulated, and presented to the title, author, study design, subject, oral condition, number of participant, study factor/exposure, outcome, test performed, results, and conclusion.
6.3. Data extraction
Data were retrieved from the articles and gathered in one document. All information such as title, authors, study design, population of study, oral conditions, number of participants, exposure and outcome, test performed, results, and conclusion were extracted. The measurement of all variables, stated confounding factors and the strategies to deal with, and statistical analysis used were extracted in detail in order to facilitate the critical appraisal performance.
6.4. Methodological quality
We assessed the quality of included articles using a standardized critical appraisal instruments as recommended by Joanna Briggs Institute. As all the studies were cross-sectional studies, the assessment of papers was carried out specifically based on “JBI Critical Appraisal Checklist for Analytical Cross-Sectional Studies'’. This standard appraisal is a set of checklists regarding the criteria of inclusion, the study subjects and setting, the measurement of exposure, the confounding factors and the strategies to deal with them, the measurement of outcome, and the statistical analysis. Any disagreements were resolved through discussion.
Declarations
Author contribution statement
T. Bramantoro: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
A. Zulfiana and W. Irmalia: Performed the experiments; Wrote the paper.
N.Hariyani, D. Setyowati and B. Purwanto: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by Universitas Airlangga, Surabaya, Indonesia, with grant number 908/UN3.14/LT/2019.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Baiju R.M., Peter E., Varghese N.O., Sivaram R. Oral health and quality of life: current concepts. J. Clin. Diagn. Res. 2017;11(6):ZE21–ZE26. doi: 10.7860/JCDR/2017/25866.10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelwan S.C., Nugraha R.A., Endaryanto A. Converging findings from linkage between periodontal pathogen with atopic and allergic immune response. Cytokine. 2019;113:89–98. doi: 10.1016/j.cyto.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 3.Cullinan M.P., Seymour G.J. Periodontal disease and systemic illness: will the evidence ever be enough? Periodontol. 2000. 2013;62(1):271–286. doi: 10.1111/prd.12007. [DOI] [PubMed] [Google Scholar]
- 4.De Souza B.C., Ribas M.E., De Oliveira Á.R., Burzlaff J.B., Haas A.N. Impact of gingival inflammation on changes of a marker of muscle injury in young soccer players during training: a pilot study. Rev. Odonto Ciência. 2012;27(4):294–299. [Google Scholar]
- 5.Abramson J.L., Vaccarino V. Relationship between physical activity and inflammation among apparently healthy middle-aged and older US adults. Arch. Intern. Med. 2002;162(11):1286. doi: 10.1001/archinte.162.11.1286. [DOI] [PubMed] [Google Scholar]
- 6.Ashley P., Di Iorio A., Cole E., Tanday A., Needleman I. Oral health of elite athletes and association with performance: a systematic review. Br. J. Sports Med. 2015;49(1):14–19. doi: 10.1136/bjsports-2014-093617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corbin C.B. A multidimensional hierarchical model of physical fitness: a basis for integration and collaboration. Quest. 1991;43(3):296–306. [Google Scholar]
- 8.Cannon J.G., Fielding R.A., Fiatarone M.A., Orencole S.F., Dinarello C.A., Evans W.J. Increased interleukin 1 beta in human skeletal muscle after exercise. Am. J. Physiol. Integr. Comp. Physiol. 1989;257(2):R451–R455. doi: 10.1152/ajpregu.1989.257.2.R451. [DOI] [PubMed] [Google Scholar]
- 9.Fielding R.A., Manfredi T.J., Ding W., Fiatarone M.A., Evans W.J., Cannon J.G. Acute phase response in exercise. III. Neutrophil and IL-1 beta accumulation in skeletal muscle. Am. J. Physiol. Integr. Comp. Physiol. 1993;265(1):R166–R172. doi: 10.1152/ajpregu.1993.265.1.R166. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong R.B. Mechanisms of exercise-induced delayed onset muscular soreness: a brief review. Med. Sci. Sports Exerc. 1984;16(6):529–538. [PubMed] [Google Scholar]
- 11.Sjuhada Oki A., Amalia N., Tantiana Wound healing acceleration in inflammation phase of post-tooth extraction after aerobic and anaerobic exercise. Sci. Sports. July 2019 [Google Scholar]
- 12.Inui A., Takahashi I., Sawada K. Teeth and physical fitness in a community-dwelling 40 to 79-year-old Japanese population. Clin. Interv. Aging. 2016;11:873–878. doi: 10.2147/CIA.S108498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leroux E., Leroux S., Maton F., Ravalec X., Sorel O. Influence of dental occlusion on the athletic performance of young elite rowers: a pilot study. Clinics (Sao Paulo) 2018;73:e453. doi: 10.6061/clinics/2017/e453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashimoto M., Yamanaka K., Shimosato T. Oral condition and health status of elderly 8020 achievers in Aichi Prefecture. Bull. Tokyo Dent. Coll. 2006;47(2):37–43. doi: 10.2209/tdcpublication.47.37. [DOI] [PubMed] [Google Scholar]
- 15.Tsakos G., Watt R.G., Rouxel P.L., De Oliveira C., Demakakos P. Tooth loss associated with physical and cognitive decline in older adults. J. Am. Geriatr. Soc. 2015;63(1):91–99. doi: 10.1111/jgs.13190. [DOI] [PubMed] [Google Scholar]
- 16.Oliveira J.A.P., Hoppe C.B., Gomes M.S., Grecca F.S., Haas A.N. Periodontal disease as a risk indicator for poor physical fitness: a cross-sectional observational study. J. Periodontol. 2015;86(1):44–52. doi: 10.1902/jop.2014.140270. [DOI] [PubMed] [Google Scholar]
- 17.Eremenko M., Pink C., Biffar R. Cross-sectional association between physical strength, obesity, periodontitis and number of teeth in a general population. J. Clin. Periodontol. 2016;43(5):401–407. doi: 10.1111/jcpe.12531. [DOI] [PubMed] [Google Scholar]
- 18.Eberhard J., Stiesch M., Kerling A. Moderate and severe periodontitis are independent risk factors associated with low cardiorespiratory fitness in sedentary non-smoking men aged between 45 and 65 years. J. Clin. Periodontol. 2014;41(1):31–37. doi: 10.1111/jcpe.12183. [DOI] [PubMed] [Google Scholar]
- 19.Shimazaki Y., Egami Y., Matsubara T. Relationship between obesity and physical fitness and periodontitis. J. Periodontol. 2010;81(8):1124–1131. doi: 10.1902/jop.2010.100017. [DOI] [PubMed] [Google Scholar]
- 20.Thai A., Papapanou P.N., Jacobs D.R., Desvarieux M., Demmer R.T. Periodontal infection and cardiorespiratory fitness in younger adults: results from continuous national health and nutrition examination survey 1999-2004. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0092441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoppe C.B., Oliveira J.A.P., Grecca F.S., Haas A.N., Gomes M.S. Association between chronic oral inflammatory burden and physical fitness in males: a cross-sectional observational study. Int. Endod. J. 2017;50(8):740–749. doi: 10.1111/iej.12686. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida M., Kikutani T., Okada G., Kawamura T., Kimura M., Akagawa Y. The effect of tooth loss on body balance control among community-dwelling elderly persons. Int. J. Prosthodont. 2015;22(2):136–139. http://www.ncbi.nlm.nih.gov/pubmed/19418857 [PubMed] [Google Scholar]
- 23.Ramírez-Vélez R., Rodrigues-Bezerra D., Correa-Bautista J.E., Izquierdo M., Lobelo F. Reliability of health-related physical fitness tests among Colombian children and adolescents: the Fuprecol study. PLoS One. 2015 doi: 10.1371/journal.pone.0140875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pilutti L.A., Sandroff B.M., Klaren R.E. Physical fitness assessment across the disability spectrum in persons with multiple sclerosis: a comparison of testing modalities. J. Neurol. Phys. Ther. 2015 doi: 10.1097/NPT.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 25.Grover V., Kapoor A., Malhotra R., Kaur G. Clinical relevance of the advanced microbiologic and biochemical investigations in periodontal diagnosis: a critical analysis. J. Oral Dis. 2014 [Google Scholar]
- 26.Needleman I., Ashley P., Fine P. Oral health and elite sport performance. Br. J. Sports Med. 2015;49(1):3–6. doi: 10.1136/bjsports-2014-093804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kosaka T., Ono T., Yoshimuta Y. The effect of periodontal status and occlusal support on masticatory performance: the Suita study. J. Clin. Periodontol. 2014;41(5):497–503. doi: 10.1111/jcpe.12241. [DOI] [PubMed] [Google Scholar]
- 28.Gaszynski T., Gaszynska E., Godala M., Szatko F. Masseter muscle tension, chewing ability, and selected parameters of physical fitness in elderly care home residents in Lodz, Poland. Clin. Interv. Aging. 2014;9:1197. doi: 10.2147/CIA.S66672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Omori S., Uchida F., Oh S. Exercise habituation is effective for improvement of periodontal disease status: a prospective intervention study. Therapeut. Clin. Risk Manag. 2018;14:565–574. doi: 10.2147/TCRM.S153397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beavers K.M., Brinkley T.E., Nicklas B.J. Effect of exercise training on chronic inflammation. Clin. Chim. Acta. 2010;411(11-12):785–793. doi: 10.1016/j.cca.2010.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park H.S., Nam H.S., Seo H.S., Hwang S.J. Change of periodontal inflammatory indicators through a 4-week weight control intervention including caloric restriction and exercise training in young Koreans: a pilot study. BMC Oral Health. 2015;15(1):1–8. doi: 10.1186/s12903-015-0094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferreira R. de O., Corrêa M.G., Magno M.B. Physical activity reduces the prevalence of periodontal disease: systematic review and meta-analysis. Front. Physiol. 2019;10:234. doi: 10.3389/fphys.2019.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toumi H., Best T.M. The inflammatory response: friend or enemy for muscle injury? Br. J. Sports Med. 2003;37(4):284–286. doi: 10.1136/bjsm.37.4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cotti E., Dessì C., Piras A., Mercuro G. Can a chronic dental infection be considered a cause of cardiovascular disease? A review of the literature. Int. J. Cardiol. 2011;148(1):4–10. doi: 10.1016/j.ijcard.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Schenkein H.A., Loos B.G. Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases. J. Clin. Periodontol. 2013;40:S51–S69. doi: 10.1111/jcpe.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tonetti M.S., Van Dyke T.E. Working group 1 of the joint EFP/AAP workshop. Periodontitis and atherosclerotic cardiovascular disease: consensus report of the Joint EFP/AAPWorkshop on Periodontitis and Systemic Diseases. J. Periodontol. 2013;84(4-s):S24–S29. doi: 10.1902/jop.2013.1340019. [DOI] [PubMed] [Google Scholar]
- 37.Enoka R.M., Duchateau J. Muscle fatigue: what, why and how it influences muscle function. J. Physiol. 2008;586(1):11–23. doi: 10.1113/jphysiol.2007.139477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noakes T.D. Fatigue is a brain-derived emotion that regulates the exercise behavior to ensure the protection of whole body homeostasis. Front. Physiol. 2012;3:82. doi: 10.3389/fphys.2012.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sunariani J., Khoswanto C., Irmalia W.R. Difference of brain-derived neurotrophic factor expression and pyramid cell count during mastication of food with varying hardness. J. Appl. Oral Sci. 2019;27:e20180182. doi: 10.1590/1678-7757-2018-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawakubo N., Miyamoto J.J., Katsuyama N. Effects of cortical activations on enhancement of handgrip force during teeth clenching: an fMRI study. Neurosci. Res. 2014;79:67–75. doi: 10.1016/j.neures.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 41.Orach C.G. Health equity: challenges in low income countries. Afr. Health Sci. 2009;9(Suppl 2):49–51. [PMC free article] [PubMed] [Google Scholar]
- 42.Hariyani N., Setyowati D., Spencer A.J., Luzzi L., Do L.G. Root caries incidence and increment in the population - a systematic review, meta-analysis and meta-regression of longitudinal studies. J. Dent. 2018;77:1–7. doi: 10.1016/j.jdent.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 43.Rühli F., van Schaik K., Henneberg M. Evolutionary medicine: the ongoing evolution of human physiology and metabolism. Physiology. 2016;31(6):392–397. doi: 10.1152/physiol.00013.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bramer W.M., Giustini D., Kleijnen J., Franco O.H. Searching Embase and MEDLINE by using only major descriptors or title and abstract fields: a prospective exploratory study. Syst. Rev. 2018 doi: 10.1186/s13643-018-0864-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Griffin S.O., Griffin P.M., Swann J.L., Zlobin N. Estimating rates of new root caries in older adults. J. Dent. Res. 2004 doi: 10.1177/154405910408300810. [DOI] [PubMed] [Google Scholar]