Summary
Amyloid are protein aggregates formed by cross β structures assemblies. Inhibiting amyloid aggregation or facilitating its disassembly are considered to be two major effective therapeutic strategies in diseases involving peptide or protein fibrillation such Alzheimer's disease or diabetes. Using thioflavin-T fluorescence, far-UV circular dichroism spectroscopy, and atomic force microscopy, we found nontoxic and biocompatible black phosphorus quantum dots (BPQDs) appear to have an exceptional capacity to inhibit insulin aggregation and to disassemble formed mature fibrils, even at an ultralow concentration (100 ng/mL). The inhibition of fibrillation persists at all stages of insulin aggregation and increases PC12 cells survival when exposed to amyloid fibrils. Molecular dynamics simulations suggest that BPQDs are able to stabilize the α-helix structure of insulin and obliterate the β-sheet structure to promote the fibril formation. These characteristics make BPQDs be promising candidate in preventing amyloidosis, disease treatment, as well as in the storage and processing of insulin.
Subject Areas: Drugs, Cell Biology
Graphical Abstract
Highlights
-
•
BPQDs inhibit insulin amyloid fibrillation at an ultralow concentration
-
•
BPQDs can depolymerize protofibrils and even mature fibers
-
•
BPQDs inhibit aggregation mainly by van der Waals' force and hydrophobic interaction
-
•
BPQDs are biocompatible and can reduce insulin fibrils-induced cytotoxicity
Drugs; Cell Biology
Introduction
Amyloid fibrils containing highly ordered β-sheet structure have been found to be one of the most prominent pathological features of a variety of diseases, including Alzheimer's disease, Parkinson's disease, Huntington's disease, prion conditions, and type II diabetes (Goedert and Spillantini, 2006, Shulman et al., 2011, Walker, 2007, Eisenberg and Jucker, 2012, Chiti and Dobson, 2006). Numerous studies have demonstrated that insoluble amyloid fibrils possess significant cytotoxicity (Pike et al., 1993, Petkova et al., 2005) and new drugs such as amyloid inhibitors could be promising therapeutic avenue.
Therapeutic strategies directed at targeting amyloidosis are divided into two main categories: the inhibition of fibrils formation or the elimination of amyloid fibrils. Depending on the strategy used, different types of inhibitors have been discovered and studied over the past decade. An emerging example of such inhibitors are nanomaterials that have demonstrated remarkable ability to interact strongly with amyloids and amyloid fibrils owing to their large surface area, unique physical properties, and small size (Yousaf et al., 2017, Wang et al., 2017). On the one hand, some nanoparticles, such as titanium dioxide nanoparticles (Wu et al., 2008), carbon nanotubes, and cerium oxide nanoparticles (Linse et al., 2007), have been reported to promote the nucleation step of amyloid fibrillation and exacerbate amyloid-related diseases. On the other hand, a number of studies have proven that nanomaterials such as carbon dots (Li et al., 2015), graphene oxide (Li et al., 2012, Wang et al., 2019), N-acetyl-L-cysteine-capped CdTe quantum dots (Xiao et al., 2010), thioglycolic acid-capped CdTe quantum dots (Yoo et al., 2011), and dihydrolipoic acid-capped CdSe/ZnS quantum dots (Thakur et al., 2011) can efficiently delay nucleation or inhibit fibrils formation. These nanomaterials have attracted a lot of attention due to their outstanding inhibitory effect (Wang et al., 2017, Cabaleiro-Lago et al., 2010). Several strategies have been suggested to further enhance nanomaterials capacity to inhibit fibrillation. For example, Xiong et al. (2017) have conjugated two peptide inhibitors (VVIA and LPFFD) onto gold nanoparticles (AuNPs) and demonstrated that the inhibition activity of the corresponding peptide@AuNPs was 50% enhanced compared with peptides alone. Nanomaterials can not only inhibit fibrillation but can also effectively destroy and clear pre-formed fibrillary structures. Recently, Kim et al. (2018) found that graphene quantum dots induced the dissociation of α-synuclein fibrils into short fragments. Yin et al. (2016) used penetratin peptide-loaded PEG-stabilized gold nanostars modified with ruthenium complex to remotely dissociate preformed fibrous Aβ fibrils under near-infrared irradiation.
Nevertheless, the toxicity of nanomaterials should not be ignored in in vivo applications. Reactive oxygen species (ROS) generation is one of the important mechanisms of nanoparticle-induced toxicity. ROS can trigger the generation of oxidative stress and even damage mitochondria, which in turn triggers a series of mitochondrial-mediated toxic effects (Wu et al., 2020, Yang et al., 2019). For example, CdTe quantum dots can not only damage mitochondria, but also exert endothelial toxicity by activating mitochondrial death pathways and inducing endothelial cell apoptosis (Yan et al., 2011). Metal ions may be released after partial biodegradation of metal nanomaterials, resulting in potential metal dyshomeostasis associated with side effects (Yang et al., 2019). In addition, the contamination of residual impurities in nanomaterials is also considered as a major risk factor. For example, nickel, yttrium, or rubidium metal impurities may be present on the surface of carbon nanotubes (Jeevanandam et al., 2018). The omnipresent toxic effect of most nanomaterials limits the therapeutic window of these materials, which lowers significantly their ability to inhibit amyloid fibrillation. Therefore, highly efficient, non-toxic, and biodegradable amyloid targeted inhibitors are still highly demanded for both defibrillation and inhibition of amyloid proteins fibrillation.
Black phosphorus quantum dot (BPQD) is a novel kind of zero-dimensional layered nanomaterial that has drawn increasing attention in recent years. Owing to the lone pair of electrons on the outer orbitals of the phosphorus atom, BPQDs are easily oxidized into non-toxic phosphate (Luo et al., 2019). OH− initiated the decomposition of BP through breaking the P-P bond and forming a P-O bond, and the degradation process of BP could be regulated by changing the pH (Zhang et al., 2019). The chemical instability of BPQDs contributes to their biocompatibility and biodegradation (Zhang et al., 2018) and has proven to benefit their biomedical application in tumor photothermal therapy, drug delivery, and therapeutic diagnostics (Luo et al., 2019, Choi et al., 2018). Pathological evaluation of tissues obtained from the lung, liver, spleen, kidney, and heart of BPQDs-treated mice at 1, 10, and 30 days post injection demonstrate that the BPQDs have good biocompatibility for these organs of the mice during the entire period (Wang et al., 2018a). Additionally, BP nanosheets have been reported to effectively capture excess Cu2+ and to form non-toxic metal complex, therefore protecting neuronal cells from ROS toxicity caused by Cu2+, a key benefit for the treatment of neurodegenerative diseases (Chen et al., 2018). Some modified BP can inhibit Aβ aggregation. For instance, the photo-excited BP@BTA (BTA: one of thioflavin-T derivatives) can generate singlet oxygen (1O2) to oxygenate Aβ under NIR laser, which inhibits the aggregation and reduces the Aβ-induced cytotoxicity (Li et al., 2019). Lim et al. (2019) found that, when the mass ratio of Aβ40 to BP is greater than 1:0.25, the titanium ligand-modified BP nanosheets can reduce total amounts of aggregated Aβ40, but they cannot delay the initiation of Aβ40 aggregation. Therefore, BP has great application potential in the field of regulating amyloid fibrosis. However, the effect of BPQDs on peptide or protein fibrils inhibition has not been clearly delineated yet.
To further investigate the interaction between BPQDs and amyloid fibrils, insulin, an important therapeutic protein for type Ⅱ diabetes condition, was chosen as a model protein (Scheme 1). The amyloidogenesis of insulin reduces the efficacy of insulin administrations and disturbs insulin delivery, which may lead to diabetic ketoacidosis, a life-threatening complication. Besides, insulin amyloid fibrillation is one of the major issues in the processes of its production, storage, and delivery (Li et al., 2015, Ratha et al., 2016). In the present study, amyloid fibrosis was monitored by thioflavin-T (ThT) fluorescence, far-UV circular dichroism (CD) spectroscopy, and atomic force microscopy (AFM). Addition of BPQDs significantly inhibited the conversion of insulin into amyloid fibrils over several days at extremely low concentrations never attained by any other nanomaterial reported so far. Molecular dynamics (MD) simulation was used to provide further insights into this remarkable effect. In addition, the cytotoxicity of BPQDs and insulin solutions exposed to different concentrations of BPQDs was evaluated using in vitro cell toxicity assays. Overall, results show that an ultralow concentration of BPQDs acts as potent and non-toxic amyloid fiber inhibitor, which offers great potential in the development of diabetes treatment and other diseases involving amyloid fibrillation.
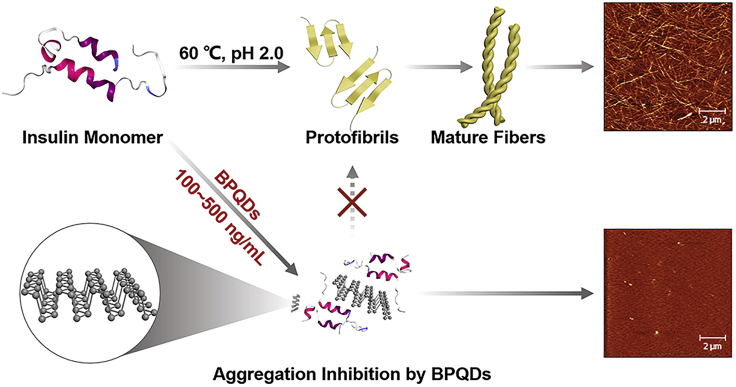
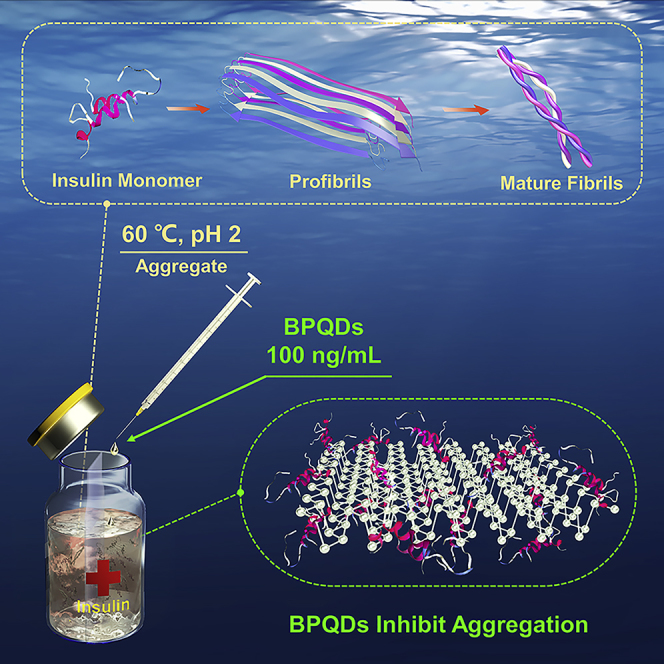
Scheme 1.
Schematic Representation of Amyloid Aggregation of Insulin in the Absence and Presence of BPQDs
The insulin monomers were all incubated at 60°C and pH 2.0. Stable amyloid fibrils made of pure insulin were formed after 2 days, whereas no significant fibrils were observed after 7 days in the presence of BPQDs. The illustrations are AFM images of insulin amyloid aggregation after 7 days.
Results
Inhibition of Insulin Aggregation and Fibrillation by BPQDs
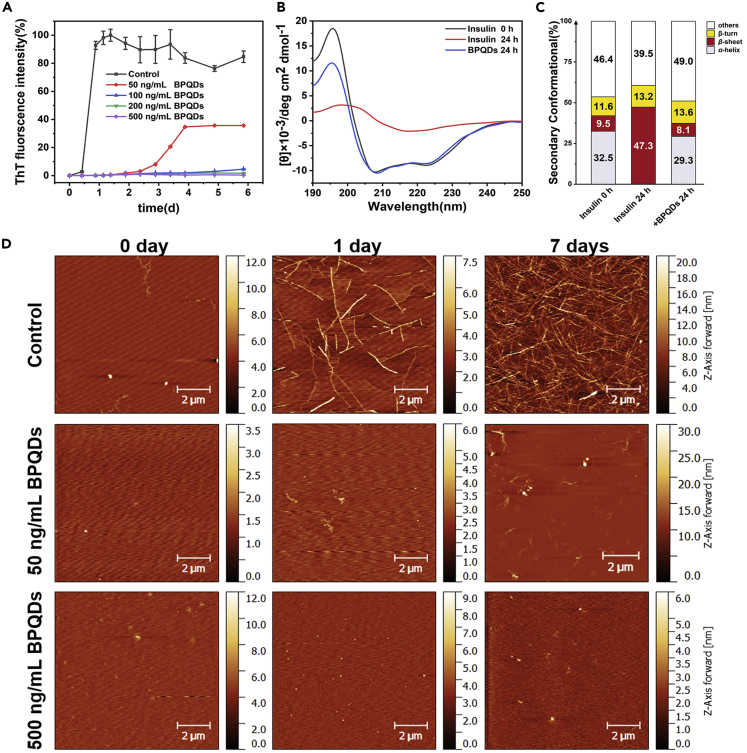
BPQDs were prepared via a liquid exfoliation method as reported before in our laboratory, and the average lateral size of BPQDs is about 3 nm (Xia et al., 2018). Accelerated insulin aggregation was triggered by heat treatment (60°C, 48 h at pH = 2) to denature α-helix structures in the protein and promote β-sheets refolding, a necessary step to aggregation and fibrillation. Different concentrations of BPQDs (0, 50, 100, 200, 500 ng/mL) were co-incubated with denatured insulin to study the inhibitory effect of BPQDs on amyloid fibrillation. The aggregation kinetics of amyloid was monitored by ThT fluorescence assay. ThT is an extrinsic fluorescent dye that can specifically bind to the β-sheet structure of amyloid fibrils, and its fluorescence intensity increases with the content of β-sheet structure (Liu et al., 2015, Niu et al., 2016, Biancalana and Koide, 2010).
As shown in Figure 1A, the aggregation kinetics (black line) of insulin alone presented three stages characteristic of a typical nucleation-dependent fibrillation process: first, a lag phase were no apparent aggregation is observed, followed by the elongation phase were aggregation and fibrillation occur simultaneously, and finally a saturation phase were mature fibrils are fully formed and monomeric insulin is derived from the medium (Lee et al., 2007, Lee et al., 2011). The lag phase was observed to be as long as 12 h in the measurement and concentration conditions used. During this phase, nucleation of small clusters from protein monomers are slowly formed (Yousaf et al., 2017, Liu et al., 2018). During the elongation phase, ThT fluorescence intensity increased rapidly indicating the quick aggregation of small clusters into amyloid fibrils. After 1-day incubation, the fluorescence intensity reached a maximum value and gradually stabilized, which demonstrated a saturation phase with the formation of mature fibrils. When incubated with BPQDs, the evolution of ThT fluorescence in denatured insulin solutions was dramatically changed. Several concentrations of BPQDs at 100, 200, and 500 ng/mL were found to totally resist the aggregation kinetics as denoted by the almost no increase in the ThT fluorescence intensity after 4 days. Even when exposed to an ultralow concentration of 50 ng/mL BPQDs (red line), a significant inhibition of insulin fibrils could be observed. The lag phase was extended to about 2.5 days and the fluorescence intensity at the final saturation phase was 60%, lower than that of insulin alone. BPQDs alone were also incubated with ThT under the same conditions to identify if the observations could have resulted from an interaction between ThT and BPQDs. As shown in Figure S1, no change in emission fluorescence was found over several days, ruling out any possible artifact from ThT and BPQDs interactions. Therefore, insulin fibrillation was effectively suppressed by BPQDs at concentrations that are much lower than previously reported from other nanomaterials (Table 1). Contrary to gold nanoparticles, whose inhibitory concentration was reported to be as low as 1 nM, BPQDs do not need to be functionalized with peptides or other functional groups (Mirsadeghi et al., 2015, Ruff et al., 2018, Xiong et al., 2017, Yin et al., 2016), or to be remotely activated (Li et al., 2017) to maintain an exceptionally high inhibitory effect.
Figure 1.
Influence of BPQDs on the Inhibition of Insulin Fibrillation Aggregation
(A) The growth kinetic curves of insulin amyloid fibrillation monitored by ThT fluorescence assay. Relative ThT fluorescence intensity varied at 485 nm with the incubation time of insulin at different BPQDs concentrations (0, 50, 100, 200, 500 ng/mL). The final concentration of insulin is 2 mg/mL. All data represent the average from three experiments. Error bars indicate ± SD.
(B) Far-UV CD spectra curves of insulin alone and in the presence of 500 ng/mL of BPQDs, as well as the corresponding secondary structure content.
(C) The primal insulin and the incubated (24 h) insulin were diluted twice and tested using a 1-nm quartz cell.
(D) AFM images of insulin amyloid fibrillation at different incubation times (0, 1, and 7days) for insulin alone and insulin-BPQDs (50 and 500 ng/mL) solutions. (AFM: tapping mode).
Table 1.
Summary of the Mass Concentration Ratios of Inhibitors and Amyloid Protein
| Protein | Protein Concentration (mg/mL) | Inhibitor | Inhibitor Concentration (ng/mL) | Mass Ratios (Inhibitor:Protein) | Inhibition ratea (%) | Reference |
|---|---|---|---|---|---|---|
| Insulin | 2.000 | Black phosphorus quantum dots | 100 | 1:20000 | 95 | This work |
| 2.000 | Silicon nanoparticles | 5,000 | 1:400 | 88 | Ma et al., 2019 | |
| 0.200 | Carbon dots | 10,000 | 1:20 | 27 | Li et al., 2015 | |
| HIAPP | 0.077 | Fluorinated graphene quantum dots | 385,000 | 1:0.2 | 95 | Yousaf et al., 2017 |
| 0.096 | Graphene quantum dots | 500,000 | 1:0.2 | 60 | Wang et al., 2018b | |
| Aβ | 0.087 | Au nanoclusters | 20,000 | 1: 4.3 | 95 | Gao et al., 2017 |
| 0.181 | bPEI-coated carbon dots | 500,000 | 1:0.4 | 75 | Chung et al., 2017 | |
| α-syn | 5.000 | Graphene quantum dots | 5,000,000 | 1:1 | 90 | Kim et al., 2018 |
For comparison, the protein and inhibitor concentrations were, respectively, converted to mass concentration.
The inhibition rate is estimated based on the results of ThT fluorescence spectroscopy.
During the aggregation process, the secondary structure of insulin was monitored using far-UV CD spectra. As shown in Figure 1B, two negative peaks were identified at 208 and 222 nm at the initial incubation of insulin, indicating the presence of α-helix conformation. After 24 h incubation of insulin alone, the negative peak intensity decreased and a single broad negative peak was observed at 218 nm, indicating that most of the secondary structure was converted to β-sheet conformation. Quantitatively, analysis of the fractional secondary structure (Figure 1C) showed that the α-helix conformation decreased from 32.5% to 0.0% and the β-sheet conformation increased from 9.5% to 47.3%. Co-incubation of insulin with BPQDs (500 ng/mL) proves that the two negative peaks observed at 208 and 222 nm in insulin solutions alone at t = 0 still remains after 24 h, demonstrating that the α-helical secondary structure was still present. After the addition of BPQDs, a small variation in the α-helix (from 32.5% to 29.3%) and the β-sheet (from 9.5% to 8.1%) fractions was detected, showing that only a small part of the α-helix and the β-sheet conformation evolved into other conformations such as random coil. These observations confirmed that the inhibitory effect of BPQDs is due to their capacity to stabilize the α-helix secondary structure of insulin, an ability that must involve specific interactions between the nanomaterial and the protein.
To further explore the inhibitory effect of BPQDs on insulin amyloid fibrillation, the morphology of insulin protofibrils at different incubation times (0, 1, and 7 days) was followed by AFM. As shown in Figure 1D, during incubation of insulin alone, a few long fibrils appeared after 1 day, whereas numerous mature fibrils were observed after 7 days. When insulin was co-incubated with 50 ng/mL of BPQDs, only a few short fibrils were detected after 7 days of incubation. When 500 ng/mL of BPQDs was used, no fibrils were detected after 7 days of incubation. Therefore, AFM data were fully consistent with the results obtained from the ThT fluorescence assay and CD spectra, further confirming the excellent capability of BPQDs in inhibiting insulin aggregation.
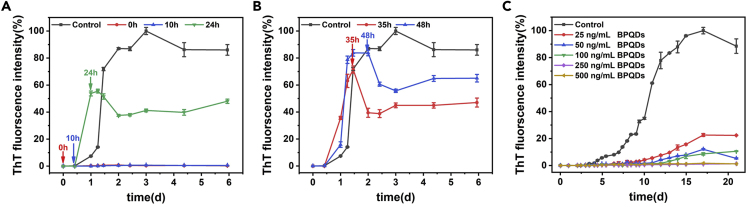
To further understand the inhibitory mechanism of BPQDs, insulin solutions were exposed to 500 ng/mL BPQDs at different stages of the aggregation process (0, 10, 24, 35, and 48 h) and ThT signal was monitored. As shown in Figures 2A and 2B, during the lag phase (0 and 10 h), the addition of BPQDs completely inhibited insulin fibrillation as demonstrated by the absence of variation in the ThT signal. When BPQDs were introduced during the elongation phase (24, 35, and 48 h), ThT fluorescence intensity decreased, indicating a loss of β-sheet structure and therefore depolymerization of protofibrils. These results suggested that BPQDs could bind not only to insulin monomers to interfere with the early stages of nucleation, but also to fibrils and stop their growth. Therefore, BPQDs have a broad impact on amyloid inhibition on various stages of the aggregation process, especially in the lag phase where almost complete inhibition was observed.
Figure 2.
ThT Fluorescence Intensity of Insulin Amyloid Fibrillation for Analyzing the Effect of BPQDs Added Time and the Existence of NaCl
The black line was treated as a control experiment that no BPQDs have been added. BPQDs (500 ng/mL) were added at different incubation times, including (A) 0 (red), 10 (blue), 24 (green), (B) 35 (red), and 48 h (blue), respectively. Arrows indicate the time of BPQDs added. All data represent the average from three experiments. Error bars indicate ± SD. (C) The growth kinetic curves of insulin under incubation conditions without NaCl monitored by ThT fluorescence assay. Relative ThT fluorescence intensity at 485 nm varied as a function of incubation time of insulin in the presence of various concentrations (0, 25, 50, 100, 250, 500 ng/mL) of BPQDs. All data represent the average from three experiments. Error bars indicate ± SD.
Since amyloid fibrillation kinetics is highly sensitive to ionic strength, a kinetic test was also performed in the absence of ions in the insulin solution (Figure 2C). Even though the kinetics of fibrillation was dramatically slowed (insulin alone took about 15 days to reach the saturation phase), BPQDs were still found to inhibit fibrillation completely at concentrations as low as 250 ng/mL.
Depolymerization Assay of Insulin Fibrils by BPQDs
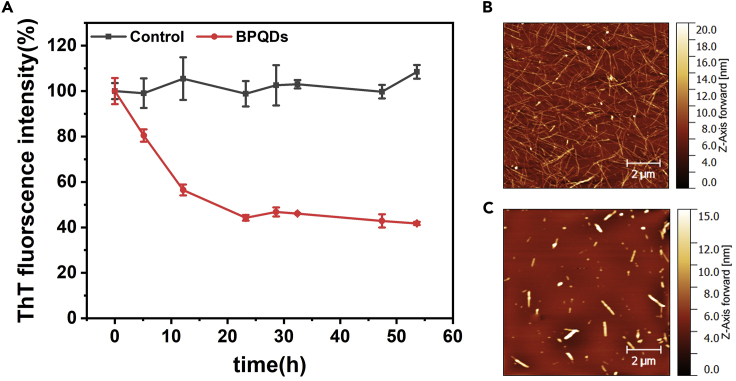
Considering the capacity of BPQDs to reduce the concentration/length of amyloid fibers during the elongation phase, the effect of BPQDs on mature fibrils was further examined in the saturation phase (Figure 3). Insulin monomer was first incubated for 4 days to ensure complete conversion to mature fibrils (Figure 3B). Then an equal amount of ultrapure water (control group) and 500 ng/mL BPQDs were added separately and ThT fluorescence was monitored. As shown in Figure 3A, the ThT fluorescence intensity gradually decreased almost 60% in the presence of BPQDs after 1-day incubation. Only short and thick fibers were observed by AFM imaging, confirming that long fibrils originally present have totally disappeared from the suspension (Figure 3C). These results confirmed the capacity of BPQDs to depolymerize mature fibers, in addition to stop their growth.
Figure 3.
Depolymerization Assay
(A) ThT fluorescence intensity in insulin solutions in absence (black) and presence (red) of BPQDs. BPQDs (500 ng/mL) were added after 5 days of fibrils maturation. Starting time (t = 0) corresponds to the instant when BPQDs were added to the mature fibrils suspension. All data points represent the average from three independent experiments. Error bars indicate ± SD.
(B and C) (B) AFM images of insulin alone after 2 days incubation, as well as the co-incubation of insulin and BPQDs (C).
Elucidation of the Interaction between BPQDs and Insulin by Molecular Dynamics Simulation
To obtain insights at the molecular level on the unique capacity of BPQDs to inhibit insulin amyloid fibrillation, MD simulations of insulin/BPQDs aggregates were performed. Since insulin fibrils are mainly formed from monomers, interaction between a single insulin molecule and a BPQD was first studied (Ahmad et al., 2005). The time evolution of root-mean-square deviation (RMSD) (Figure S2) confirmed that the BPQD/insulin complex became stable after about 40 ns, and we concentrate our analysis on the last 10 ns. Figures 4A–4D show the structures of insulin alone and in presence of BPQDs before and after 60 ns of simulation. In the absence of BPQDs, the native α-helix structure of insulin partially transformed into β conformation after 60 ns (brown segment in Figure 4C, accounting for 7.61%), which contributed to form β-sheets. Inversely, the presence of BPQDs showed to effectively maintain the α-helix structure, as no β conformation could be seen during the simulation.
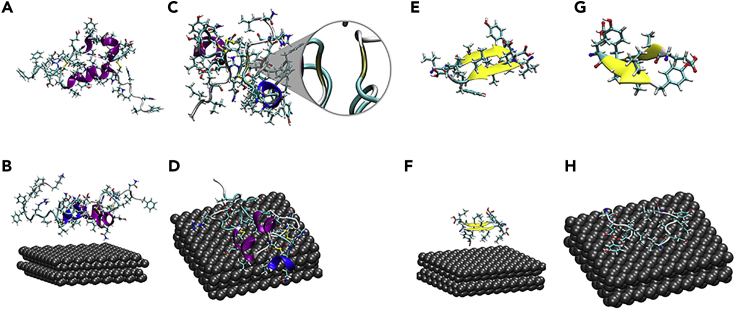
Figure 4.
Molecular Dynamics Simulation
(A) Initial structure of pure insulin.
(B) Initial structure of insulin with the BPQDs.
(C) Final structure of pure insulin after 60 ns. The enlarged brown part represents the β conformation.
(D) Final structure of insulin with the BPQDs after 60 ns.
(E) Initial β-sheet structure formed by two insulin parts (B12–B17) without BPQDs.
(F) Initial structure of the same fragment insulin (B12–B17) with the BPQDs.
(G) Final β-sheet structure formed by two insulin parts (B12–B17) without BPQDs after 60 ns.
(H) Final structure of the same fragment (B12–B17) with the BPQDs after 60 ns.
The B12–B17 segment of insulin is normally regarded as the main segment forming the spine of the fibril via anti-parallel β-strands (Groenning et al., 2009). To gain a deeper understanding of the depolymerization process of amyloid fibrils by BPQDs, the evolution of the structure of the B12–B17 (VEALYL) fragment was investigated in the presence of BPQDs. As shown in Figures 4E and 4G, the double β-strand structure of the fragment was preserved during the whole duration of the simulation when no BPQD was present. When BPQD was introduced in the simulation, BPQD was able to disrupt the β-sheets totally in the time frame of the simulation (Figures 4F and 4H).
Specifically, BPQDs interact with 16 binding sites of insulin and most of them possessed the hydrophobic residues (Ile, Phe, Leu, Tyr, and Val). There are some useful ways to visually study inter-fragment and intra-fragment interactions, and we did the weak interaction analysis with Independent Gradient Model (ICG) (Lefebvre et al., 2017) by Multiwfn, a multifunctional wave function analyzer (Lu and Chen, 2012). The green isosurface in Figure S3 represents the van der Waals' force region between a BPQD and an asparagine. For the depolymerization process, the solvent accessible surface area (SASA) of the β-sheet fragment (Figure S4A) indicates that this fragment becomes less hydrophobic after binding with BPQDs and the hydrophobic interactions attribute to the depolymerization force. At the same time, the hydrogen bonds between the β-sheet fragment and water increase, which is consistent with the SASA results (Figure S4B). The van der Waals' force region between a BPQD and β-sheet fragment (Figure S5) demonstrates that both the inhabitation and dissociation are driven chiefly by hydrophobic interactions and van der Waals' force, accompanied by structural changes.
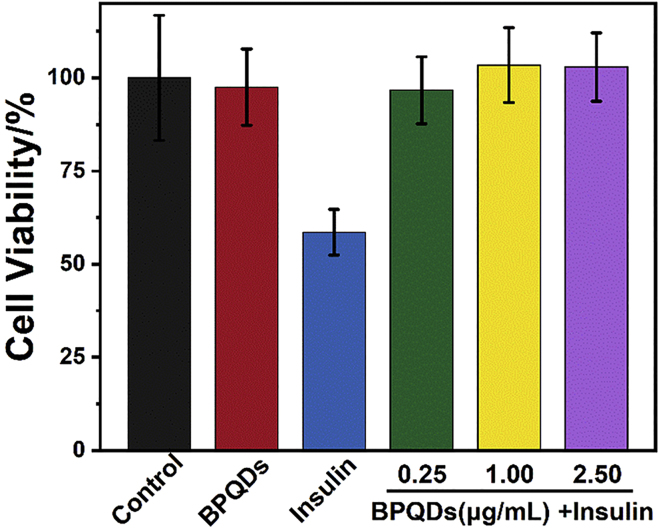
The Effect of BPQDs on Amyloid Cytotoxicity
To evaluate the effect of BPQDs on amyloid-induced cytotoxicity, PC12 cells were assayed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay to assess cellular activity. As shown in Figure 5, compared with the control test (addition of buffer), cell viability was decreased to 58% in the presence of the insulin fibrils solutions, which indicates strong cytotoxicity. However, under the same incubation conditions, the cellular activity of BPQDs alone was similar to the control test (about 97.5%), which further verified the non-toxicity of BPQDs and their potential application in the biological field. In addition, cellular activity was maintained at levels similar to that of control (95% or more of cell viability) in the co-incubated solutions of insulin and BPQDs, which illustrated the ability of BPQDs in reducing amyloid-induced cytotoxicity. The reasons for such dramatic effect was attributed to the inhibition of insulin aggregation by BPQDs, as well as the non-toxic and physical property of BPQDs (Chen et al., 2017, Luo et al., 2019, Shao et al., 2016, Sun et al., 2016).
Figure 5.
Cell Toxicity Assay of PC12 Cells
Cell viability was measured by the MTT reduction method. PC12 cells were treated with BPQDs alone, insulin alone, and insulin in the presence of BPQDs after 5 days incubation. The concentrations of BPQDs were 0.25, 1.00, and 2.50 μg/mL, respectively. The final concentration of insulin was 5 μM. The control experiment was treated without insulin fibrils. All data represent the average from six different data. Error bars indicate ± SD.
Discussion
The BPQDs can make up a drug delivery system with pH/photoresponsive release properties, and the outstanding in vivo antitumor therapeutic outcome was realized after the synergistic photodynamic/photothermal/chemotherapy with BPQDs (Chen et al., 2017). Aiming at the therapy of diseases involving protein fibrillation, we synthesize BPQDs and found it inhibits insulin aggregation completely even in a very low concentration, 50 ng/mL, which appeared as the lowest concentration of nano-inhibitor reported so far. Interestingly, compared with other nano materials, such as SiNPs and carbon dots, BPQDs had a more outstanding effect on suppressing insulin fibrillation in the lag phase, elongation phase, and saturation phase at this concentration. Furthermore, BPQDs not only inhibit insulin monomer and protofibrils further aggregation, but also depolymerize protofibrils and even mature fibers.
Molecular dynamics simulations demonstrate that BPQDs interact with insulin mainly through hydrophobic and van der Waals' interactions and the binding sites (Ile, Phe, Leu, Tyr, and Val) reserve the initial configuration of insulin, which resists the transformation from α-helix structure to β-sheet structure and open the formed β-sheet fragments. In contrast, without BPQDs, the native α-helix structure of insulin partially transformed into β conformation, which contributed to form β-sheets. BPQDs also contribute to the depolymerization process of amyloid fibrils. MD simulations reveal that BPQDs disrupt the β-strand structure of the main segment forming the spine of the fibril by interacting with individual chains and separating them.
Furthermore, without BPQDs, the C-terminal fragment of the chain B of insulin is not well ordered, which leads to the exposure of its non-polar areas, and the aromatic side chains (B24–B26, FFY) may contribute to the formation of non-native β-sheet, which resists the fibrillation (Hua and Weiss, 2004). In contrast, the aromatic ring of B24–B26 (Figure S6) was able to bind to the BPQDs and avoid the formation of β-sheets. As a consequence, in the presence of BPQDs, α-helix domains remain more stable, which stops the formation of fibrils. Upon fibrillation, insulin undergoes structural changes from a predominantly α-helical state to a β-sheet-rich conformation (Ivanova et al., 2009), but BPQDs could restrain this process by interacting with the insulin.
To explore the difference from other nanomaterials, we also performed the MD simulation for insulin and silica nanoparticles (SiNPs). Differing from BPQDs, which mainly interact with insulin by dispersion force and hydrophobic interaction, electrostatic interaction and hydrogen bond are the primary driving forces to combine the negatively charged SiNPs surface and insulin (Figures S7 and S8). Most residues of insulin are hydrophobic or neutral groups, and there are more binding sites of insulin on BPQDs than SiNPs, leading to that the BPQDs could inhibit insulin aggregation completely in a surprisingly lower concentration than SiNPs.
Cytotoxicity experiments suggested that BPQDs can reduce insulin-induced cytotoxicity. Compared with other nano-inhibitors, BPQDs demonstrate non-toxicity and very high inhibition efficiency. Therefore, BPQDs have great potential in the prevention and treatment of amyloidosis, as well as storage and processing of proteins in the pharmaceutical industry.
Limitations of the Study
Owing to the limitation of experimental conditions, the inhibition effect of BPQDs on insulin aggregation was not performed in vivo. Although the insulin incubation process was similar to that in other previous works (high temperature or high salinity), it could not demonstrate that the BPQDs possess the ability of inhibiting insulin amyloid. Thus, it is necessary to establish an in vivo mode in the future experiments. In addition, the interaction mechanism of BPQDs and insulin or denatured insulin should be stated in greater detail, such as the binding site or spatial structure effect. All of these will be needed for a further study to help us understand the inhibition or promotion mechanism of nanomaterials on protein amyloidosis.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant No. 21621004, 51473115).
Author Contributions
S.W. and R.S. conceived and designed the experiments. S.W. and Y.X. carried out the experiments. C.L. performed the computations. S.W., C.L., Y.X., X.B., and R.S. drafted the manuscript, and all authors participated in the writing and review of the final draft.
Declaration of Interests
The authors have no conflicts of interest to declare.
Published: May 22, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101044.
Data and Code Availability
All data are available from the corresponding author upon reasonable request.
Supplemental Information
References
- Ahmad A., Uversky V.N., Hong D., Fink A.L. Early events in the fibrillation of monomeric insulin. J. Biol. Chem. 2005;280:42669–42675. doi: 10.1074/jbc.M504298200. [DOI] [PubMed] [Google Scholar]
- Biancalana M., Koide S. Molecular mechanism of thioflavin-T binding to amyloid fibrils. Biochim. Biophys. Acta. 2010;1804:1405–1412. doi: 10.1016/j.bbapap.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabaleiro-Lago C., Quinlan-Pluck F., Lynch I., Dawson K.A., Linse S. Dual effect of amino modified polystyrene nanoparticles on amyloid β protein fibrillation. ACS Chem. Neurosci. 2010;1:279–287. doi: 10.1021/cn900027u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Ouyang J., Liu H., Chen M., Zeng K., Sheng J., Liu Z., Han Y., Wang L., Li J. Black phosphorus nanosheet-based drug delivery system for synergistic photodynamic/photothermal/chemotherapy of cancer. Adv. Mater. 2017;29:1603864. doi: 10.1002/adma.201603864. [DOI] [PubMed] [Google Scholar]
- Chen W., Ouyang J., Yi X., Xu Y., Niu C., Zhang W., Wang L., Sheng J., Deng L., Liu Y.N. Black phosphorus nanosheets as a neuroprotective nanomedicine for neurodegenerative disorder therapy. Adv. Mater. 2018;30:1703458. doi: 10.1002/adma.201703458. [DOI] [PubMed] [Google Scholar]
- Chiti F., Dobson C.M. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- Choi J.R., Yong K.W., Choi J.Y., Nilghaz A., Lin Y., Xu J., Lu X. Black phosphorus and its biomedical applications. Theranostics. 2018;8:1005–1026. doi: 10.7150/thno.22573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y.J., Kim K., Lee B.I., Park C.B. Carbon nanodot-sensitized modulation of alzheimer’s β-amyloid self-assembly, disassembly, and toxicity. Small. 2017;13:1700983. doi: 10.1002/smll.201700983. [DOI] [PubMed] [Google Scholar]
- Eisenberg D., Jucker M. The amyloid state of proteins in human diseases. Cell. 2012;148:1188–1203. doi: 10.1016/j.cell.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G., Zhang M., Gong D., Chen R., Hu X., Sun T. The size-effect of gold nanoparticles and nanoclusters in the inhibition of amyloid-β fibrillation. Nanoscale. 2017;9:4107–4113. doi: 10.1039/c7nr00699c. [DOI] [PubMed] [Google Scholar]
- Goedert M., Spillantini M.G. A century of Alzheimer's disease. Science. 2006;314:777–781. doi: 10.1126/science.1132814. [DOI] [PubMed] [Google Scholar]
- Groenning M., Frokjaer S., Vestergaard B. Formation mechanism of insulin fibrils and structural aspects of the insulin fibrillation process. Curr. Protein Pept. Sci. 2009;10:509–528. doi: 10.2174/138920309789352038. [DOI] [PubMed] [Google Scholar]
- Hua Q.X., Weiss M.A. Mechanism of insulin fibrillation: the structure of insulin under amyloidogenic conditions resembles a protein-folding intermediate[J] J. Biol. Chem. 2004;279:21449–21460. doi: 10.1074/jbc.M314141200. [DOI] [PubMed] [Google Scholar]
- Ivanova M.I., Sievers S.A., Sawaya M.R., Wall J.S., Eisenberg D. Molecular basis for insulin fibril assembly[J] Proc. Natl. Acad. Sci. U S A. 2009;106:18990–18995. doi: 10.1073/pnas.0910080106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeevanandam J., Barhoum A., Chan Y.S., Dufresne A., Danquah M.K. Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018;9:1050–1074. doi: 10.3762/bjnano.9.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Yoo J.M., Hwang H., Lee J., Lee S.H., Yun S.P., Park M.J., Lee M., Choi S., Kwon S.H. Graphene quantum dots prevent α-synucleinopathy in Parkinson's disease. Nat. Nanotechnol. 2018;13:812–818. doi: 10.1038/s41565-018-0179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.C., Nayak A., Sethuraman A., Belfort G., Mcrae G.J. A three-stage kinetic model of amyloid fibrillation. Biophys. J. 2007;92:3448–3458. doi: 10.1529/biophysj.106.098608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Culyba E.K., Powers E.T., Kelly J.W. Amyloid-β forms fibrils by nucleated conformational conversion of oligomers. Nat. Chem. Biol. 2011;7:602–609. doi: 10.1038/nchembio.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre C., Rubez G., Khartabil H., Boisson J.C., Contreras-Garcia J., Henon E. Accurately extracting the signature of intermolecular interactions present in the NCI plot of the reduced density gradient versus electron density. Phys. Chem. Chem. Phys. 2017;19:17928–17936. doi: 10.1039/c7cp02110k. [DOI] [PubMed] [Google Scholar]
- Li M., Yang X., Ren J., Qu K., Qu X. Using graphene oxide high near-infrared absorbance for photothermal treatment of Alzheimer's disease. Adv. Mater. 2012;24:1722–1728. doi: 10.1002/adma.201104864. [DOI] [PubMed] [Google Scholar]
- Li S., Wang L., Chusuei C.C., Suarez V.M., Blackwelder P.L., Micic M., Orbulescu J., Leblanc R.M. Nontoxic carbon dots potently inhibit human insulin fibrillation. Chem. Mater. 2015;27:1764–1771. [Google Scholar]
- Li Y., Du Z., Liu X., Ma M., Yu D., Lu Y., Ren J., Qu X. Near-infrared activated black phosphorus as a nontoxic photo-oxidant for Alzheimer’s amyloid-β peptide. Small. 2019;15:1901116. doi: 10.1002/smll.201901116. [DOI] [PubMed] [Google Scholar]
- Li M., Guan Y., Zhao A., Ren J., Qu X. Using multifunctional peptide conjugated Au nanorods for monitoring β-amyloid aggregation and chemo-photothermal treatment of Alzheimer's disease. Theranostics. 2017;7:2996–3006. doi: 10.7150/thno.18459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Y.J., Zhou W.H., Li G., Hu Z.W., Hong L., Yu X.F., Li Y.M. Black phosphorus nanomaterials regulate the aggregation of amyloid-β. ChemNanoMat. 2019;5:606–611. [Google Scholar]
- Linse S., Cabaleiro-Lago C., Xue W.F., Lynch I., Lindman S., Thulin E., Radford S.E., Dawson K.A. Nucleation of protein fibrillation by nanoparticles. Proc. Natl. Acad. Sci. U S A. 2007;104:8691–8696. doi: 10.1073/pnas.0701250104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Xu L.P., Dai W., Dong H., Wen Y., Zhang X. Graphene quantum dots for the inhibition of β amyloid aggregation. Nanoscale. 2015;7:19060–19065. doi: 10.1039/c5nr06282a. [DOI] [PubMed] [Google Scholar]
- Liu Y., Xu L.P., Wang Q., Yang B., Zhang X. Synergistic inhibitory effect of GQDs-tramiprosate covalent binding on amyloid aggregation. ACS Chem. Neurosci. 2018;9:817–823. doi: 10.1021/acschemneuro.7b00439. [DOI] [PubMed] [Google Scholar]
- Lu T., Chen F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 2012;33:580–592. doi: 10.1002/jcc.22885. [DOI] [PubMed] [Google Scholar]
- Luo M., Fan T., Zhou Y., Zhang H., Mei L. 2D Black phosphorus–based biomedical applications. Adv. Funct. Mater. 2019;29:1808306. [Google Scholar]
- Ma Y., Huang R., Qi W., Su R., He Z. Fluorescent silicon nanoparticles inhibit the amyloid fibrillation of insulin. J. Mater. Chem. B. 2019;7:1397–1403. doi: 10.1039/c8tb02964d. [DOI] [PubMed] [Google Scholar]
- Mirsadeghi S., Dinarvand R., Ghahremani M.H., Hormozi-Nezhad M.R., Mahmoudi Z., Hajipour M.J., Atyabi F., Ghavami M., Mahmoudi M. Protein corona composition of gold nanoparticles/nanorods affects amyloid β fibrillation process. Nanoscale. 2015;7:5004–5013. doi: 10.1039/c4nr06009a. [DOI] [PubMed] [Google Scholar]
- Niu L., Liu L., Xi W., Han Q., Li Q., Yu Y., Huang Q., Qu F., Xu M., Li Y. Synergistic inhibitory effect of peptide-organic coassemblies on amyloid aggregation. ACS Nano. 2016;10:4143–4153. doi: 10.1021/acsnano.5b07396. [DOI] [PubMed] [Google Scholar]
- Petkova A.T., Leapman R.D., Guo Z.H., Yau W.M., Mattson M.P., Tycko R. Self-propagating, molecular-level polymorphism in Alzheimer's β-amyloid fibrils. Science. 2005;307:262–265. doi: 10.1126/science.1105850. [DOI] [PubMed] [Google Scholar]
- Pike C.J., Burdick D., Walencewicz A.J., Glabe C.G., Cotman C.W. Neurodegeneration induced by β-amyloid peptides in vitro: the role of peptide assembly state. J. Neurosci. 1993;13:1676–1687. doi: 10.1523/JNEUROSCI.13-04-01676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratha B.N., Ghosh A., Brender J.R., Gayen N., Ilyas H., Neeraja C., Das K.P., Mandal A.K., Bhunia A. Inhibition of insulin amyloid fibrillation by a novel amphipathic heptapeptide: mechanistic details studied by spectroscopy in combination with microscopy. J. Biol. Chem. 2016;291:23545–23556. doi: 10.1074/jbc.M116.742460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff J., Hassan N., Morales-Zavala F., Steitz J., Araya E., Kogan M.J., Simon U. CLPFFD–PEG functionalized NIR-absorbing hollow gold nanospheres and gold nanorods inhibit β-amyloid aggregation. J. Mater. Chem. B. 2018;6:2432–2443. doi: 10.1039/c8tb00655e. [DOI] [PubMed] [Google Scholar]
- Shao J., Xie H., Huang H., Li Z., Sun Z., Xu Y., Xiao Q., Yu X.F., Zhao Y., Zhang H. Biodegradable black phosphorus-based nanospheres for in vivo photothermal cancer therapy. Nat. Commun. 2016;7:12967. doi: 10.1038/ncomms12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman J.M., De Jager P.L., Feany M.B. Parkinson's disease: genetics and pathogenesis. Annu. Rev. Pathol. 2011;6:193–222. doi: 10.1146/annurev-pathol-011110-130242. [DOI] [PubMed] [Google Scholar]
- Sun C., Wen L., Zeng J., Wang Y., Sun Q., Deng L., Zhao C., Li Z. One-pot solventless preparation of PEGylated black phosphorus nanoparticles for photoacoustic imaging and photothermal therapy of cancer. Biomaterials. 2016;91:81–89. doi: 10.1016/j.biomaterials.2016.03.022. [DOI] [PubMed] [Google Scholar]
- Thakur G., Micic M., Yang Y., Li W., Movia D., Giordani S., Zhang H., Leblanc R.M. Conjugated quantum dots inhibit the amyloid β (1-42) fibrillation process. Int. J. Alzheimers Dis. 2011;2011:502386. doi: 10.4061/2011/502386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker F.O. Huntington's disease. Lancet. 2007;369:218–228. doi: 10.1016/S0140-6736(07)60111-1. [DOI] [PubMed] [Google Scholar]
- Wang S.T., Lin Y., Todorova N., Xu Y., Mazo M., Rana S., Leonardo V., Amdursky N., Spicer C.D., Alexander B.D. Facet-dependent interactions of islet amyloid polypeptide with gold nanoparticles: implications for fibril formation and peptide-induced lipid membrane disruption. Chem. Mater. 2017;29:1550–1560. doi: 10.1021/acs.chemmater.6b04144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Liang Y., Liu Y., Ren G., Zhang Z., Wu S., Shen J. Ultrasmall black phosphorus quantum dots: synthesis, characterization, and application in cancer treatment. Analyst. 2018;143:5822–5833. doi: 10.1039/c8an01612g. [DOI] [PubMed] [Google Scholar]
- Wang M., Sun Y., Cao X., Peng G., Javed I., Kakinen A., Davis T.P., Lin S., Liu J., Ding F. Graphene quantum dots against human IAPP aggregation and toxicity in vivo. Nanoscale. 2018;10:19995–20006. doi: 10.1039/c8nr07180b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zhang Z., Zhang H., Li C., Chen M., Liu L., Dong M. Enhanced photoresponsive graphene oxide-modified g-C3N4 for disassembly of amyloid β fibrils. ACS Appl. Mater. Interfaces. 2019;11:96–103. doi: 10.1021/acsami.8b10343. [DOI] [PubMed] [Google Scholar]
- Wu W.H., Sun X., Yu Y.P., Hu J., Zhao L., Liu Q., Zhao Y.F., Li Y.M. TiO2 nanoparticles promote β-amyloid fibrillation in vitro. Biochem. Biophys. Res. Commun. 2008;373:315–318. doi: 10.1016/j.bbrc.2008.06.035. [DOI] [PubMed] [Google Scholar]
- Wu D., Ma Y., Cao Y., Zhang T. Mitochondrial toxicity of nanomaterials. Sci. Total Environ. 2020;702:134994. doi: 10.1016/j.scitotenv.2019.134994. [DOI] [PubMed] [Google Scholar]
- Xia Y., Wang S., Huang R., Su R., Qi W., He Z. Adsorption-desorption behavior of black phosphorus quantum dots on mucin surface. Langmuir. 2018;34:8508–8515. doi: 10.1021/acs.langmuir.8b01531. [DOI] [PubMed] [Google Scholar]
- Xiao L., Zhao D., Chan W.H., Choi M.M., Li H.W. Inhibition of β 1-40 amyloid fibrillation with N-acetyl-L-cysteine capped quantum dots. Biomaterials. 2010;31:91–98. doi: 10.1016/j.biomaterials.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Xiong N., Zhao Y., Dong X., Zheng J., Sun Y. Design of a molecular hybrid of dual peptide inhibitors coupled on AuNPs for enhanced inhibition of amyloid β-protein aggregation and cytotoxicity. Small. 2017;13:1601666. doi: 10.1002/smll.201601666. [DOI] [PubMed] [Google Scholar]
- Yan M., Zhang Y., Xu K., Fu T., Qin H., Zheng X. An in vitro study of vascular endothelial toxicity of CdTe quantum dots. Toxicology. 2011;282:94–103. doi: 10.1016/j.tox.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Yang B., Chen Y., Shi J. Nanocatalytic medicine. Adv. Mater. 2019;31:1901778. doi: 10.1002/adma.201901778. [DOI] [PubMed] [Google Scholar]
- Yin T., Xie W., Sun J., Yang L., Liu J. Penetratin peptide-functionalized gold nanostars: enhanced BBB permeability and NIR photothermal treatment of Alzheimer's disease using ultralow irradiance. ACS Appl. Mater. Interfaces. 2016;8:19291–19302. doi: 10.1021/acsami.6b05089. [DOI] [PubMed] [Google Scholar]
- Yoo S.I., Yang M., Brender J.R., Subramanian V., Sun K., Joo N.E., Jeong S.H., Ramamoorthy A., Kotov N.A. Inhibition of amyloid peptide fibrillation by inorganic nanoparticles: functional similarities with proteins. Angew. Chem. Int. Ed. 2011;50:5110–5115. doi: 10.1002/anie.201007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousaf M., Huang H., Li P., Wang C., Yang Y. Fluorine functionalized graphene quantum dots as inhibitor against hIAPP amyloid aggregation. ACS Chem. Neurosci. 2017;8:1368–1377. doi: 10.1021/acschemneuro.7b00015. [DOI] [PubMed] [Google Scholar]
- Zhang T., Wan Y., Xie H., Mu Y., Du P., Wang D., Wu X., Ji H., Wan L. Degradation chemistry and stabilization of exfoliated few-layer black phosphorus in water. J. Am. Chem. Soc. 2018;140:7561–7567. doi: 10.1021/jacs.8b02156. [DOI] [PubMed] [Google Scholar]
- Zhang S., Zhang X., Lei L., Yu X.F., Chen J., Ma C., Wu F., Zhao Q., Xing B. pH-Dependent degradation of layered black phosphorus: essential role of hydroxide ions. Angew. Chem. Int. Ed. 2019;58:467–471. doi: 10.1002/anie.201809989. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available from the corresponding author upon reasonable request.