Abstract
Since its emergence in December 2019, the virus known as severe acute respiratory syndrome coronavirus 2 has quickly caused a pandemic. This virus causes a disease now known as coronavirus disease 2019, or COVID-19. As an increasing proportion of the at-risk population becomes infected, and patients with severe illness are hospitalized, it is essential for hospitalists to remain current on how to best care for people with suspected or confirmed disease. Establishing a system for logistical planning, and accurate information sharing is strongly recommended. Infection control remains the ultimate goal. As such, health care workers should be educated on universal and isolation precautions, and the appropriate use of personal protective equipment. Social distancing should be encouraged to prevent the spread of infection, and creative and innovative ways to reduce contact may need to be considered. Moreover, it is imperative to prepare for contingencies as medical staff will inevitably get sick or become unavailable. Hospitalists have the difficult task of caring for patients while also adapting to the many logistical and social elements of a pandemic.
Keywords: COVID-19, Infection control, SARS-CoV-2
Clinical Significance.
-
•
Interventions to help a health system prepare for patients with COVID-19 include establishing a committee for logistic planning and information dissemination, creating a service dedicated to caring for patients with COVID-19, and building contingency plans for anticipated staffing needs.
-
•
Common findings of COVID-19 include fever, cough, dyspnea, lymphopenia, and normal procalcitonin.
-
•
Supportive care is the mainstay of therapy; several medications including hydroxychloroquine and remdesivir are undergoing clinical trials.
Alt-text: Unlabelled box
Introduction
Since its emergence in December 2019, the virus known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has quickly spread throughout the world. This virus is pathogenic in humans and causes what is now known as coronavirus disease 2019 (COVID-19). On March 11, 2020, the World Health Organization declared COVID-19 a pandemic. As of March 25, 2020, there have been 54,453 cases of COVID-19 in the United States1 and 414,179 cases worldwide.2 As an increasing proportion of the at-risk population becomes infected, and patients with severe illness are hospitalized, it is essential for hospitalists to remain current on how to best care for people with suspected or confirmed COVID-19.
Planning for COVID-19
As more and more of the population become infected, it will be necessary for health care systems and providers to plan for and adapt to the rapidly evolving societal and health care landscape. New information, and misinformation, manifests daily, and it is important to establish a committee focused on logistical planning and accurate information sharing. It may also be useful to form a dedicated unit for patients with suspected or confirmed COVID-19. At least initially, the hope is that this will help centralize patient care and contribute to infection control. If an institution is equipped to use order sets or note templates specific to COVID-19 patients via their electronic medical record system, this may be helpful to streamline work, and to ensure consistent patient care.
On an individual provider level, the overwhelming goal is to limit exposure to the virus. To that end, hospitalists (and other health care workers) should be educated on universal precautions, isolation precautions, and the appropriate use of personal protective equipment. Education and fit testing for respiratory protective equipment such as N95 masks and powered air-purifying respirators should be mandatory for anyone with direct patient contact.
Unnecessary contact should be avoided to prevent the spread of infection. For example, while hospital rounds are traditionally conducted as a team, contact should ideally be limited to the provider primarily responsible for the patient. Telemedicine resources, such as video chat services, can also be used by the health care staff when direct patient contact is not mandatory. A consideration can be made that some inpatient consultations may be performed entirely by chart review or with the use of video services. Moreover, policies that limit or prohibit hospital visitors should be strongly considered.
It is imperative to prepare for contingencies. Medical staff will inevitably get sick and should be educated on the signs of illness in order to appropriately triage for SARS-CoV-2 testing. Likewise, there should be contingency planning for instances when staff must leave for illness, family illness, or other similar circumstances. While quarantined, and if without symptomatic illness, hospitalists may find innovative ways to continue to work from home, such as covering triage calls, providing telemedical care, and logistic planning. Providing different forms of family support, such as childcare, can enable hospitalists to minimize absences and continue to work. Much will be asked of health care staff during this outbreak.
When to Suspect COVID-19
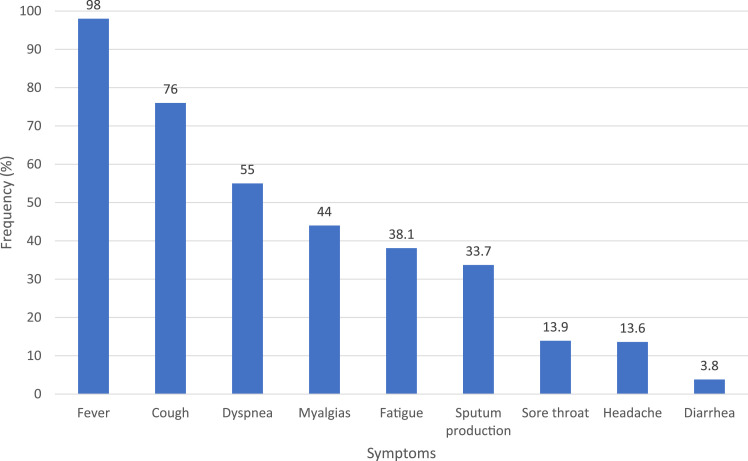
Understanding the signs, symptoms, clinical presentation, and risk factors associated with COVID-19 is essential to patient care and infection control. The most common symptoms of COVID-19 are fever, cough, fatigue, or myalgias.3 , 4 Other common symptoms include dyspnea, headache, diarrhea, and sore throat (Figure 1 ). Cough is usually dry, but not uncommonly, will be productive of sputum. Sneezing is infrequent in COVID-19 and usually indicative of other respiratory conditions rather than COVID-19.
Figure 1.
Clinical characteristics of COVID-19.
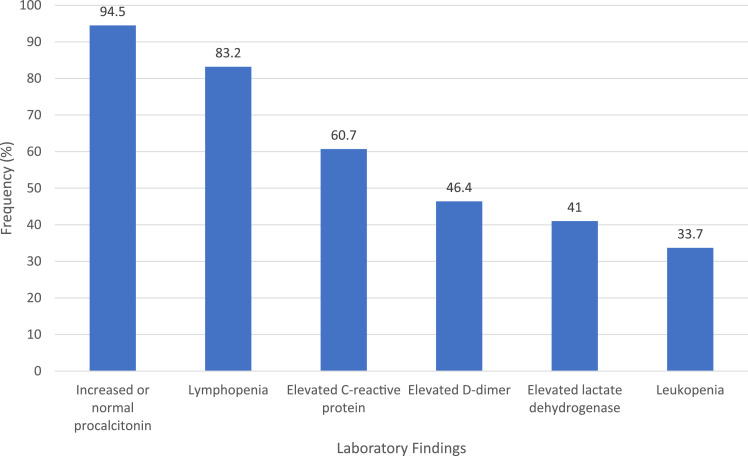
Patients with COVID-19 commonly have leukopenia, lymphopenia, an elevated D-dimer, low or normal procalcitonin, or elevated lactate dehydrogenase (Figure 2 ).3 Chest x-ray studies are abnormal in only 59.1% of patients.3 Computed tomography is more sensitive and typically shows bilateral, multifocal, ground-glass opacification.
Figure 2.
Laboratory characteristics of COVID-19.
Recommendations for SARS-CoV-2 testing are changing as the disease spreads rapidly in the general population. Currently, the Centers for Disease Control and Prevention recommends SARS-CoV-2 testing in hospitalized patients who have signs and symptoms suggestive of disease as described above.5 Considering that COVID-19 is now a pandemic, travel history is no longer a useful screening criterion. Similarly, if a patient has no known prior exposure to an individual with COVID-19, this should not dissuade clinicians from considering SARS-CoV-2 testing, especially if there is no alternative explanation for the presenting illness.
The most common presenting syndrome for COVID-19 is either an upper or lower respiratory tract infection. Severe respiratory infections are characterized by acute respiratory distress syndrome. However, it is also believed that SARS-CoV-2 may also affect the gastrointestinal tract, as diarrhea has been described as a presenting symptom, and the viral shedding in stools has been demonstrated.6 Cases of myocarditis and heart failure have also been described.7 Finally, SARS-CoV-2 may also be capable of neuroinvasive disease given its tropism, and propensity to cause headache. However, the data on this are preliminary, and require further investigation.8
Approach to Patients with Suspected COVID-19
Once a patient is suspected of having COVID-19, it is important from an infection control standpoint to initiate proper isolation. For most patients, this requires contact, and modified droplet precautions. Typically, this includes adhering to universal precautions while appropriately donning a gown, gloves, mask, and eye shield.
Airborne precautions are recommended when aerosol-generating procedures (AGPs) are performed and deemed as moderate or high risk. The highest-risk category of AGPs currently includes sputum induction; bronchoscopy; endotracheal intubation and extubation; open tracheal and nasotracheal suctioning; nasogastric tube placement; upper gastrointestinal endoscopy; transesophageal echocardiography; upper airway ear, nose, and throat procedures; and noninvasive ventilation with positive-airway pressure devices and high-flow nasal cannula. Administering nebulized medications is considered low risk in producing AGPs. However, nebulization is deemed moderate risk in producing AGPs if the patient coughs excessively with administration or it is not known how the patient tolerates the nebulized medication. Collection of nasopharyngeal specimens is considered low risk.
SARS-CoV-2 can be detected from a swab via a polymerase chain reaction-based assay. Combined nasopharyngeal and oropharyngeal swabs have better test characteristics than either alone. However, with the shortage of vital testing supplies, it is recommended to utilize the nasopharyngeal swab alone, given its characteristics comparable with combined testing.9 Sputum can be tested as well, although induced sputum is not recommended, as this is high risk for aerosol generation. It is vital to maintain a differential diagnosis while SARS-CoV-2 testing is pending. COVID-19 has nonspecific symptoms that are shared among a broad array of respiratory illnesses, such as influenza. Coinfection with influenza has been described,10 and some patients have required antibiotic treatment for presumed bacterial superinfection.3 Often, the same swab can be utilized for Influenza testing, and other investigation or empiric treatment can be considered.
Medication administration and other necessary cares should be timed together as much as possible to minimize potential exposure and conserve personal protective equipment. Providers should consider utilizing electronic forms of communication and monitoring, such as video conferencing, to reduce potential exposure.
Care of Patients with Confirmed COVID-19
Once testing returns positive for SARS-CoV-2 and COVID-19 is diagnosed, it is important to understand what is currently known about the natural history of this disease. While it is estimated that 20% of patients with COVID-19 will have severe disease requiring hospitalization, approximately 25% of these will require admission to an intensive care unit (ICU).11 The median time from symptom onset to ICU admission is estimated to be 9.5 days,12 and some of these patients may be admitted to the general medical unit with subsequent deterioration. If the patient's respiratory status is declining or compromised, transfer to an ICU should be considered. Care of patients with COVID-19 in the ICU is not within the scope of this article, and has been discussed elsewhere.13
There are several factors associated with severe disease, as summarized in Table 1 . These include older age, dyspnea, and presence of comorbidities such as hypertension, diabetes mellitus, and coronary artery disease. Laboratory findings such as leukopenia, lymphopenia, thrombocytopenia, and elevations in C-reactive protein, D-dimer, lactate dehydrogenase, aspartate aminotransferase, and alanine aminotransferase are also implicated in severe disease.3 , 14 Abnormal chest x-ray studies, or chest computed tomography findings such as consolidation, crazy-paving pattern, bronchial wall thickening, lymphadenopathy, and pleural effusion are also associated with severe disease.14
Table 1.
Associations with Severe COVID-19
| Clinical Factors | Laboratory Factors | Radiographic Factors |
|---|---|---|
| Older age, dyspnea, hypertension, diabetes mellitus, coronary artery disease | Leukopenia, lymphopenia, thrombocytopenia. Elevated C-reactive protein, procalcitonin, D-dimer, lactate dehydrogenase, aspartate aminotransferase, alanine aminotransferase | Abnormal chest x-ray study. Computed tomography findings of consolidation, crazy paving, bronchial wall thickening, lymphadenopathy, pleural effusion |
If a patient remains suitable for care on a general medical floor, proper isolation remains paramount. Providers should remain mindful of procedures that produce aerosols, and ensure that proper precautions are in place should these interventions be necessary. Remote communication remains a potential option, although it should not entirely replace face-to-face contact and examination. Supportive care is currently the cornerstone of therapy for COVID-19 patients, who in turn require proper monitoring for signs of renal or cardiac dysfunction. Patients with lower respiratory involvement are susceptible to secondary bacterial infection and may require antibiotic therapy in some cases.3 , 4 , 12 While experience with corticosteroids is limited, their routine use is not recommended due to lack of efficacy in other severe coronavirus outbreaks.15 Corticosteroid therapy should be considered only in select situations, such as refractory shock or severe acute respiratory distress syndrome.16 Angiotensin-converting enzyme 2 appears to be the receptor utilized by SARS-CoV-2, raising debate of competing risks and benefits of angiotensin receptor blocking agents.17 It remains unclear if this class of medications is beneficial or harmful. Interim guidelines recommend continuing angiotensin-converting enzyme inhibitors in patients who are on these medications, but not starting in new patients.18 Likewise, nonsteroidal anti-inflammatory drugs have not been sufficiently studied in COVID-19; however, these medications have been associated with worse outcomes in community-acquired pneumonia19 and some experts warn against their use in COVID-19.20 Management of COVID-19 is summarized in Table 2 .
Table 2.
Summary of Management of COVID-19
| Infection Control | Work-Up | Treatment | Under Investigation |
|---|---|---|---|
| Modified droplet isolation | Evaluate risk for severe disease | Continue ACEi/ARB medication if previously taking and medically appropriate | Chloroquine. Hydroxychloroquine ± azithromycin |
| Airborne isolation if recent aerosol-generating procedure | Frequent re-evaluation of respiratory status | Avoid NSAID medications | Remdesivir, lopinavir-ritonavir |
| Limit aerosol-producing procedures | Monitor for extrapulmonary complications | Consider empiric antibiotics for bacterial respiratory pathogens | Sarilumab, tocilizumab |
| Limit direct patient contact | Maintain a differential diagnosis | No routine corticosteroid use | Convalescent plasma |
ACEi = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; NSAID = nonsteroidal anti-inflammatory drug.
Treatments Under Investigation
Several drugs are under investigation for use in COVID-19. Both chloroquine and hydroxychloroquine, older antimalarial medications, have shown in vitro activity against SARS-CoV-2. Early data have shown promise for chloroquine in improving lung imaging, shortening time to viral clearance, and shortening disease course, although these data are still preliminary.21 Hydroxychloroquine and azithromycin is also being studied with early data.22 Remdesivir, a broad-spectrum antiviral drug developed to treat Ebola virus, has in vitro activity against SARS-CoV-2, and is currently being used in clinical trials to assess efficacy.23 , 24 Combination lopinavir-ritonavir medications used for human immunodeficiency virus are being trialed in select patients as well.25 Convalescent plasma is another theorized treatment due to some efficacy in SARS-CoV, without current experience in COVID-19.26 Immunomodulatory medications such as tocilizumab and sarilumab are also under investigation. Unfortunately, none of these therapies are yet to be proven efficacious in COVID-19, although enrollment in a clinical trial remains an option for some patients.
Hospital Discharge and Subsequent Quarantine
In a disease with such drastic public health implications as COVID-19, discharge can be an anxiety-provoking time for patients and providers alike. Patients should be instructed to quarantine at home, minimize any close social interactions, and wear a mask for any such interactions. Quarantine should continue until certain conditions are met. Recently, a non-test-based strategy has been adopted for in-home patients. In this strategy, patients can discontinue isolation once there have been 3 days since resolution of fever, improvement in respiratory symptoms, and 7 days have passed since symptoms began.27 A point of contact should be available for in-home patients to ensure they have met these criteria prior to discontinuing isolation. A patient may be considered for discontinuation of isolation while inpatient if they have resolution of fever without antipyretic medication, improvement in symptoms, and negative results in 2 consecutive sets of paired nasopharyngeal and oropharyngeal polymerase chain reaction swabs at least 24 hours apart.28 Their contacts should be tested for SARS-CoV-2, even if they remain asymptomatic, as shedding can still occur. The Centers for Disease Control and Prevention has a useful handout that can be provided to patients upon dismissal.29
Conclusion
SARS-CoV-2 is truly a novel virus, and much is unknown. Data from the beginning of the pandemic estimate the basic reproductive number, or the expected number of cases directly generated by one case, is estimated to be 2.2.30 The key to controlling a pandemic is reducing this rate below 1, primarily through early and effective infection control measures. Hospitalists are in a unique position to treat multiple unseen patients through effective implementation and education on infection control strategies.
Footnotes
Funding: None.
Conflict of Interest: All authors report no conflicts of interest relevant to this manuscript.
Authorship: All coauthors have seen and agree with the contents of the manuscript. This submission is not under review by other publications.
References
- 1.Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19): cases in the U.S. Available at: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed March 25, 2020.
- 2.World Health Organization. Coronavirus Disease 2019 (COVID-19) Situation Report - 65. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200325-sitrep-65-covid-19.pdf?sfvrsn=2b74edd8_2. Accessed March 25, 2020.
- 3.Guan W-J, Ni Z-Y, Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang Y, Tu M, Wang S. Clinical characteristics of laboratory confirmed positive cases of SARS-CoV-2 infection in Wuhan, China: a retrospective single center analysis [e-pub ahead of print] Travel Med Infect Dis. 2020 Feb 27 doi: 10.1016/j.tmaid.2020.101606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Criteria to guide evaluation and laboratory testing for COVID-19. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-criteria.html. Accessed March 16, 2020.
- 6.Song Y, Liu P, Shi X. SARS-CoV-2 induced diarrhoea as onset symptom in patient with COVID-19 [e-pub ahead of print] Gut. 2020 Mar 5 doi: 10.1136/gutjnl-2020-320891. [DOI] [PubMed] [Google Scholar]
- 7.Zheng Y-Y, Ma Y-T, Zhang J-Y, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y-C, Bai W-Z, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may be at least partially responsible for the respiratory failure of COVID-19 patients. J Med Virol. 2020 Mar 17 doi: 10.1002/jmv.25728. [e-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Interim guidelines for collecting, handling, and testing clinical specimens from persons for Coronavirus Disease 2019 (COVID-19). Available at:https://www.cdc.gov/coronavirus/2019-nCoV/lab/guidelines-clinical-specimens.html. Accessed March 16, 2020.
- 10.Ding Q, Lu P, Fan Y, Xia Y, Liu M. The clinical characteristics of pneumonia patients co-infected with 2019 novel coronavirus and influenza virus in Wuhan, China. J Med Virol. 2020 March 20 doi: 10.1002/jmv.25781. [e-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 12.Yang X, Yu Y, Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 Feb 24 doi: 10.1016/S2213-2600(20)30079-5. [e-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murthy S, Gomersall CD, Fowler RA. Care for critically ill patients with COVID-19. JAMA. 2020;323(15):1499–1500. doi: 10.1001/jama.2020.3633. [DOI] [PubMed] [Google Scholar]
- 14.Li K, Wu J, Wu F. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol. 2020 Feb 29 doi: 10.1097/rli.0000000000000672. [e-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alhazzani W, Møller MH, Arabi YM. Surviving Sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) [e-pub ahead of print] Crit Care Med. 2020 Mar 27 doi: 10.1097/CCM.0000000000004363. https://www.sccm.org/getattachment/Disaster/SSC-COVID19-Critical-Care-Guidelines.pdf Available at. Accessed May 1, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov [e-pub ahead of print] bioRxiv. 2020 doi: 10.1101/2020.01.26.919985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Simone G. 2020. Position statement of the ESC Council on Hypertension on ACE-Inhibitors and Angiotensin Receptor Blockers.https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang Available at. [Google Scholar]
- 19.Basille D, Plouvier N, Trouve C, Duhaut P, Andrejak C, Jounieaux V. Non-steroidal anti-inflammatory drugs may worsen the course of community-acquired pneumonia: a cohort study. Lung. 2017;195(2):201–208. doi: 10.1007/s00408-016-9973-1. [DOI] [PubMed] [Google Scholar]
- 20.Day M. Covid-19 : ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ. 2020;368:m1086. doi: 10.1136/bmj.m1086. [DOI] [PubMed] [Google Scholar]
- 21.Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14(1):72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 22.Gautret P, Lagier J, Parola P. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open- label non-randomized clinical trial [e-pub ahead of print] Int J Antimicrob Agents. 2020 Mar 20 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Holshue ML, DeBolt C, Lindquist S. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao B. A trial of remdesivir in adults with mild and moderate COVID-19. ClinicalTrials.gov. Available at: https://clinicaltrials.gov/ct2/show/NCT04252664. Accessed March 16, 2020.
- 25.Cao B, Wang Y, Wen D. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19 [e-pub ahead of print] N Engl J Med. 2020 Mar 18 doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020;2(20):19–20. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. Discontinuation of isolation for persons with COVID-19 not in healthcare settings (interim guidance). Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/disposition-in-home-patients.html. Accessed March 16, 2020.
- 28.Centers for Disease Control and Prevention. Discontinuation of transmission-based precautions and disposition of patients with COVID-19 in healthcare settings (interim guidance). Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/disposition-hospitalized-patients.html. Accessed March 16, 2020.
- 29.Centers for Disease Control and Prevention. Prevent the spread of COVID-19 if you are sick. Available at:https://www.cdc.gov/coronavirus/2019-ncov/downloads/sick-with-2019-nCoV-fact-sheet.pdf. Accessed March 16, 2020.
- 30.Li Q, Guan X, Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]