Abstract
Objectives
Coronavirus disease 2019 (COVID-19) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Umifenovir (Arbidol®) is an antiviral drug being used to treat influenza in Russia and China. This study aimed to investigate the effectiveness and safety of umifenovir for COVID-19.
Methods
A retrospective study was performed in a non-intensive care unit (ICU) ward in Jinyintan Hospital from 2 February 2020 to 20 March 2020. COVID-19 was confirmed by real-time reverse-transcriptase polymerase chain reaction (RT-PCR) assay of pharyngeal swab specimens. The confirmed patients were divided into the umifenovir group and the control group according to the use of umifenovir. The main outcomes were the rate of negative pharyngeal swab tests for SARS-CoV-2 within 1 week after admission and the time for the virus to turn negative. The negativity time of SARS-CoV-2 was defined as the first day of a negative test if the nucleic acid of SARS-CoV-2 was negative for two consecutive tests.
Results
A total of 81 COVID-19 patients were included, with 45 in the umifenovir group and 36 in the control group. Baseline clinical and laboratory characteristics were comparable between the two groups. Thirty-three out of 45 (73%) patients in the umifenovir group tested negative for SARS-CoV-2 within 7 days after admission, the number was 28/36 (78%) in the control group (p 0.19). The median time from onset of symptoms to SARS-CoV-2 turning negative was 18 days (interquartile range (IQR) 12–21) in the umifenovir group and 16 days (IQR 11–21) in the control group (p 0.42). Patients in the umifenovir group had a longer hospital stay than patients in the control group (13 days (IQR 9–17) vs 11 days (IQR 9–14), p 0.04). No deaths or severe adverse reactions were found in both groups.
Discussion
Umifenovir might not improve the prognosis or accelerate SARS-CoV-2 clearance in non-ICU patients. A randomized control clinical trial is needed to assess the efficacy of umifenovir.
Keywords: Antiviral intervention, Corona virus disease 2019, Umifenovir, Arbidol, Effectiveness
Introduction
Coronavirus disease 2019 (COVID-19) is a new form of respiratory disorder caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1,2]. There is no specific vaccine or treatment for COVID-19. As of now, symptomatic and supportive treatment are the main medical approaches for these patients.
SARS-CoV-2 is an enveloped, positive-sense RNA virus belonging to the human coronaviruses (hCoVs). The other two members of the hCoVs, namely severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome, have already caused several epidemics worldwide [3]. Previous studies have shown that the SARS-CoV-2 and SARS-CoV genome sequences are highly homologous [4,5]. Umifenovir (C22H25BrN2O3S, ethyl 6-bromo-4-[(dimethylamino)methyl]-5-hydroxy-1-methyl-2-[(phenylsulfanyl) methyl]-1H-indole-3-carboxylate) is an oral antiviral drug that was licensed for the treatment and prophylaxis of influenza A and B virus infections in Russia in 1993 (Arbidol®) and in China in 2006 [6]. Previous clinical and basic research showed that umifenovir could reduce the reproduction of the SARS virus in vitro [7,8]. However, the use of umifenovir for COVID-19 remains unclear [8,9]. It is important to evaluate the effectiveness and safety of umifenovir in COVID-19 because of the lack of antiviral treatment for this novel disease. The present retrospective study aimed to compare the clinical characteristics and outcomes between COVID-19 patients with or without umifenovir treatment.
Methods
Study design and participants
This was a single-centre retrospective study approved by the ethics committee of Jinyintan Hospital, Wuhan province. This study was conducted in a non-intensive care unit (ICU) ward at Wuhan Jinyintan Hospital from 2 February 2020 to 20 March 2020. Consecutive COVID-19 patients treated by the Fujian Medical Team to aid Hubei province were included in the study. Jinyintan Hospital, located in Wuhan, Hubei Province, China, was assigned as the designated hospital to treat COVID-19 by the government during the COVID-19 epidemic in Wuhan. The inclusion criteria were as follows: (a) patients with laboratory-identified COVID-19 [10], i.e. tested positive for the nucleic acid of SARS-CoV-2 in a pharyngeal swab specimen using real-time reverse-transcriptase polymerase chain reaction (RT-PCR); (b) over 18 years old; (c) with complete clinical data. Exclusion criterion was patients using other medications with potential antiviral activity.
The following data were collected from electronic medical records: age, sex, chronic medical illness, symptoms, laboratory findings, chest computed tomographic (CT) scans, main treatment, time of symptom onset, time of first negative result of pharyngeal swab and the length of hospital stay. Onset of disease was defined as the first day the symptoms occurred. The negativity time of SARS-CoV-2 was defined as the first day of a negative test if the nucleic acid of SARS-CoV-2 was negative for two consecutive pharyngeal swab tests using RT-PCR. The data were collected and reviewed by a trained team of designated physicians. Baseline data referred to the clinical data at the time of admission and laboratory data within the first 24 hr after admission, as some biochemical indexes were tested and reported on the second day of hospitalization.
CT image acquisition and scoring
The images of CT scans within 2 days after admission and 7 days after treatment were collected. CT scans were reviewed and scored by two physicians specializing in respiratory medicine. The scores of the CT scans were assessed based on previous reports (Table S1) [11]. There was a score of 0–5 for each lobe of the lung, with a total possible score of 25.
Outcomes
The main outcomes were the rate of negative pharyngeal swab tests for SARS-CoV-2 within 1 week after admission and the time for virus to turn negative. The second outcome was the change in CT scores after treatment.
Treatment and grouping
All patients received symptomatic treatment, including appropriate supportive care and regular clinical and laboratory monitoring. The standard protocol for monitoring COVID-19 patients in this hospital was as follows: the SARS-CoV-2 pharyngeal swab nucleic acid test was done every 2 days in patients with normal body temperature and improved symptoms after admission. The chest CT examination was performed within 2 days of admission and on the seventh day after hospitalization. According to treatment with or without umifenovir, patients were divided into the umifenovir group and the control group. Patients in the umifenovir group received umifenovir at 0.2 g three times a day. The levels of ALT (alanine transaminase), AST (aspartate aminotransferase) and creatinine after treatment were collected on the tenth day after hospitalization.
In order to further clarify the impact of umifenovir on patients with different severity of disease, we divided the patients into moderate and severe subgroups according to the criteria of the diagnosis and treatment programme for COVID-19 [12]. Patients were defined as having severe COVID-19 if they met any of the following criteria: (a) respiratory distress, breathing frequency ≥30 breaths/min; (b) mean oxygen saturation ≤93% in resting state; (c) arterial blood oxygen partial pressure/oxygen concentration ≤300 mmHg (1 mmHg = 0.133 kPa). The others were included in moderate group. Patients who needed mechanical ventilation or vasopressors were defined as critically ill patients.
Statistical analysis
All statistical analyses were performed using SPSS 22.0 (SPSS Inc., College Station, TX). Categorical variables were described as percentages, and continuous variables were described using mean ± standard deviation or median (interquartile range). Means for continuous variables were compared using independent group t tests when the data were normally distributed; otherwise, the Mann–Whitney test was used. Categorical variables were compared using the chi-square test; the Fisher exact test was used when the data were limited. Statistical significance was recognized at P < 0.05.
Results
Population and baseline data
A total of 108 patients with COVID-19 were hospitalized in the ward between 2 February 2020 and 20 March 2020. The reasons for 27 subjects being excluded from the final analysis were as follows: treatment with lopinavir/ritonavir (11); treatment with remdesivir (8); treatment with traditional Chinese medicine (2); incomplete clinical data (6). Finally, a total of 81 cases (umifenovir group, 45 cases; control group, 36 cases) with confirmed COVID-19 were included in this study. Patients with mild disease were not hospitalized thus were not included in this study. The median age was 60 years and 45/81 (56%) were male. Fifty-one out of 81 (63%) patients were moderate COVID-19 and 30/81 (37%) patients were severe COVID-19. No patients needed invasive mechanical ventilation on admission. The most common symptoms were fever 56/81 (69%), cough 56/81 (69%) and dyspnoea 41/81 (51%). All patients had decreased levels of lymphocytes and elevated levels of C-reactive protein and erythrocyte sedimentation rate. There were no statistically differences between the two groups in age, sex, underlying diseases, symptoms, vital signs and laboratory examination findings. Oxygen inhalation, antibiotic and systemic corticosteroid treatments were similar between the two groups (all p > 0.05). Noticeably, the values of CT scores in the umifenovir group were higher than those in control group (10 (IQR 7–14) vs 8 (IQR 5–10), p < 0.05, Table 1 ).
Table 1.
Clinical characteristics of non-ICU hospitalized patients with COVID-19.
| Overall (n = 81) | Umifenovir group (n = 45) | Control group (n = 36) | p | |
|---|---|---|---|---|
| Age (years) | 60 (49–66) | 58 (50–66) | 63 (49–66) | 0.76 |
| Male sex | 45 (56) | 28 (62) | 17 (47) | 0.26 |
| Severe cases | 30 (37) | 12 (27) | 18 (50) | 0.05 |
| Chronic medical illness | ||||
| Hypertension | 14 (17) | 9 (20) | 5 (14) | 0.67 |
| Diabetes | 8 (10) | 4 (9) | 4 (11) | 1.00 |
| CHD | 7 (9) | 4 (9) | 3 (8) | 1.00 |
| Onset time (days) | 11 (10–15) | 10 (9–14) | 13 (10–15) | 0.19 |
| Symptoms | ||||
| Fever | 56 (69) | 31 (69) | 25 (69) | 0.58 |
| Cough | 56 (69) | 30 (67) | 26 (72) | 0.77 |
| Expectoration | 22 (27) | 14 (31) | 8 (22) | 0.26 |
| Dyspnoea | 41 (51) | 23 (51) | 18 (50) | 1.00 |
| Diarrhoea | 8 (10) | 5 (11) | 3 (8) | 0.18 |
| Vital signs | ||||
| Temperature (°C) | 38.5 (38.0–39.0) | 38.5 (38.0–39.0) | 38.7 (38.1–39.3) | 0.25 |
| Respiratory rates | 22 (20–23) | 21 (20–23) | 23 (22–23) | 0.06 |
| Heart rates | 80 (79–89) | 80 (79– 89) | 80 (79–89) | 0.06 |
| SpO2 (%) | 97 (96–98) | 97 (96–98) | 97 (96–98) | 0.28 |
| Laboratory examination | ||||
| White blood cell count ( × 10⁹ cells per L) | 5.17 (4.24–6.94) | 5.15 (4.35–6.72) | 5.3 (4.17–7.81) | 0.66 |
| Lymphocyte count ( × 10⁹ cells per L) | 3.68 (2.80–5.32) | 3.64 (2.67–4.92) | 4.14 (3.09–, 5.65) | 0.18 |
| Neutrophil count ( × 10⁹ cells per L) | 1.2 (0.9–1.6) | 1.1 (0.9–1.4) | 1.4 (1.0–1.8) | 0.07 |
| Total bilirubin (μmol/L) | 13.6 (9.0–16.8) | 13.9 (9.1–17.6) | 12 (8.9–15.5) | 0.46 |
| ALT (IU/L) (baseline) | 34 (18–54) | 36 (19–56) | 30 (17–39) | 0.10 |
| ALT (IU/L) (after treatment) | 33 (30–35) | 33 (30–36) | 32 (30–34) | 0.80 |
| AST (IU/L) (baseline) | 33 (30–36) | 33 (30–36) | 34 (31–36) | 0.40 |
| AST (IU/L) (after treatment) | 30 (27–32) | 30 (28–32) | 30 (26–33) | 0.46 |
| Alb (g/L) | 26.5 (19–79) | 26 (19–104) | 27 (18–37) | 0.15 |
| γ-GT (IU/L) | 5.2 (4.2–6.9) | 5.2 (4.4–6.7) | 5.3 (4.2–7.8) | 0.66 |
| ALP (IU/L) | 3.7 (2.8–5.3) | 3.6 (2.7–4.9) | 4.1 (3.1–5.7) | 0.18 |
| Creatinine (μmol/L) (baseline) | 70 (59–809) | 70 (61–80) | 69 (56–79) | 0.62 |
| Creatinine (μmol/L) (after-treatment) | 62 (58–67) | 62 (59–66) | 63 (55–67) | 0.64 |
| C-reactive protein (mg/L) | 13.9 (3.6–52.1) | 19.2 (4.0–63.0) | 8.4 (1.9–34.2) | 0.22 |
| ESR (mm/h)a | 42.9 ± 21.2 | 46.3 ± 19.7 | 38.7 ± 22.5 | 0.18 |
| Fibrinogen (g/dL) | 4.0 (3.1–5.0) | 4.2 (3.2–5.3) | 3.8 (2.5–4.6) | 0.16 |
| CT score | 9.0 (6.0–11.0) | 10.0 (7.0–14.0) | 8.0 (5.0–10.0) | 0.01 |
| Treatments | ||||
| Oxygen inhalation | 21 (26) | 11 (24) | 10 (28) | 0.46 |
| Antibiotics | 12 (15) | 5 (11) | 7 (19) | 0.23 |
| Corticosteroid | 26 (32) | 14 (31) | 12 (33) | 0.51 |
Data are presented as n (%) or median (IQR) unless otherwise indicated. COVID-19, coronavirus disease 2019; CHD, coronary heart disease; AST, aspartate aminotransferase; ALT, alanine transaminase; γ-GT, gamma-glutamyltransferase; ALP, alkaline phosphatase; ESR, erythrocyte sedimentation rate; CT, computed tomography.
a Presented as mean ± standard deviation.
The effects of umifenovir in non-ICU patients with COVID-19
The majority of patients were given umifenovir on admission; the others were given umifenovir within the first 24 hr after admission, because the SARS-CoV-2 nucleic acid was tested on admission and reported on the second day during hospitalization. Eight out of 45 (18%) patients were given umifenovir for 5 days and the remainder of the patients (37/45; 82%) for 7–10 days. After 1 week of treatment, the negative rate of SARS-CoV-2 in pharyngeal swabs between the two groups was similar (33/45 (73%) vs 28/36 (78%)). The median time from admission to the first negative test for SARS-CoV-2 was longer in the umifenovir group than in the control group (6 days (IQR 4–8) vs 3 days (IQR 1–7), p < 0.05). However, the median time from onset of disease to the date of the first negative result was comparable between the two groups (18 days (IQR 12–21) vs 16 days (IQR 11–21), p > 0.05). The CT scores remained higher in the umifenovir group after 1 week of treatment, but the changes in CT scores within 1 week were not different between the two groups. The length of hospital stay was longer in the umifenovir group than in the control group (13 days (IQR 9–17) vs 11 (IQR 9–14) days, p 0.04) (Table 2 ).
Table 2.
Effectiveness of umifenovir in patients with COVID-19.
| Umifenovir group (n = 45) | Control group (n = 36) | p | |
|---|---|---|---|
| CT score (after treatment) | 7 (5–9) | 5 (2–6) | 0.01 |
| CT score dif | 3 (1–7) | 3 (1–4) | 0.52 |
| Time from admission to first negative test of SARS-CoV-2 (days) | 6 (4–8) | 3 (1–7) | 0.01 |
| Time from onset of symptoms to first negative test of SARS-CoV-2 (days) | 18 (12–21) | 16 (11–21) | 0.42 |
| Negative rate of pharyngeal swab test for SARS-CoV-2 within 1 week | 33 (73) | 28 (78) | 0.19 |
| Length of hospital stay (days) | 13 (9–17) | 11 (9–14) | 0.04 |
Data are presented as n (%) or median (IQR). COVID-19, coronavirus disease 2019; CT score dif, CT score (within 2 days of admission); CT score, at the seventh day after hospitalization; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
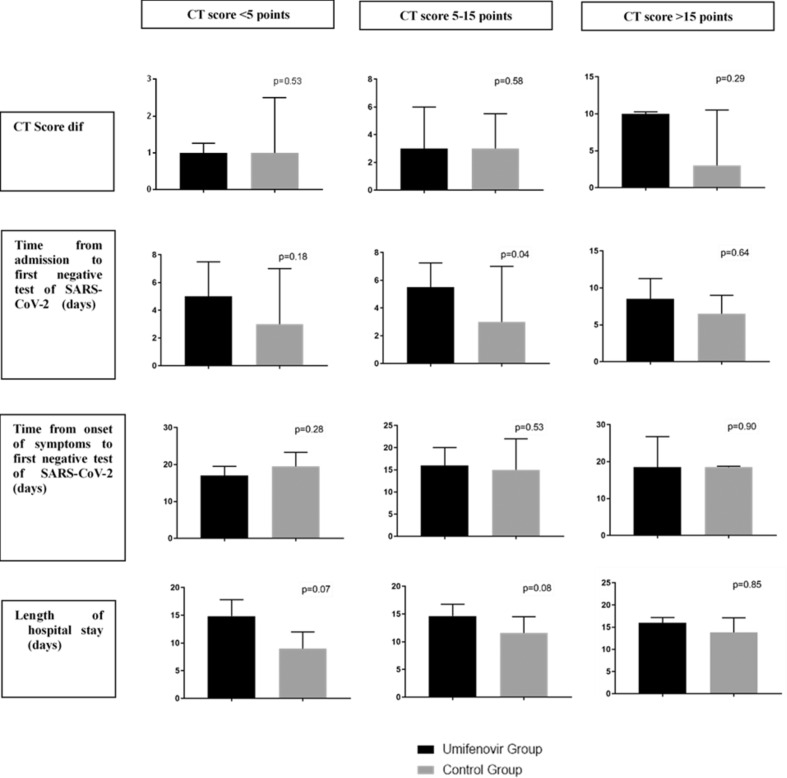
Patients with severe disease who received umifenovir did not fare better than severe patients in the control group (Table 3 ). Since the baseline CT scores of the two groups were different, we performed a subgroup analysis based on CT scores (<5, 5–15 and > 15 points). The results were similar (Fig. 1 ).
Table 3.
Effectiveness of umifenovir in patients with moderate and severe COVID-19.
| Moderate |
Severe |
|||||
|---|---|---|---|---|---|---|
| Umifenovir (n = 33) | Control (n = 18) | p | Umifenovir (n = 12) | Control (n = 18) | p | |
| CT score (post treatment) | 9 (6–10) | 6 (3–7) | 0.05 | 6 (5–8) | 3 (1–5) | 0.01 |
| CT score dif | 3 (1–7) | 3 (1–4) | 0.77 | 3 (1–8) | 3 (2–6) | 0.28 |
| Time from admission to first negative test of SARS-CoV-2 (days) | 5 (3–7) | 1 (4–7) | 0.24 | 7 (5–9) | 2 (1–7) | 0.02 |
| Time from onset of symptoms to first negative test of SARS-CoV-2 (days) | 17 (13–20) | 17 (11–23) | 0.93 | 19 (16–22) | 17 (13–21) | 0.19 |
| Negative rate of pharyngeal swab's test for SARS-CoV-2 within 1 week | 26 (79) | 15 (83) | 0.50 | 7 (64) | 13 (72) | 0.34 |
| Length of hospital stay (days) | 14 (9–17) | 11 (8–14) | 0.04 | 12 (10–15) | 10 (9–15) | 0.34 |
Data are presented as n (%) or median (IQR). COVID-19, coronavirus disease 2019; CT score dif = CT score (within 2 days of admission)- CT score (at the seventh day after hospitalization); SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Fig. 1.
Effectiveness of umifenovir in patients with COVID-19, a subgroup analysis based on computed tomography scores. Data are presented as median (interquartile range). COVID-19, coronavirus disease 2019; CT Score dif = CT score (within 2 days of admission) - CT score (at the seventh day after hospitalization); SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
One patient out of 45 (2.2%) in the umifenovir group and 1/36 (2.7%) in the control group had disease progression and needed mechanical ventilation during hospitalization. There were no deaths in either group.
The side effects of umifenovir
During the treatment period, 5/45 (11%) patients in the umifenovir group and 3/36 (8%) patients in the control group demonstrated digestive symptoms, including diarrhoea and nausea (p 0.49). No patients discontinued treatment because of adverse effects. No severe impairment of liver function and kidney function was found. After treatment, the levels of ALT, AST and creatinine between the two groups were comparable (Table 1).
Discussion
This retrospective study found that umifenovir treatment did not shorten the negativity time of SARS-CoV-2, or the length of hospital stay in non-ICU hospitalized patients with COVID-19. No severe side effects were found in umifenovir treatment.
No vaccine is available for COVID-19 for now, so it is urgent that an effective medicine against COVID-19 is found [8]. Umifenovir, as a broad-spectrum antiviral agent, has been proposed as a potential medication for COVID-19 [12]. An in vitro study showed that umifenovir can significantly inhibit SARS-CoV replication in GMK-AH-1 cells [7,13]. Although umifenovir was recommended by China's prevention and treatment guidelines and widely used in clinical treatment of COVID-19, its effectiveness has been questioned [12,14,15].
In this study, the median time from onset of the disease to the first negative result of a pharyngeal swab for SARS-CoV was not different between the two groups, suggesting that umifenovir cannot accelerate viral clearance. As the baseline, CT scores were higher in the umifenovir group; stratified analysis based on CT scores was performed to correct this bias between the two groups. The results of stratified analysis showed that umifenovir therapy was not superior than supportive therapy treatment in terms of radiology improvement. The results were consistent with a previous study which found that 5 days of umifenovir treatment did not increase the negative rate of pharyngeal swab tests within 1 week [15]. In our study, even the patients who had longer treatment duration (7–10 days) with umifenovir did not experience better outcomes.
Deng et al. found umifenovir combined with lopinavir/ritonavir had an apparent favourable clinical response compared with lopinavir/ritonavir alone [9]. Umifenovir is a haemagglutinin inhibitor, which can specifically inhibit fusion of the virus with the host cell membrane and inhibit the synthesis of viral DNA and RNA. It can also induce the production of interferon and play a role in regulation of the immune system. Combined with drugs with different antiviral mechanisms, umifenovir may show an improved efficacy, but as a trade-off the potential adverse effects may increase during treatment [12].
There are several limitations in the study. First of all, this was a single-centre, retrospective study with a small sample size. Observational studies have bias and the conclusion could be subjective. Second, pharyngeal swabs were not collected every day due to the limited medical resources, and pathogenic nucleic acids were not quantified either. Third, this study only included patients with moderate and severe COVID-19, so the effectiveness of umifenovir in mild and critical patients cannot be confirmed in this study.
In conclusion, compared with symptomatic and supportive treatment, additional umifenovir has not been found to shorten the duration of SARS-CoV-2 negativity time and improve the prognosis in non-ICU patients. This conclusion needs to be further verified in randomized control clinical trials.
Transparency declaration
The authors disclose no conflicts of interest. This work was supported by the Natural Science Foundation of Fujian Province, China (No. 2019J01440) and Startup Fund for Scientific Research, Fujian Medical University, China (NO.2017XQ1080).
Author contributions
Qichang Lin design the study and revised the manuscript. Ningfang Lian and Xie Hansheng analysed data and prepared the manuscript. Jianming Zhao collected data and drafted the manuscript. Jiefeng Huang searched the literature and analysed data. Su Lin reviewed the results and made critical comments on the manuscript.
Editor: L. Leibovici
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2020.04.026.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.El Zowalaty M.E., Järhult J.D. From SARS to COVID-19: a previously unknown SARS- related coronavirus (SARS-CoV-2) of pandemic potential infecting humans – call for a One Health approach. One Health (Amsterdam, Netherlands) 2020;9:100124. doi: 10.1016/j.onehlt.2020.100124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He F., Deng Y., Li W. Coronavirus disease 2019: what we know? J Med Virol. 2020 doi: 10.1002/jmv.25766. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behzadi M.A., Leyva-Grado V.H. Overview of current therapeutics and novel candidates against influenza, respiratory syncytial virus, and Middle East Respiratory syndrome coronavirus infections. Front Microbiol. 2019;10:1327. doi: 10.3389/fmicb.2019.01327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun P., Qie S., Liu Z., Ren J., Li K., Xi J. Clinical characteristics of 50466 hospitalized patients with 2019-nCoV infection. J Med Virol. 2020 doi: 10.1002/jmv.25735. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Proskurnina E.V., Izmailov D.Y., Sozarukova M.M., Zhuravleva T.A., Leneva I.A., Poromov A.A. Antioxidant potential of antiviral drug umifenovir. Molecules. 2020;25 doi: 10.3390/molecules25071577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khamitov R.A., Loginova S., Shchukina V.N., Borisevich S.V., Maksimov V.A., Shuster A.M. [Antiviral activity of arbidol and its derivatives against the pathogen of severe acute respiratory syndrome in the cell cultures] Vopr Virusol. 2008;53:9–13. [PubMed] [Google Scholar]
- 8.Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci Trends. 2020;14:69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- 9.Deng L., Li C., Zeng Q., Liu X., Li X., Zhang H. Arbidol combined with LPV/r versus LPV/r alone against corona virus disease 2019: a retrospective cohort study. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.002. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization . 2020. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance.https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected Available from: [Google Scholar]
- 11.Chang Y.C., Yu C.J., Chang S.C., Galvin J.R., Liu H.M., Hsiao C.H. Pulmonary sequelae in convalescent patients after severe acute respiratory syndrome: evaluation with thin-section CT. Radiology. 2005;236:1067–1075. doi: 10.1148/radiol.2363040958. [DOI] [PubMed] [Google Scholar]
- 12.Novel coronavirus pneumonia prevention and control program (7th ed.) 2020. http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml [in Chinese]. Available from: [Google Scholar]
- 13.Barnard D.L., Kumaki Y. Recent developments in anti-severe acute respiratory syndrome coronavirus chemotherapy. Future Virol. 2011;6:615–631. doi: 10.2217/fvl.11.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov Ther. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 15.Chen J., Ling Y., Xi X.H., Liu P., Li F., Li T. Efficacy of lopinavir, ritonavir and arbidol for the treatment of new coronavirus pneumonia. Chin J Infect Dis. 2020;38(2):86–89. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.