Abstract
The 2019-2020 coronavirus pandemic elucidated how a single highly infectious virus can overburden health care systems of even highly economically developed nations. A leading contributor to these concerning outcomes is a lack of available intensive care unit (ICU) beds and mechanical ventilation support. Poorer health is associated with a higher risk for severe respiratory complications from the coronavirus. We hypothesize that impaired respiratory muscle performance is an underappreciated factor contributing to poor outcomes unfolding during the coronavirus pandemic. Although impaired respiratory muscle performance is considered to be rare, it is more frequently encountered in patients with poorer health, in particular obesity. However, measures of respiratory muscle performance are not routinely performed in clinical practice, including those with symptoms such as dyspnea. The purpose of this article is to discuss the potential role of respiratory muscle performance from the perspective of the coronavirus pandemic. We also provide a theoretical patient management model to screen for impaired respiratory muscle performance and intervention, if identified, with the goal of unburdening health care systems during future pandemic crises.
Keywords: Exercise training, Mechanical ventilation, Muscle force production, Pandemic, Rehabilitation
Clinical Significance.
-
•
Poor baseline health increases the risk of acute respiratory distress syndrome as a result of coronavirus infection and poorer outcomes.
-
•
Patients with poorer baseline health, notably obesity, are more likely to demonstrate impaired respiratory muscle performance and poorer outcomes following intensive care unit admission and mechanical ventilation.
-
•
This relationship indicates respiratory muscle performance may contribute to outcomes related to acute respiratory distress syndrome from coronavirus infection in patients with poor baseline health.
Alt-text: Unlabelled box
Introduction
The 2019-2020 coronavirus (COVID-19) pandemic elucidated how a single highly infectious virus can overburden health care systems of even highly economically developed nations.1, 2, 3, 4, 5 Of the serious complications that have arisen from this pandemic,3 the burden placed on intensive care units (ICUs) and mechanical ventilation as a result of acute respiratory distress syndrome is particularly concerning.1, 2, 3, 4, 5 Some patients are unable to receive otherwise necessary and routine surgeries because of a lack of available ICU and mechanical ventilation resources due to COVID-19.6 This is notwithstanding the mortality rate of patients with acute respiratory distress syndrome resulting from COVID-19 who were able to receive ICU and mechanical ventilation support.2, 3, 4, 5
Even prior to the COVID-19 pandemic, ICU and mechanical ventilation resources were heavily resourced.7 Despite increases in total ICU beds over the past decade, occupancy rates in the United States are approximately 66%-68%7 at any given time; some facilities report as high as 82.1% occupation.7 Currently, mechanical ventilation is used in approximately 33% of ICU admissions7 and is projected to increase 2.3% per year.8 The number of patients requiring prolonged mechanical ventilation is also expected to increase, especially for older adults and patients with comorbidities.9 There also appear to be surges in mechanical ventilation use because of seasonal influenza.10 Previous research indicates that United States could accommodate a surge of 26,200 to 56,300 cases requiring mechanical ventilation with extensive pre-pandemic preparation.11 With the surge in cases of acute respiratory distress syndrome as a result of the COVID-19 pandemic, vulnerabilities to the continuity of our health care system is apparent. Importantly, the availability of these resources varies between states and geographical areas, and as such, some areas may be more heavily impacted than others.12
COVID-19 and other viral infections can cause significant damage to the lungs and airways, potentially resulting in acute respiratory distress syndrome and, if severe enough, respiratory failure.13 There are certain patient demographics that appear to be associated with a higher susceptibility to developing severe respiratory complications from COVID-19 requiring ICU admission and mechanical ventilation.2, 3, 4, 5 , 14 These conditions include obesity, older age, and smoking as well as cardiometabolic and lung diseases.2, 3, 4, 5 Patients with these characteristics and preexisting medical conditions are also at higher risk for serious complications from seasonal influenza.13 , 15 , 16 Of note, obesity was present in nearly one-third of hospitalizations and fatal cases during the 2009 H1N1 pandemic.13 However, not all patients sharing these characteristics experience similar outcomes during this pandemic or even became infected. There are also people who do not possess these characteristics and require mechanical ventilation and ICU admissions as a result of the COVID-19 pandemic.4 , 5 , 17, 18, 19, 20, 21, 22, 23 As such, there are other, underappreciated factors contributing to the risk for poor outcomes resulting from the COVID-19 pandemic observed in patients who were admitted beyond the aforementioned risk factors.
We hypothesize that one of the outcomes associated with these demographics is impaired respiratory muscle performance. Although impaired respiratory muscle performance is considered to be rare,24 , 25 it is more frequently encountered in patients possessing poor health characteristics, in particular obesity. Measures of respiratory muscle performance are also not routinely performed in clinical practice, even in patients presenting with dyspnea.26, 27, 28 The purpose of this article is to discuss the potential role of respiratory muscle performance regarding the COVID-19 pandemic. We also provide a theoretical model to screen for impaired respiratory muscle performance and intervene when identified with the goal of unburdening the health care system during future pandemic crises.
Respiratory Muscle Physiology and Pathophysiology
In healthy individuals with normal total respiratory system compliance, the opening pressure required to fully inflate the alveoli is approximately 40 cm H2O.29, 30, 31, 32, 33 The ability to recruit the lungs is necessary for pulmonary hygiene behaviors such as yawning and coughing.34 Tidal breathing in healthy individuals only requires ≈5-10 cm H2O.29, 30, 31, 32, 33 The average maximal inspiratory pressure generated by a healthy adult ages 18-29 years is 128 cm H2O (116.3-139.5 cm H2O) for males and 97 cm H2O (88.6-105.4 cm H2O) for females. By comparison, the average maximal inspiratory pressure for a healthy adult ages 70-83 years is 76.2 cm H2O (66.1-86.4 cm H2O) for males and 65.3 cm H2O (57.8-72.7 cm H2O) for females.24 In healthy adults, the pressure needed for both full breaths and quiet breathing represents a small fraction of the respiratory muscles’ maximal pressure generating capacity24 (Table 1 ). The disparity between the pressure demands of breathing and peak-pressure-generating capacity of the respiratory musculature allows spontaneous breathing to occur with high efficiency (ie, low energy costs and a large reserve).
Table 1.
Respiratory Muscle Performance: Normative Values and Threshold for Weakness*
| Normal Maximal inspiratory pressure (units in cm H2O) | ||
|---|---|---|
| Age | Men | Women |
| 18-29 | 128 (116.3-139.5) | 97 (88.6-105.4) |
| 30-39 | 128.5 (118.3-138.7) | 89 (84.5-93.5) |
| 40-49 | 117.1 (104.9-129.2) | 92.9 (78.4-107.4) |
| 50-59 | 108.1 (98.7-117.6) | 79.7 (74.9-84.9) |
| 60-69 | 92.7 (84.6-100.8) | 75.1 (67.3-82.9) |
| 70-83 | 76.2 (66.1-86.4) | 65.3 (57.8-72.7) |
| Maximal inspiratory pressure values associated with “higher” likelihood of inspiratory muscle weakness (units in cm H2O) | ||
|---|---|---|
| Age | Men | Women |
| <40 | 63 | 58 |
| 40-60 | 55 | 50 |
| 61-80 | 47 | 43 |
| >80 | 42 | 38 |
Opening pressure to fully recruit alveoli in normal healthy lung = 40 cm H2O and = 55 cm H2O in a diseased lung.
Adapted from Laveneziana P, Albuquerque A, Aliverti A, et al. ERS statement on respiratory muscle testing at rest and during exercise. Eur Respir J. 2019;53(6):1801214.36
Several factors decrease respiratory muscle performance (eg, aging, obesity, physical inactivity, smoking, and chronic disease).35, 36, 37, 38, 39 In patients with obesity and chronic lung diseases, in addition to a reduction in respiratory muscle strength, the demand imposed on the respiratory muscle also increases as a result of changes in airway resistance and chest wall mechanics.35, 36, 37 Quiet breathing accounts for 1%-3% of total oxygen consumption in healthy individuals of a normal weight.35 , 40 In individuals who are obese, the demand imposed on the respiratory muscle increases more than 3-fold,40 increasing total respiratory muscle oxygen consumption to more than 14%.35 , 40 In an acutely diseased lung (eg, acute respiratory distress syndrome), the pressure required to breathe further increases.41 Therefore, patients who are obese sustaining an acute viral infection can experience further imbalances between respiratory muscle-force-generating capacity and imposed demands required for breathing, increasing the risk for respiratory failure.41
In patients with severe acute respiratory distress syndrome and respiratory failure, mechanical ventilation may be required to offload the respiratory musculature. However, mechanical ventilation has been shown to induce rapid atrophy and profound weakness of the respiratory musculature.19 , 42 , 43 Adverse changes in the respiratory muscles as a result of mechanical ventilation have been defined as ventilator-induced diaphragm dysfunction (VIDD) and may occur after only 18 hours of mechanical ventilation.19 , 42 , 43 These effects of VIDD makes it difficult for patients to successfully wean from mechanical ventilation by creating an imbalance between respiratory muscle-force-generating capabilities and the demand imposed for spontaneous breathing.19 , 21 , 22 , 44 , 45 Lower diaphragm thickness even at the outset of mechanical ventilation is associated with delayed weaning from mechanical ventilation and a higher risk of complications.46 Unsuccessful weaning from mechanical ventilation due to VIDD may be exacerbated in patients with baseline respiratory muscle performance impairments as many are also risk factors for failing to wean.17 , 18 , 20 , 22 , 23 This relationship is especially concerning in patients who are obese.17 , 18 , 20 Obesity has been shown to increase the length of stay and need for mechanical ventilation even because of influenza infection16 and results in poorer outcomes.15
Current Respiratory Muscle Testing in Clinical Practice
Effective preventive strategies are contingent on the use of valid and reliable screening tools and assessments in patients with higher pretest probability.41 It is generally not recommended that all patients receive the same screening tests to minimize costly false-positives.41 Respiratory system screening tools are recommended for patients with symptoms consistent with respiratory muscle impairment, most notably dyspnea.26, 27, 28
In patients with dyspnea, the US Preventive Services Task Force and Joint Guidelines of the American College of Physicians, American College of Chest Physicians, American Thoracic Society (ATS), and the European Respiratory Society (ERS) recommend the use of spirometry and other examinations for screening and eventual diagnosis.26, 27, 28 However, these guidelines and statements do not include assessment of the respiratory musculature.26, 27, 28 Even in the Practice Advisory for Pre-anesthesia Evaluation by the American Society of Anesthesiologists, respiratory muscle assessment is not included in routine preoperative assessment, even for higher-risk patient populations.47
The omission of respiratory muscle performance testing in guidelines for dyspnea assessment and respiratory disease screening is surprising. The ATS and ERS have produced guidelines on respiratory muscle testing procedures,36 , 37 and normative values for respiratory muscle performance exist.24 , 25 Moreover, criteria and thresholds for defining respiratory muscle weakness have been proposed.24 , 25 , 36 , 37
Available evidence does indicate that respiratory muscle weakness is rare in the general population.24 , 25 The threshold for respiratory muscle weakness in a younger healthy adult is also fairly low (ie, >40 years of age: 63 cm H2O).25 However, respiratory muscle weakness is associated with dyspnea,28 and there are certain populations where respiratory muscle weakness is more likely.17 , 48 , 49 Even in healthy older individuals, the maximal inspiratory pressure produced by the respiratory musculature decreases.24 However, because of the normal age-related changes in lung compliance and muscle strength, the threshold for respiratory muscle weakness with aging is also lower (ie, >80 years: 42 cm H2O).25 It is important to acknowledge that these age-related reductions are in reference to healthy individuals. As described previously, in patients with multimorbidity, the risk of respiratory muscle weakness may increase or potentially compound these age-related reductions in respiratory muscle performance.35 , 38 Although routinely screened measures of lung volume and flow rate are associated with respiratory muscle performance,36 , 37 changes in respiratory muscle performance may occur independently of these values and may be detected prior to changes in lung volume.36 , 37 , 49 , 50
Respiratory Muscle Performance Testing
The use of noninvasive handheld manometers to assess maximal static inspiratory pressure quantifies respiratory muscle strength across multiple populations with varying characteristics36 , 37 , 51 with excellent reliability.52 The ATS and ERS have provided standardized protocols testing when using handheld devices,36 , 37 which are affordable and easily accessible.
To assess maximal inspiratory pressure, individuals are instructed to fully expire and then perform a maximal inspiratory effort for at least 1.5 seconds.36 , 37 The peak negative pressure sustained for at least 1 second during that inspiratory maneuver is considered the maximal inspiratory pressure.36 , 37 A minimum of 3 trials are performed with 1 minute of rest between trials and the highest value recorded defining maximal inspiratory pressure.36 , 37 Acceptable values for each trial should be within 10% of each other.
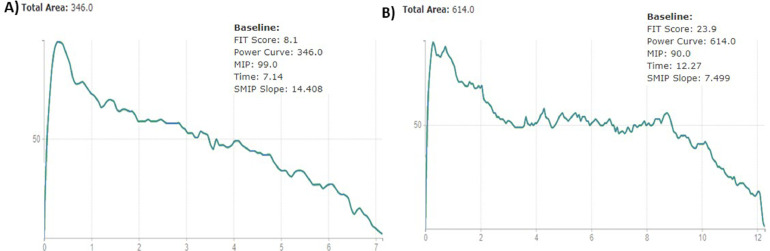
Another test to assess respiratory muscle performance is the test of incremental respiratory endurance (TIRE).53 The TIRE requires the patient to breathe in through the manometer from residual volume to total lung capacity and to sustain that breath for as long as possible.53 Because of the nature of this test the TIRE provides maximal inspiratory pressure and several other measurements of respiratory muscle performance. Inspiratory duration, which is measured in seconds, represents the duration of maximal sustained inhalation (ie, endurance).53 Sustained maximal inspiratory pressure is expressed in pressure time units or joules and represents single-breath work capacity.53 Both the sustained maximal inspiratory pressure and the slope of its curve are emerging clinical measures across several populations.47 , 54 The combination of these values provide a more comprehensive assessment that may identify characteristics of respiratory muscle weakness or fatiguability that may be missed when only measuring maximal inspiratory pressure.54 An example of this is demonstrated in Figure 1 ; an individual with a higher maximal inspiratory pressure may conversely demonstrate a much lower capacity for producing work over time. At this time, there is only a single study that has proposed normative values for TIRE.55 In addition, reliability and validity for the TIRE have only been reported in patients with chronic obstructive pulmonary disease.47 Additional research is needed to further assess the validity and reliability of the TIRE, as well as further establish normative values for comparison in patient populations.
Figure 1.
Comparison of patients with same maximal inspiratory pressure but vastly different performance in other characteristics of respiratory muscle performance. This image represents the results of the test of incremental respiratory endurance (TIRE) performed on 2 separate patients who were obese. The maximal inspiratory pressure in patient A is higher (A: 99 cm H2O vs B: 90 cm H2O), but when plotted over time, the peak work capacity is much lower than patient B (A: 346 PTU vs B: 614 PTU). This demonstrates the additional information regarding respiratory muscle performance that can be gleaned from the TIRE compared to assessing maximal inspiratory pressure alone.
Respiratory Muscle Training
Respiratory muscle training improves respiratory muscle strength, exercise capacity, diaphragm muscle thickness, and dyspnea in several patient populations.56, 57, 58, 59, 60 Patients exhibiting the most profound baseline respiratory muscle weakness tend to receive the greatest benefit from respiratory muscle training. Those in poor health and at risk for viral infection (eg, influenza) may demonstrate respiratory muscle weakness and, thus, be at increased risk of a poorer clinical trajectory if acute respiratory distress syndrome ensues. However, even in healthy individuals without dyspnea, respiratory muscle training has also been shown to provide modest benefits on exercise performance.61 Conventional respiratory muscle training protocols require breathing through a device with valve set to a pressure threshold. Once enough pressure is generated, the valve opens and air flows through the device. A percentage of the maximal inspiratory pressure is used as the training load based on the type of protocol. Training protocols typically use resistive loads ranging between 30% and 80% of maximal inspiratory pressure. One of the unique advantages of respiratory muscle training is that it can be implemented in shorter intervals (30 breaths, 2 times/d). Training effects from respiratory muscle training have been observed for multiple protocols lasting only 4 weeks.59 , 62 , 63 For patients with dyspnea or who are sedentary, these training characteristics may promote adherence and compliance to training.64 , 65
Currently, no studies have directly investigated the role of respiratory muscle training to reduce the risk of respiratory failure as a result of viral infection. Preoperative respiratory muscle training has been shown to reduce the risk of postoperative respiratory complications.44 , 66, 67, 68 Mans et al demonstrated that preoperative respiratory muscle training may reduce the risk of postoperative pulmonary complications following cardiothoracic or abdominal surgery by half.44 A recent study by Chen et al found that a 5-day intensive preoperative respiratory muscle training protocol resulted in significant reductions in both pulmonary complications and length of hospitalization.69 Four weeks of respiratory muscle training at 50% of maximal inspiratory pressure has been shown to reduce hospital length of stay, mortality, and the risk of intubation in patients at risk for prolonged hospitalization.70 In patients requiring mechanical ventilation, respiratory muscle training improves weaning outcomes71 and reduces hospital length of stay.72 Evidence regarding the beneficial effects of respiratory muscle training is strong, and the connection among impaired respiratory muscle performance, mechanical ventilation, and respiratory complications is also apparent. Although the role of respiratory muscle training to mitigate the respiratory complications of viral infection in susceptible patients has yet to be investigated, currently available evidence supports further exploration of respiratory muscle training to reduce the risk of severe complications during a viral infection.
Theoretical Risk Reduction Model
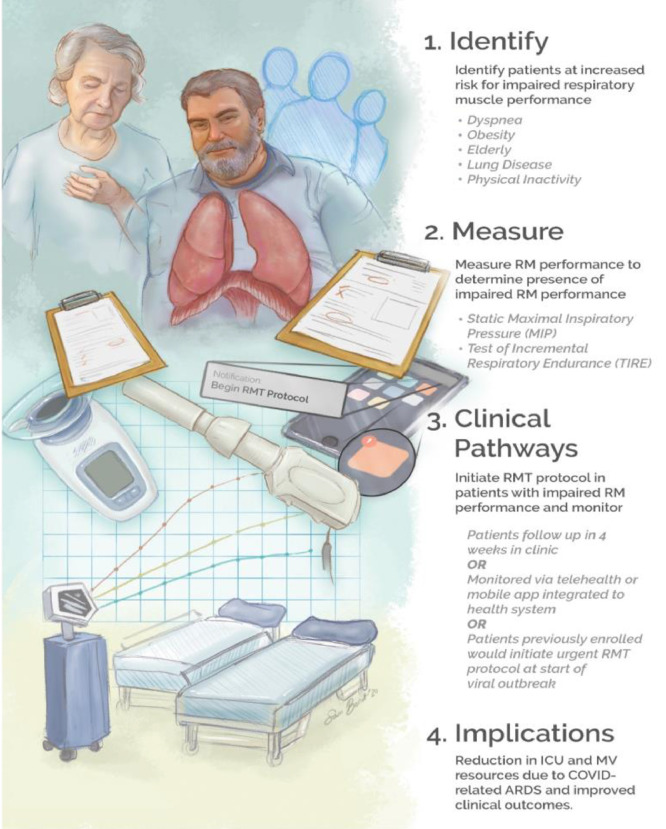
A model to improve outcomes and reduce the burden of future viral pandemics may involve respiratory muscle performance screening. This model and its potential downstream effects on available mechanical ventilation and ICU resources are illustrated in Figure 2 . Patients with increased risk for acute respiratory distress syndrome and respiratory failure resulting from viral infection would receive this screening. If impaired performance is identified, patients could then be prescribed a respiratory muscle training program to implement at home. Patients would receive follow up after 4 weeks of training to assess progress. A telehealth or mobile app-based model would allow for the opportunity for real-time remote monitoring of compliance and assessment. Telehealth and home-based models for respiratory muscle training have been studied with similar effects.73, 74, 75
Figure 2.
Theoretical Risk Reduction Model. This model describes our theoretical patient management model which includes 4 components: 1) Identify patients at increased risk for impaired respiratory muscle (respiratory muscle) performance; 2) measure respiratory muscle performance in patients screened for high risk of impaired respiratory muscle performance using either static maximal inspiratory pressure or the test of incremental respiratory endurance (TIRE); 3) clinical pathways for respiratory muscle training (respiratory muscle training) in patients with impaired respiratory muscle performance, which involve either follow-ups every 4 weeks in clinic, home-based monitoring using telehealth or mobile apps, or patients previously enrolled would initiate an urgent 5-week protocol at the start of an outbreak by receiving a notification on their mobile app; and 4) the downstream benefits would involve reducing the use of intensive care unit and mechanical ventilation resources as a result of COVID-related acute respiratory distress syndrome and improving clinical outcomes.
The timing of this initial screening could occur during routine annual follow up. Another approach could be to implement this screening when at-risk patients receive vaccinations before flu season with hopes of “inoculating” patients from severe respiratory complications resulting from viral infection, at the same clinical “touchpoint” for seasonal vaccinations. With rapidly advancing mobile technology, at-risk patients who have already received their device could even perform their own monitoring at home. Long-term participation in respiratory muscle training may be necessary for maintenance of improvements following training. Even with 12 weeks of respiratory muscle training, the effects gradually decline after 1 year if patients do not perform maintenance respiratory muscle training76 or participate in vigorous aerobic exercise.77 , 78 However, because the effects of respiratory muscle training have been shown to occur even within 5 days of training,69 at the first outbreak of a viral pandemic, at-risk patients could be advised to initiate an urgent prophylactic respiratory muscle training protocol. Initiating respiratory muscle training following ICU admission requiring mechanical ventilation support due to acute respiratory distress syndrome could also help accelerate recovery and discharge home.
This model could potentially result in substantial downstream benefits by reducing the number of infectious cases requiring ICU and mechanical ventilation during a pandemic. This would also make ICU and mechanical ventilation resources more available for care not related to pandemics.
Conclusion
In times of crisis, it is important to remember our experiences and lessons learned from COVID-19; from a health care perspective, a primary lesson is we need to be better prepared moving forward. This preparation for the future entails exploring and testing plausible solutions that have not been considered. As described, it appears diminished respiratory muscle performance, in conjunction with other disconcerting characteristics (eg, obesity, chronic diseases), may contribute to the overwhelming burden imposed on health care systems due to viral pandemics. What is more concerning is that frequency of viral pandemics and the prevalence of the global population in poor health associated with impaired respiratory muscle performance are potentially both at the tipping point. In nations with high economic development, poor baseline health is now, unfortunately, the norm; obesity alone is present in 42.4% of the adult population in the United States and continues to increase.79 Another troubling trend is the presence of multimorbidity in older adults,80 a population that also continues to increase.81
Given how these issues intersect in a multitude of ways, we propose that screening for respiratory muscle impairment in patients with dyspnea or characteristics associated with increased risk of severe respiratory complication as a result of viral infection may be advantageous. Furthermore, in patients identified as having respiratory muscle impairments, respiratory muscle training may prove valuable in mitigating the health impact of future pandemics.
Footnotes
Funding: RS doctoral studies supported by the Foundation for Physical Therapy, PODS II Scholarship; RA, CJL, SB, SAP report none.
Conflicts of Interest: None.
Authorship: All authors had access to the data and a role in writing this manuscript.
References
- 1.Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. StatPearls Publishing; Treasure Island, FL: 2020. Features, Evaluation and Treatment Coronavirus (COVID-19) Available at: http://www.ncbi.nlm.nih.gov/pubmed/32150360. Accessed March 20, 2020. [PubMed] [Google Scholar]
- 2.Zhou F, Yu T, Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China : a retrospective cohort study. Lancet. 2020;6736(20):1–9. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.Arentz M, Yim E, Klaff L. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Livingston E, Bucher K. Coronavirus Disease 2019 (COVID-19) in Italy. JAMA. 2020;323(14):1335. doi: 10.1001/jama.2020.4344. [DOI] [PubMed] [Google Scholar]
- 6.US News. Cancer, heart surgeries delayed as coronavirus alters care | Health News. Available at:https://www.usnews.com/news/health-news/articles/2020-03-18/cancer-heart-surgeries-delayed-as-coronavirus-alters-care?fbclid=IwAR040guIT2cHYx1KscmoyayYuiqjFTn9MGnWhyDYBmGJBSE4JYLgqlKOzgI. Accessed March 20, 2020.
- 7.Wunsch H, Wagner J, Herlim M, Chong DH, Kramer AA, Halpern SD. ICU occupancy and mechanical ventilator use in the united states. Crit Care Med. 2013;41(12):2712–2719. doi: 10.1097/CCM.0b013e318298a139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Needham DM, Bronskill SE, Calinawan JR, Sibbald WJ, Pronovost PJ, Laupacis A. Projected incidence of mechanical ventilation in Ontario to 2026: Preparing for the aging baby boomers. Crit Care Med. 2005;33(3):574–579. doi: 10.1097/01.CCM.0000155992.21174.31. [DOI] [PubMed] [Google Scholar]
- 9.Zilberberg MD, De Wit M, Pirone JR, Shorr AF. Growth in adult prolonged acute mechanical ventilation: implications for healthcare delivery. Crit Care Med. 2008;36(5):1451–1455. doi: 10.1097/CCM.0b013e3181691a49. [DOI] [PubMed] [Google Scholar]
- 10.King JC, Ajao A, Lichenstein R, Magder LS. Surge in hospitalizations associated with mechanical ventilator use during influenza outbreaks. Disaster Med Public Health Prep. 2014;8(2):136–142. doi: 10.1017/dmp.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ajao A, Nystrom SV, Koonin LM. Assessing the capacity of the US health care system to use additional mechanical ventilators during a large-scale public health emergency. Disaster Med Public Health Prep. 2015;9(6):634–641. doi: 10.1017/dmp.2015.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubinson L, Vaughn F, Nelson S. Mechanical ventilators in US acute care hospitals. Disaster Med Public Health Prep. 2010;4(3):199–206. doi: 10.1001/dmp.2010.18. [DOI] [PubMed] [Google Scholar]
- 13.Honce R, Schultz-Cherry S. Impact of obesity on influenza A virus pathogenesis, immune response, and evolution. Front Immunol. 2019;10(10):1071. doi: 10.3389/fimmu.2019.01071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai H. Sex difference and smoking predisposition in patients with COVID-19. Lancet Respir Med. 2020;8(4):e20. doi: 10.1016/S2213-2600(20)30117-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fezeu L, Julia C, Henegar A. Obesity is associated with higher risk of intensive care unit admission and death in influenza A (H1N1) patients: a systematic review and meta-analysis. Obes Rev. 2011;12(8):653–659. doi: 10.1111/j.1467-789X.2011.00864.x. [DOI] [PubMed] [Google Scholar]
- 16.Martin ET, Archer C, Mcroberts J. Epidemiology of severe influenza outcomes among adult patients with obesity in Detroit, Michigan, 2011. Influenza Other Respi Viruses. 2013;7(6):1004–1007. doi: 10.1111/irv.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anzueto A, Frutos-Vivar F, Esteban A. Influence of body mass index on outcome of the mechanically ventilated patients. Thorax. 2011;66(1):66–73. doi: 10.1136/thx.2010.145086. [DOI] [PubMed] [Google Scholar]
- 18.Chao CM, Lai CC, Cheng AC. Establishing failure predictors for the planned extubation of overweight and obese patients. PLoS One. 2017;12(8) doi: 10.1371/journal.pone.0183360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin AD, Smith BK, Gabrielli A. Mechanical ventilation, diaphragm weakness and weaning: A rehabilitation perspective. Respir Physiol Neurobiol. 2013;189(2):377–383. doi: 10.1016/j.resp.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlucci A, Ceriana P, Prinianakis G, Fanfulla F, Colombo R, Nava S. Determinants of weaning success in patients with prolonged mechanical ventilation. Crit Care. 2009;13(3):R97. doi: 10.1186/cc7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zilberberg MD, Luippold RS, Sulsky S, Shorr AF. Prolonged acute mechanical ventilation, hospital resource utilization, and mortality in the United States. Crit Care Med. 2008;36(3):724–730. doi: 10.1097/CCM.0B013E31816536F7. [DOI] [PubMed] [Google Scholar]
- 22.Purro A, Appendini L, De Gaetano A, Gudjonsdottir M, Donner CF, Rossi A. Physiologic determinants of ventilator dependence in long-term mechanically ventilated patients. Am J Respir Crit Care Med. 2000;161(4 I):1115–1123. doi: 10.1164/ajrccm.161.4.9812160. [DOI] [PubMed] [Google Scholar]
- 23.De Jong A, Chanques G, Jaber S. Mechanical ventilation in obese ICU patients: from intubation to extubation. Crit Care. 2017;21(1):63. doi: 10.1186/s13054-017-1641-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pessoa IMS, Parreira VF, Fregonezi GA, Sheel AW, Chung F, Reid WD. Reference values for maximal inspiratory pressure: A systematic review. Can Respir J. 2014;21(1):43. doi: 10.1155/2014/982374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodrigues A, Da Silva ML, Berton DC. Maximal inspiratory pressure: does the choice of reference values actually matter. Chest. 2017;152(1):32–39. doi: 10.1016/j.chest.2016.11.045. [DOI] [PubMed] [Google Scholar]
- 26.Qaseem A. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155(3):179. doi: 10.7326/0003-4819-155-3-201108020-00008. [DOI] [PubMed] [Google Scholar]
- 27.US Preventive Services Task Force. Draft recommendation statement: chronic obstructive pulmonary disease: screening. Available at:https://www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement159/chronic-obstructive-pulmonary-disease-screening. Accessed March 20, 2020.
- 28.Parshall MB, Schwartzstein RM, Adams L. An official American thoracic society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435–452. doi: 10.1164/rccm.201111-2042ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gold WM, Koth LL. Pulmonary function testing. In: Broaddus VC, Mason RJ, Ernst JD, et al., eds. Murray and Nadel's Textbook of Respiratory Medicine. 6th ed. Philadelphia, PA: Elsevier; 2016:407-435.e18.
- 30.Venkataraman ST. Mechanical ventilation and respiratory care. In: Fuhrman BP, Zimmerman JA, Carcillo JA, et al., eds. Pediatric Critical Care. 4th ed. Philadelphia, PA: Elsevier Inc; 2011:657-688.
- 31.Galetke W, Feier C, Muth T, Ruehle KH, Borsch-Galetke E, Randerath W. Reference values for dynamic and static pulmonary compliance in men. Respir Med. 2007;101(8):1783–1789. doi: 10.1016/j.rmed.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 32.Albert SP, DiRocco J, Allen GB. The role of time and pressure on alveolar recruitment. J Appl Physiol. 2009;106(3):757–765. doi: 10.1152/japplphysiol.90735.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tusman G, Böhm SH, Vazquez De Anda GF, Do Campo JL, Lachmann B. Vol. 82. 1999. Clinical investigations “alveolar recruitment strategy” improves arterial oxygenation during general anaesthesia; pp. 8–13. (BJA: Br J Anaesth). [DOI] [PubMed] [Google Scholar]
- 34.Smith JA, Aliverti A, Quaranta M. Chest wall dynamics during voluntary and induced cough in healthy volunteers. J Physiol. 2012;590(3):563–574. doi: 10.1113/jphysiol.2011.213157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sood A. Altered resting and exercise respiratory physiology in obesity. Clin Chest Med. 2009;30(3):445–454. doi: 10.1016/j.ccm.2009.05.003. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laveneziana P, Albuquerque A, Aliverti A. ERS statement on respiratory muscle testing at rest and during exercise. Eur Respir J. 2019;53(6) doi: 10.1183/13993003.01214-2018. [DOI] [PubMed] [Google Scholar]
- 37.ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166(4):518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- 38.Pereira FD, Batista WO, Fuly P dos SC, Alves Junior E de D, Silva EB da. Physical activity and respiratory muscle strength in elderly: a systematic review. Fisioter em Mov. 2014;27(1):129–139. doi: 10.1590/0103-5150.027.001.ar01. [DOI] [Google Scholar]
- 39.Kress JP, Pohlman AS, Alverdy J, Hall JB. The impact of morbid obesity on oxygen cost of breathing VO2 at rest. Am J Respir Crit Care Med. 1999;160(3):883–886. doi: 10.1164/ajrccm.160.3.9902058. [DOI] [PubMed] [Google Scholar]
- 40.Kallet RH, Hemphill JC, Dicker RA. The spontaneous breathing pattern and work of breathing of patients with acute respiratory distress syndrome and acute lung injury. Respir Care. 2007;52(8):989–995. [PubMed] [Google Scholar]
- 41.Levine S, Nguyen TE, Taylor N. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008;35813358:1327–1335. doi: 10.1056/NEJMoa070447. [DOI] [PubMed] [Google Scholar]
- 42.Berger D, Bloechlinger S, von Haehling S. Dysfunction of respiratory muscles in critically ill patients on the intensive care unit. J Cachexia Sarcopenia Muscle. 2016;7(4):403–412. doi: 10.1002/jcsm.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vassilakopoulos T, Zakynthinos S, Roussos C. The tension-time index and the frequency/tidal volume ratio are the major pathophysiologic determinants of weaning failure and success. Am J Respir Crit Care Med. 1998;158(2):378–385. doi: 10.1164/ajrccm.158.2.9710084. [DOI] [PubMed] [Google Scholar]
- 44.Martin AD, Smith BK, Davenport PD. Inspiratory muscle strength training improves weaning outcome in failure to wean patients: A randomized trial. Crit Care. 2011;15(2):R84. doi: 10.1186/cc10081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sklar MC, Dres M, Fan E. Association of low baseline diaphragm muscle mass with prolonged mechanical ventilation and mortality among critically ill adults. JAMA Netw open. 2020;3(2) doi: 10.1001/jamanetworkopen.2019.21520. [DOI] [PubMed] [Google Scholar]
- 46.Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology. 2012;116(3):522–538. doi: 10.1097/ALN.0b013e31823c1067. [DOI] [PubMed] [Google Scholar]
- 47.Formiga MF, Campos MA, Cahalin LP. Inspiratory muscle performance of former smokers and nonsmokers using the test of incremental respiratory endurance. Respir Care. 2018;63(1):86–91. doi: 10.4187/respcare.05716. [DOI] [PubMed] [Google Scholar]
- 48.Kim NS, Seo JH, Ko MH, Park SH, Kang SW, Won YH. Respiratory muscle strength in patients with chronic obstructive pulmonary disease. Ann Rehabil Med. 2017;41(4):659–666. doi: 10.5535/arm.2017.41.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hill K, Jenkins SC, Philippe DL. High-intensity inspiratory muscle training in COPD. Eur Respir J. 2006;27(6):1119–1128. doi: 10.1183/09031936.06.00105205. [DOI] [PubMed] [Google Scholar]
- 50.Arena R, Cahalin LP. Evaluation of cardiorespiratory fitness and respiratory muscle function in the obese population. Prog Cardiovasc Dis. 2014;56:457–464. doi: 10.1016/j.pcad.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 51.Jalan NS, Daftari SS, Retharekar SS, Rairikar SA, Shyam AM, Sancheti PK. Intra- and inter-rater reliability of maximum inspiratory pressure measured using a portable capsule-sensing pressure gauge device in healthy adults. Can J Respir Ther. 2015;51(2):39–42. [PMC free article] [PubMed] [Google Scholar]
- 52.Cahalin LP, Arena R. Novel methods of inspiratory muscle training via the test of incremental respiratory endurance (TIRE) Exerc Sport Sci Rev. 2015;43(2):84–92. doi: 10.1249/JES.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 53.Formiga MF, Roach KE, Vital I. Reliability and validity of the test of incremental respiratory endurance measures of inspiratory muscle performance in COPD. Int J COPD. 2018;13:1569–1576. doi: 10.2147/COPD.S160512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cahalin LP, Garcia C, Denis T, Colas-Salgado S, Formiga M, Cohen M. Normative values for the test of incremental respiratory endurance (TIRE) Am J Respir Crit Care Med. 2016;193:A6363. [Google Scholar]
- 55.Lin S-J, McElfresh J, Hall B, Bloom R, Farrell K. Inspiratory muscle training in patients with heart failure: a systematic review. Cardiopulm Phys Ther J. 2012;23(3):29–36. doi: 10.1097/01823246-201223030-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gosselink R, De Vos J, Van Den Heuvel SP, Segers J, Decramer M, Kwakkel G. Impact of inspiratory muscle training in patients with COPD: what is the evidence. Eur Respir J. 2011;37(2):416–425. doi: 10.1183/09031936.00031810. [DOI] [PubMed] [Google Scholar]
- 57.Pazzianotto-Forti EM, Mori T, Zerbetto R. Effects of inspiratory muscle training on respiratory muscle strength, physical fitness and dyspnea in obese women. Eur Resp J. 2019;54:PA2204. doi: 10.1183/13993003.congress-2019.pa2204. [DOI] [Google Scholar]
- 58.Edwards AM, Maguire GP, Graham D, Boland V, Richardson G. Four weeks of inspiratory muscle training improves self-paced walking performance in overweight and obese adults: a randomised controlled trial. J Obes. 2012;2012 doi: 10.1155/2012/918202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Souza H, Rocha T, Pessoa M. Effects of inspiratory muscle training in elderly women on respiratory muscle strength, diaphragm thickness and mobility. J Gerontol A Biol Sci Med Sci. 2014;69(12):1545–1553. doi: 10.1093/gerona/glu182. [DOI] [PubMed] [Google Scholar]
- 60.Illi SK, Held U, Frank I, Spengler CM. Effect of respiratory muscle training on exercise performance in healthy individuals. Sport Med. 2012;42(8):707–724. doi: 10.1007/bf03262290. [DOI] [PubMed] [Google Scholar]
- 61.Langer D, Charususin N, Jácome C. Efficacy of a novel method for inspiratory muscle training in people with chronic obstructive pulmonary disease. Phys Ther. 2015;95(9):1264–1273. doi: 10.2522/ptj.20140245. [DOI] [PubMed] [Google Scholar]
- 62.Chiappa GR, Roseguini BT, Vieira PJC. Inspiratory muscle training improves blood flow to resting and exercising limbs in patients with chronic heart failure. J Am Coll Cardiol. 2008;51(17):1663–1671. doi: 10.1016/j.jacc.2007.12.045. [DOI] [PubMed] [Google Scholar]
- 63.Hulzebos EHJ, Helders PJM, Favié NJ, De Bie RA, De La Riviere AB, Van Meeteren NLU. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high-risk patients undergoing CABG surgery: A randomized clinical trial. J Am Med Assoc. 2006;296(15):1851–1857. doi: 10.1001/jama.296.15.1851. [DOI] [PubMed] [Google Scholar]
- 64.Katsura M, Kuriyama A, Takeshima T, Fukuhara S, Furukawa TA. Preoperative inspiratory muscle training for postoperative pulmonary complications in adults undergoing cardiac and major abdominal surgery. Cochrane Database Syst Rev. 2015;2015(10) doi: 10.1002/14651858.CD010356.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Karanfil EOT, Møller AM. Preoperative inspiratory muscle training prevents pulmonary complications after cardiac surgery – a systematic review. Dan Med J. 2018;65(3):A5450. [PubMed] [Google Scholar]
- 66.Mans CM, Reeve JC, Elkins MR. Postoperative outcomes following preoperative inspiratory muscle training in patients undergoing cardiothoracic or upper abdominal surgery: a systematic review and meta analysis. Clin Rehabil. 2015;29(5):426–438. doi: 10.1177/0269215514545350. [DOI] [PubMed] [Google Scholar]
- 67.Chen X, Hou L, Zhang Y. The effects of five days of intensive preoperative inspiratory muscle training on postoperative complications and outcome in patients having cardiac surgery: a randomized controlled trial. Clin Rehabil. 2019;33(5):913–922. doi: 10.1177/0269215519828212. [DOI] [PubMed] [Google Scholar]
- 68.Nepomuceno BRV, de Sá Barreto M, Almeida NC, Guerreiro CF, Xavier-Souza E, Neto MG. Safety and efficacy of inspiratory muscle training for preventing adverse outcomes in patients at risk of prolonged hospitalisation. Trials. 2017;18(1):626. doi: 10.1186/s13063-017-2372-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Elkins M, Dentice R. Inspiratory muscle training facilitates weaning from mechanical ventilation among patients in the intensive care unit: A systematic review. J Physiother. 2015;61(3):125–134. doi: 10.1016/j.jphys.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 70.Nikoletou D, Man WDC, Mustfa N. Evaluation of the effectiveness of a home-based inspiratory muscle training programme in patients with chronic obstructive pulmonary disease using multiple inspiratory muscle tests. Disabil Rehabil. 2016;38(3):250–259. doi: 10.3109/09638288.2015.1036171. [DOI] [PubMed] [Google Scholar]
- 71.Langer D, Charususin N, Jácome C. Efficacy of a Novel method for inspiratory muscle training in people with chronic obstructive pulmonary disease. Phys Ther. 2015;95(9):1264–1273. doi: 10.2522/ptj.20140245. [DOI] [PubMed] [Google Scholar]
- 72.Sørensen D, Svenningsen H. Adherence to home-based inspiratory muscle training in individuals with chronic obstructive pulmonary disease. Appl Nurs Res. 2018;43:75–79. doi: 10.1016/j.apnr.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 73.Weiner P, Magandle R, Beckerman M, Weiner M, Berar-Yanay N. Maintenance of inspiratory muscle training in COPD patients: one year follow-up. Eur Respir J. 2004;23(1):61–65. doi: 10.1183/09031936.03.00059503. [DOI] [PubMed] [Google Scholar]
- 74.Chlif M, Chaouachi A, Ahmaidi S. Effect of aerobic exercise training on ventilatory efficiency and respiratory drive in obese subjects. Respir Care. 2017;62(7):936–946. doi: 10.4187/respcare.04923. [DOI] [PubMed] [Google Scholar]
- 75.O'Donnell DE, McGuire M, Samis L, Webb KA. General exercise training improves ventilatory and peripheral muscle strength and endurance in chronic airflow limitation. Am J Respir Crit Care Med. 1998;157(5 PART I):1489–1497. doi: 10.1164/ajrccm.157.5.9708010. [DOI] [PubMed] [Google Scholar]
- 76.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: united states, 2017-2018 key findings data from the National Health and Nutrition Examination Survey, 2017. Available at: https://www.cdc.gov/nchs/products/index.htm. Accessed March 21, 2020.
- 77.Ward BW, Schiller JS, Goodman RA. Multiple chronic conditions among us adults: a 2012 update. Prev Chronic Dis. 2014;11(4):E62. doi: 10.5888/pcd11.130389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Colby SL, Ortman JM.Population estimates and projections current population reports, 2015. Available at: www.census.gov. Accessed March 21, 2020.
- 79.Centers for Disease Control and Prevention. Current cigarette smoking among adults in the United States. Available at:https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm#nation. Accessed March 21, 2020.
- 80.Hammond D, Reid JL, Rynard VL. Prevalence of vaping and smoking among adolescents in Canada, England, and the United States: Repeat national cross sectional surveys. BMJ. 2019;365:l2219. doi: 10.1136/bmj.l2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Layden JE, Ghinai I, Pray I. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin — preliminary report. N Engl J Med. 2020;382(10):903–916. doi: 10.1056/nejmoa1911614. [DOI] [PubMed] [Google Scholar]