Abstract
Epithelial-mesenchymal transitions (EMTs), the acquisition of mesenchymal features from epithelial cells, occur during some biological processes and are classified into three types: the first type occurs during embryonic development, the second type is associated with adult tissue regeneration, and the third type occurs in cancer progression. EMT occurring during embryonic development in gastrulation, renal development, and the origin and fate of the neural crest is a highly regulated process, while EMT occurring during tumor progression is highly deregulated. EMT allows the solid tumors to become more malignant, increasing their invasiveness and metastatic activity. Secondary tumors frequently maintain the typical histologic characteristics of the primary tumor. These histologic features connecting the secondary metastatic tumors to the primary is due to a process called mesenchymal-epithelial transition (MET). MET has been demonstrated in different mesenchymal tumors and is the expression of the reversibility of EMT. EMT modulation could constitute an approach to avoid metastasis. Some of the targeted small molecules utilized as antiproliferative agents have revealed to inhibit EMT initiation or maintenance because EMT is regulated through signaling pathways for which these molecules have been designed.
Introduction
Epithelial cell maintains apical-basal polarity and contact with adjacent cells through adherens junctions, tight junctions, and desmosomes. Mesenchymal cells on the other hand are separated with each other by the extracellular matrix, do not have a basal lamina separating them from adjacent tissue, and do not have the distinctive apical-basolateral polarity as epithelial cells.
Greenburg and Hay were the first to show that when epithelial cells derived from embryonic and adult anterior lens were maintained in three-dimensional conditions using a collagen gel culture system, they lose their polarity and acquire mesenchymal properties, and termed this phenomenon “epithelial to mesenchymal transformation” [1]. In 1985, Stocker and Perryman [2] demonstrated that the supernatant derived by fibroblast culture induced the migration of epithelial Madin-Darly canine kidney. In the 1990s, the scattering activity was attributed to the hepatocyte growth factor (HGF).
Epithelial-mesenchymal transitions (EMTs), the acquisition of mesenchymal features from epithelial cells, occur during some biological processes and are classified into three types [3,4]: the first type occurs during embryonic development, the second type is associated with adult tissue regeneration, and the third type occurs in cancer progression. EMT occurring during embryonic development in gastrulation, renal development, and the origin and fate of the neural crest is a highly regulated process, while EMT occurring during tumor progression s highly deregulated.
Biological Mechanisms of EMT
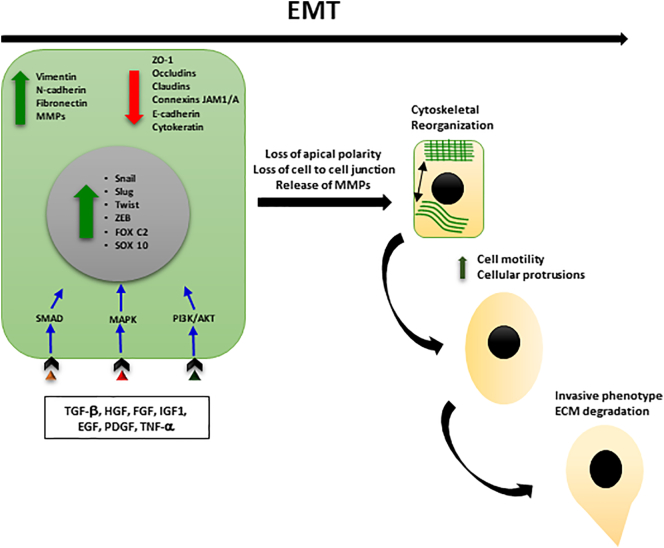
The events occurring during EMT include the loss of adherents junctions and the downregulation of cytokeratins and E-cadherin, epithelial specific markers, and by the increase of mesenchymal markers, such as fibronectin, N-cadherin, and vimentin, the gaining of a fibroblastoid invasive phenotype, as well as the anoikisis/apoptosis resistance [[5], [6], [7]]. EMT markers are summarized in Table 1. EMT is modulated through complex molecular pathways that involve microRNAs and epigenetic and posttranslational regulators along with alternative splicing events [8].
Table 1.
EMT Markers
| Increased proteins | |
|---|---|
| N-cadherin | |
| Vimentin | |
| Fibronectin | |
| Snail 1 (Snail) | |
| Snail 2 (Slug) | |
| Twist | |
| FOX C2 | |
| SOX 10 | |
| MMP-2, MMP-3, MMP-9 | |
| N-cadherin | |
| Decreased proteins | |
| E-cadherin | |
| Desmoplakin | |
| Cytokeratin | |
| Occludin | |
| Functional markers | |
| Increased migration | |
| Increased invasion | |
| Increased scattering | |
| Elongation of cell shape | |
| Resistance to anoikis |
Transcription factors including Snail1/Snail, Snail2/Slug, Twist, and ZEB1 are involved in the orchestration of EMT [9]. Snail1-induced EMT involves the loss of E-cadherin and claudins with concomitant upregulation of vimentin and fibronectin, among other biomarkers [10]. Snail2-deficient mice show delayed mammary gland tubule growth and precocious branching morphogenesis [11]. Twist overexpression was correlated with hepatocellular carcinoma metastasis through induction of EMT changes and hepatocellular cell invasiveness [12]. In invasive ductal and lobular breast cancer, upregulation of ZEB1 was coupled to cancer cell dedifferentiation [13]. During EMT, increased expression of the mesenchymal markers vimentin and N-cadherin and the downregulation of the epithelial marker E-cadherin, a powerful suppressor of tumor cell invasion and metastasis, have been observed [14,15]. However, loss of E-cadherin alone might not be sufficient to elicit EMT associated changes [16]. In fact, complete loss of E-cadherin expression observed in lobular carcinoma in situ of the breast is not associated to an aggressive phenotype, but on the other hand, the invasive type of the same breast carcinoma has a favorable outcome with respect to invasive ductal breast carcinoma that instead expresses E-cadherin [17]. β-Catenin molecule, which forms an important membrane complex with E-cadherin, often detaches the cell membrane and translocates to the nucleus to participate in the induction of EMT signaling events [18]. As cytosolic levels of β-catenin increase, the protein is often found to accumulate in the nucleus, where β-catenin can interact with members of the LEF/TCF family of transcription factors to promote EMT [19]. During gastrulation, β-catenin forms a complex with LEF-1 to bind and inhibit the transcription of CDH1 and induce EMT [20].
EMT in Cancer
In the early 80s, the correlation between EMT and cancer was reported. The benign tumor cells acquire infiltrating and metastasizing properties during the tumor progression due to EMT. The vast majority of tumors undergo EMT during tumor progression, so much so that cancers derived from epithelia are those in which the EMT process is determinant [21]. The only exception is the carcinosarcoma, in which a precursor cell develops both epithelial and mesenchymal compartments able to coexist [22]. Carcinosarcomas are rare and extremely aggressive tumors resulting in short survival of patients and are characterized by high malignancy grade in both epithelial and mesenchymal components.
After activation of EMT, tumor epithelial cells lose their cell polarity and cell-cell adhesion and gain migratory and invasive properties, becoming mesenchymal cells [21]. It has been demonstrated that transforming growth factor beta (TGF-β)/Smads pathway is the strongest EMT inducer by the upregulation of EMT-related transcription factors [23].
The role of EMT in the tumorigenesis of different cancers including prostate, lung, liver, pancreatic, and breast cancers has been demonstrated [24,25]. The decrease or loss of E-cadherin and catenins expression is considered as an unfavorable prognostic factor in non–small cell lung cancer (NSCLC) [26,27]. In addition, vimentin and Snail have also been associated with the malignant phenotype of NSCLC [[26], [27], [28]]. It has been demonstrated that the invasive phenotype prostate cancer cell is associated with the decreased expression of E-cadherin influencing grade, local invasiveness, dissemination into the blood, and tumor relapse after therapy [[29], [30], [31]].
In human carcinomas, among the transcription factors involved in EMT, Snail has the major role as inducer, while Twist and Zeb ½ are principally involved in retaining the invasive mesenchymal phenotype [32]. In particular, it was found that Snail 1 is specifically expressed at the invasive front of colon carcinoma. In addition, the Snail 1 expression is correlated both to the malignancy of experimentally induced breast tumors and to an increased possibility of tumor relapse and poor survival in human breast cancer [33]. Vimentin is expressed at high levels in many epithelial tumors, including breast cancer, prostate cancer, melanoma, and lung cancer, and its expression is determinant for tumor growth, invasion, and poor prognosis and serves as potential target for cancer therapy [34] Overexpression of vimentin in MCF7 cells increased cell stiffness, elevated cell motility and directional migration, reoriented microtubule polarity, and increased EMT phenotypes due to the increased β1-integrin and the loss of junction protein E-cadherin [35]. A relation between hypoxia and β-catenin in human prostate cancer has been found. In this tumor, both the expression and the nuclear activity of β-catenin are associated with the concentration of hypoxia inducible factor 1 alpha (HIF-1α)–induced EMT [36]. This process can also be potentiated by Wnt3a/β-catenin pathway in hepatocellular carcinoma [37].
EMT and Stemness
EMT activation has been associated to the generation of cancer stem cells (CSCs) [38]. Several studies have demonstrated a link between EMT, stemness, and the metastatic initiating potential of tumor cells. Induction of EMT in transformed epithelial cells was shown to culminate in endowing cells with stem-like traits [39,40]. These stem-like traits promoted the initiation of primary tumors and accelerated metastasis [41,42]. For example, EMT-TF Zeb1 promotes stemness and inhibits epithelial differentiation by repressing miR-200 family members [43]. Carcinoma CSCs express at the same time phenotypic characteristics of epithelial and mesenchymal cells, and this allows CSCs to move in alternative states during EMT. Moreover, CSCs are involved in tumor growth, metastatic process, drug resistance, and tumor relapse after therapy. Experimental evidences showed that a subpopulation of CSCs included in a human mammary epithelial cell culture if stimulated by TGF-β or through Snail1/Twist1 inducer undergoes EMT and develops mammospheres [44]. In pancreatic cancer cells, Notch signaling is involved in the acquisition of EMT and cancer stem-like phenotypes [45]. Since the initial discovery of the connection between breast cancer cells that have undergone an EMT and their entrance into a stem-cell like state [46], a number of studies have reported acquisition of stemness following the activation of an EMT program in multiple cancer types including pancreatic, prostate, colorectal, and ovarian cancer [[47], [48], [49], [50]].CCL21/CCR7 axis regulated EMT progress and promoted the stemness of oral squamous cell carcinoma by activating the JAK2/STAT3 signaling pathway [51]. A stemness- and EMT-based gene expression signature identifies phenotypic plasticity and is a predictive but not prognostic biomarker for breast cancer [52].Using different murine pancreatic ductal adenocarcinoma and pancreatitis models, Rhim et al. demonstrated that cells which have undergone a partial EMT and express E-cadherin and Zeb1 exhibit stem cell properties [53]. CD133, a surface antigen associated with CSCs in human pancreatic ductal adenocarcinoma, mediates EMT through the regulation of Snai2 in human pancreatic cells [54].
Overall, these studies demonstrated that EMT, along with the resulting acquisition of stem cell-like properties, facilitates dissemination and consequently the outgrowth of tumor cells at distant organs [55].
EMT and Cytokines
Different cytokines are involved in EMT induction, including HGF, epidermal growth factor (EGF), and fibroblast growth factor (FGF) [[56], [57], [58]]. FGF acts as a chemoattractant for epithelial cells which express FGF receptors, and FGF is able to induce epithelial cell growth. Cancer cells that undergo EMT secrete cytokines such as TGF-β, interleukin-10 (IL-10), and thrombospondin-1 (TSP-1) that result in a generally immunosuppressive tumor microenvironment [59]. Cytokines such as IL-8, IL-6, and tumor necrosis factor alpha (TNF-α), often secreted by tumor stroma, can also promote EMT [[60], [61], [62]].
In human NSCLC, IL-27 through a STAT1 dominant pathway increases the expression of epithelial markers and inhibits cell migration and the angiogenic activity [63]. Moreover, when lung cancer cells are stimulated with IL-27, they increased the epithelial marker expression (E-cadherin and γ-catenin) and reduced the expression of both Snail (transcriptional repressor of E-cadherin) and mesenchymal markers (N-cadherin and vimentin). It has been also observed that IL-27 inhibited in vitro tumor cell migration [63]. The negative effect of IL-6 on E-cadherin expression in breast cancer cell lines was also demonstrated [64]. Sullivan et al. [61] demonstrated that the ectopic expression of IL-6 in MCF7 cells activated the JAK2/STAT3 pathway and Twist, leading to the acquisition of mesenchymal markers and properties.
EMT and Stromal Tumor Cells
Epithelial-mesenchymal interactions within the tumor microenvironment integrate several important signaling molecules that are critical for tumor growth and metastasis, including integrins, cytokines, and growth factors [65]. The presence of cytokines such as HGF, EGF, platelet derived growth factor (PDGF), and TGF-β produced and released by the tumor stroma acts by inducing EMT and favoring processes such as metastasis [66,67], and they can activate a number of transcription factors of genes that promote EMT, such as Snail, Slug, ZEB1, and Twist, to transmit EMT promotion signals [3,68]. The activation of specific genes induce EMT in breast cancer cell lines when they were co-cultured with mesenchymal stem cells (MSCs) and decrease the expression of genes related to epithelial differentiation [69]. Many signals received from the tumor microenvironment can initiate EMT including TGFβ, HIF-1α, EGF, WNTs, and Notch. Different signals trigger the expression of these transcription factors including heterotypic interactions with neighboring cancer cells and interactions with adjacent tumor-associated stromal cells.
Cancer-associated fibroblasts (CAFs) activate the EMT program in nearby carcinoma cells. CAFs from human prostate cancers induced EMT in co-cultured PC-3 human prostate carcinoma cells via secretion of matrix metalloproteinases (MMPs) [70]. The conditioned culture medium of CAFs isolated from invasive breast tumors induces EMT-like changes in multiple human breast cancer cell lines through TGFβ secreted by CAFs [71]. Tumor-associated macrophages can contribute to the activation of EMT in carcinoma cells. Depletion of macrophages from mice bearing F9 teratocarcinoma allografts results in the epithelial differentiation of tumor cells, thus indicating the critical role of macrophages in maintaining the mesenchymal properties of the tumor cells. This EMT effect is mediated, in part, by the secretion of TGFβ by macrophages [72].
EMT and Metastasis
Invasion of cells into the extracellular matrix is considered one of the first steps in metastatic cascade. The cells acquiring the ability to migrate and invade matrix have long been considered a hallmark of EMT and have been used as a surrogate to describe the role of EMT in metastasis [73]. Distinct mechanisms are involved including cytoskeletal reorganization, altered expression of cell adhesion molecules, degradation of basement membrane through activation of MMP-2 and MMP-9 [74], as well as sustained autocrine growth factor signaling to evade apoptosis and/or anoikis [75]. Studies using mouse models of breast and skin cancers have demonstrated that activation of an EMT program is important for primary tumor cells to disseminate into the lungs, while the disseminated cells need to subsequently reverse the EMT program and gain epithelial characteristics in order to efficiently form macroscopic metastases [76,77]. In multiple carcinomas, experimental activation of the EMT program results in a remarkable increase in the ability of these cells to extend filopodium-like protrusions, allowing these EMT-activated cells to proliferate following extravasation, ultimately enabling them to seed metastases [78].
EMT and Circulating Tumor Cells (CTCs)
CTCs are constituted by carcinoma cells penetrated into the blood vessels (intravasated) and reaching distant sites where they form new metastatic colonies. CTCs show an incomplete EMT activation and express both epithelial and mesenchymal markers. CTCs are considered as precursors of metastasis, and the molecular attributes of these cells will provide a useful tool to clarify the mechanisms underlying malignant spread. A higher number of mitotic CTCs have been found in advanced metastatic breast cancer patients, and the mitotic state of CTCs correlated with shorter survival in these patients [79]. Mesenchymal CTCs have been found to be higher in patients affected by progressive tumors [80]. The presence of CTCs during primary prostatic cancer has been associated to the alteration of E-cadherin expression [29].
EMT and Tumor Angiogenesis
EMT and angiogenesis are two critical factors influencing tumor growth and metastasis. Angiogenesis is a crucial event in tumor progression and metastasis formation, allowing the transition from an avascular steady state of tumor growth to a vascularized phase through the so-called angiogenic switch. In the promotion of tumorigenesis, EMT and angiogenesis have been revealed as integral processes [81]. It has been demonstrated that levels of vascular endothelial growth factor (VEGF) and EGF receptor (EGFR) are associated with hangs in Twist2 expression and reduction of E-cadherin levels [82]. The activation of VEGF pathways in hypoxic tumors stimulates endothelial mesenchymal transition in angiogenic tumor endothelial cells [83]. The administration of VEGF in xenograft experimental models of preinvasive cells induced the expression of EMT markers [84,85]. VEGF receptor-1 (VEGFR-1) activation results in EMT, tumor cell motility, and invasiveness in human pancreatic carcinoma cells [86]. The cross talk between Notch and VEGF pathways in the context of hypoxic tumors promotes endothelial mesenchymal transition in angiogenic tumor endothelial cells [87]. Studies on xenografts in preinvasive cells demonstrated that the addition of VEGF induces the appearance of EMT markers [88,89].
EMT and Tumor Dormancy
Dormancy of the early disseminated tumor cells may display an EMT/mesenchymal-to-epithelial transition (MET) transient state leaning to a more mesenchymal phenotype, resulting in CSC-like traits responsible for their quiescence. Snail, one of EMT transcription factors, could dramatically impair cell-cycle progression by repressing the transcription of cyclin D2 whose activity was required for cell division [90]. Moreover, Snail could suppress tumor cell proliferation through binding to flanking region of proliferating cell nuclear antigen gene to decrease its expression [91]. However, how these tumor cells remain and exit dormancy has not been verified, and the dynamic changes of the cellular phenotype in tumor progression have not been shown in vivo [53,92]. EMT of dormant MCF-7 cells expressing LOXL2 was required for their CSC-like properties and their transition to metastatic outgrowth. Moreover, increase in LOXL2 mRNA levels correlates with increase in the mRNA levels of EMT and stem cells markers, and is also associated with decrease in relapse-free survival of breast cancer patients [93].
EMT and Immune Interactions
The link between EMT and immune recognition and killing of cancer cells is well established. EMT contributes to immune escape of tumors as it has been demonstrated in the human mammary carcinoma model MCF7 which underwent EMT, following stable expression of Snail or after prolonged exposure to TNF-α, and exhibited reduced susceptibility to tumor lymhocytes-mediated lysis [94]. High levels of the EMT-related factor Brachyury reduced the susceptibility of carcinoma cells not only to tumor lymphocytes but also to natural killer (NK) cells, lymphokine-activated killer, FAS, and TRAIL-induced cell death [95]. Exposure to inflammatory cytokines can endow cancer cells undergoing EMT with a number of immunomodulatory effects, including interference with proliferation, differentiation, and apoptosis of NK, T-, and B-cell populations [96]. A correlation between high EMT score and high expression of several immune checkpoints including PD1, PD-L1, PD-L2, B7-H3, OX40, OX40L, CD137, TIM3, LAG3, and CTLA4 has been demonstrated [97]. A CXCL1-LCN2 paracrine network was demonstrated in prostate cancer tissue samples, which was correlated with the recurrence of prostate cancer. CXCL1-LCN2 axis activates Src signaling, triggers the EMT, and promotes the migration of prostate cancer cells, leading to enhanced tumor metastasis [98]. Neuroblastoma MSCs exhibited greater immunosuppressive capacity on activated T lymphocytes compared with bone marrow MSCs, and transcriptomic profiling results indicated that neuroblastoma MSCs were enriched with EMT genes compared to bone marrow MSCs [99]. Analysis of primary tumors from patients with NSCLC revealed a positive correlation among intratumoral macrophage densities, EMT markers, TGF-β levels, and tumor grade [100]. In colon and breast cancer, platelets promote extravasation of cancer cells by inducing EMT through direct contact and release of TGF-β [101]. Various cytokines, including IL4, IL6, IL10, TNF-α, and TGF-β1, secreted by activated macrophages could induce EMT by altering the expression of EMT-related genes in human cholangiocarcinoma [102].
EMT and Chemoresistance
Two reports provide convincing evidence linking the EMT to cancer drug resistance, particularly favoring the multidrug resistance phenotype but also radioresistance, which may be caused by an enhancement of cancer cell survival, cell fate transition, and/or upregulation of drug resistance-related genes [103,104]. Moreover, the context-dependent stemness of the transformed cells and their mesenchymal status, the dysregulation of particular transcription factors, as well as relevant signaling cascades influencing major antitumor barriers in cells, i.e., senescence and various forms of cell death, might be involved [[105], [106], [107], [108], [109]].
The levels of SPARC allow to distinguish high-grade breast cancer with improved EMT, resistance to treatment, and poor prognosis. The induction of EMT by SPARC is associated to the localization and suppressive function of myeloid cells, and the administration of amino-bisphosphonates could revert EMT through the inhibition of the suppression activity in myeloid-derived stem cells [110] .
The identification of the EMT as a common regulator of the CSC phenotype across various carcinoma types has provided us to investigate, at the molecular level, how CSCs and therapeutic resistance are linked via EMT programs. An analysis of responses to chemotherapy in patients with breast cancer revealed a close association between therapeutic resistance and increased expression of genes that are usually expressed primarily by the stromal cells; this transcriptional upregulation seems to be caused by activation of the EMT program within carcinoma cells [111]. In NSCLC and ovarian cancer, the EMT switches the dependence of carcinoma cells from the EGFR to the AXL receptor tyrosine kinase, thereby yielding resistance to EGFR-targeted therapy [112,113]. Moreover, EMT contributes to the establishment of an immunosuppressive tumor microenvironment and thereby confers resistance to immunotherapies [114]. In breast cancer model, mesenchymal cancer cells within a tumor were able to induce the immunosuppressive microenvironment and protect the more epithelial cancer cells residing in the same tumor from immune attack [115].
EMT and Senescence
Increasing evidence suggests that the two processes that seem to operate independently, EMT and senescence, are in fact intertwined. For example, several transcription factors can both inhibit senescence and induce EMT. Activation of EMT is linked to suppression of cellular senescence, as it has been demonstrated in human epithelial cells, in which whereas ectopically expressed ErbB2 induces senescence, overexpression of both Twist and ErbB2 triggers EMT and allows for senescence bypass [116], and also in the context of another EMT regulator, Zeb1 [117]. Moreover, when cells were locked in a senescent state by activation of p53, TGFβ was no longer able to induce EMT, raising the possibility that senescent cells cannot undergo EMT [ [118]. Culture media from senescent cells decreased overall and cell surface β-catenin and E-cadherin, and reduced cytokeratin expression [119], consistent with a mesenchymal transition. In the meantime, senescent cells secrete chemokines that can create a gradient to promote cell migration and invasion. In breast cancer, the high levels of IL-6 and IL-8 secreted by senescent fibroblasts enhanced the invasiveness of cancer cell lines in cell culture [119,120]. Furthermore and consistent with a SASP-induced EMT, culture media from senescent, but not nonsenescent, cells stimulated premalignant and malignant cancer cells to invade a basement membrane [119]. Senescent-associated phenotype derived from senescent fibroblasts induces EMT in neighboring epithelial cells and contributes to EMT in nonaggressive human breast cancer cell lines [121].
EMT and Inflammation
Inflammatory mediators, including soluble factors, oxidative stress, or hypoxia, can foster the acquisition of EMT-like features in cancer cells [122]. The number of tumor-associated macrophages has been correlated with EMT-like features in gastric cancer [123], NSCLC [72], or head and neck cancer [124]. In hepatocellular carcinoma, macrophages induce EMT in cancer cells in co-culture experiments in an IL-8–dependent fashion [125] or in a TGF-β–dependent fashion [126,127]. The induction of EMT by TNF-α, in synergy with TGF-β or other inflammatory factors, has been described [128]. The link between IL-8 and EMT has established a form of a mutual loop in which IL-8 and EMT programs sustain each other in tumor microenvironment [129]. EMT in association with inflammation has also been correlated with higher stages of cancer progression. In patients with inflammatory breast cancer, a correlation exists between immune activation and the presence of circulating tumor cells with EMT characteristics [130].
Mesenchymal-Epithelial Transition
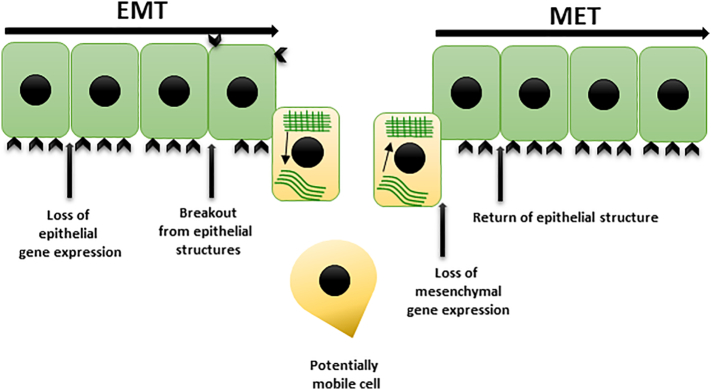
Following the metastatic tumor cells arriving at distant sites, they undergo MET, reversing the conversion into metastatic lesions [21]. Multiple transition states have been demonstrated in tumors, suggesting that the tumor cells could frequently express a mix of both epithelial and mesenchymal genes [131]. The histologic features connecting the secondary metastatic tumors to the primary is due to a process called MET that allows the cancer tumor cells to colonize secondary distant sites (Figure 1). MET has been demonstrated in different mesenchymal tumors and is expression of the reversibility of EMT [132]. Comparing expression of epithelial junctional proteins including E-cadherin, β-catenin, and connexin in primary tumor and matched distant metastases in lung, liver, and brain of cancer patients shows equal or increased epithelial cells in metastases, indicating that circulating mesenchymal tumor cells undergo MET [133]. However, the exact mechanisms underlying MET, including where and how MET takes place and how it facilitates the formation of metastases, remain largely elusive, and compared to EMT, molecular mechanisms mediating MET are relatively less characterized [134,135]. GRHL2, a transcription factor that activates E-cadherin and Claudin-4, and OVOL1/2 can repress EMT-associated transcription factors and drive MET [[136], [137], [138]]. However, the overexpression of OVOL2, GRHL2, or E-cadherin may not always be sufficient to drive complete MET [[139], [140], [141], [142]].
Figure 1.
Epithelial to mesenchymal transition (EMT) and mesenchymal to epithelial transition (MET).
Concluding Remarks and Future Perspectives
EMT is of extreme importance in tumor microenvironment in the regulation of tumor growth, progression, and metastatic cascade. Context-dependent signaling transduction pathways and microenvironment signals, such as hypoxia, oxidative stress, nutrient deprivation, or inflammation, and EMT transcription factors such as Snail1/Snail2, ZEB1/ZEB2, and Twist1 are responsible to induce and sustain the mesenchymal phenotype (Figure 2).
Figure 2.
Main events in EMT.
The EMT process within the tumor context is highly dynamic, implying transient and reversible states, thus resembling embryonic development where EMT and the reverse MET processes occur as necessary steps for early embryogenesis and morphogenesis.
EMT stimulates the tumor angiogenesis, but the role of EMT on the interaction between tumor cells and the tumor microenvironment has not been deepened even though the mechanisms inducing EMT and their association to improved invasive properties of tumor cells have been extensively studied.
The induction of EMT confers therapy resistance in tumor cells [143] that leads to a reduction of proliferation rate and increased expression of both antiapoptotic proteins and transporters belonging to ATP binding cassette that are responsible of drug efflux. EMT has a role in the establishment of an immunosuppressive tumor microenvironment and induces also immunotherapies resistance. In this context, an increased immunosuppressive regulatory T cells infiltration in tumor microenvironment when EMT is stimulated by Snail in melanoma cells was found [144].
Intermediate states between epithelial and mesenchymal phenotypes might occur at different steps of the metastatic cascade with cells transitioning through hybrid states during tumor dissemination. EMT modulation could constitute an approach to avoid metastasis. For example, some of the targeted small molecules utilized as antiproliferative agents have been revealed to inhibit EMT initiation or maintenance because EMT is regulated through signaling pathways for which these molecules have been designed [145]. The modulation of the signaling processes involved in inducing and maintaining mesenchymal characteristics can inhibit EMT. Experimental works established that extracellular vesicles, including exosomes, play a role in EMT and metastasis [[146], [147], [148]]. Thus, exosomes are considered as potential doxorubicin delivery system for tumor tissue to inhibit tumor growth with a reduced toxicity [149].
One of the challenges is the characterization of a number of genes or proteins that could be studied in human samples to predict the establishment or acquisition of EMT or hybrid states, along with the detection of the reverse MET process. These specific signatures could be used as new tools for understanding tumor cell plasticity as well as for diagnosis and prognosis. Additional studies using innovative genetically engineered animal models to trace and analyze tumor cells responsible for seed metastasis, together with higher-resolution in vivo intravital imaging microscopy, would contribute to better understand the biological relevance of EMT and plasticity processes to metastasis in different tumors. Finally, the emergence of targeted therapies against signaling regulators of EMT might lead toward clinical benefits due to the specific targeting of cancer cells undergoing EMT.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by the European Union Seventh Framework Program OPTATIO (FP7/2007- 2013) under grant agreement n°278570.
References
- 1.Greenburg G., Hay E.D. Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J Cell Biol. 1982;95(1):333–339. doi: 10.1083/jcb.95.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stoker M., Perryman M. An epithelial scatter factor released by embryo fibroblasts. J Cell Sci. 1985;77:209–223. doi: 10.1242/jcs.77.1.209. [DOI] [PubMed] [Google Scholar]
- 3.Kalluri R., Weinberg R.A. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeisberg M., Neilson E.G. Biomarkers for epithelial-mesenchymal transitions. Journal of Clinical Investigation. 2009;119(6):1429–1437. doi: 10.1172/jci36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garside V.C., Chang A.C., Karsan A., Hoodless P.A. Co-ordinating Notch, BMP, and TGF-β signaling during heart valve development. Cellular and Molecular Life Sciences. 2012;70(16):2899–2917. doi: 10.1007/s00018-012-1197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Micalizzi D.S., Farabaugh S.M., Ford H.L. Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. Journal of Mammary Gland Biology and Neoplasia. 2010;15(2):117–134. doi: 10.1007/s10911-010-9178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wells A., Yates C., Shepard C.R. E-cadherin as an indicator of mesenchymal to epithelial reverting transitions during the metastatic seeding of disseminated carcinomas. Clinical & Experimental Metastasis. 2008;25(6):621–628. doi: 10.1007/s10585-008-9167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skrypek N., Goossens S., De Smedt E., Vandamme N., Berx G. Epithelial-to-mesenchymal transition: epigenetic reprogramming driving cellular plasticity. Trends in Genetics. 2017;33(12):943–959. doi: 10.1016/j.tig.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Stemmler M.P., Eccles R.L., Brabletz S., Brabletz T. Non-redundant functions of EMT transcription factors. Nat Cell Biol. 2019;21(1):102–112. doi: 10.1038/s41556-018-0196-y. [DOI] [PubMed] [Google Scholar]
- 10.Kaufhold S., Bonavida B. Central role of Snail1 in the regulation of EMT and resistance in cancer: a target for therapeutic intervention. J Exp Clin Cancer Res. 2014;33:62. doi: 10.1186/s13046-014-0062-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radice G.L., Ferreira-Cornwell M.C., Robinson S.D., Rayburn H., Chodosh L.A., Takeichi M. Precocious mammary gland development in P-cadherin–deficient mice. J Cell Biol. 1997;139(4):1025–1032. doi: 10.1083/jcb.139.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee T.K., Poon R.T., Yuen A.P., Ling M.T., Kwok W.K., Wang X.H. Twist overexpression correlates with hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clin Cancer Res. 2006;12(18):5369–5376. doi: 10.1158/1078-0432.CCR-05-2722. [DOI] [PubMed] [Google Scholar]
- 13.Aigner K., Dampier B., Descovich L., Mikula M., Sultan A., Schreiber M. The transcription factor ZEB1 (deltaEF1) promotes tumour cell dedifferentiation by repressing master regulators of epithelial polarity. Oncogene. 2007;26(49):6979–6988. doi: 10.1038/sj.onc.1210508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin K., Baritaki S., Militello L., Malaponte G., Bevelacqua Y., Bonavida B. The role of B-RAF mutations in melanoma and the induction of EMT via dysregulation of the NF- B/Snail/RKIP/PTEN Circuit. Genes & Cancer. 2010;1(5):409–420. doi: 10.1177/1947601910373795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura M., Tokura Y. Epithelial-mesenchymal transition in the skin. Journal of Dermatological Science. 2011;61(1):7–13. doi: 10.1016/j.jdermsci.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Navarro P., Lozano E., Cano A. Expression of E- or P-cadherin is not sufficient to modify the morphology and the tumorigenic behavior of murine spindle carcinoma cells. Possible involvement of plakoglobin. J Cell Sci. 1993;105(Pt 4):923–934. doi: 10.1242/jcs.105.4.923. [DOI] [PubMed] [Google Scholar]
- 17.Chu K., Boley K.M., Moraes R., Barsky S.H., Robertson F.M. The paradox of E-cadherin: role in response to hypoxia in the tumor microenvironment and regulation of energy metabolism. Oncotarget. 2013;4(3) doi: 10.18632/oncotarget.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klymkowsky M.W. β-catenin and its regulatory network. Human Pathology. 2005;36(3):225–227. doi: 10.1016/j.humpath.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Logan C.Y., Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 20.Kemler R., Hierholzer A., Kanzler B., Kuppig S., Hansen K., Taketo M.M. Stabilization of beta-catenin in the mouse zygote leads to premature epithelial-mesenchymal transition in the epiblast. Development. 2004;131(23):5817–5824. doi: 10.1242/dev.01458. [DOI] [PubMed] [Google Scholar]
- 21.Thiery J.P., Acloque H., Huang R.Y.J., Nieto M.A. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Thompson L., Chang B., Barsky S.H. Monoclonal origins of malignant mixed tumors (carcinosarcomas) The American Journal of Surgical Pathology. 1996;20(3):277–285. doi: 10.1097/00000478-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Xu J., Lamouille S., Derynck R. TGF-β–induced epithelial to mesenchymal transition. Cell Research. 2009;19(2):156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hugo H., Ackland M.L., Blick T., Lawrence M.G., Clements J.A., Williams E.D. Epithelial-mesenchymal and mesenchymal-epithelial transitions in carcinoma progression. Journal of Cellular Physiology. 2007;213(2):374–383. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 25.Lee T.K., Poon R.T.P., Yuen A.P., Ling M.T., Kwok W.K., Wang X.H. Twist overexpression correlates with hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clinical Cancer Research. 2006;12(18):5369–5376. doi: 10.1158/1078-0432.ccr-05-2722. [DOI] [PubMed] [Google Scholar]
- 26.Kase S., Sugio K., Yamazaki K., Okamoto T., Koga T., Ondo K. Expression of E-cadherin and beta-catenin in human non-small cell lung cancer: clinical significance and prognosis. Lung Cancer. 2000;29(1):196. doi: 10.1016/s0169-5002(00)80667-4. [DOI] [PubMed] [Google Scholar]
- 27.Pirinen R.T. Reduced expression of alpha-catenin, beta-catenin, and gamma-catenin is associated with high cell proliferative activity and poor differentiation in non-small cell lung cancer. Journal of Clinical Pathology. 2001;54(5):391–395. doi: 10.1136/jcp.54.5.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato M., Shames D.S., Hasegawa Y. Emerging evidence of epithelial-to-mesenchymal transition in lung carcinogenesis. Respirology. 2012;17(7):1048–1059. doi: 10.1111/j.1440-1843.2012.02173.x. [DOI] [PubMed] [Google Scholar]
- 29.Loric S., Paradis V., Gala J.L., Berteau P., Bedossa P., Benoit G. Abnormal E-cadherin expression and prostate cell blood dissemination as markers of biological recurrence in cancer. European Journal of Cancer. 2001;37(12):1475–1481. doi: 10.1016/s0959-8049(01)00143-5. [DOI] [PubMed] [Google Scholar]
- 30.Mason M.D., Davies G., Jiang W.G. Cell adhesion molecules and adhesion abnormalities in prostate cancer. Critical Reviews in Oncology/Hematology. 2002;41(1):11–28. doi: 10.1016/s1040-8428(01)00171-8. [DOI] [PubMed] [Google Scholar]
- 31.Ray M.E., Mehra R., Sandler H.M., Daignault S., Shah R.B. E-cadherin protein expression predicts prostate cancer salvage radiotherapy outcomes. Journal of Urology. 2006;176(4):1409–1414. doi: 10.1016/j.juro.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 32.Peinado H., Olmeda D., Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nature Reviews Cancer. 2007;7(6):415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 33.Moody S.E., Perez D., Pan, T.-c., Sarkisian, C.J., Portocarrero, C.P., Sterner, C.J., et al. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell. 2005;8(3):197–209. doi: 10.1016/j.ccr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 34.Satelli A., Li S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cellular and Molecular Life Sciences. 2011;68(18):3033–3046. doi: 10.1007/s00018-011-0735-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu, C.Y., Lin, H.H., Tang, M.J., and Wang, Y.K. (2015). Vimentin contributes to epithelial-mesenchymal transition cancer cell mechanics by mediating cytoskeletal organization and focal adhesion maturation. Oncotarget 6(18), 15966-15983. doi: 10.18632/oncotarget.3862. [DOI] [PMC free article] [PubMed]
- 36.Jiang Y.G., Luo Y., He D.L., Li X., Zhang L.L., Peng T. Role of Wnt/beta-catenin signaling pathway in epithelial-mesenchymal transition of human prostate cancer induced by hypoxia-inducible factor-1alpha. Int J Urol. 2007;14(11):1034–1039. doi: 10.1111/j.1442-2042.2007.01866.x. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Q., Bai X., Chen W., Ma T., Hu Q., Liang C. Wnt/β-catenin signaling enhances hypoxia-induced epithelial-mesenchymal transition in hepatocellular carcinoma via crosstalk with hif-1α signaling. Carcinogenesis. 2013;34(5):962–973. doi: 10.1093/carcin/bgt027. [DOI] [PubMed] [Google Scholar]
- 38.Ye X., Weinberg R.A. Epithelial-mesenchymal plasticity: a central regulator of cancer progression. Trends in Cell Biology. 2015;25(11):675–686. doi: 10.1016/j.tcb.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mani S.A., Guo W., Liao M.J., Eaton E.N., Ayyanan A., Zhou A.Y. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.May C.D., Sphyris N., Evans K.W., Werden S.J., Guo W., Mani S.A. Epithelial-mesenchymal transition and cancer stem cells: a dangerously dynamic duo in breast cancer progression. Breast Cancer Res. 2011;13(1):202. doi: 10.1186/bcr2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo W., Keckesova Z., Donaher J.L., Shibue T., Tischler V., Reinhardt F. Slug and Sox9 cooperatively determine the mammary stem cell state. Cell. 2012;148(5):1015–1028. doi: 10.1016/j.cell.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scheel C., Weinberg R.A. Phenotypic plasticity and epithelial-mesenchymal transitions in cancer and normal stem cells? Int J Cancer. 2011;129(10):2310–2314. doi: 10.1002/ijc.26311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Korpal M., Ell B.J., Buffa F.M., Ibrahim T., Blanco M.A., Celia-Terrassa T. Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nat Med. 2011;17(9):1101–1108. doi: 10.1038/nm.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mani S.A., Guo W., Liao M.-J., Eaton E.N., Ayyanan A., Zhou A.Y. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Z., Li Y., Kong D., Banerjee S., Ahmad A., Azmi A.S. Acquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the Notch signaling pathway. Cancer Research. 2009;69(6):2400–2407. doi: 10.1158/0008-5472.can-08-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morel A.P., Lievre M., Thomas C., Hinkal G., Ansieau S., Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008;3(8) doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fan F., Samuel S., Evans K.W., Lu J., Xia L., Zhou Y. Overexpression of snail induces epithelial-mesenchymal transition and a cancer stem cell-like phenotype in human colorectal cancer cells. Cancer Med. 2012;1(1):5–16. doi: 10.1002/cam4.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kong D., Banerjee S., Ahmad A., Li Y., Wang Z., Sethi S. Epithelial to mesenchymal transition is mechanistically linked with stem cell signatures in prostate cancer cells. PLoS One. 2010;5(8) doi: 10.1371/journal.pone.0012445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Long, H., Xiang, T., Qi, W., Huang, J., Chen, J., He, L., et al. (2015). CD133+ ovarian cancer stem-like cells promote non-stem cancer cell metastasis via CCL5 induced epithelial-mesenchymal transition. Oncotarget 6(8), 5846-5859. doi: 10.18632/oncotarget.3462. [DOI] [PMC free article] [PubMed]
- 50.Rasheed Z.A., Yang J., Wang Q., Kowalski J., Freed I., Murter C. Prognostic significance of tumorigenic cells with mesenchymal features in pancreatic adenocarcinoma. J Natl Cancer Inst. 2010;102(5):340–351. doi: 10.1093/jnci/djp535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen Y., Shao Z., Jiang E., Zhou X., Wang L., Wang H. CCL21/CCR7 interaction promotes EMT and enhances the stemness of OSCC via a JAK2/STAT3 signaling pathway. J Cell Physiol. 2020 doi: 10.1002/jcp.29525. [DOI] [PubMed] [Google Scholar]
- 52.Akbar M.W., Isbilen M., Belder N., Canli S.D., Kucukkaraduman B., Turk C. A stemness and EMT based gene expression signature identifies phenotypic plasticity and is a predictive but not prognostic biomarker for breast cancer. J Cancer. 2020;11(4):949–961. doi: 10.7150/jca.34649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rhim A.D., Mirek E.T., Aiello N.M., Maitra A., Bailey J.M., McAllister F. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148(1-2):349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ding Q., Miyazaki Y., Tsukasa K., Matsubara S., Yoshimitsu M., Takao S. CD133 facilitates epithelial-mesenchymal transition through interaction with the ERK pathway in pancreatic cancer metastasis. Mol Cancer. 2014;13:15. doi: 10.1186/1476-4598-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oskarsson T., Batlle E., Massague J. Metastatic stem cells: sources, niches, and vital pathways. Cell Stem Cell. 2014;14(3):306–321. doi: 10.1016/j.stem.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grünert S., Jechlinger M., Beug H. Opinion: diverse cellular and molecular mechanisms contribute to epithelial plasticity and metastasis. Nature Reviews Molecular Cell Biology. 2003;4(8):657–665. doi: 10.1038/nrm1175. [DOI] [PubMed] [Google Scholar]
- 57.Hay E.D. An overview of epithelio-mesenchymal transformation. Cells Tissues Organs. 1995;154(1):8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- 58.Huber M.A., Kraut N., Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Current Opinion in Cell Biology. 2005;17(5):548–558. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 59.Yaguchi T., Sumimoto H., Kudo-Saito C., Tsukamoto N., Ueda R., Iwata-Kajihara T. The mechanisms of cancer immunoescape and development of overcoming strategies. Int J Hematol. 2011;93(3):294–300. doi: 10.1007/s12185-011-0799-6. [DOI] [PubMed] [Google Scholar]
- 60.Fernando R.I., Castillo M.D., Litzinger M., Hamilton D.H., Palena C. IL-8 signaling plays a critical role in the epithelial-mesenchymal transition of human carcinoma cells. Cancer Res. 2011;71(15):5296–5306. doi: 10.1158/0008-5472.CAN-11-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sullivan N.J., Sasser A.K., Axel A.E., Vesuna F., Raman V., Ramirez N. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene. 2009;28(33):2940–2947. doi: 10.1038/onc.2009.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu Y., Deng J., Rychahou P.G., Qiu S., Evers B.M., Zhou B.P. Stabilization of snail by NF-kappaB is required for inflammation-induced cell migration and invasion. Cancer Cell. 2009;15(5):416–428. doi: 10.1016/j.ccr.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kachroo P., Lee M.-H., Zhang L., Baratelli F., Lee G., Srivastava M.K. IL-27 inhibits epithelial-mesenchymal transition and angiogenic factor production in a STAT1-dominant pathway in human non-small cell lung cancer. Journal of Experimental & Clinical Cancer Research. 2013;32(1):97. doi: 10.1186/1756-9966-32-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Asgeirsson K.S., Olafsdottir K., Jonasson J.G., Ogmundsdottir H.M. The effects of IL-6 on cell adhesion and e-cadherin expression in breast cancer. Cytokine. 1998;10(9):720–728. doi: 10.1006/cyto.1998.0349. [DOI] [PubMed] [Google Scholar]
- 65.Tlsty T.D., Coussens L.M. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 66.Kong D., Wang Z., Sarkar S.H., Li Y., Banerjee S., Saliganan A. Platelet-derived growth factor-D overexpression contributes to epithelial-mesenchymal transition of PC3 prostate cancer cells. Stem Cells. 2008;26(6):1425–1435. doi: 10.1634/stemcells.2007-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thompson E.W., Newgreen D.F., Tarin D. Carcinoma invasion and metastasis: a role for epithelial-mesenchymal transition? Cancer Res. 2005;65(14):5991–5995. doi: 10.1158/0008-5472.CAN-05-0616. discussion 5995. [DOI] [PubMed] [Google Scholar]
- 68.Thiery J.P. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 69.Hass R., von der Ohe J., Ungefroren H. Potential role of MSC/cancer cell fusion and EMT for breast cancer stem cell formation. Cancers (Basel) 2019;11(10) doi: 10.3390/cancers11101432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Giannoni E., Bianchini F., Masieri L., Serni S., Torre E., Calorini L. Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer Res. 2010;70(17):6945–6956. doi: 10.1158/0008-5472.CAN-10-0785. [DOI] [PubMed] [Google Scholar]
- 71.Yu Y., Xiao C.H., Tan L.D., Wang Q.S., Li X.Q., Feng Y.M. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of breast cancer cells through paracrine TGF-beta signalling. Br J Cancer. 2014;110(3):724–732. doi: 10.1038/bjc.2013.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bonde A.K., Tischler V., Kumar S., Soltermann A., Schwendener R.A. Intratumoral macrophages contribute to epithelial-mesenchymal transition in solid tumors. BMC Cancer. 2012;12:35. doi: 10.1186/1471-2407-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kang Y., Massagué J. Epithelial-mesenchymal transitions. Cell. 2004;118(3):277–279. doi: 10.1016/j.cell.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 74.Wick W., Platten M., Weller M. Glioma cell invasion: regulation of metalloproteinase activity by TGF-beta. J Neurooncol. 2001;53(2):177–185. doi: 10.1023/a:1012209518843. [DOI] [PubMed] [Google Scholar]
- 75.Kalluri R., Weinberg R.A. The basics of epithelial-mesenchymal transition. Journal of Clinical Investigation. 2009;119(6):1420–1428. doi: 10.1172/jci39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ocana O.H., Corcoles R., Fabra A., Moreno-Bueno G., Acloque H., Vega S. Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer Cell. 2012;22(6):709–724. doi: 10.1016/j.ccr.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 77.Tsai J.H., Donaher J.L., Murphy D.A., Chau S., Yang J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell. 2012;22(6):725–736. doi: 10.1016/j.ccr.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shibue T., Brooks M.W., Weinberg R.A. An integrin-linked machinery of cytoskeletal regulation that enables experimental tumor initiation and metastatic colonization. Cancer Cell. 2013;24(4):481–498. doi: 10.1016/j.ccr.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Adams D.L., Adams D.K., Stefansson S., Haudenschild C., Martin S.S., Charpentier M. Mitosis in circulating tumor cells stratifies highly aggressive breast carcinomas. Breast Cancer Research. 2016;18(1) doi: 10.1186/s13058-016-0706-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yu M., Bardia A., Wittner B.S., Stott S.L., Smas M.E., Ting D.T. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339(6119):580–584. doi: 10.1126/science.1228522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sánchez-García I. The crossroads of oncogenesis and metastasis. New England Journal of Medicine. 2009;360(3):297–299. doi: 10.1056/nejmcibr0808031. [DOI] [PubMed] [Google Scholar]
- 82.Rojas-Puentes L., Cardona A.F., Carranza H., Vargas C., Jaramillo L.F., Zea D. Epithelial-mesenchymal transition, proliferation, and angiogenesis in locally advanced cervical cancer treated with chemoradiotherapy. Cancer Medicine. 2016;5(8):1989–1999. doi: 10.1002/cam4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Holderfield M.T., Hughes C.C.W. Crosstalk between vascular endothelial growth factor, notch, and transforming growth factor-β in vascular morphogenesis. Circulation Research. 2008;102(6):637–652. doi: 10.1161/circresaha.107.167171. [DOI] [PubMed] [Google Scholar]
- 84.Desai S., Laskar S., Pandey B.N. Autocrine IL-8 and VEGF mediate epithelial–mesenchymal transition and invasiveness via p38/JNK-ATF-2 signalling in A549 lung cancer cells. Cellular Signalling. 2013;25(9):1780–1791. doi: 10.1016/j.cellsig.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 85.Gonzalez-Moreno O., Lecanda J., Green J.E., Segura V., Catena R., Serrano D. VEGF elicits epithelial-mesenchymal transition (EMT) in prostate intraepithelial neoplasia (PIN)-like cells via an autocrine loop. Experimental Cell Research. 2010;316(4):554–567. doi: 10.1016/j.yexcr.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 86.Yang A.D., Camp E.R., Fan F., Shen L., Gray M.J., Liu W. Vascular endothelial growth factor receptor-1 activation mediates epithelial to mesenchymal transition in human pancreatic carcinoma cells. Cancer Research. 2006;66(1):46–51. doi: 10.1158/0008-5472.can-05-3086. [DOI] [PubMed] [Google Scholar]
- 87.Holderfield M.T., Hughes C.C. Crosstalk between vascular endothelial growth factor, notch, and transforming growth factor-beta in vascular morphogenesis. Circ Res. 2008;102(6):637–652. doi: 10.1161/CIRCRESAHA.107.167171. [DOI] [PubMed] [Google Scholar]
- 88.Desai S., Laskar S., Pandey B.N. Autocrine IL-8 and VEGF mediate epithelial-mesenchymal transition and invasiveness via p38/JNK-ATF-2 signalling in A549 lung cancer cells. Cell Signal. 2013;25(9):1780–1791. doi: 10.1016/j.cellsig.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 89.Gonzalez-Moreno O., Lecanda J., Green J.E., Segura V., Catena R., Serrano D. VEGF elicits epithelial-mesenchymal transition (EMT) in prostate intraepithelial neoplasia (PIN)-like cells via an autocrine loop. Exp Cell Res. 2010;316(4):554–567. doi: 10.1016/j.yexcr.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 90.Vega S., Morales A.V., Ocana O.H., Valdes F., Fabregat I., Nieto M.A. Snail blocks the cell cycle and confers resistance to cell death. Genes Dev. 2004;18(10):1131–1143. doi: 10.1101/gad.294104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Emanuele M.J., Ciccia A., Elia A.E., Elledge S.J. Proliferating cell nuclear antigen (PCNA)-associated KIAA0101/PAF15 protein is a cell cycle-regulated anaphase-promoting complex/cyclosome substrate. Proc Natl Acad Sci U S A. 2011;108(24):9845–9850. doi: 10.1073/pnas.1106136108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Blick T., Widodo E., Hugo H., Waltham M., Lenburg M.E., Neve R.M. Epithelial mesenchymal transition traits in human breast cancer cell lines. Clin Exp Metastasis. 2008;25(6):629–642. doi: 10.1007/s10585-008-9170-6. [DOI] [PubMed] [Google Scholar]
- 93.Weidenfeld, K., Schif-Zuck, S., Abu-Tayeh, H., Kang, K., Kessler, O., Weissmann, M., et al. (2016). Dormant tumor cells expressing LOXL2 acquire a stem-like phenotype mediating their transition to proliferative growth. Oncotarget 7(44), 71362-71377. doi: 10.18632/oncotarget.12109. [DOI] [PMC free article] [PubMed]
- 94.Akalay I., Janji B., Hasmim M., Noman M.Z., Andre F., De Cremoux P. Epithelial-to-mesenchymal transition and autophagy induction in breast carcinoma promote escape from T-cell–mediated lysis. Cancer Res. 2013;73(8):2418–2427. doi: 10.1158/0008-5472.CAN-12-2432. [DOI] [PubMed] [Google Scholar]
- 95.Hamilton, D.H., Litzinger, M.T., Jales, A., Huang, B., Fernando, R.I., Hodge, J.W., et al. (2013). Immunological targeting of tumor cells undergoing an epithelial-mesenchymal transition via a recombinant brachyury-yeast vaccine. Oncotarget 4(10), 1777-1790. doi: 10.18632/oncotarget.1295. [DOI] [PMC free article] [PubMed]
- 96.Ricciardi M., Zanotto M., Malpeli G., Bassi G., Perbellini O., Chilosi M. Epithelial-to-mesenchymal transition (EMT) induced by inflammatory priming elicits mesenchymal stromal cell-like immune-modulatory properties in cancer cells. Br J Cancer. 2015;112(6):1067–1075. doi: 10.1038/bjc.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mak M.P., Tong P., Diao L., Cardnell R.J., Gibbons D.L., William W.N. A Patient-derived, pan-cancer EMt signature identifies global molecular alterations and immune target enrichment following epithelial-to-mesenchymal transition. Clin Cancer Res. 2016;22(3):609–620. doi: 10.1158/1078-0432.CCR-15-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lu Y., Dong B., Xu F., Xu Y., Pan J., Song J. CXCL1-LCN2 paracrine axis promotes progression of prostate cancer via the Src activation and epithelial-mesenchymal transition. Cell Commun Signal. 2019;17(1):118. doi: 10.1186/s12964-019-0434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pelizzo G., Veschi V., Mantelli M., Croce S., Di Benedetto V., D'Angelo P. Microenvironment in neuroblastoma: isolation and characterization of tumor-derived mesenchymal stromal cells. BMC Cancer. 2018;18(1):1176. doi: 10.1186/s12885-018-5082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gao D., Vahdat L.T., Wong S., Chang J.C., Mittal V. Microenvironmental regulation of epithelial-mesenchymal transitions in cancer. Cancer Res. 2012;72(19):4883–4889. doi: 10.1158/0008-5472.CAN-12-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Labelle M., Begum S., Hynes R.O. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20(5):576–590. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Techasen A., Loilome W., Namwat N., Dokduang H., Jongthawin J., Yongvanit P. Cytokines released from activated human macrophages induce epithelial mesenchymal transition markers of cholangiocarcinoma cells. Asian Pac J Cancer Prev. 2012;13(Suppl):115–118. [PubMed] [Google Scholar]
- 103.Fischer K.R., Durrans A., Lee S., Sheng J., Li F., Wong S.T. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527(7579):472–476. doi: 10.1038/nature15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zheng X., Carstens J.L., Kim J., Scheible M., Kaye J., Sugimoto H. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527(7579):525–530. doi: 10.1038/nature16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bao Y., Lu Y., Wang X., Feng W., Sun X., Guo H. Eukaryotic translation initiation factor 5A2 (eIF5A2) regulates chemoresistance in colorectal cancer through epithelial mesenchymal transition. Cancer Cell Int. 2015;15:109. doi: 10.1186/s12935-015-0250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hu T., Li Z., Gao C.Y., Cho C.H. Mechanisms of drug resistance in colon cancer and its therapeutic strategies. World J Gastroenterol. 2016;22(30):6876–6889. doi: 10.3748/wjg.v22.i30.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tato-Costa J., Casimiro S., Pacheco T., Pires R., Fernandes A., Alho I. Therapy-induced cellular senescence induces epithelial-to-mesenchymal transition and increases invasiveness in rectal cancer. Clin Colorectal Cancer. 2016;15(2):170–178. doi: 10.1016/j.clcc.2015.09.003. e173. [DOI] [PubMed] [Google Scholar]
- 108.Tsoumas, D., Nikou, S., Giannopoulou, E., Champeris Tsaniras, S., Sirinian, C., Maroulis, I., et al. (2018). ILK expression in colorectal cancer is associated with EMT, cancer stem cell markers and chemoresistance. Cancer Genomics Proteomics 15(2), 127-141. doi: 10.21873/cgp.20071. [DOI] [PMC free article] [PubMed]
- 109.Yang Y., Wang G., Zhu D., Huang Y., Luo Y., Su P. Epithelial-mesenchymal transition and cancer stem cell-like phenotype induced by Twist1 contribute to acquired resistance to irinotecan in colon cancer. Int J Oncol. 2017;51(2):515–524. doi: 10.3892/ijo.2017.4044. [DOI] [PubMed] [Google Scholar]
- 110.Sangaletti S., Tripodo C., Santangelo A., Castioni N., Portararo P., Gulino A. Mesenchymal transition of high-grade breast carcinomas depends on extracellular matrix control of myeloid suppressor cell activity. Cell Reports. 2016;17(1):233–248. doi: 10.1016/j.celrep.2016.08.075. [DOI] [PubMed] [Google Scholar]
- 111.Farmer P., Bonnefoi H., Anderle P., Cameron D., Wirapati P., Becette V. A stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancer. Nat Med. 2009;15(1):68–74. doi: 10.1038/nm.1908. [DOI] [PubMed] [Google Scholar]
- 112.Byers L.A., Diao L., Wang J., Saintigny P., Girard L., Peyton M. An epithelial-mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance. Clin Cancer Res. 2013;19(1):279–290. doi: 10.1158/1078-0432.CCR-12-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gjerdrum C., Tiron C., Hoiby T., Stefansson I., Haugen H., Sandal T. Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. Proc Natl Acad Sci U S A. 2010;107(3):1124–1129. doi: 10.1073/pnas.0909333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Terry S., Savagner P., Ortiz-Cuaran S., Mahjoubi L., Saintigny P., Thiery J.P. New insights into the role of EMT in tumor immune escape. Mol Oncol. 2017;11(7):824–846. doi: 10.1002/1878-0261.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dongre A., Rashidian M., Reinhardt F., Bagnato A., Keckesova Z., Ploegh H.L. Epithelial-to-mesenchymal transition contributes to immunosuppression in breast carcinomas. Cancer Res. 2017;77(15):3982–3989. doi: 10.1158/0008-5472.CAN-16-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ansieau S., Bastid J., Doreau A., Morel A.P., Bouchet B.P., Thomas C. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell. 2008;14(1):79–89. doi: 10.1016/j.ccr.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 117.Liu Y., El-Naggar S., Darling D.S., Higashi Y., Dean D.C. Zeb1 links epithelial-mesenchymal transition and cellular senescence. Development. 2008;135(3):579–588. doi: 10.1242/dev.007047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ohashi S., Natsuizaka M., Wong G.S., Michaylira C.Z., Grugan K.D., Stairs D.B. Epidermal growth factor receptor and mutant p53 expand an esophageal cellular subpopulation capable of epithelial-to-mesenchymal transition through ZEB transcription factors. Cancer Res. 2010;70(10):4174–4184. doi: 10.1158/0008-5472.CAN-09-4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Coppe J.P., Patil C.K., Rodier F., Sun Y., Munoz D.P., Goldstein J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6(12):2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yuan A., Chen J.J., Yao P.L., Yang P.C. The role of interleukin-8 in cancer cells and microenvironment interaction. Front Biosci. 2005;10:853–865. doi: 10.2741/1579. [DOI] [PubMed] [Google Scholar]
- 121.Laberge R.M., Awad P., Campisi J., Desprez P.Y. Epithelial-mesenchymal transition induced by senescent fibroblasts. Cancer Microenviron. 2012;5(1):39–44. doi: 10.1007/s12307-011-0069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lopez-Novoa J.M., Nieto M.A. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med. 2009;1(6-7):303–314. doi: 10.1002/emmm.200900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yan Y., Zhang J., Li J.H., Liu X., Wang J.Z., Qu H.Y. High tumor-associated macrophages infiltration is associated with poor prognosis and may contribute to the phenomenon of epithelial-mesenchymal transition in gastric cancer. Onco Targets Ther. 2016;9:3975–3983. doi: 10.2147/OTT.S103112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hu Y., He M.Y., Zhu L.F., Yang C.C., Zhou M.L., Wang Q. Tumor-associated macrophages correlate with the clinicopathological features and poor outcomes via inducing epithelial to mesenchymal transition in oral squamous cell carcinoma. J Exp Clin Cancer Res. 2016;35:12. doi: 10.1186/s13046-015-0281-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fu X.T., Dai Z., Song K., Zhang Z.J., Zhou Z.J., Zhou S.L. Macrophage-secreted IL-8 induces epithelial-mesenchymal transition in hepatocellular carcinoma cells by activating the JAK2/STAT3/Snail pathway. Int J Oncol. 2015;46(2):587–596. doi: 10.3892/ijo.2014.2761. [DOI] [PubMed] [Google Scholar]
- 126.Deng Y.R., Liu W.B., Lian Z.X., Li X., Hou X. Sorafenib inhibits macrophage-mediated epithelial-mesenchymal transition in hepatocellular carcinoma. Oncotarget. 2016;7(25):38292–38305. doi: 10.18632/oncotarget.9438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fan Q.M., Jing Y.Y., Yu G.F., Kou X.R., Ye F., Gao L. Tumor-associated macrophages promote cancer stem cell-like properties via transforming growth factor-beta1-induced epithelial-mesenchymal transition in hepatocellular carcinoma. Cancer Lett. 2014;352(2):160–168. doi: 10.1016/j.canlet.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 128.Bates R.C., Mercurio A.M. The epithelial-mesenchymal transition (EMT) and colorectal cancer progression. Cancer Biol Ther. 2005;4(4):365–370. doi: 10.4161/cbt.4.4.1655. [DOI] [PubMed] [Google Scholar]
- 129.Long X., Ye Y., Zhang L., Liu P., Yu W., Wei F. IL-8, a novel messenger to cross-link inflammation and tumor EMT via autocrine and paracrine pathways (Review) Int J Oncol. 2016;48(1):5–12. doi: 10.3892/ijo.2015.3234. [DOI] [PubMed] [Google Scholar]
- 130.Cohen E.N., Gao H., Anfossi S., Mego M., Reddy N.G., Debeb B. Inflammation mediated metastasis: immune induced epithelial-to-mesenchymal transition in inflammatory breast cancer cells. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0132710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nieto M.A., Huang Ruby Y.-J., Jackson Rebecca A., Thiery Jean P. EMT: 2016. Cell. 2016;166(1):21–45. doi: 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 132.Thiery J.P. Epithelial-mesenchymal transitions in tumour progression. Nature Reviews Cancer. 2002;2(6):442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 133.Chao Y., Wu Q., Acquafondata M., Dhir R., Wells A. Partial mesenchymal to epithelial reverting transition in breast and prostate cancer metastases. Cancer Microenviron. 2012;5(1):19–28. doi: 10.1007/s12307-011-0085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pei D., Shu X., Gassama-Diagne A., Thiery J.P. Mesenchymal-epithelial transition in development and reprogramming. Nat Cell Biol. 2019;21(1):44–53. doi: 10.1038/s41556-018-0195-z. [DOI] [PubMed] [Google Scholar]
- 135.Yao D., Dai C., Peng S. Mechanism of the mesenchymal-epithelial transition and its relationship with metastatic tumor formation. Mol Cancer Res. 2011;9(12):1608–1620. doi: 10.1158/1541-7786.MCR-10-0568. [DOI] [PubMed] [Google Scholar]
- 136.Chung V.Y., Tan T.Z., Tan M., Wong M.K., Kuay K.T., Yang Z. GRHL2-miR-200-ZEB1 maintains the epithelial status of ovarian cancer through transcriptional regulation and histone modification. Sci Rep. 2016;6:19943. doi: 10.1038/srep19943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Frisch S.M., Farris J.C., Pifer P.M. Roles of Grainyhead-like transcription factors in cancer. Oncogene. 2017;36(44):6067–6073. doi: 10.1038/onc.2017.178. [DOI] [PubMed] [Google Scholar]
- 138.Roca H., Hernandez J., Weidner S., McEachin R.C., Fuller D., Sud S. Transcription factors OVOL1 and OVOL2 induce the mesenchymal to epithelial transition in human cancer. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0076773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chung V.Y., Tan T.Z., Ye J., Huang R.L., Lai H.C., Kappei D. The role of GRHL2 and epigenetic remodeling in epithelial-mesenchymal plasticity in ovarian cancer cells. Commun Biol. 2019;2:272. doi: 10.1038/s42003-019-0506-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jolly M.K., Ware K.E., Xu S., Gilja S., Shetler S., Yang Y. E-cadherin represses anchorage-independent growth in sarcomas through both signaling and mechanical mechanisms. Mol Cancer Res. 2019;17(6):1391–1402. doi: 10.1158/1541-7786.MCR-18-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Qi X.K., Han H.Q., Zhang H.J., Xu M., Li L., Chen L. OVOL2 links stemness and metastasis via fine-tuning epithelial-mesenchymal transition in nasopharyngeal carcinoma. Theranostics. 2018;8(8):2202–2216. doi: 10.7150/thno.24003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Somarelli J.A., Shetler S., Jolly M.K., Wang X., Bartholf Dewitt S., Hish A.J. Mesenchymal-epithelial transition in sarcomas is controlled by the combinatorial expression of microRNA 200s and GRHL2. Mol Cell Biol. 2016;36(19):2503–2513. doi: 10.1128/MCB.00373-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shibue T., Weinberg R.A. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nature Reviews Clinical Oncology. 2017;14(10):611–629. doi: 10.1038/nrclinonc.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kudo-Saito C., Shirako H., Takeuchi T., Kawakami Y. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell. 2009;15(3):195–206. doi: 10.1016/j.ccr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 145.Thiery J.P., Sleeman J.P. Complex networks orchestrate epithelial–mesenchymal transitions. Nature Reviews Molecular Cell Biology. 2006;7(2):131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 146.dos Anjos Pultz B., Andrés Cordero da Luz F., Socorro Faria S., Peixoto Ferreira de Souza L., Cristina Brígido Tavares P., Alonso Goulart V. The multifaceted role of extracellular vesicles in metastasis: priming the soil for seeding. International Journal of Cancer. 2017;140(11):2397–2407. doi: 10.1002/ijc.30595. [DOI] [PubMed] [Google Scholar]
- 147.Kim J., Kim T.Y., Lee M.S., Mun J.Y., Ihm C., Kim S.A. Exosome cargo reflects TGF-β1-mediated epithelial-to-mesenchymal transition (EMT) status in A549 human lung adenocarcinoma cells. Biochemical and Biophysical Research Communications. 2016;478(2):643–648. doi: 10.1016/j.bbrc.2016.07.124. [DOI] [PubMed] [Google Scholar]
- 148.Wang, Z., von Au, A., Schnölzer, M., Hackert, T., and Zöller, M. (2016). CD44v6-competent tumor exosomes promote motility, invasion and cancer-initiating cell marker expression in pancreatic and colorectal cancer cells. Oncotarget 7(34). doi: 10.18632/oncotarget.10580. [DOI] [PMC free article] [PubMed]
- 149.Tian Y., Li S., Song J., Ji T., Zhu M., Anderson G.J. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35(7):2383–2390. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]