Abstract
Objective
Roux-en-Y gastric bypass surgery (RYGB) can achieve long-term remission of type 2 diabetes. However, the specific molecular mechanism through which this occurs has remained largely elusive. Bile acid signaling through the nuclear hormone receptor farnesoid X receptor (FXR) exerts beneficial effects after sleeve gastrectomy (VSG), which has similar effects to RYGB. Therefore, we investigated whether FXR signaling is necessary to mediate glycemic control after RYGB.
Methods
RYGB or sham surgery was performed in high-fat diet-induced obese FXR−/− (knockout) and FXR+/+ (wild type) littermates. Sham-operated mice were fed ad libitum (S-AL) or by weight matching (S-WM) to RYGB mice via caloric restriction. Body weight, body composition, food intake, energy expenditure, glucose tolerance tests, insulin tolerance tests, and homeostatic model assessment of insulin resistance were performed.
Results
RYGB surgery decreases body weight and fat mass in WT and FXR-KO mice. RYGB surgery has similar effects on food intake and energy expenditure independent of genotype. In addition, body weight-independent improvements in glucose control were attenuated in FXR −/− relative to FXR +/+ mice after RYGB. Furthermore, pharmacologic blockade of the glucagon-like peptide-1 receptor (GLP-1R) blunts the glucoregulatory effects of RYGB in FXR +/+ but not in FXR −/− mice at 4 weeks after surgery.
Conclusions
These results suggest that FXR signaling is not required for the weight loss up to 16 weeks after RYGB. Although most of the improvements in glucose homeostasis are secondary to RYGB-induced weight loss in wild type mice, FXR signaling contributes to glycemic control after RYGB in a body weight-independent manner, which might be mediated by an FXR-GLP-1 axis during the early postoperative period.
Keywords: Roux-en-Y gastric Bypass, Bariatric surgery, Type 2 diabetes mellitus, Glucose homeostasis, Farnesoid X receptor
Highlights
-
•
The reduction in body weight after RYGB is independent of FXR, which is mainly due to a decrease in net energy intake.
-
•
RYGB prevents the weight loss-induced decrease observed in nonsurgical weight-matched mice in both genotypes.
-
•
FXR signaling contributes to glycemic control after RYGB in a body weight-independent manner.
-
•
The early body weight-independent improvements in glucose homeostasis after RYGB might be mediated by an FXR-GLP-1 axis.
1. Introduction
The increasing worldwide prevalence of type 2 diabetes mellitus (T2DM) has become one of the greatest threats to human health and is related to rising numbers of obese individuals [1,2]. Currently, bariatric surgery is recommended as the most effective procedure for obese patients with T2DM [3]. The most common bariatric surgeries performed globally are Roux-en-Y gastric bypass (RYGB) and vertical sleeve gastrectomy (VSG) [4]. While alterations in gut hormones, circulation of bile acids, and gut microbiota have been proposed as potential mechanisms, the specific molecular mechanisms governing the beneficial effects of bariatric surgery have remained largely elusive.
Bile acids are a family of steroid molecules that can be synthesized from cholesterol and conjugated to taurine or glycine in the liver, secreted into the bile, and discharged into the duodenum after a meal. Approximately 95% of bile acids are reabsorbed in the terminal ileum and colon and then recirculated to the liver by portal vein blood [5]. A growing body of evidence suggests that an increase in circulating levels of bile acids is observed after bariatric surgery and that this strongly correlates with positive metabolic effects [[6], [7], [8], [9], [10]]. Bile acids have recently been recognized as versatile signaling molecules that can modulate G-protein-coupled receptors (GPCRs), such as TGR5, and several nuclear hormone receptors, including the farnesoid X receptor (FXR). Bile acids regulate metabolic improvement and glucose homeostasis via activation of these signaling pathways [5,11,12]. There are three studies [[13], [14], [15]] that performed bariatric surgery (2 VSG and 1 RYGB) in TGR5-deficient mice, and the outcomes of these studies in terms of weight loss and glucose homeostasis are contradictory. While Ding et al. reported that TGR5-signaling is required for VSG-induced weight loss [13], McGavigan et al. and Hao et al. found that it is not required [14]. Furthermore, although TGR5 contributes to the glucoregulatory benefits of VSG surgery [13,14], similar RYGB-induced improvement of glycemic control in both wild type (WT) and TGR5-deficient mice was reported [15]. Therefore the mechanisms by which bariatric surgeries produce metabolic improvements are likely complex. Moreover, FXR was recently identified as a potential molecular target of VSG-induced weight loss and improved glucose tolerance [16]. However, there is a lack of information focusing on the role of FXR signaling in RYGB-induced beneficial effects in mice. Based on these facts, we speculate that FXR may be an effector of the metabolic improvement observed after the RYGB procedure. In addition, although previous studies revealed that enhanced postprandial glucagon-like peptide-1 (GLP-1) secretion was related to improved glucose tolerance after bariatric surgeries [17,18], mice with genetic deficiency of the GLP-1 receptor (GLP-1R) respond normally to VSG [19] and RYGB [20,21] in terms of improvements in glucose regulation. Therefore the contribution of GLP-1 signaling following bariatric surgery remains to be fully elucidated.
In our study, we performed RYGB surgery in high-fat diet (HFD)-induced FXR knockout mice and their WT littermates to explore whether FXR signaling participates in the post-operative regulation of weight loss, energy expenditure, and glucose control. In addition, pharmacologic blockade of GLP-1R was performed before oral glucose tolerance tests (OGTTs) in animal models to explore the effect of GLP-1-signaling on postprandial glucose excursion after RYGB.
2. Methods
2.1. Animals and diets
Heterozygous FXR+/− breeding pairs generated on a C57BL/6J background (B6/JGpt-Nr1h4em1Cd/Gpt) were obtained from GemPharmatech Co., Ltd. (Nanjing, China) and a breeding colony was established. Another group of 4–6-week-old C57BL/6J male mice served as WT controls and were purchased from SLAC Laboratory Animal Co., Ltd. (Shanghai, China). All mice were housed in a specific pathogen-free environment. All procedures involving animals were conducted according to Shanghai Jiaotong University guidelines, with the approval of Shanghai Jiaotong University Animal Care and Utilization Committee.
At approximately 6 weeks of age, genotyped male FXR−/− (KO) and FXR+/+ (WT) mice were placed on an HFD (kcal%: carb, 20; fat, 60; prot, 20, Diet D12492, Research Diets, New Brunswick, NJ) for 10 weeks before surgery. RYGB, or sham surgery, was performed in HFD-induced obese FXR KO and WT littermates. Sham-operated mice were fed ad libitum (S-AL) or by weight matching (S-WM) to RYGB mice via caloric restriction. S-WM mice were included to assess the body weight-independent effects of RYGB. Mice of each genotype were stratified into three groups. The final group numbers were 9 S-AL WT, 8 RYGB WT, 10 S-WM WT, 10 S-AL KO, 9 RYGB KO, and 10 S-WM KO. All animals were kept in climate-controlled rooms at 22 ± 2 °C with a 12/12 h light/dark cycle (lights on from 07:00 to 19:00) except for the metabolic chambers.
2.2. RYGB, sham surgery, and weight matching
The surgical protocol used was described previously [22]. The main procedure involved making a small gastric pouch with about 5% of the total gastric volume and leaving the Roux and biliopancreatic limb both 5–6 cm long (the average length of the small intestine was 22 ± 2 cm). The jejunum was transected, and the cut ends were sterilized with 5% povidone iodine. The distal end of the jejunum was then anastomosed with the stomach pouch in an end-to-end manner, and the jejuno-jejunostomy was accomplished with an end-to-side anastomosis. All anastomoses were made by interrupted stitches with 11-0 nylon sutures. In the sham group, mice underwent laparotomy without stomach and jejunum transection. A simple continuous suture was used on the anterior wall of the stomach.
All mice were fasted for 24 h with free access to tap water and then were fed a liquid diet ad libitum (10% Ensure, Abbott Laboratories, Columbus, USA) for 2 days after surgery. After 3 days, the mice were returned to a 60% HFD until the end of the experiment. Mice weight-matched to the RYGB group were restricted to approximately 50%–60% of the food intake of the RYGB group once per day in the light.
2.3. Measurement of body weight, body composition, and food intake
Body weight and food intake were measured twice a week in RYGB and S-AL groups, and daily rations of food for mice of the S-WM group were recorded. Total food intake (in kcal) was determined from the intake of the HFD (5.24 kcal/g), taking spillage into account. Body fat content was measured before the experiment began and every 4 weeks thereafter with a nuclear magnetic resonance minispec (LF 90 NMR Analyzer; Bruker Optics, Billerica, MA, USA).
2.4. Measurement of energy expenditure
Mice of each group were individually placed in an Oxymax indirect calorimeter 13 weeks after surgery (Columbus Instruments, OH, USA). All mice were acclimatized for 24 h prior to measurements. Carbon dioxide output (VCO2) and oxygen uptake (VO2) were recorded during the day and night. Mice were maintained at 25 °C for 2 days under a 12 h light/dark cycle with free access to food and water. Energy expenditure in kcal was calculated based on VO2 and VCO2 by company-supplied software.
2.5. Oral glucose and insulin tolerance tests
All mice were fasted for 6 h with free access to water the morning before OGTTs and insulin tolerance tests (ITTs). Blood glucose was measured through the tail vein using the OneTouch UltraEasy Blood Glucose Monitoring System (Lifescan, Milpitas, CA, USA) 0, 15, 30, 60, and 120 min after an oral gavage of d-glucose (1.0 g/kg) at postoperative weeks 4 and 14. Blood glucose was measured 0, 15, 30, 60, and 120 min after intraperitoneal injection with human insulin (0.8 U/kg Humulin R; Novo Nordisk) at postoperative week 15. In additional experiments, fasted mice were intraperitoneally injected with sterile saline (0.9% NaCl) with or without the GLP-1R antagonist Ex-9 (50 μg; Tocris Biosciences, Bristol, UK) 120 min before the OGTT in each group at postoperative weeks 4 and 14.
2.6. Glucose-stimulated insulin and GLP-1 secretion
For the insulin secretion test, mice were fasted overnight and then gavaged with d-glucose (1.5 g/kg). Blood was collected (40 μL) by retro-orbital venipuncture under isoflurane anesthesia at postoperative weeks 4 and 14. Next, serum insulin levels were measured using an enzyme-linked immunosorbent assay (ELISA) kit (Shanghai Enzyme-linked Biotechnology Co., Shanghai, China). Plasma GLP-1 was measured after an overnight fast and 0, 15, and 30 min after an oral gavage of d-glucose (1.0 g/kg) at postoperative weeks 4 and 14. Blood was collected (80 μL) by retro-orbital venipuncture under isoflurane anesthesia at the indicated times and treated with EDTA and a protease inhibitor cocktail (DPP-IV inhibitor, protease inhibitor, and Pefabloc SC). Plasma was collected by centrifugation, and GLP-1 levels were assessed using ELISA kits (Millipore Corporation, Billerica, MA, USA) according to the manufacturer's instructions.
2.7. Plasma glucose and hormone assays
All mice were euthanized 16 weeks following surgery after an overnight fast. A few drops of trunk blood were collected, and blood glucose was immediately tested by a glucometer, as mentioned above. An additional 500 μL of trunk blood was collected and treated with EDTA and a protease inhibitor cocktail (DPP-IV inhibitor, Protease inhibitor, and Prefabloc SC). The plasma was then separated by centrifugation (3000 rpm) at 4 °C for 15 min. Plasma aliquots were frozen in liquid nitrogen before storage at −80 °C for further analysis. Serum insulin levels were determined using ELISA kits (Shanghai Enzyme-linked Biotechnology Co., Shanghai, China). The homeostasis model assessment ratio (HOMA-R) was used to evaluate insulin resistance. Formula: HOMA-R = fasting glucose (mmol/L) × fasting insulin (μU/mL)/22.5.
2.8. Statistics and data analysis
We performed statistical analysis using GraphPad Prism software version 7.02 (San Diego, CA). Differences in initial body weight and blood glucose levels between WT and KO mice were analyzed with Student's t-tests. Differences in body fat mass and adiposity index changes between S-WM and RYGB mice were analyzed with Student's t-tests. Total energy expenditure was analyzed with an adjusted ANCOVA using body weight and lean mass as covariates. Body weight, body weight change, fat mass, adiposity index, food intake, feeding efficiency, glucose and insulin tolerance area under the curve (AUC), fasting plasma assays, and HOMA-IR were analyzed with a two-way ANOVA using the treatment group and genotype as between-subject variables, followed by Tukey's post-hoc test. Where appropriate, time was used as within-subjects repeated measure ANOVA. Data are presented as the mean ± SEM. Statistical significance was defined as P < 0.05.
3. Results
3.1. Mortality and complications
One of the S-AL WT mice exhibited a lack of appetite and a steep decline in body weight and was euthanized 3 weeks after surgery. Also, one of the FXR-KO mice developed moderate jaundice and was euthanized 4 weeks after the RYGB procedure. Their autopsy reports were inconclusive. In addition, two WT mice died within the first week after RYGB surgery. The autopsy showed the obstruction of the alimentary limb at the level of the jejunojejunostomy and anastomotic leak of the jejunojejunum end-to-side anastomosis. None of these four mice were included in the analyses. The rest of the mice appeared completely healthy and required no special diets throughout the study.
3.2. Baseline measurements in WT and FXR-KO mice
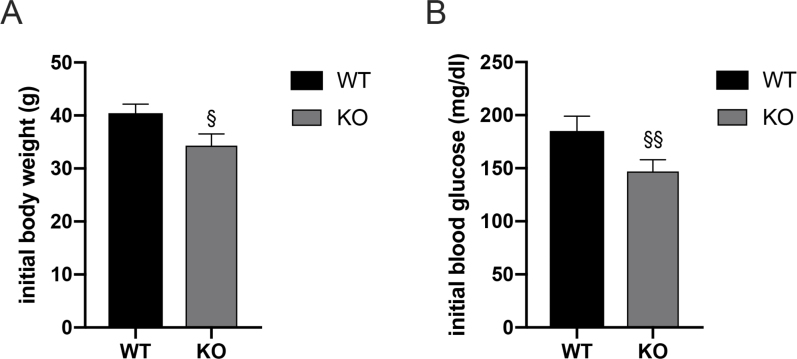
FXR-deficiency has previously been shown to attenuate weight gain and improve glucose homeostasis [23]. Our results are consistent with these observations (Supplementary Figure S1A-B). We maintained all mice on a 60% HFD for 10 weeks prior to surgery. This approach ensured that their body weights were more than 30 g and that the fasting blood glucose levels were more than 120 mg/dl prior to surgery in both WT and FXR KO mice.
3.3. RYGB surgery decreases body weight and fat mass in WT and FXR-KO mice
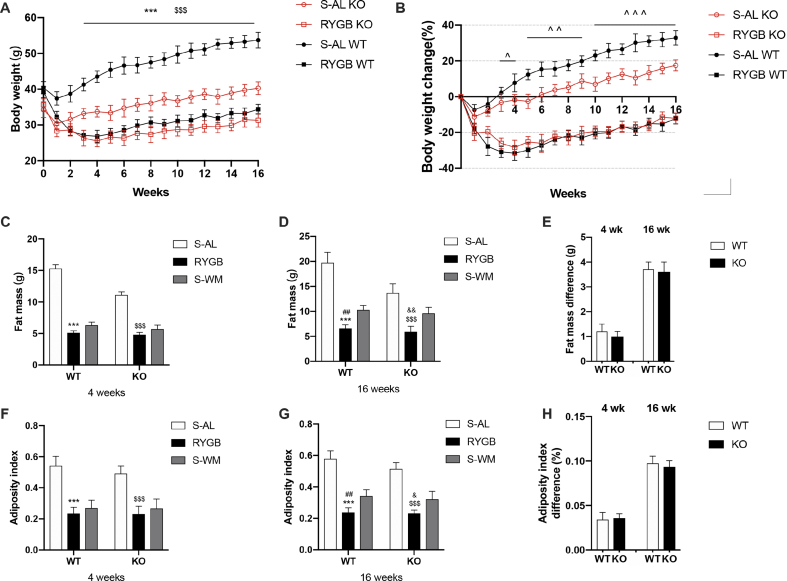
WT and KO mice displayed rapid weight loss in the first 4 weeks after RYGB surgery, while the respective sham-operated controls demonstrated only a small and transient loss in week one (Figure 1A). There was a difference in body weight among the groups from the third week after surgery. Compared with sham-operated controls, RYGB led to more weight loss from week 3 to week 16 in both genotypes, and the body weight change relative to preoperative body weight did not differ between WT and KO mice (Figure 1A–B). Different from our observations in RYGB groups, sham-operated WT mice exhibited substantial weight gain compared with KO mice (Figure 1A–B). These data suggest that although FXR-deficient mice are obesity-resistant, RYGB results in the maintenance of weight loss independent of genotype. Because a nadir of body weight occurred 4 weeks after RYGB, we analyzed the body fat and adiposity index of each group at weeks 4 and 16. Compared with the S-AL controls, the fat mass and adiposity index of RYGB mice were significantly reduced at 4 and 16 weeks in both genotypes, while the differences between S-WM and RYGB mice were only observed at postoperative week 16 (Figure 1C-D-F-G). These results suggest that RYGB significantly reduces fat mass compared with both S-AL and S-WM controls in both genotypes. The fat mass and adiposity index did not differ between RYGB mice genotypes (Figure 1C-D-F-G) or between S-WM and RYGB groups in both genotypes (Figure 1E–H). Taken together, these results suggest that FXR signaling is not required to maintain the reduction in fat mass and adiposity index achieved by RYGB.
Figure 1.
Effect of RYGB, S-AL, or S-WM on body weight, fat mass, and adiposity index in FXR−/− (KO) and wild-type (WT) mice. (A) Body weight of mice after surgery. (B) Body weight change in mice after surgery. (C) Body fat mass 4 weeks after surgery. (D) Body fat mass 16 weeks after surgery. (E) Fat mass difference between RYGB and S-WM at 4 and 16 weeks after surgery in both genotypes. (F) Adiposity index 4 weeks after surgery. (G) Adiposity index 16 weeks after surgery. (H) Adiposity index difference between RYGB and S-WM at 4 and 16 weeks after surgery in both genotypes. ∗ = P < 0.05, ∗∗ = P < 0.01, ∗∗∗ = P < 0.001 S-AL vs. RYGB in WT mice, ˆ = P < 0.05, ˆ ˆ = P < 0.01, ˆ ˆ ˆ = P < 0.001 WT vs. KO in S-AL mice, # = P < 0.05, ## = P < 0.01 S-WM vs. RYGB in WT mice, $ = P < 0.05, $$ = P < 0.01 S-AL vs. RYGB in KO mice, & = P < 0.05, && = P < 0.01 S-WM vs. RYGB in KO mice by Student's t-tests (E and H) and two-way ANOVA with Tukey's post hoc test (A, B, C, D, F, and G). Data are shown as mean ± SEM (n = 8–10 per group). S-AL: sham-operated animals fed ad libitum; S-WM: sham-operated animals weight matched to RYGB-operated mice; RYGB: Roux-en-Y gastric bypass surgery; KO, knockout; WT, wild type.
3.4. RYGB surgery has similar effects on food intake and energy expenditure in WT and FXR-KO mice
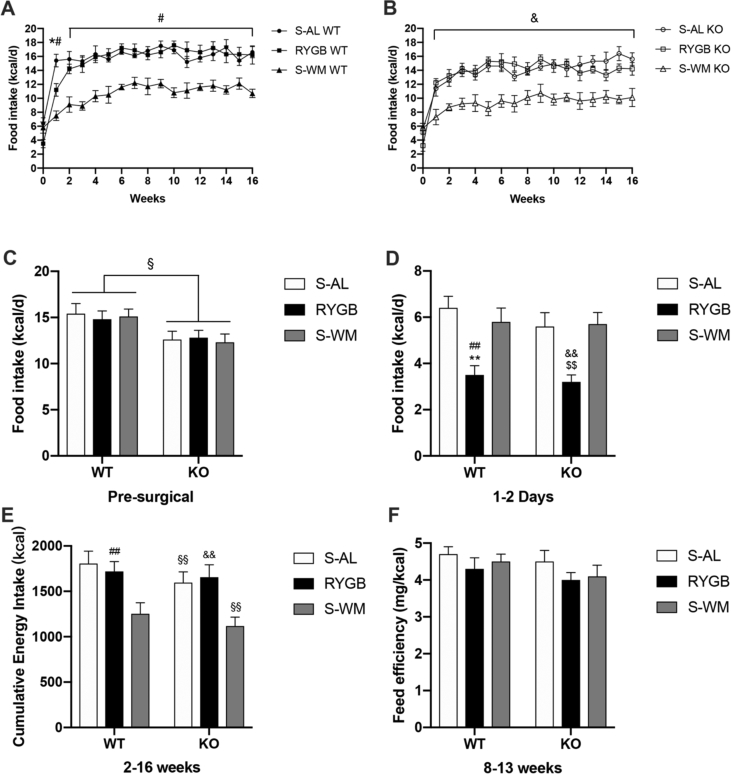
As a result of the difference in preoperative body weight, food intake was generally slightly lower in FXR−/− compared with WT mice before surgery (Figure 2C). Food intake was suppressed in all groups within the first week and recovered 2–3 weeks after surgery. Because all mice were fed 10% Ensure ad libitum within 2 days after the operation, food intake was significantly lower in RYGB compared with sham mice of both genotypes (Figure 2D). RYGB food intake during the first week appeared to be significantly lower in WT but not in KO mice compared with corresponding S-AL groups. After the first week, food intake after RYGB approached S-AL levels in both genotypes. The amount of food weight-matched mice needed to maintain body weight was significantly less than that required by the respective RYGB groups in WT and KO mice (Figure 2A–B).
Figure 2.
Effect of RYGB, S-AL, or S-WM on food intake and feed efficiency in FXR−/− (KO) and wild-type (WT) mice. (A) Food intake in WT mice. (B) Food intake in KO mice. (C) Presurgical food intake. (D) Ensure intake during the first 2 days. (E) Cumulative energy intake between 2 and 16 weeks. (D) Feed efficiency between 8 and 13 weeks in both WT and KO mice. ∗ = P < 0.05, ∗∗ = P < 0.01 S-AL vs. RYGB in WT mice, # = P < 0.05, ## = P < 0.01 S-WM vs. RYGB in WT mice, § = P < 0.05, §§ = P < 0.01 genotype comparison of mice with the same treatment conditions, $ = P < 0.05, $$ = P < 0.01 S-AL vs. RYGB in KO mice, & = P < 0.05, && = P < 0.01 S-WM vs. RYGB in KO mice by two-way ANOVA with Tukey's post hoc test. Data are shown as mean ± SEM (n = 8–10 per group). S-AL: sham-operated animals fed ad libitum; S-WM: sham-operated animals weight matched to RYGB-operated mice; RYGB: Roux-en-Y gastric bypass surgery; KO, knockout; WT, wild type.
Interestingly, although RYGB mice could achieve substantial weight loss and fat mass reduction compared with S-AL mice, cumulative food intake was similar in S-AL and RYGB groups of both genotypes (Figure 2E). Furthermore, the cumulative energy intake was significantly reduced in KO–S-AL mice compared with WT-S-AL groups. The main reason for this is that KO and WT mice had different weights during our experiment, which could have lead to differences in the amount of food required to maintain body weight. To eliminate this difference, we used feed efficiency (defined as weight gain per kcal consumed) to measure the relationship between body weight and energy intake. Our results confirmed that there was no significant difference in feed efficiency between WT and KO mice within the three different treatment groups when their weight gain had plateaued (weeks 8–13, Figure 2F). This suggests that FXR is not required for the changes in food intake after RYGB surgery.
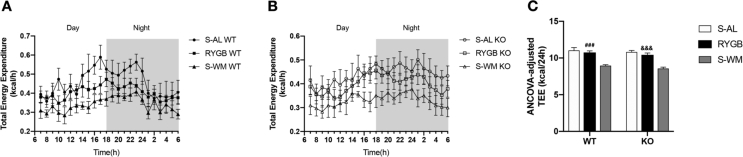
Because S-AL mice have a much higher body weight and adiposity than mice of the S-WM and RYGB groups, their total energy expenditure might differ. Therefore we analyzed energy expenditure by an adjusted ANCOVA using body weight and lean mass as covariates (Figure 3). RYGB mice had similar energy expenditure compared to S-AL mice, but significantly higher energy expenditure compared with S-WM mice, and this effect was similar in both genotypes (Figure 3C). This suggests that FXR does not contribute to RYGB-induced relative increases in energy expenditure.
Figure 3.
Effect of RYGB, S-AL, or S-WM on energy expenditure in FXR−/− (KO) and wild-type (WT) mice 13 weeks after surgery in metabolic chambers. (A) TEE per hour in WT mice. (B) TEE per hour in KO mice. (C) ANCOVA-adjusted TEE using body weight and lean mass as covariates in both genotypes. ### = P < 0.001 S-WM vs. RYGB in WT mice, &&& = P < 0.001 S-WM vs. RYGB in KO mice by two-way ANOVA with Tukey's post hoc test. Data are shown as mean ± SEM (n = 8–10 per group). S-AL: sham-operated animals fed ad libitum; S-WM: sham-operated animals weight matched to RYGB-operated mice; RYGB: Roux-en-Y gastric bypass surgery; KO, knockout; WT, wild type. TEE: total energy expenditure.
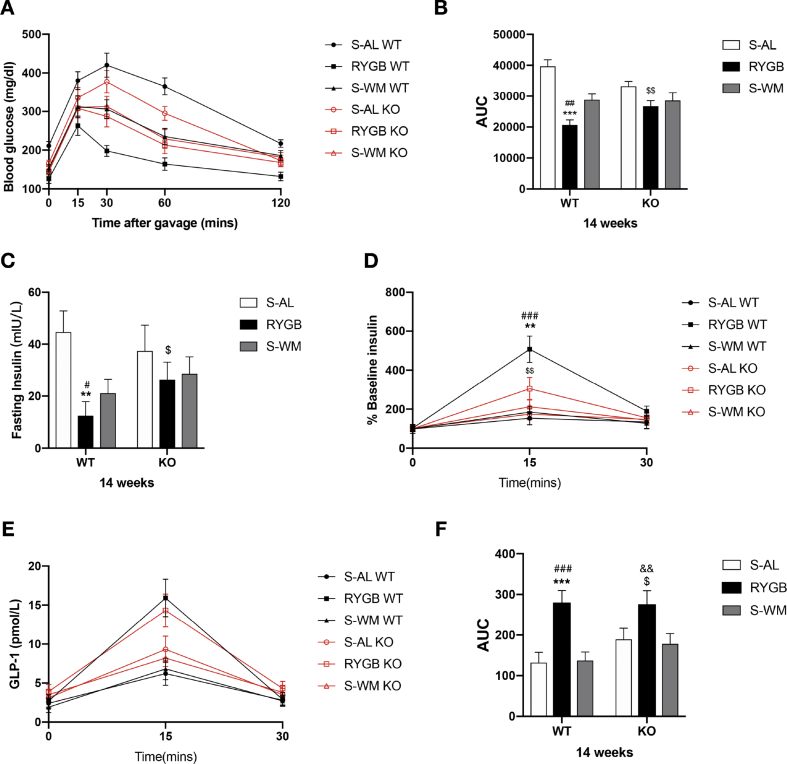
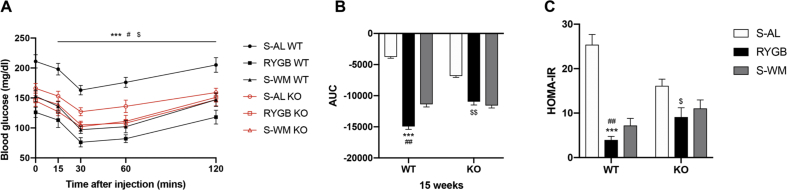
3.5. FXR signaling contributes to improvements in glycemic control after RYGB
To investigate the role of FXR signaling in glycemic control after RYGB surgery, OGTTs, ITTs, and fasting and postprandial insulin levels were analyzed in all surgical cohorts.
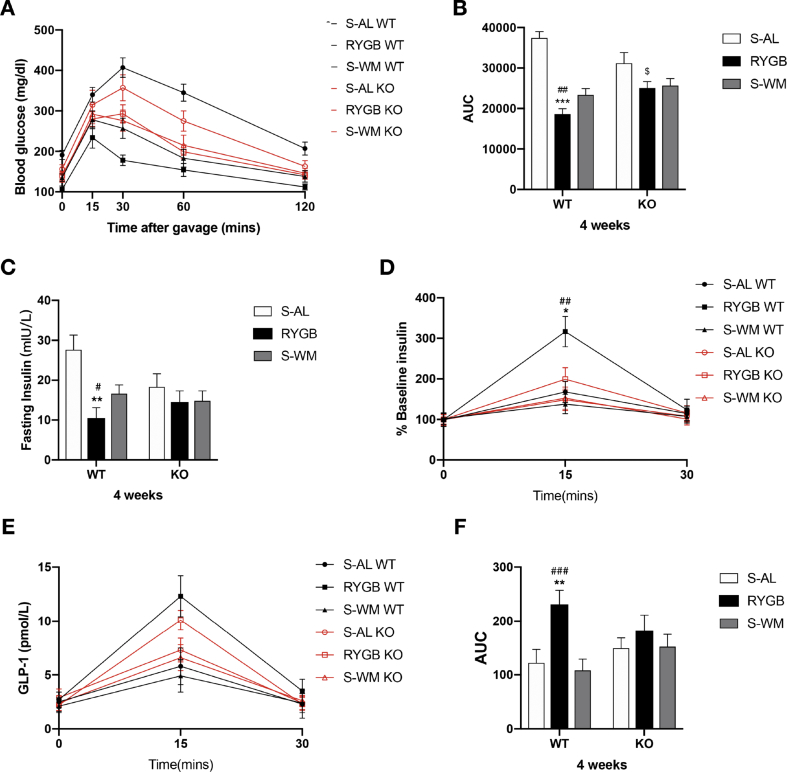
Fasting blood glucose concentrations were lower in FXR-deficient mice prior to RYGB surgery (Supplementary Figure S1 B). Insulin and glucose tolerance were significantly improved in WT-RYGB compared with their respective S-AL and S-WM groups, but were similarly reduced in both RYGB and S-WM relative to S-AL in FXR knockout mice (Figure 4, Figure 5, Figure 6A–B). In addition, compared with their respective S-WM groups, HOMA-IR was significantly improved in RYGB mice with a WT genetic background, but not in mice with an FXR knockout genetic background (Figure 6C). This suggests that RYGB-induced body weight-independent improvements in glucose control are impaired in the absence of FXR signaling.
Figure 4.
Effect of RYGB, S-AL, or S-WM on oral glucose tolerance tests and glucose-stimulated insulin and GLP-1 secretion in FXR−/− (KO) and wild-type (WT) mice at 4 weeks after surgery. Oral glucose tolerance test (A), glucose AUC 0–120min (B), fasting insulin (C), the ratio of secreted insulin compared with the baseline (% baseline insulin) (D), glucose-stimulated GLP-1 secretion (E) and GLP-1 AUC 0–30min (F). ∗ = P < 0.05, ∗∗ = P < 0.01, ∗∗∗ = P < 0.001 S-AL vs. RYGB in WT mice, # = P < 0.05, ## = P < 0.01, ### = P < 0.001 S-WM vs. RYGB in WT mice, $ = P < 0.05, $$ = P < 0.01 S-AL vs. RYGB in KO mice by two-way ANOVA with Tukey's post hoc test. Data are shown as mean ± SEM (n = 8–10 per group). S-AL: sham-operated animals fed ad libitum; S-WM: sham-operated animals weight matched to RYGB-operated mice; RYGB: Roux-en-Y gastric bypass surgery; KO, knockout; WT, wild type. AUC: area under curve.
Figure 5.
Effect of RYGB, S-AL, or S-WM on oral glucose tolerance tests and glucose-stimulated insulin and GLP-1 secretion in FXR−/− (KO) and wildtype (WT) mice at 14 weeks after surgery. Oral glucose tolerance test (A), glucose AUC 0–120min (B), fasting insulin (C), the ratio of secreted insulin compared with the baseline (% baseline insulin) (D), glucose-stimulated GLP-1 secretion (E) and GLP-1 AUC 0–30min (F). ∗ = P < 0.05, ∗∗ = P < 0.01, ∗∗∗ = P < 0.001 S-AL vs. RYGB in WT mice, # = P < 0.05, ## = P < 0.01, ### = P < 0.001 S-WM vs. RYGB in WT mice, $ = P < 0.05, $$ = P < 0.01 S-AL vs. RYGB in KO mice, && = P < 0.01 S-WM vs. RYGB in KO mice by two-way ANOVA with Tukey's post hoc test. Data are shown as mean ± SEM (n = 8–10 per group). S-AL: sham-operated animals fed ad libitum; S-WM: sham-operated animals weight matched to RYGB-operated mice; RYGB: Roux-en-Y gastric bypass surgery; KO, knockout; WT, wild type. AUC: area under curve.
Figure 6.
Effect of RYGB, S-AL, or S-WM on insulin tolerance tests and insulin sensitivity in FXR−/− (KO) and wildtype (WT) mice after surgery. Insulin tolerance test at 15 weeks after surgery (A). Glucose AUC 0–120min at 15 weeks after surgery (B). HOMA-IR (C) at 16 weeks after surgery. ∗ = P < 0.05, ∗∗ = P < 0.01, ∗∗∗ = P < 0.001 S-AL vs. RYGB in WT mice, # = P < 0.05, ## = P < 0.01 S-WM vs. RYGB in WT mice, $ = P < 0.05, $$ = P < 0.01 S-AL vs. RYGB in KO mice by two-way ANOVA with Tukey's post hoc test. Data are shown as mean ± SEM (n = 8–10 per group). S-AL: sham-operated animals fed ad libitum; S-WM: sham-operated animals weight matched to RYGB-operated mice; RYGB: Roux-en-Y gastric bypass surgery; KO, knockout; WT, wild type. AUC: area under curve. HOMA-IR: the homeostasis model assessment ratio.
Fasting plasma insulin decreased significantly in WT-RYGB compared with their respective S-AL and S-WM groups at 4 and 14 weeks after surgery. In contrast, fasting insulin level did not differ among FXR KO groups at 4 weeks and was only significantly lower in KO-RYGB relative to KO–S-AL at 14 weeks after surgery (Figure 4, Figure 5C). This suggests that the body weight-independent reductions in fasting plasma insulin after RYGB appear to rely, in part, on FXR signaling.
The percent increase in plasma insulin concentrations from fasting to peak values increased significantly in RYGB-operated animals relative to their corresponding S-AL and S-WM controls at postoperative weeks 4 and 14. Although a remarkable increase was observed in KO-RYGB compared with KO–S-AL mice at 14 weeks, the percentage of peak insulin values did not significantly differ between KO-RYGB and KO–S-WM postoperatively (Figure 4, Figure 5D). This suggests that the increase in glucose-stimulated insulin secretion achieved by the RYGB procedure was partially via FXR signaling.
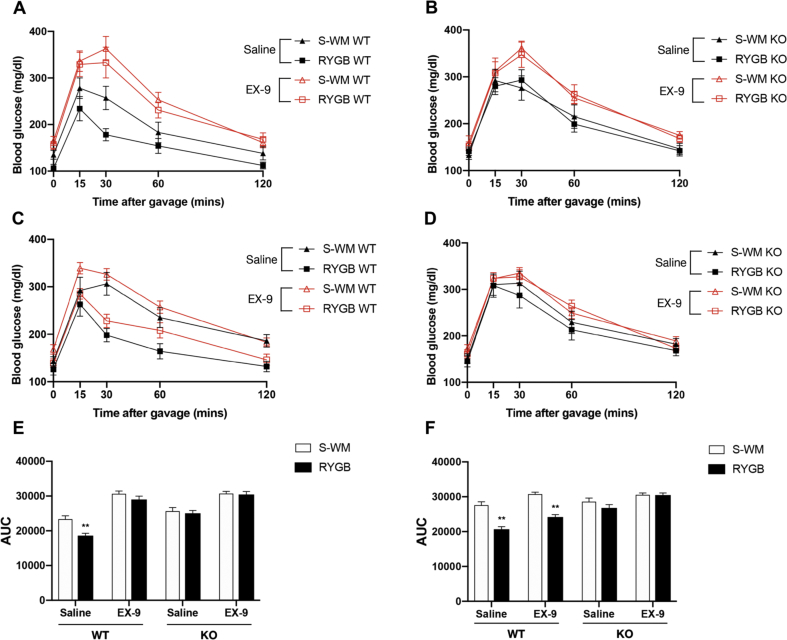
3.6. GLP-1R blockade blunts the glucoregulatory benefits of RYGB in WT but not in FXR KO mice at 4 weeks after surgery
To investigate the role of GLP-1 signaling in the glucoregulatory benefits after RYGB surgery, we next assessed glucose-stimulated GLP-1 secretion and glucose tolerance after Ex-9 pretreatment in experimental cohorts.
Compared with their respective sham controls, the glucose-stimulated GLP-1 secretion was significantly increased in RYGB mice, and the GLP-1 AUC did not differ between WT S-AL and S-WM mice. However, the enhancement of GLP-1 secretion in RYGB-operated mice relative to their corresponding sham controls was blunted in FXR KO groups at 4 weeks but not at 14 weeks post-surgery (Figure 4, Figure 5E–F). This suggests that FXR signaling might contribute to postoperative increases in GLP-1 secretion at early time points.
At 4 weeks after the operations, glucose tolerances after saline intervention were significantly improved in WT-RYGB mice compared with their respective S-WM groups (Figure 7A–E). However, the postprandial glycemic response of Ex-9 infusion was similar between WT RYGB and S-WM mice (Figure 7A–E). Interestingly, compared with OGTT with saline intervention, pharmacologic blockade of GLP-1R did not change the pattern of the increase in postprandial blood glucose excursions between FXR KO RYGB and S-WM mice. (Figure 7B–E). This suggests that early interruption of GLP-1R signaling blunts the glucoregulatory effects of RYGB in WT but not FXR KO mice.
Figure 7.
Effect of pharmacologic blockade of GLP-1R on glucose control in FXR−/− (KO) and wild-type (WT) mice at postoperative weeks 4 and 14. RYGB and S-WM mice underwent OGTT. The glucose excursion curve after sterile saline with or without Ex-9 administered by intraperitoneal injection in fasted WT (A) and KO (B) mice at postoperative week 4. The glucose excursion curve after similar treatment in WT (C) and KO (D) mice at postoperative week 14. Glucose AUC 0–120min with saline or Ex-9 pretreatment in both WT and KO mice at postoperative weeks 4 (E) and 14 (F). ∗∗ = P < 0.01 S-WM vs. RYGB in WT mice by two-way ANOVA with Tukey's post hoc test. Data are shown as mean ± SEM. S-WM: sham-operated animals weight matched to RYGB-operated mice; RYGB: Roux-en-Y gastric bypass surgery; KO, knockout; WT, wild type. OGTT: oral glucose tolerance tests, AUC: area under curve.
Although the postprandial GLP-1 levels were significantly increased in RYGB groups compared with their respective sham controls in both genotypes 14 weeks after the operations (Figure 5E–F), pharmacologic blockade of GLP-1R did not change the pattern of the increase in postprandial glycemic excursions and glucose AUC between RYGB and S-WM mice in both genotypes (Figure 7C-D-F). This suggests that pharmacologic blockade of GLP-1R was unable to negate the effects of RYGB on glucose homeostasis in both genotypes at 14 weeks post-surgery.
4. Discussion
Accumulating evidence [6,7,[24], [25], [26], [27]] has shown that metabolic improvements following bariatric surgeries have been attributed to favorable changes in the concentrations and composition of bile acids. In addition, bile acids exert metabolic improvements mainly through activating their receptors, FXR and TGR5 [12]. Indeed, plasma bile acid levels positively correlate with GLP-1 after bariatric surgery in humans [6,28] and mice [29]. The direct roles of TGR5 in the metabolic improvements after VSG have also been demonstrated through genetic research in mice [13,14]. However, the beneficial effects of RYGB were independent of TGR5 [15]. Furthermore, GLP-1 receptor activity is not necessary for weight loss and glucose homeostasis improvements induced by RYGB [21,30] and VSG surgery [19]. Therefore the contribution of increased circulating bile acids and their related signaling pathways to the metabolic improvements observed following bariatric surgery remains to be fully elucidated. Ryan et al. showed that the absence of FXR actually prevented the beneficial effects of VSG [16]. Our results contradict the findings by Ryan et al. with regard to the effects on weight loss and extend the findings that FXR signaling contributes to the improvement of glycemic control in a body weight-independent manner following RYGB. Furthermore, our results suggest that the improved glucose control is dependent on GLP-1 in the early postoperative period.
Many well-designed studies have shown that the improvement of glycemic control achieved by bariatric surgery is partially independent of weight loss [31,32]. To eliminate the effect of body weight changes on glucose metabolism, we used mice weight-matched to the RYGB group. Body weight change from baseline and fat mass difference between S-WM and RYGB groups did not differ between the genotypes. Therefore we were able to assess the body weight-independent contributions of FXR signaling to the hypoglycemic benefits of RYGB. Again, FXR-deficiency has previously been shown to attenuate body weight increases and improve glucose homeostasis [23]. To avoid the impact of and account for different body weights between the two genotypes, we used feed efficiency and ANCOVA-adjusted energy expenditure to eliminate this difference between the genotypes. In addition, to control for the effects of different fasting blood glucose levels, comparisons were only conducted within each genotype for the three different treatment conditions. These approaches allowed us to control these variables and better compare metabolic parameters in both genotypes after surgery. Our data show that RYGB is superior to S-WM controls in improving glucose tolerance and insulin sensitivity/resistance in WT mice, while similar effects were observed in FXR knockout mice for both treatment conditions. Taken together, these results suggest that FXR signaling facilitates the improvement of glycemic control after RYGB in a body weight-independent manner.
Although the release of GLP-1 has been reported to be modulated by TGR5 in the small intestine, a recent study showed a small attenuation of postprandial GLP-1 secretion following bariatric surgery in global TGR5 knockout mice [14]. Interestingly, Trabelsi et al. demonstrated that the inhibition of FXR improves glucose disposal via the GLP-1 pathway in both cultured cells and FXR-deficient mice. In addition, bile acid signaling increased intestinal GLP-1 production via FXR signaling [33]. Our study further confirmed that the level of postprandial GLP-1 in FXR KO mice was higher than that in WT mice, and the level in RYGB groups was higher than that in their corresponding sham controls, except that there was no significant difference in postprandial GLP-1 levels among KO-RYGB mice and their respective sham groups at 4 weeks post-surgery.
Previous functional studies that focused on the contribution of GLP-1 signaling to improved glucose tolerance after bariatric surgery have produced mixed results. Mice with genetic deficiency of the GLP-1R respond normally to VSG [19] and RYGB [20,21] in terms of improvements in glucose regulation, while the glucoregulatory effects of biliary diversions to ileum (GB-IL), a new surgical procedure imitating the alterations of bile delivery to the distal small intestine caused by RYGB, were lost in global GLP-1R-deficient mice [33]. The discrepancy between these studies could be explained by the difference in the timing and feeding conditions after surgeries. First, the types of diet feed were different. An HFD containing 40% fat was used by Wilson-Perez et al., a 60% HFD by Mokadem et al. a two-choice diet consisting of an HFD and regular chow by Boland et al. and a chow diet containing 4.5% fat was used by Albaugh et al. Second, the time of blood glucose monitoring also ranged from 18 days to 8 weeks after surgery. Third, glucose tolerances were implemented in different ways, including oral gavage of a standard liquid diet [19], d-glucose [20,33], and intraperitoneal injection with d-glucose [21]. The mice in our study were maintained on a 60% HFD after surgery, and oral gavage of d-glucose was conducted at postoperative weeks 4 and 14. Our results indicated that blockade of the GLP-1R with Ex-9 abolished the glucoregulatory improvements in FXR+/+ but not in FXR−/− RYGB mice compared with their respective S-WM groups at 4 weeks post-operation. In other words, the FXR-mediated improvement in glucose metabolism was dependent of GLP-1 signaling at 4 weeks but independent of the GLP-1R at 14 weeks after RYGB.
We have not measured plasma bile acid levels in the diet-induced obese mice after surgery. However, several studies show that both RYGB [[6], [7], [8], [9]] and VSG [10] can result in increased levels of serum bile acids, which are involved in improved glucose metabolism in obese individuals with T2DM. This finding is also supported by experiments on rodents [6,7,[24], [25], [26], [27]]. Additionally, Bordenstein et al. showed that increased levels of bile acids and improved glycemic control occur within 4 weeks of surgery, suggesting that bile acids might mediate early glucoregulatory benefits in post-bariatric patients [34]. Furthermore, GB-IL [7,33,35,36] induced the most substantial weight loss and glucose tolerance improvement, similar to RYGB. These studies indicate that bile acids can exert beneficial effects in the hindgut independently of surgical intervention of gastrointestinal anatomy. Based on these findings, our results may shed light on the physiological mechanisms, which can now be applied to future research.
In terms of the beneficial effects on body weight, a previous study found that FXR signaling was required for longer term weight maintenance after VSG [16]. In that study, they found that WT-VSG mice maintained the initial weight loss for the duration of the experiment, while KO-VSG animals regained the weight within 5 weeks compared with their sham controls. However, our results show that relative to the sham-operative groups of both genotypes, RYGB can achieve remarkable weight loss throughout the experiment. Body weight changes from the baseline were similar between genotypes after the RYGB procedure, suggesting that weight loss maintenance after RYGB is independent of FXR signaling.
The disparity between different studies focusing on body weight may be attributed to the different types of surgery performed. Interestingly, a recent study reported that VSG was less effective at consistently suppressing body weight than RYGB [37]. Similar to the study conducted by Ryan et al. the degree of weight regain reaches preoperative levels approximately 10 weeks after VSG [16], while body weight following RYGB was consistently lower than preoperative levels at all time points in our study. The differences in surgical body weight outcomes have also been reported in other studies [[13], [14], [15],38]. The mechanisms of weight loss might be different between the two surgical procedures, mainly due to food intake and energy expenditure [37]. Genetic studies focusing on the effect of metabolic improvement among different surgical procedures in mice are needed to fully elucidate the mechanistic differences.
The food intake of RYGB or VSG groups is consistently significantly lower than that of their corresponding sham counterparts in the early postoperative period, after which the surgical mice compensate for the initial reduced intake [13,15,16,37]. In our study, we selected the postoperative period in which food intake and body weight were relatively stable for analysis to better imitate the long-term changes after RYGB in humans. We found that there was no significant difference in cumulative food intake (2–16 weeks) and feed efficiency (8–13 weeks) between RYGB and S-AL groups of both genotypes. However, when taking into account the greater energy lost in the feces of RYGB mice reported previously [39], the net energy intake in RYGB animals was intermediate to that of S-AL and S-WM controls. In addition, when energy expenditure is adjusted for body weight and lean mass, RYGB mice consume as much energy as S-AL groups, but more than S-WM controls. Taken together, the weight loss in response to RYGB is mainly due to a decrease in net energy intake independent of genotype. In addition, RYGB does not increase energy expenditure, but instead prevents the weight loss-induced decrease observed in nonsurgical weight-matched mice (metabolic adaptation to weight loss) in both genotypes.
There are some limitations to the present study. First, although we carefully designed the experiment by introducing sham-weight matching controls, using ANCOVA-adjusted methods, and only comparing data within each genotype for the three different treatment conditions, it is undeniable that KO mice had lower body weight and fat mass at the time of surgery, which would more or less affect the comparison of experimental results. For this reason, in future studies, we could deeply explore mice of the same body weight at different ages, the same ages but different body weight, or even the same body weight at the same age through different high-fat feeding schemes. Second, we did not measure the pool of circulating bile acids. The bile acid levels and sub-type profiles might be important and relevant to the changes in glucose homeostasis. Third, because FXR may have different roles in the liver and intestines, tissue-specific knockout mice should be used to explore the tissue-specific effects of FXR on metabolism after RYGB. Lastly, hyperinsulinemic-euglycemic and hyperglycemic clamps are the gold standard for assessing insulin sensitivity and pancreas islet function, but we did not use them in this study.
5. Conclusion
We demonstrated that RYGB surgery decreases body weight and fat mass and has similar effects on food intake and energy expenditure independent of genotype. Moreover, the observed body weight reduction in response to RYGB is mainly due to a decrease in net energy intake, and RYGB does not increase energy expenditure but instead prevents the weight loss-induced decrease observed in nonsurgical weight-matched mice (metabolic adaptation to weight loss). Although most of the improvements in glucose homeostasis are secondary to RYGB-induced weight loss in WT mice, FXR signaling facilitated improved glycemic control in a body weight-independent manner after the RYGB procedure, which might be mediated by an FXR-GLP-1 axis in the early postoperative period.
Statement of informed consent
Informed consent does not apply to this study.
Statement of human and animal rights
All applicable institutional and national guidelines for the care and use of animals were followed.
Acknowledgments
This study was supported by Fundamental Research Program Funding of Ninth People's Hospital affiliated to Shanghai Jiao Tong University School of Medicine (JYZZ022), Ph.D. Innovation Program Funding of Shanghai Jiao Tong University School of Medicine (BXJ201934), Clinical Research Program of Ninth People's Hospital affiliated to Shanghai Jiao Tong University School of Medicine (JYLJ022), Research Project of Pudong New Area Health and Family Planning Commission (PW2018D-01) and Clinical Research MDT Program of Ninth People's Hospital affiliated to Shanghai Jiao Tong University School of Medicine (201701008).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2020.100980.
Contributor Information
Zengmin Miao, Email: zengminmiao@126.com.
Yousheng Li, Email: guttx@hotmail.com.
Conflict of interest
The authors declare that they have no conflicts of interest.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Figure 1.
Initial body weight and blood glucose levels in mice. (A) All body weights were more than 30 g, and (B) fasting blood glucose levels were more than 120 mg/dl prior to surgery in both WT and FXR KO mice. § = P < 0.05, §§ = P < 0.01 genotype comparison of mice. Student's t-tests were used for comparisons. Data are shown as mean ± SEM (n = 8–10 per group). S-AL: sham-operated animals fed ad libitum; S-WM: sham-operated animals weight matched to RYGB-operated mice; RYGB: Roux-en-Y gastric bypass surgery; KO, knockout; WT, wild type.
References
- 1.Zheng Y., Ley S.H., Hu F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nature Reviews Endocrinology. 2018;14:88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 2.Ogurtsova K., da Rocha Fernandes J.D., Huang Y., Linnenkamp U., Guariguata L., Cho N.H. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Research and Clinical Practice. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 3.Mingrone G., Panunzi S., De Gaetano A., Guidone C., Iaconelli A., Nanni G. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet (London, England) 2015;386:964–973. doi: 10.1016/S0140-6736(15)00075-6. [DOI] [PubMed] [Google Scholar]
- 4.Angrisani L., Santonicola A., Iovino P., Vitiello A., Zundel N., Buchwald H. Bariatric surgery and endoluminal procedures: IFSO worldwide survey 2014. Obesity Surgery. 2017;27:2279–2289. doi: 10.1007/s11695-017-2666-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chavez-Talavera O., Tailleux A., Lefebvre P., Staels B. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology. 2017;152:1679–1694. doi: 10.1053/j.gastro.2017.01.055. e1673. [DOI] [PubMed] [Google Scholar]
- 6.Patti M.E., Houten S.M., Bianco A.C., Bernier R., Larsen P.R., Holst J.J. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring, Md) 2009;17:1671–1677. doi: 10.1038/oby.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pournaras D.J., Glicksman C., Vincent R.P., Kuganolipava S., Alaghband-Zadeh J., Mahon D. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology. 2012;153:3613–3619. doi: 10.1210/en.2011-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerhard G.S., Styer A.M., Wood G.C., Roesch S.L., Petrick A.T., Gabrielsen J. A role for fibroblast growth factor 19 and bile acids in diabetes remission after Roux-en-Y gastric bypass. Diabetes Care. 2013;36:1859–1864. doi: 10.2337/dc12-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Werling M., Vincent R.P., Cross G.F., Marschall H.U., Fandriks L., Lonroth H. Enhanced fasting and post-prandial plasma bile acid responses after Roux-en-Y gastric bypass surgery. Scandinavian Journal of Gastroenterology. 2013;48:1257–1264. doi: 10.3109/00365521.2013.833647. [DOI] [PubMed] [Google Scholar]
- 10.Jahansouz C., Xu H., Hertzel A.V., Serrot F.J., Kvalheim N., Cole A. Bile acids increase independently from hypocaloric restriction after bariatric surgery. Annals of Surgery. 2016;264:1022–1028. doi: 10.1097/SLA.0000000000001552. [DOI] [PubMed] [Google Scholar]
- 11.Thomas C., Pellicciari R., Pruzanski M., Auwerx J., Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nature Reviews Drug Discovery. 2008;7:678–693. doi: 10.1038/nrd2619. [DOI] [PubMed] [Google Scholar]
- 12.Fiorucci S., Distrutti E. Bile acid-activated receptors, intestinal microbiota, and the treatment of metabolic disorders. Trends in Molecular Medicine. 2015;21:702–714. doi: 10.1016/j.molmed.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Ding L., Sousa K.M., Jin L., Dong B., Kim B.W., Ramirez R. Vertical sleeve gastrectomy activates GPBAR-1/TGR5 to sustain weight loss, improve fatty liver, and remit insulin resistance in mice. Hepatology (Baltimore, Md) 2016;64:760–773. doi: 10.1002/hep.28689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGavigan A.K., Garibay D., Henseler Z.M., Chen J., Bettaieb A., Haj F.G. TGR5 contributes to glucoregulatory improvements after vertical sleeve gastrectomy in mice. Gut. 2017;66:226–234. doi: 10.1136/gutjnl-2015-309871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hao Z., Leigh Townsend R., Mumphrey M.B., Gettys T.W., Yu S., Munzberg H. Roux-en-Y gastric bypass surgery-induced weight loss and metabolic improvements are similar in TGR5-deficient and wildtype mice. Obesity Surgery. 2018;28:3227–3236. doi: 10.1007/s11695-018-3297-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryan K.K., Tremaroli V., Clemmensen C., Kovatcheva-Datchary P., Myronovych A., Karns R. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509:183–188. doi: 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garibay D., McGavigan A.K., Lee S.A., Ficorilli J.V., Cox A.L., Michael M.D. Beta-Cell glucagon-like peptide-1 receptor contributes to improved glucose tolerance after vertical sleeve gastrectomy. Endocrinology. 2016;157:3405–3409. doi: 10.1210/en.2016-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Svane M.S., Bojsen-Moller K.N., Nielsen S., Jorgensen N.B., Dirksen C., Bendtsen F. Effects of endogenous GLP-1 and GIP on glucose tolerance after Roux-en-Y gastric bypass surgery. American Journal of Physiology. Endocrinology and Metabolism. 2016;310:E505–E514. doi: 10.1152/ajpendo.00471.2015. [DOI] [PubMed] [Google Scholar]
- 19.Wilson-Perez H.E., Chambers A.P., Ryan K.K., Li B., Sandoval D.A., Stoffers D. Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like Peptide 1 receptor deficiency. Diabetes. 2013;62:2380–2385. doi: 10.2337/db12-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mokadem M., Zechner J.F., Margolskee R.F., Drucker D.J., Aguirre V. Effects of Roux-en-Y gastric bypass on energy and glucose homeostasis are preserved in two mouse models of functional glucagon-like peptide-1 deficiency. Molecular Metabolism. 2014;3:191–201. doi: 10.1016/j.molmet.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boland B.B., Mumphrey M.B., Hao Z., Townsend R.L., Gill B., Oldham S. Combined loss of GLP-1R and Y2R does not alter progression of high-fat diet-induced obesity or response to RYGB surgery in mice. Molecular Metabolism. 2019;25:64–72. doi: 10.1016/j.molmet.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hao Z., Zhao Z., Berthoud H.R., Ye J. Development and verification of a mouse model for Roux-en-Y gastric bypass surgery with a small gastric pouch. PloS One. 2013;8 doi: 10.1371/journal.pone.0052922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prawitt J., Abdelkarim M., Stroeve J.H., Popescu I., Duez H., Velagapudi V.R. Farnesoid X receptor deficiency improves glucose homeostasis in mouse models of obesity. Diabetes. 2011;60:1861–1871. doi: 10.2337/db11-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Myronovych A., Kirby M., Ryan K.K., Zhang W., Jha P., Setchell K.D. Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight-loss-independent manner. Obesity (Silver Spring, Md) 2014;22:390–400. doi: 10.1002/oby.20548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohli R., Kirby M., Setchell K.D., Jha P., Klustaitis K., Woollett L.A. Intestinal adaptation after ileal interposition surgery increases bile acid recycling and protects against obesity-related comorbidities. American Journal of Physiology Gastrointestinal and Liver Physiology. 2010;299:G652–G660. doi: 10.1152/ajpgi.00221.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang M., Wu Q., Xie H., Shao Y., Zhong M., Zhang X. Effects of sleeve gastrectomy on serum 12alpha-hydroxylated bile acids in a diabetic rat model. Obesity Surgery. 2017;27:2912–2918. doi: 10.1007/s11695-017-2714-6. [DOI] [PubMed] [Google Scholar]
- 27.Wu Q., Zhang X., Zhong M., Han H., Liu S., Liu T. Effects of bariatric surgery on serum bile acid composition and conjugation in a diabetic rat model. Obesity Surgery. 2016;26:2384–2392. doi: 10.1007/s11695-016-2087-2. [DOI] [PubMed] [Google Scholar]
- 28.Steinert R.E., Peterli R., Keller S., Meyer-Gerspach A.C., Drewe J., Peters T. Bile acids and gut peptide secretion after bariatric surgery: a 1-year prospective randomized pilot trial. Obesity (Silver Spring, Md) 2013;21:E660–E668. doi: 10.1002/oby.20522. [DOI] [PubMed] [Google Scholar]
- 29.Bhutta H.Y., Rajpal N., White W., Freudenberg J.M., Liu Y., Way J. Effect of Roux-en-Y gastric bypass surgery on bile acid metabolism in normal and obese diabetic rats. PloS One. 2015;10 doi: 10.1371/journal.pone.0122273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ye J., Hao Z., Mumphrey M.B., Townsend R.L., Patterson L.M., Stylopoulos N. GLP-1 receptor signaling is not required for reduced body weight after RYGB in rodents. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2014;306:R352–R362. doi: 10.1152/ajpregu.00491.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chambers A.P., Jessen L., Ryan K.K., Sisley S., Wilson-Perez H.E., Stefater M.A. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology. 2011;141:950–958. doi: 10.1053/j.gastro.2011.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nannipieri M., Mari A., Anselmino M., Baldi S., Barsotti E., Guarino D. The role of beta-cell function and insulin sensitivity in the remission of type 2 diabetes after gastric bypass surgery. Journal of Clinical Endocrinology & Metabolism. 2011;96:E1372–E1379. doi: 10.1210/jc.2011-0446. [DOI] [PubMed] [Google Scholar]
- 33.Albaugh V.L., Banan B., Antoun J., Xiong Y., Guo Y., Ping J. Role of bile acids and GLP-1 in mediating the metabolic improvements of bariatric surgery. Gastroenterology. 2019;156:1041–1051. doi: 10.1053/j.gastro.2018.11.017. e1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bordenstein S.R., Guo Y., Wasserman D.H., Abumrad N.N., Albaugh V.L., Flynn C.R. Early increases in bile acids post roux-en-Y gastric bypass are driven by insulin-sensitizing, secondary bile acids. Nature Communications. 2015;100:E1225–E1233. doi: 10.1210/jc.2015-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kohli R., Setchell K.D., Kirby M., Myronovych A., Ryan K.K., Ibrahim S.H. A surgical model in male obese rats uncovers protective effects of bile acids post-bariatric surgery. Endocrinology. 2013;154:2341–2351. doi: 10.1210/en.2012-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flynn C.R., Albaugh V.L. Bile diversion to the distal small intestine has comparable metabolic benefits to bariatric surgery. Nature Communication. 2015;6:7715. doi: 10.1038/ncomms8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hao Z., Townsend R.L., Mumphrey M.B., Morrison C.D., Munzberg H., Berthoud H.R. RYGB produces more sustained body weight loss and improvement of glycemic control compared with VSG in the diet-induced obese mouse model. Obesity Surgery. 2017;27:2424–2433. doi: 10.1007/s11695-017-2660-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pucci A., Batterham R.L. Mechanisms underlying the weight loss effects of RYGB and SG: similar, yet different. Journal of Endocrinological Investigation. 2019;42:117–128. doi: 10.1007/s40618-018-0892-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liou A.P., Paziuk M., Luevano J.-M., Machineni S., Turnbaugh P.J., Kaplan L.M. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Science Translational Medicine. 2013;5 doi: 10.1126/scitranslmed.3005687. 178ra141. [DOI] [PMC free article] [PubMed] [Google Scholar]