Abstract
The cytoplasmic dynein light chain 1 (DYNLL1) is an important constituent of motor proteins complex. In human it is encoded by DYNLL1 gene. It is involved in cargo transport functions and interacts with many viral proteins with the help of short linear consensus motif sequence (K/R) XTQT. Viral proteins bind to DYNLL1 through its consensus short linear motif (SLiM) sequence to reach the target site in the cell and cause different infections in the host. It is still unknown if bacterial proteins also contain the same conserved SLiMs sequence through which they bind to this motor protein and cause infections. So, it is important to investigate the role of DYNLL1 in human bacterial infections. The interaction partner proteins of DYNLL1 against conserved viral motif sequences were predicted through PDBSum. Pairwise sequence alignment, between viral motif sequence and that of predicted proteins, was performed to identify conserved region in predicted interaction partners. Docking between the DYNLL1 and new pathogenic interaction partners was performed, by using PatchDock, to explore the protein-protein binding quality. Interactions of docked complexes were visualized by DimPlot. Three pathogenic bacterial proteins i.e., enterochelin esterase (3MGA), protective antigen (3J9C) and putative lipoprotein (4KT3) were selected as candidate interaction partners of DYNLL1. The putative lipoprotein (4KT3) showed low quality binding with DYNLL1. So, enterochelin esterase (3MGA) and protective antigen (3J9C) were speculated to be involved in human bacterial infections by using DYNLL1 to reach their target sites.
Keywords: Motor protein, Protein-peptide docking, Human pathogenic bacteria, Protein-protein interaction, Short linear motifs
1. Introduction
Dynein is a family of motor proteins in cell cytoplasm. These proteins provide energy for cell transportation processes by converting the chemical energy of ATP to mechanical energy. This family is broadly classified into two main groups of proteins i.e., cytoplasmic and axonemal dyneins. Axonemal dynein is involved in movement of cilia and flagella by sliding of axoneme. Cytoplasmic dynein is found in animal cells. It is the part of a large multiprotein complex involved in transport of cargoes along cytoplasmic microtubules. Dynein protein consists of three chains: heavy, intermediate and light. Heavy chains are involved in ATP hydrolysis while light and intermediate chains play important role in cargo binding process (Bader and Vaughan, 2010). Among these chains dynein light chain 1 (DYNLL1) is highly conserved and discovered for the first time as a subunit of dynein of axoneme in Chlamydomonas involved in diverse functions (Pfister et al., 1982).
The Cytoplasmic dynein light chain 1 (DYNLL1) is an important constituent of motor proteins complex. In human it is encoded by DYNLL1 gene (Pfister et al., 2005). Many viral proteins like Adenain, p54, E4, P Protein and VP35 have been reported to travel in cells with the help of DYNLL1 (Jacob et al., 2000, Mabit et al., 2002, Jouvenet et al., 2004, Schneider et al., 2011). Binding motifs of DYNLL1 have been divided into two classes: K/R-XTQT or (K)3 X)2 T)1 Q0 T1 X2) and G-I/V-QVD or [X)3 G)2 (I/V))1 Q0 V1 D2] (Döhner et al., 2005, Barbar, 2008, Rapali et al., 2011). The central Gln residue present at 0 position covers N-terminal of the second alpha helix in protein. The binding residues are present in side chains at different positions, i.e., +1, 1 and 3 and interact with inner side of binding groove. There are some DYNLL1 interaction partners which contain non-consensus binding motif sequence and do not contain conserved Gln residue. Generally the interaction topology of these peptides is like those of the recognized ones.
Dynein light chain 1 consists of two β sheets which are antiparallel and play important role in dimerization. Each β sheet consists of five strands, out of which four belong to one monomer and one to other monomer. The β sheets are surrounded by four α helices at opposite faces of dimer. The bound ligands of DYNLL1 lie in two identical parallel grooves formed at the two edges of the dimerization interface. The bound peptides form an extra antiparallel β strand augment the central β sheets (Espindola et al., 2000; Benison et al., 2007).
Human cytoplasmic dynein light chain 1 homodimers are found to bind with other proteins through conserved motif sequence KETQTP. Most of its interacting viral and cellular proteins generally display related recognition amino acid sequences in the form of short linear motifs (Merino-Gracia et al., 2011).
Usually viruses use active transport for movement through cytoplasm but most of the viral particles are too large to diffuse freely in the crowded cytoplasm environment. The viral particles with more than 50 mm diameter prefer to use microtubules for movements in a retrograde manner by using cell dynein or some of its components (Merino-Gracia et al., 2011). It has been experimentally proved that the compatibility of microtubules and viral proteins is necessary for causing infection. Most of the human pathogenic viruses like herpes virus (Mabit et al., 2002), Mokola virus, Papilloma virus (Schneider et al., 2011), Adenovirus (Mabitet al., 2002) and African swine fever virus (Jouvenet et al., 2004) use microtubules for reaching their target to cause infection. Viruses use two approaches for intracellular transport: (a) hijack cytoplasmic membrane traffic (b) interact directly with the cytoskeletal transport machinery (Döhner et al., 2005). Most of the viruses utilize cell cytoskeletal transport machinery to facilitate virions nucleoprotein complexes to travel long distances in cytoplasm from cell surface to replication and transcription site of viral DNA and induce infection.
On the basis of already reported viral interaction partners of DYNLL1 we identified some new pathogenic bacterial proteins as potential interaction partners. We predicted computationally that the bacterial proteins can also interact with DYNLL1 and cause infections in humans. The findings of present study provide directions to identify new drug targets against pathogenic bacterial diseases. Thus, our study highlighted a unique and revolutionary area of medical research by predicting important role of dynein light chain 1 in bacterial infections. These findings can be used in further research works.
2. Materials and methods
2.1. Data set
The experimentally determined three dimensional structure of the human cytoplasmic dynein light chain 1 (DYNLL1) was retrieved from PDB (Protein Data Bank) under PDB ID: 1CMI (Bernstein et al., 1977) (Fig. 1). Binding motifs of its interaction partner proteins were selected through extensive literature search (Jacob et al., 2000, Raux et al., 2000, Alonso et al., 2001, Rodriguez-Crespo et al., 2001, Martínez-Moreno et al., 2003, Kubota et al., 2009, García-Mayoral et al., 2011) (Table1).
Fig. 1.
3D structure of human dynein light chain 1 (1CMI).
Table 1.
Experimentally verified binding motifs (bold and underlined) of human cytoplasmic dynein light chain 1 interaction partners.
2.2. Identification of human cytoplasmic dynein light chain 1interaction proteins
Homology search, on the basis of particular consensus short linear motifs (SLiMs) sequence (K/R) XTQT for DYNLL1 interaction partners, was done against an online available database, PDBsum (Laskowski et al., 1997). The viral DYNLL1 SLiMs sequences, i.e., KSTQT, TASQT and SQTQT were used as query sequence to search the identical SLiMs sequences. The SLiMs sequences showing more than 65% sequence identity with the query sequence were selected as interaction partners of DYNLL1. The SLiMs from previously reported proteins were excluded from data set while those from new proteins were selected for further analysis.
2.3. Pairwise sequence alignment
The SLiMs of new selected interaction proteins of DYNLL1 were retrieved from UniProt. Pairwise sequence alignment was performed, between the query virus SLiMs sequence and those of new interaction proteins, using EMBOSS water tool. The purpose of performing pairwise sequence alignment was to identify the regions with high similarity which show structural, functional or evolutionary relationship between query protein and predicted interaction partners.
2.4. Selection of candidate proteins
From the results of pairwise sequence alignment proteins with highest motif residual contribution were short listed. The interaction partner proteins of pathogenic organism were selected as candidate proteins for further study.
2.5. Candidate protein structure retrieval
Three dimensional structures of candidate proteins: protective antigen (PDB ID: 3J9C), enterochelin esterase (PDB ID: 3MGA) and putative lipoprotein (PDB ID: 4KT3) were retrieved from protein data bank (Bernstein et al., 1977).
2.6. Motif structure
The structures of candidate proteins were opened in UCSF chimera (Pettersen et al., 2004). Structure of SLiMs residues was cut from the original structure and saved. The resulted small motif structure was used for molecular docking.
2.7. Molecular docking analysis of human cytoplasmic dynein light chain1 with new interaction partners
Molecular docking analysis of DYNLL1 with selected interaction partner proteins i.e., protective antigen, enterochelin esterase and putative lipoprotein, was performed using PatchDock. FireDock was used for rescoring and refinement (Schneidman-Duhovny et al., 2005, Andrusier et al., 2007). The input for PatchDock consisted of PDB files of DYNLL1 and respective candidate proteins with set default parameters. This process was repeated for each candidate protein. Geometric fit, atomic desolvation energy, and scoring functions were used for the evaluation of candidate proteintrans formations.
2.8. Protein-protein interactions
Protein-protein interaction was calculated using Ligplus (Laskowski et al., 2011). The selected models of DYNLL1- Enterochelin esterase, DYNLL1-Putative lipoprotein and DYNLL1- Protective antigen were opened in DIMPLOT (Laskowski et al., 2011) and their interactions were viewed.
3. Results
3.1. Selection of candidate interaction partner proteins of human cytoplasmic dynein light chain 1
The proteins having maximum motif residues in alignment were short listed on the basis of pairwise sequence alignment scores. Previously reported interaction partners of DYNLL1 were excluded while new and pathogenic interaction partners, Putative lipoprotein (Pseudomonas fluorescens: 4KT3), Enterochelin esterase (Salmonella typhimurium: 3MGA) and Protective Antigen (Bacillus anthracis: 3J9C), were selected as candidate proteins (Table 2).
Table 2.
Predicted interaction partners of human cytoplasmic dynein light chain 1.
| Query |
Candidate |
|||||
|---|---|---|---|---|---|---|
| Organism | Protein | Motif sequence* | Organism | Protein** | Conserved motif sequence | Identity with query sequence (%) |
| Adenovirus | Adenain | CITLVKSTQTV | Bacillus anthracis | Protective Antigen (3J9C) | TDSQT | 77.80 |
| ASF virus | p54 | VTTQNTASQTM | Homo Sapiens | C-C motif chemokine7 (1BO0) | KKTQT | 77.80 |
| Papillomavirus | E4 | DHHQDKQTQTP | Salmonella typhimurium | Enterochelin esterase (3MGA) | AQPQT | 70.00 |
| Rabies Virus | P Protein | RSSEDKSTQTT | Pseudomonas fluorescens | Putative lipoprotein (4KT3) | YQLQR | 66.70 |
| Mokola Virus | P Protein | KSTEDKSTQTP | Helicobacter Pylori | 2-dehydro-3-deoxyphosphooctonate aldolase (4Z1D) | KSIQS | 66.70 |
| Ebola virus | VP35 | PKTRNSQTQTD | Homo Sapiens | C-C motif chemokine7 (1BO0) | KKTQT | 85.70 |
Bold and underlined sequences are conserved.
Bold candidate pathogenic proteins were selected for studies.
3.2. Structure and motif retrieval of candidateproteins
The structures of selected proteins were retrieved from PDB (Fig. 2a, Fig. 3a, Fig. 4a). The motif structure was cut upto11 residues to extend the binding pockets for docking by using UCSF Chimera (Fig. 2b, Fig. 3b, Fig. 4b).
Fig. 2a.

3D structure of protective antigen (3J9C).
Fig. 3a.

3D structure of enterochelin esterase (3MGA).
Fig. 4a.
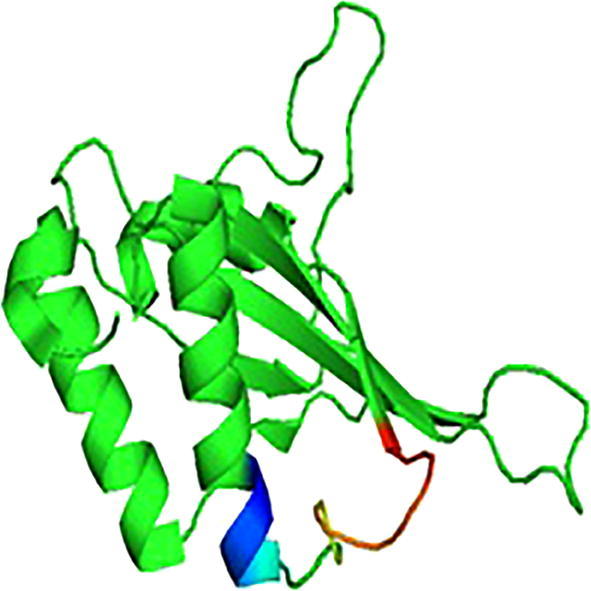
3D structure of putative lipoprotein (4KT3).
Fig. 2b.

Motif structure of protective antigen3J9C).
Fig. 3b.
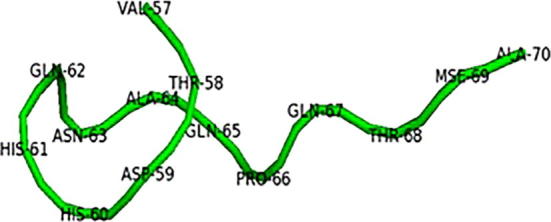
Motif structure of enterochelin esterase (3MGA).
Fig. 4b.

Motif structure of putative lipoprotein (4KT3).
3.3. Molecular docking analysis of candidate proteins withhuman cytoplasmic dynein light chain 1
PatchDock was used for performing molecular docking between candidate protein and DYNLL1. The results produced near native conformation of DYNLL1-candidate protein complexes which assured the reliability on the interaction mode. Ten best solutions from the results of PatchDock were screened for further refinement and rescoring by using FireDock algorithm. Final results of selected candidate proteins are presented in Table 3. In case of Enterochelin esterase (3MGA) global energy for the best docking model was −32.18 kcal/mol with atomic contact energy −6.19 kcal/mol which showed good binding quality.
Table 3.
Docking results of pathogenic bacterial proteins with human dynein light chain 1.
| Candidate Protein | Organism | Global Energy (kcal/mol) | Attractive VDW* | Repulsive VDW* | ACE** (kcal/mol) |
|---|---|---|---|---|---|
| Enterochelin esterase (3MGA) | Salmonella typhimurium | −32.18 | −27.04 | 17.65 | −6.19 |
| Protective Antigen (3J9C) | Bacillus anthracis | −25.75 | −26.44 | 6.08 | 5.78 |
| Putative lipoprotein (4KT3) | Pseudomonas fluorescens | −0.58 | −3.90 | 1.46 | −0.38 |
VDW = Vander Walls.
ACE = Atomic contact energy.
The global energy for the best docking model DYNLL1-Protective antigen (3J9C) was −25.75 kcal/mol with atomic contact energy of −5.78 kcal/mol, which depicted good feasibility of binding. The best docking model DYNLL1-Putative lipoprotein (4KT3) had −0.58 kcal/mol global energy and −0.38 kcal/mol atomic contact energy which reflects weaker binding quality. The interacting residues of the representative docking models out of the largest clusters were analyzed by DIMPLOT (Laskowski et al., 2011) (Fig. 5, Fig. 6, Fig. 7).
Fig. 5.
DYNLL1- protective antigen interaction complex.
Fig. 6.
DYNLL1- enterochelin esterase interaction complex.
Fig. 7.
DYNLL1- putative lipoprotein interaction complex.
4. Discussion
In the present study, we tried to find some novel and pathogenic interaction partners of human cytoplasmic dynein light chain 1 (DYNLL1). Homology based search was performed, using viral DYNLL1-binding short linear motif (K/R)XTQT, to find some new interaction partner of DYNLL1 sharing closely related viral short linear motifs (SLiMs) sequence. SLiMs are the short stretches of amino acid sequences which facilitate the protein-protein interaction (Neduva and Russell, 2006, Diella et al., 2008). Three pathogenic bacterial proteins enterochelin esterase (Salmonella typhimurium) putative lipoprotein (Pseudomonas fluorescens) and protective antigen (Bacillus anthracis) showed good pairwise sequence alignment with viral protein and were selected, as candidate interaction partners of DYNLL1, for further analysis. Short linear motifs (SLiMs) of candidate protein were docked to find the interaction with DYNLL1 and their interactions were visualized.
Only one motif residue, Thr68, from enterochelin esterase (Salmonella typhimurium) made hydrogen bond with the DYNLL1 binding pocket residue, Gln80, with hydrogen donor and acceptor distance of 2.28 Å. The preferred interaction region for a hydrogen bond between carbonyl oxygen and amide nitrogen is 2.5–3.5 Å (Hubbard et al., 2010), which confirms the strong interaction between DYNLL1 and enterochelin esterase. Remaining motif residues were involved in hydrophobic interactions. Dashed lines represent the hydrogen bonding but residues shown as an arc with spokes represent hydrophobic interactions (Liu et al., 2008). The interaction between these hydrophobic regions of the binding site with the ligand is responsible to provide the driving force for binding (Kelly and Mancera, 2005). It showed the lowest binding energy (−32.18), which reflects the strong and stable interaction (Ajay and Murko, 1995). All these factors contribute to select enterochelin esterase of S. typhimurium as the best interaction partner of human DYNLL1.
Salmonella typhimurium is a gram-negative, flagellated facultative anaerobic bacterium generally present in mammalian gastrointestinal tract (Morpeth et al., 2009). It causes mucosal inflammation in immune-compromised patients (Madi et al., 2010). The infection occurs when high concentration of bacteria is ingested through contaminated food, which results in food poisoning. Enterochelin esterase is the virulence factor of S. typhimurium (Robert and Shelly, 1979). The assimilation of protein bound iron in the cell is the key source for multiplication of S. typhimurium (Bullen et al., 1971). Enterochelin protein in enterobacterial pathogens act as iron chelator and it is crucial for multiplication (Rogers, 1973, Miles and Khimji, 1975). Outer membrane receptors of host cell give entry to enterochelin to the cell (Braun, 1976) and TonB helps for its translocation to the cytoplasmic membrane (Kadner and McElhaney, 1978). The results of current study showed that this pathogenic protein reaches its target site by binding with DYNLL1 and travel the long distance in cytoplasm.
Second strongest interaction partner (−25.75 kJ/mol global energy) of DYNLL1 was protective antigen of Bacillus anthracis. Three motif residues, i.e., Thr282, Thr286 and Thr288 were involved in hydrogen bonding with DYNLL1 binding pocket residue Ser64, Tyr77 and Gln80. B. anthracis produces toxin called anthrax which causes Anthrax, a serious bacterial infection in human. It is an A-B type toxin consisting of A and B subunits. B subunit is called protective antigen (PA), which is involved in cell and A subunit binding. A subunits consists of two factors, i.e., lethal factor (LF) and edema factor (EF), performing enzymatic toxic activities (Collier and Young, 2003). PA undergoes heptamerization and converted to a new complex called PAheptamer which is able to bind three molecules of LF and/or EF (Collier and Young, 2003). PAheptamer acquires some conformational changes and help LF and EF to insert into endosomal and delivered to cytoplasm (Collier and Young, 2003). The subsequent delivery of LF to the cytoplasm occurs later in the endocytic pathway (Laurence et al., 2004). The mechanism of movement of PA in the cytoplasm to reach its target site is still unknown. The findings of current study showed that PA gets attached to the DYNLL1 in the cytoplasm and travels to its target site.
Putative lipoprotein is a virulence factor of pathogenic bacteria, Pseudomonas fluorescens. In Putative lipoprotein motif residues Gln57, Leu58, Arg60 were involved in making interactions with the DYNLL1binding pocket residues Tyr75 and Ser64. Although the number of hydrogen bonds formed are highest but the global energy (−0.58 kJ/mol) was very poor. So, we cannot claim putative lipoprotein of P. fluorescens as a strong interaction partner of DYNLL1. In a previous study Kausar et al. (2013) found P. aeruginosa as the strongest interaction partner of DYNLL1. P. aeruginosa is involved in life threatening infections especially in pneumonia patients (Giantsou and Manolas, 2011), but in current study we could not find good interaction of P. fluorescens with human DYNLL1.
Enterochelin esterase (Salmonella typhimurium) and protective antigen (Bacillus anthraci) showed lower global energies. Fernández-Recio et al. (2004) found that low energy regions in the docked complex corresponded to actual binding sites of the proteins. So we speculate that these two proteins could be the potential interaction partners of DYNLL1. Enterochelin esterase (Salmonella typhimurium) and protective antigen (Bacillus anthraci) might use this motor protein to reach their target sites in human cell and result in severe bacterial infections. These results open up an area of further research to study the role of DYNLL1 in bacterial infections mechanism.
Declaration of Competing Interest
The authors declare no conflict of interest
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Qudsia Yousafi, Email: qudsia@cuisahiwal.edu.pk.
Muhammad Saad Khan, Email: saad.khan@cuisahiwal.edu.pk.
Asim Mehmood, Email: assim_324@cuisahiwal.edu.pk.
Shahzad Saleem, Email: shahzadsaleem@cuisahiwal.edu.pk.
Muhammad Wasim Sajid, Email: muhammad.wasim@cuisahiwal.edu.pk.
Abrar Hussain, Email: abrar.hussain@cuisahiwal.edu.pk.
Mohammad Amjad Kamal, Email: prof.ma.kamal@gmail.com.
References
- Ajay, Murcko M.A. Computational methods to predict binding free energy in ligand-receptor complexes. J. Med. Chem. 1995;38:4953–4967. doi: 10.1021/jm00026a001. [DOI] [PubMed] [Google Scholar]
- Alonso C., Miskin J., Hernáez B., Fernandez-Zapaterom P., Soto L., Cantó C., Crespo I., Dixon L., Escribano J.M. African swine fever virus protein p54 interacts with the microtubular motor complex through direct binding to light-chain dynein. J. Virol. 2001;75:9819–9827. doi: 10.1128/JVI.75.20.9819-9827.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrusier N., Nussinov R., Wolfson H.J. FireDock: fast interaction refinement in molecular docking. Proteins Struct. Funct. Genet. 2007;69:139–159. doi: 10.1002/prot.21495. [DOI] [PubMed] [Google Scholar]
- Bader J.R., Vaughan K.T. Dynein at the kinetochore: timing, interactions and functions. Semin. Cell. Dev. Biol. 2010;21:269–275. doi: 10.1016/j.semcdb.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbar E. Dynein light chain LC8 is a dimerization hub essential in diverse protein networks. Biochemistry. 2008;47:503–508. doi: 10.1021/bi701995m. [DOI] [PubMed] [Google Scholar]
- Benison G., Karplus P.A., Barbar E. Structure and dynamics of LC8 complexes with KXTQT-motif peptides: swallow and dynein intermediate chain compete for a common site. J. Mol. Biol. 2007;371:457–468. doi: 10.1016/j.jmb.2007.05.046. [DOI] [PubMed] [Google Scholar]
- Bernstein F.C., Koetzle T.F., Williams G.J.B., Meyer E.F., Jr, Brice M.D., Rodgers J.R., Kennard O., Shimanouchi T., Tasumi M. The protein data bank. Eur. J. Biochem. 1977;80:319–324. doi: 10.1111/j.1432-1033.1977.tb11885.x. [DOI] [PubMed] [Google Scholar]
- Braun V., Hancock R.E.W., Hantke K., Hartmann A. Functional organization of the outer membraneof Escherichia coli Phage and colicin receptors as components of iron uptake systems. J. Supramol. Struct. 1976;5:37–58. doi: 10.1002/jss.400050105. [DOI] [PubMed] [Google Scholar]
- Bullen J.J., Rogers H.J., Lewin J.E. The bacteriostatic effect of serum on Pasteurellasepticaand its abolition by iron compounds. Immunology. 1971;20:391–406. [PMC free article] [PubMed] [Google Scholar]
- Collier R.J., Young J.A. Anthrax toxin. Annu. Rev. Cell Dev. Biol. 2003;19:45–70. doi: 10.1146/annurev.cellbio.19.111301.140655. [DOI] [PubMed] [Google Scholar]
- Diella F., Haslam N., Chica C., Budd A., Michael S., Brown N.P., Travé G., Gibson T.J. Understanding eukaryotic linear motifs and their role in cell signaling and regulation. Front. Biosci. 2008;13:6580–6603. doi: 10.2741/3175. [DOI] [PubMed] [Google Scholar]
- Döhner K., Sodeik B., Dohner K. The role of the cytoskeleton during viral infection. Curr. Top. Microbiol. Immunol. 2005;285:67–108. doi: 10.1007/3-540-26764-6_3. [DOI] [PubMed] [Google Scholar]
- Espindola F.S., Suter D.M., Partata L.B.E., Cao T., Wolenski J.S., Cheney R.E., King S.M., Mooseker M.S. The light chain composition of chicken brain myosin-Va: calmodulin, myosin-II essential light chains, and 8-kDa dynein light chain/PIN. Cell Motil. Cytoskeleton. 2000;47:269–281. doi: 10.1002/1097-0169(200012)47:4<269::AID-CM2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Fernandez-Recio J., Totrov M., Abagyan R. Identification of protein–protein interaction sites from docking energy landscapes. J. Mol. Biol. 2004;335:843–865. doi: 10.1016/j.jmb.2003.10.069. [DOI] [PubMed] [Google Scholar]
- García-Mayoral M.F., Rodríguez-Crespo I., Bruix M. Structural models of DYNLL1 with interacting partners: African swine fever virus protein p54 and postsynaptic scaffolding protein gephyrin. FEBS Lett. 2011;585:53–57. doi: 10.1016/j.febslet.2010.11.027. [DOI] [PubMed] [Google Scholar]
- Giantsou E., Manolas K. Superinfections in Pseudomonas aeruginosa ventilator-associated pneumonia. Minerva Anestesiol. 2011;77:964–970. [PubMed] [Google Scholar]
- Hubbard, Roderick E., Kamran H.M. Encyclopedia of Life Sciences (ELS) John Wiley & Sons, Ltd; Chichester: 2010. Hydrogen bonds in proteins: role and strength. [DOI] [Google Scholar]
- Jacob Y., Badrane H., Ceccaldi P.E., Tordo N. Cytoplasmic dynein LC8 interacts with lyssavirus phosphoprotein. J. Virol. 2000;74:10217–10222. doi: 10.1128/jvi.74.21.10217-10222.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouvenet N., Monaghan P., Way M., Wileman T. Transport of African swine fever virus from assembly sites to the plasma membrane is dependent on microtubules and conventional kinesin. J. Virol. 2004;78:7990–8001. doi: 10.1128/JVI.78.15.7990-8001.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadner R.J., McElhaney G. Outer membrane-dependent transport systems in Escherichia coli: turnover of TonB function. J. Bacteriol. 1978;134:1020–1029. doi: 10.1128/jb.134.3.1020-1029.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kausar S., Asif M., Bibi N., Rashid S. Comparative molecular docking analysis of cytoplasmic dynein light chain DYNLL1 with pilin to explore the molecular mechanism of pathogenesis caused by Pseudomonas aeruginosa PAO. PLoS ONE. 2013;8:1–14. doi: 10.1371/journal.pone.0076730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M.D., Mancera R.L. A new method for estimating the importance of hydrophobic groups in the binding site of a protein. J. Med. Chem. 2005;48:1069–1078. doi: 10.1021/jm049524q. [DOI] [PubMed] [Google Scholar]
- Kubota T., Matsuoka M., Chang T., Bray M., Jones S., Tashiro M., Kato A., Ozato K. Ebolavirus VP35 Interacts with the cytoplasmic dynein light chain 8. J. Virol. 2009;83:6952–6956. doi: 10.1128/JVI.00480-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski A.R., Hutchinson E.G., Michie A.D., Wallace A.C., Jones M.L., Thornton J.M. PDBsum: a Web-based database of summaries and analyses of all PDB structures. Trends Biochem. Sci. 1997;22:488–490. doi: 10.1016/s0968-0004(97)01140-7. [DOI] [PubMed] [Google Scholar]
- Laskowski R.A., Swindells M.B. LigPlot +: multiple ligand À protein interaction diagrams for drug discovery. Chem. Inf. Model. 2011;51:2778–2786. doi: 10.1021/ci200227u. [DOI] [PubMed] [Google Scholar]
- Laurence A., Margaret L., Robert G.P., Leppla S.H., Goot F.G. Membrane insertion of anthrax protective antigen and cytoplasmic delivery of lethal factor occur at different stages of the endocytic pathway. J. Cell Biol. 2004;166:645–651. doi: 10.1083/jcb.200312072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Wang G., Li Z., Wang R. Geometrical preferences of the hydrogen bonds on protein–ligand binding interface derived from statistical surveys and quantum mechanics calculations. J. Chem. Theory Comput. 2008;4:1959–1973. doi: 10.1021/ct800267x. [DOI] [PubMed] [Google Scholar]
- Mabit H., Nakano M.Y., Prank U., Saam B., Dohner K., Sodeik B., Greber U.F. Intact microtubules support adenovirus and herpes simplex virus infections. J. Virol. 2002;76(19):9962–9971. doi: 10.1128/JVI.76.19.9962-9971.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madi A., Lakhdari O., Blottière H.M., Guyard-Nicodème M., Roux K.L., Groboillot A., Svinareff P., Doré J., Orange N., Feuilloley M.G.J., Connil N. The clinical Pseudomonas fluorescens MFN1032 strain exerts a cytotoxic effect on epithelial intestinal cells and induces Interleukin-8 via the AP-1 signaling pathway. BMC Microbiol. 2010;10:215–222. doi: 10.1186/1471-2180-10-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Moreno M., Navarro-Lérida I., Roncal F., Albar J.P., Alonso C., Gavilanes F., Crespo I. Recognition of novel viral sequences that associate with the dynein light chain LC8 identified through a pepscan technique. FEBS Lett. 2003;544:262–267. doi: 10.1016/s0014-5793(03)00516-7. [DOI] [PubMed] [Google Scholar]
- Merino-Gracia J., García-Mayoral M.F., Rodríguez-Crespo I. The association of viral proteins with host cell dynein components during virus infection. FEBSJ. 2011;278:2997–3011. doi: 10.1111/j.1742-4658.2011.08252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles A.A., Khimji P.L. Enterobacterial chelators of iron: their occurrence, detection, and relation to pathogenicity. J. Med. Microbiol. 1975;8:477–490. doi: 10.1099/00222615-8-4-477. [DOI] [PubMed] [Google Scholar]
- Morpeth S.C., Ramadhani H.O., Crump J.A. Invasive non-TyphiSalmonella disease in Africa. Clin. Infect. Dis. 2009;49:606–611. doi: 10.1086/603553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neduva V., Russell R.B. Peptides mediating interaction networks: new leads at last. Curr. Opin. Biotechnol. 2006;17:465–471. doi: 10.1016/j.copbio.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera - A visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Pfister K.K., Fay R.B., Witman G.B. Purification and polypeptide composition of dynein ATPases from chlamydomonas flagella. Cell Motil. 1982;2:525–547. doi: 10.1002/cm.970020604. [DOI] [PubMed] [Google Scholar]
- Pfister K.K., Fisher E.M., Gibbons I.R., Hays T.S., Holzbaur E.L., McIntosh J.R., Porter M.E., Schroer T.A., Vaughan K.T., Witman G.B., King S.M., Vallee R.B. Cytoplasmic dynein nomenclature. J. Cell Biol. 2005;171:411–413. doi: 10.1083/jcb.200508078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapali P., Szenes Á., Radnai L., Bakos A., Pál G., Nyitray L. DYNLL/LC8: a light chain subunit of the dynein motor complex and beyond. FEBS J. 2011;278:2980–2996. doi: 10.1111/j.1742-4658.2011.08254.x. [DOI] [PubMed] [Google Scholar]
- Raux H., Flamand A., Blondel D. Interaction of the rabies virus P protein with the LC8 dynein light chain interaction of the rabies virus P protein with the LC8 dynein light chain. J. Virol. 2000;74:1–6. doi: 10.1128/jvi.74.21.10212-10216.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert J.Y., Shelly A.L.B., Charles E.L. Enterochelin (Enterobactin): virulence factor for Salmonella typhimurium. Infect. Immun. 1979;24:174–180. doi: 10.1128/iai.24.1.174-180.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Crespo I., Yélamos B., Roncal F., Albar J.P., Ortiz de Montellano P.R., Gavilanes F. Identification of novel cellular proteins that bind to the LC8 dynein light chain using a pepscan technique. FEBS Lett. 2001;503:135–141. doi: 10.1016/s0014-5793(01)02718-1. [DOI] [PubMed] [Google Scholar]
- Rogers H.J. Iron binding catechols and virulence in Escherichia coli. Infect. Immun. 1973;7:445–456. doi: 10.1128/iai.7.3.445-456.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M.A., Spoden G.A., Florin L., Lambert C. Identification of the dynein light chains required for human papillomavirus infection. Cell Microbiol. 2011;13:32–46. doi: 10.1111/j.1462-5822.2010.01515.x. [DOI] [PubMed] [Google Scholar]
- Schneidman-Duhovny D., Inbar Y., Nussinov R., Wolfson H.J. PatchDock and SymmDock: servers for rigid and symmetric docking. Nucleic Acids Res. 2005;33:363–367. doi: 10.1093/nar/gki481. [DOI] [PMC free article] [PubMed] [Google Scholar]






