Abstract
Introduction
Body height and axial length (AL) increase during childhood with excessive axial elongation resulting in myopia. There is no consensus regarding the association between body growth and AL during refractive development. This study explored the association between change in body height, AL and refractive status over 4-years in children and young adults.
Material and Methods
Measures were collected biennially (timepoints: t1, t2, t3) (t1 n = 140, aged 5-20years). Non-cycloplegic autorefraction was obtained using the Shin-Nippon openfield autorefractor. AL, corneal curvature (CC) and anterior chamber depth (ACD) were measured by IOL Master. Body height (cm) was measured using a wall mounted tape measure. Refractive status was classified using spherical equivalent refraction (SER): persistent emmetropes (PE) (-0.50D to +1.00D), persistent myopes (PM) (≤-0.50D), progressing myopes (PrM) (increase of ≤-0.50D between timepoints), incident myopes (IM) (subsequent SER≤-0.50D) and persistent hyperopes (PH) (>+1.00D).
Results
Change in AL and change in height were correlated in the PE (all t:p ≤ 0.003) and the IM (t1-t2 p = 0.04). For every increase in body height of 1 cm: t1-t2: AL increased by 0.03 mm in the PE, 0.15 in the PM, 0.11 mm in the IM, 0.14 mm in the PrM, -0.006 mm in the PH. T2-t3: AL increased by 0.02 mm in the PE, 0.06 in the PM, 0.16 mm in the PrM, 0.12 mm in the IM and -0.03 mm in the PH.
Conclusions
In emmetropia body growth and axial elongation are correlated. In participants with myopia, body growth appears to stabilise whilst axial elongation continues at a much faster rate indicating dysregulation of normal ocular growth.
Keywords: Myopia, Axial length, Body height
Resumen
Introducción
La estatura y la longitud axial (LA) se incrementan durante la infancia, derivando en miopía el exceso de elongación axial. No existe consenso acerca de la asociación entre crecimiento corporal y LA durante el desarrollo refractivo. Este estudio exploró la asociación entre los cambios de estatura, LA y estatus refractivo a lo largo de un periodo de cuatro años en niños y adultos jóvenes.
Material y Métodos
Las medidas se recopilaron bianualmente (puntos temporales: t1, t2, t3) (t1 n = 140, edades de 5 a 20 años). Se obtuvo autorefracción no ciclopléjica utilizando el autorrefractor de campo abierto Shin-Nippon. Se midieron LA, curvatura de la córnea (CC) y profundidad de la cámara anterior (ACD) utilizando IOL Master. La estatura (cm) se midió utilizando una cinta medidora montada en la pared. El estatus refractivo se clasificó utilizando la refracción equivalente esférica (SER): emétropes persistentes (EP) (de -0,50D a +1D), miopes persistentes (MP) (≤-0,5D), miopes progresivos (MPr) (incremento de ≤-0,5D entre puntos temporales), miopes incidentales (MI) (SER subsiguiente ≤-0,5D) e hipermétropes persistentes (HP) (>+1D).
Resultados
Los cambios en cuanto a LA y estatura se correlacionaron en los sujetos EP todos los t:p ≤ 0,003 y los MI t1-t2 p = 0,04. Para cada incremento de estatura de 1 cm: t1-t2: LA se incrementó en 0,03 mm en los sujetos EP, 0,15 en los MP, 0,11 mm en los MI, 0,14 mm en los MPr, y -0,006 mm en los HP. T2-t3: LA se incrementó en 0,02 mm en los sujetos EP, 0,06 en los MP, 0,16 mm en los MPr, 0,12 mm en los MI y -0,03 mm en los HP.
Conclusiones
En los sujetos emétropes, el crecimiento corporal y la elongación axial se correlacionan. En los participantes miopes, el crecimiento corporal parece estabilizarse, mientras que la elongación axial se sigue produciendo a una tasa mucho más rápida, lo cual indica desregulación del crecimiento ocular normal.
Palabras clave: Miopía, Longitud axial, Estatura.
Introduction
The prevalence of myopia is increasing globally and even low levels of myopia are associated with an increased risk of ocular pathology in comparison to emmetropia.1 Myopic growth arises primarily from excessive axial elongation but the underlying mechanism is unknown. Axial length (AL) increases at the same time as body height in children, most rapidly during puberty, and compensatory mechanisms by other ocular structures such as lens thinning prevent the development of myopia in the emmetropic eye.2, 3 Various genetic and environmental influences including less time spent outdoors and greater time spent on near work4, 5 have been identified as risk factors for myopia but the precise mechanism is unclear. An interaction between genetics and education level is evident in European adults.6 Those with a high genetic risk in addition to a high level of education have a greater risk of myopia. This additive effect of education is likely to be attributed to less time outdoors and more time on intense near work. A large European study, the E3 study, has reported a significant relationship between higher education level and greater prevalence of myopia.7 Other studies show that the combined effect of high amounts of near work and low amounts of time spent outdoors lead to a greater risk of myopia in Australian children.8
Axial elongation and change in spherical equivalent refraction (SER) occur most rapidly during growth spurts in puberty.9 Children with an earlier peak in their body height exhibit earlier onset myopia than those with a later peak in height. The time of peak growth also occurs earlier in females than males.9 Although a global meta-analysis reported a higher prevalence of myopia in female children,10 myopic progression did not vary by gender in children of puberty age.11 The association between gender and myopia is conflicting with some studies reporting no difference by gender.12, 13
Anthropometric measures and refractive error were explored in Australian adult twins to determine if a ‘myopia body stature’ exists.14 The study reported that females of a heavier weight (greater than or equal to 80 kg) were at greater risk of myopia than those in the lightest quartile of weight.14 As time spent outdoors and physical activity were not analysed in this study, it is unclear if these factors influenced this association. Greater height is associated with a longer AL but not with refractive error in Asian adults.15, 16 In Asian children, a greater prevalence of myopia in subject groups with higher height has been reported.17, 18, 19 However, in Taiwanese children and in Danish conscripts, height was not independently associated with myopia.20, 21
A study in the UK explored growth trajectories during childhood (2.5–10 years) and resulting refractive error by the age of 11 and 15 years.22 Body growth and axial elongation were independently associated even after accounting for factors such as parental myopia, time spent outdoors and time spent reading for pleasure. The authors suggest this indicates that the scaling of eye and body size is controlled by a shared growth mechanism. Although participants with a greater than average rate of height increase were more myopic, this model predicted less than 1% of variation in refractive error in the cohort. Additionally, genetic markers for height were associated with corneal curvature (CC) but not associated with refractive error or AL by the age of 15 years. Conversely, a study exploring body growth patterns during pregnancy and early childhood reports that after accounting for gender and ethnicity, body growth parameters including greater height and greater head circumference were significant predictors for greater AL and greater CC by six years of age.23 Although there was a causal association between birth height, weight and greater AL, the genetic risk for height contributed to only 0.2% of the variance in AL.
To date, literature exploring the association between body growth, AL and refractive error is conflicting and further research is required to determine if greater body growth is associated with greater AL and myopia.
The aim of this study was to explore the association between change in body height, change in ocular biometry and change in refractive status over a four-year period in Caucasian children and young adults.
Material and methods
Ethical approval was granted by the Ethics Committee of the School of Life Sciences at Glasgow Caledonian University. A total of 140 participants (aged 5–20 years) were recruited from the patient database of an optometric practice in Duisburg, Germany. Duisburg has close to half a million residents and lies in the densely populated Ruhr Area in the Western part of Germany. The climate in Duisburg is temperate and rainfall occurs most months of the year. Children of 6 years of age attend primary school for 4 years. This is followed by secondary school for between 5–9 years.
Participants were selected from patient records and appointments were made for an initial study visit. All participants underwent a routine comprehensive eye examination during which the additional parameters required for the study were also determined.
Inclusion criteria included visual acuity of 6/6 or better in each eye. Exclusion criteria included those with ocular or systemic disease, strabismus, astigmatism greater than two dioptres or anisometropia greater than two dioptres. Existing or previous rigid gas permeable contact lens wearers were also excluded from the study. Participants wearing soft contact lenses removed contact lenses prior to measures. None of the participants were receiving myopia control treatment.
All measures were collected biennially over three timepoints (t1, t2 and t3) over a four-year period. Non-cycloplegic autorefraction with the Shin Nippon NVision-K 5001 infrared binocular open field autorefractor was used to measure refractive error. As the use of cycloplegia is not permitted in optometric practice in Germany, a Fresnel fogging lens (+3D) was mounted at the viewing window of the autorefractor to relax accommodation in hyperopic participants. Literature reports that fogging lenses produce a similar effect to cycloplegia in those with hyperopia and also reports no significant difference between cycloplegic and non-cycloplegic measures in those with myopia.24 Participants viewed a distance fixation target at 5.35 m and 5 measurements of refractive error were obtained from both eyes. Subjective refraction was also undertaken for every patient. At baseline the mean subjective refractive error (−0.41D (1.52D)) was more myopic than the mean non-cycloplegic autorefraction result (−0.21D (1.50D)) indicating that the use of the fogging lens in this study adequately controlled accommodation.
Mean SER was calculated as sphere + (1/2 cyl). Only values from the right eye were used in analysis due to the high correlation between right eye and left eye SER (r = 0.97, p ≤ 0.01).
Change in refractive status between t1 and t2 and between t2 and t3 in those with myopia and those without myopia was classified (Table 1), as described by Wong et al.25
Table 1.
Definition of refractive groups using change in spherical equivalent refraction (SER) between timepoints.
| Refractive group | Spherical Equivalent Refraction |
|---|---|
| Persistent emmetropesa | Between -0.50D to +1.00D |
| Persistent myopes | ≤-0.50D at each timepoint |
| Progressing myope | Initial SER of ≤-0.50D and increase of ≤-0.50D between timepoints |
| Incident myopia | Resulting SER of ≤-0.50D |
| Persistent hyperope | >+1.00D at each timepoint |
| Emmetropizing hyperopes | Initial SER>+1.00D and subsequent SER between -0.50D and +1.00D |
Mean change in the persistent emmetrope group was -0.11D (maximum change of −0.83D) over the four-year study period.
The Zeiss IOL Master was used to measure AL, CC and anterior chamber depth (ACD). Four measures of AL were taken and the average of these measures used in analysis. The average of at least three measures of CC and ACD were used in analysis.
Body height was used as an index of general growth.26 Body height was measured in centimetres using a standard protocol.27 The measurement was performed without shoes and with participants standing next to a wall mounted tape measure. A plate was placed on their head and body height measured.
Statistical analysis
The Skewness/Kurtosis test in addition to histogram inspection were used to determine if data were normal.
Longitudinal analyses
The association between change in height and change in AL between t1 and t2 and between t2 and t3 was explored. As these data were non-parametric, Wilcoxon-Mann-Whitney and Spearmans correlation (r) were used.
Cross sectional analyses
The association between height and ocular biometry by refractive group at t2 and at t3 was explored using a one-way ANOVA as these data were normal. Tukey post hoc test was used to explore pairwise comparisons. Due to the low number of emmetropizing hyperopes (EH) at t2 (n = 1) and t3 (n = 4) this group was excluded from analyses.
Parametric data are described using mean and standard deviation (SD). Non-parametric data are described using median and interquartile range (IQR).
Results
The cohort was predominantly Caucasian (99%). The majority of participants were from the city of Duisburg and the surrounding area. The dropout rate between t1 and t3 was 25%. There was no significant difference between participants who dropped out and remaining participants when compared by age at baseline (p = 0.29), SER at baseline (p = 0.25) or gender (X2 = 0.15, p = 0.70). Males and females were of a similar age at baseline (mean 12.9 (4.3) years vs 13.1 (3.8) years, respectively) (p = 0.70).
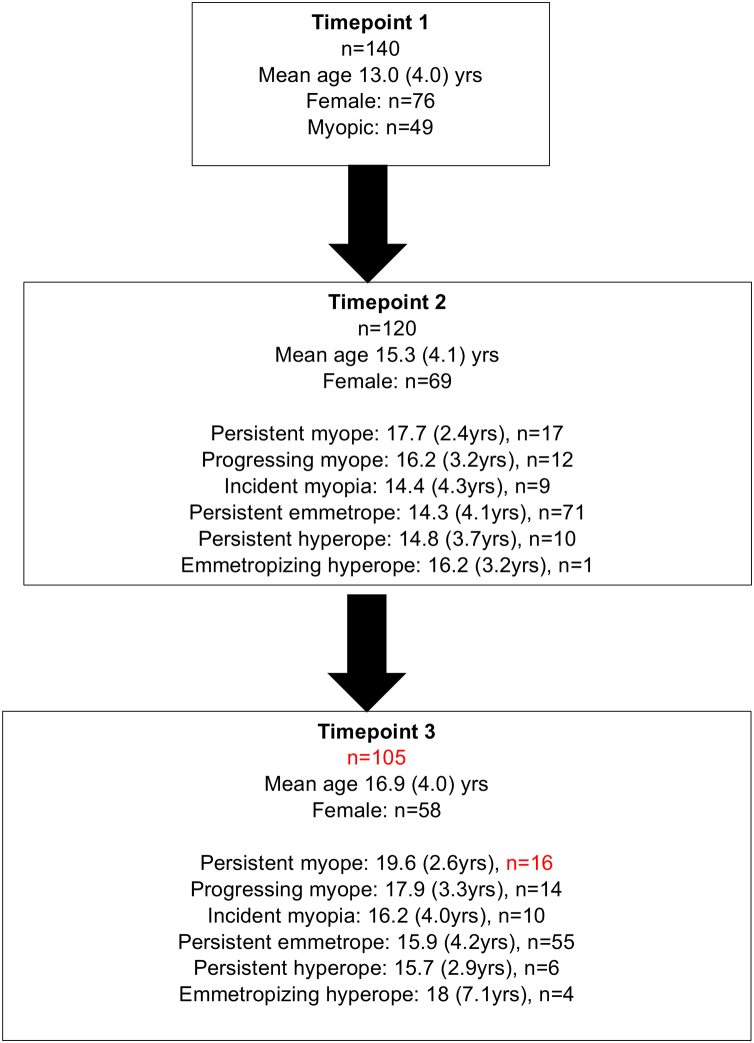
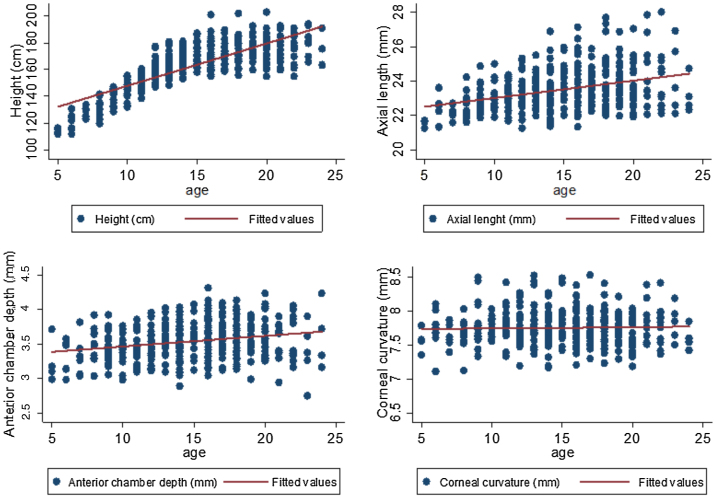
Change in height over the study period (between t1 and t3) was significantly greater in males (10 cm (IQR: 1.5 cm–23.5 cm) than in females (4 cm (IQR:1.0–17.5 cm) (p ≤ 0.01). Change in AL between t1 and t3 was not significantly different between males (0.15 mm (IQR: 0.06-0.43 mm)) and females (0.24 mm (IQR:0.09-0.57 mm) (p = 0.32). There was no significant difference in refractive group between genders at t2 (p = 0.65) or at t3 (p = 0.18). Fig. 1 illustrates the participant characteristics over the study period and Fig. 2 illustrates age and biometry (height, AL, ACD and CC) for all data combined.
Fig. 1.
Flow chart illustrating the number of participants and their means age (standard deviation (SD)) at each timepoint.
Fig. 2.
Age (years) and biometry (height, axial length, anterior chamber depth and corneal curvature) for all timepoints combined.
Change in axial length and height
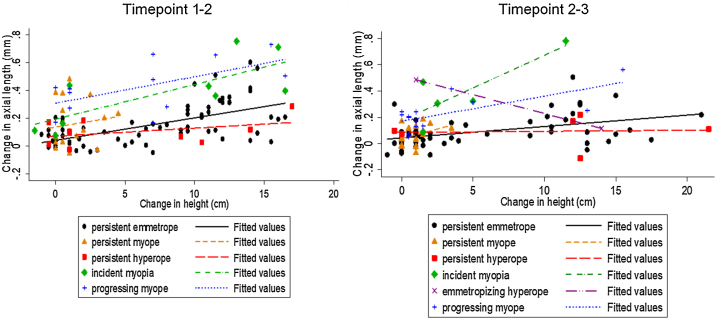
Change in AL and change in height between t1 and t2 were positively correlated in the persistent emmetrope (PE) (r2 = 0.59, p < 0.001) (y = 0.04 + 0.016x) and in the incident myope (IM) (r2 = 0.70 p = 0.04) (y = 0.19 + 0.025x) groups only (Fig. 3). Between t2 and t3, change in AL and change in height were significantly correlated in the PE group only (r2 = 0.40, p = 0.003) (y = 0.065 + 0.009x). A small number of participants appeared to have slight decreases in body height. This will be considered in the discussion.
Fig. 3.
Change in axial length (mm) and change in height (cm) between timepoints 1 and 2 and between timepoints 2 and 3 by refractive group.
Between t1 and t2, for every increase in body height of 1 cm AL increased by 0.03 mm in the PE group, 0.15 mm in the persistent myope (PM) group, 0.11 mm in the IM group, 0.14 mm in the progressing myope (PrM) group, -0.006 mm in the persistent hyperope (PH) group. Between t2 and t3, for every increase in body height of 1 cm, AL increased by 0.02 mm in the PE group, 0.06 in the PM group, 0.12 mm in the IM group, 0.16 mm in the PrM group and -0.03 mm in the PH group.
Change in anterior chamber depth and height
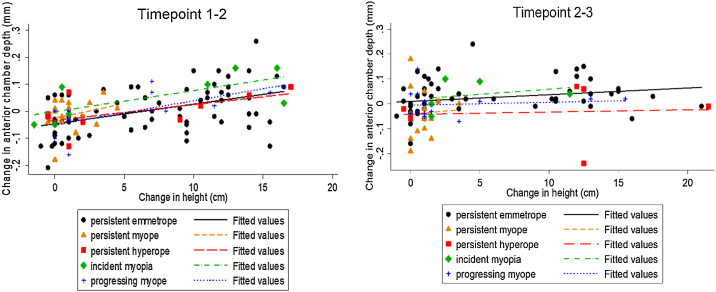
Change in ACD and change in height between t1 and t2 were significantly correlated in the PE (r2 = 0.45, p < 0.001) (y=-0.046 + 0.0072x) and the PrM (r2 = 0.70, p = 0.01) (y=-0.050 + 0.0089x) groups only (Fig. 4). Between t2 and t3 this correlation was only present in the PE group (r2 = 0.40, p = 0.003) (y = 0.011 + 0.0027×).
Fig. 4.
Change in anterior chamber depth (mm) and change in height (cm) between timepoints 1–2 and timepoints 2–3 by refractive group.
Between t1 and t2, for every increase in body height of 1 cm, ACD increased by 0.004 mm in the PE group, −0.02 mm in the PM group, 0.05 mm in the IM group, 0.006 mm in the PrM group and 0.01 mm in the PH group. Between t2 and t3, for every increase in body height of 1 cm, ACD increased by 0.02 mm in the PE group, −0.001 mm in the PrM group, −0.03 mm in the PM group, 0.04 mm in the IM group and −0.03 mm in the PH group.
CC did not change substantially over the study period. Change in CC and change height were not significantly correlated in any group (all p ≥ 0.17) (Fig. 5).
Fig. 5.
Change in corneal curvature (mm) and change in height (cm) between timepoint 1–2 and timepoint 2–3 by refractive group.
Cross-sectional analyses
There was a statistically significant difference in height between refractive groups at t2 (F(5,114) = 4.2, p < 0.05) and at t3 (F(5,90) = 3.69, p < 0.05) (Table 2). Pair wise comparison analysis indicated that PM were significantly taller than PE at t2 (p < 0.001) and at t3 (p < 0.001) (mean difference:4.41 cm). There was no significant difference between any other group comparisons (all p ≥ 0.17).
Table 2.
Summary statistics and one-way ANOVA analysis exploring the association between height, ocular biometry and refractive group at timepoint 2 and timepoint 3. Height, spherical equivalent refraction (SER), axial length (AL) and anterior chamber depth (ACD) significantly differed by refractive group. Corneal curvature (CC) was not associated with refractive group.
| Timepoint 2 |
Timepoint 3 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Height | AL | ACD | CC | SER | n | Height | AL | ACD | CC | SER | |
| Persistent myope | 17 | 177 (10.0) | 24.8 (1.1) |
3.7 (0.22) | 7.8 (0.24) |
−2.4 (1.2) |
16 | 180 (6.5) |
25.4 (1.0) | 3.8 (0.22) | 7.9 (0.25) | −3.0 (1.5) |
| Progressing myope | 12 | 173 (14.4) | 25.3 (1.1) |
3.85 (0.19) | 7.75 (0.29) |
−3.7 (1.43) | 14 | 173 (13.7) | 25.1 (1.1) | 3.8 (0.22) | 7.7 (0.18) | −3.73 (1.7) |
| Incident myopia | 9 | 164 (13.1) | 23.8 (1.3) |
3.53 (0.27) | 7.78 (0.34) |
−0.88 (0.30) | 10 | 163 (14.4) | 24.5 (1.1) | 3.8 (0.27) | 7.7 (0.20) | −1.9 (1.7) |
| Persistent emmetrope | 71 | 161 (16.1) | 23.0 (0.71) |
3.45 (0.25) | 7.74 (0.25) |
+0.37 (0.31) | 55 | 165 (13.2) | 23.1 (0.69) | 3.5 (0.23) | 7.8 (0.25) | 0.35 (0.33) |
| Persistent hyperope | 10 | 162 (15.4) | 22.4 (0.50) |
3.3 (1.2) |
7.8 (0.21) |
+1.8 (0.72) | 6 | 169 (11.4) | 22.4 (0.62) | 3.3 (0.23) | 7.8 (0.27) | 2.0 (0.77) |
| Emmetropizing hyperope | 1 | 150.00 |
22.3 | 3.43 | 7.56 | 0.93 | 4 | 173 (28.6) | 23.4 (0.82) | 3.3 (0.19) | 7.9 (0.32) | 0.48 (0.22) |
| One way ANOVA | F5,114 = 4.2 P = 0.002 |
F5,114 = 27.0 P<0.001 |
F5,11410.6 P<0.001 |
F5,114 = 0.29 P = 0.92 |
F5,114 = 113 P<0.001 |
F5,90 = 3.69 p = 0.004 | F5,97 = 29.4 P<0.001 |
F5,97 = 12.6 P<0.001 |
F5,97 = 1.7 P = 0.15 |
F5,97 = 63.4 P<0.001 |
||
There was a significant difference in AL between refractive groups at t2 (F(5,114) = 27, p < 0.001) and at t3 (F(5,97) = 29.4, p < 0.001). At t2, pairwise comparisons between PrM, PM and those without myopia were significantly different (all p ≤ 0.01). AL was significantly greater in the IM group in comparison to the PH group (p = 0.01) but there was no significant difference between the IM group and PE group (p = 0.12). At t3, pairwise comparisons between PrM, PM and those without myopia were significantly different (all p ≤ 0.002). Those with IM did not significantly differ from those without myopia (all p ≥ 0.07).
There was a significant difference in ACD between refractive groups at t2 (F(5,114) = 10.6, p < 0.001) and at t3 (F(5,97) = 29.4, p < 0.001). The PrM and PM groups had a significantly greater ACD those without myopia (all p ≤ 0.03). The IM group did not significantly differ from those without myopia (all p ≥ 0.18). CC did not significantly differ by refractive group at t2 (all p ≥ 0.96) or at t3 (all p ≥ 0.30).
Discussion
This study in Caucasian children and young adults reports that axial elongation and body growth are correlated in those with PE in agreement with research in UK children.22 Participants who became myopic exhibited a rate of axial elongation relative to body growth which was much greater than PE. The data indicate that those with PE would be expected to exhibit an increase in AL of 0.03 mm on average for every 1 cm increase in body height. This differs greatly in comparison to those with PrM who would be expected to exhibit an increase in AL of 0.15 mm on average for every 1 cm increase in body height. Although some of the group sizes were small, this intergroup comparison agrees with previous work on growth curves25 and reports that the scaling of body height and AL in myopia is dysregulated.
PM and IM groups were taller than non-myopes at t2 but the difference was not statistically significant. This may be due to the smaller sample size in these groups. Additionally, the PM group was older at baseline and did not exhibit as great an increase in height as the other refractive groups. Although myopia was not associated with greater body height in European adults,28, 29 AL increases at a greater rate in those of a younger age possibly attributed to puberty9 and therefore comparison with studies in adults may be misleading. A previous UK study in children reported that increasing body height was not a significant contributor to myopic growth by age 15 years.22 However, body height was measured between birth and 10 years of age when the cohort is unlikely to have reached puberty and the period of rapid body growth.
Change in height between t2 and t3 was not as great as change in height between t1 and t2 which is possibly due to the coincidence with the end of puberty and slowing of normal growth in the mid to late teenage years. Despite this, the proportion of participants who became myopic was greater between the last two timepoints. This again points to a breakdown of any coordinated growth between height and AL in those who became myopic. However, additional factors such as the time spent outdoors, near work and emmetropization are also likely to have affected this association but were not accounted for in this study.
Factors such as genetics, environment and diet are also likely to have influenced body growth and eye growth.30 Genetics contribute to 80% of variance in height with environmental factors such as diet also reported as contributing factors.31 Research exploring the association between genetics, height and AL is conflicting. A genetic link between height and AL has been reported in Asian twins32 but a genetic link between height and axial elongation was not reported in UK children.22 It is unclear why a mismatch between axial elongation and an increase in height in those with myopia occurs, most notably during growth spurts.
Although non-cycloplegic autorefraction was used to define refractive status, the effect of accommodation on refractive errors was minimised by using binocular open field distance refraction and fogging. Furthermore, the non-cycloplegic results did not significantly differ from subjective refraction. Northstone et al.22 report that non-cycloplegic autorefraction in a cohort just slightly older than this study would have resulted in only a small bias -0.22D (SD 0.84D) evident at each measurement phase in the current study. There was a small sample size in some of the group comparisons yet, when the data were combined into myopic and non-myopic groups to increase group numbers, myopes remained significantly taller than non-myopes at both timepoints (all p ≤ 0.001).
Males had a greater increase in height over the study period than females in agreement with previous research.33 There was a minority of participants who exhibited a small decrease in body height between timepoints. This may be due to variations in intra-observer measures which are similar to that reported in literature.27 The mean difference of the participants with a decrease in body height (0.63 cm) is within clinical tolerances.27 There was also a minority of participants who exhibited a modest decrease in AL most of which were in the PE groups. These participants also had a slight decrease in ACD indicating that an increase in lens thickness may have contributed to this anomaly.
Conclusion
This study reports that body height and axial elongation are correlated in those with stable emmetropia. AL increases at a greater rate than body height in myopia. This indicates that at a time when body growth is stabilising, axial elongation is unregulated.
Funding acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The Authors declare that there is no conflict of interest.
Acknowledgements
The Authors would like to acknowledge Dr Wolfgang Cagnolati and Carl Zeiss Meditec for their support with study equipment.
References
- 1.Flitcroft D.I. The complex interactions of retinal, optical and environmental factors in myopia aetiology. Prog Retinal Eye Res. 2012;31:622–660. doi: 10.1016/j.preteyeres.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Ojaimi E., Morgan I.G., Robaei D. Effect of stature and other anthropometric parameters on eye size and refraction in a population-based study of Australian children. Invest Ophthalmol Vis Sci. 2005;46:4424–4429. doi: 10.1167/iovs.05-0077. [DOI] [PubMed] [Google Scholar]
- 3.Saw S., Chua W., Hong C. Height and its relationship to refraction and biometry parameters in Singapore Chinese children. Invest Ophthalmol Vis Sci. 2002;43:1408–1413. [PubMed] [Google Scholar]
- 4.French A.N., Morgan I.G., Mitchell P., Rose K.A. Risk factors for incident myopia in Australian schoolchildren: The sydney adolescent vascular and eye study. Ophthalmology. 2013;120:2100–2108. doi: 10.1016/j.ophtha.2013.02.035. [DOI] [PubMed] [Google Scholar]
- 5.Sherwin J.C., Reacher M.H., Keogh R.H. The association between time spent outdoors and myopia in children and adolescents: A systematic review and meta-analysis. Ophthalmology. 2012;119:2141–2151. doi: 10.1016/j.ophtha.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 6.Verhoeven V.J., Buitendijk G.H. Consortium for Refractive Error and Myopia (CREAM) et al. Education influences the role of genetics in myopia. Eur J Epidemiol. 2013;28:973–980. doi: 10.1007/s10654-013-9856-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams K.M., Bertelsen G., Cumberland P. Increasing prevalence of myopia in europe and the impact of education. Ophthalmology. 2015;122:1489–1497. doi: 10.1016/j.ophtha.2015.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rose K.A., Morgan I.G., Ip J. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. 2008;115:1279–1285. doi: 10.1016/j.ophtha.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 9.Yip V.C., Pan C., Lin X. The relationship between growth spurts and myopia in Singapore children. Invest Ophthalmol Vis Sci. 2012;53:7961–7966. doi: 10.1167/iovs.12-10402. [DOI] [PubMed] [Google Scholar]
- 10.Rudnicka A.R., Kapetanankis V.V., Wathern A.K. Global variations and time trends in the prevalence of childhood myopia, a systematic review and quantitative meta-analysis: Implications for aetiology and prevention. Br J Ophthalmol. 2016;100:882–890. doi: 10.1136/bjophthalmol-2015-307724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Öner V., Bulut A., Oruç Y., Özgür G. Influence of indoor and outdoor activities on progression of myopia during puberty. Int Ophthalmol. 2016;36:121–125. doi: 10.1007/s10792-015-0091-5. [DOI] [PubMed] [Google Scholar]
- 12.Fan D.S.P., Lam D.S.C., Lam R.F. Prevalence, incidence, and progression of myopia of school children in Hong Kong. Invest Ophthalmol Vis Sci. 2004;45(4):1071–1075. doi: 10.1167/iovs.03-1151. [DOI] [PubMed] [Google Scholar]
- 13.Junghans B.M., Crewther S.G. Prevalence of myopia among primary school children in eastern sydney. Clin Exp Optom. 2003;86(5):339–345. doi: 10.1111/j.1444-0938.2003.tb03130.x. [DOI] [PubMed] [Google Scholar]
- 14.Dirani M., Islam A., Baird P.N. Body stature and myopia-the genes in myopia (GEM) twin study. Ophthalmic Epidemiol. 2008;15:135–139. doi: 10.1080/09286580801957751. [DOI] [PubMed] [Google Scholar]
- 15.Wong T.Y., Foster P.J., Johnson G.J., Klein B.E., Seah S.K. The relationship between ocular dimensions and refraction with adult stature: The Tanjong Pagar Survey. Invest Ophthalmol Vis Sci. 2001;42:1242. [PubMed] [Google Scholar]
- 16.Wu H.M., Gupta A., Newland H.S., Selva D., Aung T., Casson R.J. Association between stature, ocular biometry and refraction in an adult population in rural myanmar: The meiktila eye study. Graefes Arch Clin Exp Ophthalmol. 2007;35:834–839. doi: 10.1111/j.1442-9071.2007.01638.x. [DOI] [PubMed] [Google Scholar]
- 17.Chen J., Chen Z., Lin S. Correlation analysis for school-age children’s height and refractive errors. Adv Clin Exp Med. 2018;27:1125–1130. doi: 10.17219/acem/78773. [DOI] [PubMed] [Google Scholar]
- 18.Qian D., Zhong H., Li J., Niu Z., Yuan Y., Pan C. Myopia among school students in rural china (yunnan) Ophthalmic Physiol Opt. 2016;36:381–387. doi: 10.1111/opo.12287. [DOI] [PubMed] [Google Scholar]
- 19.Rim T.H., Kim S., Lim K.H., Kim H.Y., Baek S. Body stature as an age-dependent risk factor for myopia in a south korean population. Semin Ophthalmol. 2017;32:326–336. doi: 10.3109/08820538.2015.1088554. [DOI] [PubMed] [Google Scholar]
- 20.Huang C., Hou C., Lin K., Lee J., Yang M. Relationship of lifestyle and body stature growth with the development of myopia and axial length elongation in Taiwanese elementary school children. Indian J Ophthalmol. 2014;62:865–869. doi: 10.4103/0301-4738.141047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobsen N., Jensen H., Goldschmidt E. Prevalence of myopia in danish conscripts. Acta Ophthalmol (Copenh) 2007;85:165–170. doi: 10.1111/j.1600-0420.2006.00789.x. [DOI] [PubMed] [Google Scholar]
- 22.Northstone K., Guggenheim J.A., Howe L.D. Body stature growth trajectories during childhood and the development of myopia. Ophthalmology. 2012;120:1064–1073. doi: 10.1016/j.ophtha.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tideman J.W.L., Polling J.R., Jaddoe V.W.V., Vingerling J.R., Klaver C.C.W. Growth in foetal life, infancy, and early childhood and the association with ocular biometry. Ophthalmic Physiol Opt. 2019;39:245–252. doi: 10.1111/opo.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Queirós A., González-Méijome J., Jorge J. Influence of fogging lenses and cycloplegia on open-field automatic refraction. Ophthalmic Physiol Opt. 2008;28:387–392. doi: 10.1111/j.1475-1313.2008.00579.x. [DOI] [PubMed] [Google Scholar]
- 25.Wong H., Machin D., Tan S., Wong T., Saw S. Ocular component growth curves among Singaporean children with different refractive error status. Invest Ophthalmol Vis Sci. 2010;51:1341–1347. doi: 10.1167/iovs.09-3431. [DOI] [PubMed] [Google Scholar]
- 26.Leung J.T., Brown B. Progression of myopia in Hong Kong chinese schoolchildren is slowed by wearing progressive lenses. Optom Vis Sci. 1999;76:346–354. doi: 10.1097/00006324-199906000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Berkson S.S., Espinola J., Corso K.A., Cabral H., McGowan R., Chomitz V.R. Reliability of height and weight measurements collected by physical education teachers for a school-based body mass index surveillance and screening system. J Sch Health. 2013;83:21–27. doi: 10.1111/j.1746-1561.2012.00743.x. [DOI] [PubMed] [Google Scholar]
- 28.Jacobsen N., Jensen H., Goldschmidt E. Prevalence of myopia in danish conscripts. Acta Ophthalmol (Copenh) 2007;85:165–167. doi: 10.1111/j.1600-0420.2006.00789.x. [DOI] [PubMed] [Google Scholar]
- 29.Rosner M., Laor A., Belkin M. Myopia and stature: Findings in a population of 106,926 males. Eur J Ophthalmol. 1995;5:1–6. doi: 10.1177/112067219500500101. [DOI] [PubMed] [Google Scholar]
- 30.Roberts J.L., Stein A.D. The impact of nutritional interventions beyond the first 2 years of life on linear growth: A systematic review and meta-analysis. Adv Nutr. 2017;8:323–336. doi: 10.3945/an.116.013938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McEvoy B.P., Visscher P.M. 2009, genetics of human height. Econ Hum Biol. 2009;7:294–306. doi: 10.1016/j.ehb.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J., Hur Y., Huang W., Ding X., Feng K., He M. Shared genetic determinants of axial length and height in children: The Guangzhou twin eye study. Arch Ophthalmol. 2011;129:63–68. doi: 10.1001/archophthalmol.2010.323. [DOI] [PubMed] [Google Scholar]
- 33.Bong Y.B., Shariff A.A., Mohamed A.M., Merican A.F. Growth curves for school children from kuching, sarawak: A methodological development. Asia Pac J Public Health. 2015;27:1217–1227. doi: 10.1177/1010539512446959. [DOI] [PubMed] [Google Scholar]