Abstract
This study emphasis the production of yellow pigment from endolichenic Bacillus sp. isolated from the lichen Dirinaria aegialita (Afzel. ex Ach.) B.J. Moore. Yellow pigment-producing twenty different strains were investigated. The hyperactive pigment-producing bacterial strain was identified as Bacillus gibsonii based on 99 % sequence similarity. Maximum bacterial pigment production appeared in Luria Bertani medium. Methanol extraction of the pigment and its partial purification using TLC was carried out. Furthermore, isolated pigments were characterized using UV-visible spectroscopy, FTIR spectroscopy, and GC-MS results related to the possibility of the carotenoid occurrence. The pigment also exhibited efficient antifungal activity against selected fungal pathogens of economic importance. Likewise, the pigment extract evaluated for the total antioxidant potential using Phosphomolybdenum and Ferric reducing antioxidant power assay and the results represented in Ascorbic Acid Equivalent (AAE)- 21.45 ± 1.212 mg/mL. The SC50 of the pigment extract found to be 75.125 ± 0.18 µg/ml determined by the ABTS assay.
Keywords: Pigment, Lichens, Bacillus sp., Antifungal activity, FTIR, GC-MS
1. Introduction
Recently, human well-being and environmental conservation have gained importance, leading to concerns regarding food safety issues, such as those arising due to indiscriminate use of food colors. Color additives are the main ingredients of many products that make it look attractive and tasty. FDA is responsible for ensuring that all foods containing color additives are safe for consumption. Various synthetic pigments applied in food materials, cosmetics and pharmaceutical industries have different harmful side effects (Venil and Lakshmanaperumalsamy, 2009).
To counteract these ill impacts of synthetic colorants, there is a global attention in making pigments from natural sources. Basically, natural pigments (NP) are colored, water-insoluble, and organic solid powders. They are available in a wide range of colors and are not have any side effects with the substrate in which they are added. The demand for utilization of NP in the aforementioned industries is increasing currently (Unagul et al., 2005). Sources of these NP can be either plants or microbes. The approved NP mined from plants have frequent negatives: instability, low-water solubility, and limited availability during the year. Thus, recent research focuses on pigment production from microorganisms. The reasons for high interest in using microbes as pigment sources are as follows: fast and easy growth rate of microbes using low-cost culture medium, independent in the conditions of weather, and wide range of available color(s). So, production of the pigments using microbes is one of the major emerging research field having demonstrated its latent for several industrial applicants (Parekh et al., 2000).
Pigments such as Quinones, Carotenoids, Violacein, Indigo and Melanins are produced from microbes (Dufossé, 2006). Carotenoids are among the most diverse natural products. Because of antioxidant properties, microbial carotenoids are known for their nutrition, which prevents degenerative diseases to enhance the immune response in both the humans and animals (Kirakosyan et al., 2003). There are many sources of pigment-producing bacteria, but soil and marine sources being the most common ones. The unique organization of lichens provides unexplored sources of bacterial communities (Grube et al., 2009), particularly pigmented bacteria. Lichens are composite, symbiotic systems, consisting of two or more closely interacting organisms: a fungus, and one or more partners, called photobionts (Grube and Berg, 2009). Lichens provide a stable consortium and habitat for other micro-organisms (i.e. bacteria, fungi) (Panosyan, 1966), which resides beneath the tissue at epidermal cell-layer, without affecting any damage to the host (Stone et al., 2000). The diversity of endophytes is high, and they grow relatively fast on routinely used laboratory media, producing large amounts of novel compounds. Recent research proves that endophytic microorganisms are the new, potential sources of novel natural products (Stone et al., 2000). At present, limited studies exist for standardized pigment production from endolichenic bacteria. Hence, this investigation focused on isolating pigmented bacteria from lichen, harnessing their potentially bioactive compounds, followed by their separation. The pigment isolated here also exhibited efficient antifungal activity against selected fungal pathogens of economic importance.
2. Materials and methods
2.1. Microorganisms and chemicals
An endolichenic bacterium (DBS4 strain) was isolated from the lichen Dirinaria aegialita from the terrestrial region of Manjeri, Kerala, and India. Analytical graded chemicals were used.
2.2. Morphological and biochemical properties
The biochemical properties of the DBS4 strain was determined by carbohydrate fermentation, hydrolysis of starch, lipid and gelatin, indole, methyl red, citrate, urease, oxidase, and catalase tests, according to the standard microbiological methods. The morphology and motility of the strain were observed microscopically.
2.3. DBS4 identification using MALDI-TOF MS analysis
The isolates DBS 4 subjected to MALDI-TOF MS (Bruker Daltonik GmbH, German) analysis for the species level identification. Initially, the direct transfer of single colony forming unit (CFU) of the DBS4 to the MALDI target plate. Followed by the coating with the matrix solution 10 mg/mL of alpha-cyano-4-hydroxy cinnamic acid solution in acetonitrile: trifluoro-acetic acid (50:2.5%) and allowed them to dry. The mass spectral analysis was performed using the software Bruker BioTyper 3.1 (Germany). E. coli DH5 alpha used for external calibration of mass sectra. The MALDI target plate was placed inside the microflex LT MALDI-TOF mass spectrophotometer for the spectral analysis and data interpretation. The identification results represented in logarithmic score ranging from 0 to 3.0 by comparing with the peaks profile of the known organism available in the Bruker database. However, the MALDI Biotyper score ranging from 2.3 to 3.0 represent high probability species identification (+++), range between 2.0 and 2.299 genus-level identification, probable species identification (++), range 1.7–1.999 are probable genus identification (+) and ranges between 0 and 1.699 are considered as not reliable for identification (−).
2.4. Analysis of 16S rRNA gene
Genomic DNA was extracted by following the procedure described by Alharbi, 2015 (Alharbi et al., 2015). The DNA amplification of pigmented Bacillus sp. DBS4 was performed using PCR thermocycler (Applied Biosystem). Study of 16s rRNA gene and its amplification performed using specific primer 27F/1492R oligonucleotides for the bacterial consensus region. With the help of Montage PCR kit (Millipore, US) purification was performed for the amplified PCR products. Sanger-Sequencing was performed. (Alharbi et al., 2015, Batais et al., 2019). With the help of NCBI program (https:// www.ncbi.nlm.nih.gov/BLAST), sequence data were arranged to analyze-identify the bacterium. Percentages of sequence resemblances with closest known species were compared. As per ClustalW program, sequences were aligned and using the MEGA6 software, the phylogenic tree was constructed.
2.5. Pigment production media
Three different media were used in this investigation to study the production of bacterial pigment. Luria Bertani (LB) Yeast extract from broth (5 g/L), Peptone-10 g/L and Nacl2-5 g/L. The NB will be defined as nutrient broth as beef extract-3 g/L; Peptone (5 g/L) and Nacl2-6 g/L and minimal medium such as NA3PO4-6 g/L; KH2PO4-3 g/L; Nacl2-0.5 g/L; NH4Cl-1 g/L; MgSO4 (1 M), CaCl2 (0.1 M), 10% glucose (w/v). To test the effect of salinity, LB broth was used to grow the DBS4 strains in 16% salinity. A 250 mL of conical flasks consists of 100 mL of sterile media was used to grow the bacterial inocula and placed in a incubating shaker at 100 RPM-30 °C for 48 h and placed in static for 3–4 days.
2.6. Pigment extraction
Solvents like acetone, ethanol, methanol and petroleum ether were scrutinized to extract the intercellular water-insoluble pigment. Pellets were mixed with the most effective solvent, methanol, then vigorously vortex for 1 min and finally incubated at 60 °C for 15 min in a water bath. Until unless the extracted pigments were visible and then centrifuged at 6000 rpm – 15 min. By applying the Whatmann filter paper, colored supernatant was dispersed (Sasidharan et al., 2013). This process will be completed until the pellet removes color. A similar protocol will be applied for the remaining solvents.
2.7. Pigment separation
Active crude pigment in TLC was separated using Silica gel (5 g silica and 0.1 g calcium sulfate in 10 mL sterile distilled water, dimensions 12 cm × 4 cm) as the stationary phase. For standardization of mobile system for TLC, following solvent systems were selected for facilitating proper separation of pigment components: petroleum and acetone (1:1), petroleum ether and ethyl acetate (9:1), petroleum and benzene (49:1), benzene and acetone (2:1) (Godinho and Bhosle, 2008), hexane and methanol (2:3), (Khaneja et al., 2010) and chloroform and methanol (4: 1) (Girija et al., 2014). The yellow-pigmented band was scraped from the silica plate and eluted with methanol.
2.8. Characterization of pigments
2.8.1. Ultraviolet–visible spectroscopy
UV–Visible Spectrophotometer (Eppendorf, USA) was used to characterize the pigments. The data was recorded between the 250–700 nm wavelengths. The absorption spectra of the same samples were also measured at 450 nm to compare with the standard β-carotene pigment, and methanol was used as a blank.
2.8.2. Fourier transformed infrared spectrum
The infra-red spectra of both crude and purified methanolic extracts of pigment were analyzed by liquid FTIR using a Shimadzu Irtracer-100 spectrometer at the International Research Centre [IRC], Kalasalingam University, Krishnankovil, Tamil-Nadu, India.
2.8.3. GC-MS analysis
The technique GC-MS was performed with the extracted pigments using Perkin-Elmer GC clarus system armed with an Elite-I capillary coloumn coated with a silica. 70 eV of Ionizing energy was used. Maximum (99.99%) of Helium gas was used 2 µL of injection volume with 10:1 of split ratio along with the temperatures of injector (250 °C) and Ion (280 °C). The programmed oven temperature was 110 °C, 200 °C and 280 °C at various degrees/mins. The total time duration for succeeding the reaction was 36 min. Similarly, the relative peak area (peak %) of each compound was calculated by comparing its average of individual peak area to the total areas, using the software Turbomass, designed especially to analyze the mass spectra and peak chromatograms. Based on NIST referenced database, GC-MS was implemented.
2.9. Antimicrobial activity of methanolic extract of pigment
Pigment was tested using the agar well-diffusion method (Grammer, 1976). Different bacteria i.e., Staphylococcus aureus, Bacillus cereus, Pseudomonas aeruginosa, and Cuproidus alkaliferus were used for the antibacterial activity. Similarly, streptomycin (1 mg/mL, 100 µL) and methanol were used as positive and negative controls respectively. At the same time, The antifungal activity against different fungi, namely R. solani, F. oxysporum, and S. rolfsi, were tested. Terbinafine hydrochloride (1 mg/mL) commercial antifungal agent was used as a positive control and methanol (100 µL) as a negative control.
2.10. Antioxidant activity of the pigment extract
2.10.1. Total antioxidant assay
Total antioxidant activity of methanolic extract of pigment was determined as follows; 0.05 mL of test solution (range 20–200 µg) was added to the 0.95 mL of reagent solution (0.6 m/L−1 sulphuric acid; 28 mM/L sodium phosphate and 4 mM/L ammonium molybdate). The reaction combination was incubated at 95 °C for 1 h 30 min and the absorbance was read at 695 nm. Instead of the test sample methanol was used as negative control and all the reaction was carried out in triplicate. The calibration curve for the standard ascorbic acid was formed (Prieto et al., 1999). The total antioxidant activity of the test sample was shown as ascorbic acid equivalents per gram of dry weight of the extracted pigment.
2.10.2. Reducing power assay
Ferric reducing antioxidant power assay was performed as described by Oktay et al. (2003). The pigment extract was taken in various concentrations (0.1–5 mg/mL) were added to the 100 µL-phosphate buffer and 150 µL-potassium ferricyanide was incubated at 50 °C for 20 min. Followed with the addition of 150 µL Trichloroacetic acid the mixture was centrifuged at 3000 rpm for 10 min. Top layer of the mixture was collected and mixed with 1.5 mL of ferric chloride solution. Similarly, the blank was set using the above-mentioned mixture without the pigment extract and finally the absorbance was read at 700 nm. Ascorbic acid with varying concentrations used as antioxidant standard.
2.10.3. ABTS radical scavenging assay
The ABTS diammonium salt radical based decolorization assay was used to study the antioxidant property of the pigment extract. Initially, an equal volume of 7 mM ABTS with 2 mM potassium persulphate was mixed and kept in dark condition at 37 °C for 16 h. The solution was further diluted with methanol until the absorbance reaches 1.1 ± 0.05 at 734 nm. The ABTS free radical scavenging property was evaluated by mixing 1 mL ABTS solution with different concentrations of methanolic extract of pigment. The final absorbance of the reaction mixture was read at 734 nm (Sowndhararajan and Kang, 2013). The free radical scavenging activity was represented in inhibition percentage using the formula:
The IC50 value of the pigment was calculated from the sample concentration required to scavenge 50% of ABTS radicals.
3. Results and discussion
3.1. Morphological and biochemical properties
The bacterium DBS4 appeared as yellow colored colony on the nutrient agar medium. After four days of incubation, the color of the colonies changed to bright yellow. The bacterial strain properties were determined to be gram-positive, non-motile, rod-shaped, forming flat colonies on the nutrient agar surface. Indra Arulselvi et al. (2014) reported a similar yellow pigment-producing bacteria which showed morphological characteristics similar to bacterium DBS4. Similarly, Bhat et al. (2013) isolated 13 different yellow pigments producing, non-motile, Gram-positive bacteria from various foodstuffs, soil, and air samples. Results of several biochemical tests confirmed that the strain DBS4 able to assimilate glucose and fructose as carbon source. They can hydrolyze starch, lipid, and gelatin, and showed positive results for citrate and catalase tests (Table 1). Sasidharan et al. (2013) isolated catalase-positive, indole and methyl red-negative yellow-pigmented bacteria from soil samples, and Manachini et al. (1985) reported glucose- and fructose-utilizing Bacillus spp. isolated from compost.
Table 1.
Biochemical characteristics of strain DBS4.
| Biochemical characteristics | Strain DBS4 |
|---|---|
| Assimilation of carbon source | |
| Glucose | + |
| Fructose | + |
| Maltose | – |
| Mannitol | – |
| Starch | – |
| Extracellular enzymatic activity | |
| Starch | – |
| Lipid | + |
| Gelatin | + |
| Other biochemical activities | |
| Indole | – |
| Methyl Red | – |
| Citrate | + |
| Urease | – |
| Oxidase | – |
| Catalase | + |
3.2. Molecular characterization
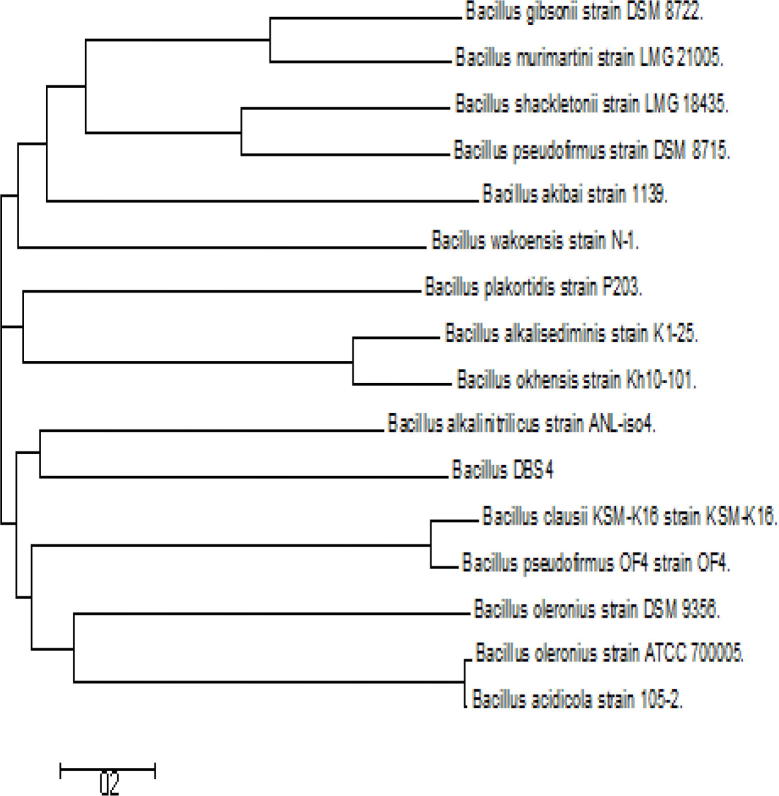
In MALDI TOF MS based identification of DBS4 shows less congruency with 16S rRNA sequence result. Though the DBS4 strain identified as Bacillus gibsonii in both the identification method still the relativeness of confirming the strain up to genus level through MALDI TOF MS (score value 1.464, Rank quality “–”) is found to be not effective as compared to the 16S rRNA sequencing (Fig. 1). Whereas, the strain DBS4 was identified as Bacillus spp., based on homology alignment of the 16S rRNA sequence from the GenBank database, which showed 99% homology with Bacillus gibsonii. The phylogenetic tree based on the16S rRNA gene sequence is displayed in Fig. 2, and it shows the homology relationships of the DBS4 strain with another different Bacillus spp.
Fig. 1.
DBS4 strain identification using MALDi-TOF MS analysis.
Fig. 2.
Phylogenetic tree showing the relationship between the isolate DBS4 and related microorganisms in the Bacillus sp.
3.3. Pigment production and extraction
The production of pigment in media mainly depends on the media components. Pigment production and biomass were higher in LB media, as compared to that with NB media and minimal media, ascertained by color intensity tests. The formation of pigments depends on nutrient availability in the media (Grossart et al., 2009). The growth of the bacteria and its pigment synthesis increased at 5% of NaCl concentration, and further increase in NaCl concetration resulted a decreasing trend. Mohana et al., 2013 studied the influence of NaCl on pigment synthesis of Micrococcus spp. up to a concentration of 7%. B. gibsonii DBS4 also produced maximum pigment in medium containing 5% NaCl. Bhat et al. (2013) demonstrate the effect of salt concentration over yellow pigment-producing bacteria isolated from different foods. It was observed that 8% NaCl resulted in high pigment production. The influence of salinity in production of pigment may vary with organisms and it could also depend on their location of isolation and existence (Grossart et al., 2009).
Among various solvents with different poloarity tested for extraction of pigment, methanol was found to be an effective solvent as compared to others. This may be due to differences in polarities of other solvents and nature of the pigment itself. Manachini et al. (1985) reported extraction of the pigment using different solvents. They used methanol, ethanol, ethyl acetate, chloroform, and petroleum ether and found that maximum pigment yield using methanol extraction.
3.4. Pigment purification
In TLC, the pigments were separated with resolving solvent mixture contains chloroform and methanol at 4:1 ratio. Three different bands were observed on TLC plate. Fraction 1 has Rf value 0.83, which was similar to the Rf value of carotenoid hydrocarbon. The Rf value for fraction 2 was 0.77, corresponding to carotenoid ketones, while that of fraction 3 was 0.69, corresponding to hydroxylated carotenoid (Godinho and Bhosle, 2008). The Rf value of β-carotene was reported as 0.84 by Jagannadham et al. (1991). The absorption spectra showed a single peak for fraction 1 at 450 nm and for fraction 2 at 421 nm. The TLC plate showed pigment separation bands under normal white light, UV light, and on exposure to iodine vapors. However, spraying the plates with 10% alcoholic sulfuric acid reagent and 0.1% alcoholic ferric chloride did not produce any bands. These results were concurrent with the earlier studies of Girija et al. (2014).
3.5. Characterization of pigment
3.5.1. Ultraviolet –Visible spectroscopy
The absorption spectra of methanolic extract of pigment showed three peaks at 450 nm, 427 nm, and 343 nm. The maximum absorption was at 450 nm can be correlated with the absorption spectrum of carotenoid derivatives. The maximum absorption of the pigment between wavelengths of 300–600 nm indicates the presence of carotenoids (Gross, 2012). A typical pattern of absorption spectrum of alloxanthin was also discussed by (D'souza and Gauns, 2018). Moreover, the earlier research reported similar absorption spectra of carotenoids related derivatives from bacterial isolates (Sahin, 2011, Takaichi et al., 2003, Zaibunnisa et al., 2011).
4. FTIR spectroscopy
4.1. Solid Fourier transform infrared spectrum of crude sample
The characteristic transmission peaks of the crude sample were observed at 3423.65, 2924.09, 1625.99, 1390.68, 1076.28, 914.26, and 727.16 cm−1. The peak at 3423.65 cm−1 is due to the presence of OH bond in phenols, one of the typical features of β-carotene (Köcher et al., 2009). The peak at 2924.09 cm−1 indicates the presence of C-H aliphatic bond.
4.1.1. Liquid FTIR spectroscopy
The crude pigment extract showed similar results in liquid FTIR as those in the solid FTIR. FTIR result of purified pigment was also analyzed (Fig. 3). The main bonds identified in both the results are listed in Table 2. It shows some bond stretches that are also present in carotenoids. Accurate characteristics of carotenoids are the intense coloration results through the chain of conjugation of double bonds, act as chromospheres. Color variation in the carotenoids rises through enormous at their specific number of conjugated double bonds (Perez-Fons et al., 2011) (Table 3).
Fig. 3.
Liquid Fourier Transformed Infrared spectrum for crude (a) and purified pigment (b).
Table 2.
Bonds in crude pigment extract identified by liquid FTIR.
| Serial no. | Functional group (crude) | Frequency (cm−1) | Functional group (purified) | Frequency cm−1 |
|---|---|---|---|---|
| 1 | –OH bonds in phenols | 3664.753657.90 | –OH bonds in phenols | 3630.663614.60 |
| 2 | –C=O in ß-diketons | 1635.64 | –C–H aliphatic bonds | 2935.66 |
| 3 | –C–H aliphatic bonds | 2910 | C–H aromatic | 846.75800.46 |
| 4 | C–H aromatic Alkenes | 846.75669.30 | C–C=O bond | 586.21 |
| 5 | C–C=O in aldehyde | 563.21 |
Table 3.
Antioxidant activity of Bacillus sp. DBS4 pigment methanolic extract.
| Sample | Total antioxidant activity | Ferric reducing anti-oxidant power assay | Percentage of ABTS scavenging activity (SC50) (µg/ml) |
|---|---|---|---|
| Ascorbic Acid Equivalent (AAE) (mg/mL) | |||
| MeOH extract of pigment | 21.45 ± 1.212* | 2.92 ± 0.33 | 75.125 ± 0.18 |
Phosphomolybdenum assay, Data are presented as a mean ± standard deviation of (n = 3).
4.1.2. GC-MS analysis
The chemical composition of methanol extract of crude and purified pigment was analyzed using GC-MS and the results are shown in Fig. 4. The major components were Fumaric acid and 2-ethylacridine. Other compounds such as tetrasiloxane, decamethyl, coumarin, and 2-nonadecanol, were also reported in the crude pigment extract. However, GC-MS analysis of purified pigment showed several compounds. Majorly, siloxane, octasiloxane, heptasiloxane, and hexasiloxane are reported in many bacteria, and their antioxidant and antimicrobial activities have been investigated (Mukherjee and Singh, 2011, Schauer et al., 2005, Boo et al., 2012, Konzen et al., 2006, Venil et al., 2013). Sometimes the presence of polyunsaturated fatty acid (PUFA) like complex molecules get substituted with one or more hydroxyl and aldehyde groups to give a new form of pigment derivative such as laetiporic acid isolated wood rotting basidiomycete (Davoli et al., 2005). Also, the pigment of our interest may have interlinked with siloxane, octasiloxane like fatty acid methyl esters. Since the carotenoids can be found in both free and esterified forms. However, the peak at m/z 470 (M-106) was characteristic of carotenoid (Armstrong et al., 1990). Some other peaks at M-105 and M-104 were also present at negligible percentages. Therefore, the presence of carotenoid pigments in methanolic extract was validated.
Fig. 4.
Gas Chromatography Mass Spectrum for crude (a) and purified (b) pigment extract.
4.2. Antimicrobial activity of pigment extract
After incubation at 37 °C for 24 h, the plates showed no zones of inhibition around the agar wells. Therefore, we concluded that the methanolic extract of pigment does not have any antibacterial activity. The antifungal activity was analyzed based on the inhibition of mycelial growth in fungus. Here, the antifungal activities of both crude and purified pigments were observed after three days of incubation against three fungi: R. solani, F. oxysporum, and S. rolfsi. The fungal growth at the center of the test Petri plate with pigment extract was restricted, while there was no inhibition in the control plate (Fig. 5). The activity of pigment extract near each fungus was different. The pigment extract showed effective inhibition against S. rolfsi and R. solani. The length of the mycelia was reduced, as compared to that in the control, along with shrinkage of hyphae. Curling and lysis of fungal mycelia were also observed in the tested fungi (Fig. 5). These results showed that the pigment does not have any antibacterial activity, but shows effective antifungal activity. The similar findings of Liu et al. (2008) reported the soil-based bacillus subtilis G8 producing antifungal volatile organic compounds (VOCs) prevent the growth of soil-borne fungal pathogen up to 93% inhibition. Similarly Bacillus amyliliquefaciens SH-B10 isolates of sea sediment reported for producing potent antifungal agent fengycin A and aminobuturic acid (Chen et al., 2010). Likewise, yellow pigmented caratenoid produced by alkaliphilic Bacillus sp. and their biological activity was discussed elsewhere (Aono and Horikoshi, 1991).
Fig. 5.
Antifungal activities of pigment extract on third day of incubation.
4.3. Antioxidant potential of pigment
Generally, pigments are well known for antioxidative potential. However, the colored pigment compounds are potential pharmaceutical ingredients that provides several health benefits.
4.3.1. Total antioxidant activity
The total antioxidant potential of the pigment extract was evaluated using Phospomolybdenum test. In which the reduction of Mo(VI) to Mo(V) by the formation of green phosphate/Mo(V) complex was quantitatively estimated for total antioxidant activity which is expressed as the number of equivalents of ascorbic acid (Prieto et al., 1999). The pigment has the total antioxidant activity of 21.45 ± 1.212 mg of ascorbic acid equivalents per gram of sample.
4.3.2. Reducing power assay
The reducing activity of methanolic extract of pigment was analyzed. The pigment gives inhibition result in terms of activity of ascorbic acid standard. It was found to be 2.92 ± 0.33 mg of ascorbic acid equivalent per gram of sample. In the presence of antioxidants, it act as strong reducing agent causes a reduction of Fe3+ to Fe2+ ions. Thus the absorbance of the reaction mixture at 700 nm increase which inferred the antioxidant properties are associated increased in reducing power as described by Oktay et al. (2003).
4.3.3. ABTS radical scavenging activity
ABTS scavenging assay has several advantages over other methods. ABTS is soluble in wide range of solvents and helpful in evaluating the antioxidant potential in various assay media. Similarly the ABTS reaction with sample is rapid, require less time to reach the steady-state condition. The ABTS scavenging activity of methanolic extract of pigment was analyzed. The concentration of pigment where the inhibition is reduced by half (IC50 Value) was found to be 75.125 µg/ml. from this result it is inferred that the pigment could result less scavenging activity. In ABTS assay, the measurement of decrease in ABTS radical based decolorization in the presence of anti-oxidants is the quickest method to screen antioxidant activity (Sowndhararajan and Kang, 2013). Our results correlate with the findings of Kai Zhong et al. (Zhong et al., 2011) who reported the yellow-colored pigment obtained from rhizosphere associated Streptomyces sp. possess ABTS radical scavenging activity of 172.43 ± 20.19 µg/mL. From these we conclude the microbial pigments are considered to be potential antioxidant compound that detoxifies free radicals and protect various cell molecules from oxidative damages.
5. Conclusion
In this study, we were able to isolate the yellow pigment producing Bacillus sp. from the lichen D. aegialita. The polyphasic characterization of this bacterium reveals that this strain showed similarity with B. gibsonii. The maximum pigmentation was observed in LB medium with 5% salinity. Methanol was confirmed as an appropriate solvent for extracting this bacterial pigment. Pigment separation was carried out using TLC with chloroform and methanol (4:1) as a standardized solvent system. The absorption spectrum and FTIR spectrum of the pigment compared relatively with the spectral pattern of carotenoid. Further the GC-MS results of pigments revealed all possible compounds in the combination. The antimicrobial and antioxidant activity of the pigment was studied, and the pigment was found to possess antifungal and antioxidant activity. Future studies can be focused on the process design for large-scale production of the pigment for industrial use.
Acknowledgments
Acknowledgments
The authors express their sincere gratitude to the Researchers Supporting Project number (RSP-2019/70), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare no conflict of interest
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Shine Kadaikunnan, Email: sshine@ksu.edu.sa.
Shyam Kumar Rajaram, Email: kingshyam2003@gmail.com.
References
- Venil C.K., Lakshmanaperumalsamy P. An insightful overview on microbial pigment, prodigiosin. Electron. J. Biol. 2009;5(3):49–61. [Google Scholar]
- Unagul P., Wongsa P., Kittakoop P., Intamas S., Srikitikulchai P., Tanticharoen M. Production of red pigments by the insect pathogenic fungus Cordyceps unilateralis BCC 1869. J. Ind. Microbiol. Biotechnol. 2005;32(4):135–140. doi: 10.1007/s10295-005-0213-6. [DOI] [PubMed] [Google Scholar]
- Parekh S., Vinci V.A., Strobel R.J. Improvement of microbial strains and fermentation processes. Appl. Microbiol. Biotechnol. 2000;54(3):287–301. doi: 10.1007/s002530000403. [DOI] [PubMed] [Google Scholar]
- Dufossé L. Microbial production of food grade pigments. Food Technol. Biotechnol. 2006;44(3):313–323. [Google Scholar]
- Kirakosyan A., Seymour E., Kaufman P.B., Warber S., Bolling S., Chang S.C. Antioxidant capacity of polyphenolic extracts from leaves of Crataegus laevigata and Crataegus monogyna (Hawthorn) subjected to drought and cold stress. J. Agric. Food. Chem. 2003;51(14):3973–3976. doi: 10.1021/jf030096r. [DOI] [PubMed] [Google Scholar]
- Grube M., Cardinale M., de Castro Jr J.V., Müller H., Berg G. Species-specific structural and functional diversity of bacterial communities in lichen symbioses. ISME J. 2009;3(9):1105. doi: 10.1038/ismej.2009.63. [DOI] [PubMed] [Google Scholar]
- Grube M., Berg G. Microbial consortia of bacteria and fungi with focus on the lichen symbiosis. Fungal Biol. Rev. 2009;23(3):72–85. [Google Scholar]
- Panosyan A, Nikogosyan VJANAS, Biol Zhurn Armen. The presence of Azotobacter in lichens. 1966;19:3–11.
- Stone, J.K., Bacon, C.W. and White Jr, J.F., 2000. An overview of endophytic microbes: endophytism defined. In: Microbial endophytes (pp. 17–44). CRC Press.
- Alharbi K.K., Kashour T.S., Al-Hussaini W., Nbaheen M.S., Hasanato R.M., Mohamed S., Tamimi W., Khan I.A. Screening for genetic mutations in LDLR gene with familial hypercholesterolemia patients in the Saudi population. Acta Biochimica Polonica. 2015;62(3) doi: 10.18388/abp.2015_1015. [DOI] [PubMed] [Google Scholar]
- Batais M.A., Almigbal T.H., Shaik N.A., Alharbi F.K., Alharbi K.K., Khan I.A. Screening of common genetic variants in the APOB gene related to familial hypercholesterolemia in a Saudi population: a case–control study. Medicine. 2019;98(4) doi: 10.1097/MD.0000000000014247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasidharan P., Raja R., Karthik C., Ranandkumar S., Indra Arulselvi P. Isolation and characterization of yellow pigment producing Exiguobacterium sps. J. Biochem. Tech. 2013;4(4):632–635. [Google Scholar]
- Godinho, A., Bhosle, S., 2008. Carotenes produced by alkaliphilic orange-pigmented strain of Microbacterium arborescens-AGSB isolated from coastal sand dunes. 37 (3), 307–312.
- Khaneja R., Perez-Fons L., Fakhry S., Baccigalupi L., Steiger S., To E., Sandmann G., Dong T.C., Ricca E., Fraser P.D., Cutting S.M. Carotenoids found in Bacillus. J. Appl. Microbiol. 2010;108(6):1889–1902. doi: 10.1111/j.1365-2672.2009.04590.x. [DOI] [PubMed] [Google Scholar]
- Girija, S., Duraipandiyan, V., Kuppusamy, P.S., Gajendran, H. and Rajagopal, R., 2014. Chromatographic characterization and GC-MS evaluation of the bioactive constituents with antimicrobial potential from the pigmented ink of Loligo duvauceli. International Scholarly Research Notices, 2014. [DOI] [PMC free article] [PubMed]
- Grammer, A., 1976. Antibiotic sensitivity and Assay test. In: Microbiological methods, Collins, C. H and Lyne, PM. Butterworths, London.
- Prieto P., Pineda M., Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal. Biochem. 1999;269(2):337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- Oktay M., Gülçin İ., Küfrevioğlu Ö.İ. Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. LWT-Food Sci. Technol. 2003;36(2):263–271. [Google Scholar]
- Sowndhararajan K., Kang S.C. Evaluation of in vitro free radical scavenging potential of Streptomyces sp. AM-S1 culture filtrate. Saudi. J. Biol. Sci. 2013;20(3):227–233. doi: 10.1016/j.sjbs.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indra Arulselvi P., Umamaheswari S., Ranandkumar S.G., Karthik C., Jayakrishna C. Screening of yellow pigment producing bacterial isolates from various eco-climatic areas and analysis of the carotenoid produced by the isolate. J. Food Process. Technol. 2014;2014(5):1. [Google Scholar]
- Bhat S.V., Khan S.S., Amin T. Research article isolation and characterization of pigment producing bacteria from various foods for their possible use as biocolours. Int. J. Rec. Sci. Res. 2013;4(10):1605–1609. [Google Scholar]
- Manachini P.L., Fortina M.G., Parini C., Craveri R. Bacillus thermoruber sp. nov., nom. rev., a red-pigmented thermophilic bacterium. Int. J. Syst. Evol. Microbiol. 1985;35(4):493–496. [Google Scholar]
- Grossart, H.P., Thorwest, M., Plitzko, I., Brinkhoff, T., Simon, M. and Zeeck, A., 2009. Production of a blue pigment (glaukothalin) by marine Rheinheimera spp. Int. J. Microbiol. 2009. [DOI] [PMC free article] [PubMed]
- Jagannadham M.V., Rao V.J., Shivaji S. The major carotenoid pigment of a psychrotrophic Micrococcus roseus strain: purification, structure, and interaction with synthetic membranes. J. Bacteriol. 1991;173(24):7911–7917. doi: 10.1128/jb.173.24.7911-7917.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J. Springer Science & Business Media; 2012. Pigments in Vegetables: Chlorophylls and Carotenoids. [Google Scholar]
- D'souza, A.M., Gauns, M.U., 2018. Temporal variability in copepod gut pigments over the central western continental shelf of India. J. Mar. Biol. Assoc. U. K, 98(1), 149–159.
- Sahin N. Significance of absorption spectra for the chemotaxonomic characterization of pigmented bacteria. Turkish J. Biol. 2011;35(2):167–175. [Google Scholar]
- Takaichi S., Oh-oka H., Maoka T., Jung D.O., Madigan M.T. Novel carotenoid glucoside esters from alkaliphilic heliobacteria. Arch. Microbiol. 2003;179(2):95–100. doi: 10.1007/s00203-002-0504-5. [DOI] [PubMed] [Google Scholar]
- Zaibunnisa, A.H., Aini Marhanna, M.N.A., Ainun Atirah, M., 2011. Characterisation and solubility study of γ-cyclodextrin and Β-carotene complex. Int. Food Res. J. 18(3).
- Köcher S., Breitenbach J., Müller V., Sandmann G. Structure, function and biosynthesis of carotenoids in the moderately halophilic bacterium Halobacillus halophilus. Arch. Microbiol. 2009;191(2):95–104. doi: 10.1007/s00203-008-0431-1. [DOI] [PubMed] [Google Scholar]
- Perez-Fons L., Steiger S., Khaneja R., Bramley P.M., Cutting S.M., Sandmann G., Fraser P.D. Identification and the developmental formation of carotenoid pigments in the yellow/orange Bacillus spore-formers. Biochimica et Biophysica Acta (BBA) – Mol. Cell Biol. Lipids. 2011;1811(3):177–185. doi: 10.1016/j.bbalip.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Mohana D.C., Thippeswamy S., Abhishek R.U. Antioxidant, antibacterial, and ultraviolet-protective properties of carotenoids isolated from Micrococcus spp. Radiat. Prot. Environ. 2013;36:168–174. doi: 10.4103/0972-0464.142394. [DOI] [Google Scholar]
- Mukherjee G., Singh S.K. Purification and characterization of a new red pigment from Monascus purpureus in submerged fermentation. Process Biochem. 2011;46(1):188–192. [Google Scholar]
- Schauer N., Steinhauser D., Strelkov S., Schomburg D., Allison G., Moritz T., Lundgren K., Roessner-Tunali U., Forbes M.G., Willmitzer L., Fernie A.R. GC–MS libraries for the rapid identification of metabolites in complex biological samples. FEBS Lett. 2005;579(6):1332–1337. doi: 10.1016/j.febslet.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Boo H.O., Hwang S.J., Bae C.S., Park S.H., Heo B.G., Gorinstein S. Extraction and characterization of some natural plant pigments. Ind. Crops Prod. 2012;40:129–135. [Google Scholar]
- Konzen M., De Marco D., Cordova C.A., Vieira T.O., Antônio R.V., Creczynski-Pasa T.B. Antioxidant properties of violacein: possible relation on its biological function. Bioorg. Med. Chem. 2006;14(24):8307–8313. doi: 10.1016/j.bmc.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Venil C.K., Zakaria Z.A., Ahmad W.A. Bacterial pigments and their applications. Process Biochem. 2013;48(7):1065–1079. [Google Scholar]
- Davoli P., Mucci A., Schenetti L., Weber R.W. Laetiporic acids, a family of non-carotenoid polyene pigments from fruit-bodies and liquid cultures of Laetiporus sulphureus (Polyporales, Fungi) Phytochemistry. 2005;66(7):817–823. doi: 10.1016/j.phytochem.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Armstrong G.A., Schmidt A., Sandmann G., Hearst J.E. Genetic and biochemical characterization of carotenoid biosynthesis mutants of Rhodobacter capsulatus. J. Biol. Chem. 1990;265(14):8329–8338. [PubMed] [Google Scholar]
- Liu W., Mu W., Zhu B., Liu F. Antifungal activities and components of VOCs produced by Bacillus subtilis G8. Curr. Res. Bacteriol. 2008;1:28–34. [Google Scholar]
- Chen L., Wang N., Wang X., Hu J., Wang S. Characterization of two anti-fungal lipopeptides produced by Bacillus amyloliquefaciens SH-B10. Bioresource Technol. 2010;101(22):8822–8827. doi: 10.1016/j.biortech.2010.06.054. [DOI] [PubMed] [Google Scholar]
- Aono R., Horikoshi K. Carotenes produced by alkaliphilic yellow-pigmented strains of Bacillus. Agric. Biol. Chem. 1991;55(10):2643–2645. [Google Scholar]
- Zhong K., Gao X.L., Xu Z.J., Gao H., Fan S., Yamaguchi I., Li L.H., Chen R.J. Antioxidant activity of a novel Streptomyces strain Eri 12 isolated from the Rhizosphere of Rhizoma curcumae longae. Cur. Res. Bacteriol. 2011;4(2):63–72. [Google Scholar]