Abstract
To investigate the effects of knocking out the Sperm associated antigen6 (Spag6) gene on the auditory system of mice, the heterozygous type Spag6 knockout mouse model built in the previous period was used for mating and breeding, and homozygous type Spag6 gene knockout mouse (Spag−/−), heterozygous type Spag6 gene knockout mouse (Spag+/−) and wild type mouse (Spag+/+) were obtained. PCR technology was used to verify mouse models with different genotypes. After verification, the hearing threshold responses of Spag+/+ and Spag−/− genotype mice were detected. The localization of Spag6 gene in the basal membrane of the cochlea of the inner ear was detected by immunofluorescence staining. The changes of middle ear tissues were observed by H.E. staining sections. The relative expression of Prestin gene and Pgrn gene in different age mice was detected by fluorescence quantitative PCR. The relative expression of Prestin gene was detected by western blot. The results showed that Spag−/− mice had hearing impairment compared with Spag+/+ mice. And Spag6 protein is distributed in different genotypes of mouse hair cells; Spag−/− mice showed otitis media. The expression of Prestin mRNA and protein in Spag−/− mice was significantly higher than that in Spag+/+ mice (P < 0.01). The expression of Pgrn gene in Spag+/+ mice was significantly higher than that in Spag−/− mice (P < 0.05). It indicates that the loss of Spag6 gene would lead to the decline of hearing sense in mice. It is likely that the Spag6 gene could affect hearing by regulating the expression of Prestin gene. And the absence of the Spag6 gene causes otitis media in mice. The results of this study can lay a theoretical foundation for the follow-up studies of Spag6 gene in deafness diseases.
Keywords: Spag6 genes, Knockout mice, Hearing impairment, Prestin genes, Pgrn genes, Otitis media
1. Introduction
Deafness has the highest incidence of diseases with sensory or functional defects in humans, and there are many factors causing deafness, and the causes are also very complex (Godinho et al., 2017). Otitis media is a disease that occurs frequently in children. Severe otitis media can lead to permanent neurological deafness. But overall, deafness is caused by both genetic and environmental factors, with genetic factors accounting for the main cause. At present, hundreds of genes have been found to be closely related to the occurrence and development of deafness (Hoberman et al., 2017). In mammals, the perception of sound is that the external sound enters the inner ear after passing through the external auditory canal and middle ear canal. The vibration of hair bundles on the surface of hair cells and the basement membrane transforms the sound signal into electrical signal, and finally transmits it to the brain for sound perception. Hair cells are very important auditory related cells, which can quickly regulate their own morphology and respond to sound stimulation, and this response mechanism originates from a gene specifically expressed in the lateral wall of outer hair cells (Guevar et al., 2018).
The structure of Prestin gene is basically consistent with that of the members of the Slc26a protein family, which can amplify electromotor and auditory effects in hair cells (Alonso et al., 2017), but it is highly susceptible to external factors such as noise and line of defence irradiation (Okanoya et al., 2018). Progranulin (Pgrn) is a new secreted growth factor, which can promote the recruitment of fibroblasts and macrophages and speed up the repair of wounds. Therefore, it has received extensive attention in inflammatory diseases (Pickett and Raible, 2019). However, Spag6 gene is a gene encoding cilia-related protein associated with male infertility, and its mRNA is highly expressed in the testicles of mammals. In addition to being closely related to the reproductive system, Spag gene has been found to be related to human respiratory diseases, the regulation of neurons, the occurrence of some cancers and the development of the inner ear. So presumably the Spag6 gene is playing an important role in the hair cells of the inner ear.
The Spag6 gene knockout mice was used as the research object in this research, and the differences in hearing were detected and analysed between homozygous Spag6 gene knockout and wild type mice. Immunofluorescence staining of the basal membrane of cochlea and hematoxylin - eosin staining of the middle ear were performed. The relative expression levels of Prestin gene and Pgrn gene mRNA in the ear tissues of these two kinds of mice were detected by fluorescence quantitative PCR. The relative expression of Prestin gene in the ear tissues of these two kinds of mice was detected by western blot. Based on the molecular level, the influence mechanism of hearing in mice was preliminarily discussed, aiming to lay a theoretical foundation for the follow-up study of Spag6 gene in deafness disease.
2. Materials and methods
2.1. Animals for test
The Spag6 gene knockout mouse model was built by members of the previous laboratory. After cage feeding of the sexually mature hybrid Spag6 gene knockout mice, homozygous Spag6 gene knockout mice, heterozygous Spag6 gene knockout mice and wild type Spag6 gene mice were obtained. In the experiment, all mice were raised in the laboratory room with free feeding and drinking water, constant temperature and humidity, and no specific pathogen. The knockout mice used in this study have been approved by the ethics committee of our hospital, and all the tests were conducted in accordance with the rules and regulations formulated by the ethics committee.
2.2. Identification of genotype
The whole genome DNA of the new wild type Spag6 mouse (Spag6 +/+), the heterozygous Spag6 gene knockout mouse (Spag6 +/−) and the homozygous Spag6 gene knockout mouse (Spag6 −/−) was extracted from the 0.3 cm tail tissues according to the instructions of the animal tissue DNA extraction kit (Thermo Fisher, USA). Specific primers for the exogenous introduction fragment of the Spag6 gene and knockout mouse were designed, and the extracted Spag6 +/+ mouse, Spag6 +/− mouse and Spag6 −/− mouse genomic DNA were used as templates for PCR verification, and the mice were divided into three groups according to their genotypes. Primer Premier5.0 software was used to design Primer amplification. The designed Primer was synthesized by Shanghai Sangon Biological Engineering co., LTD. The Primer amplification information was shown in Table 1.
Table 1.
PCR amplified primer information.
| The name of the gene | Primer sequence |
|---|---|
| Spag6 | F: GACTTAGCAGAAGCAGTCG |
| R: CGGAGAGAAGCTGCTACC | |
| Neo | F: CGTGTTCCGGCTGTCAGCGCA |
| R: CAACGCTATGTCCTGATAGCG | |
| GAPDH | F: CCACTCTCCACCTTTGAC |
| R: ACCCTGTTGCTGTAGCCA |
2.3. The listening test
Mice from different groups were raised to the age of 3 weeks and weighed, and then anesthetization were injected intraperitoneally to the Spag6 −/− and Spag6 +/+ mice. After the mice were completely anesthetized, different electrodes were placed under the skin of different parts of the mice. The recording electrode was located in the centre of the head, the reference electrode was located next to the auricle, and the grounding electrode was located in the back. After that, the hearing threshold of mice was recorded with different frequencies and the intensity of the sound was the same as that of the previous gradient descent. After anesthetizing the Spag6 −/− and Spag6 +/+ mice, different electrodes were placed under the skin of different parts of the mice, with the recording electrode in the ear window alcove, the reference electrode in the neck, and the ground electrode in the back. After that, 300–3000 Hz pass filter was used for stimulation, and the changes of hearing threshold in mice were recorded.
2.4. Immunofluorescence staining of mouse ear tissue
After the mice were anesthetized as above, the mice were killed by drug heart injection. The cochlear basal membrane tissues were taken, and the tissues were rinsed with 0.1 mol/LPBS solution for 3 times. The tissues were rinsed with 1% tritonx-100 for about 1 h before PBS rinsing. Donkey serum was added to the shaker, sealed at room temperature for 1 h, and then washed with PBS. Add the anti-mouse Spag6 antibody of sheep with concentration of 1:300 dilution or 1:200 dilution, incubate it in a shaking table at room temperature for 1 h and incubate it overnight at 4 °C, then rinse it with PBS. Add donkey anti-rabbit fluorescence secondary antibody, incubate at room temperature for 2 h in dark, and rinse with PBS.DAPI dye was added at room temperature for 30 min staining, and then washed with PBS. The processed tissue was placed on the slide and sealed. The results of tissue staining were observed and recorded under the microscope.
2.5. Preparation and staining of mouse ear tissue sections
After the mice were anesthetized and killed as above, the mouse auditory alveolar tissue was taken under the microscope and soaked in 4% paraformaldehyde at room temperature for 1d, and then decalcified for 3d with 10% EDTA solution at 4 °C. The tissues were dehydrated with 70%, 80%, 95% and 100% ethanol and xylene solutions, and the soaking time of ethanol and xylene solutions of different concentrations was 30 min. The dehydrated tissues were soaked in soft paraffin for 30 min and hard paraffin for 2 times for 1 h each. Finally, the embedded tissue was prepared into 4 µm thick slices by using a microtome. Paraffin sections were stained with Hematoxylin and eosin staining (H.E.). The sections were baked in a 60 oven for 30 min and dewaxed with turpentine twice, 30 min each time. After that, 100%, 90%, 80% and 70% ethanol solution were used to soak the tissues for 5 min, and then washed for 5 min. Stain with Hematoxylin solution for 2 min, wash with 1% alcohol hydrochloride for 10 s, and put it in water for 5 min. After that, eosin solution was added for dyeing for 2 min. After washing, 70%, 80%, 90% and 100% ethanol solution were used for gradient dehydration. After drying, the neutral gum is used for sealing. The stained sections were dried and observed under a microscope.
2.6. The difference detection of MRNA and protein expression in mice with different genotypes
After the anesthetization above, the basal membrane tissues and middle ear tissues of cochlear in the labyrinth of the inner ear of mice were fully ground and mixed with 1 mL Trizol solution for 15 min, followed by 200 µm chloroform shock and centrifugation at 12000 rpm for 10 min. The supernatant was added to isopropyl alcohol of equal volume, and the mixture was allowed to stand at room temperature for 10 min and centrifuged at low temperature at 12000 rpm for 10 min. Discard the supernatant and add 800 mu L 75% ethanol to wash and precipitate twice. After drying at room temperature for 15 min, ultrapure water without RNA enzyme was added to dissolve RNA precipitation. After the concentration and purity of RNA were detected, the reverse transcription kit was used for cDNA reverse transcription. Fluorescence quantitative PCR kit was used to detect the expression of Prestin gene in outer hair cells of basal membrane tissues of cochlea and Pgrn gene in middle ear tissues. GAPDH was used as internal reference gene and the relative expression difference of Prestin gene was calculated with 2-delta Ct. Quantitative primer information was shown in Table 2.
Table 2.
Fluorescence quantitative primer information of PCR.
| The name of the gene | Primer sequence |
|---|---|
| Prestin | F: GCCGGGATTGTGAAAGAATA |
| R: AAGTGACGCTGTGGCTTCTT | |
| Pgrn | F: ACATGTACGGTCGAGACT |
| R: CATTCCCGTTGGCTGTCT |
After the mice were anesthetized as above, the labyrinth tissue of the inner ear bone was taken from the mice, and the basal membrane tissue of cochlea was obtained. RIPA lysate was added, and the tissue was fully ground to mix well with the lysate. After 30 min of ice bath, the tissue was centrifuged at a low temperature of 12000 rpm for 10 min. The supernatant is taken and the appropriate amount of 5 × SDS-page sample loading buffer is added, and both of them are put in water bath at 95 °C for 8 min. 10% SDS-page separation adhesive and 5% SDS-page concentrated adhesive are configured. The protein to be measured and Marker were added into the separation gel, followed by 80 V electrophoresis for 30 min and 110 V electrophoresis for 90 min. The target fragment was transferred to PVDF membrane at room temperature for 100 min and sealed for 1 h. At room temperature, 1:200 diluted sheep anti-mouse Prestin antibody was added and incubated in a shaker at room temperature for 1 h, then incubated at 4 °C overnight, and TBST was used for washing. At room temperature, the horseradish peroxidase-tagged donkey anti-rabbit secondary antibody is added and incubated for 2 h, then washed with TBST. The protein colour is observed and recorded according to the instructions of ECL luminescence kit.
2.7. Statistics and analysis
All the data in the experiment were expressed as mean ± standard deviation. The independent sample t-test in SPSS19.0 software was used for statistical analysis, and it shows that there was a statistical difference between the two groups when P < 0.05.
3. Results
3.1. Genotype test results of mice
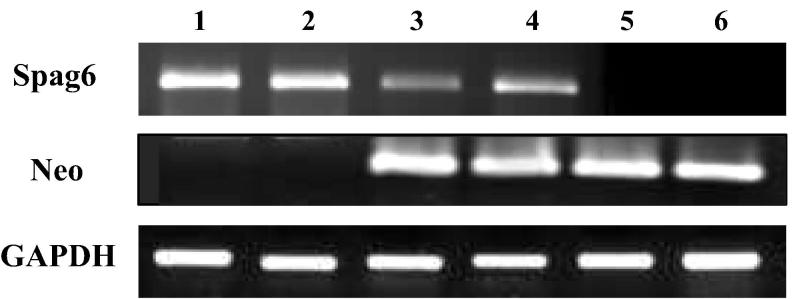
The extracted Spag6 +/+ mouse, Spag6 +/− mouse and Spag6 −/− mouse genomic DNA were used as templates for PCR verification, and the detection results were shown in Fig. 1. It showed that the target fragment of internal reference gene GAPDH can be successfully amplified in different groups of mice, indicating that the whole genomic DNA extracted in the previous stage is qualified, and the PCR amplification system is more suitable. The homozygous Spag6 +/+ mouse and the heterozygous Spag6 +/− mouse, which were obtained by the mating of the female and male heterozygous Spag6 +/− mouse, could amplify the target fragment of the Spag6 gene, but could not amplify the exogenous Neo marker fragment used for knocking out the Spag6 gene. The homozygous Spag6 −/− mouse and the heterozygous Spag6 +/− mouse were able to amplify the exogenous introduction of the Neo tagged fragment, but the homozygous Spag6 −/− mouse was unable to amplify the target fragment of the Spag6 gene.
Fig. 1.
PCR product detection results of target genes in mice with different genotypes (holes 1 and 2 are Spag6 +/+ mice; holes 3 and 4 are Spag6 +/− mice; holes 5 and 6 are Spag6 −/− mice).
3.2. Hearing test results in mice
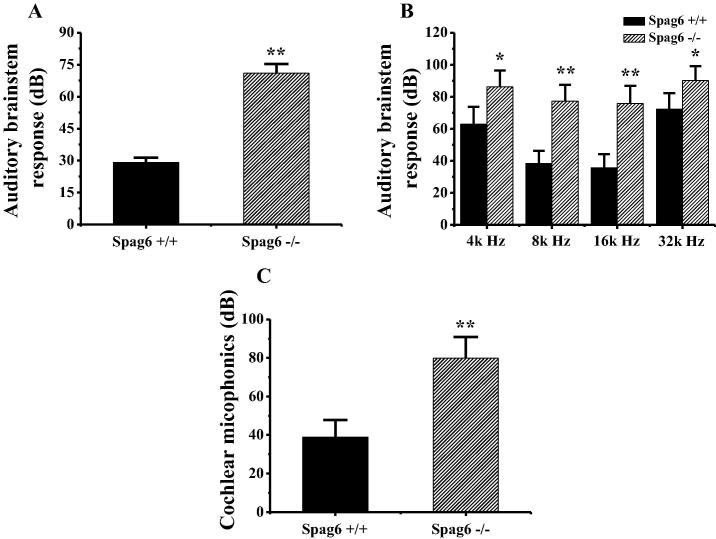
The aural detection results of the Spag6 +/+ mouse and the Spag6 −/− mouse auditory brainstem response and cochlear microphonic potential are shown in Fig. 2. According to Fig. 2A, the average hearing threshold of the Spag6 −/− mouse auditory brainstem response is significantly larger than that of the Spag6 +/+ mouse (P < 0.01). According to Fig. 2B, after different frequencies of sound stimulation are used to stimulate the two groups of mice, Spag6 +/+ and Spag6 −/− mice hearing thresholds were an average of more than 90 dB, and Spag6 −/− mice in 4 KHZ and 32 KHZ sound under the stimulus of the hearing threshold was significantly higher than that of Spag6 +/+ mice (P < 0.05) in 8 KHZ and 16 KHZ sound under the stimulus of hearing threshold was significantly higher than that of Spag6 +/+ mice (P < 0.01). The result of hearing detection of cochlear microphonic potential in mouse Fig. 2C shows that the average threshold derived from the Spag6 −/− mouse cochlear microphonic potential is significantly larger than that of the Spag6 +/+ mouse (P < 0.01).
Fig. 2.
Hearing test results of Spag6+/+ and Spag6−/− mice (A is the result of auditory brainstem response under Click sound stimulation; B is the result of sound stimulation of different frequencies; C is the detection result of cochlear microphonic potential stimulated by 80 dB intensity. * indicates significant difference between the two groups of mice (P < 0.05); ** means the difference between the two groups of mice is very significant (P < 0.01)).
3.3. Immunofluorescence staining of mouse ear tissue
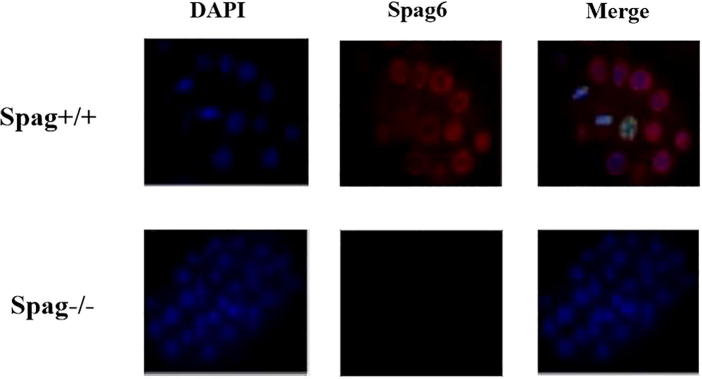
Immunofluorescence staining was performed on the cochlea basilar tissues of the Spag6 +/+ mouse and the Spag6 −/− mouse, as shown in Fig. 3. It indicated that the nuclei of the hair cells of both Spag6 +/+ and Spag6−/− mouse hair tissues were stained by DAPI staining. The result of Spag6 protein staining showed that there was a significant red fluorescence point only in the hair cells of the basal membrane of the cochlea of the Spag6 +/+ mouse, and the fluorescence point of the Spag6 protein was negative in the Spag6 −/− mouse, and no fluorescence point was found in the outer hair cells of the basal membrane of the cochlea of the mouse. The result of Merge staining showed that the distribution of Spag6 protein was found in the Spag6 +/+ mouse hair cells, but the distribution of the Spag6 gene was not found in the Spag6 −/− mouse hair cells.
Fig. 3.
Spag6 protein distribution in different genotypes of mouse hair cells.
3.4. Results of H.E. staining of mouse ear tissue sections
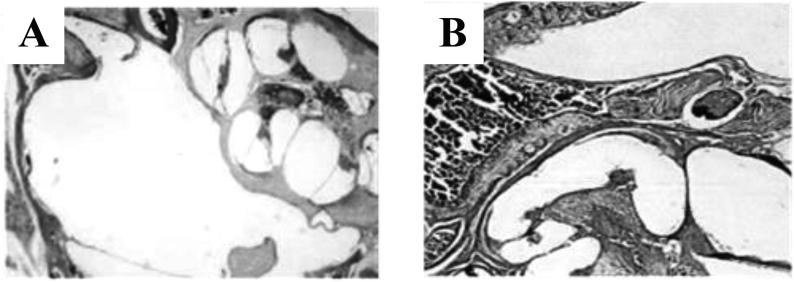
In order to study the influence of Spag6 gene knockout on the middle ear tissues of mice, the middle ear tissues of Spag6 +/+ mice and Spag6 −/− mice were collected in the research and made tissue slices for staining observation. The results are shown in Fig. 4. According to Fig. 4A, the middle ear tissues of the Spag6 +/+ mouse showed fluid seepage and infiltration of some inflammatory cells, accompanied by the trend of proliferation of some tissues. As can be seen from Fig. 4B, the middle ear tissues of the Spag6 −/− mouse were normal, and no similar inflammation was observed as that of the Spag6 +/+ mouse.
Fig. 4.
Results of H.E. staining in middle ear sections of mice with different genotypes (A is the histology section of Spag6 +/+ mouse middle ear; B is the histology section of the middle ear of Spag6 −/− mouse).
3.5. Results of mRNA differential expression of related genes
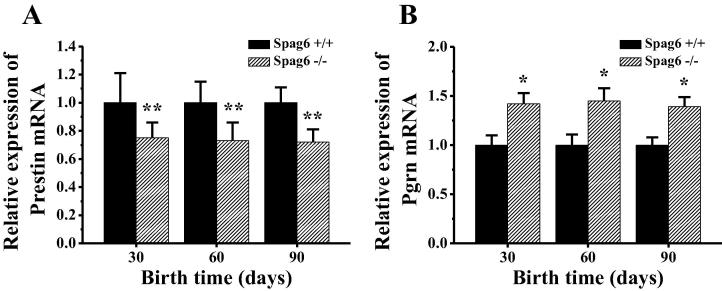
The relative expressions of Prestin gene and Pgrn gene mRNA in outer hair cells of basal membrane of cochlea of different day-age Spag6 +/+ mice and Spag6 −/− mice were detected by fluorescence quantitative PCR, and the detection results were shown in Fig. 5. As can be seen from Fig. 5A, the relative expression of Prestin gene in the outer hair cells of Spag6 +/+ mouse and Spag6 −/− mouse showed little difference with the increase of mouse day age. However, the relative expression of Prestin gene in the outer hair cells of the 30-day, 60-day and 90-day Spag6 −/− mice was significantly lower than that of the Spag6 +/+ mice (P < 0.01). As can be seen from Fig. 5B, with the increase of mouse day age, the relative expression of Pgrn gene in the outer hair cells of Spag6 +/+ mouse and Spag6 −/− mouse showed little difference. However, the relative expression of Prgn gene in the middle ear tissues of the 30-day-old, 60-day-old and 90-day-old Spag6 −/− mice was significantly higher than that of the Spag6 +/+ mice (P < 0.05).
Fig. 5.
Expression difference of Prestin and Pgrn gene mRNA in mice with different genotypes at different day ages (* indicates that the differences are significant compared with the Spag6+/+ mouse (P < 0.05); ** indicates that the differences are very significant compared with the Spag6+/+ mouse (P < 0.01)).
3.6. Results of Prestin and Pgrn gene protein differential expression
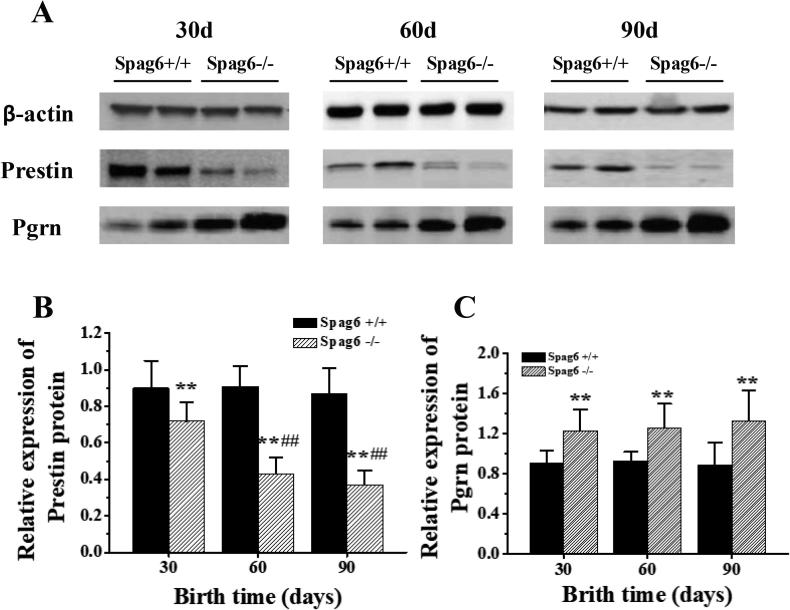
The difference in protein expression of Prestin and Pgrn gene in the outer hair cells of cochlea basal membrane tissues of different day-age Spag6 +/+ mice and Spag6 −/− mice was detected by using western blot technique, as shown in Fig. 6. As shown in Fig. 6A, the expression of internal reference gene beta-actin was balanced in the outer hair cells of mice of different age and genotype, indicating that the experimental results were true and reliable. The Prestin gene was evenly expressed in the outer hair cells of the 30-day-old, 60-day-old and 90-day-old Spag6 +/+ mice, but the expression of the Prestin gene was significantly decreased in the outer hair cells of the Spag6 −/− mice; the expression of Pgrn protein in outer hair cells of Spag6−/− mice was significantly higher than that of Spag+/+ mice. After calculating Prestin relative expression of gene protein, as shown in Fig. 6B, Prestin gene protein expression in the age of 30, 60 days of age and 90 days of age Spag6 in outer hair cells −/− mice is significantly lower than the same day age Spag6 expression of +/+ mice (P < 0.01), and 60 days of age and 90 days of age Spag6 −/− mice Prestin gene protein expression in outer hair cells is significantly less than 30 days of age Spag6 −/− mice expression (P < 0.01). And the differences between 60-day old and 90-day old Spag6 −/− mice were not significant (P > 0.05). As concluded from Fig. 6C, the expression of Pgrn gene protein in the outer hair cells of Spag−/− mice was significantly higher than that of Spag+/+ mice at 30d, 60d and 90d (P < 0.01); however, there was no significant difference in Pgrn gene protein expression in outer hair cells between different Spag−/− mice at 30d, 60d, and 90d (P > 0.05).
Fig. 6.
The Prestin and Pgrn gene expression difference in different genotypes of mice of different age (A is the electrophoresis of Prestin and Pgrn gene. B is the relative expression of Prestin gene. C is the relative expression of Pgrn gene. ** means that the difference is very significant compared with the Spag6+/+ mouse (P < 0.01); compared with the 30-day old Spag6−/− mouse, the difference is very significant (P < 0.01)).
4. Discussion
The PCR technology was used to detect the genotypes of homozygous Spag6 gene knockout mice (Spag6 −/−), heterozygous Spag6 gene knockout mice (Spag6 +/−), and propagated wild type Spag6 mice (Spag6 +/+) in the research. After inserting the Lac-z fragment labeled by neomycin (Neo) into the nucleotide chain of the Spag6 gene that was not coding, the gene was found to be expressed in Spag−/− mice, and the results showed that the homozygous Spag6 gene knockout mice were successfully constructed in the previous experiment, which laid a foundation for subsequent research. The listening tests of the Spag6 +/+ mouse and the Spag6 −/− mouse auditory brainstem response and cochlear microphonic potential were conducted, and it was found that the listening of the Spag6 −/− mouse in the same nest decreased significantly compared with that of the Spag6 +/+ mouse (P < 0.05), indicating that the Spag6 gene plays an important role in the hearing of the mouse. The immunofluorescence detection results showed that the Spag6 protein was widely distributed in the Spag6 +/+ mouse hair cells, but no Spag6 protein was distributed in the Spag6 −/− mouse, which was basically consistent with the research results of ERDOĞAN et al. (Erdoğan et al., 2017). This indicates that Spag6 protein is an important component of hair cells and may be closely related to the physiological structure of hair cells. The expression of Spag6 protein is most obvious in the cilia of hair cells of Spag6 −/− knockout mice, suggesting that it may be related to the growth and arrangement of cilia. When the Spag6 +/+ mouse and the Spag6 −/− mouse middle ear tissue staining sections were made and observed, inflammatory cells were found in the Spag6 −/− mouse, and the epithelium of the middle ear tissue was thickened, whereas the Spag6 +/+ mouse middle ear tissue was normal. This indicates that the deletion of the Spag6 gene may increase the incidence of otitis media disease in mice and may be passed on to future generations (Santossacchi and Tan, 2018).
After using fluorescence quantitative PCR technology and protein imprinting technology to further confirm the effect of on hearing, it was found that different day age Spag6 −/− mice ear basement membrane organization of Prestin gene mRNA and protein expression was significantly lower than Spag6 +/+ mice (P < 0.01), and the lack of Prestin gene protein expression with Spag6 growth witnessed a gradual decrease in mice. Prestin gene is closely related to the physiological function of hair cells, and the loss of Spag6 gene will cause the decrease of mRNA and protein expression of Prestin gene, so it is speculated that Spag6 gene may be one of the components of the cell membrane of hair cells, and Spag6 gene may participate in the regulation of the transcription of Prestin gene (Zhou et al., 2017). Pgrn is a kind of cytokines involved in the inflammatory response, this research showed that in middle ear, Spag6 −/− mice tissues Pgrn gene mRNA expression was significantly higher than that of Spag6 +/+ mice, which shows that knockout Spag6 gene in mice after middle ear tissue inflammation may occur, which agrees with H.E. stain of middle ear tissue biopsy results. It prompted that after inflammation, Pgrn gene mRNA expression increased to inhibit the inflammatory reaction of otitis media.
To sum up, it was found in the study that the absence of the Spag6 gene led to stunted development of the cochlea tissue in the inner ear of mice and the loss of outer hair cells, and the absence of the Spag6 gene reduced the expression of the Prestin gene and increased the expression of the Pgrn gene, which suggests that the Spag6 gene might interact with the Prestin and Pgrn genes to affect the mice's hearing. Only the effects of the absence of the Spag6 gene on the inner ear and outer hair cells of mice are analyzed in the study, while the hearing of mice is not tested. The number of samples needs to be expanded to investigate the effect of the Spag6 gene deletion on the hearing and middle ear tissues of mice in the future study. The results of this study can lay a theoretical foundation for finding molecular markers of deafness.
Acknowledgement
This research was jointly supported by Zhejiang Provincial Natural Science Foundation of China and Wenzhou basic scientific research project under Grant No.LY19H130003, LY19H130004 and Y20180091.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alonso N., Caleropaniagua I., Pinomontes J.D. Clinical and genetic advances in paget’s disease of bone: a review. Clin. Rev. Bone Mineral Metab. 2017;15(1):1–12. doi: 10.1007/s12018-016-9226-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdoğan B., Metin B., Levent Ö. DNA methylation of the prestin gene and outer hair cell electromotileresponse of the cochlea in salicylate administration. Turkish J. Med. Sci. 2017;47(5):1626. doi: 10.3906/sag-1604-137. [DOI] [PubMed] [Google Scholar]
- Godinho I., Gameiro J., Jorge S. Diabetes, deafness and renal disease. Clin. Kidney J. 2017;10(4):487. doi: 10.1093/ckj/sfx021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevar J., Olby N.J., Meurs K.M. Deafness and vestibular dysfunction in a Doberman Pinscher puppy associated with a mutation in the PTPRQ gene. J. Vet. Intern. Med. 2018;32(2):665–669. doi: 10.1111/jvim.15060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoberman A., Paradise J.L., Rockette H.E. Shortened antimicrobial treatment for acute otitis media in young children. N. Engl. J. Med. 2017;375(25):2446–2456. doi: 10.1056/NEJMoa1606043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okanoya K., Yosida S., Barone C.M. Auditory-vocal coupling in the naked mole-rat, a mammal with poor auditory thresholds. J. Comp. Physiol. A: Neuroethology, Sensory, Neural, Behavioral Physiol. 2018;204(11):905–914. doi: 10.1007/s00359-018-1287-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett S.B., Raible D.W. Water waves to sound waves: using zebrafish to explore hair cell biology. J. Assoc. Res. Otolaryngol. 2019;20(1):1–19. doi: 10.1007/s10162-018-00711-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santossacchi J., Tan W. The frequency response of outer hair cell voltage-dependent motility is limited by kinetics of prestin. J. Neurosci. 2018;38(24) doi: 10.1523/JNEUROSCI.0425-18.2018. 425–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D., Li S., Li W. Increased expression of PGRN protein in follicular fluid and mRNA in granulosa cells in overweight patients with polycystic ovary syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017;218:106–112. doi: 10.1016/j.ejogrb.2017.09.017. [DOI] [PubMed] [Google Scholar]