Abstract
Metabolic syndrome (Mets) is a major health hazard. The syndrome is strongly linked to cardiovascular disease. The objective of the current study was to address whether or not crocin could protect against experimentally-induced MetS in rats as well as the possible underlying mechanisms. Animals were allocated into 4 groups. The first one served as control and was kept on regular food pellets and drinking water. The other three groups were subjected to experimental MetS. Induction of MetS was achieved by keeping rats on food pellets containing 3% NaCl; and drinking water enriched with 10% fructose. The first and second groups were daily gavaged with saline. The third and fourth groups were daily administered crocin 5 and 10 mg/kg, respectively. The treatment continued for 12 consecutive weeks. Crocin significantly reduced body weight gain and adiposity index as compared to MetS group. Also, crocin protected against the occurrence of hyperglycemia and insulin resistance as indicated by controlled values of HOMA-IR. Crocin protected against MetS-induced dyslipidemia as well as the rise blood pressure. These beneficial effects were accompanied by enhanced serum levels of PPARγ & AMPK and inhibited serum levels of IL-6 and TNF-α. In conclusion, crocin protects against experimentally-induced MetS. This can be attributed, at least partly, to activation of PPARγ and AMPK as well as inhibiting inflammation.
Keywords: Crocin, Metabolic syndrome, PPAR-gamma, AMPK, Rats
1. Introduction
Metabolic syndrome (MetS) embraces different metabolic defects. These comprise central fatness, hypertension, insulin resistance and dyslipidemia. The syndrome poses a significant risk for cardiovascular disease (CVD) (Rochlani et al., 2017, Sherling et al., 2017). Unfortunately, the worldwide incidence of MetS is riskily high (Aguilar et al., 2015, Ansarimoghaddam et al., 2018). Pathogenesis of MetS encompasses genetic and epigenetic factors (Stančáková and Laakso, 2014, Kuneš et al., 2015) that are linked to initiation of several inflammation pathways and eventually lead to CVD. Molecular mechanisms that mediate MetS have been reviewed (Armani et al., 2017). However, peroxisome proliferator-activated receptors-gamma (PPARγ) regulators and adenosine monophosphate-activated protein kinase (AMPK) activators have been proposed for the treatment and management of MetS (Stump et al., 2015, Sharma and Kumar, 2016). Pharmacological management of MetS includes statins, ACE inhibitors, metformin, canagliflozin, exenatide and drugs belonging to their classess (Rask Larsen et al., 2018). However, the increasingly rate of incidence MetS and obesity indicates that effectiveness of the available medications is unsatisfactory and necessitates the search of new therapies. In this regard, natural products represent a safe and effective alternative.
Crocus sativus L– known as Saffron – has been exploited as a coloring agent and food spice culinary purposes. In addition, the plant is widely used in traditional remedy as anti-spasmodic, sedative, stomachic, carminative, expectorant, and aphrodisiac (Borch-Johnsen, 2007). Crocin is a major apo-carotenoid of saffron (Bukhari et al., 2018). Experimentally, crocin has been shown to possess antinociceptive (Erfanparast et al., 2015), learning and memory enhancing (Lin et al., 2019), neuroprotective (Mozaffari et al., 2019), nephroprotective (Jalili et al., 2019), hepatoprotective (Kalantar et al., 2019), cardioprotective (Feidantsis et al., 2018) and antiosteoporotic (Algandaby, 2019) activities. Further, crocin has been shown to combat elements of MetS in different animal models. It significantly reduced weight gain and alleviated experimentally obesity in rats induced by high fat content in animal diet (Algandaby, 2019). In experimentally-induced diabetes, crocin amended insulin resistance and dyslipidemia (Shirali et al., 2013). Also, crocin prevented hypertension induced by angiotensin II in rats (Shafei et al., 2017). The objective of this work was to address whether or not crocin could prevent experimentally-induced MetS in rats as well as the probable causal mechanisms.
2. Materials and methods
2.1. Chemicals
Crocin, fructose and sodium chloride were bought from Merck, Taufkirchen, Germany. Other used chemicals were highly pure (>98%).
2.2. Animals and animal treatment
Twenty-four Wistar rats (male gender, weight range 140–170 g) were got from the local vivarium (College of Pharmacy, University of King Abdulaziz, Saudi Arabia). Daily, rats were retained at fixed temperature of 23 ± 2 °C and 12-h dark-light succession. Animal handling in the current study was endorsed in advance by Biomedical Ethics Unit at the College of Medicine, University of King Abdulaziz (Ref. # 329–16). Animals were grouped into 4 sets (6 each). The control (group 1) received regular drinking water and standard food pellets. Second third and fourth groups were exposed to investigational MetS. Induction of MetS was achieved by giving the animals food pellets containing 3% NaCl; and drinking water enriched with 10% fructose. Exposure to high salt food pellets and high fructose drinking water continued for 12 weeks (Abdallah et al., 2016). The first and second group were orally administered saline on a daily basis, while groups 3 & 4 were gavaged with crocin at two daily doses (5 or 10 mg/kg); respectively. Doses of crocin are supported by a preliminary experiment and in harmony with previous animal studies (Zhang et al., 2017, Algandaby, 2019). Crocin treatments were repeated for 5 days per week during the 12-week experimental period. Animals weights were recorded every 3 weeks. Blood pressure was assessed at the last day of week 12. This was followed by collecting blood samples from tail veins for determining glucose levels. At the first day of week 13, rats were allowed to inhale ether in a closed chamber for anesthesia. Then, samples of blood were collected from the plexus located retro-orbitally and allowed to clot. This was followed by centrifugation to get sera that were stored at −80 °C for subsequent analyses. Abdomens were quickly opened and visceral fat from each animal was collected and weighed. Finally, animals were sacrificed by decapitation.
2.3. Blood pressure measurement
Rats were restrained, kept in a warming chamber and subjected to conditioning for 10–20 min daily for 3 consecutive days. Then, blood pressure (BP) was determined as previously described (El-Bassossy et al., 2014) in conscious, moderately restrained rats using the tail cuff method. Measurements were executed between 8:00 and 11:00 AM. Routinely, inflation–deflation cycles were repeated 10 times, separated by 5 and 10 min of stabilization in at 35 °C in a warm chamber. Blood pressure was calculated as the average of 6 determinations within a narrow range of 6–10 mmHg. Mean arterial pressure (MAP) was computed as follows:
2.4. Blood analysis
Glucose levels (Fasting and postprandial) in tail vein blood were measured by an appropriate glucometer (ACCU-CHEK obtained from Roche, Mannheim, Germany). Levels of blood insulin in fasted animals were assessed using rat insulin ELISA Kit (Cayman, Ann Arbor, MI, USA) and expressed as µU/L. HOMA-IR was computed according to Harati et al. (2003)
Total cholesterol (TC), triglycerides (TG) and high density lipoprotein cholesterol (HDL-C) were determined using commercially available kits obtained from Biodiagnostics® (Giza, Egypt). Additionally, cholesterol of the type very low density lipoprotein was computed as VLDL-C = TG/5. Low density lipoprotein cholesterol known as LDL-C was computed as follows: LDL = TC − (VLDL + HDL) (Friedewald et al., 1972). Serum PPARγ was determined by means of ELISA Kit (Bioassay Technology Laboratory Company, Shanghai, China). Similarly, serum level of PAMPK was assessed by a commercially available ELISA kit (MyBioSource, San Diego, USA). Finally, serum content of IL-6 and TNF-α was evaluated by ELISA kits (Jiancheng, Nanjing, China) according to the producer's instructions.
2.5. Statistical analyses
Means ± SD were used to present collected data. Significant differences between means were tested using one-way ANOVA. Bonferroni was used as a post hoc test (GarphPad Instat software, version 3.1, San Diego, CA, USA). The level of 0.05 was used as a cut-off value of significance (p < 0.05).
3. Results
3.1. Effect of crocin on weight gain and adiposity index
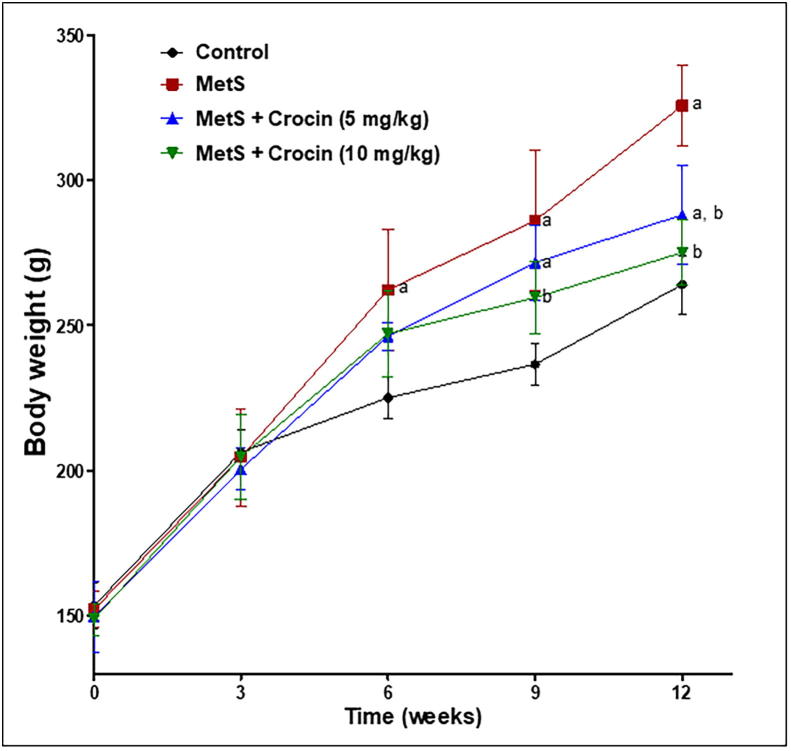
Overweight is one of the major determinants of MetS. Therefore, body weight and fatty changes were assessed in the current animal model of MetS. As shown in (Fig. 1), animals suffering from MetS started to show a significant increase in their body weights at the sixth week after induction, compared to the control rats. Treatment with crocin (5 mg/kg) failed to demonstrate any significant decrease in body weight from MetS group except at the last week of experiment (week 12). However, crocin (10 mg/kg) was more efficient and significantly reduced the body weight from MetS group, starting from the ninth week of model induction. These findings were further confirmed by calculating weight gain percentage as indicated in Table 1. Rats with MetS showed a significant rise in their weight gain by 53%, in comparison to the control group. Unfortunately, crocin (5 mg/kg) caused insignificant reduction of weight gain compared to MetS group. Yet, crocin (10 mg/kg) significantly reduced weight gain by about 23% as compared to MetS rats. Other markers of fatty changes like visceral fat weight and adiposity index have added to the obtained outcomes, as specified in Table 1. Weight of visceral fat was increased significantly in MetS group by about 80% in comparison to control animals. Crocin at both lower and higher doses successfully induced a significant decrease of visceral fat as compared to MetS group, in a dose-related manner. Moreover, crocin higher dose (10 mg/kg) was more effective than the lower dose and almost restored visceral fat to the control value. Similar pattern of activity has been shown with the adiposity index, in which MetS caused about 45% increase, compared to the control animals, while crocin showed a dose-related decrease of adiposity index, in comparison to MetS animals.
Fig. 1.
Influence of crocin on weight gain in metabolic syndrome (MetS)-induced rats. Data are displayed as Mean ± S.D. Statistical analysis was carried out using one-way ANOVA followed by Tukey’s post-hoc test. (a) Significantly different from control at p < 0.05. (b) Significantly different from MetS group at p < 0.05.
Table 1.
Influence of crocin on weight gain and adiposity index in metabolic syndrome (MetS) rats.
| Initial body weight (g) | Final body weight (g) | Weight gain (%) | Visceral fat (g) | Adiposity index | |
|---|---|---|---|---|---|
| Control | 153.3 ± 7.8 | 264 ± 10.0 | 72.4 ± 6.0 | 10.7 ± 0.7 | 4.06 ± 0.3 |
| MetS | 151.8 ± 6.7 | 325.6a ± 15.1 | 110.8a ± 12.7 | 19.3a ± 0.8 | 5.9a ± 0.3 |
| MetS + Crocin 5 mg/kg | 149.5 ± 12.1 | 288.0a, b ± 17.0 | 93.7 ± 18.9 | 13.8a, b ± 1.0 | 4.8a, b ± 0.3 |
| MetS + Crocin 10 mg/kg | 148.7 ± 5.5 | 275.0b ± 11.2 | 85.4b ± 13.1 | 11.9b, c ± 0.8 | 4.4b ± 0.4 |
Data are displayed as Mean ± S.D.
Statistical analysis was carried out using one-way ANOVA followed by Tukey’s post-hoc test.
Significantly different from control at p < 0.05.
Significantly different from MetS group at p < 0.05.
Significantly different from crocin (5 mg/kg) at p < 0.05.
3.2. Influence of crocin on FBG, insulin concentrations, HOMA-IR and OGTT
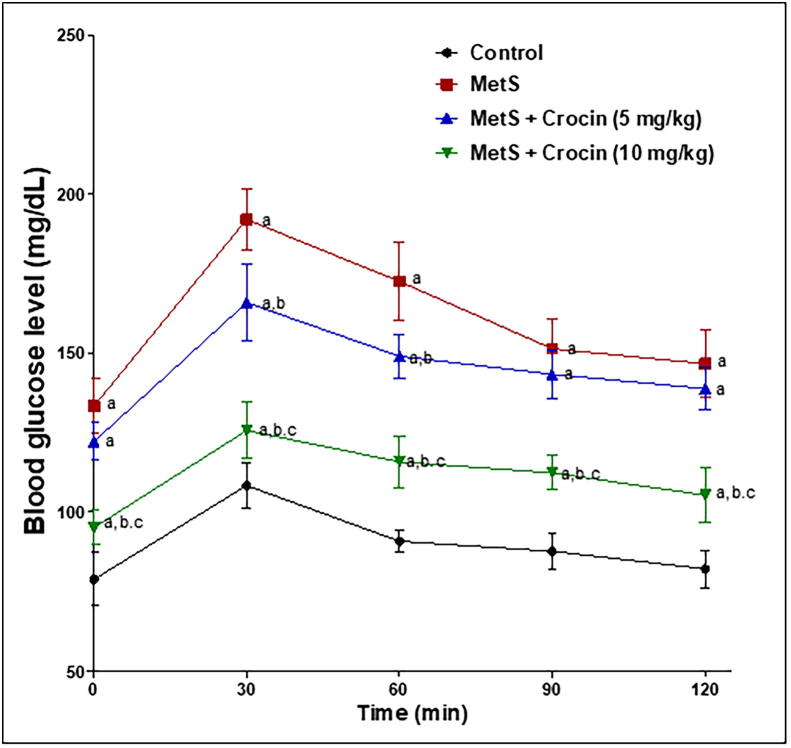
Because overweight is usually connected to glucose intolerance in MetS, FBG and serum insulin levels were assessed. This was followed by calculation of HOMA-IR as a marker of insulin resistance, as indicated in Table 2. Levels of both FBG and of serum insulin were significantly raised in MetS rats by about 70% and 36% respectively, compared to the corresponding control groups. Animals given crocin (5 mg/kg) showed significant decrease of both markers by about 15% and 23% respectively, compared to the corresponding MetS groups. Moreover, crocin (10 mg/kg) markedly improved glucose tolerance, being able to ameliorate MetS-induced elevations of fasting blood glucose and serum insulin, so that no significant differences were detected compared to the corresponding controls. These finding were reflected on insulin resistance measured as HOMA-IR that was increased by more than 2-folds in MetS group, compared to control animals. Meanwhile, crocin (5 and 10 mg/kg) was capable of decreasing HOMA-IR values by about 34% and 51% respectively, compared to MetS group. Oral glucose tolerance test further confirmed the previous results as shown in Fig. 2. Rats with MetS showed glucose intolerance manifested by significantly high blood glucose levels over 120 min, compared to the control group. Again, crocin improved glucose tolerance in a dose-related with the ability of crocin (10 mg/kg) dose to significantly reduce blood glucose level at all-time intervals, compared to both MetS group and the other one given crocin at the lower dose (5 mg/kg).
Table 2.
Influence of crocin on FBG and insulin concentrations, and HOMA-IR in metabolic syndrome (MetS)-induced rats.
| FBG (mg/dL) | Fasting serum insulin (μU/L) | HOMA-IR | |
|---|---|---|---|
| Control | 83.82 ± 6.35 | 12.30 ± 1.30 | 2.54 ± 0.26 |
| MetS | 142.87a ± 10.67 | 16.74a ± 2.02 | 5.92a ± 0.94 |
| MetS + Crocin (5 mg/kg) | 122.00a, b ± 12.33 | 12.90b ± 1.40 | 3.90a, b ± 0.68 |
| MetS + Crocin (10 mg/kg) | 94.00b, c ± 11.77 | 12.40b ± 1.50 | 2.90b ± 0.65 |
Data are displayed as Mean ± S.D.
Statistical analysis was carried out using one-way ANOVA followed by Tukey’s post-hoc test
Significantly different from control at p < 0.05.
Significantly different from MetS group at p < 0.05.
Significantly different from crocin (5 mg/kg) at p < 0.05.
Fig. 2.
Influence of crocin on OGTT in metabolic syndrome (MetS)-induced rats. Data are displayed as Mean ± S.D. Statistical analysis was carried out using one-way ANOVA followed by Tukey’s post-hoc test. (a) Significantly different from control at p < 0.05. (b) Significantly different from MetS group at p < 0.05. (c) Significantly different from crocin (5 mg/kg) at p < 0.05.
3.3. Influence of crocin on metabolic syndrome (MetS)-induced dyslipidemia
It is worthy noted that hyperlipidemia and hypertension are common characteristics of MetS, therefore, lipid profile and blood pressure changes were evaluated in the current animal model. As indicated in Table 3, MetS induced hyperlipidemia manifested by significant elevations of TG, LDL-C and TC accompanied with a fall in HDL-C, in comparison to corresponding control groups. Crocin at 5 mg/kg significantly reduced only TG and LDL-C, compared to corresponding MetS groups. However, crocin (10 mg/kg) could significantly reduce TC in addition to TG and LDL-C, compared to their corresponding MetS groups. Both doses of crocin caused non-significant increases of HDL-C level compared to MetS group.
Table 3.
Effect of crocin on metabolic syndrome (MetS)-induced dyslipidemia in rats.
| TG (mg/dL) | HDL-C (mg/dL) | LDL-C (mg/dL) | TC (mg/dL) | |
|---|---|---|---|---|
| Control | 91.20 ± 8.1 | 21.2 ± 3.8 | 23.5 ± 3.3 | 83.41 ± 5.3 |
| MetS | 223.2 a ± 14.1 | 14.6 a ± 2.5 | 35.9 a ± 2.93 | 140.4 a ± 7.8 |
| MetS + Crocin (5 mg/kg) | 173.9a, b ± 16.3 | 16.8 ± 2.3 | 27.7b ± 3.4 | 128.9a ± 11.5 |
| MetS + Crocin (10 mg/kg) | 133.7a, b, c ± 15.8 | 18.8 ± 2.7 | 26.4b ± 2.3 | 125.0a, b ± 5.8 |
Data are displayed as Mean ± S.D.
Statistical analysis was carried out using one-way ANOVA followed by Tukey’s post-hoc test.
Significantly different from control at p < 0.05.
Significantly different from MetS group at p < 0.05.
Significantly different from crocin (5 mg/kg) at p < 0.05.
3.4. Influence of crocin on blood pressure
Regarding blood pressure changes, MetS induced significant increases in systolic, diastolic and mean arterial pressures by about 28%, 25% and 26% respectively, compared to the corresponding control groups, as shown in Table 4. Crocin at the lower dose (5 mg/kg) could not reduce systolic pressure, compared to MetS group. However, crocin (10 mg/kg) could obviously ameliorate MetS-induced hypertension, being able to normalize systolic, diastolic and mean arterial pressures as manifested by absence of any statistical differences from their corresponding controls.
Table 4.
Influence of Crocin on blood pressure of metabolic syndrome (MetS)-induced rats.
| Systolic BP (mmHg) | Diastolic BP (mmHg) | Mean arterial BP (mmHg) | |
|---|---|---|---|
| Control | 108.1 ± 7.6 | 75.3 ± 5.5 | 86.2 ± 5.5 |
| MetS | 138.3a ± 10.1 | 93.9a ± 6.0 | 108.7a ± 6.7 |
| MetS + Crocin (5 mg/kg) | 129.4a ± 5.6 | 79.9b ± 6.5 | 96.4a, b ± 4.5 |
| MetS + Crocin (10 mg/kg) | 119.7b ± 10.0 | 77.1b ± 5.6 | 91.3b ± 6.2 |
Data are displayed as Mean ± S.D.
Statistical analysis was carried out using one-way ANOVA followed by Tukey’s post-hoc test.
Significantly different from control at p < 0.05.
Significantly different from MetS group at p < 0.05.
3.5. Influence of crocin on serum levels of PPARγ, pAMPK, IL-6 and TNF-α
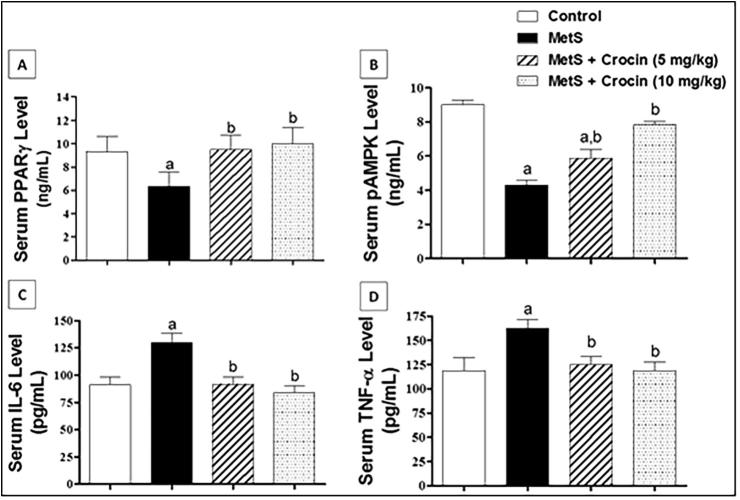
In order to investigate how crocin improved experimentally-induced MetS in rats, serum levels of PPARγ, phosphor-AMPK, IL-6 and TNF-α were quantified. As shown in Fig. 3A, MetS-induced a significant drop of PPARγ level, when compared with control values. Crocin at both doses improved PPARγ level when compared to values of MetS animals with no significant difference was detected from the control group. A similar pattern was observed with phosphor-AMPK that represents the active form of AMPK enzyme, as shown in Fig. 3B. Though, the effect of crocin was dose-related, where only (10 mg/kg) dose could restore phospho-AMPK level to the control value. Regarding the inflammatory biomarkers IL-6 and TNF-α, they had the same pattern as demonstrated in Fig. 3C and D. Animals suffering from MetS had significant increases of both markers, in comparison to the corresponding control groups. Contrariwise, crocin at doses of 5 and 10 mg/kg significantly ameliorated such changes, being able to reduce IL-6 and TNF-α to normal values with no significant differences from the corresponding controls.
Fig 3.
Influence of Crocin on serum levels of PPARγ, pAMPK, IL-6 and TNF-α in metabolic syndrome (MetS) rats. Data are displayed as Mean ± S.D. Statistical analysis was carried out using one-way ANOVA followed by Tukey’s post-hoc test. (a) Significantly different from control at p < 0.05. (b) Significantly different from MetS group at p < 0.05.
4. Discussion
The current study aimed at exploring the influence of crocin on the progress of MetS induced by high fructose-drinking water and high salt-food in rats. The used model for induction of MetS is a standardized experimental approach and it results in development of all signs of the disease as reviewed by Pan and Kong (2018). Administration of crocin (5 and 10 mg/kg) significantly inhibited weight gain. This is in accordance with previous investigations highlighting the anti-obesity properties in animals exposed to 10-week-long high-fat diet (HFD) (Hazman et al., 2016). Further, crocin has been reported to protect against complications of obesity as fatty liver in obese rats (Mashmoul et al., 2016). In addition, our data indicated that crocin significantly prevented the rise in adopsity index. In humans, crocin improved appetite accumulation of central obesity in patients with coronary artery disease (Abedimanesh et al., 2017). Anti-obesity activity of crocin was proposed to encompass AMPK-mediated inhibition of adipocyte differentiation in addition to enhancement of lipolysis (Gu et al., 2018).
Our results indicate that crocin protected against MetS-induced hyperglycemia. This was confirmed by controlled rise in blood glucose levels in OGGT. The potential of crocin to combat hyperglycemia in experimentally-induced diabetes has been previously described (Rajaei et al., 2013). Further, crocin has been shown to protect against several diabetic complications experimentally. These include nephropathy (Yaribeygi et al., 2018), compromised memory and learning (Tamaddonfard et al., 2013), elevated HbA1c & microalbuminuria (Shirali et al., 2013), peripheral neuropathy (Farshid and Tamaddonfard, 2015) and impaired spermatogenesis (Roshankhah et al., 2019). Additionally, crocin treatment has been reported to inhibit apoptotic changes in pancreatic β-islets and pancreatic atrophy in experimental diabetes in rats (Bayatpoor et al., 2019, Samaha et al., 2019). Our data also illustrated that crocin treatment significantly prevented against hyperinsulinemia and insulin resistance as indicated by ameliorating the rise in HOMA-IR. This observation is in line with earlier studies showing crocin’s ability to improve insulin resistance in diabetic rats (Shirali et al., 2013). Also, crocin improved HOMA-IR in type 2 diabetes in rats induced by streptozotocin/high fat diet (Ghorbanzadeh et al., 2016).
Induction of MetS in rats was associated with dyslipidemia. However, crocin significantly prevented the rise in serum TG, LDL-C & TC and the decline in HDL-C. This finding gains support by an earlier reports indicating the ability of crocin to prevent the rise of the same parameters in diabetic animals (Shirali et al., 2013). In rats, crocin ameliorated the rise in levels of hyperlipidemic markers via inhibition of lipase enzyme (Sheng et al., 2006). Also, crocin ameliorated diazinon-induced hyperlipemia in rats. The mechanism was suggested to involve modulation of ERK pathway and increase of LDL receptor upragulation (Lari et al., 2014). Complications of dyslipidemia as atherosclerosis were prevented by crocin in rats kept on high cholesterol diet for 2 months (Xu et al., 2005).
Our data indicated that crocin significantly protected against MetS-induced rise in PB. Experimentally, crocin was previously shown to prevent hypertension induced by deoxycorticosterone acetate in rats (Imenshahidi et al., 2010, Imenshahidi et al., 2014). It has been suggested that crocin's antihypertensive activities involve inhibition of renin-angiotensin system (RAS). This is supported by the ability of crocin to prevent acute hypertension that was induced by angiotensin II in sedated rats (Shafei et al., 2017). Also, protection by crocin against endothelial injury (Xu et al., 2006) may be included. Additionally, modulation of intracellecular Ca2+ levels in vascular smooth muscles cannot be excluded. Crocin has been shown to impede both Ca2+ influx from extracellular compartments along with the release of intracellular Ca2+ in cells isolated from bovine aortic smooth muscles (He et al., 2004).
The obtained results in this study clearly indicate crocin capability to protect against MetS. This gains support by a report suggesting saffron and its constituents are effective in attenuating MetS complications including obesity, hyperglycemia, dyslipidemia and hypertension (Kermani et al., 2017, Shafiee et al., 2017). In humans, saffron prevented MetS in psychotic patients on olanzapine treatment (Fadai et al., 2014). The possible underlying mechanisms of the observed activities of crocin against MetS were explored. Our data indicate that crocin significantly enhanced serum PPARγ levels. Peroxisome proliferator-activated receptors (PPARs) possess significant impact on metabolic regulation of blood lipids, blood glucose, and abdominal adiposity (Botta et al., 2018). In general, PPAR agonists have been suggested to combat MetS (Mansour, 2014). In particular, PPARγ activators have proven potent in combating hypertension and MetS (Stump et al., 2015). These reports lend support to our findings. Further, the role of AMPK in fighting diabetes and MetS has been reported (Zhang et al., 2009). Also, AMPK activators are effective in preventing insulin resistance and MetS (Ruderman et al., 2013). These reports give support to our observations of enhancement blood AMPK levels by crocin. Due to their safety profile, natural AMPK activators have been recommended for preventing MetS (Sharma and Kumar, 2016). In this regard, crocin has been previously reported to activate AMPK signaling leading to enhanced β-oxidation of fatty acids and inhibited lipogenesis in mice (Luo et al., 2019). Also, our results highlighted anti-inflammatory activities of crocin as indicated by preventing the elevation of blood IL-6 and TNF-α. These data are consistent with the observed activation of PPARγ and AMPK (Martin, 2010, Lyons and Roche, 2018). Inflammation has been shown to link obesity, diabetes and MetS (Esser et al., 2014). Therefore, targeting inflammation has been give attention to fight MetS (Welty et al., 2016). In conclusion, crocin protects against experimentally-induced MetS. This can be attributed, at least partly, to activation PPARγ and AMPK as well as inhibiting inflammation.
Acknowledgments
Acknowledgements
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, Saudi Arabia, under grant No. G: 240-130-1439. The author, therefore, acknowledges with thanks DSR for technical and financial support.
Moreover, the author is grateful to Ashraf B. Abdel-Naim, Department of Pharmacology & Toxicology, King Abdulaziz University, for his help in animal treatments.
Declaration of Competing Interest
The author declares no conflicts of interest.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdallah H.M., El-Bassossy H.M., Mohamed G.A., El-Halawany A.M., Alshali K.Z., Banjar Z.M. Phenolics from Garcinia mangostana alleviate exaggerated vasoconstriction in metabolic syndrome through direct vasodilatation and nitric oxide generation. BMC Complement. Altern. Med. 2016;16:359. doi: 10.1186/s12906-016-1340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abedimanesh N., Bathaie S.Z., Abedimanesh S., Motlagh B., Separham A., Ostadrahimi A. Saffron and crocin improved appetite, dietary intakes and body composition in patients with coronary artery disease. J. Cardiovasc. Thorac. Res. 2017;9:200–208. doi: 10.15171/jcvtr.2017.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar M., Bhuket T., Torres S., Liu B., Wong R.J. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA. 2015;313:1973–1974. doi: 10.1001/jama.2015.4260. [DOI] [PubMed] [Google Scholar]
- Algandaby M.M. Crocin attenuates metabolic syndrome-induced osteoporosis in rats. J. Food Biochem. 2019;43 doi: 10.1111/jfbc.12895. [DOI] [PubMed] [Google Scholar]
- Ansarimoghaddam A., Adineh H.A., Zareban I., Iranpour S., HosseinZadeh A., Kh F. Prevalence of metabolic syndrome in Middle-East countries: Meta-analysis of cross-sectional studies. Diabetes Metab. Syndr. Clin. Res. Rev. 2018 doi: 10.1016/j.dsx.2017.11.004. [DOI] [PubMed] [Google Scholar]
- Armani A., Berry A., Cirulli F., Caprio M. Molecular mechanisms underlying metabolic syndrome: The expanding role of the adipocyte. FASEB J. 2017 doi: 10.1096/fj.201601125RRR. [DOI] [PubMed] [Google Scholar]
- Bayatpoor M.E., Mirzaee S., Karami Abd M., Mohammadi M.T., Shahyad S., Bahari Z., Raouf Sarshoori J. Crocin treatment decreased pancreatic atrophy, LOX-1 and RAGE mRNA expression of pancreas tissue in cholesterol-fed and streptozotocin-induced diabetic rats. J. Complement. Integr. Med. 2019 doi: 10.1515/jcim-2019-0117. [DOI] [PubMed] [Google Scholar]
- Borch-Johnsen K. The metabolic syndrome in a global perspective. The public health impact–secondary publication. Dan. Med. Bull. 2007;54:157–159. [PubMed] [Google Scholar]
- Botta M., Audano M., Sahebkar A., Sirtori C.R., Mitro N., Ruscica M. PPAR agonists and metabolic syndrome: an established role? Int. J. Mol. Sci. 2018 doi: 10.3390/ijms19041197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari S.I., Manzoor M., Dhar M.K. A comprehensive review of the pharmacological potential of Crocus sativus and its bioactive apocarotenoids. Biomed. Pharmacother. 2018;98:733–745. doi: 10.1016/j.biopha.2017.12.090. [DOI] [PubMed] [Google Scholar]
- El-Bassossy H.M., Dsokey N., Fahmy A. Characterization of vascular complications in experimental model of fructose-induced metabolic syndrome. Toxicol. Mech. Methods. 2014;24(9):536–543. doi: 10.3109/15376516.2014.945109. [DOI] [PubMed] [Google Scholar]
- Erfanparast A., Tamaddonfard E., Taati M., Dabbaghi M. Effects of crocin and safranal, saffron constituents, on the formalin-induced orofacial pain in rats. Avicenna J. Phytomed. 2015;5:392–402. [PMC free article] [PubMed] [Google Scholar]
- Esser N., Legrand-Poels S., Piette J., Scheen A.J., Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014 doi: 10.1016/j.diabres.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Fadai F., Mousavi B., Ashtari Z., Ali Beigi N., Farhang S., Hashempour S., Shahhamzei N., Zahra Bathaie S. Saffron aqueous extract prevents metabolic syndrome in patients with schizophrenia on olanzapine treatment: a randomized triple blind placebo controlled study. Pharmacopsychiatry. 2014;47:156–161. doi: 10.1055/s-0034-1382001. [DOI] [PubMed] [Google Scholar]
- Farshid A.A., Tamaddonfard E. Histopathological and behavioral evaluations of the effects of crocin, safranal and insulin on diabetic peripheral neuropathy in rats. Avice2nna. J. Phytomed. 2015;5:469–478. [PMC free article] [PubMed] [Google Scholar]
- Feidantsis K., Mellidis K., Galatou E., Sinakos Z., Lazou A. Treatment with crocin improves cardiac dysfunction by normalizing autophagy and inhibiting apoptosis in STZ-induced diabetic cardiomyopathy. Nutr. Metab. Cardiovasc. Dis. 2018;28:952–961. doi: 10.1016/j.numecd.2018.06.005. [DOI] [PubMed] [Google Scholar]
- Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- Ghorbanzadeh V., Mohammadi M., Dariushnejad H., Chodari L., Mohaddes G. Effects of crocin and voluntary exercise, alone or combined, on heart VEGF-A and HOMA-IR of HFD/STZ induced type 2 diabetic rats. J. Endocrinol. Invest. 2016;39:1179–1186. doi: 10.1007/s40618-016-0456-2. [DOI] [PubMed] [Google Scholar]
- Gu M., Luo L., Fang K. Crocin inhibits obesity via AMPK-dependent inhibition of adipocyte differentiation and promotion of lipolysis. Biosci. Trends. 2018;12:587–594. doi: 10.5582/bst.2018.01240. [DOI] [PubMed] [Google Scholar]
- Harati M., Ani M., Messripour M. Effect of vanadyl sulfate on fructose-induced insulin resistance. Iran. Biomed. J. 2003;7:179–182. [Google Scholar]
- Hazman Ö., Aksoy L., Büyükben A. Effects of crocin on experimental obesity and type-2 diabetes. Turkish J. Med. Sci. 2016;46:1593–1602. doi: 10.3906/sag-1506-108. [DOI] [PubMed] [Google Scholar]
- He S., Qian Z., Tang F. Effect of crocin on intracellular calcium concentration in cultured bovine aortic smooth muscle cells. Yao Xue Xue Bao. 2004;39:778–781. [PubMed] [Google Scholar]
- Imenshahidi M., Hosseinzadeh H., Javadpour Y. Hypotensive effect of aqueous saffron extract (Crocus sativus L.) and its constituents, safranal and crocin, in normotensive and hypertensive rats. Phytother. Res. 2010;24:990–994. doi: 10.1002/ptr.3044. [DOI] [PubMed] [Google Scholar]
- Imenshahidi M., Razavi B.M., Faal A., Gholampoor A., Mousavi S.M., Hosseinzadeh H. Effects of chronic crocin treatment on desoxycorticosterone acetate (doca)-salt hypertensive rats. Iran. J. Basic Med. Sci. 2014;17:9–13. [PMC free article] [PubMed] [Google Scholar]
- Jalili C., Ghanbari A., Roshankhah S., Salahshoor M.R. Toxic effects of methotrexate on rat kidney recovered by crocin as a consequence of antioxidant activity and lipid peroxidation prevention. Iran. Biomed. J. 2019;24(1):39–46. doi: 10.29252/ibj.24.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalantar M., Kalantari H., Goudarzi M., Khorsandi L., Bakhit S., Kalantar H. Crocin ameliorates methotrexate-induced liver injury via inhibition of oxidative stress and inflammation in rats. Pharmacol. Rep. 2019;71:746–752. doi: 10.1016/j.pharep.2019.04.004. [DOI] [PubMed] [Google Scholar]
- Kermani T., Kazemi T., Molki S., Ilkhani K., Sharifzadeh G., Rajabi O. The efficacy of crocin of saffron (Crocus sativus L.) on the components of metabolic syndrome: a randomized controlled clinical trial. J. Res. Pharm. Pract. 2017;6:228. doi: 10.4103/jrpp.jrpp_17_26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuneš J., Vaněčková I., Mikulášková B., Behuliak M., Maletínská L., Zicha J. Epigenetics and a new look on metabolic syndrome. Physiol. Res. 2015;64(5):611–620. doi: 10.33549/physiolres.933174. [DOI] [PubMed] [Google Scholar]
- Lari P., Rashedinia M., Abnous K., Hosseinzadeh H. Crocin improves lipid dysregulation in subacute diazinon exposure through ERK1/2 pathway in rat liver. Drug Res. (Stuttg) 2014;64:301–305. doi: 10.1055/s-0033-1357196. [DOI] [PubMed] [Google Scholar]
- Lin L., Liu G., Yang L. Crocin improves cognitive behavior in rats with Alzheimer’s disease by regulating endoplasmic reticulum stress and apoptosis. Biomed. Res. Int. 2019;2019:1–9. doi: 10.1155/2019/9454913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L., Fang K., Dan X., Gu M. Crocin ameliorates hepatic steatosis through activation of AMPK signaling in db/db mice 11 medical and health sciences 1103 clinical sciences. Lipids Health Dis. 2019;18 doi: 10.1186/s12944-018-0955-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons C.L., Roche H.M. Nutritional modulation of AMPK-impact upon metabolic-inflammation. Int. J. Mol. Sci. 2018 doi: 10.3390/ijms19103092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour M. Progress in Molecular Biology and Translational Science. Elsevier B.V.; 2014. The roles of peroxisome proliferator-activated receptors in the metabolic syndrome; pp. 217–266. [DOI] [PubMed] [Google Scholar]
- Martin H. Role of PPAR-gamma in inflammation. Prospects for therapeutic intervention by food components. Mutat. Res. – Fundam. Mol. Mech. Mutagen. 2010 doi: 10.1016/j.mrfmmm.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Mashmoul M., Azlan A., Mohtarrudin N., Mohd Yusof B.N., Khaza’ai H., Khoo H.E., Farzadnia M., Boroushaki M.T. Protective effects of saffron extract and crocin supplementation on fatty liver tissue of high-fat diet-induced obese rats. BMC Complement. Altern. Med. 2016;16 doi: 10.1186/s12906-016-1381-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffari S., Yasuj S.R., Motaghinejad M., Motevalian M., Kheiri R. Crocin acting as a neuroprotective agent against methamphetamine-induced neurodegeneration via CREB-BDNF signaling pathway. Iran. J. Pharm. Res. 2019;18:745–758. doi: 10.22037/ijpr.2019.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Kong L.-D. High fructose diet-induced metabolic syndrome: pathophysiological mechanism and treatment by traditional Chinese medicine. Pharmacol. Res. 2018;130:438–450. doi: 10.1016/j.phrs.2018.02.020. [DOI] [PubMed] [Google Scholar]
- Rajaei Z., Hadjzadeh M.A.R., Nemati H., Hosseini M., Ahmadi M., Shafiee S. Antihyperglycemic and antioxidant activity of crocin in streptozotocin-induced diabetic rats. J. Med. Food. 2013;16:206–210. doi: 10.1089/jmf.2012.2407. [DOI] [PubMed] [Google Scholar]
- Rask Larsen J., Dima L., Correll C.U., Manu P. The pharmacological management of metabolic syndrome. Expert Rev. Clin. Pharmacol. 2018 doi: 10.1080/17512433.2018.1429910. [DOI] [PubMed] [Google Scholar]
- Rochlani Y., Pothineni N.V., Kovelamudi S., Mehta J.L. Metabolic syndrome: pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 2017 doi: 10.1177/1753944717711379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshankhah S., Jalili C., Salahshoor M. Effects of crocin on sperm parameters and seminiferous tubules in diabetic rats. Adv. Biomed. Res. 2019;8:4. doi: 10.4103/abr.abr_124_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman N.B., Carling D., Prentki M., Cacicedo J.M. AMPK, insulin resistance, and the metabolic syndrome. J. Clin. Invest. 2013;123:2764–2772. doi: 10.1172/JCI67227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha M.M., Said E., Salem H.A. A comparative study of the role of crocin and sitagliptin in attenuation of STZ-induced diabetes mellitus and the associated inflammatory and apoptotic changes in pancreatic β-islets. Environ. Toxicol. Pharmacol. 2019;72:103238. doi: 10.1016/j.etap.2019.103238. [DOI] [PubMed] [Google Scholar]
- Shafei M.N., Faramarzi A., Khajavi Rad A., Anaeigoudari A. Crocin prevents acute angiotensin II-induced hypertension in anesthetized rats. Avicenna J. Phytomed. 2017;7:345–352. [PMC free article] [PubMed] [Google Scholar]
- Shafiee M., Moghaddam N.S.A., Nosrati M., Tousi M., Avan A., Ryzhikov M., Parizadeh M.R., Fiuji H., Rajabian M., Bahreyni A., Khazaei M., Hassanian S.M. Saffron against components of metabolic syndrome: current status and prospective. J. Agric. Food Chem. 2017 doi: 10.1021/acs.jafc.7b03762. [DOI] [PubMed] [Google Scholar]
- Sharma H., Kumar S. Natural AMPK activators: an alternative approach for the treatment and management of metabolic syndrome. Curr. Med. Chem. 2016;24:1007–1047. doi: 10.2174/0929867323666160406120814. [DOI] [PubMed] [Google Scholar]
- Sheng L., Qian Z., Zheng S., Xi L. Mechanism of hypolipidemic effect of crocin in rats: crocin inhibits pancreatic lipase. Eur. J. Pharmacol. 2006;543:116–122. doi: 10.1016/j.ejphar.2006.05.038. [DOI] [PubMed] [Google Scholar]
- Sherling D.H., Perumareddi P., Hennekens C.H. Metabolic syndrome. J. Cardiovasc. Pharmacol. Ther. 2017;22:365–367. doi: 10.1177/1074248416686187. [DOI] [PubMed] [Google Scholar]
- Shirali S., Zahra Bathaie S., Nakhjavani M. Effect of crocin on the insulin resistance and lipid profile of streptozotocin-induced diabetic rats. Phyther. Res. 2013;27:1042–1047. doi: 10.1002/ptr.4836. [DOI] [PubMed] [Google Scholar]
- Stančáková A., Laakso M. Genetics of metabolic syndrome. Rev. Endocr. Metab. Disord. 2014;15:243–252. doi: 10.1007/s11154-014-9293-9. [DOI] [PubMed] [Google Scholar]
- Stump M., Mukohda M., Hu C., Sigmund C.D. PPARγ regulation in hypertension and metabolic syndrome. Curr. Hypertens. Rep. 2015 doi: 10.1007/s11906-015-0601-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaddonfard E., Farshid A.A., Asri-Rezaee S., Javadi S., Khosravi V., Rahman B., Mirfakhraee Z. Crocin improved learning and memory impairments in streptozotocin-induced diabetic rats. Iran. J. Basic Med. Sci. 2013;16:91–100. [PMC free article] [PubMed] [Google Scholar]
- Welty F.K., Alfaddagh A., Elajami T.K. Targeting inflammation in metabolic syndrome. Transl. Res. 2016 doi: 10.1016/j.trsl.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G., Yu S., Gong Z., Zhang S. Study of the effect of crocin on rat experimental hyperlipemia and the underlying mechanisms. Zhongguo Zhong Yao Za Zhi. 2005;30:369–372. [PubMed] [Google Scholar]
- Xu G.L., Qian Z.Y., Yu S.Q., Gong Z.N., Shen X.C. Evidence of crocin against endothelial injury induced by hydrogen peroxide in vitro. J. Asian Nat. Prod. Res. 2006;8:79–85. doi: 10.1080/10286020500044732. [DOI] [PubMed] [Google Scholar]
- Yaribeygi H., Mohammadi M.T., Rezaee R., Sahebkar A. Crocin improves renal function by declining Nox-4, IL-18, and p53 expression levels in an experimental model of diabetic nephropathy. J. Cell. Biochem. 2018;119:6080–6093. doi: 10.1002/jcb.26806. [DOI] [PubMed] [Google Scholar]
- Zhang B.B., Zhou G., Li C. AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009 doi: 10.1016/j.cmet.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Zhang X., Fan Z., Jin T. Crocin protects against cerebral- ischemia-induced damage in aged rats through maintaining the integrity of blood-brain barrier. Restor. Neurol. Neurosci. 2017;35:65–75. doi: 10.3233/RNN-160696. [DOI] [PubMed] [Google Scholar]