Abstract
Asian citrus psyllid is a most damaging insect pest of citrus. In this field study, the efficacy of seven insecticides (emamectin benzoate, bifenthrin, chlorfenapyr, fipronil, imidacloprid, pyriproxyfen and thiamethoxam) was evaluated against Diaphorina citri Kuwayama in the citrus orchard of Kinnow mandarin, Citrus reticulata Blanco. The insecticides revealed a differential and substantial relative efficacy against D. citri compared to the untreated plants. The insecticidal effect attributed as percent reduction in insect population was more prominent after three days of spray: highest reduction values were recorded with thiamethoxam (50.89%), imidacloprid (44.27%) and bifenthrin (42.94%) after first spray, and thiamethoxam (83.36%), imidacloprid (73.20%) and bifenthrin (72.66%) after second spray. Thus, neonicotinoids (thiamethoxam and imidacloprid) and pyrethroid (bifenthrin) resulted as highly effective against D. citri at three days after both sprays. At seven days, imidacloprid (63.53%) and fipronil (62.47%) presented relatively higher population reduction after first spray, and thiamethoxam (92.66%) and chlorfenapyr (89.59%) after second spray. At 12 days, the insecticidal effect on insect population became significantly at par after each spray except chlorfenapyr that reflected high population reduction (93.17%) only after second spray. It is also obvious from the data that there is need of regular monitoring to suppress the psyllids population below threshold level by timely application of the second insecticidal spray.
Keywords: Insecticides, Efficacy, Citrus, Asian citrus psyllid, Population reduction
1. Introduction
Diaphorina citri Kuwayama (Homoptera: Psyllidae) commonly known as Asian citrus psyllid is one of the important insect pest of all citrus varieties and cause severe losses (82-95%) to citrus plants in many countries over the glob (Shivankar et al., 2000, FAO, 2002, Childers and Rogers, 2005, Grafton-Cardwell et al., 2006, Sharma, 2008, Mead and Fasulo, 2011). It was first reported from Taiwan in 1908, and then widespread in Asia and other countries of the world (Halbert and Manjunath, 2004, Singh and Yadav, 2018) including Pakistan (Farmanullah and Gul, 2005, Khan et al., 2013, Mahmood et al., 2014).
Both immature nymph and mature adult insects suck the cell sap from flowers, buds, leaves and young shoots (Singh and Yadav, 2018). It causes leaf distortion, curling of leaves, complete defoliation or shedding of flower and leaves (Mead and Fasulo, 2011, Grafton-Cardwell et al., 2013). Young plants especially under the age of four years are more susceptible due to presence of fresh leaves, shoots and buds that attract psyllids (Bográn et al., 2012). D. citri also carries gram negative phloem-limited bacterium, Candidatus Liberibactor asiaticus (species; Alphaproteobacteria) that causes Huanglongbing (HLB) disease with yellowing and chlorosis of leaves (Yang et al., 2006, Batool et al., 2008, Razi et al., 2011, Mann et al., 2013). The infested plant produces less number of reduced, de-shaped, curved, bitter and salty taste fruits, and may lead to 20% trees loss in poorly managed orchards (Shokrollah et al., 2011, Khan et al., 2012a). The commercially cultivated citrus species are vulnerable to HLB disease that leads to reduction in citrus production and loss in revenue over the globe (Grafton-Cardwell et al., 2013, Jones et al., 2013).
Citrus are favored refreshing fruit due to their delicious and nutritional value throughout the world (Shivankar et al., 2000, Liu et al., 2012). Pakistan is among the major citrus growing countries in South East Asia (Ladaniya, 2008, Iqbal et al., 2009, FAO, 2016, Memon, 2017). Kinnow mandarin (Citrus reticulata Blanco) is widely cultivated variety of citrus in Pakistan (Altaf et al., 2008, Khan, 2010, Razi et al., 2011) due to its attractive color, easy peel-able, savory fragrance, good size, unique delightful flavor and high juice content (44–47.5%) (Ahmed et al., 2008, Din et al., 2012, Memon, 2014). Over 95% of total kinnow production in Pakistan come from Punjab province (Khan et al., 2012a, Khan et al., 2012b, Memon, 2012, Memon, 2014, Tahir, 2014).
The appropriate and timely management of D. citri is necessary to avoid direct and indirect damages to fruit quality and yield. Insecticides are broadly used against variety of insect pests due to their rapid knockdown effect. Numerous studies reported the effectiveness of insecticides against citrus psyllid (McKenzie et al., 2004, Khan et al., 2013, Monzo et al., 2014), and development of insecticidal resistance in psyllids (Naeem et al., 2016). Nevertheless, the extensive use of insecticides exerts a pressure for selection of most suitable insecticides against D. citri in citrus growing areas (Singh and Yadav, 2018). Therefore, this field study assessed the comparative efficacy of commonly available insecticides of different chemical groups against citrus psyllid on kinnow mandarin plants.
2. Materials and methods
2.1. Site selection and experimental layout
Field experiments in randomized complete block design were executed at Horticulture farm, Ayub Agricultural Research Institute, Faisalabad, Pakistan (31.24 °N, 73.03 °E). Eight treatments including one control were applied with three replications and a single plant of 10 years old was characterized as one replication (Farmanullah and Gul, 2005, Sharma, 2008). The size of experimental area was 6156 m2 and the distance within each plant and row was 6 m. One plant was left untreated between each treatment and replication as a buffer line.
3. Application of insecticides
Seven insecticides (emamectin, bifenthrin, chlorfenapyr, fipronil, imidacloprid, pyriproxyfen and thiamethoxam) (Table 1) were sprayed at their recommended doses on respective Kinnow mandarin (C. reticulata Blanco) plants during May-June 2014. The control plants were treated with water only. Two successive sprays were performed using spray-gun fitted knapsack sprayer at economic threshold level (ETL) of citrus psyllid (6 nymphs or adults per leaf) (GOP, 2010). The sprayer was calibrated using the water on non-experimental plants and by calculating the consumed water per plant. The calculated dose of insecticide was applied on the experimental plants. Knapsack sprayer was washed each time with water before the next insecticidal spray.
Table 1.
Insecticides and their field doses sprayed against Diaphorina citri.
| Insecticide treatments | Trade name | Chemical group | Manufacturer | Dose per100 liter of water |
|---|---|---|---|---|
| Emamectin benzoate | Gold® 1.8 EC | Avermectin | Swat Agro. | 80 ml |
| Bifenthrin | Talstar® 10 EC | Pyrethroids | FMC | 50 ml |
| Chlorfenapyr | Pirate® 360 SC | Pyrazole | Swat Agro. | 70 ml |
| Fipronil | Fipryte® 5 SC | Pyrazole | Jaffer Agro. | 100 ml |
| Imidacloprid | Confidor® 200 SL | Neonicotinoids | Bayer Crop. | 40 ml |
| Pyriproxyfen | Rolex® 10.8 EC | IGRs | HELB | 100 ml |
| Thiamethoxam | Actara® 25 WP | Neonicotinoids | Syngenta | 10 g |
| Water | Control | – | – | – |
3.1. Determination of percent population reduction
The post-treatment data regarding insect population was recorded from twenty-five randomly selected young leaves from all sides of the plant (Sharma, 2008) at 3, 7 and 12 days after spray (DAS). Both adults and nymphs were counted from both sides of the leaf by visual observation. The percentage population change (reduction) was figured with the formula of Henderson and Tilton (1955).
(where n = insect population; T = treated; Co = control)
3.2. Statistical analysis
The population data were analyzed statistically using Analytical Software (Statistix 8.1) and subjected to the ANOVA (Analysis of variance). Means were separated using Least Significance Difference test (Steel et al., 1997, Ahmed et al., 2004) at P ≤ 0.05.
4. Results and discussion
4.1. Population reduction of Diaphorina citri
D. citri is a serious threat to citrus plants and causing huge losses to citrus yield in Pakistan (Batool et al., 2008). The present study assessed the efficacy of seven different insecticides against D. citri on Kinnow mandarin (C. reticulata) under field conditions. The tested insecticides revealed a substantial relative efficacy against D. citri at different days (3, 7 and 12) after spray (DAS). The population reduction was highly prominent after 3 days of each insecticidal spray. However, the insecticidal effect gradually reached significantly at par in later days after spray. Our results are harmonized with previous studies where insecticides were found effective to control citrus psyllid (Dahiya et al., 1994, Tiwari et al., 2012, Grafton-Cardwell et al., 2013, Jones et al., 2013, Chen et al., 2018).
Table 2 showed a significant difference in insect population reduction (%) after first spray of insecticides (3 DAS: F = 5.62; P = 0.0009), (7 DAS: F = 29.77; P = 0.0000) and (12 DAS: F = 44.46; P = 0.0000). After 3 days of first spray, highest reduction (%) in psyllid population was noticed in plants treated with thiamethoxam (50.89%) followed by imidacloprid (44.27%). A moderate pest population reduction was observed in plants sprayed with bifenthrin (42.94%), emamectin benzoate (36.05%) and chlorfenapyr (35.07%). The plants sprayed with fipronil displayed a low pest reduction (28.24%), whereas plants treated with pyriproxyfen showed lowest pest reduction (24.24%) at 3 DAS. Imidacloprid (63.53%) and fipronil (62.47%) elicited highest population reduction at 7 DAS followed by bifenthrin, thiamethoxam, chlorfenapyr and pyriproxyfen (60.49%, 59.86%, 56.22%, 54.52%) that were significantly at par with each other except emamectin benzoate with least population reduction (50.93%). After 12 DAS, the population reduction was above 60% and significantly at par with all insecticides.
Table 2.
Population reduction of Diaphorina citri after first Spray of insecticides.
| Insecticide treatments | Pre-treatment (mean population per leaf) |
Post-treatment (Percent population reduction* ± SEM) |
||
|---|---|---|---|---|
| 1 DBS | 3 DAS | 7 DAS | 12 DAS | |
| Emamectin benzoate | 16.54 | 36.05 ± 4.03 abc | 50.93 ± 4.89b | 60.20 ± 6.30 a |
| Bifenthrin | 15.91 | 42.94 ± 13.14 abc | 60.49 ± 7.44 ab | 72.00 ± 5.24 a |
| Chlorfenapyr | 16.62 | 35.07 ± 8.99 abc | 56.22 ± 7.76 ab | 60.87 ± 7.41 a |
| Fipronil | 15.66 | 28.24 ± 13.27 bc | 62.47 ± 5.28 a | 66.22 ± 7.68 a |
| Imidacloprid | 16.92 | 44.27 ± 15.20 ab | 63.53 ± 7.39 a | 70.87 ± 5.92 a |
| Pyriproxyfen | 16.40 | 24.24 ± 9.68c | 54.52 ± 3.84 ab | 60.29 ± 7.06 a |
| Thiamethoxam | 15.73 | 50.89 ± 5.54 a | 59.86 ± 3.96 ab | 66.85 ± 4.90 a |
| Control (water) | 17.04 | 0.00 ± 0.00 d | 0.00 ± 0.00c | 0.00 ± 0.00b |
| F, P value | 5.62, 0.0009 | 29.77, 0.0000 | 44.46, 0.0000 | |
| LSD | 19.59 | 11.34 | 12.21 | |
Means sharing the common letters are not significantly different from each other. LSD = least significance difference; DBS = days before spray; DAS = days after spray; SEM = standard error of mean. Pre-treatment data indicated the mean psyllid population/ leaf collected from twenty-five randomly selected leaves of a plant. Pre-treatment population was above the economic threshold level (6 nymphs or adults per leaf).
Table 3 revealed the population reduction after second insecticidal spray that was launched when the insect population again approached to ETL. This time period was two weeks after last observation of first spray. The population reduction was significantly different among treatments after second spray (3 DAS: F = 23.45; P = 0.0000), (7 DAS: F = 35.31; P = 0.0000) and (12 DAS: F = 32.18; P = 0.0000).
Table 3.
Population reduction of Diaphorina citri after second spray of insecticides.
| Insecticide treatments | Pre-treatment (mean population per leaf) |
Post-treatment (Percent population reduction* ± SEM) |
||
|---|---|---|---|---|
| 1 DBS | 3 DAS | 7 DAS | 12 DAS | |
| Emamectin benzoate | 19.67 | 60.11 ± 5.24 bc | 56.14 ± 11.18c | 76.79 ± 7.44b |
| Bifenthrin | 16.36 | 72.66 ± 11.17 ab | 85.99 ± 4.85 ab | 89.50 ± 3.48 ab |
| Chlorfenapyr | 17.88 | 71.35 ± 9.59 ab | 89.59 ± 5.12 a | 93.17 ± 2.52 a |
| Fipronil | 17.26 | 64.11 ± 12.40 bc | 83.92 ± 4.21 ab | 89.96 ± 2.72 ab |
| Imidacloprid | 16.94 | 73.20 ± 3.37 ab | 85.58 ± 3.15 ab | 90.18 ± 1.72 ab |
| Pyriproxyfen | 18.11 | 55.21 ± 11.12c | 71.89 ± 12.16b | 88.93 ± 0.91 ab |
| Thiamethoxam | 17.28 | 83.36 ± 2.69 a | 92.66 ± 1.42 a | 85.60 ± 9.24 ab |
| Control (water) | 18.19 | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00c |
| F, P value | 23.45, 0.0000 | 35.31, 0.0000 | 32.18, 0.0000 | |
| LSD | 15.65 | 15.29 | 13.82 | |
Means sharing the common letters are not significantly different from each other. LSD = least significance difference; DBS = days before spray; DAS = days after spray; SEM = standard error of mean. Pre-treatment data indicated the mean psyllid population/ leaf collected from twenty-five randomly selected leaves of a plant. Pre-treatment population was above the economic threshold level (6 nymphs or adults per leaf).
At 3 days of second spray, highest population reduction was noted in plants treated with thiamethoxam (83.36%) followed by imidacloprid (73.20%), bifenthrin (72.66%) and chlorfenapyr (71.35%). A moderate population reduction was observed in plants sprayed with fipronil (64.11%) and emamectin benzoate (60.11%). Whereas, pyriproxyfen treated plants exhibited least population reduction (55.21%) at 3 DAS. Highest population reduction was recorded with spray of thiamethoxam (92.66%) and chlorfenapyr (89.59%) after 7 days of second spray. Pest population reduction was significantly at par on plants treated with bifenthrin (85.99%), imidacloprid (85.58%), fipronil (83.92%) and pyriproxifen (71.89%). Whereas, emamectin benzoate sprayed plants exhibited the least population reduction (56.14%) at 7 DAS.
Chlorfenapyr treated plants provided highest reduction (93.17%) in pest population at 12 DAS. The pest population reduction with other insecticides (imidacloprid, fipronil, bifenthrin, pyriproxifen and thiamethoxam) were significantly at par with values 90.18%, 89.96%, 89.50%, 88.93% and 85.60%, respectively. The least population reduction (76.79%) was recorded with the spray of emamectin benzoate at 12 DAS.
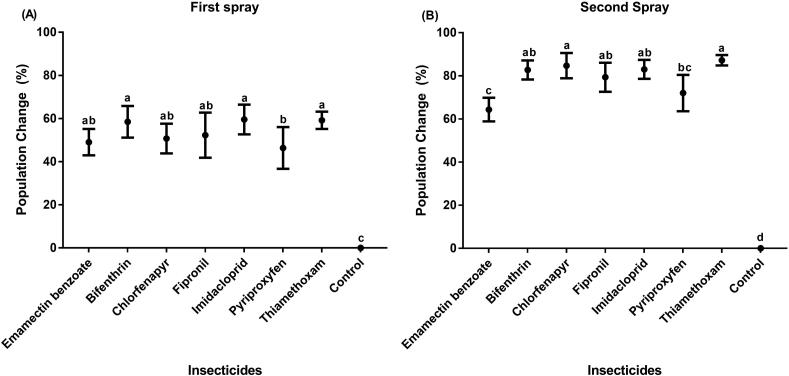
The pooled average data after first spray (Fig. 1A) presented the significant difference (F = 24.96; P = 0.0000) in the mean insect population reduction with the order of insecticidal efficacy as imidacloprid (59.56%), thiamethoxam (59.20%) and bifenthrin (58.48%) followed by fipronil (52.31%), chlorfenapyr (50.72%) and emamectin benzoate (49.06%). The minimum population reduction was noted after plants treated with pyriproxifen (46.35%). Likewise, the pooled average data after second spray (Fig. 1B) revealed the significant difference (F = 58.91; P = 0.0000) in the mean insect population reduction with the order of insecticidal efficacy as thiamethoxam (87.21%) and chlorfenapyr (84.70%) followed by imidacloprid (82.99%), bifenthrin (82.71%) and fipronil (79.33%). The values of population reduction with pyriproxifen were (72.01%) and emamectin benzoate gave minimum population reduction (64.34%). It is evident from the pooled data that the relatively higher reduction percentages of psyllid population were observed after second spray than first spray which is in line with the observations of Farmanullah and Gul (2005).
Fig. 1.
Mean reduction percentage of psyllids population (A) after first spray of insecticides (B) after second spray of insecticides. Means sharing the common letters in the graphs are not significantly different from each other.
In present data, thiamethoxam was found very effective exhibiting highest population reduction range (50–92%) of D. citri at 3–7 days after first and second insecticidal sprays which is in accordance with the findings of Sharma (2008) regarding very high population reduction (91–100%) with thiamethoxam and imidacloprid in the same time range. Farmanullah and Gul (2005) stated thiamethoxam as highly effective to decrease D. citri population as compared to thiodan, lufenuron, methidathion flufenoxuron and α-cyhalothrin. In another study, thiamethoxam showed the maximum percent of mortality (97.7%) of D. citri in comparison with dimethoate and acetamiprid (Abbaszadeh et al., 2011). Imidacloprid and thiamethoxam showed highest statistically at par nymphal population reduction of citrus psyllid up to 7 days after spraying (Arora and Sharma, 2011).
Imidacloprid was the second effective insecticide after thiamethoxam against D. citri at 3 DAS with 44.27% and 73.20% population reduction after first and second spray, respectively. Imidacloprid exhibited 100% mortality of D. citri after 4 days in comparison with methomyl, lambda-cyhalothrin and neem (Azadirachta indica) based solutions (Khan et al., 2012b). The laboratory tests with direct spray of imidacloprid on D. citri also revealed 94.33–100% mortality (Khan et al., 2013, Qasim and Hussian, 2015). Imidacloprid significantly control the attack of D. citri in the leaf tissue of grape fruit Citrus paradisi after two weeks of its soil treatment (Setamou et al., 2010). Ahmed et al. (2004) found that all insecticides (methamidophos, dimethoate and imidacloprid) equally reduced the D. citri population on Kinnow and other citrus species. Imidacloprid is known to affect the growth, life cycle and landing of adult females for egg laying of D. citri on citrus plant, (Leong et al., 2012, Boina and Bloomquist, 2015) that might be the potential reason for its noted efficacy in present study. However, high resistance was found in field population of D. citri against imidacloprid (Naeem et al., 2016) which contradicts our results.
Bifenthrin was the third most effective insecticide that gave 42.94% and 72.66% population control at 3 DAS after first and second spray, respectively. Bifenthrin exhibited highest mortality (93.83%) when used as spray in the laboratory studies (Qasim and Hussian, 2015). Fipronil and chlorfenapyr were also effective at 7 DAS which is in accord with results of Deng et al. (2011), who stated high efficiency of chlorfenapyr during 2–15 days after treatment.
5. Conclusions
Chemical insecticides can provide fast and accurate control of D. citri. The neonicotinoids (thiamethoxam, imidacloprid) and pyrethroid (bifenthrin) insecticides are relatively more effective against D. citri. It is also suggested that D. citri population should be monitored regularly to launch timely second spray in case the population regain ETL. The farmers may associate two timely sprays of these insecticides in their citrus management program.
Acknowledgments
The authors extend sincere appreciation to the Deanship of Scientific Research at King Saud University, Saudi Arabia for supporting the research work through College of Food and Agriculture Sciences Research Center.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abbaszadeh G., Ameri A., Torabizadeh M. Evaluation of different groups of insecticides on Asian citrus Psylla, Diaphorina citri K. Pestic. Res. J. 2011;23(1):52–54. [Google Scholar]
- Ahmed M., Ahmad A., Chatha Z.A., Dilshad S.M.R. Studies on preparation of ready to serve mandarin (Citrus reticulata) diet drink. Pak. J. Agri. Sci. 2008;45(4):470–476. [Google Scholar]
- Ahmed S., Ahmad N., Khan R.R. Studies on population dynamics and chemical control of citrus psylla, Diaphorina citri. Int. J. Agric. Biol. 2004;6(6):970–973. [Google Scholar]
- Altaf N., Khan A., Hussain J. Fruit variability in Kinnow mandarin (Citrus reticulata) Pak. J. Bot. 2008;40(2):599–604. [Google Scholar]
- Arora P.K., Sharma D.R. Bio-efficacy of some neonicotinoids against citrus psylla, Diaphorina citri Kuwayama on kinnow mandarin. J. Insect Sci. 2011;24(4):399–401. [Google Scholar]
- Batool A., Iftikhar Y., Mughal S.M., Khan M.M., Jaskani M.J., Abbas M., Khan I.A. Citrus Greening Disease - A major cause of citrus decline in the world: A Review. Hort. Sci. 2008;34(4):159–166. doi: 10.17221/1897-hortsci. [DOI] [Google Scholar]
- Bográn C.E., Villanueva R.T., Setamou M. Texas AgriLife Extension Service; USA: 2012. Identification and management of the Asian citrus psyllid and citrus greening disease in Texas nurseries; pp. 1–4. [Google Scholar]
- Boina D.R., Bloomquist J.R. Chemical control of the Asian citrus psyllid and of huanglongbing disease in citrus. Pest Manag. Sci. 2015;71(6):808–823. doi: 10.1002/ps.3957. [DOI] [PubMed] [Google Scholar]
- Chen X.D., Ashfaq M., Stelinski L.L. Susceptibility of Asian citrus psyllid, Diaphorina citri (Hemiptera: Liviidae), to the insecticide afidopyropen: a new and potent modulator of insect transient receptor potential channels. Appl. Entomol. Zool. 2018;53(4):453–461. doi: 10.1007/s13355-018-0574-8. [DOI] [Google Scholar]
- Childers C.C., Rogers M.E. Chemical control and management approaches of the Asian citrus psyllid, Diaphorina citri kuwayama (Homoptera: psyllidae) in Florida citrus. Proc. Fla State Hort. Soc. 2005;118:49–53. [Google Scholar]
- Dahiya K., Lakra R., Dahiya A., Singh S. Bioefficacy of some insecticides against citrus psylla, Diaphorina citri Kuw, (Psyllidae: Homoptera) Crop. Res. 1994;8(1):137–140. [Google Scholar]
- Deng M.-X., Qin X., Tan Y.-L., Zhai G.-Y., Gan G.-Y., Jiang J.-J., Lan B.-T., Wei W. The Field Efficacy Trials of Chlorfenapyr 10% SC on Diaphorina Citri Kuwayama and Phyllocnistis Citrella Stainton and other citrus pests. Southern Hort. 2011;2011(6):1–6. [Google Scholar]
- Din A., Asghar M., Parveen S., Azhar Ali M. Evaluation of kinnow mandarin as influenced by pre-harvest management practices. J. Agri. Res. 2012;50(3):381–392. [Google Scholar]
- FAO . Food and Agriculture Organization; United Nations, Rome: 2002. Saudi citrus industry thrives in desert. [Google Scholar]
- FAO . Food and Agriculture Organization; United Nations, Rome: 2016. Citrus fruit – Fresh and processed statistical bulletin; pp. 1–77. [Google Scholar]
- Farmanullah H.B., Gul R. Evaluation of six different groups of insecticides for the control of citrus psylla Diaphorina citri (Hemiptera: Psyllidae) Songklanakarin J. Sci. Technol. 2005;27(1):17–23. [Google Scholar]
- GOP . Directorate General of Pest Warning and Quality Control of Pesticides; Punjab, Lahore, Pakistan: 2010. Economic threshold level (ETL) of different insect pests and diseases. [Google Scholar]
- Grafton-Cardwell E.E., Godfrey K.E., Rogers M.E., Childers C.C., Stansly P.A. University of California, Agriculture and Natural Resources; USA: 2006. Asian citrus psyllid; pp. 1–8. [Google Scholar]
- Grafton-Cardwell E.E., Stelinski L.L., Stansly P.A. Biology and management of Asian citrus psyllid, vector of the huanglongbing pathogens. Annu. Rev. Entomol. 2013;58(1):413–432. doi: 10.1146/annurev-ento-120811-153542. [DOI] [PubMed] [Google Scholar]
- Halbert S.E., Manjunath K.L. Asian citrus psyllids (Sternorrhyncha: Psyllidae) and greening disease in citrus: a literature review and assessment of risk in Florida. Fla. Entomol. 2004;87(3):330–353. [Google Scholar]
- Henderson C.F., Tilton E.W. Tests with acaricides against the brown wheat mite. J. Econ. Entomol. 1955;48(2):157–161. doi: 10.1093/jee/48.2.157. [DOI] [Google Scholar]
- Iqbal S., Maqbool H.S., Hussain Z. Technical efficiency of citrus production in Sargodha district. Punjab. Int. J. Agric. Appl. Sci. 2009;1(2):68–75. [Google Scholar]
- Jones M.M., Stansly P.A., Russo J. Extension model to improve Asian citrus psyllid control in citrus health management areas (CHMAs) Proc. Fla State Hort. Soc. 2013;126:68–71. [Google Scholar]
- Khan A.A., Afzal M., Raza A.M., Khan A.M., Iqbal J., Tahir H.M., Qureshi J.A., Khaliq A., Zia-ul-Haq M., Aqeel M.A. Toxicity of botanicals and selective insecticides to Asian citrus psylla, Diaphorina citri K. (Homoptera: Psyllidae) in laboratory conditions. Jokull J. 2013;63(8):52–72. [Google Scholar]
- Khan F.U., Khan N., Abdullah Z. Impact of kinnow (Mandarin) yield losses on socio-economic conditions of the farming community in Punjab, Pakistan. Pakistan J. Agric. Res. 2012;25(1):63–68. [Google Scholar]
- Khan I., Zahid M., Khan G.Z. Toxicity of botanic and synthetic pesticide residues to citrus psyllid Diaphorina citri Kuwayama and Chrysoperla carnea (Stephens) Pak. J. Zool. 2012;44(1):197–201. [Google Scholar]
- Khan M.A. United Nations Industrial Development Organization; 2010. Report on enterprise based survey of horticulture sector, Trade Related Technical Assistance (TRTA) Programme Pakistan; pp. 1–48. [Google Scholar]
- Ladaniya M.S. Citrus fruit production and prospects. In: Ladaniya M.S., editor. Citrus fruit: Biology, technology and evaluation. Academic Press, Elsevier; UK: 2008. pp. 1–12. [Google Scholar]
- Leong S.C.T., Abang F., Beattie A., Kueh R.J.H., Wong S.K. Impacts of horticultural mineral oils and two insecticide practices on population fluctuation of Diaphorina citri and spread of huanglongbing in a citrus orchard in Sarawak. Sci. World J. 2012;2012:1–7. doi: 10.1100/2012/651416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Heying E., Tanumihardjo S.A. History, global distribution, and nutritional importance of citrus fruits. Comp. Rev. Food Sci. Food Safety. 2012;11(6):530–545. doi: 10.1111/j.1541-4337.2012.00201.x. [DOI] [Google Scholar]
- Mahmood R., Rehman A., Ahmad M. Prospects of biological control of citrus insect pests in Pakistan. J. Agri. Res. 2014;52(2):229–244. [Google Scholar]
- Mann R.S., Rouseff R.L., Smoot J., Rao N., Meyer W.L., Lapointe S.L., Robbins P.S., Cha D., Linn C.E., Webster F.X., Tiwari S., Stelinski L.L. Chemical and behavioral analysis of the cuticular hydrocarbons from Asian citrus psyllid, Diaphorina citri. Insect. Sci. 2013;20(3):367–378. doi: 10.1111/j.1744-7917.2012.01541.x. [DOI] [PubMed] [Google Scholar]
- McKenzie C.L., Brock G., Murph B.C. Insecticidal control of Asian citrus psyllid and citrus leafminer on Hamlin oranges, 2003. Arthropod Manage. Tests. 2004;29(1):D8. doi: 10.1093/amt/29.1.D8. [DOI] [Google Scholar]
- Mead F.W., Fasulo T.R. University of Florida, IFAS Extension; USA: 2011. Asian Citrus Psyllid, Diaphorina citri Kuwayama (Insecta: Hemiptera: Psyllidae) pp. 1–7. [Google Scholar]
- Memon N.A. Fruits exports show an increase of 22% in 2010–11. Pak. Food J. 2012;2:20–21. [Google Scholar]
- Memon N.A. Market potential for Pakistani citrus fruit (Kinnow) in world. Pak. Food J. 2014;1:41–42. [Google Scholar]
- Memon N.A. Citrus Fruit (Kino): Punjab produced 98% of production. Pak. Food J. 2017;1:29–31. [Google Scholar]
- Monzo C., Qureshi J.A., Stansly P.A. Insecticide sprays, natural enemy assemblages and predation on Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae) Bull. Entomol. Res. 2014;104(5):576–585. doi: 10.1017/s0007485314000315. [DOI] [PubMed] [Google Scholar]
- Naeem A., Freed S., Jin F.L., Akmal M., Mehmood M. Monitoring of insecticide resistance in Diaphorina citri Kuwayama (Hemiptera: Psyllidae) from citrus groves of Punjab, Pakistan. Crop. Prot. 2016;8662–68 doi: 10.1016/j.cropro.2016.04.010. [DOI] [Google Scholar]
- Qasim M., Hussian D. Efficacy of insecticides against citrus psylla (Diaphorina Citri Kuwayama) in field and laboratory conditions. Cercet. Agron. Mold. 2015;48(2):91–97. doi: 10.1515/cerce-2015-0033. [DOI] [Google Scholar]
- Razi M.F., Khan I.A., Jaskani M.J. Citrus plant nutritional profile in relation to Huanglongbing prevalence in Pakistan. Pak. J. Agri. Sci. 2011;48(4):299–304. [Google Scholar]
- Setamou M., Rodriguez D., Saldana R., Schwarzlose G., Palrang D., Nelson S.D. Efficacy and uptake of soil-applied imidacloprid in the control of Asian citrus psyllid and a citrus leafminer, two foliar-feeding citrus pests. J. Econ. Entomol. 2010;103(5):1711–1719. doi: 10.1603/ec09371. [DOI] [PubMed] [Google Scholar]
- Sharma D.R. Population dynamics in relation to abiotic factors and management of citrus psylla in Punjab. Indian J. Hort. 2008;65(4):417–422. [Google Scholar]
- Shivankar V.J., Rao C.N., Singh S. Studies on citrus psylla, Diaphorina citri Kuwayama: A review. Agric. Rev. 2000;21(3):199–204. [Google Scholar]
- Shokrollah H., Lee Abdullah T., Sijam K., Abdullah S.N.A. Identification of physical and biochemical characteristic of mandarin (Citrus reticulata) fruit infected by huanglongbing (HLB) Aust. J. Crop Sci. 2011;5(2):181. [Google Scholar]
- Singh P.V., Yadav G.S. Bio-ecology and Management of Citrus psylla, Diaphorina citri Kuwayama on Citrus - A Review. Int. J. Curr. Microbiol. App. Sci. 2018;7(4):3091–3107. doi: 10.20546/ijcmas.2018.704.351. [DOI] [Google Scholar]
- Steel R.G.D., Torrie J.H., Dickey D.A. 3rd ed. McGraw-Hill; USA: 1997. Principles and procedures of statistics: A biometrical approach. [Google Scholar]
- Tahir A. Forecasting citrus exports in Pakistan. Pakistan J. Agric. Res. 2014;27(1):64–68. [Google Scholar]
- Tiwari S., Clayson P.J., Kuhns E.H., Stelinski L.L. Effects of buprofezin and diflubenzuron on various developmental stages of Asian citrus psyllid, Diaphorina citri. Pest. Manage. Sci. 2012;68(10):1405–1412. doi: 10.1002/ps.3323. [DOI] [PubMed] [Google Scholar]
- Yang Y., Huang M.C., Beattie G.A., Xia Y., Ouyang G., Xiong J. Distribution, biology, ecology and control of the psyllid Diaphorina citri Kuwayama, a major pest of citrus: A status report for China. Int. J. Pest. Manage. 2006;52(4):343–352. doi: 10.1080/09670870600872994. [DOI] [Google Scholar]