Abstract
Irrigation of industrial effluents may end in the bioaccumulation of various toxic metals and consequent genetic changes in contaminated food crops. To test this hypothesis and extent of genetic modifications, Allium cepa test was performed to food crops viz. tomato (Lycopersicum esculentum) and chili (Capsicum annum) as Allium cepa test is a useful tool to assess genetic variations in plants. Prior to A. cepa test, the plants were exposed to various metal concentrations 125–1000 mg/L in the synthetic wastewater. The extracts of harvested plants were used to grow the root of A. cepa following its standard method. The root tips were fixed, stained and examined under compound microscope (almost 300–400 dividing cells) to check the extent of chromosomal variations during various stages of mitosis. The results revealed various chromosomal abnormalities including laggards, stickiness, vagrant chromosomes, binucleated cells, nuclear lesions, giant cells and c-mitosis at different level of treatment. On the whole, aberrations were increasing with the increasing doses along the positive control. In comparison, chili crop had higher level of aberrations depicting the higher chromosomal changes. Lower mitotic index (MI) with increasing level of doses was also describing the hampered cell division due to increased metal stress. The study is showing that the cell division was ceased with increasing metal stress thus increasing the rate of cell aberrations.
Keywords: Allium cepa, Chromosomal aberrations, Edible crops, Metals, Mitotic index
1. Introduction
Over last decades, the environmental pollution of soils with toxic metals has become a global issue owing to the rapid urbanization and industrialization (Sharma et al., 2012). Noxious metals and metalloids pose detrimental effects to ecosystems and also affect human health owing to their toxic, non-degradable nature and easy bioaccumulation (Li et al., 2018, Ngole-Jeme and Fantke, 2017, Nriagu, 1990, Yi et al., 2011); and therefore, could be a potential risk for food security (Lei et al., 2015, Zhang et al., 2015). Metal accumulation in various edible plants may be harmful for human health (Hussain et al., 2013, Roy and McDonald, 2015, Singh et al., 2010). Numerous anthropogenic sources, including mining and metal-ore processing, industrial effluents, vehicle emissions and agricultural activities may result in metal contamination of soils (Dietrich et al., 2019, Luo et al., 2015). However, contamination of food plants is quite common due to wastewater irrigation now a day (Chary et al., 2008, Iqbal et al., 2016, Khan et al., 2008).
Freshwater scarcity and economic demands have compelled wastewater irrigation practices, particularly in arid areas of the globe (Hamilton et al., 2007, Wiener et al., 2016). One tenth of the global human population depends on food grown from wastewater irrigation (Corcoran, 2010). According to another estimate, 200 million farmers practice various forms of wastewater irrigation on 20 million ha of land around the world (Harvest, 2001, Raschid-Sally and Jayakody, 2009, Scott et al., 2004). Although wastewater possess considerable amount of valuable plant nutrients that can reduce the need for addition of artificial fertilizers (Jiménez and Asano, 2008, Schwartz and Boyd, 1994, Siebe, 1998), long-term wastewater irrigation could result in metal accumulation and bio-magnification which cause potential health hazards (Christou et al., 2017, Farahat and Linderholm, 2015). Alterations in genetic material like chromosomal aberrations, ploidy and point mutations may be anticipated as a consequence of metals accumulation (Haq et al., 2017, Silveira et al., 2017). Previously, different techniques have been developed to evaluate the ecotoxicological effects of environmental pollutants (Baderna et al., 2011). Among these, Allium cepa test is widely employed and is very sensitive assay to explore chromosomal aberrations caused by various chemical toxins (Grant, 1999, Maluszynska and Juchimiuk, 2005, Timothy et al., 2014). The test has a long history in scientific literature i.e. developed and described by Levan (1938) and later modified for environmental monitoring (Fiskesjö, 1985, Rank, 2003). A. cepa has low number of chromosomes (2n = 16) which is handy to assess the genetic changes at chromosomal scale appearing after plants are exposed to toxic elements. This test has been employed to investigate the harmful effects of toxins on normality of cellular division. The test is a convenient technique to promote the intricate knowledge on how toxic substances alone or in mixture bring chromosomal alterations (Andrade et al., 2010, Palmieri et al., 2016, Aragão et al., 2015, Freitas et al., 2016).
Since 26% of vegetables grown in Pakistan originate from wastewater irrigation of agricultural lands (Ensink et al., 2004), the risk of metal bioaccumulation is very high. Wastewater irrigation practice is widespread in the whole world. Water scarcity, mismanagement of freshwater resources, relaxation mode and illiterate farmers in Pakistan has led to extensive use of wastewater irrigation. Soil pollution with heavy metals (HMs) due to discharge of untreated urban and industrial wastewater is a major threat to ecological integrity and human well-being. Heavy metals are naturally occurring in the earth’s crust but anthropogenic and industrial activities have led to drastic environmental pollutions in distinct areas. Although, HMs are natural elements of soils and occur naturally in the environment but yet, is of major concern worldwide due to their excessive concentrations. When wastewater originates from industrial estates, it have toxic substances like heavy metals, recalcitrant organics and other undesirable pollutants which may deposit in the edible parts of food crops and pose serious threats to the human life. Trace metals, such as arsenic, cadmium, lead, chromium, nickel and mercury are important environmental pollutants, particularly in urban areas.
Therefore, we hypothesized that vegetables (i.e., tomatoes and chilies) irrigated with industrial wastewater can accumulate heavy metals. Further, metal accumulation may cause genetic changes, and subsequently the food safety can be affected. The present investigation was aimed at assessing the chromosomal aberrations in onion root tips caused by metals accumulation from wastewater irrigation of tomatoes and chilies using A. cepa tests.
2. Methodology
2.1. Treatment and plant extract
The plant materials viz. tomato (Lycopersicum esculentum) and chili (Capsicum annum) were taken after the metal’s exposure throughout their life cycle. The plants were grown with the synthetic wastewater having known concentrations of metals including Cr, Cd, Fe, Pb and Ni. The metal concentrations were given during active growth period of plants under investigation. All the desired agronomic practices were applied to raise these plants (Gurmu and Mano, 2016). The metals concentrations were set according to the observed values in accordance with the field samples collected adjoining to the Hattar Industrial Estate. There are around 117 operational unit in Hattar that are mainly composed of food and beverage, textile, crockery, paper printing, cement, publishing, chemical, rubber and leather products, etc. The lab experiment was conducted in the greenhouse under controlled conditions. The plant samples (leaves) were collected from different treatments, washed and pat dried with tissue napkins. Before reducing to powder form, the samples were air dried first and later dried in oven at 50 °C for 72 h. A grinder accomplished grinding of the dried samples. Approximately, 400 mL of methanol was used to extract 100 g powder through maceration at constant agitation for four days. Later, a muslin cloth filtered the mixture prior to filtering it twice with Whatman filter paper No.1. The rotatory evaporator concentrated the filtrate further in vacuum at 50 °C and oven accomplished drying at 60 °C in the glass petri dish. Almost 17% of crude extract was obtained and subsequently placed in the Petri-dishes sealed with parafilm in a dark cupboard. The extract was used later by dissolving in methanol to get the desired concentrations.
2.2. Allium cepa test
Average sized (purple) healthy, young A. cepa bulbs were obtained from local bazaar in Abbottabad. The outer dead scales of the bulb were removed without damaging the root primordia to promote the growth of new roots. The bulbs were grown for 3 days at 25 °C in the nutrient solution of known composition given by Feretti et al. (2007) in darkness. Within 3–4 days, the roots grew up to a length of 2–4 cm and were ready to treat with crude extract of known metal concentrations (i.e., 125, 250, 500, 1000 μg/mL) as a positive control. The metal exposure was based on the metal concentrations found in various types of real industrial wastewaters. A parallel experiment was also conducted in dimethyl sulfoxide (DMSO) with same concentrations (i.e., 125, 250, 500, 1000 μg/mL). Additionally, A. cepa was also grown in water as a negative control (Yuet Ping et al., 2012). All experiments were conducted in darkness and the beakers were completely covered with aluminum foil to protect from light.
After a growth period of another couple of days, root tips of plants were harvested, fixed in the Carnoy’s fixative (1:3 acetic acid: alcohol) for 24 h and subsequently stored at 4 °C for several days before the observation of abnormalities (Nefic et al., 2013). These fixed roots were then placed in the petri dish and hydrolyzed with 1 N HCl and later heated in an oven at 60 °C for five minutes, intermittently, in order to dissolve the cell wall (Rajneet et al., 2014) and washed with distilled water thrice and stored (Odeigah et al., 1997). The roots were transferred on a glass slide and a small section of each root (1–2 mm) was separated by cutting with a surgical blade and then dipped in a drop of 2% acetoorcein for 2 min. The roots tips are then a glass rod squashed the root tip by adding another drop of acetoorcein for at least 2 min. The slide cover was carefully placed over the slide by avoiding the entry of air bubbles. Finally, pressed the section of slide containing stained root tip by thumb pressure by wrapping the slide with blotting paper which helped to absorb extra stain. The edges of slide cover glass were sealed with clear nail varnish for preservation (Grant, 1982, Sharma, 1983, Yuet Ping et al., 2012).
For every treatment, 6 slides were prepared and, on every slide, almost 1000 cells including 300–400 dividing cells, were observed in all the slides at 1000× magnification for different phases of cell divisions and the chromosomal aberrations using the trinocular microscope Euromax (Euromex Microscopen Spain) and the photomicrographs were also taken from the attached digital camera. The mitotic index and the chromosomal aberrations were calculated according to the standard method described by (Bakare et al., 2000, Fiskesjö, 1985) whereby the total number of aberrant cells per total number of cells of each treatment was calculated. The mitotic index was obtained as follows (Eq. (1)):
| (1) |
The percentage of aberrant cells can be calculated by eq. (2).
| (2) |
3. Results
3.1. A. cepa assay
The results suggested that all the leaf extracts of the of L. esculentum and C. anuum containing metal concentrations caused significant abnormalities in cell division in comparison to negative control and positive control. The quantitative analyses of various chromosomal aberrations were shown in Table 1.
Table 1.
Cytogenetic analysis of A. cepa root tips exposed to different concentrations of DMSO, chili and tomato extracts.
| Concentration | Bridges | Lagging | Stickiness | Vagrant | Binucleated | Nuclear Lesions | Giant cell | c-mitosis | Aberrations (%) |
|---|---|---|---|---|---|---|---|---|---|
| DMSO-125 | 1.8 (0.7) | 2.6 (1.14) | 1.6 (0.5) | 3.2 (0.8) | 0.8 (0.8) | 2.8 (0.83) | 3.4 (0.5) | 2.6 (0.5) | 7 |
| DMSO-250 | 4.8 (0.8) | 6.2 (1.15) | 3.8 (0.6) | 3.8 (0.9) | 1.6 (0.9) | 4.2 (0.84) | 4.2 (0.6) | 6 (0.6) | 12 |
| DMSO-500 | 1.8 (0.9) | 8 (1.16) | 4.8 (0.7) | 2 (0.1) | 3.2 (0.1) | 4 (0.85) | 5.2 (0.7) | 8.6 (0.7) | 15 |
| DMSO-1000 | 7 (0.1) | 9.4 (1.17) | 7.6 (0.8) | 6.2 (0.11) | 5.6 (0.11) | 9 (0.86) | 2.8 (0.8) | 15.6 (0.8) | 48 |
| Chili-125 | 4.8 (0.11) | 4.4 (1.18) | 2.2 (0.9) | 3.8 (0.12) | 2.2 (0.12) | 2.2 (0.87) | 1.6 (0.9) | 2 (0.9) | 10 |
| Chili-250 | 1.8 (0.12) | 6.6 (1.19) | 3.8 (0.1) | 4.8 (0.13) | 3 (0.13) | 4.4 (0.88) | 5 (0.1) | 3.6 (0.1) | 13 |
| Chili-500 | 2.6 (0.13) | 9.6 (1.2) | 6.8 (0.11) | 2.8 (0.14) | 4.6 (0.14) | 4.2 (0.89) | 6.8 (0.11) | 4.4 (0.11) | 21 |
| Chili-1000 | 10.2 (0.14) | 15 (1.21) | 10 (0.12) | 4.8 (0.15) | 7.2 (0.15) | 8.6 (0.9) | 9.4 (0.12) | 12.8 (0.12) | 50 |
| Tomato-125 | 1.8 (0.15) | 3 (1.22) | 2.2 (0.13) | 4.8 (0.16) | 2.2 (0.16) | 2 (0.91) | 2.8 (0.13) | 1.4 (0.13) | 8 |
| Tomato-250 | 3.8 (0.16) | 3.6 (1.23) | 3 (0.14) | 1.4 (0.17) | 4.4 (0.17) | 5.6 (0.92) | 3.6 (0.14) | 3.2 (0.14) | 12 |
| Tomato-500 | 4.4 (0.17) | 5.8 (1.24) | 21.4 (0.15) | 3.6 (0.18) | 3.4 (0.18) | 6.8 (0.93) | 5.8 (0.15) | 17 (0.15) | 23 |
| Tomato-1000 | 9.2 (0.18) | 7 (1.25) | 26 (0.16) | 4.2 (0.19) | 7.8 (0.19) | 7 (0.94) | 9.2 (0.16) | 19 (0.16) | 38 |
It is evident that MI for tomato and chilies was in the range of 16–20 and 12–19 for the concentration of 125–1000 mg, respectively. Further, the MI gradually decreased with increasing metal exposure in the growth medium (Table 1). At the highest metal concentration (1000 mg), the MI for chilies was recorded as 12; whereas, at same concentration of metal exposure tomato was less affected with MI value of 16. Cytological analysis showed various types of abnormalities which included chromosomal bridges, laggards, stickiness, vagrants, binucleated cells, nuclear lesions, giant cells and c-mitosis. The range of chromosomal bridges was 2–7 in DMSO treated cells. For chilies and tomatoes, the chromosomal bridges were in range of 2–5 and 2–10, respectively (Table 1). The number of laggard chromosomes increased with increasing concentration of the metal extract and ranged from 4 to 15. The majority of laggards were observed in chilies (3–7). Similarly, the stickiness character also increased with increasing concentration of metal concentration and recorded in range of 2–26 in tomatoes, 2–10 in chilies and 2–8 in DMSO respectively. Binucleated cells were also recorded highest for tomato extract at 1000 mg (i.e., 8) followed by chilies (i.e., 7) at maximum treatment affecting the cytokinesis. The number of giant cells gradually increased with increasing metal concentration for chilies and tomatoes. For tomatoes, C-mitosis was recorded as 17 and 19 at concentration of 500 and 1000 mg respectively. In case of chilies and DMSO, it was 16 and 13 respectively at highest concentration. Similarly, the highest aberrant cells were observed for tomato extract. The decreased MI also showed a strong trend of inhibition of the cell division along higher metal concentration in the L. esculentum and C. anuum (Table 1).
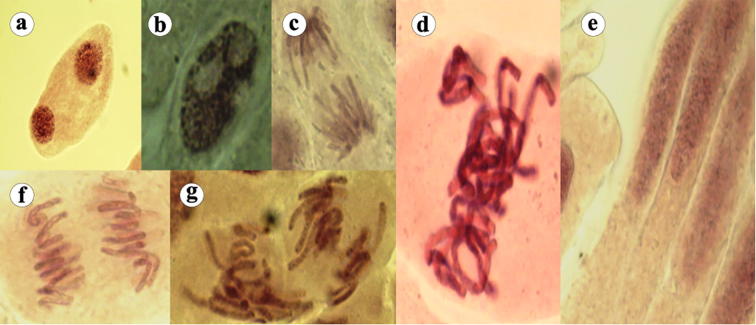
Various cytological observations were shown in Fig. 1, Fig. 2, Fig. 3a, Fig. 3b, Fig. 3c. Fig. 1 demonstrated normal stages of mitotic division. Fig. 3a−c showed various kinds of chromosomal aberrations observed in onion root tip cells after metal exposure.
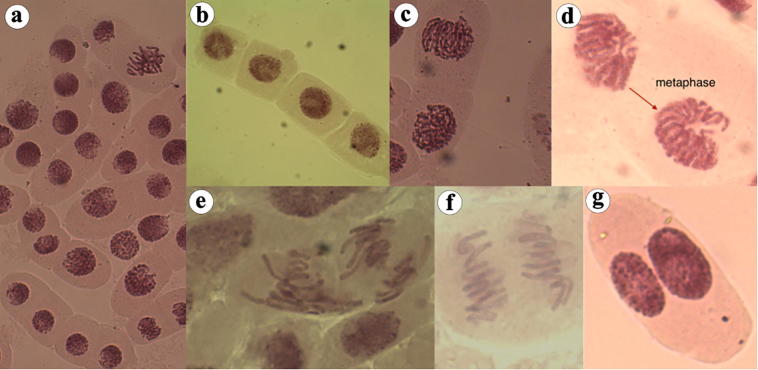
Fig. 1.
Different normal mitotic phases are shown here. a-b: prophase, c-d: metaphase, e: interphase, f: anaphase and g: telophase.
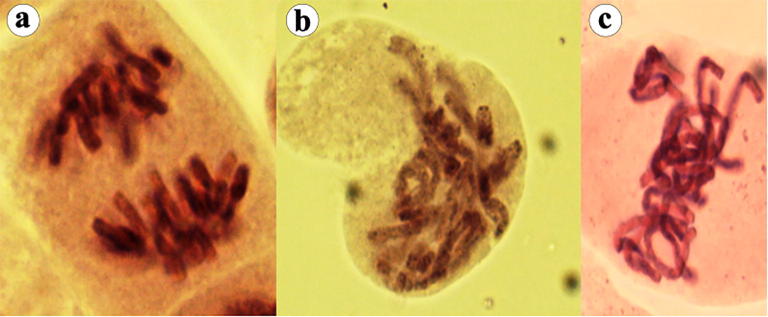
Fig. 2.
Various types of chromosomal aberrations observed. (a) Sticky anaphase with chromosome fragments, (b) sticky anaphase, and (c) laggards.
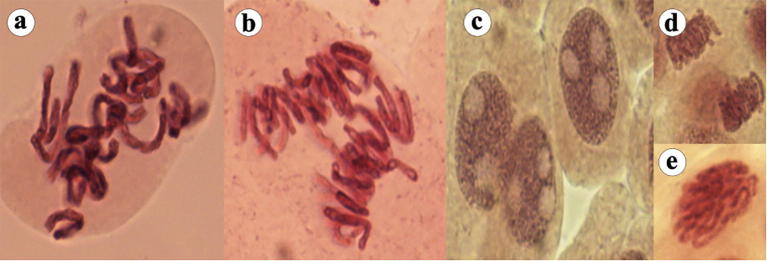
Fig. 3a.
Some other aberrations in onion root tips under the influence of metals, (a) prolong prophase and abnormal cytokinesis, (b) chromosome bridge and disturbance in metaphasic spindle, (c) Nuclear lesions, (d) micronucleus in prophase and stickiness, and € stickiness.
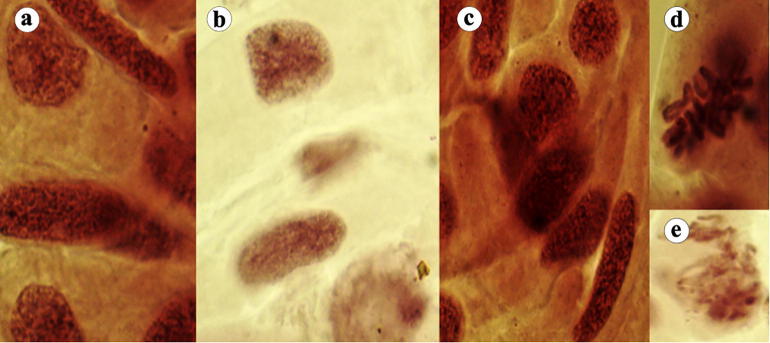
Fig. 3b.
In this figure, (a–c) Giant cells, (d) stickiness and (e) sticky chromosomes.
Fig. 3c.
In this figure, (a) Binucleated, (b) nuclear lesions, (c) bridge and laggard chromosome, (d) vagrant, c-mitosis, (e) giant nucleus, (f) multipolar anaphase and spindle disturbance and (g) stickiness.
4. Discussion
Onion has been considered as apposite material for genotoxicity investigations. It offers many advantages like clarity of mitotic phases, visible, diverse and low chromosome number, stability of karyotype; quick reaction to the toxic materials etc. (Therefore, A. sepa assay is well documented to determine effects of various genotoxic materials. This is the pioneer study which evaluated the effects of metal bioaccumulation in tomato and chili and their subsequent A. cepa test. The onion root tips which are undergoing growth may offer a good plant portion which can be investigated for the genotoxic effects of pollutants like metals. Onion plant is useful in studying genotoxic effects such as chromosomal aberrations (CA), effects on karyokinesis and cytokinesis, nuclear alterations, disorders in the mitotic cycle, and the existence of micronuclei in meristem cells.
The results showed that mitotic index (MI) for tomato extracts decreased from 20 to 16 while for chilies extracts it decreased from 19 to 12 for the concentration of 125–1000 mg. It implied that MI in chili extracts was significantly affected by metals exposure as compared to tomato extracts. The decrease in the MI of A. cepa meristem cells indicated that the presence of high concentrations of heavy metals in the soil caused cytotoxic effects in both plants under investigation (Nefic et al., 2013). CAs includes changes either in chromosomal structure or in chromosomal number. Alterations in the chromosomal structure can be caused by breaks of DNA, DNA synthesis inhibition and modification in the DNA replication. Clastogenic action is indicated by various CA including chromosomal breaks and bridges. Aneuploidy and polyploidy are numerical CA caused by abnormal chromosomal segregation (adherence, C-metaphases, delays, chromosome losses and multipolarity) under the influence of aneugenic agents or spontaneously. In the absence of telomeres, chromosomes turn out to be “sticky” which may join to other fragmented chromosomal ends (Nefic et al., 2013). Cytotoxic effects are mainly responsible for the altered MI in comparison to control whereby karyokinesis is affected (Leme and Marin-Morales, 2009). C-mitosi (D’Amato1950) is characterized by the advancement of metaphase to anaphase and therefore leading to polyploidy.
Variable numbers of chromosomal bridges were also observed for various metal treatments. The chromosomal bridges result when chromosomes become sticky and their separation is delayed; chromosomes remain connected by bridges which move freely. Chromosomal bridges mainly develop because of the non-disjunction of sticky chromosome or breakage and reunion during separation at anaphase (Feretti et al., 2007). The current study also showed increased number of lagging chromosomes along higher metal concentration in the extract (Table 1). Lagging chromosomes arise when chromosomes fail to remain connected with spindle fiber which may move to either of poles (Khanna and Sharma, 2013). Stickiness is caused by either increased contraction or condensation of chromosomes or DNA depolymerization and partial dissolution of nucleoproteins. It reflects toxic effects which are usually irreversible and might lead to cellular deaths. Our results confirm previous findings dealing with the effects of various on living systems (Türkoğlu, 2007).
A number of cells having c-mitosis and few vagrant chromosomes were also observed. The failure of the spindle apparatus organization and its normal function is the cause of c-mitosis and vagrant chromosomes (Levan, 1938). C-mitosis indicates the presence of toxic chemicals in the growth medium of plants (Bonciu et al., 2018). A number of cells with nuclear lesions were found to be increasing with increasing extract doses in all the treatments except in the control. The number of the nuclear lesions had linear relation with the increasing extract concentration. The mitotic index and the cell division were normal in the control cells. The results also showed that the chromosomal aberrations in the roots treated with the specific aqueous leaf extracts had showed marked difference from the control. It was apparent that metals caused genetic changes in the experimental plants which were not observed in the control. The observation of cells with laggards, chromosomal bridges, giant cells, c-mitosis and bridges indicted that genetic changes occurred especially at higher metal concentrations. In vagrant chromosome/s, a chromosome moves ahead of its associated chromosomal group toward poles and leads to the unequal separation of chromosomes in the daughter cells. Vagrant chromosomes have been observed by many workers in different studies (Sondhi et al., 2008). An aberrant spindle division during early anaphase or failure of cytokinesis after telophase creates binucleated cells. Giant cells are usually polyploid arising as a consequence of endomitosis or endoreplication (Bonea et al., 2018).
The number of giant cells gradually increased with the increasing amount of metals in the extract. Endoreplication or endomitosis may result in polyploidy and giant cells which may be polyploid. The CAs are categorized into clastogenic and aneugenic based on a break occurring at the chromosomal level or any issues with mitotic spindle affecting chromosomal separation along inhibition of cytokinesis. The clastogenic CA include chromosome breaks chromosome and bridges; C-metaphase, laggard chromosomes, chromosomal losses, multi- polar divisions and chromosomal stickiness induced by aneugenic agents (Silveira et al. 2017). Chromosomal bridges and breaks (clastogenic aberrations) are the most significant ones to consider including chromosome loss, delays, adherence, multi-polarity (Leme and Marin-Morales, 2009).
Thus, the chromosomal aberrations observed in the onion root tip cells may be anticipated in the vegetable crops exposed to these metals. The consumption of genetically modified food crop may behave like genetically engineered/modified GEM crops, whose consumption is still debatable due to their potential deleterious nature and undesirable consequences in human consuming them.
5. Conclusion
The current study on the effects of plant extracts exposed to various metal concentrations on chromosomal aberrations in onion root tips revealed that variable nature of chromosomal abnormalities were observed. The metals induced aberrations like chromosomal bridges, lagging chromosomes, vagrants, binucleated cells, nuclear lesions, giant cells, and c-mitosis in onion root tips. The presence of such abnormalities indicates the interference of metals in the normal growth of plants exposed to industrial effluents. Plants with such abnormalities may transfer altered genetic makeup to not only to their offspring but also to human when consumed as food thereby causing further complications.
Declaration of Competing Interest
No conflict of interest existed for this manuscript.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Qaisar Mahmood, Email: mahmoodzju@gmail.com, drqaisar@cuiatd.edu.pk.
Naeem Shahid, Email: naeemshahid@ciitvehari.edu.pk.
References
- Andrade L.F., Davide L.C., Gedraite L.S. The effect of cyanide compounds, fluorides and inorganic oxides present in spent pot Liner on germination and root tip cells of Lactuca sativa. Ecotoxicology Environmental Safety. 2010;73:626–631. doi: 10.1016/j.ecoenv.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Aragão F.B., Andrade-Vieira L.F., Ferreira A., Costa A.V., Queiroz V.T., Pinheiro P.F. Phytotoxic and cytotoxic effects of Eucalyptus essential oil on Lactuca sativa L. Allelopathy Journal. 2015;35:259–272. [Google Scholar]
- Baderna D., Maggioni S., Boriani E., Gemma S., Molteni M., Lombardo A., Colombo A., Bordonali S., Rotella G., Lodi M. A combined approach to investigate the toxicity of an industrial landfill’s leachate: chemical analyses, risk assessment and in vitro assays. Environ. Res. 2011;111:603–613. doi: 10.1016/j.envres.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Bakare A., Mosuro A., Osibanjo O. Effect of simulated leachate on chromosomes and mitosis in roots of Allium cepa(L.) J. Environ. Biol. 2000;21:263–271. [Google Scholar]
- Bonciu E., Firbas P., Fontanetti C.S., Wusheng J., Karaismailoğlu M.C., Liu D., Menicucci F., Pesnya D.S., Popescu A., Romanovsky A.V. An evaluation for the standardization of the Allium cepa test as cytotoxicity and genotoxicity assay. Caryologia. 2018;71:191–209. [Google Scholar]
- Chary N.S., Kamala C., Raj D.S.S. Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer. Ecotoxicol. Environ. Saf. 2008;69:513–524. doi: 10.1016/j.ecoenv.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Christou A., Karaolia P., Hapeshi E., Michael C., Fatta-Kassinos D. Long-term wastewater irrigation of vegetables in real agricultural systems: concentration of pharmaceuticals in soil, uptake and bioaccumulation in tomato fruits and human health risk assessment. Water Res. 2017;109:24–34. doi: 10.1016/j.watres.2016.11.033. [DOI] [PubMed] [Google Scholar]
- Corcoran, E., 2010. Sick water? the central role of wastewater management in sustainable development: a rapid response assessment. UNEP/Earthprint.
- Dietrich M., Wolfe A., Burke M., Krekeler M.P. The first pollution investigation of road sediment in Gary, Indiana: Anthropogenic metals and possible health implications for a socioeconomically disadvantaged area. Environ. Int. 2019;128:175–192. doi: 10.1016/j.envint.2019.04.042. [DOI] [PubMed] [Google Scholar]
- Ensink J.H., Mahmood T., Van der Hoek W., Raschid-Sally L., Amerasinghe F.P. A nationwide assessment of wastewater use in Pakistan: an obscure activity or a vitally important one? Water Policy. 2004;6:197–206. [Google Scholar]
- Farahat E., Linderholm H.W. The effect of long-term wastewater irrigation on accumulation and transfer of heavy metals in Cupressus sempervirens leaves and adjacent soils. Sci. Total Environ. 2015;512:1–7. doi: 10.1016/j.scitotenv.2015.01.032. [DOI] [PubMed] [Google Scholar]
- Feretti D., Zerbini I., Zani C., Ceretti E., Moretti M., Monarca S. Allium cepa chromosome aberration and micronucleus tests applied to study genotoxicity of extracts from pesticide-treated vegetables and grapes. Food Addit. Contam. 2007;24:561–572. doi: 10.1080/02652030601113602. [DOI] [PubMed] [Google Scholar]
- Fiskesjö G. The Allium test as a standard in environmental monitoring. Hereditas. 1985;102:99–112. doi: 10.1111/j.1601-5223.1985.tb00471.x. [DOI] [PubMed] [Google Scholar]
- Freitas A.S., Cunha I.M.F., Andrade-Vieira L.F., Techio V.H. Effect of SPL (Spent Pot Liner) and its main components on root growth, mitotic activity and phosphorylation of Histone H3 in Lactuca sativa L. Ecotoxicology and Environmental Safety. 2016;124:426–434. doi: 10.1016/j.ecoenv.2015.11.017. [DOI] [PubMed] [Google Scholar]
- Gormu, L., Mano,Y., 2016. Training Manual on Agronomic Practice of Selected Vegetable for Development Agents and Farmers at Kenbata Tembaro Zone, Mendel University Project, Ethiopia.
- Grant W.F. Chromosome aberration assays in Allium: A report of the US Environmental Protection Agency gene-tox program. Mutat. Res./Rev. Genet. Toxicol. 1982;99:273–291. doi: 10.1016/0165-1110(82)90046-x. [DOI] [PubMed] [Google Scholar]
- Grant W.F. Higher plant assays for the detection of chromosomal aberrations and gene mutations—a brief historical background on their use for screening and monitoring environmental chemicals. Mutat. Res./Fundament. Mol. Mech. Mutagenesis. 1999;426:107–112. doi: 10.1016/s0027-5107(99)00050-0. [DOI] [PubMed] [Google Scholar]
- Hamilton A.J., Stagnitti F., Xiong X., Kreidl S.L., Benke K.K., Maher P. Wastewater irrigation: the state of play. Vadose Zone J. 2007;6:823–840. [Google Scholar]
- Haq I., Kumar S., Raj A., Lohani M., Satyanarayana G. Genotoxicity assessment of pulp and paper mill effluent before and after bacterial degradation using Allium cepa test. Chemosphere. 2017;169:642–650. doi: 10.1016/j.chemosphere.2016.11.101. [DOI] [PubMed] [Google Scholar]
- Harvest F. Consultative Group on International Agricultural Research; Washington, DC: 2001. Wastewater Irrigation: Economic necessity or threat to health and environment? [Google Scholar]
- Hussain A., Alamzeb S., Begum S. Accumulation of heavy metals in edible parts of vegetables irrigated with waste water and their daily intake to adults and children, District Mardan, Pakistan. Food Chem. 2013;136:1515–1523. doi: 10.1016/j.foodchem.2012.09.058. [DOI] [PubMed] [Google Scholar]
- Iqbal H.H., Taseer R., Anwar S., Qadir A., Shahid N. Human health risk assessment: Heavy metal contamination of vegetables in Bahawalpur, Pakistan. Bull. Environ. Stud. 2016;1:10–17. [Google Scholar]
- Jiménez, B., Asano, T., 2008. Water reuse: an international survey of current practice, issues and needs. IWA London.
- Khan S., Cao Q., Zheng Y., Huang Y., Zhu Y. Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ. Pollut. 2008;152:686–692. doi: 10.1016/j.envpol.2007.06.056. [DOI] [PubMed] [Google Scholar]
- Khanna N., Sharma S. Allium cepa root chromosomal aberration assay: a review. Indian J. Pharm. 2013;1:3. [Google Scholar]
- Lei M., Tie B.-Q., Song Z.-G., Liao B.-H., Lepo J.E., Huang Y.-Z. Heavy metal pollution and potential health risk assessment of white rice around mine areas in Hunan Province, China. Food Security. 2015;7:45–54. [Google Scholar]
- Leme D.M., Marin-Morales M.A. Allium cepa test in environmental monitoring: a review on its application. Mutat. Res./Rev. Mutat. Res. 2009;682:71–81. doi: 10.1016/j.mrrev.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Levan A. The effect of colchicine on root mitoses in Allium. Hereditas. 1938;24:471–486. [Google Scholar]
- Li F., Cai Y., Zhang J. Spatial characteristics, health risk assessment and sustainable management of heavy metals and metalloids in soils from central China. Sustainability. 2018;10:91. [Google Scholar]
- Luo X.-S., Xue Y., Wang Y.-L., Cang L., Xu B., Ding J. Source identification and apportionment of heavy metals in urban soil profiles. Chemosphere. 2015;127:152–157. doi: 10.1016/j.chemosphere.2015.01.048. [DOI] [PubMed] [Google Scholar]
- Maluszynska J., Juchimiuk J. Plant genotoxicity: a molecular cytogenetic approach in plant bioassays. Arhiv za higijenu rada i toksikologiju. 2005;56:177–184. [PubMed] [Google Scholar]
- Nefic H., Musanovic J., Metovic A., Kurteshi K. Chromosomal and nuclear alterations in root tip cells of Allium cepa L. induced by alprazolam. Med. Arch. 2013;67:388. doi: 10.5455/medarh.2013.67.388-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngole-Jeme V.M., Fantke P. Ecological and human health risks associated with abandoned gold mine tailings contaminated soil. PLoS ONE. 2017;12:e0172517. doi: 10.1371/journal.pone.0172517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nriagu J.O. Global metal pollution: poisoning the biosphere? Environ. Sci. Pol. Sustain. Dev. 1990;32:7–33. [Google Scholar]
- Odeigah P.G.C., Nurudeen O., Amund O.O. Genotoxicity of oil field wastewater in Nigeria. Hereditas. 1997;126:161–167. [Google Scholar]
- Palmieri M.J., Andrade-Vieira L.F., Trento M.V.C., Eleuterio M.W.F., Luber J., Davide L.C., Marcussi S. Cytogenotoxic effects of Spent Pot Liner (SPL) and its main components on human leukocytes and meristematic cells of Allium cepa. Water Air Soil Pollution. 2016;227:1–10. [Google Scholar]
- Rank J. The method of Allium anaphase-telophase chromosome aberration assay. Ekologija. 2003;1:38–42. [Google Scholar]
- Rajneet K.S., Jatinder K.K., Avinash N. Allium cepa Root Chromosomal Aberration Assay: An Efficient Test System for Evaluating Genotoxicity of Agricultural Soil. International Journal of Science and Research (IJSR) 2014;3:245–250. [Google Scholar]
- Raschid-Sally, L., Jayakody, P., 2009. Drivers and characteristics of wastewater agriculture in developing countries: Results from a global assessment. IWMI.
- Roy M., McDonald L.M. Metal uptake in plants and health risk assessments in metal-contaminated smelter soils. Land Degrad. Dev. 2015;26:785–792. [Google Scholar]
- Schwartz M.F., Boyd C.E. Effluent quality during harvest of channel catfish from watershed ponds. Progressive Fish-Culturist. 1994;56:25–32. [Google Scholar]
- Scott C.A., Faruqui N.I., Raschid-Sally L. Wastewater use in irrigated agriculture: management challenges in developing countries. In: Publishing C.A.B.I., editor. Wastewater Use in Irrigated Agriculture: Confronting the Livelihood and Environmental Realities. Wallingford; UK: 2004. pp. 1–10. [Google Scholar]
- Sharma C. Plant meristems as monitors of genetic toxicity of environmental chemicals. Curr. Sci. 1983:1000–1002. [Google Scholar]
- Sharma P., Bihari V., Agarwal S.K., Verma V., Kesavachandran C.N., Pangtey B.S., Mathur N., Singh K.P., Srivastava M., Goel S.K. Groundwater contaminated with hexavalent chromium [Cr (VI)]: a health survey and clinical examination of community inhabitants (Kanpur, India) PLoS ONE. 2012;7:e47877. doi: 10.1371/journal.pone.0047877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebe C. Nutrient inputs to soils and their uptake by alfalfa through long-term irrigation with untreated sewage effluent in Mexico. Soil Use Manage. 1998;14:119–122. [Google Scholar]
- Silveira, G.L., Lima, M.G.F., Reis, G.B.d., Palmieri, M.J., Andrade-Vieria, L.F., 2017. Toxic effects of environmental pollutants: Comparative investigation using Allium cepa L. and Lactuca sativa L. Chemosphere 178, 359–367. [DOI] [PubMed]
- Singh A., Sharma R.K., Agrawal M., Marshall F.M. Health risk assessment of heavy metals via dietary intake of foodstuffs from the wastewater irrigated site of a dry tropical area of India. Food Chem. Toxicol. 2010;48:611–619. doi: 10.1016/j.fct.2009.11.041. [DOI] [PubMed] [Google Scholar]
- Sondhi N., Bhardwaj R., Kaur S., Kumar N., Singh B. Isolation of 24-epibrassinolide from leaves of Aegle marmelos and evaluation of its antigenotoxicity employing Allium cepa chromosomal aberration assay. Plant Growth Regulation. 2008;54:217–224. [Google Scholar]
- Timothy O., Idu M., Olorunfemi D., Ovuakporie-Uvo O. Cytotoxic and genotoxic properties of leaf extract of Icacina trichantha Oliv. S. Afr. J. Bot. 2014;91:71–74. [Google Scholar]
- Türkoğlu Ş. Genotoxicity of five food preservatives tested on root tips of Allium cepa L. Mutat. Res./Genet. Toxicol. Environ. Mutagenesis. 2007;626:4–14. doi: 10.1016/j.mrgentox.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Wiener M.J., Jafvert C.T., Nies L.F. The assessment of water use and reuse through reported data: a US case study. Sci. Total Environ. 2016;539:70–77. doi: 10.1016/j.scitotenv.2015.08.114. [DOI] [PubMed] [Google Scholar]
- Yi Y., Yang Z., Zhang S. Ecological risk assessment of heavy metals in sediment and human health risk assessment of heavy metals in fishes in the middle and lower reaches of the Yangtze River basin. Environ. Pollut. 2011;159:2575–2585. doi: 10.1016/j.envpol.2011.06.011. [DOI] [PubMed] [Google Scholar]
- Yuet Ping K., Darah I., Yusuf U.K., Yeng C., Sasidharan S. Genotoxicity of Euphorbia hirta: an Allium cepa assay. Molecules. 2012;17:7782–7791. doi: 10.3390/molecules17077782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zhong T., Liu L., Ouyang X. Impact of soil heavy metal pollution on food safety in China. PLoS ONE. 2015;10:e0135182. doi: 10.1371/journal.pone.0135182. [DOI] [PMC free article] [PubMed] [Google Scholar]