Abstract
The most common reasons for revision of metal-on-metal hip arthroplasty are aseptic loosening and metal reaction. Failure of a metal-on-metal implant due to the aggressive destruction of periprosthetic tissues may require extensive reconstruction procedures. The aim of this case report is to describe the treatment in an asymptomatic patient with high levels of chromium and cobalt, using chelation therapy. The rational use of N-acetyl-cysteine (NAC) involves thiol groups to chelate sites for metals. More than 10 years after the metal-on-metal hip arthroplasty, the patient did not have to undergo revision surgery; the levels of the ions in the blood were considerably lowered (chromium from 4.51 mcg/L to 1.85 mcg/L; cobalt from 7.78 UG/L to 0.8 UG/L) after using NAC without adverse effects.
Keywords: Metallosis, Metal-on-metal hip arthroplasty, N-Acetyl-cysteine, Chelation therapy, Adverse reaction to metal debris
Introduction
Total hip replacement is a universally recognized treatment for the final stage of arthrosis [1]. The production of wear debris, which is primarily generated by the contact surface of the prosthetic components, represents the major causal factor of periprosthetic osteolysis and therefore of implant survival. For this reason, a number of alternative bearings have been developed, based on both new and traditional concepts. Current alternatives are represented by metal-on-metal (MoM) couplings, ceramic-on-ceramic, and a combination of ceramic-coated heads with polyethylene. Since 1996, more than 1 million total hip arthroplasties with a metal-on-metal bearing couple have been implanted in the United States and worldwide [2]. Despite the theoretical advantages associated with lower wear rates and the greater relative stability of large diameter components, factors that have made this bearing combination favorable option in younger and more active patients have not yet been shown to have long-term superiority over other bearing choices. The deposition of metal ions into the periprosthetic space can lead to a wide spectrum of soft-tissue reactions including massive sterile effusions, necrosis, corrosive osteolysis, and both cystic and solid periprosthetic masses. The abnormal soft-tissue reaction to MoM bearing surfaces results from the deposition of cobalt-chrome particulate debris in the surrounding tissues. The contact between the cobalt-chrome femoral head and metal liner leads to release of metal ions; furthermore, this frictional torque is the subject of recent controversy and skepticism owing to the occurrence of failures caused by adverse tissue reactions to the metal-on-metal surface prosthetics [3]. After the withdrawal from the world market in 2010 of surface prosthetics, Durom (Zimmer Biomet), and ASR (DePuy Johnson & Johnson), as well as the large diameter XL heads (DePuy Johnson & Johnson), metal-on-metal bearings have been a subject of contention in the scientific literature [4].
Although most patients with this type of prosthesis do not show problems, there is evidence that in some cases, metallosis generates local and systemic reactions, owing to an increased release of cobalt and chromium ions in the joint space [5]. Furthermore, these wear particles can be generated not only by the prosthetic joint interline but also from corrosion and fretting phenomena of the modular head-neck junction (morse taper) or modular neck-prosthesis stem with stem [6].
Release of both metal ions and nanoparticles occurs after abnormal wear of MoM articulations. Metal particulate debris has increased bioactivity, quantity of particles, and surface area compared with the classic larger particles from polyethylene wear. The metal particles are then phagocytosed by giant cells and macrophages, which leads to a release of intracellular metal ions and subsequent cell death. Macroscopically, this can manifest as soft tissue destruction, aseptic loosening, and osteolysis. Local metal debris is also associated with increased serum ion levels. These levels may become grossly elevated with progressive implant loosening [3].
In 2012, the Hip Society proposed an algorithmic approach to diagnosis and management of metal-on-metal arthroplasty to minimize complications and standardize treatment in patients with this type of prosthetic implants [7].
One of the major questions currently presented to the orthopaedic surgeon is how to evaluate and monitor patients with a MoM implant over time and how to treat patients with prosthetic malfunctions or complications ascribable to metallosis. The aim of this case report is to describe the treatment of a patient with elevated blood chromium and cobalt levels in the absence of systemic manifestations using a chelating therapy with N-acetyl-cysteine (NAC); in fact, thiol groups in NAC may provide chelating sites for metals.
Case report
In July 2007, a 49-year-old man, with a negative medical history, was referred to our department because of pain in his right hip. During the medical examination, the patient complained of right coxalgia without a history of trauma. The pain was constant, present even at night, and worse with weight bearing. The reported Harris Hip Score was 48, whereas the Oxford Hip Score was 18 [8,9].
After speaking with the patient, we decided to perform total hip replacement for severe end-stage osteoarthritis using MoM total hip implantation (Durom Metasul® Acetabular Component 52, cementless stem CLS 9/135° and head 48) using a posterolateral approach. The postoperative clinical course was good with normal range of movement and no major or minor complications. After 6 years without clinical symptoms, the patient returned to our attention to perform contralateral hip replacement surgery (Fig. 1). During the preoperative routine examinations, in consideration of the previous metal-on-metal implant on the right hip, blood examinations were performed, highlighting an increase in chromium and cobalt ions (Cr 1.6 mcg/L, Co 3.5 UG/L). The values of the 2 ions have increased constantly and magnetic resonance imaging was also performed showing fluid collection near the iliopsoas muscle (Fig. 2). Owing to the persistently elevated Co/Cr blood levels, following the previously mentioned guidelines [7], the patient was suggested to undergo revision surgery. The patient rejected this option, as it is completely asymptomatic (Harris Hip score 92), and for this reason, we opted for chelation therapy with oral high-dose NAC (1200 mg/die) starting in March 2017. The dosage involves taking 2 tablets a day (600 mg per tablet) every 12 hours. We decided on NAC therapy in light of the promising results shown in animal and in vitro studies; moreover, NAC can be considered a potential Co chelator in the case of severe arthroprosthetic cobaltism. Finally, we decided on NAC because of the lack of toxicity. In less than a year, the chromium values went from 3.27 mcg/L to 1.69 mcg/L, whereas cobalt decreased from 7.78 UG/L to 2.5 UG/L without any side effects (Fig. 3). Blood examination was repeated every 6 months showing an important improvement, especially regarding cobalt. In fact, in May 2018, the value of chromium was 1.84 mcg/L; after 6 months, 2.14 mcg/L, whereas in November 2019, 1.85 mcg/L. As regards cobalt, the level in May 2018 was 2.80 UG/L; in November 2018, 2.70 UG/L, whereas at the last follow-up, 0.8 UG/L. Detailed results are reported in Table 1.
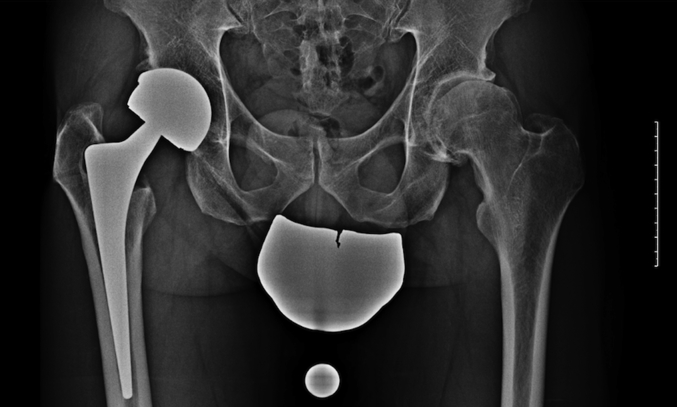
Figure 1.
Plain anteroposterior radiograph 6 years after surgery shows well-fixed components.
Figure 2.
Axial (A) and coronal (B) magnetic resonance imaging of the right hip show fluid collection in the right iliopsoas about 5 cm × 2 cm × 2 cm.
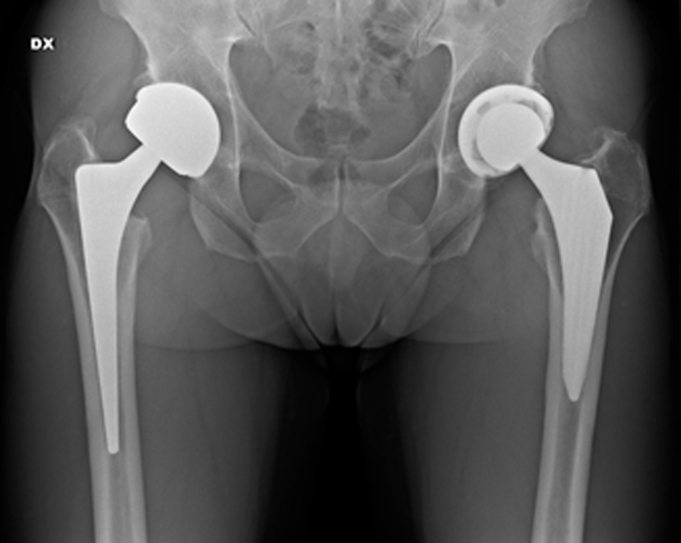
Figure 3.
Plain anteroposterior radiograph at the last follow-up showing no worsening of the right hip.
Table 1.
Value of chrome and cobalt over the years. From March 2017, the patient started the oral consumption of NAC 1200 mg/die.
| Follow-up | Chromium (mcg/L) | Cobalt (UG/L) |
|---|---|---|
| November 2013 | 1.60 | 3.50 |
| November 2014 | 1.80 | 4.15 |
| November 2015 | 2.69 | 4.31 |
| November 2016 | 4.51 | 7.07 |
| March 2017a | 3.27a | 7.78a |
| November 2017 | 1.69 | 2.50 |
| May 2018 | 1.84 | 2.80 |
| November 2018 | 2.14 | 2.70 |
| November 2019 | 1.85 | 0.8 |
NAC, n-acetyl-cysteine therapy.
Starting of NAC, at a dosage of 1200 mg/day.
At the last follow-up, a radiograph of the pelvis and hips was also taken showing stable positioning of the prosthesis without signs of loosening or osteolysis.
The patient remains well, without local or systemic issues related to the use of NAC or to metallosis.
Discussion
MoM bearings were introduced as a potentially favorable option in younger and more active patients, while ceramic-on-ceramic and metal-on-crosslinked polyethylene have also demonstrated encouraging results at up to 10 years of follow-up [10].
MoM wear particles measure between 20 and 80 nm and are substantially smaller than those found with polyethylene particulate debris [11,12]. The number of particles produced per year has been estimated at around 6.7 × 1012 to 2.5 × 1014 which corresponds to 13-500 times the quantity produced in the case of metal-on-polyethylene bearing combinations [13]. This large aggregate of particles can have local and systemic effects. In the case of metal-on-metal prostheses, the local tissue reaction, quantified as per the number of histiocytes, is approximately one order of magnitude lower than in the case of metal-on-polyethylene prostheses [14]. Given the smaller size of metal particles compared to those derived from polyethylene, the number of histiocytes recruited to store the particles is lower [15]. The metallic particles enter the histiocytes through pinocytosis and not by phagocytosis as for polyethylene, and this could alter the cellular response.
Periprosthetic tissues demonstrate a distinct pattern of inflammation with two histologic features including a perivascular lymphocytic infiltrate and an accumulation of plasma cells in association with macrophages containing variable amounts of metallic wear particles. The synovial lining in patients with MoM implants is more frequently ulcerated when compared with other types of implants. This unique lymphocytic perivascular infiltration has been termed aseptic lymphocytic vasculitis–associated lesion [16].
In addition, the Co-Cr particles have a greater cytotoxic potential, and as a consequence, the cells may be unable to generate the same inflammatory response [13,15]. The release of these particles also results in the increase in serum values of chromium and cobalt in erythrocytes, serum, and urine [17]. In vitro studies demonstrate a dose-dependent response to metal particles: low or moderate levels stimulate the release of cytokines that can induce osteolysis [18]. High concentrations are cytotoxic leading to cell death and therefore tissue necrosis [19]. In general, posteolysis associated with metallic particulates is lower than that induced by polyethylene particles. Cr and Co particles have been shown to induce malignant tumors in animal models, fueling concern about the possibility of similar effects in humans [18,19]. In this regard, there is ambiguity in clinical evidence. Few studies report cases of periprosthetic tumors, most of which were malignant histiocytomas. Another study reports 4 cases of soft-tissue sarcoma in the areas adjacent to a prosthetic implant [20,21]. Epidemiologic studies about malignant tumors at a distance from the implants show an increased risk of lymphoma and leukemias related to old MoM implants [20,21]. Precisely because of these risks, different treatments have been reported, which take advantage of chelation therapy, as in our case, to treat metallosis phenomena. In the literature, there are some studies that use edetate calcium disodium (EDTA), sodium 2,3-dimercaptopropane sulfonate (DMPS), and dimercaprol as chelating agents [[22], [23], [24], [25]]. EDTA is used to bind metal ions in the practice of chelation therapy mainly for mercury and lead poisoning. For chromium toxicity, EDTA failed to show any benefit in increasing urinary elimination, whereas for cobalt, EDTA has been the predominant chelator in limited human experience. It was used as adjuvant therapy in a case in which a prosthetic hip was leaching cobalt, providing short-term lowering of blood cobalt measurements. However, cobalt concentrations rebounded in a matter of days [22].
Dimercaprol and DMPS are chelating agents principally involved in the treatment of poisoning by arsenic and polonium-210, respectively. For the treatment of chromium, DMPS failed to show any increase in chromium excretion [26].
Only 1 other case report describes the use of NAC in 2 patients, reporting that in the first case, Co/Cr blood concentrations were reduced by 86% and 87% of the prechelation levels, whereas in the other one, a decrease of 45% and 24% of the prechelation levels was reported [27]. The rational use of NAC involves thiol groups to chelate sites for metals [27]. Promising results have already been described in animal studies supported also by in vitro studies [28]. NAC has been reported not only as an effective and safe Co-chelating agent in some animal models but also as a potential Co chelator in humans in case of severe arthroprosthetic cobaltism [[27], [28], [29]]. In 1 animal study, NAC showed an increase in urinary chromium clearance. Of note, this was not due to any increase in concentration in the urine, but rather due to maintenance of the critical factors of adequate urine volume and output. In the study, Cr was taken in the form of potassium dichromate and animals treated with NAC reported a dramatica increase in Cr in the urine on the first day of therapy but on all days, the increase was statistically significant [30]. NAC is also able to reduce chromium hypersensitivity dermatologic reaction [22]. In chronic-cobalt exposure, NAC is the only chelating agent that reduces tissue cobalt concentrations in the liver and spleen [31] Moreover, the distinguishing feature of NAC is that it does not show any toxicity or adverse effect [32].
Conclusions
Clinical management of metallosis in patients with hip arthroplasty remains a significant challenge that involves several specialists including orthopaedic surgeons and toxicology experts. Promising results have been reported using chelating agents, and in our experience, an oral high dose of NAC resulted in a decrease of Co/Cr blood levels with no adverse effects. Further research is needed to determine the role of chelation therapy in asymptomatic metal-on-metal total hip arthroplasty.
Conflict of interests
The authors declare there are no conflicts of interest.
Acknowledgments
Informed consent: Informed consent was waived from all patients for being included in the study.
Animal and human right statements: All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Footnotes
For full disclosure statements refer to https://doi.org/10.1016/j.artd.2020.03.010.
Appendix A. Supplementary data
References
- 1.D'Ambrosi R., Marciandi L., Frediani P.V., Facchini R.M. Uncemented total hip arthroplasty in patients younger than 20 years. J Orthop Sci. 2016;2:500. doi: 10.1016/j.jos.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Bozic K.J., Kurtz S., Lau E. The epidemiology of bearing surface usage in total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91:1614. doi: 10.2106/JBJS.H.01220. [DOI] [PubMed] [Google Scholar]
- 3.Chang J.S., Haddad F.S. Revision total hip arthroplasty for metal-on-metal failure. J Clin Orthop Trauma. 2020;11:9. doi: 10.1016/j.jcot.2019.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haddad F.S., Konan S. Current controversies in hip surgery: a report on the proceedings of the London Hip Meeting 2011. J Bone Joint Surg Br. 2012;94:297. doi: 10.1302/0301-620X.94B3.28362. [DOI] [PubMed] [Google Scholar]
- 5.Kiran M., Armstrong C., Shivarathre D., Peter V.K. Blood metal ion levels have limited utility in the surveillance of asymptomatic large-head metal-on-metal total hip arthroplasties. J Arthroplasty. 2017;32:3685. doi: 10.1016/j.arth.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 6.Akrawi H., Hossain F.S., Niculescu S., Hashim Z., Ng A.B., Shetty A. Midterm results of 36 mm metal-on-metal total hip arthroplasty. Indian J Orthop. 2016;50:256. doi: 10.4103/0019-5413.181786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lombardi A.V., Jr., Barrack R.L., Berend K.R. The Hip Society: algorithmic approach to diagnosis and management of metal-on-metalarthroplasty. J Bone Joint Surg Br. 2012;94:14. doi: 10.1302/0301-620X.94B11.30680. [DOI] [PubMed] [Google Scholar]
- 8.Harris W.H. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737. [PubMed] [Google Scholar]
- 9.Dawson J., Fitzpatrick R., Carr A., Murray D. Questionnaire on the perceptions of patients about total hip replacement. J Bone Joint Surg Br. 1996;78:185. [PubMed] [Google Scholar]
- 10.Atrey A., Ward S.E., Khoshbin A. Ten-year follow-up study of three alternative bearing surfaces used in total hip arthroplasty in young patients: a prospective randomised controlled trial. Bone Joint J. 2017;99-B:1590. doi: 10.1302/0301-620X.99B12.BJJ-2017-0353.R1. [DOI] [PubMed] [Google Scholar]
- 11.Fehring K.A., Fehring T.K. Modes of failure in metal-on-metal total hip arthroplasty. Orthop Clin North Am. 2015;46:185. doi: 10.1016/j.ocl.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Xia Z., Ricciardi B.F., Liu Z. Nano-analyses of wear particles from metal-on-metal and non-metal-on-metal dual modular neck hip arthroplasty. Nanomedicine. 2017;13:1205. doi: 10.1016/j.nano.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Kovochich M., Fung E.S., Donovan E., Unice K.M., Paustenbach D.J., Finley B.L. Characterization of wear debris from metal-on-metal hip implants during normal wear versus edge-loading conditions. J Biomed Mater Res B Appl Biomater. 2018;106:986. doi: 10.1002/jbm.b.33902. [DOI] [PubMed] [Google Scholar]
- 14.Cerilli L.A. Images in pathology. Histiocytic lymphadenopathy secondary to metallosis following hip replacement. Int J Surg Pathol. 2006;14:329. doi: 10.1177/1066896906292536. [DOI] [PubMed] [Google Scholar]
- 15.Reito A., Parkkinen J., Puolakka T., Pajamäki J., Eskelinen A. Diagnostic utility of joint fluid metal ion measurement for histopathological findings in metal-on-metalhip replacements. BMC Musculoskelet Disord. 2015;16:393. doi: 10.1186/s12891-015-0851-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis D.L., Morrison J.J. Hip arthroplasty pseudotumors: pathogenesis, imaging, and clinical decision making. J Clin Imaging Sci. 2016;6:17. doi: 10.4103/2156-7514.181493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bizzotto N., Sandri A., Trivellin G. Chromium-induced diffuse dermatitis with lymph node involvement resulting from Langerhans cell histiocytosis after metal-on-metal hip resurfacing. Br J Dermatol. 2015;172:1633. doi: 10.1111/bjd.13517. [DOI] [PubMed] [Google Scholar]
- 18.Catelas I., Campbell P.A., Dorey F., Frausto A., Mills B.G., Amstutz H.C. Semi-quantitative analysis of cytokines in MM THR tissues and their relationship to metal particles. Biomaterials. 2003;24:4785. doi: 10.1016/s0142-9612(03)00378-8. [DOI] [PubMed] [Google Scholar]
- 19.Zahiri C.A., Schmalzried T.P., Ebramzadeh E. Lessons learned from loosening of the McKee-Farrar metal-on-metal total hip replacement. J Arthroplasty. 1999;14:326. doi: 10.1016/s0883-5403(99)90059-1. [DOI] [PubMed] [Google Scholar]
- 20.McCarthy C.L., Uchihara Y., Vlychou M., Grammatopoulos G., Athanasou N.A. Development of malignant lymphoma after metal-on-metal hip replacement: a case report and review of the literature. Skeletal Radiol. 2017;46:831. doi: 10.1007/s00256-017-2612-y. [DOI] [PubMed] [Google Scholar]
- 21.Lalmohamed A., MacGregor A.J., de Vries F., Leufkens H.G., van Staa T.P. Patterns of risk of cancer in patients with metal-on-metal hip replacements versus other bearing surface types: a record linkage study between a prospective joint registry and general practice electronic health records in England. PLoS One. 2013;8:e65891. doi: 10.1371/journal.pone.0065891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pazzaglia U.E., Apostoli P., Congiu T., Catalani S., Marchese M., Zarattini G. Cobalt, chromium and molybdenum ions kinetics in the human body: data gained from a total hip replacement with massive third body wear of the head and neuropathy by cobalt intoxication. Arch Orthop Trauma Surg. 2011;131:1299. doi: 10.1007/s00402-011-1268-7. [DOI] [PubMed] [Google Scholar]
- 23.Pelclova D., Sklensky M., Janicek P., Lach K. Severe cobalt intoxication following hip replacement revision: clinical features and outcome. Clin Toxicol (Phila) 2012;50:262. doi: 10.3109/15563650.2012.670244. [DOI] [PubMed] [Google Scholar]
- 24.Gilbert C.J., Cheung A., Butany J. Hip pain and heart failure: the missing link. Can J Cardiol. 2013;29:639.e1. doi: 10.1016/j.cjca.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 25.Dahms K., Sharkova Y., Heitland P., Pankuweit S., Schaefer J.R. Cobalt intoxication diagnosed with the help of Dr House. Lancet. 2014;383:574. doi: 10.1016/S0140-6736(14)60037-4. [DOI] [PubMed] [Google Scholar]
- 26.Torres-Alanis O., Garza-Ocanas L., Bernal M.A., Pineyro-Lopez A. Urinary excretion of trace elements in humans after sodium 2, 3-dimercaptopropane-1-sulfonate challenge test. J Toxicol Clin Toxicol. 2000;38:697. doi: 10.1081/clt-100102382. [DOI] [PubMed] [Google Scholar]
- 27.Giampreti A., Lonati D., Ragghianti B. N-Acetyl-Cysteine as effective and safe chelating agent in metal-on-metal hip implanted patients: two cases. Case Rep Orthop. 2016;2016:8682737. doi: 10.1155/2016/8682737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luczak M.W., Zhitkovich A. Role of direct reactivity with metals in chemoprotection by N-acetylcysteine against chromium (VI), cadmium (II), and cobalt (II) Free Radic Biol Med. 2013;65:262. doi: 10.1016/j.freeradbiomed.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Unice K.M., Kerger B.D., Paustenbach D.J., Finley B.L., Tvermoes B.E. Refined biokinetic model for humans exposed to cobalt dietary supplements and other sources of systemic cobalt exposure. Chem Biol Interact. 2014;216:53. doi: 10.1016/j.cbi.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Banner W., Jr., Koch M., Capin D.M., Hopf S.B., Chang S., Tong T.G. Experimental chelation therapy in chromium, lead, and boron intoxication with N-acetylcysteine and other compounds. Toxicol Appl Pharmacol. 1986;83:142. doi: 10.1016/0041-008x(86)90331-5. [DOI] [PubMed] [Google Scholar]
- 31.Llobet J.M., Domingo J.L., Corbella J. Comparative effects of repeated parenteral administration of several chelators on the distribution and excretion of cobalt. Res Commun Chem Pathol Pharmacol. 1988;60:225. [PubMed] [Google Scholar]
- 32.Smith S.W. The role of chelation in the treatment of other metal poisonings. J Med Toxicol. 2013;9:355. doi: 10.1007/s13181-013-0343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.