Abstract
Extraction can be carried out at ambient temperature or high temperature to accelerate the extraction process of secondary metabolites from simplicia. This study aimed to determine the effectiveness of extraction methods on antioxidant activity of secondary metabolites of papery skin extracts and fractions of Maja Cipanas onion (Allium cepa L. var. ascalonicum). Extraction methods were maceration, percolation, reflux, and Soxhlet method, and then, concentrated extracts were fractionated by liquid-liquid extraction based on the polarity of secondary metabolites. Antioxidant activity was determined by the DPPH (2,2-diphenyl-1-picrylhydrazyl) method. The phytochemical screening showed that onion papery skin contained alkaloids, polyphenols, flavonoids, and tannins. The IC50 value of the extract, ethyl acetate fraction, and water fraction of the four extraction methods in the concentration range 25–400 μg/mL were in the range of 55.62–107.08, 31.31–84.06, and 126.05–139.82 μg/mL, respectively, while the IAA value was in the ranges of 0.25–0.49, 0.32–0.86, and 0.19–0.21, respectively. Variation in IC50 and IAA values indicate that the extraction method affects antioxidant activity, due to extracted secondary metabolites from simplicia. The highest antioxidant activity was an ethyl acetate fraction by the reflux method, while the lowest was water fraction by the percolation method.
1. Introduction
The production of onion (Allium cepa L., Liliaceae) in Indonesia was 1,470,155 tons in 2017 and increased to 1,503,436 tons in 2018, about 2.26% [1]. Onion production is fluctuating because of the imbalance of production between in-season and off-season harvest [2]. This is due to the high intensity of pest and disease if planted in out of season [3, 4].
The used part of the onion is the bulbs, while the papery skin is thrown away. The onion papery skin has not been utilized, although it contains anthocyanins [5]. Previous studies showed that Maja Cipanas onion had higher anthocyanin content than Bima Brebes onion [6] and the production of Maja Cipanas onion (10.9 tons/ha) was higher than Bima Brebes onion (9.9 tons/ha) in 2015 [1]. Anthocyanins are flavonoid derivate, which have antioxidant activity [7]. This study aimed to determine the effectiveness of extraction methods on antioxidant activity of secondary metabolites of papery skin extracts and fractions of Maja Cipanas onion (Allium cepa L. var. ascalonicum).
2. Materials and Methods
2.1. Materials
Maja Cipanas Onion (Allium cepa L. var. ascalonicum) bulbs were collected from the Gede Bage market, West Java Province, Indonesia in July 2016. Bulbs were identified by the School of Biological Sciences and Technology, Bandung Institute of Technology, Indonesia, with No. 912/II.CO2.2/PL/2016. All chemical reagents are of analytical grade (Merck, Germany), i.e., ethanol, hydrochloride acid, sodium hydroxide, n-buthanol, acetic acid, potassium chloride, potassium chloride, and sodium acetate.
2.2. Water Determination
Onion papery skin was peeled, washed, and dried at 40 C. Two grams of onion papery skin were dried on 105°C at an atmospheric pressure for 5 h and then weighed. Drying and weighing continued, every 1 h, until a constant weight [8].
2.3. Extraction
Each of 30 g onion papery skin was extracted with 300 mL of a mixture of 70% ethanol and 2 N hydrochloride acid, with a pH of 1.0 in a macerator, percolator, and reflux and Soxhlet apparatus. The extraction was repeated for three cycles with solvent replacement. Each cycle was 24 h for maceration, 8 h for percolation, and 2 h for reflux and Soxhlet method [6]. All extracts were collected and concentrated with a rotary evaporator at 40°C, and then, the yield was calculated.
2.4. Fractionation
The concentrated extract was dissolved in 40 mL of 60°C water and then put into a separating funnel, and 20 mL of n-hexane was added. The separating funnel was closed and shaken and then allowed to separate. The two fractions were separated into two different containers. The water fraction was put back into the separating funnel, and then, another 20 mL of n-hexane was added and carried out as above. Repeat the fractionation until uncolored n-hexane fraction is obtained. The n-hexane fraction is then held in the same container. The water fraction was placed into a separating funnel and fractioned using ethyl acetate in the same way as fractioned with n-hexane. Ethyl acetate and water fraction were placed in different containers and then concentrated, and the yield was calculated [9].
2.5. Phytochemical Screening
Fransworth method was conducted for phytochemical screening to simplicia, extracts, and fraction [10].
2.6. Antioxidant Activity Assay
About 40 μg/mL DPPH solution was added to 96% ethanol and allowed to stand for 20 min in a dark place. The absorbance was measured in 400–700 nm. Ascorbic acid standard, extract, and fraction were dissolved in 96% ethanol and diluted into five concentrations. Each sample (1 mL) was added to 2 ml of DPPH solution and incubated for 20 min. Absorbance was measured at maximum wavelength, and % inhibition was calculated using equation (1). The IC50 value was calculated from linear regression between % inhibition and concentration. The antioxidant activity category based on the IC50 value is very strong when IC50 < 50 μg/mL, strong when IC50 is between 50 and 100 μg/mL, moderate when IC50 is between 100 and 150 μg/mL, and low when IC50 > 150 μg/mL [11]. The antioxidant activity index was calculated using equation (2). The antioxidant activity category based on the AAI value is low when AAI <0.5, moderate when AAI is between 0.5 and 1.0, strong when AAI is between 1.0 and 2.0, and very strong when AAI >2.0 [12]:
| (1) |
| (2) |
2.7. Statistical Analysis
Results are presented as the mean ± standard deviation (SD). One-way ANOVA followed by Student's t-test was used to analyze the data comparisons between groups. Values were considered statistically significant at p < 0.05.
3. Results and Discussion
3.1. Water Determination
Maja Cipanas onion is resistant to tuber blight due to Botrytis allii and potential for growing in Indonesia. A total of 5 kg of Maja Cipanas onion was produced from 36 g of dry papery skin onion with 0.72% of yield. The simplicia moisture content was 4.2 ± 0.5%, which met the requirement, i.e., less than 8%. High water content causes secondary metabolite degradation due to microorganism growth [13]. Maja Cipanas onion has pinky red papery skin [1], which suggested anthocyanins [14]. The best solvent for anthocyanin extraction is acetic acid or hydrochloric acid-contained solvent [15]. Our previous study showed that the mixture of 70% ethanol and HCl with pH 1.0 was best solvent for anthocyanin extraction [6]. All extracts of papery skin Maja Cipanas onion were red, due to an oxonium form of anthocyanin [14], with various yields and intensity because of the different extraction methods (Table 1).
Table 1.
The yield of extraction.
| Method | Simplicia (g) | Extract (g) | Yield (%) | Color |
|---|---|---|---|---|
| Maceration | 30 | 9.58 | 31.92 | Red |
| Percolation | 30 | 11.88 | 39.6 | Dark red |
| Reflux method | 30 | 9.70 | 32.34 | Red |
| Soxhlet method | 30 | 8.81 | 34.05 | Light red |
The concentrated extracts were produced from cold extraction (maceration and percolation) and hot extraction (reflux and Soxhlet extraction). The efficiency of maceration and reflux extraction, i.e., the soaking method, depends on the effective diffusion and solubility of secondary metabolites [16]. The yield of maceration was lower than reflux extraction, because heating in reflux extraction increases diffusion [17]. The efficiency of percolation and Soxhlet extraction, i.e., the flowing solvent method, depends on repeated cycle extraction with a fresh solvent until all the solutes are dissolved in simplicia [18]. The yield of Soxhlet extraction was lower than percolation, due to anthocyanin instability to temperature [14]. Percolation was the extraction method with the highest yield (Table 1). This result showed that secondary metabolites can be completely extracted using flowing acidified solvents at ambient temperature.
The yield of water fractions was higher than of the ethyl acetate fractions (Table 2), due to the polarity of acidified ethanol as the extraction solvent, in which polar secondary metabolites extracted higher than nonpolar ones. The n-hexane fractions were not obtained because n-hexane is a nonpolar solvent, while the extraction solvent is polar acidified ethanol. It is suggested that no nonpolar secondary metabolites were extracted.
Table 2.
The yield of fractionation.
| Method | Water fraction | Ethyl acetate fraction |
|---|---|---|
| Maceration | 67.88 | 23.69 |
| Percolation | 66.67 | 23.08 |
| Reflux method | 66.82 | 22.47 |
| Soxhlet method | 57.27 | 26.36 |
Alkaloids in sample solution were reacted with Dragendorff's reagent to produce an orange to orange-red precipitate, which accorded with the literature [19]. Polyphenols in sample solution were reacted with iron III chloride (FeCl3) to produce the green solution. These results were accorded with the literature, in which complexes of polyphenols-FeCl3 are blue, green, red, or purple color [20]. Tannins in sample solution were reacted with a gelatin solution containing sodium chloride to produce white precipitate, which accorded with the literature [20]. Flavonoids in sample solution were reacted with magnesium powder and concentrated hydrochloric acid to produce pink to red color. These results were accorded with the literature, in which flavonoids produce pink or red color with this reagent [21]. The phytochemical screening showed that simplicia, extracts, ethyl acetate fractions, and water fractions from the maceration, percolation, reflux, and Soxhlet method contain alkaloids, polyphenols, flavonoids, and tannins. These results show that all extraction methods can dissolve the same secondary metabolites in different quantities, which can be observed from the extract color (Table 1).
3.2. Antioxidant Activity Assay
DPPH free radicals are organic compounds with unstable nitrogen, due to unpaired electrons [22]. DPPH solution is dark purple with a maximum wavelength of 518 nm (Figure 1). The oxidant compounds give the hydrogen atom to the DPPH free radical, which reduced to DPPH-H (1,1-diphenyl-2-picrylhydrazine) [22]. The absorbances of extract and fraction of Maja Cipanas onion were able to donate its hydrogen atoms in sufficient quantities to scavenging the DPPH free radicals (Table 3).
Figure 1.
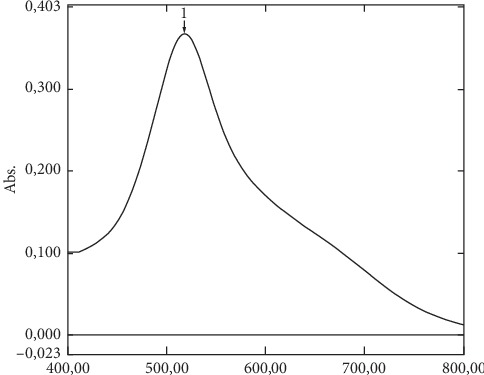
Maximum wavelength of DPPH solution. The purple DPPH solution has a maximum wavelength at 518 nm.
Table 3.
Antioxidant activity of sample to DPPH solution (n = 3).
| Extraction method | Sample (n = 3) | Concentration (μg/mL) | % Scavenging | Linear equation | IC50 value (μg/mL) | AAI |
|---|---|---|---|---|---|---|
| None | Ascorbic acid | 2 | 45.51 ± 0.03 |
y = 6.52x + 33.00 R2 = 0.998 |
2.60 | 10.38 |
| 4 | 59.12 ± 0.08 | |||||
| 6 | 72.90 ± 0.04 | |||||
| 8 | 85.74 ± 0.06 | |||||
| 10 | 97.45 ± 0.07 | |||||
|
| ||||||
| Maceration | Extract | 25 | 43.86 ± 0.04 |
y = 0.083x + 42.86 R2 = 0.9966 |
86.28 | 0.31 |
| 50 | 47.86 ± 0.05 | |||||
| 100 | 51.17 ± 0.03 | |||||
| 200 | 59.86 ± 0.08 | |||||
| 400 | 75.72 ± 0.06 | |||||
| Ethyl acetate fraction | 25 | 48.28 ± 0.06 |
y = 0.083x + 47.11 R2 = 0.9975 |
34.92 | 0.77 | |
| 50 | 52.00 ± 0.03 | |||||
| 100 | 55.31 ± 0.04 | |||||
| 200 | 64.00 ± 0.05 | |||||
| 400 | 79.87 ± 0.07 | |||||
| Water fraction | 25 | 37.10 ± 0.08 | y = 0.106x + 36.04 R2 = 0.9919 | 131.69 | 0.21 | |
| 50 | 40.97 ± 0.04 | |||||
| 100 | 47.86 ± 0.06 | |||||
| 200 | 59.03 ± 0.03 | |||||
| 400 | 77.38 ± 0.05 | |||||
|
| ||||||
| Percolation | Extract | 25 | 47.12 ± 0.05 |
y = 0.067x + 46.12 R2 = 0.9907 |
55.62 | 0.49 |
| 50 | 50.85 ± 0.03 | |||||
| 100 | 53.05 ± 0.06 | |||||
| 200 | 58.47 ± 0.04 | |||||
| 400 | 77.73 ± 0.07 | |||||
| Ethyl acetate fraction | 25 | 49.15 ± 0.04 |
y = 0.069x + 47.08 R2 = 0.992 |
41.91 | 0.64 | |
| 50 | 50.85 ± 0.05 | |||||
| 100 | 54.41 ± 0.08 | |||||
| 200 | 59.32 ± 0.03 | |||||
| 400 | 75.59 ± 0.06 | |||||
| Water fraction | 25 | 34.07 ± 0.05 |
y = 0.129x + 31.95 R2 = 0.993 |
139.82 | 0.19 | |
| 50 | 37.46 ± 0.04 | |||||
| 100 | 45.76 ± 0.08 | |||||
| 200 | 60.17 ± 0.03 | |||||
| 400 | 82.37 ± 0.07 | |||||
|
| ||||||
| Reflux method | Extract | 25 | 46.63 ± 0.03 | y = 0.064x + 45.02 R2 = 0.9934 | 78.07 | 0.35 |
| 50 | 49.19 ± 0.05 | |||||
| 100 | 50.93 ± 0.04 | |||||
| 200 | 56.74 ± 0.07 | |||||
| 400 | 71.05 ± 0.08 | |||||
| Ethyl acetate fraction | 25 | 73.84 ± 0.05 |
y = 0.066x + 47.95 R2 = 0.9923 |
31.31 | 0.86 | |
| 50 | 61.98 ± 0.03 | |||||
| 100 | 53.84 ± 0.04 | |||||
| 200 | 52.21 ± 0.08 | |||||
| 400 | 48.72 ± 0.06 | |||||
| Water fraction | 25 | 38.84 ± 0.08 |
y = 0.089x + 37.94 R2 = 0.9919 |
134.88 | 0.20 | |
| 50 | 42.09 ± 0.07 | |||||
| 100 | 47.91 ± 0.04 | |||||
| 200 | 57.33 ± 0.05 | |||||
| 400 | 72.79 ± 0.03 | |||||
|
| ||||||
| Soxhlet method | Extract | 25 | 38.62 ± 0.09 | y = 0.116x + 37.64 R2 = 0.9929 | 107.08 | 0.25 |
| 50 | 45.75 ± 0.06 | |||||
| 100 | 49.31 ± 0.03 | |||||
| 200 | 60.57 ± 0.04 | |||||
| 400 | 84.25 ± 0.05 | |||||
| Ethyl acetate fraction | 25 | 45.50 ± 0.09 | y = 0.081x + 43.40 R2 = 0.9955 | 84.06 | 0.32 | |
| 50 | 48.51 ± 0.05 | |||||
| 100 | 51.26 ± 0.07 | |||||
| 200 | 58.51 ± 0.04 | |||||
| 400 | 76.44 ± 0.03 | |||||
| 50 | 48.51 ± 0.05 | |||||
| Water fraction | 25 | 40.69 ± 0.07 |
y = 0.086x + 39.13 R2 = 0.9948 |
126.05 | 0.21 | |
| 50 | 42.76 ± 0.04 | |||||
| 100 | 48.51 ± 0.03 | |||||
| 200 | 57.82 ± 0.05 | |||||
| 400 | 73.10 ± 0.06 | |||||
Three of the extracts were strong antioxidant (55.62–86.28 μg/mL), except the Soxhlet extract was moderate antioxidant (107.08 μg/mL). All ethyl acetate fractions were strong antioxidant (31.31–84.06 μg/mL) and all water fractions were moderate antioxidant (126.05–139.82 μg/mL). The AAI value was calculated with equation (2) [12], and the final DPPH concentration was 30.75 μg/mL, resulting in a constant for each extract and fraction. In this work, we considered that all extracts and water fractions from all extraction methods showed low antioxidant (0.19–0.49), while all ethyl acetate fractions, except for the reflux method, were moderate antioxidant (0.64–0.86). The difference in the strength of antioxidant activity was due to differences in the content and quantity of extracting secondary metabolites in extracts and fractions. Antioxidant activity calculated by AAI is lower than the IC50 value, but the AAI value is more accurate, due to independent on DPPH and sample concentrations [12].
Anthocyanins have antioxidant activity [7]. In this study, total anthocyanin contents were not compared with antioxidant activity (Table 4). The total anthocyanin content of the extract was significantly different in various extraction methods (p=3.62 × 10−6). This showed that the antioxidant activity was given not only by anthocyanins, but mixed secondary metabolites in papery skin onion, i.e., alkaloids, polyphenols, flavonoids, and tannins. Alkaloids and polyphenols, including flavonoids and tannins, are the major antioxidants with antioxidant activities in natural products [23, 24].
Table 4.
The IC50 value and anthocyanin content (n = 3).
| Extraction method | IC50 value (μg/mL) | Total anthocyanin content [6] | ||
|---|---|---|---|---|
| Extract | Ethyl acetate fraction | Water fraction | ||
| Maceration | 86.28 | 34.92 | 131.69 | 1.181 ± 0.008 |
| Percolation | 55.62 | 41.91 | 139.82 | 0.597 ± 0.015 |
| Reflux method | 78.07 | 31.31 | 134.88 | 1.449 ± 0.013 |
| Soxhlet method | 107.08 | 84.06 | 126.05 | 0.342 ± 0.022 |
The antioxidant activity of extracts and fractions was significantly different (p=9.77 × 10−3). The best antioxidant activity was extract from percolation (p=3.55 × 10−9), ethyl acetate fraction from the reflux method (p=4.78 × 10−10), and the water fraction from the Soxhlet method (p=2.41 × 10−7). This showed that the extraction method affects the extracted secondary metabolites from simplification, thus affecting the extracted secondary metabolites into the ethyl acetate and water fractions.
4. Conclusions
The extraction method affects extracted secondary metabolites, due to antioxidant activity.
Acknowledgments
The authors thank Irma Erika Herawati, Niasti Devi Maharani, Qoorina As Shafa, Oktavia Khomala Sari, and Siti Nurjanah for technical assistance.
Data Availability
The data in this study are available from the corresponding author. Anyone who needs the data in this study can ask the corresponding author by email.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1. https://www.pertanian.go.id/home/?show=page&act=view&id=61.
- 2.Baswarsiati L., Rosmahani E., Korlina E. P., et al. Proceeding of Seminar of Research Result/Assessment of BPTP Karangploso; 1996. Adaptation of some off-season onion varieties. [Google Scholar]
- 3.Duriat A. S., Soetiarso T. A., Prabaningrum L., et al. Onion Production Technology. Jakarta, Indonesia: Puslitbanghorti; 1994. Application of integrated pest and disease control on red onion cultivation. [Google Scholar]
- 4.Hadisoeganda W. W. E., Suryaningsih, Moekasa T. K. Onion Production Technology. Jakarta, Indonesia: Puslitbanghorti; 1995. Onion diseases and pests and how to control them. [Google Scholar]
- 5.Frankly A A. Food and Chemistry. 2nd ed. New York, USA: Mc Graw Hill Book Camp; 1983. [Google Scholar]
- 6.Saptarini N. M., Herawati I. E. Extraction methods and varieties affect total anthocyanins content in acidified extract of papery skin of onion (Allium cepa L.) Drug Invention Today. 2018;10(4):471–474. [Google Scholar]
- 7.Wang J., Mazza G. Effects of anthocyanins and other phenolic compounds on the production of tumor necrosis factor alpha in LPS/IFN-gamma-activated RAW 264.7 macrophages. J Agric Food Chem. 2002;50(41):83–89. doi: 10.1021/jf011613d. [DOI] [PubMed] [Google Scholar]
- 8.The United States Pharmacopeial Convention. US Pharmacopeia. 40th. Rockville, MD, USA: Water Determination; 2010. https://hmc.usp.org/sites/default/files/documents/HMC/GCs-Pdfs/c921.pdf. [Google Scholar]
- 9.Saptarini N. M., Wardati Y., Juliawati R. Antioxidant activity of extract and fraction of yellow passion fruit (Passiflora flavicarpa) leaves. International Journal of Pharmacy and Pharmaceutical Sciences. 2013;5(2):194–196. [Google Scholar]
- 10.Fransworth N. R. Biological and phytochemycal screening of plants. Journal of Pharmaceutical Sciences. 1996;1:55–59. doi: 10.1002/jps.2600550302. [DOI] [PubMed] [Google Scholar]
- 11.Molyneux P. The use of the stable free radical diphenylpicrylhydrazil (DPPH) for estimating antioxidant activity. Songklankarin J Sci Tech. 2004;26:211–219. [Google Scholar]
- 12.Scherer R., Godoy H. T. Antioxidant activity index (AAI) by the 2,2-diphenyl-1-picrylhydrazyl method. Food Chemistry. 2009;112(3):654–658. doi: 10.1016/j.foodchem.2008.06.026. [DOI] [Google Scholar]
- 13.Agoes G. Book of Natural Materials Technology (Serial of Pharmaceutical Industry) Bandung, Indonesia: Penerbit Institut Teknologi Bandung; 2009. [Google Scholar]
- 14.Wrolstad R. E., Acree T. E., Decker E. A., et al. Handbook of Food Analytical Chemistry: Pigments, Colorants, Flavors, Texture, and Bioactive Food Components. Hoboken, NJ, USA: John Wiley & Sons; 2005. [Google Scholar]
- 15.Harborne J. B. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis. London, UK: ” Thompson Science; 1998. [Google Scholar]
- 16.Giusti M. M., Wrolstad R. E. Characterization and measurement of anthocyanins by UV-vis spectroscopy. Current Protocols in Food Analytical Chemistry. 2001;2:1–13.306240097 [Google Scholar]
- 17.Cheok C. Y., Salman H. A. K., Sulaiman R. Extraction and quantification of saponins: a review. Food Research International. 2014;59:16–40. doi: 10.1016/j.foodres.2014.01.057.S096399691400074X [DOI] [Google Scholar]
- 18.Azmir J., Zaidul A., Rahman A., et al. Techniques for extraction of bioactive compounds from plant materials: a review. Journal of Food Engineering. 2013;117:426–436.S0260877413000277 [Google Scholar]
- 19.Nayeem A. A., Khatun A., Rahman M. S., et al. Evaluation of phytochemical and pharmacological properties of Mikania cordata (Asteraceae) leaves. Journal of Pharmacognosy and Phytotherapy. 2011;3(8):118–123.236295917 [Google Scholar]
- 20.Trease G. E., Evans W. E. Trease and Evan’s Textbook of Pharmacognosy. 13th. Vol. 546. London, UK: Cambridge University Press; 1989. [Google Scholar]
- 21.Yisa J. Phytochemical analysis and antimicrobial activity of Scoparia dulcis and Nymphaea lotus. Australian Journal of Basic and Applied Sciences. 2009;3(4):3975–3979.266495058 [Google Scholar]
- 22.Reynertson K. A., Yang H., Jiang B., et al. Quantitative analysis of antiradical phenolic constituents from fourteen edible Myrtaceae fruits. Food Chemistry. 2008;109(4):883–890. doi: 10.1016/j.foodchem.2008.01.021.21340048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pu F., Ren X.-L., Zhang X.-P. Phenolic compounds and antioxidant activity in fruits of six Diospyros kaki genotypes. European Food Research and Technology. 2013;237(6):923–932. doi: 10.1007/s00217-013-2065-z.s00217-013-2065-z [DOI] [Google Scholar]
- 24.Yin W. Q., Duan S. Q., Zhang Y., et al. Antioxidant activities of different solvents extracts and alkaloids of Uncaria rhynchophylla (Miq.) Jacks. Journal of Guangxi Normal University. 2010;28(1):31–34.20103165819 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data in this study are available from the corresponding author. Anyone who needs the data in this study can ask the corresponding author by email.


