Abstract
Purpose
We evaluated the protective effect of PRP on ovarian function in female rats with cyclophosphamide (Cy)-induced ovarian damage.
Methods
Thirty-two adult female Sprague–Dawley rats were randomly divided into four groups. Group 1 (control-sodium chloride 0.9%; 1 mL/kg, single-dose ip injection), group 2 (Cy); 75 mg/kg, single-dose ip injection and sodium chloride 0.9% (1 mL/kg, single-dose ip injection), group 3 Cy plus PRP, Cy (75 mg/kg, single-dose and PRP (200 μl, single-dose) ip injection), group 4 (PRP, 200 μl, single-dose ip injection). Primordial, antral, and atretic follicle counts; serum anti-Müllerian hormone (AMH) levels; AMH-positive granulosa cells; and gene expression analysis of Ddx4 were assessed.
Results
Serum AMH levels were significantly lower in group 2 compared to groups 1, 3, and 4 (p < 0.01, p < 0.01, and p = 0.04, respectively). A significant difference was found in the primordial, primary, secondary, antral, and atretic follicle counts between all groups (p < 0.01). There was a statistically significant difference in AMH-positive staining primary, secondary, and antral follicles count between the groups (p < 0.01). There was a statistically significant difference in primary, secondary, and antral AMH positive staining follicle intensity score between the groups (p < 0.01). Ddx4 expression in group 4 was highest compared to other groups.
Conclusion
Our study may provide evidence that PRP could protect ovarian function against ovarian damage induced by Cy. It could lead to improved primordial, primary, secondary, and antral follicle numbers.
Keywords: Follicle, Ovarian failure, Ovary, Platelet-rich plasma, Stereology
Introduction
Premature ovarian insufficiency (POI) affects approximately 1–3% of women younger than 40 years of age, although the prevalence of POI is actually less certain [1, 2]. The causes of POI are clearly heterogeneous, with a wide spectrum of causes. The majority of POIs are classified as idiopathic, but there are several known mechanisms related to the development of POI, such as genetic, autoimmune, mitochondrial dysfunction, inflammatory, chemotherapeutic treatments, endocrine, psychological, paracrine, or metabolic factors [3, 4]. POI leads to the reduction of the number of oocytes in the ovaries because of especially accelerated atresia. Early loss of ovarian function results in significant health problems and infertility. Women with POI have only a 5–10% chance of conceiving but the likelihood of recovery of ovarian function is not possible to predict. There are various medical therapies to restore ovarian function and/or treat fertility and/or achieve pregnancy in women with POI, such as immunomodulating therapies, apoptotic inhibitors, antioxidant therapies, IVF, and embryo transfer using donor oocytes [5]. There are no proven treatment methods, and trials have generally failed to demonstrate any significant improvement in ovulation or pregnancy rates in women with POI by these recommended treatment options.
Effective screening, early diagnosis, and enhanced cancer therapies contribute to an increase in long-term survival for cancer patients. One of the most crucial long-term side effects of chemotherapeutic agents in female cancer survivors is infertility due to chemotherapy-induced ovarian damage and subsequent iatrogenic POI. New treatment modalities, which cope with the risk of subsequent infertility in young cancer patients with chemotherapy-induced POI, are absolutely needed to restore ovarian function for future fertility. The chemotherapeutic agents are generally categorized as high-risk, medium-risk, and low-risk agents according to their gonadotoxicity [6]. The alkylating agents, including cyclophosphamide, chlorambucil, busulfan, and procarbazine, are considered to be high-risk chemotherapeutic agents. The chemotherapeutic agents decrease ovarian reserve and induce POI; alkylating agents induce the most severe damage with an odds ratio of almost 4.4 [7, 8]. Therefore, in the present study, cyclophosphamide (Cy) was used to induce ovarian failure in the animal model. Cy primarily causes the inhibition of DNA synthesis and function and the induction of DNA damage through DNA cross-linking and double-strand breaks. It can also result in the initiation of apoptosis via the activation of an intrinsic mitochondrial pathway leading to a release of cytochrome c into the cytosol and the activation of endoplasmic reticulum stress. The primary action of Cy in ovarian damage appears to diminish primordial follicles, oocytes, and granulosa cells by the induction of apoptosis and to decrease ovarian blood by the inhibition of angiogenesis and the induction of ovarian atrophy [9–11].
Platelet-rich plasma (PRP) was first described by Marx et al. in 1998 [12]. It is an autologous product rich in growth factors, such as platelet-derived growth factor (PDGF), transforming growth factor (TGF)-β1, and vascular endothelial growth factor (VEGF), obtained from a blood sample. The growth factors in PRP improve tissue repair by stimulating chemotaxis, proliferation, and differentiation of stem cells and angiogenesis [13]. Mesenchymal stem cells and PRP are used to the repair and regeneration of damaged tissues as regenerative medicine for the treatment of many serious diseases. The locally regenerative and angiogenic effect of PRP has clearly been demonstrated in numerous studies in the field of bone, muscle, tendon, cartilage, and skin growth [14–17]. PRP also has several advantages, including being an inexpensive product, easy to obtain, and an autologous product, as well as having an antimicrobial action [18, 19]. Likewise, PRP administrated into the ovaries could improve ovarian function and serve as a regenerative product by releasing growth factors known to have protective effects on ovarian failure in the patients with diminished ovarian reserve and POI.
The aim of this study is to investigate the protective effect of PRP on ovarian function in female rats with Cy-induced ovarian damage by measuring primordial, antral, and atretic follicle counts, serum anti-Müllerian hormone (AMH) levels, AMH-positive granulosa cells, and gene expression analysis of Ddx4, the key indicator of the existence of active germ stem cells, which is secreted by human primordial follicles. In this study, we focused on evaluating whether PRP improved ovarian function in a Cy-induced ovarian-damaged animal model.
Materials and methods
Animals
Thirty-two adult female Sprague–Dawley rats (weight 200–250 g; age 65–75 days), provided by the Bezmialem University Animal Reproduction Center and housed in the Animal Laboratory of Bezmialem University, were used for the study. The rats were maintained under standard housing conditions with a 12-h light–dark cycle with ad-libitum access to food and water. The experimental protocol was approved by the Institutional Animal Care and Use Committee of Bezmialem University. All procedures were carried out in accordance with the National Academy of Science’s Guide for Care and Use of Laboratory Animals (1996).
Experimental design
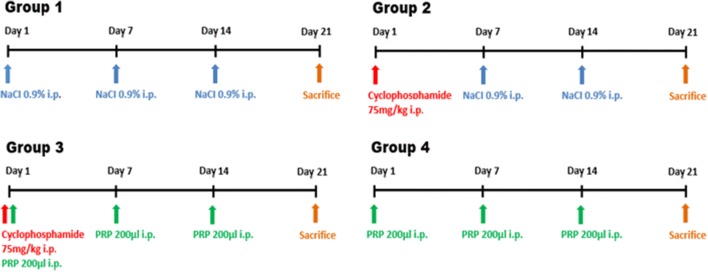
The rats were randomly divided into four groups (eight rats per group) (Fig. 1).
Fig. 1.
The time line of the experiment (the rats were randomly divided into four groups; eight rats per group)
Group 1 (control, n = 8) received a sodium chloride 0.9% (1 mL/kg, single dose) intraperitoneal (ip) injection on the first, seventh, and fourteenth days.
Group 2 (cyclophosphamide, n = 8) received a Cy (75 mg/kg, single dose) ip injection on the first day and a sodium chloride 0.9% (1 mL/kg, single dose) ip injection on the seventh and fourteenth days.
Group 3 (cyclophosphamide plus PRP, n = 8) received a Cy (75 mg/kg, single dose) [20] on the first day and a PRP (200 μl, single dose) ip injection on the first, seventh, and fourteenth days.
Group 4 (PRP, n = 8) received a PRP (200 μl, single dose) ip injection on the first, seventh, and fourteenth days.
Preparing platelet-rich plasma
Eight mature male Sprague–Dawley rats were used to prepare PRP. The blood samples were collected from the right ventricle through cardiac puncture under anesthesia and the samples were transferred into test tubes including 3.2% sodium citrate (Merck, Darmstadt, Germany) at blood/citrate ratio of 9/1. The blood samples were centrifuged at 400×g for 10 min and then the upper portion of the plasma with platelets and buffy coat was transferred into another tube and centrifuged again at 800×g for 10 min. This tube contained platelet sediments and some red blood cells (an erythrocyte-platelet clump). The top 2/3 of supernatant containing the platelet-poor plasma was removed and then the remaining layer (1/3) was considered to be PRP. The final fraction containing 2.1 × 106 platelets/ml was about 3,8 times greater than the number of the blood platelet (550,000 platelets/μl). We used fresh PRP per administration.
Sample collection
On day 21 of the experiment, all the rats were weighed and anesthetized by an intramuscular administration of 50 mg/kg ketamine hydrochloric acid (Ketalar; Eczacibasi Warner-Lambert Ilac Sanayi, Levent, Istanbul, Turkey) and 7 mg/kg xylazine hydrochloric acid (Rompun, Bayer Sisli, Istanbul, Turkey). After immobilizing the rats on a standard surgery board, blood samples were collected to measure serum AMH level. The aseptic technique was used to make a ventral midline incision to expose the reproductive organs and the ovaries were removed. The right ovary was fixed in 10% neutral buffered formaldehyde solution for histopathological examination. The fixed specimens were embedded in paraffin blocks, sectioned at 5-μm thickness, and stained with hematoxylin and eosin. Histopathologic examination was carried out by a pathologist unaware of the treatment allocation. To evaluate the gene expression analysis of Ddx4 by PCR, the left ovary of each rat was immediately stored at − 80 °C.
Histological analysis and ovarian follicle count
The histopathologic examination was performed by a histologist blinded to the groups. The ovaries of the rats were taken out and fixed in 10% neutral formalin for 72 h. After fixation, the ovaries were rinsed with water and were gradually dehydrated by increasing concentrations of alcohol (70%, 90%, 96%, and 100%) and cleared in xylene. Thereafter, the samples were submerged in paraffin overnight at 60 °C. From the paraffin blocks, 5-μm-thick sections were obtained and placed on slides. The paraffin sections were stained with hematoxylin & eosin (H&E) for histomorphometric analysis and their follicle count examined under a photomicroscope (Nikon Eclipse i5, Tokyo, Japan). Ten sections were taken at 100-μm intervals to determine the follicle counts for each ovary. Only follicles that had an oocyte nucleus were scored. The follicles were classified into five stages as follows: primordial, primary, secondary, antral, and atretic follicles. Follicles whose oocyte was surrounded by a single layer of squamous granulosa cells were classified as primordial follicles. If a single layer of cuboidal granulosa cells was observed, follicles were classified as primary follicles. Secondary follicles had multiple layers of cuboidal granulosa cells. If an antrum was observed in the granulosa cell layers, the follicle was classified as an antral follicle. Atretic follicles were counted only when a degenerated oocyte and multiple layers of pycnotic granulosa cells were observed [21].
AMH immunohistochemistry
Paraffin sections were incubated overnight at 37 °C. After deparaffinization with xylene and rehydration in descendant grades of ethanol, the sections were incubated in 3% hydrogen peroxide in methanol for 10 min to inhibit endogenous enzyme blockage and then washed with tap and distilled water. Then, the sections were microwaved at 200 W with a citrate buffer (pH 6.1) for 20 min for antigen retrieval. The slices were cooled to room temperature. After they were washed with phosphate-buffered saline (PBS), they were incubated in blocking solution for 10 min and then incubated in mouse anti-Mullerian hormone (AMH) antibody (1:20, GeneTex, Cat: GTX42794) at 4 °C overnight. Secondary antibody staining was performed using the Histostain®-Plus 3rd Gen IHC Detection Kit (Cat: 85-9073, Invitrogen, CA, USA) following the manufacturer’s protocol. After washing, sections were incubated with streptavidin–peroxidase (ready-to-use) for 10 min at room temperature, followed by incubation with 3, 3′-diaminobenzidine (DAB) for 5 min. Slides were finally counterstained with Mayer’s hematoxylin and covered with mounting medium. The number and intensity of the AMH positive stained primary, secondary, and antral follicles were evaluated on three serial sections taken at 100-μm intervals. In each section, AMH-positive follicles were evaluated by staining intensity (0 to 3 as follows: 0 = no staining, 1 = weak staining, 2 = moderate staining, and 3 = strong staining) and the distribution of staining (0 or 1, as follows: 0 = ≤ 50% of the structure staining and 1 = ≥ 50% of the structure staining) semi-quantitatively. The total score was calculated as 4 points and a score greater than or equal to 2 was considered positive for AMH [22] (Table 1).
Table 1.
Comparison of the antral and atretic follicle counts, number of AMH-positive follicles, and intensity of staining of the follicles positive for AMH and serum concentrations of AMH of all groups
| Variables | Group I (control, n = 8) |
Group II (cy), n = 8) |
Group III (cy plus PRP, n = 8) |
Group IV (PRP, n = 8) |
p value |
|---|---|---|---|---|---|
| Primary AMH positive staining follicle counts | 26.83 ± 6.14 | 11.83 ± 2.99 | 22 ± 5.44 | 25.83 ± 5.56 | < 0.01* |
| Secondary AMH positive staining follicle counts | 7.71 ± 2.62 | 3.42 ± 1.13 | 6.75 ± 1.38 | 7.71 ± 3.35 | < 0.01* |
| Antral AMH positive staining follicle counts | 7.42 ± 1.51 | 4.28 ± 1.11 | 7.12 ± 1.8 | 8.75 ± 1.83 | < 0.01* |
| Primary AMH positive staining follicle intensity score | 3 ± 0.21 | 1 ± 0.21 | 191 ± 0.14 | 3 ± 0.33 | < 0.01* |
| Secondary AMH positive staining follicle intensity score | 2.89 ± 0.23 | 0.98 ± 0.21 | 2.49 ± 0.47 | 3.09 ± 0.22 | < 0.01* |
| Antral AMH positive staining follicle intensity score | 3.07 ± 0.26 | 1.16 ± 0.34 | 2.37 ± 0.37 | 3.19 ± 0.11 | < 0.01* |
| Primordial follicle counts | 154.7 ± 50.11 | 51.5 ± 11.54 | 121.4 ± 43.8 | 159.4 ± 57.28 | < 0.01* |
| Primary follicle counts | 88.5 ± 22.02 | 40.33 ± 9.89 | 73.83 ± 16.71 | 85.67 ± 18.17 | < 0.01* |
| Secondary follicle counts | 23.43 ± 7.23 | 12.29 ± 2.49 | 19.75 ± 4.4 | 24.43 ± 9.18 | < 0.01* |
| Antral follicle counts | 23.14 ± 4.59 | 12.14 ± 2.73 | 20.38 ± 6.5 | 26.5 ± 5.31 | < 0.01* |
| Atretic follicle counts | 4.14 ± 2.96 | 9.28 ± 4.42 | 3.75 ± 2.6 | 3.5 ± 2.56 | < 0.01* |
|
Serum concentrations of AMH (ng/ml) |
1.87 ± 0.524 | 0.7 ± 0.284.5.6 | 1.25 ± 0.245 | 1.66 ± 0.36 | < 0.01* |
| Ddx4 expression | 1 ± 0.0 | 4.34 ± 1.02 | 7.21 ± 1.52 | 9.86 ± 4.39 | < 0.01* |
All values are expressed as mean ± SD
*p < 0.01 significant difference, comparison of all groups
Comparison of primordial follicle counts; group 1 vs 2 p < 0.01; group 2 vs 4 p < 0.01; group 2 vs 3 p = 0.04
Comparison of primary follicle counts; group 1 vs 2 p < 0.01; group 2 vs 4 p < 0.01; group 2 vs 3 p = 0.01
Comparison of secondary follicle counts; group 1 vs 2 p = 0.01; group 2 vs 4 p < 0.01; group 2 vs 3 p = 0.12
Comparison of antral follicle counts, group 1 vs 2 p < 0.01; group 2 vs 4 p < 0.01; group 2 vs 3–0.02
Comparison of atretic follicle counts, group 1 vs 2 p = 0.02; group 2 vs 4 p < 0.01; group 2 vs 3 p = 0.01
Comparison of primary AMH positive staining follicle counts, group 1 vs 2 p < 0.01; group 2 vs 4 p < 0.01; group 2 vs 3 p = 0.01
Comparison of secondary AMH positive staining follicle counts, group 1 vs 2 p < 0.01; group 2 vs 4 p < 0.01; group 2 vs 3 p = 0.04
Comparison of antral AMH positive staining follicle counts, group 1 vs 2 p < 0.01; group 2 vs 4 p < 0.01; group 2 vs 3 p = 0.01
Comparison of primary AMH positive staining follicle intensity score, group 1 vs 2 p < 0.01; group 2 vs 4 p < 0.01; group 2 vs 3 p < 0.01
Comparison of secondary AMH positive staining follicle intensity score, group 1 vs 2 p < 0.01; group 2 vs 4 p < 0.01; group 2 vs 3 p < 0.01
Comparison of antral AMH positive staining follicle intensity score, group 1 vs 2 p < 0.01; group 2 vs 4 p < 0.01; group 2 vs 3 p < 0.01
Comparison of serum concentrations of AMH, group 1 vs 2 p < 0.01; group 2 vs 4 p < 0.01; group 2 vs 3 p = 0.04
Serum AMH concentrations
Serum AMH levels were measured by USCN Life Science enzyme-linked immunosorbent assays (ELISA). The assay range was 0.31–20 ng/mL, and the minimum detectable dose of this assay was less than 0.078 ng/mL. The technician was unaware of the treatment allocation.
Quantitative RT-PCR
Ovary tissues were homogenized using tissue homogenizer (Next Advance, USA) and RNA was isolated with the PureLink RNA Mini Kit (Invitrogen, USA) according to the manufacturer instructions. RNA samples were reverse transcribed with an iScript cDNA Synthesis Kit (Biorad, USA) for 1 μg total RNA in each reaction. For determining expression changes of DDX-4 genes, quantitative RT-PCR (qPCR) was performed on Biorad CFX Connect using iTaq Universal SYBR Green Supermix (Biorad, USA) with the primer pairs listed in Table 2. Non-template controls for each primer pair were included in each experiment. Relative expression levels of each transcript were normalized against the housekeeping gene GAPDH, and expression fold changes were calculated relative to group 1.
Table 2.
Forward and reverse primer sequences used in qPCR
| Gene | Forward (5′-- > 3′) | Reverse (5′-- > 3′) |
|---|---|---|
| ddx4 | TGTCAGATGCTCAACAGGATGT | CAGTGTGTTCTTGCCCTGGA |
| gapdh | AGTGCCAGCCTCGTCTCATA | GAGGTCAATGAAGGGGTCGTT |
Statistical analysis
Based on power analysis, 5 rats in each group were required to assess statistical significance (power of 0.95 and α = 0.05). Power calculation was based on antral follicle counts [20]. The results were analyzed using GraphPad Prism 6.0 (GraphPad Software, Inc. La Jolla, CA, USA). Data were reported as mean ± standard deviation (SD), number or percentage. Data were analyzed by one-way ANOVA followed by Tukey test. p < 0.05 was considered statistically significant.
Results
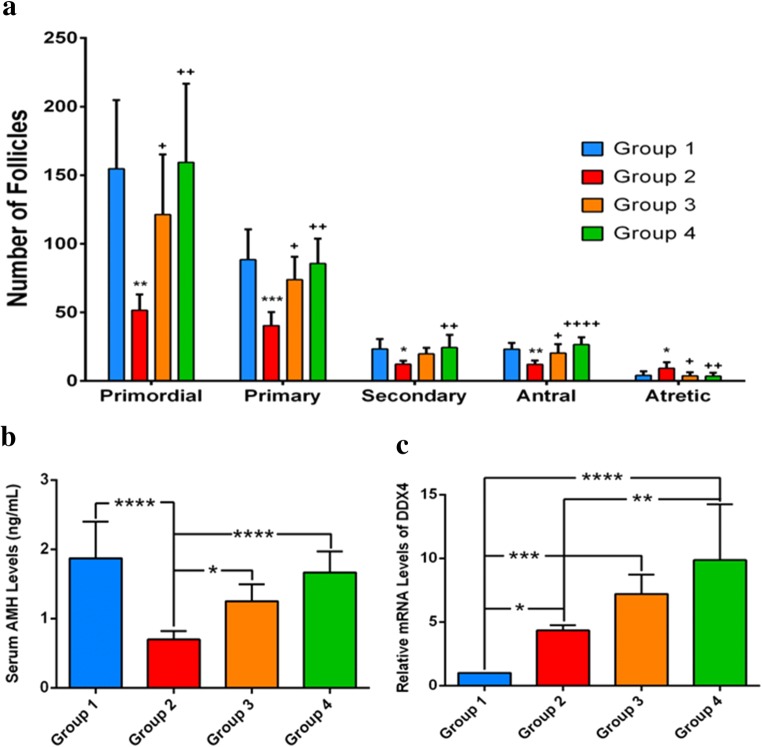
The mean serum AMH concentrations were 1.87 ± 0.52, 0.7 ± 0.28, 1.25 ± 0.24, and 1.66 ± 0.36 ng/mL in groups 1, 2, 3, and 4, respectively (p < 0.01) (Table 1, Fig. 2). Serum AMH levels were significantly lower in group 2 compared to groups 1, 3, and 4 (p < 0.01, p < 0.01, and p = 0.04, respectively). There was no significant difference between groups 3 and 4 (p = 0.16).
Fig. 2.
a Number of follicles. Primordial, primary, secondary, antral, and atretic follicle counts of ovaries in the all groups. * p < 0.05, ** p < 0.01, *** p < 0.001, compared to group 1; +p < 0.05, ++p < 0.01, ++++p < 0.0001, compared to group 2. b Serum concentrations of AMH, c Ddx4 expression
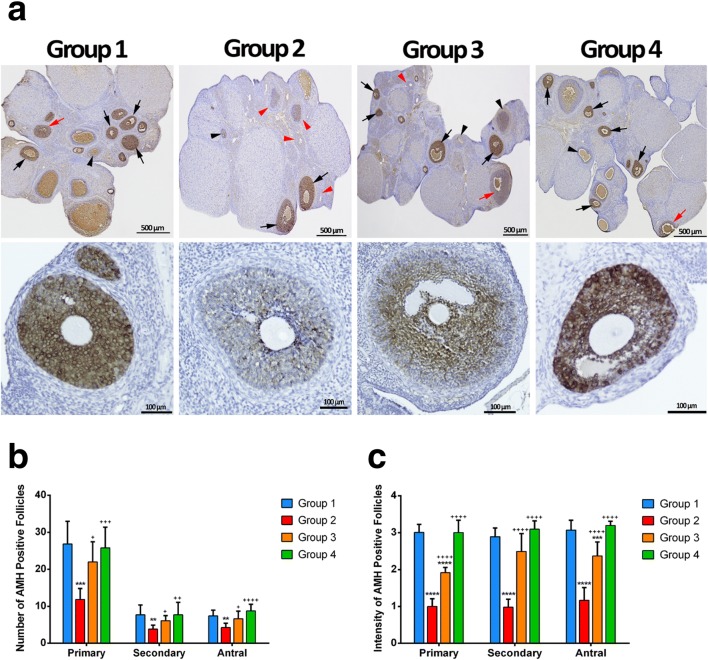
Primary, secondary, and antral AMH positive staining follicle counts and primary, secondary and antral AMH positive staining follicle intensity scores in all groups are shown in Table 1 and Figs. 2 and 3. There was a statistically significant difference in AMH-positive staining of primary, secondary, and antral follicle counts between the groups (p < 0.01) (Table 1). Group 2 showed the lowest AMH-positive staining in primary, secondary, and antral follicle count. AMH-positive staining of primary, secondary, and antral follicle count was significantly lower in group 2 compared to group 3 (p = 0.01, p = 0.04, and p = 0.01, respectively). There was no significant difference between groups 1 vs 3 and 3 vs 4 in terms of AMH-positive staining of primary, secondary, and antral follicle count. There was a statistically significant difference in primary, secondary, and antral AMH positive staining follicle intensity score between the groups (p < 0.01) (Table 1). Group 2 showed the lowest primary, secondary, and antral AMH positive staining follicle intensity score. Group 4 had the highest primary, secondary, and antral AMH positive staining follicle intensity score. Primary, secondary, and antral AMH positive staining follicle intensity scores were significantly lower in group 2 compared to group 3 (p < 0.01 for all).
Fig. 3.
Immunohistochemistry of AMH in the ovary. In the upper panel, AMH positive-stained follicles are seen in the ovary sections. In groups 1and 4, both strong (black arrow) and moderate (red arrow)-stained follicles were observed, whereas strong (black arrow), weak (black arrowhead), or non-stained (red arrowhead) follicles were observed in group 2. In group 3, all follicles stained at different intensities were observed. a In the bottom panel, different staining intensity of follicles is seen in the all groups. Primary, secondary, and antral AMH positive staining follicle counts in the all groups. b ** p < 0.01, *** p < 0.001, compared to group 1; + p < 0.05, ++ p < 0.01, +++ p < 0.01, ++++ p < 0.01, compared to group 2. Primary, secondary and antral AMH positive staining follicle intensity score in the all groups. c *** p < 0.01, **** p < 0.01, compared to group 1; ++++ p < 0.01, compared to group 2
The primordial, primary, secondary, antral, and atretic follicle counts of all groups are shown in Table 1 and Fig. 2. A significant difference was found in the primordial, primary, secondary, antral, and atretic follicle counts between all groups (p < 0.01). Group 2 has the lowest primordial, primary, secondary, and antral and the highest atretic follicle count. The atretic follicle count was significantly lower in group 3 compared to group 2 (p = 0.01). The primordial, primary, and antral follicle counts were significantly higher in group 3 compared with group 2 (p = 0.04, p = 0.01, and p = 0.02, respectively). There was no significant difference between groups 1 vs 3 and 3 vs 4 in terms of primordial, primary, antral, and atretic follicle count.
Expression of Ddx4 as an indicator of the existence of active germ stem cells was examined to confirm protective effect. Ddx4 expression in group 4 was highest compared to other groups (9.86 ± 4.39). Ddx4 was expressed at a higher level in group 4 than in group 3 but did not achieve statistically significant difference (9.86 ± 4.39 vs 7.21 ± 1.52) (p = 0.2). Ddx4 had greater expression in group 3 than group 2 that failed to reach statistical significance (7.21 ± 1.52 vs 4.34 ± 1.02) (p = 0.1) (Table 1, Fig. 2).
Discussion
The incidence of POI is recently increasing and its prevalence is up to 1–3% of females [23]. Currently, POI is one of the main diseases causing female infertility and threatening women’s health. Unfortunately, to recover ovarian function and achieve pregnancy in women with POI remains extremely controversial. Although several treatment strategies have been suggested for POI management, an optimal treatment strategy has not been clearly defined. Moreover, chemotherapy-induced POI, also known as iatrogenic POI, is a common long-term sequel of cancer treatment. Many of the mechanisms behind how chemotherapy damages the ovary remain largely unclear but it involves multiple mechanisms [24]. Chemotherapeutic drugs have different levels of ovarian toxicity and they target follicles at different developmental stages. Alkylating agents such as Cy show ovarian toxicity. They induce the depletion of ovarian reserve with their primary and secondary effect on primordial follicles [25]. Cy negatively affects angiogenesis by damaging the micro-vessel network [9, 26]. Cy may also cause diminishing primordial follicles via follicle burn out by over-activation of the dormant primordial follicles as a result of the upregulation in the PI3K/PTEN/Akt signaling pathway [27–30]. Furthermore, it results in the induction of DNA damage and/or oxidative stress that triggers the activation of apoptosis in primordial follicles [31, 32]. Eventually, the effects of Cy administration on an ovary may be associated with age-related dysfunction. Therefore, it is also required to develop new strategies for restoring damaged ovarian tissue of women with chemotherapy-induced POI effectively.
Germ cells are stored as primordial follicles in human ovarian tissue and thus, these primordial follicles may be activated under physiological conditions [33]. The activation of these primordial follicles may be the key to successful management of women with chemotherapy-induced POI [34]. Although the regenerative and repair processes of PRP in somatic tissues remain largely unclear, the growth factors produced from PRP might have multiple critical roles in ovaries via the physiologically local effects such as cell growth, proliferation, differentiation, chemotaxis, angiogenesis, the formation of the extracellular matrix, and even controlling the release of other growth factors in very close proximity to their release site [35, 36]. These growth factors may repair and recover the ovarian function. PRP may allow the follicles to self-repair after chemotherapy because ovaries have the potential for spontaneous repair [37]. Additionally, PRP elements might induce precursor cell differentiation into a mature oocyte by supplying the requisite signals. Given these mechanisms, PRP administrated into the ovaries might protect and/or restore ovarian function in Cy-induced POI and reserve as a regenerative product by releasing growth factors known to have protective effects on ovarian failure in induced POI. Therefore, PRP may have a protective role when administered together with a gonadotoxic.
Huang et al. evaluated the effect of G-CSF-mobilized peripheral blood mononuclear cells (PBMCs) combined with PRP on the restoration of ovarian function in Cy-induced POI rats. They hypothesized to accelerate the restoration of ovarian function in Cy-induced POI rats by the synergistic activation of PBMCs and PRP. Their results showed that either PBMCs in combination with PRP or PRP alone were able to restore ovarian function in the Cy-induced rat POI model, but this effect was better for PBMCs in combination with PRP due to the addictive effect of PBMCs. Therefore, they suggest that either PBMCs in combination with PRP or PRP alone may have therapeutic potential to restore ovarian function in POI women [38]. Dehghani et al. evaluated the positive effect of PRP on structural impairment of rat testis in infertile rat models induced by busulfan [39]. According to their results, PRP significantly increases the number of spermatogenic stem cells, count, motility, and tail length of the sperm and testosterone level in infertile rat models induced by busulfan. They concluded that PRP can improve the structural and functional impairment of the testis [20]. An experimental animal stereological study investigated the effect of PRP on ovarian structures in Cy-induced ovarian failure indicated that PRP has a protective effect on ovarian failure in the infertile female rat model [20]. Nevertheless, they used stereological methods to show this protective effect and they used frozen aliquots of PRP, whereas we used fresh PRP, and we also evaluated serum anti-Müllerian hormone (AMH) levels, AMH-positive granulosa cells, and gene expression analysis of Ddx4 in the current study. These study results show that PRP had a dominant positive effect on the ovarian cortex volume, pre-antral follicles number, and antral follicle diameter.
Another POI animal study assessed the effect of mesenchymal stem cell transplantation with/without the supplementation of PRP on the maintenance of ovarian function [40]. For 2 months following the transplantation, they evaluated AMH and estradiol blood levels, follicle counts by hematoxylin-eosin staining, and immunofluorescence staining, and gene expression analyses of CXCL12, BMP-4, TGF-β, and IGF-1 to show ovarian regeneration. This study demonstrated MSCs +/− PRP transplantation after POI supports recovery of the ovarian function, but they also show that a single administration of PRP was not sufficient for ovarian recovery. The co-transplantation of MSCs and PRP had better results for follicular regeneration when compared to only MSC treatment. Pantos et al. presented a case series with menopausal and POI patients. This case series included two women with POI aged 40 and 27 years and one menopausal woman aged 46 years. These patients achieved pregnancy through natural conception within 2–6 months following PRP treatment [41].
In the current study, we focused mainly on the potential protective effect of PRP on ovarian function against ovarian damage induced by Cy. In the present study, hormonal, histopathological, and immunohistochemical methods and gene expression analysis were used to detect the potential protective effect of PRP on ovarian damage induced by the Cy model. When using PRP in combination with Cy, the AMH serum level, primordial, primary, secondary and antral follicles and AMH-positive follicles dramatically increased, whereas the atretic follicle count significantly decreased. Primordial germ cells have been shown to express the specific pluripotency markers indicative of germ cell formation such as Ddx4, also known as Vasa [42]. It is expressed and localized in the plasma membrane of putative germ stem cells of postnatal ovaries. Thus, it can be used to isolate putative ovarian germ stem cells [43].
We utilized Ddx4 to detect Ddx4 positive germ cells to show the recovery of ovarian function in terms of the pool of primordial follicles. However, according to our results, the differences between group 3 and group 2, in terms of Ddx4 expression, did not achieve statistical significance. Two potential causal mechanisms are offered to explain this striking observation. The first possible reason for this may be early performed analysis following an acute exposure, while the animals were still under the effect of Cy. If the ovaries of animals were removed a few weeks after the last administration of PRP, the results could better reflect the possible beneficial effect of PRP on Ddx4 expression due to better long-term recovery. The second possible reason may be explained by challenges in dosing and schedule of PRP administration. Improvements in PRP administration, such as increasing the dose, longer duration of treatment, and/or earlier initiation of PRP administration prior to Cy exposure, may better demonstrate the possible protective role of PRP. These outcomes suggest that PRP could be effective in protecting ovarian function against ovarian damage induced by Cy. We show that a three-dose administration of PRP is sufficient for the protection of ovarian function.
In conclusion, our study may provide evidence that PRP could protect ovarian function against ovarian damage induced by Cy. It could lead to improved primordial, primary, secondary, and antral follicle numbers. Other experimental studies ought to be designed to determine the optimum dosage, schedule, and duration of PRP treatment to enhance its protective effects. Moreover, the data obtained using an experimental animal model may not predict accurate results on human reproduction directly, and future studies are needed to investigate the effect of PRP on human ovaries.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Woad KJ, Watkins WJ, Prendergast D, Shelling AN. The genetic basis of premature ovarian failure. Aust N Z J Obstet Gynaecol. 2006;46:242–244. doi: 10.1111/j.1479-828X.2006.00585.x. [DOI] [PubMed] [Google Scholar]
- 2.Shelling AN. Premature ovarian failure. Reproduction. 2010;140:633–641. doi: 10.1530/REP-09-0567. [DOI] [PubMed] [Google Scholar]
- 3.Sheikhansari G, Aghebati-Maleki L, Nouri M, Jadidi-Niaragh F, Yousefi M. Current approaches for the treatment of premature ovarian failure with stem cell therapy. Biomed Pharmacother. 2018;102:254–262. doi: 10.1016/j.biopha.2018.03.056. [DOI] [PubMed] [Google Scholar]
- 4.Sukur YE, Kivancli IB, Ozmen B. Ovarian aging and premature ovarian failure. J Turk-German Gynecol Assoc. 2014;15:190–196. doi: 10.5152/jtgga.2014.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Kasteren Y. Treatment concepts for premature ovarian failure. J Soc Gynecol Investig. 2001;8:58–S59. [PubMed] [Google Scholar]
- 6.Blumenfeld Z. Chemotherapy and fertility. Best Pract Res Clin Obstet Gynaecol. 2012;26(3):379–390. doi: 10.1016/j.bpobgyn.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Meirow D, Biederman H, Anderson RA, Wallace WH. Toxicity of chemotherapy and radiation on female reproduction. Clin Obstet Gynecol. 2010;53:727–739. doi: 10.1097/GRF.0b013e3181f96b54. [DOI] [PubMed] [Google Scholar]
- 8.Meirow D. Reproduction post-chemotherapy in young cancer patients. Mol Cell Endocrinol. 2000;169:123–131. doi: 10.1016/s0303-7207(00)00365-8. [DOI] [PubMed] [Google Scholar]
- 9.Soleimani R, Heytens E, Darzynkiewicz Z, Oktay K. Mechanisms of chemotherapy-induced human ovarian aging: double strand DNA breaks and microvascular compromise. Aging. 2011;3(8):782–793. doi: 10.18632/aging.100363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou L, Xie Y, Li S, Liang Y, Qiu Q, Lin H, et al. Rapamycin prevents cyclophosphamide-induced over-activation of primordial follicle pool through PI3K/Akt/mTOR signaling pathway in vivo. J Ovar Res. 2017;10(1):56. doi: 10.1186/s13048-017-0350-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuksel A, Bildik G, Senbabaoglu F, Akın N, Arvas M, Unal F, et al. The magnitude of gonadotoxicity of chemotherapy drugs on ovarian follicles and granulosa cells varies depending upon the category of the drugs and the type of granulosa cells. Hum Reprod. 2015;30:2926–2935. doi: 10.1093/humrep/dev256. [DOI] [PubMed] [Google Scholar]
- 12.Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85(6):638–646. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 13.Pintat J, Silvestre A, Magalon G, Gadeau AP, Pesquer L, Perozziello A, et al. Intra-articular injection of mesenchymal stem cells and platelet-rich plasma to treat patellofemoral osteoarthritis: preliminary results of a long-term pilot study. J Vasc Interv Radiol. 2017;28(12):1708–1713. doi: 10.1016/j.jvir.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Lee KS, Wilson JJ, Rabago DP, Baer GS, Jacobson JA, Borrero CG. Musculoskeletal applications of platelet-rich plasma: fad or future? AJR Am J Roentgenol. 2011;196(3):628–636. doi: 10.2214/AJR.10.5975. [DOI] [PubMed] [Google Scholar]
- 15.Bir SC, Esaki J, Marui A, Yamahara K, Tsubota H, Ikeda T, et al. Angiogenic properties of sustained release platelet-rich plasma: characterization in-vitro and in the ischemic hind limb of the mouse. J Vasc Surg. 2009;50(4):870–879. doi: 10.1016/j.jvs.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 16.Sheth U, Simunovic N, Klein G, Fu F, Einhorn TA, Schemitsch E, Ayeni OR, Bhandari M. Efficacy of autologous platelet-rich plasma use for orthopaedic indications: a meta-analysis. J Bone Joint Surg Am. 2012;94(4):298–307. doi: 10.2106/JBJS.K.00154. [DOI] [PubMed] [Google Scholar]
- 17.Li W, Enomoto M, Ukegawa M, Hirai T, Sotome S, Wakabayashi Y, Shinomiya K, Okawa A. Subcutaneous injections of platelet-rich plasma into skin flaps modulate proangiogenic gene expression and improve survival rates. Plast Reconstr Surg. 2012;129(4):858–866. doi: 10.1097/PRS.0b013e3182450ac9. [DOI] [PubMed] [Google Scholar]
- 18.Bielecki TM, Gazdzik TS, Arendt J, Szczepanski T, Krol W, Wielkoszynski T. Antibacterial effect of autologous platelet gel enriched with growth factors and other active substances: an in vitro study. J Bone Joint Surg Br Volüme. 2007;89(3):417–420. doi: 10.1302/0301-620X.89B3.18491. [DOI] [PubMed] [Google Scholar]
- 19.Cieslik-Bielecka A, Bielecki T, Gazdzik TS, Arendt J, Krol W, Szczepanski T. Autologous platelets and leukocytes can improve healing of infected high-energy soft tissue injury. Transfusion Apheresis Sci: official journal of the World Apheresis Association: official journal of the European Society for Haemapheresis. 2009;41(1):9–12. doi: 10.1016/j.transci.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Dehghani F, Aboutalebi H, Esmaeilpour T, Panjehshahin MR, Bordbar H. Effect of platelet-rich plasma (PRP) on ovarian structures in cyclophosphamide-induced ovarian failure in female rats: a stereological study. Toxicol Mech Methods. 2018;28(9):653–659. doi: 10.1080/15376516.2018.1491662. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen T, Peters H. Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil. 1968;17:555–557. doi: 10.1530/jrf.0.0170555. [DOI] [PubMed] [Google Scholar]
- 22.Yeh J, Kim BS, Peresie J. Protection against cisplatin-induced ovarian damage by the antioxidant sodium 2-mercaptoethanesulfonate (mesna) in female rats. Am J Obstet Gynecol. 2008;198(4):463.e1–463.e6. doi: 10.1016/j.ajog.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 23.Christin-Maitre S, Braham R. General mechanisms of prematüre ovarian failure and clinical check-up. Gynecol Obstet Fertil. 2008;36:857–861. doi: 10.1016/j.gyobfe.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Morgan S, Anderson RA, Gourley C, Wallace WH, Spears N. How do chemotherapeutic agents damage the ovary? Hum Reprod Update. 2012;18:525–535. doi: 10.1093/humupd/dms022. [DOI] [PubMed] [Google Scholar]
- 25.Hao X, Anastácio A, Liu K, Rodriguez-Wallberg KA. Ovarian follicle depletion induced by chemotherapy and the investigational stages of potential fertility-protective treatments-a review. Int J Mol Sci. 2019;23(19):20. doi: 10.3390/ijms20194720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ben-Aharon I, Meizner I, Granot T, Uri S, Hasky N, Rizel S, et al. Chemotherapy-induced ovarian failure as a prototype for acute vascular toxicity. Oncologist. 2012;17:1386–1393. doi: 10.1634/theoncologist.2012-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jayasinghe YL, Wallace WHB, Anderson RA. Ovarian function, fertility and reproductive lifespan in cancer patients. Expert Rev Endocrinol Metab. 2018;13:125–136. doi: 10.1080/17446651.2018.1455498. [DOI] [PubMed] [Google Scholar]
- 28.Ronnes H, Kashi O, Meirow D. Prevention of chemotherapy-induced ovarian damage. Fertil Steril. 2016;105:20–29. doi: 10.1016/j.fertnstert.2015.11.043. [DOI] [PubMed] [Google Scholar]
- 29.Kalich-Philosoph L, Roness H, Carmely A, Fishel-Bartal M, Ligumsky H, Paglin S, et al. Cyclophosphamide triggers follicle activation causing ovarian reserve ‘burn out’; AS101 prevents follicle loss and preserves fertility. Sci Transl Med. 2013;5:185ra62. doi: 10.1126/scitranslmed.3005402. [DOI] [PubMed] [Google Scholar]
- 30.Bedoschi G, Navarro PA, Oktay K. Chemotherapy-induced damage to ovary: mechanisms and clinical impact. Future Oncol. 2016;12:2333–2344. doi: 10.2217/fon-2016-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonfloni S, Di Tella L, Caldarola S, Cannata SM, Klinger FG, Di Bartolomeo C, et al. Inhibition of the c-Abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nat Med. 2009;15:1179–1185. doi: 10.1038/nm.2033. [DOI] [PubMed] [Google Scholar]
- 32.Ganesan S, Keating AF. The ovarian DNA damage repair response is induced prior to phosphoramide mustard-induced follicle depletion, and ataxia telangiectasia mutated inhibition prevents PM-induced follicle depletion. Toxicol Appl Pharmacol. 2016;292:65–74. doi: 10.1016/j.taap.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 33.Leng L, Tan Y, Gong F, Hu L, Ouyang Q, Zhao Y, Lu G, Lin G. Differentiation of primordial germ cells from induced pluripotent stem cells of primary ovarian insufficiency. Hum Reprod. 2015;30(3):737–748. doi: 10.1093/humrep/deu358. [DOI] [PubMed] [Google Scholar]
- 34.Bukovsky A. Novel methods of treating ovarian infertility in older and POF women, testicular infertility, and other human functional diseases. Reprod Biol Endocrinol. 2015;13:10. doi: 10.1186/s12958-015-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fabi S, Sundaram H. The potential of topical and injectable growth factors and cytokines for skin rejuvenation. Facial Plast Surg. 2014;30:157–171. doi: 10.1055/s-0034-1372423. [DOI] [PubMed] [Google Scholar]
- 36.Babu M, Wells A. Dermal-epidermal communication in wound healing. Wounds. 2001;13:183–189. [Google Scholar]
- 37.Herraiz S, Buigues A, Diaz-Garcia C, Romeu M, Martinez S, Gomez-Segui I, et al. Fertility rescue and ovarian follicle growth promotion by bone marrow stem cell infusion. Fertil Steril. 2018;109:908–918. doi: 10.1016/j.fertnstert.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 38.Huang Q, Liu B, Jiang R, Liao S, Wei Z, Bi Y, Liu X, Deng R, Jin Y, Tan Y, Yang Y, Qin A. G-CSF-mobilized peripheral blood mononuclear cells combined with platelet-rich plasma accelerate restoration of ovarian function in cyclophosphamide-induced POI rats. Biol Reprod. 2019;101:91–101. doi: 10.1093/biolre/ioz077. [DOI] [PubMed] [Google Scholar]
- 39.Dehghani F, Sotoude N, Bordbar H, Panjeshahin MR, Karbalay-Doust S. The use of platelet-rich plasma (PRP) to improve structural impairment of rat testis induced by busulfan. Platelets. 2019;30(4):513–520. doi: 10.1080/09537104.2018.1478400. [DOI] [PubMed] [Google Scholar]
- 40.Vural B, Duruksu G, Vural F, Gorguc M, Karaoz E. Effects of VEGF + mesenchymal stem cells and platelet-rich plasma on inbred rat ovarian functions in cyclophosphamide-induced premature ovarian insufficiency model. Stem Cell Rev. 2019;15(4):558–573. doi: 10.1007/s12015-019-09892-5. [DOI] [PubMed] [Google Scholar]
- 41.Pantos K, Simopoulou M, Pantou A, Rapani A, Tsioulou P, Nitsos N, et al. A case series on natural conceptions resulting in ongoing pregnancies in menopausal and prematurely menopausal women following platelet-rich plasma treatment. Cell Transplant. 2019;4:963689719859539. doi: 10.1177/0963689719859539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anderson RA, Fulton N, Cowan G, Coutts S, Saunders PT. Conserved and divergent patterns of expression of DAZL, VASA and OCT4 in the germ cells of the human fetal ovary and testis. BMC Dev Biol. 2007;7:136. doi: 10.1186/1471-213X-7-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo K, Li CH, Wang XY, He DJ, Zheng P. Germ stem cells are active in postnatal mouse ovary under physiological conditions. Mol Hum Reprod. 2016;22(5):316–328. doi: 10.1093/molehr/gaw015. [DOI] [PMC free article] [PubMed] [Google Scholar]