Abstract
Purpose
To identify the disease gene in 40 patients with female infertility due to oocyte maturation arrest.
Methods
Genomic DNA was extracted from peripheral blood of 40 patients and their family members. Whole-exome sequencing was performed on the patients, and the PATL2 mutations were identified and confirmed by Sanger sequencing. Harmfulness of the mutations was analyzed by SIFT, Polyphen-2, Mutation Taster, and M-CAP software, and we used western immunoblotting analysis to check the effect of mutations on PATL2 protein expression in vitro.
Results
Two novel missense mutations c.1528C>A (p.Pro510Thr) and c.1376C>A (p.Ser459Tyr) in PATL2 were identified in three patients (7.5%) from two consanguineous families in our cohort. We found that mutations in PATL2 resulted in variable oocyte phenotypes, including GV arrest, MI arrest, and morphologic abnormalities. Western immunoblotting analysis showed that the expression levels of the two novel mutant PATL2 proteins decreased significantly.
Conclusions
We identified two novel PATL2 mutations that caused oocyte maturation arrest and abnormal morphology, and variable phenotypes in patients.
Keywords: PATL2, Female infertility, Oocyte maturation arrest, GV arrest
Introduction
In 1978, the first IVF baby was born in Oldham General Hospital [1], and assisted reproductive technology has been widely used ever since in the treatment of infertility. Infertility is usually used to describe as a couple who cannot become pregnant after 12 months of regular unprotected sexual intercourse and affects approximately 15% of all couples [2, 3]. Oocyte maturation arrest is of the principal causes of primary female infertility, including GV arrest, MI arrest, MII arrest, and mixed arrest [1]. Genetic mutations constitute one of the factors in oocyte maturation arrest, although enzyme and hormonal changes can also cause maturation arrest in oocytes. Recently, it was reported that ZP1 mutations are responsible for female infertility due to a missing zona pellucida [4, 5]. TUBB8 mutations cause female infertility due to arrest at metaphase I, fertilization, or early embryonic development [6]. PATL2 mutations as a cause of female infertility are characterized by GV arrest, mixed arrest, and/or morphologic abnormalities [7–10]. Mutations in WEE2 cause female infertility and fertilization failure [11]. PANX1 channelopathies are responsible for human oocyte death [12]. However, the genetic etiology of most patients with oocyte maturation arrest remains largely unknown.
PATL2 is a mRNA-binding protein specifically expressed in immature oocytes that inhibits the post-transcriptional translation process in cells; and as the oocyte matures, the expression of PATL2 gradually disappears. The amount of PATL2 was greatly impaired by PATL2 mutations in patients with oocyte maturation arrest [8, 9], and overexpression of a PATL2 ortholog (also named as P100) in Xenopus oocytes also repressed mRNA translation and resulted in maturation arrest [13]. These results indicate that temporal control of PATL2 expression levels is critical for the normal maturation of oocytes.
Herein, we analyzed 40 unrelated Chinese patients with oocyte maturation arrest and found two novel homozygous mutations, c.1528C>A (p.Pro510Thr) and c.1376C>A (p.Ser459Tyr), in PATL2 in three patients (7.5%). Noticeably, the phenotypes of two patients from the same family carrying the same mutation (p.Pro510Thr) were different. In vitro evidence also showed that the two novel mutations in PATL2 decreased PATL2 protein content. This present study provides additional evidence that PATL2 is very important in oocyte maturation, expanding the current mutational spectrum.
Materials and methods
Study subjects
Forty individuals with primary infertility due to oocyte maturation arrest from 36 families were recruited from the Reproductive Medicine Center, Tongji Hospital, Huazhong University of Science and Technology. Peripheral blood from affected individuals and their family members were collected to extract genomic DNA using standard methods.
Evaluation of oocyte phenotypes
After obtaining oocytes from the affected individuals, we performed oocyte phenotype analysis under a light microscope to evaluate oocyte morphology and the degree of maturity. We characterized oocytes with a complete germinal vesicle (GV) to be at the GV stage, and oocytes that contained no GV or polar body to be at the metaphase I (MI) stage, and oocytes with the first polar body extruded were at metaphase II (MII) stage. Oocytes in GV and MI stages were characterized as immature [8].
Mutation analysis
We performed whole-exome capture (Agilent) and sequencing (NovaSeq 6000, Illumina) in affected individuals, and the sequencing coverage rate and target mean depth were 98.77% and 166.65, respectively. We mapped the raw FASTQ files to the human reference sequence (NCBI Genome build GRCh37), and SNP and InDel were searched using software such as SAMtools and GATK, and then annotated with ANNOVAR software to determine the genetic information, functional information, and harmfulness of the mutation site. The conservation of the affected amino acid residues of the PATL2 protein was analyzed among multiple species using MEGA software; the ExAC and 1000 Genomes databases were used to search for the frequency of the corresponding mutation, and we analyzed the harmfulness of mutations by using Sorting Intolerant From Tolerant (SIFT, http://sift.bii.a-star.edu.sg/), Polymorphism Phenotyping (Polyphen-2, http://genetics.bwh. harvard.edu/pph2/), Mutation Taster (http://www.mutaiontaster.org/), and Mendelian Clinically Applicable Pathogenicity (M-CAP, http://bejerano.stanford.edu/MCAP). We amplified all exons and exon-intron boundaries of PATL2 by PCR using reference primers [9]. PCR products were purified using a Gel Extraction Kit (Beijing CoWin Biotech Co., Ltd., China), and then Sanger sequencing was carried out using an ABI PRISM 3500 Genetic Analyzer (Applied Biosystems, Foster City, CA) for all exons and exon-intron boundaries of PATL2.
Expression constructs
The wild-type PATL2 cDNA (a gift from Qing Sang at Fudan University) was inserted into the pEGFP-C1 vector for transient expression in HEK293T cells. The P510T and S459Y mutant plasmids were generated using the Mut Express II Fast Mutagenesis Kit V2 (Vazyme Biotech Co., Ltd) according to the manufacturer’s protocol. All expression constructs were confirmed by Sanger DNA sequencing.
Western blotting analysis
The P510T, S459Y mutant, and wild-type PATL2 expression plasmids were transiently transfected into HEK293T cells using Lipofectamine 2000 (Invitrogen, USA) to evaluate the effects of the mutations on PATL2 protein expression. Thirty-six hours after transfection, total proteins were extracted from cell lysates by grinding in a protease inhibitor cocktail (Roche, Germany), and separated by 10% SDS/PAGE gels in a running buffer of Tris, glycine, and SDS. We transferred proteins onto a PVDF membrane (Merck KGaA, Germany). Membranes were blocked in 5% skim milk and washed 3 times in Tris-buffered saline-Tween 20 (TBS-T). The membranes were incubated with mouse anti-GFP-Tag primary antibodies (AB clonal, China) at a 1:2000 dilution overnight at 4 °C. The membranes were then washed in TBS-T and incubated with goat anti-mouse IgG HRP-linked secondary antibodies (Beijing CoWin Biotech Co., Ltd., China) at a dilution of 1:10000. We then captured the results using a ChemiDoc XRS+ System (Bio-Rad, USA) and scanned the gray values of the protein band with Quantity One software, using GAPDH as an internal control to quantify PATL2 expression. We repeated this experiment 8 times. The P values were detected using one-way ANOVA with a multiple-comparison test with 95% confidence.
Subcellular localization in HEK293T cells and real-time quantitative PCR
The P510T, S459Y mutant, and wild-type EGFP-tagged PATL2 expression constructs were transiently transfected in HEK293T cells, and after 36 h, the cells were fixed in 4% formaldehyde for 10 min at room temperature. The nuclei of cells were stained with DAPI for 10 min at room temperature and washed in phosphate-buffered saline (PBS). We ultimately captured images using a confocal microscope FV1000 (Olympus, Japan).
Total RNA was extracted using the RNAiso Plus kit (TaKaRa, China), followed by reverse-transcription with a HiScript 1st Strand cDNA Synthesis Kit (Vazyme, China), and we used ChamQ Universal SYBR qPCR Master Mix (Vazyme, China) to perform real-time quantitative fluorescence PCR. GAPDH was used as an internal control to quantify the expression of PATL2 mRNA according to the 2-ΔΔCt method.
Ethical approval
All participants in the study signed informed consent to participate in our investigation, and all studies on human subjects were approved by the Ethics Committee of Huazhong University of Science and Technology.
Results
Clinical phenotypes of patients
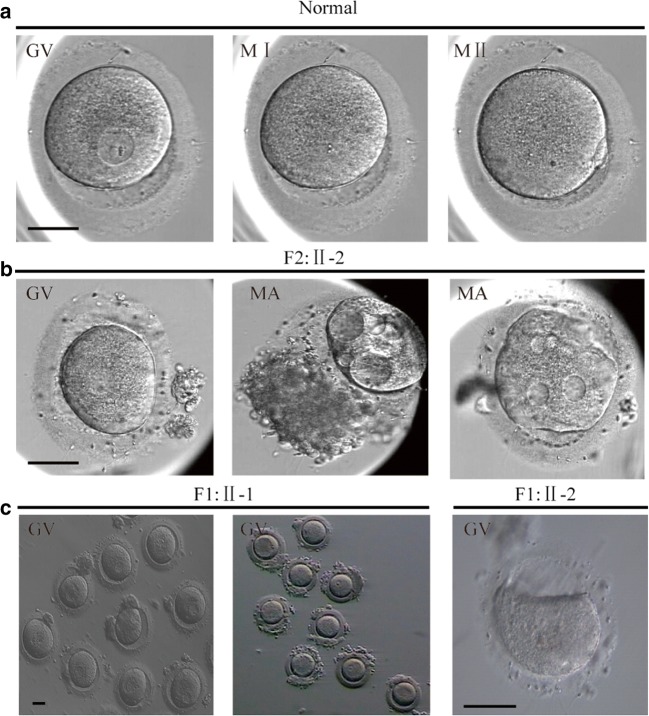
In consanguineous family 1 (F1), there were two sisters (II-1 and II-2, currently 39 and 37 years old, respectively) who both have primary infertility. Affected individual II-1 had a 15-year history of primary infertility and underwent two failed IVF cycles, with 71 oocytes retrieved: 62 were arrested at the GV stage and the remainder at MI stage, with large vacuoles, and signs of degeneration. All oocytes were immature even after 48 h in vitro. The woman’s baseline LH and FSH concentrations were 2.09 IU/L and 5.34 IU/L, respectively. Her sister (II-2) had a 13-year history of primary infertility, undergoing three failed IVF cycles, with 18 oocytes retrieved: eight were arrested at the GV stage, eight at the MI stage, and the remainder showed morphologic abnormalities. Mature oocytes were not obtained in extended culture (Fig. 1c).
Fig. 1.
Phenotypes of oocytes from patients and fertile control. a Morphology of GV, MI, and MII oocytes from a fertile control individual. b Oocytes arrested at the GV stage and morphologic abnormalities from II-2 (F2). c GV stage oocytes from II-1 and II-2 (F1). Scale bar, 50 μm. F2, Family2; F1, Family1; GV, Germinal Vesicle; MI, Metaphase I; MII, Metaphase II; MA, morphology abnormality
In consanguineous family 2 (F2), patient II-2 was a 30-year-old woman with a 2-year history of primary infertility. In May 2019, she underwent 1 IVF cycle, and seven oocytes were retrieved: two were arrested at the GV stage and five showed morphologic abnormalities (Fig. 1b). The oocytes were cultured in vitro for 48 h without maturation (Table 1).
Table 1.
Oocyte characteristics of three patients
| Age (years) | Duration of infertility (years) | IVF cycles | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Date | Oocytes retrieved (n) | GV (n) | MI (n) | MII (n) | MA (n) | Fertilized oocytes (n) | |||
| Family 1 (II-1) | 39 | 15 | 2008. 11 | 42 | 37 | 1 | 0 | 4 | 0 |
| 2009. 3 | 29 | 25 | 1 | 0 | 3 | 0 | |||
| Family 1 (II-2) | 37 | 13 | 2018. 3 | 9 | 4 | 4 | 0 | 1 | 0 |
| 2018. 8 | 4 | 3 | 0 | 0 | 1 | 0 | |||
| 2018. 10 | 5 | 1 | 4 | 0 | 0 | 0 | |||
| Family 2 (II-2) | 30 | 2 | 2019. 5 | 7 | 2 | 0 | 0 | 5 | 0 |
GV, germinal vesicle; MI, metaphase I; MII, metaphase II; MA, morphology abnormalities
All three patients manifested regular menstrual cyclicity did not possess Nessler’s cysts and showed a normal karyotype (46, XX), and the respective husbands exhibited normal seminal parameters. All patients’ case data were from the Reproductive Medicine Center, Tongji Hospital, Huazhong University of Science and Technology.
Identification of PATL2 mutations
We performed whole-exome sequencing in the patients from the two consanguineous families described above. As the pattern of inheritance is recessive in patients from consanguineous families, the infertile condition will be caused by homozygous mutations in the disease gene. We applied stringent screening criteria according to mutation types, allele frequency (< 1%) and gene function [4, 8]; only found a homozygous mutation in a known disease gene PATL2 in three patients. There were no mutations identified in any other disease genes related to female infertility or oocyte development.
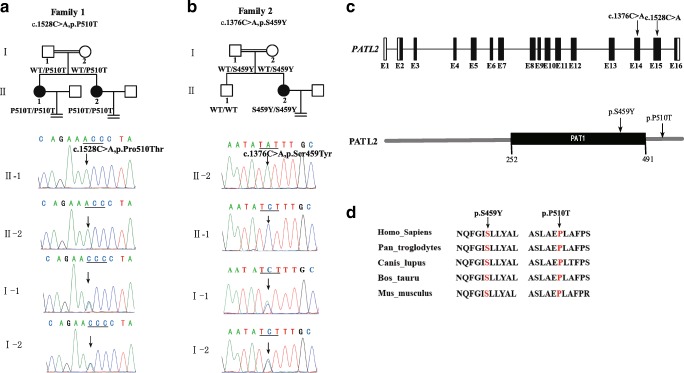
We found a novel homozygous missense mutation (c.1528C>T; p.P510T) in PATL2 in two patients (II-1 and II-2) in consanguineous F1, with genotype and phenotype co-segregated in F1 (Fig. 2a). This mutation was located at the C-terminal of the PATL2 protein (Fig. 2c) and may affect protein stability.
Fig. 2.
Identification of mutations in PATL2. The patients in family1 (a) and family2 (b) carried 2 different missense mutations: c.1528C>T (p.P510T) and c.1376C>A (p.S459Y), respectively. Black circles represent patients. c Location of 2 novel homozygous missense mutations in the PATL2 gene and protein. We predicted the domain of the PATL2 protein using SMART software. d The conservation of P510 and S459 among multiple species
In consanguineous F2, we identified another novel homozygous missense mutation in PATL2 (c.1376C>A; p.S459Y) using whole-exome sequencing, and this was confirmed by Sanger sequencing (Fig. 2b). We did not find mutations in any other known disease gene related to female infertility, nor did we find mutations in other genes that are known to be involved in oocyte development. S459 is located in the PAT1 domain (Fig. 2c) involved in RNA binding, and the mutation might affect this binding. The patient’s mother and grandmother (not shown in the study) are heterozygous for the mutation. P510 and S459 are also known to be highly conserved among multiple species (Fig. 2d). The allele frequencies of two mutational sites were not found in the ExAc or 1000 Genomes databases. The SIFT, Polyphen-2, Mutation Taster, and M-CAP predictions indicated that these two mutations were harmful (Table 2).
Table 2.
Summary of PATL2 mutation analysis in two consanguineous families
| Chromosome 15 | Mutation | Exon | Mutation type | ExAc allele frequency | SIFTa | Polyphen-2a | Mutation Tastera | M-CAPa | |
|---|---|---|---|---|---|---|---|---|---|
| Family 1 (II-1 and II-2) | 44958675 | c.1528C>T p.P510T | 15 | Missense | Not found | D | P | D | D |
| Family 2 (II-2) | 44959391 | c.1376C>A p.S459Y | 14 | Missense | Not found | D | P | D | D |
aAnalysis of the harmfulness of mutations by using Sorting Intolerant From Tolerant (SIFT), Polymorphism Phenotyping (Polyphen-2), Mutation Taster, and Mendelian Clinically Applicable Pathogenicity (M-CAP). D, damaging; P, probably damaging
Expression level of PATL2 protein and subcellular localization in HEK293T cells
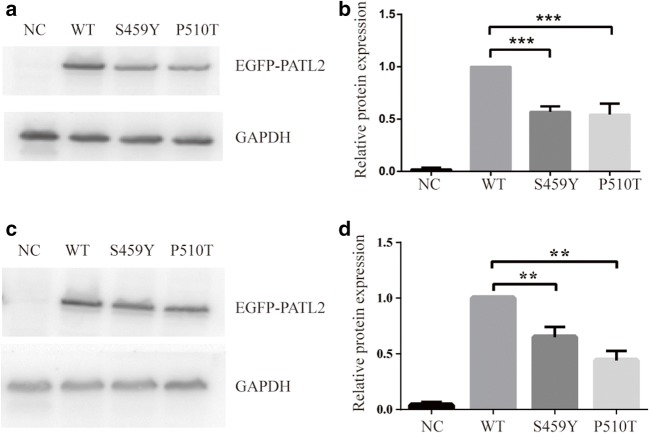
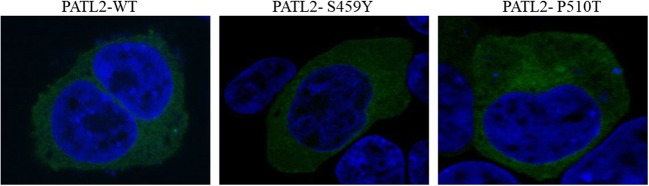
To assess whether the two novel mutations affected the expression of PATL2 protein in vitro, the P510T, S459Y mutant, and wild-type EGFP-tagged PATL2 expression constructs were transiently expressed in HEK293T and COS-7 cells. When we evaluated the expression of the protein by western blotting analysis (Fig. 3a and c), we showed that P510T and S459Y mutations in PATL2 significantly decreased the expression of PATL2 protein (Fig. 3b and d). By RT-PCR, we also found that there was no significant difference in mutation levels of PATL2 mRNA compared to WT PATL2 in transfected HEK293T cells (36 h) (Fig. 5). Therefore, the mutation in P510T and S459Y did not affect mRNA, while affecting the level of PATL2 protein expression. There was no difference in subcellular localization patterns of the WT and PATL2 mutation, which were both widely distributed in the cytoplasm of transfected HEK293T cells (Fig. 4).
Fig. 3.
Expression level of PATL2 protein in vitro. Western immunoblotting analysis showed the expression levels of WT and mutant PATL2 protein in HEK293T cells (a) and COS-7 cells (c). The relative expression levels of WT, P510T, and S459Y PATL2 protein in HEK293T (b) and COS-7 cells (d). ***P < 0.0001, **P < 0.001, n = 8 (b) and n = 3 (d). NC, negative control
Fig. 5.
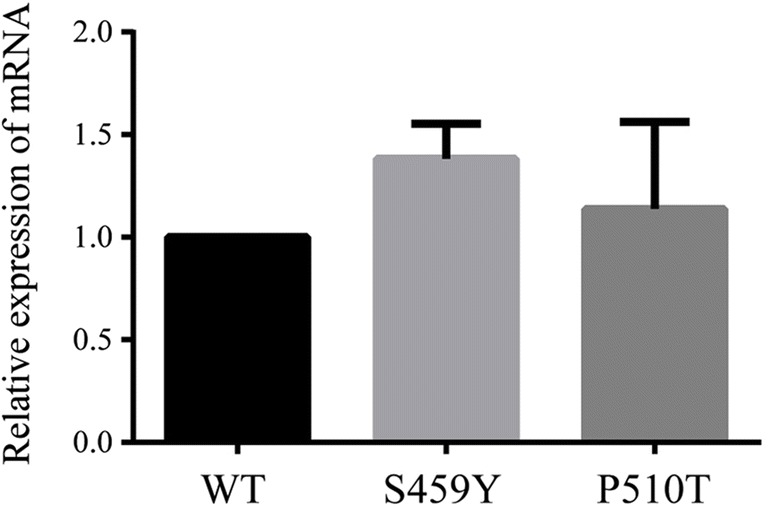
The relative expression levels of PATL2 mRNA. Real-time quantitative fluorescence PCR was performed to detect the relative expression levels of WT, S459Y, and P510T PATL2 mRNAs 36-h post-transfection in HEK293T cells
Fig. 4.
The subcellular localization patterns of PATL2 protein. PEGFP-C1-PATL2 (WT, P510T, and S459Y) plasmids were transfected into HEK293T cells. After 36 h, cells were fixed in 4% formaldehyde, and then the nuclei of the cells were stained with DAPI (blue color)
Discussion
In the present study, we identified two novel homozygous missense mutations (P510T and S459Y) in the PATL2 gene, in three patients from two consanguineous families that exhibited oocyte maturation arrest. P510 and S459 are highly conserved among multiple species. Chen et al. reported that the PATL2 heterozygous mutations L189R and Y217N resulted in significant attenuation in the expression of PATL2 protein in oocytes [8]. These authors also demonstrated that the I318T mutation resulted in significantly diminished expression of PATL2 protein in cultured cell lines [8], most likely due to decreased stability of the PATL2 protein, resulting in its degradation. In our study, S459Y and P510T mutations in PATL2 (similar to that for I318T) likely led to PATL2 protein degradation due to reduced stability of the protein, leading to a reduced level of PATL2 expression [8], and ultimately resulting in oocyte maturation arrest. S459Y and P510T mutations in PATL2, however, did not affect the levels of PATL2 mRNA or the subcellular localization patterns of the PATL2 protein (Figs. 4 and 5). We therefore speculate that the main mechanism of mutations in the PATL2 gene reduces the level of expression protein PATL2. Our three patients demonstrated significant differences in phenotypes, which might be explained by phenotypic heterogeneity.
A PATL2 ortholog (also called P100) is specifically expressed in immature Xenopus oocytes, and gradually disappears during oocyte maturation [13]. Overexpression of P100 in Xenopus oocytes inhibited the accumulation of c-Mos and cyclin B1 (which are involved in cell cycle regulation), and led to oocyte maturation arrest [13]. Female PATL2 knock-out mice were fertile but fertility was significantly reduced; GV stage oocytes were significantly smaller than those from wild-type mice, and significant morphologic abnormalities were also observed in MII oocytes [14]. RNA-seq indicated a significant down-regulation of gene expression associated with oocyte maturation, including CDC25a and SohIh2, in PATL2-deficient mouse oocytes [14]. Human PATL2 (similar to P100) was also abundantly expressed in immature human oocytes, and some PATL2 mutations in patients with oocyte maturation arrest resulted in decreased amounts of PATL2 protein [8]. The precise regulation of PATL2 expression levels is important to oocyte maturation. For example, in the present study, the P510T and S459Y mutations decreased PATL2 protein expression in vitro potentially leading to a cascade of abnormal expression of genes involved in the normal maturation of oocytes resulting in arrest of oocyte maturation. However, the exact molecular mechanism whereby a PATL2 mutation causes oocyte maturation arrest remains largely unknown and must be investigated further in the future.
To our knowledge, there are only five extant publications reported that describe mutations in PATL2 causing female infertility with oocyte maturation arrest [7–10, 14]. Sixty-eight percent of the disease-causing mutations in PATL2 are concentrated in the PAT1 domain (amino acids [aa] 252-491) of the PATL2 protein, responsible for mRNA binding; 32% of mutations exist in the N-terminal region (aa 1-252), and no mutations have been reported at the C-terminus (aa 491-543) (Fig. 2c). Two novel mutations were reported in our study, one of which was located in the PAT1 domain and the other mutation (P510T) is the first mutation reported at the C-terminus. Although many genes have been reported to be involved in oocyte maturation arrest in mice and Xenopus, there are no reports of homologous mutations in human genes (including Cdc25b, Pde3a, CDK1, MLH1, MEI1, CKS2, LFNG, and CCNB3) [15–22]. Additionally, we did not find genetic causes for the remaining 92.5% of patients in our cohort, as there are very likely other novel disease-causing genes involved in female infertility.
In conclusion, we identified two novel mutations in the PATL2 gene and found that patients who carried the same PATL2 mutation (P510T) also showed different phenotype: GV arrest (F1, II-1), and GV arrest and MI arrest (F1, II-2). We expect that our work will prove to be very useful in the diagnosis of certain infertile conditions, and to provide a basis for future treatment.
Acknowledgments
We would like to thank Sang Qing and Lei Wang from Fudan University for providing the gift of PATL2 cDNA. We also thank all patients for their participation.
Funding information
This work was supported by grants from the National Natural Science Foundation of China (81000079, 81170165, and 81870959 to X.Z.) and supported by Program for HUST Academic Frontier Youth Team (2016QYTD02).
Compliance with ethical standards
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhenxing Liu, Lixia Zhu, Jiarui Wang and Geng Luo contributed equally to this work.
Contributor Information
Lei Jin, Email: leijintongjih@qq.com.
Xianqin Zhang, Email: xqzhang04@hust.edu.cn.
References
- 1.Beall S, Brenner C, Segars J. Oocyte maturation failure: a syndrome of bad eggs. Fertil Steril. 2010;94:2507–2513. doi: 10.1016/j.fertnstert.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, et al. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril. 2013;99:1324–1331. doi: 10.1016/j.fertnstert.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gurunath S, Pandian Z, Anderson RA, Bhattacharya S. Defining infertility-a systematic review of prevalence studies. Hum Reprod Update. 2011;17:575–588. doi: 10.1093/humupd/dmr015. [DOI] [PubMed] [Google Scholar]
- 4.Zhou Z, Ni C, Wu L, Chen B, Xu Y, Zhang Z, Mu J, Li B, Yan Z, Fu J, Wang W, Zhao L, Dong J, Sun X, Kuang Y, Sang Q, Wang L. Novel mutations in ZP1, ZP2, and ZP3 cause female infertility due to abnormal zona pellucida formation. Hum Genet. 2019;138:327–337. doi: 10.1007/s00439-019-01990-1. [DOI] [PubMed] [Google Scholar]
- 5.Huang HL, Lv C, Zhao YC, Li W, He XM, Li P, Sha AG, Tian X, Papasian CJ, Deng HW, Lu GX, Xiao HM. Mutant ZP1 in familial infertility. N Engl J Med. 2014;370:1220–1226. doi: 10.1056/NEJMoa1308851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng R, Sang Q, Kuang Y, Sun X, Yan Z, Zhang S, Shi J, Tian G, Luchniak A, Fukuda Y, Li B, Yu M, Chen J, Xu Y, Guo L, Qu R, Wang X, Sun Z, Liu M, Shi H, Wang H, Feng Y, Shao R, Chai R, Li Q, Xing Q, Zhang R, Nogales E, Jin L, He L, Gupta ML Jr, Cowan NJ, Wang L. Mutations in TUBB8 and human oocyte meiotic arrest. N Engl J Med. 2016;374:223–232. doi: 10.1056/NEJMoa1510791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maddirevula S, Coskun S, Alhassan S, Elnour A, Alsaif HS, Ibrahim N, Abdulwahab F, Arold ST, Alkuraya FS. Female infertility caused by mutations in the oocyte-specific translational repressor PATL2. Am J Hum Genet. 2017;101:603–608. doi: 10.1016/j.ajhg.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen B, Zhang Z, Sun X, Kuang Y, Mao X, Wang X, Yan Z, Li B, Xu Y, Yu M, Fu J, Mu J, Zhou Z, Li Q, Jin L, He L, Sang Q, Wang L. Biallelic mutations in PATL2 cause female infertility characterized by oocyte maturation arrest. Am J Hum Genet. 2017;101:609–615. doi: 10.1016/j.ajhg.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu L, Chen H, Li D, Song D, Chen B, Yan Z, Lyu Q, Wang L, Kuang Y, Li B, Sang Q. Novel mutations in PATL2: expanding the mutational spectrum and corresponding phenotypic variability associated with female infertility. J Hum Genet. 2019;64:379–385. doi: 10.1038/s10038-019-0568-6. [DOI] [PubMed] [Google Scholar]
- 10.Huang L, Tong X, Wang F, Luo L, Jin R, Fu Y, Zhou G, Li D, Song G, Liu Y, Zhu F. Novel mutations in PATL2 cause female infertility with oocyte germinal vesicle arrest. Hum Reprod. 2018;33:1183–1190. doi: 10.1093/humrep/dey100. [DOI] [PubMed] [Google Scholar]
- 11.Sang Q, Li B, Kuang Y, Wang X, Zhang Z, Chen B, Wu L, Lyu Q, Fu Y, Yan Z, Mao X, Xu Y, Mu J, Li Q, Jin L, He L, Wang L. Homozygous mutations in WEE2 cause fertilization failure and female infertility. Am J Hum Genet. 2018;102:649–657. doi: 10.1016/j.ajhg.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sang Q, Zhang Z, Shi J, Sun X, Li B, Yan Z, et al. A pannexin 1 channelopathy causes human oocyte death. Sci Transl Med. 2019;11:485. doi: 10.1126/scitranslmed.aav8731. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura Y, Tanaka KJ, Miyauchi M, Huang L, Tsujimoto M, Matsumoto K. Translational repression by the oocyte-specific protein P100 in Xenopus. Dev Biol. 2010;344:272–283. doi: 10.1016/j.ydbio.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Christou-Kent M, Kherraf ZE, Amiri-Yekta A, Le Blévec E, Karaouzène T, Conne B, et al. PATL2 is a key actor of oocyte maturation whose invalidation causes infertility in women and mice. EMBO Mol Med. 2018;10:e8515. doi: 10.15252/emmm.201708515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lincoln AJ, Wickramasinghe D, Stein P, Schultz RM, Palko ME, De Miguel MP, et al. Cdc25b phosphatase is required for resumption of meiosis during oocyte maturation. Nat Genet. 2002;30:446–449. doi: 10.1038/ng856. [DOI] [PubMed] [Google Scholar]
- 16.Masciarelli S, Horner K, Liu C, Park SH, Hinckley M, Hockman S, Nedachi T, Jin C, Conti M, Manganiello V. Cyclic nucleotide phosphodiesterase 3A-deficient mice as a model of female infertility. J Clin Invest. 2004;114:196–205. doi: 10.1172/JCI21804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frank-Vaillant M, Jessus C, Ozon R, Maller JL, Haccard O. Two distinct mechanisms control the accumulation of cyclin B1 and Mos in Xenopus oocytes in response to progesterone. Mol Biol Cell. 1999;10:3279–3288. doi: 10.1091/mbc.10.10.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woods LM, Hodges CA, Baart E, Baker SM, Liskay M, Hunt PA. Chromosomal influence on meiotic spindle assembly: abnormal meiosis I in female Mlh1 mutant mice. J Cell Biol. 1999;145:1395–1406. doi: 10.1083/jcb.145.7.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Libby BJ, De La Fuente R, O'Brien MJ, Wigglesworth K, Cobb J, Inselman A, et al. The mouse meiotic mutation mei1 disrupts chromosome synapsis with sexually dimorphic consequences for meiotic progression. Dev Biol. 2002;242:174–187. doi: 10.1006/dbio.2001.0535. [DOI] [PubMed] [Google Scholar]
- 20.Spruck CH, de Miguel MP, Smith AP, Ryan A, Stein P, Schultz RM, Lincoln AJ, Donovan PJ, Reed SI. Requirement of Cks2 for the first metaphase/anaphase transition of mammalian meiosis. Science. 2003;300:647–650. doi: 10.1126/science.1084149. [DOI] [PubMed] [Google Scholar]
- 21.Hahn KL, Johnson J, Beres BJ, Howard S, Wilson-Rawls J. Lunatic fringe null female mice are infertile due to defects in meiotic maturation. Development. 2005;132:817–828. doi: 10.1242/dev.01601. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Wang L, Zhang L, He Z, Feng G, Sun H, Wang J, Li Z, Liu C, Han J, Mao J, Li P, Yuan X, Jiang L, Zhang Y, Zhou Q, Li W. Cyclin B3 is required for metaphase to anaphase transition in oocyte meiosis I. J Cell Biol. 2019;218:1553–1563. doi: 10.1083/jcb.201808088. [DOI] [PMC free article] [PubMed] [Google Scholar]