Abstract
Purpose
The majority of data regarding oocyte cryopreservation (OC) outcomes focuses on healthy women. We compare trends, cycle characteristics, and outcomes between women freezing oocytes for fertility preservation due to cancer versus elective and other medical or fertility-related diagnoses.
Methods
Retrospective cohort using national surveillance data includes all autologous OC cycles between 2012 and 2016. Cycles were divided into 4 distinct groups: cancer, elective, infertility, and medically indicated. We calculated trends and compared cycle and outcome characteristics between the 4 groups. We used multivariable log-binomial models to estimate associations between indication and gonadotropin dose, hyperstimulation, and cancelation and used Poisson regression models to estimate associations between indication and oocyte yield and maturity.
Results
The study included 29,631 autologous OC cycles. Annual total (2925 to 8828) and cancer-related (177 to 504) cycles increased over the study period; the proportions remained constant. Compared to elective, cancer-related cycles were more likely to be performed among women < 35 years old, with higher BMI, living in the South, using an antagonist protocol. Compared to elective OC cycles, gonadotropin dose (aRR 0.89, 95%CI 0.80–0.99), cancelation (aRR 0.90, 95%CI 0.70–1.14), and hyperstimulation (aRR 1.46, 95%CI 0.77–2.29) were not different for cancer-related cycles. Oocyte yield and percent maturity were comparable in both groups.
Conclusion
The number of OC cycles among women with cancer has increased; however, the percentage OC cycles for cancer have remained stable. While patient demographic characteristics were different among those undergoing OC for cancer indication, cycle outcomes were comparable to elective OC. The outcomes of the subsequent oocyte thaw, fertilization, and embryo transfer cycles remain unknown.
Electronic supplementary material
The online version of this article (10.1007/s10815-020-01715-8) contains supplementary material, which is available to authorized users.
Keywords: Cancer, Fertility preservation, Oocyte (egg) freezing, Oocyte maturity, Outcomes
Introduction
With increasing awareness of the importance of future fertility counseling at the time of cancer diagnosis and the availability of oocyte cryopreservation (OC) as a fertility preservation option, more reproductive-aged women diagnosed with cancer have the opportunity to consider freezing oocytes [1, 2], and the total number of oocyte cryopreservation cycles in the USA has increased annually [3].
Unlike embryo freezing, OC allows women to preserve fertility independent of relationship status and broadens the pool of individuals who may be candidates for fertility preservation. As OC is not covered by insurance in most states and may require a delay in cancer treatment, women considering oocyte freezing must actively weigh the risks and benefits of moving forward with preservation. Currently, the majority of data regarding stimulation outcomes for oocyte cryopreservation focuses on women electively freezing eggs for nonmedical indications, termed as planned fertility delay [4, 5]. However, there is limited information on whether women with active cancer respond differently to controlled ovarian stimulation compared to their healthy counterparts.
Studies published to date that aim to better characterize oocyte freezing among cancer patients are limited by relatively small sample sizes and heterogeneous comparison groups [5]. A 2018 meta-analysis that included 10 studies (713 women with cancer who underwent 722 cycles) revealed no impact of cancer diagnosis on oocyte yield between women diagnosed with cancer and controls [6]. However, the majority of the comparison groups in the included studies were comprised of women undergoing in vitro fertilization (IVF) for tubal factor or male factor infertility rather than women electively freezing eggs [6]. A recent small single-center study compared outcomes between newly diagnosed breast cancer patients and elective OC cycles and found no differences in oocyte yield [7]. In contrast, another recent small retrospective cohort study comparing outcomes between oocyte cryopreservation for cancer and IVF for male factor infertility found cancer-associated cycles to be associated with higher medication doses and increased likelihood of cancelation [8]. Data regarding outcomes following oocyte thaw and fertilization are scant; a small single-center study from 2016 revealed comparable live birth rates following frozen embryo transfers using cryopreserved oocytes comparing those who cryopreserved for cancer versus those who cryopreserved electively [9].
In order to better assess this question, we used national surveillance data to report trends in cancer-related and elective OC from 2012, when national surveillance of oocyte cryopreservation cycle collection began, through 2016. We compare cycle and outcome characteristics between women freezing oocytes due to a recent cancer diagnosis and those freezing eggs for elective reasons. We also compare cancer-related OC cycle outcomes to those performed for other medical or fertility-related diagnoses.
Materials and methods
The data used for this study were derived from the Society for Assisted Reproductive Technology Clinical Outcomes Reporting System (SART CORS), a national surveillance system that has been collecting assisted reproductive technology (ART) patient and cycle characteristics and outcomes data since 1985 [10]. Reporting of all ART procedures in the USA to the Centers for Disease Control and Prevention has been federally mandated since 1992 [11]. Currently, SART CORS data encompasses 80% of all fertility clinics in the USA, and approximately 95% of all ART cycles performed in the USA. Data are validated annually with select clinics undergoing in-person on-site chart review. The primary purpose of SART CORS is pregnancy outcome surveillance. As a result, the most recent complete cycles are from 2016.
We included autologous oocyte cryopreservation cycles (ovarian stimulation, oocyte retrieval, and oocyte cryopreservation only) from SART CORS that occurred between 2012 and 2016. Oocyte thaw, fertilization, and subsequent embryo transfers were not included. Donor oocyte cryopreservation cycles and embryo cryopreservation cycles were excluded. Included cycles were divided into 4 distinct groups by indication: (1) cancer only, (2) elective only (planned fertility delay), (3) infertility-indicated, and (4) medically indicated. If more than one indication was reported, the cycle was placed in the most heterogeneous group; both the cancer only and elective groups are as clean as possible in that these patients did not have a secondary diagnosis. Cancer only cycles specifically indicated a cancer diagnosis or “banking prior to gonadotoxic treatment” as the reason for cryopreservation. Elective only cycles were specifically indicated as having been performed for elective, social family planning reasons. Infertility-related cycles are those in which an infertility diagnosis, such as ovulatory disorder or tubal factor, was assigned. The medically indicated group included medical reasons for preserving fertility such as Fragile X carrier status, history of prior oophorectomy, and BRCA carrier status (see Table 1). Multiple imputations were used to characterize cycles with a missing indication for fertility preservation (32.3%), race/ethnicity (47.2%), and body mass index (22.0%). Five imputed datasets were used, utilizing the fully conditional specification method. The distribution of those missing an indication for OC is shown in Supplemental Table 1. Variables for age at cryopreservation, body mass index, stimulation protocol, geographic region of residence, and race/ethnicity were included in the imputation procedure. The robustness of the multiple imputation approach has been previously validated [12, 13].
Table 1.
Group categorization
| Group | Name | Description |
|---|---|---|
| 1 | Cancer | Cancer group* |
| 2 | Elective/family planning preservation | Elective, planned fertility delay preservation group |
| 3 | Infertility | Infertility diagnosis listed including endometriosis, ovulatory dysfunction, polycystic ovary syndrome, tubal disease or occlusion, recurrent pregnancy loss, functional hypothalamic amenorrhea, male factor infertility, unexplained infertility, need for preimplantation genetic testing, premature ovarian failure, secondary amenorrhea, uterine septum, or other malformation |
| 4 | Medical | Medical indication listed, including diminished ovarian reserve, Turner syndrome, Fragile X, prior oophorectomy, BRCA, diabetes, hyperprolactinemia, lupus, same sex couple, gender dysphoria, seizure disorder, history of organ transplantation, Mayer-Rokitansky-Küster-Hauser syndrome, HIV discordance, migraine, IgA nephropathy, nephrotic kidney disease, thalassemia, cervical stenosis, hypothyroidism, balanced translocation or other genetic mutation, aplastic anemia, heart disease, autoimmune hepatitis, Addison’s disease, Fanconi’s anemia, multiple sclerosis, psoriatic arthritis, unspecified immunological disease, antiphospholipid syndrome without RPL or other thrombophilia, history of hysterectomy, bipolar disorder, Wegener’s granulomatosis, Wilson’s disease, stiff person syndrome, chronic fatigue syndrome, sickle cell disease, history of fetal anomaly, and unspecified medical egg freeze |
•Top 2 most common indications in bold
*The most common cancer diagnoses were (1) unspecified cancer – “pretreatment before chemotherapy,” “cancer,” “oncofertility” (n ~ 800). (2) Breast cancer (~550). (3) Lymphoma/leukemia (~ 250)
Trends in absolute number and proportion of cycles within each group were analyzed over time using linear regression. Chi-squared analyses were used to compare cycle and outcome characteristics between the 4 groups. Subsequently, multivariable log-binomial models were used to estimate pooled unadjusted and adjusted risk ratios (aRRs) and 95% confidence intervals (CIs) for associations between indication for cryopreservation and ovarian hyperstimulation syndrome, gonadotropin dose, and cycle cancelation. Poisson regression models were used to estimate oocyte yield among non-canceled cycles. Models controlled for age at cryopreservation, body mass index (BMI), stimulation protocol, geographic region of residence, and race/ethnicity. Variables were selected a priori based on clinical evidence that each could impact outcomes. Elective cryopreservation cycles were the referent group as these cycles included women without known infertility or disease. p values < 0.05 where considered statistically significant. SAS 9.4 software was used for all analyses.
This study was reviewed and deemed exempt by the Emory University Institutional Research Board.
Results
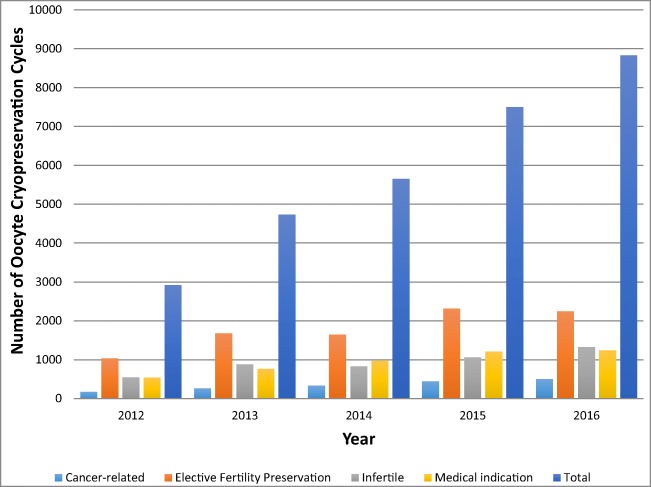
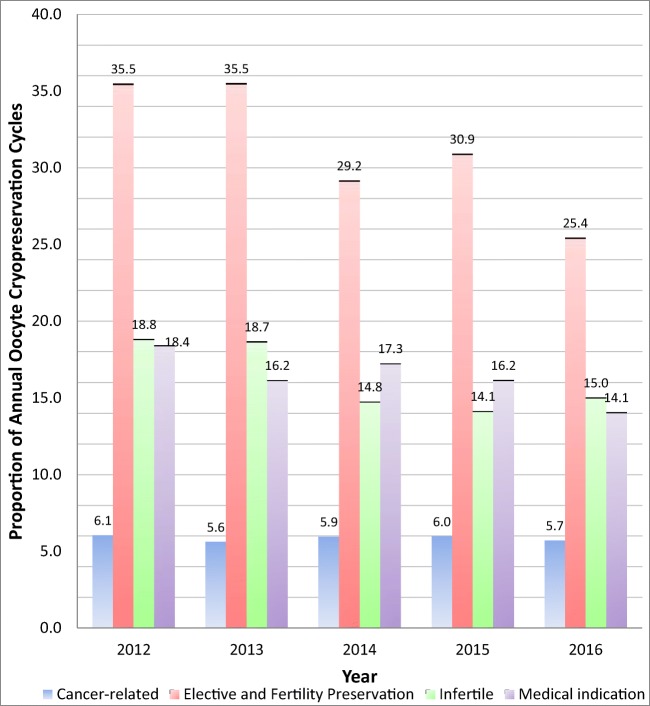
Between 2012 and 2016, 29,631 autologous oocyte cryopreservation cycles were reported to SART CORS. The total number of OC cycles performed for any indication increased from 2925 to 8828 cycles from 2012 to 2016 (Fig. 1). The number of cancer-related cycles increased from 177 to 504 cycles and comprised a similar proportion (range 5.6–6.1% annually) of all OC cycles performed in the USA over the study period (Fig. 2). Elective/Social family planning cycles increased from 1038 to 2246 but comprised a significantly smaller proportion of all OC cycles performed, decreasing from 35.5 to 25.4% (p = 0.03). Medically indicated cycles increased from 539 to 1242 in the same period. There was a nonsignificant decrease in the proportion of cycles performed for a medical indication from 18.4% to 14.1% of total cycles (p = 0.07). Combined non-cancer indications (groups 2–4) increased nonsignificantly from 2209 (75.5%) to 7082 (80.2%) cycles (p = 0.09).
Fig. 1.
National trends in absolute number of oocyte cryopreservation by indication, 2012–2016
Fig. 2.
National trends in proportion of oocyte cryopreservation by indication, 2012–2016
Compared to purely elective OC cycles, cycles completed for a cancer diagnosis were more likely to be performed among women under 35 years old, with a higher BMI, living in the South, and were more likely to use an antagonist protocol (Table 2, Supplemental Table 1). Compared to all other groups, OC cycles performed for medical indications were more likely to be in individuals > 38 years, in individuals who reside in the northeast, to use a non-gonadotropin protocol, to be canceled, and to retrieve 5 or fewer eggs. Cycles performed in individuals with an infertility diagnosis were generally similar to the elective family planning group but did tend to yield fewer oocytes.
Table 2.
Oocyte cryopreservation patient and cycle characteristics: cancer-related versus elective versus infertile versus medically indicated cycles, 2012–2016 (imputed data)a
| Cancer (group 1) | Elective and social family planning (group 2) | Other infertile (group 3) | Medical indication (group 4) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | p valueb | |
| 2715 | 9.16 | 13,626 | 45.99 | 6863 | 23.16 | 6427 | 21.69 | ||
| Female Characteristics | |||||||||
| Age (years) | < 0.0001 | ||||||||
| < 35 | 1722 | 63.43 | 4086 | 29.99 | 1794 | 26.14 | 896 | 13.94 | |
| 35–37 | 591 | 21.78 | 4898 | 35.95 | 2160 | 31.46 | 1494 | 23.24 | |
| 38–40 | 310 | 11.42 | 3609 | 26.49 | 19,145 | 27.90 | 1997 | 31.07 | |
| 41–42 | 61 | 2.25 | 753 | 5.52 | 542 | 7.89 | 935 | 14.55 | |
| >42 | 30 | 1.12 | 280 | 2.05 | 453 | 6.59 | 1105 | 17.19 | |
| Body Mass Index (kg/m2) | 0.020 | ||||||||
| <18.5 | 144 | 5.31 | 639 | 4.69 | 313 | 4.56 | 267 | 4.15 | |
| 18.5–24.9 | 1637 | 60.30 | 9429 | 69.20 | 4466 | 65.07 | 4606 | 71.66 | |
| 25–29.9 | 578 | 21.28 | 2441 | 17.91 | 1386 | 20.20 | 1086 | 16.89 | |
| > 30.0 | 356 | 13.11 | 1117 | 8.20 | 698 | 10.17 | 469 | 7.29 | |
| Geographic Location (defined by US Census data) | < 0.0001 | ||||||||
| Northeast | 828 | 30.48 | 5199 | 38.16 | 2571 | 37.47 | 3253 | 50.62 | |
| South | 836 | 30.81 | 3008 | 22.08 | 1501 | 21.86 | 1100 | 17.11 | |
| West | 683 | 25.16 | 4198 | 30.81 | 2330 | 33.96 | 1603 | 24.95 | |
| Midwest | 368 | 13.55 | 1220 | 8.96 | 461 | 6.71 | 471 | 7.32 | |
| Race/ethnicity | 0.0074 | ||||||||
| Non-Hispanic White | 2008 | 73.97 | 9450 | 69.35 | 4353 | 63.43 | 4077 | 63.44 | |
| Non-Hispanic Black | 191 | 7.03 | 819 | 6.01 | 575 | 8.38 | 457 | 7.11 | |
| Asian/Pacific Islander | 329 | 12.12 | 2475 | 18.16 | 1460 | 21.28 | 1453 | 22.61 | |
| Hispanic/Latino | 139 | 5.13 | 560 | 4.11 | 328 | 4.79 | 296 | 4.61 | |
| Other c | 47 | 1.74 | 322 | 2.36 | 146 | 2.13 | 143 | 2.23 | |
| Cycle Characteristics | |||||||||
| Stimulation Protocol | < 0.0001 | ||||||||
| Antagonist | 2235 | 82.31 | 10,273 | 75.39 | 4557 | 66.40 | 3307 | 51.46 | |
| Standard agonist | 123 | 4.55 | 1014 | 7.44 | 533 | 7.77 | 319 | 4.96 | |
| Agonist flare | 68 | 2.51 | 1011 | 7.42 | 489 | 7.13 | 611 | 9.51 | |
| Mixed/other d | 289 | 10.63 | 1329 | 9.75 | 1283 | 18.70 | 2190 | 34.07 | |
| Gonadotropin dose (International units) | < 0.0001 | ||||||||
| None | 91 | 3.36 | 702 | 5.15 | 1100 | 16.03 | 1305 | 20.31 | |
| 1–2000 | 565 | 20.82 | 2343 | 17.19 | 1137 | 16.57 | 1147 | 17.85 | |
| 2001–4000 | 1354 | 49.85 | 6230 | 45.72 | 2572 | 37.48 | 1814 | 28.23 | |
| 4001–6000 | 582 | 21.45 | 3459 | 25.39 | 1563 | 22.78 | 1523 | 23.70 | |
| > 6000 | 123 | 4.52 | 893 | 6.55 | 490 | 7.15 | 637 | 9.92 | |
| Cancelation | < 0.0001 | ||||||||
| Yes | 127 | 4.66 | 759 | 5.57 | 470 | 6.84 | 624 | 9.71 | |
| No | 2588 | 95.34 | 12,867 | 94.43 | 6393 | 93.16 | 5803 | 90.29 | |
| Oocyte yield (n)e | < 0.0001 | ||||||||
| ≤ 5 | 272 | 10.50 | 1801 | 14.00 | 1339 | 20.94 | 2569 | 44.28 | |
| 6–10 | 530 | 20.47 | 3243 | 25.20 | 1679 | 26.26 | 1579 | 27.22 | |
| 11–20 | 991 | 38.29 | 4959 | 38.54 | 2198 | 34.37 | 1204 | 20.74 | |
| 21–30 | 556 | 21.48 | 1995 | 15.51 | 808 | 12.64 | 337 | 5.80 | |
| > 30 | 240 | 9.26 | 869 | 6.76 | 370 | 5.79 | 114 | 1.97 | |
| Hyperstimulation | 0.076 | ||||||||
| Yes | 17 | 0.63 | 42 | 0.31 | 15 | 0.22 | 10 | 0.15 | |
| No | 2698 | 99.37 | 13,584 | 99.69 | 6848 | 99.78 | 6417 | 99.85 | |
BMI body mass index, FSH follicle stimulating hormone
aAll N’s and percentages are calculated as an average among the 5 imputed datasets. All N’s are rounded to the nearest whole number
bAll p values are calculated using combined chi-squared test from the five imputed datasets
cOther Race includes American Indian, Alaskan Native, or mixed race including two or more selected races
dMixed/other stimulation protocol includes Clomid ± FSH, aromatase inhibitors ± FSH, unstimulated cycle, mixed cycle incorporating more than one protocol
eOnly includes cycles that were not canceled
Compared to elective OC, rates of cycle cancelation (aRR 0.90, 95% CI 0.70–1.14) and hyperstimulation (aRR 1.46, 95% CI 0.77–2.29) were not different for cancer-related cycles (Table 3). Gonadotropin dose was marginally lower among cancer cycles than elective OC cycles (aRR 0.89, CI 0.80–0.99). Compared to elective OC, there was no difference in oocyte yield for cycles completed for a cancer diagnosis (Beta coefficient 0.02, 95% CI 0.00–0.04). The estimated adjusted oocyte yield, approximately 16 (95% CI 14.5–16.5), and percent maturity, approximately 80% (95% CI 79.3–82.7), were comparable in both groups. Oocyte cryopreservation performed for medical indications was associated with higher gonadotropin dose (aRR 1.22, 95% CI 1.12–1.33), higher likelihood of cancelation (aRR 1.68, 95% CI 1.46–1.92), and lower oocyte yield (Beta coefficient − 0.30, 95% CI − 0.33, − 0.26) compared to elective OC.
Table 3.
Medication dose, cancelation, oocyte yield, and cancelation between cancer-related oocyte cryopreservation cycles and elective, infertility-related, and medically indicated oocyte cryopreservation cycles, 2012–2016
| Unadjusted | Adjusted | |||||||
|---|---|---|---|---|---|---|---|---|
| Cancer | Elective fertility preservation | Other infertile | Medical | Cancer | Elective fertility preservation | Other infertile | Medical | |
| RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | aRR (95% CI) | aRR (95% CI) | aRR (95% CI) | aRR (95% CI) | |
| Gonadotropin dose > 4000 IUa,b | 0.75 (0.67, 0.83) | Referent | 0.91 (0.85, 0.98) | 1.08 (1.00, 1.16) | 0.89 (0.80, 0.99) | Referent | 0.97 (0.90, 1.1) | 1.22 (1.12, 1.33) |
| Cycle cancellationc | 0.83 (0.66, 1.04) | Referent | 1.25 (1.10, 1.41) | 1.82 (1.61, 2.06) | 0.90 (0.70, 1.14) | Referent | 1.19 (1.05, 1.35) | 1.68 (1.46, 1.92) |
| Hyperstimulationc | 2.06 (1.11, 3.81) | Referent | 0.71 (0.32, 1.56) | 0.48 (0.20, 1.13) | 1.46 (0.77, 2.79) | Referent | 0.79 (0.35, 1.78) | 0.71 (0.29, 1.74) |
| Total oocytes retrieved | Predictedd | Predictedd | ||||||
| Poisson beta coefficient for number of eggs retrieved | 0.13 (0.11, 0.14) | Referent | − 0.11 (− 0.13, − 0.09) | − 0.55 (− 0.58, − 0.52) | 0.02 (0.00, 0.04) | Referent | − 0.04 (− 0.07, − 0.01) | − 0.30 (− 0.33, − 0.26) |
| Percentage mature oocytese | 79.8 (79.3, 80.3) | 80.2 (79.6, 80.9) | 80.9 (80.4, 81.5) | 80.2 (79.7, 80.7) | 81.1 (80.1, 82.0) | 82.6 (80.6, 82.7) | 82.2 (81.2, 83.2) | 81.7 (80.8, 82.6) |
IU International Units, RR risk ratio, aRR adjusted risk ratio, 95% CI 95% confidence interval
aAdjusted for age, body mass index, stimulation protocol, geographic region, and race
bCompared to those who received 0–4000 IU gonadotropins
cAdjusted for age, body mass index, stimulation protocol, geographic region, gonadotropin dose, and race
dPoisson model for predicted number of oocytes retrieved excludes cycles that were canceled
eAnalysis for percent mature does not include those who had cycle canceled or those who had 0 eggs retrieved
Discussion
The number of oocyte cryopreservation cycles among women with a cancer diagnosis has increased over the past 5 years; however, the percentage OC cycles for cancer has remained stable as the total number of oocyte cryopreservation cycles increased over the study period. While patient demographic characteristics were different among those freezing eggs for fertility preservation due to cancer, the cycle outcomes were comparable to elective cryopreservation cycles after controlling for potential confounding.
Our findings, specifically related to oocyte yield and percentage maturity, are reassuring to women freezing eggs for fertility preservation due to an active cancer diagnosis requiring cancer treatment and delay of conception. The findings affirm those of prior smaller studies that found no difference in oocyte yield between individuals with and without cancer [7], even among women with BRCA gene mutations [14]. Admittedly, the study would be strengthened by inclusion of outcome data regarding subsequent thaw, fertilization, and live birth. Such a study will be more feasible in the future when more time has elapsed from time of oocyte cryopreservation. Women treated for cancer in 2012 that required chemotherapy, surgery, and possible continued endocrine therapy are likely just now approaching a time when conception may be feasible. A portion of these women may conceive on their own without using previously frozen oocytes. A small single-center 2016 study did suggest comparable live birth rate between women with and without cancer at time of OC [9]. A future larger study on the topic could affirm the generalizability and accuracy of the findings.
Previous studies have hypothesized that women with cancer will have a poor response to ovarian stimulation due to the inflammatory burden of their disease [15]; the results of our study suggest that the response to medication appears similar to women without cancer. In fact, the total gonadotropin dose among cancer patients was lower than that in the elective group, perhaps as a result of the overall younger age or potentially out of a heightened effort to avoid ovarian hyperstimulation. Our results may even underestimate the similarity between cancer and non-cancer outcomes as the cancer group could, in theory, include women with previous history of cancer treated with gonadotoxic treatment that lessened their potential response.
The increased prevalence of obesity in the cancer group may reflect the increased risk of cancer among overweight women and may also reflect clinic policies to allow for the use of ART independent of BMI in women with cancer. Many clinics have BMI cutoffs that may be waived for cancer patients given the urgency and finality of the opportunity to freeze oocytes in a narrow timeframe. In all groups, the majority of patients undergoing treatment were non-Hispanic Whites; an opportunity remains to expand access to all fertility treatment, particularly fertility preservation for cancer; while 13% of the US population self-identifies as non-Hispanic Black, only 7% of the study population identified as such [16, 17].
Women who underwent oocyte cryopreservation due to medical illness were more likely to require higher medication doses and to be canceled. Several of the medically indicated cryopreservation diagnoses, such as Fragile X carrier status and Turner Syndrome, are associated with diminished ovarian reserve and may have contributed to the findings. The group is, however, heterogeneous and also contains individuals with medical diagnoses that are not associated with diminished ovarian reserve.
The study is strengthened by its generalizability; it is larger in number and geographic breadth than previous studies aiming to compare outcomes between cancer and non-cancer oocyte cryopreservation. The ability to correct for potential confounders including age, body mass index, stimulation protocol, geographic region, gonadotropin dose, and race/ethnicity also strengthens the study. The use of multiple imputation to account for missing data allowed for analysis of the complete set of autologous oocyte cryopreservation cycles; multiple imputation has been shown to be a valid approach even when the proportion of missing data is large [12, 13]. The results are limited by the retrospective nature of the analysis, the reliance on the accuracy of individual clinic data entry, the inability to account for clustering by clinic, the evolution of indication specificity over time, the inability to control for anti-mullerian hormone level (though diagnosis of diminished ovarian reserve was included in the model), the lack of subsequent birth outcomes, and the potential for residual confounding, most likely due to age given the younger age of the women in the cancer group. When interpreting trends in oocyte cryopreservation indication over time, it is important to consider that those cycles missing an indication were not included in the trends analysis.
In our study, women freezing oocytes for oncologic reasons had similar oocyte yield and maturity as compared to those of women freezing oocytes electively. The outcomes of the subsequent egg thaw, fertilization, and transfer cycles remain unknown and warrant future study.
Electronic supplementary material
(DOCX 21.4 kb)
Acknowledgements
SART wishes to thank all of its members for providing clinical information to the SART CORS database for use by patients and researchers. Without the efforts of our members, this research would not have been possible.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ethics Committee of the American Society for Reproductive Medicine. Electronic address Aao Fertility preservation and reproduction in patients facing gonadotoxic therapies: an ethics committee opinion. Fertil Steril. 2018;110(3):380–386. doi: 10.1016/j.fertnstert.2018.05.034. [DOI] [PubMed] [Google Scholar]
- 2.Oktay K, Harvey BE, Partridge AH, Quinn GP, Reinecke J, Taylor HS, Wallace WH, Wang ET, Loren AW. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018;36(19):1994–2001. doi: 10.1200/JCO.2018.78.1914. [DOI] [PubMed] [Google Scholar]
- 3.Hipp HS, Shandley LM, Schirmer A, McKenzie L, Kawwass JF. Oocyte cryopreservation in adolescent women. J Pediatr Adolesc Gynecol. 2019;32(4):377–82. 10.1016/j.jpag.2019.03.001. [DOI] [PubMed]
- 4.Practice Committees of American Society for Reproductive M. Society for Assisted Reproductive T Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99(1):37–43. doi: 10.1016/j.fertnstert.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Velasco JA, Domingo J, Cobo A, Martinez M, Carmona L, Pellicer A. Five years' experience using oocyte vitrification to preserve fertility for medical and nonmedical indications. Fertil Steril. 2013;99(7):1994–1999. doi: 10.1016/j.fertnstert.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Turan V, Quinn MM, Dayioglu N, Rosen MP, Oktay K. The impact of malignancy on response to ovarian stimulation for fertility preservation: a meta-analysis. Fertil Steril. 2018;110(7):1347–1355. doi: 10.1016/j.fertnstert.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 7.Quinn MM, Cakmak H, Letourneau JM, Cedars MI, Rosen MP. Response to ovarian stimulation is not impacted by a breast cancer diagnosis. Hum Reprod. 2017;32(3):568–574. doi: 10.1093/humrep/dew355. [DOI] [PubMed] [Google Scholar]
- 8.Dolinko AV, Farland LV, Missmer SA, Srouji SS, Racowsky C, Ginsburg ES. Responses to fertility treatment among patients with cancer: a retrospective cohort study. Fertility Research and Practice. 2018;4:3. doi: 10.1186/s40738-018-0048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pereira N, Hancock K, Cordeiro CN, Lekovich JP, Schattman GL, Rosenwaks Z. Comparison of ovarian stimulation response in patients with breast cancer undergoing ovarian stimulation with letrozole and gonadotropins to patients undergoing ovarian stimulation with gonadotropins alone for elective cryopreservation of oocytesdagger. Gynecol Endocrinol. 2016;32(10):823–826. doi: 10.1080/09513590.2016.1177013. [DOI] [PubMed] [Google Scholar]
- 10.Technology SfAR. In: history of IVF. 2019. https://www.sart.org/patients/history-of-ivf/. Accessed July 10 2019.
- 11.Public Law 102–493 - Fertility Clinic Success Rate and Certification Act of 1992. 1992. [PubMed]
- 12.Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pedersen AB, Mikkelsen EM, Cronin-Fenton D, Kristensen NR, Pham TM, Pedersen L, Petersen I. Missing data and multiple imputation in clinical epidemiological research. Clin Epidemiol. 2017;9:157–166. doi: 10.2147/CLEP.S129785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunnala V, Fields J, Irani M, D'Angelo D, Xu K, Schattman G, Rosenwaks Z. BRCA carriers have similar reproductive potential at baseline to noncarriers: comparisons in cancer and cancer-free cohorts undergoing fertility preservation. Fertil Steril. 2019;111(2):363–371. doi: 10.1016/j.fertnstert.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Lekovich J, Lobel ALS, Stewart JD, Pereira N, Kligman I, Rosenwaks Z. Female patients with lymphoma demonstrate diminished ovarian reserve even before initiation of chemotherapy when compared with healthy controls and patients with other malignancies. J Assist Reprod Genet. 2016;33(5):657–662. doi: 10.1007/s10815-016-0689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ethics Committee of the American Society for Reproductive M Disparities in access to effective treatment for infertility in the United States: an ethics committee opinion. Fertil Steril. 2015;104(5):1104–1110. doi: 10.1016/j.fertnstert.2015.07.1139. [DOI] [PubMed] [Google Scholar]
- 17.Commerce UDo. US census. 2018. https://www.census.gov/quickfacts/fact/table/US/PST045218. Accessed July 11 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 21.4 kb)