Abstract
Introduction
Interphasic DNA has a constant three-dimensional conformation, which is particularly striking for spermatic DNA, with distinct chromosomal territories and a constant chromosomal conformation. We hypothesized that this organization is fragile, and that an excess or a lack of chromosomal segments could hinder the genomic structure as a whole.
Methods
Five human male chromosomal translocation carriers and five controls were included. Spermatic DNA spatial organization was studied, in both balanced and unbalanced spermatozoa, with two-dimensional fluorescent in situ hybridization (FISH) via analysis of chromosomes not implicated in the cases’ translocations, compared to that of normal controls. Two parameters were studied: the distance between the two telomeric ends of chromosome 1, and the area of the chromosomal territories of chromosomes 1 and 17.
Results
Sperm FISH analysis of rearrangement carriers revealed changes in the nuclear architecture compared to that of controls. Inter-telomeric distance and chromosomal territories areas were both significantly increased.
Discussion
We show that an excess or lack of chromosomal segments can hinder the normal spatial nuclear architecture in sperm. These results show that nuclear architecture is a fragile assembly, and that local chromosomal abnormalities may impact the nucleus as a whole. This suggests a potential avenue for selection of spermatozoa prior to in vitro fertilization, not only in rearrangement carriers but also in the infertile population at large. Furthermore, we suggest that 2D-FISH could possibly be a useful tool in assessing spermatic nuclear organization in a way to evaluate male fertility.
Keywords: Sperm nucleus, Nuclear architecture, Chromosomal translocation
Introduction
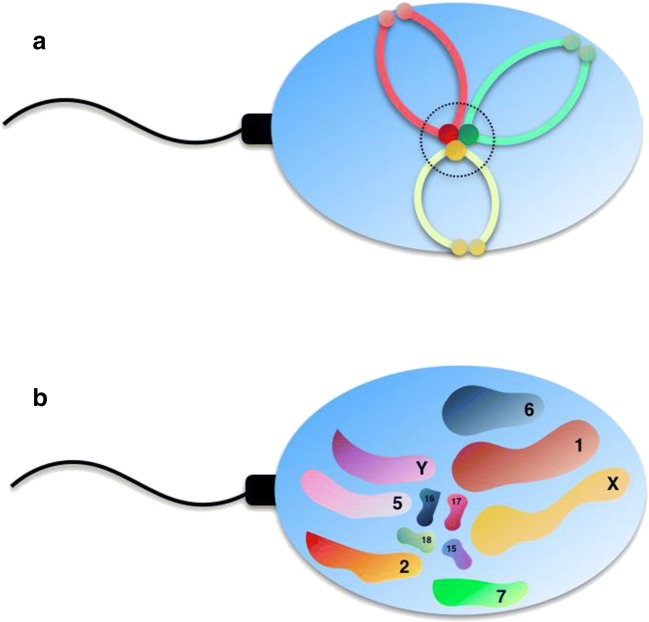
During interphase, chromosomes occupy distinct locations within the nucleus, known as chromosomal territories [1]. Each chromosomal territory has a preferential intranuclear position, with gene-poor chromosomes favoring peripheral locations, in contrast to the internal locations of gene-rich chromosomes [2, 3]. Certain regions tend to be spatially associated, forming topologically associated domains, or TADs, forming what has been described as a compartmentalization of the genome [4]. This nuclear architecture is retained in spermatozoa, in which preferential chromosome positioning has been established for a number of chromosomes [5]. Longitudinally, from acrosome to flagellum, chromosomes are located in the following order: X, 7, (6, 15, 16, 17), 1, (Y, 18), 2, 5. Radially, chromosomes 7 and 6 tend to be the most peripheral, and 16 and 18 the most internal [6], (Fig. 1). This organization has consequences on the timing of chromosomal exposure to the ooplasm during fertilization, and could have implications on embryo development [5].
Fig. 1.
a Chromosomal conformation in sperm nuclei. Chromosomes are bent at their centromeres, with their telomeres covalently linked to each other and to the nuclear membrane, with their centromeres gathered at the center, forming a structure called chromocenter. b Chromosomal territories in the super nuclei follow a non-random organization from Zalensky et al. (2007)
In spermatozoa, that is to say after meiosis II, the chromosomes themselves have a specific spatial conformation. Telomeres are coupled as dimers, and are bound to the nuclear membrane [7], while the centromeres are centrally located, giving the chromosome a looped hairpin configuration [8]. The region of the nucleus containing the centromeres has been coined “chromocenter” [9].
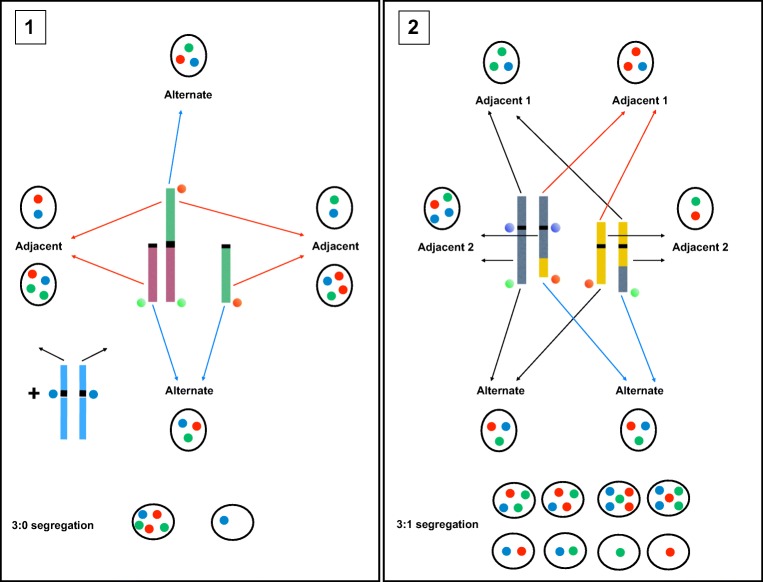
Balanced chromosomal rearrangements, such as translocations or inversions, are found in the general population with a prevalence of approximately 1/500. Though affected individuals are often asymptomatic, apart from a possible infertility, they risk the transmission of an unbalanced karyotype to their offspring via fertilization of an unbalanced gamete. Indeed, in such patients, a certain proportion of gametes contain an unbalanced combination of chromosomal segments, which is related to an abnormal segregation during meiosis (Fig. 2). The fertilization of such gametes is associated with spontaneous abortions, fetal malformations, and sometimes with the birth of an affected child presenting with dysmorphic features, malformations, and intellectual deficiency. The fertilization of balanced gametes however, either carrying the translocation or not, lead to the birth of phenotypically normal children [10].
Fig. 2.
Chromosomal segregation modes during meiosis in chromosomal rearrangement carriers. All modes are unbalanced except the alternate mode. In Robertsonian translocation carriers (1), the unbalanced modes are the adjacent and the 3:0 modes. In reciprocal translocation carriers (2) the unbalanced modes are the adjacent 1, adjacent 2, and 3:1 modes
In males, it has been shown that chromosomally unbalanced spermatozoa have larger nuclei [11] and lower density [12]. This is thought to be related to incomplete nuclear condensation, due to an abnormal three-dimensional chromosomal architecture.
In this study, we used chromosomal rearrangement human carriers as a model for studying the effects of chromosomal abnormalities upon sperm nuclear architecture. We hypothesized that the three-dimensional spermatic nuclear conformation is fragile, and likely to be altered when containing an abnormal chromosomal carriage. Indeed, the existence of chromosomal segments in excess or in shortage could hinder the appropriate formation of the normal tridimensional architecture. In order to study these effects, we conducted two-dimensional FISH (fluorescent in situ hybridization) experiments on spermatozoa from five individuals, known to be chromosomal rearrangements carriers, by using probes coding for chromosomes not involved in the rearrangement. We then analyzed two chromosomal parameters: the distance between the telomeric ends of a given chromosome and the area of the chromosomal territories of two arbitrarily chosen chromosomes, and compared them to that of controls with a normal karyotype. Chromosomal carriers have both balanced and unbalanced spermatozoa. The aforementioned parameters were studied on both, regardless of their chromosomal content, since there was no way to study both the segregation mode and nuclear architecture, with just one FISH procedure.
Materials and methods
We conducted a retrospective study on five reciprocal translocation carriers who consulted our center over the past 3 years. These patients were referred to our clinic after a translocation was diagnosed as part of a miscarriage workup. Their cytogenetic formula, semen parameters (as per WHO guidelines [13]), and proportion of unbalanced spermatozoa are shown in Table 1. Five controls were included as well. They took part in ART procedures, and had all normal semen parameters, which was also the case for the cases. Importantly, the controls all had a normal karyotype. This retrospective study was conducted in the course of normal medical care for these patients, without any specific additional intervention. The Fédération Française des CECOS Ethics Committee considered this study to be exempted from ethical approval since no intervention was carried out specifically on the patients. Informed consent was obtained from each patient.
Table 1.
The 5 subjects and 5 controls were included in the study, with their cytogenetic formula and sperm parameters
| Cytogenetic formula | Sperm concentration | Sperm motility (progressive motility) | % morphologically normal sperm | % chromosomally unbalanced sperm | |
|---|---|---|---|---|---|
| S1 | t(3;6)(q29;q27) | 175 M/ml | 60% | 28 | 56 |
| S2 | t(7;20)(p13;q12) | 60 M/ml | 65% | 10 | 49 |
| S3 | t(4;10)(q31.3;q26.1) | 50 M/ml | 50% | 15 | 70 |
| S4 | t(5;10)(q34;p12.1) | 100 M/ml | 56% | 14 | 65 |
| S5 | t(7;20)(q21.1;p12) | 40 M/ml | 50% | 20 | 49 |
| C1 | 46,XY | 34 M/ml | 45% | 10 | NA |
| C2 | 46,XY | 90 M/ml | 50% | 18 | NA |
| C3 | 46,XY | 50 M/ml | 50% | 15 | NA |
| C4 | 46,XY | 60 M/ml | 60% | 18 | NA |
| C5 | 46,XY | 110 M/ml | 60% | 20 | NA |
Semen samples were obtained by masturbation after an abstinence period ranging from 1 to 5 days (average abstinence for subjects, 3.4 days; SD, 0.4 days; average abstinence for controls, 3.1 days; SD, 0.5 days). Semen analysis was performed for cases and samples as per WHO guidelines (5th edition, 2010). The samples were incubated at 37 °C before being washed in PBS buffer (Eurobio, France). Spermatozoa were then fixated in methanol and acetic acid (3:1) for 30 min at room temperature, and spread on microscope slides.
FISH was conducted on all patients and controls by using two different types of probes: in-house contiguous telomeric probes and whole-chromosome painting mixes (Abbott Molecular, USA). Hybridization efficiency was evaluated on metaphase chromosomes prior to the study (data not shown). In order to separate the effect of the translocation on sperm nucleus architecture from the translocation itself, the chromosomes studied were not involved in any of the carriers’ rearrangements. These arbitrarily-chosen chromosomes were chromosome 1 (whole chromosome painting, telomeric ends) and chromosome 17 (whole chromosome painting). FISH was conducted as has been previously described [12]. After fixation, the spermatozoa underwent a nuclear decondensation step in NaOH, and then hybridization at 70 °C for 2 min and 30 s with the aforementioned fluorescent probes. In order to increase fluorescence, the slides were left to incubate overnight in a SlideBooster (Beckman Coulter, FL, USA) device.
Moreover, the proportion of unbalanced spermatozoa for each case was assessed through FISH as well, as previously described [12]. For each chromosomal rearrangement, a set of three specific fluorescent probes was used: one for the telomeric end of one of the involved chromosomes, one of the telomeric end of the other chromosomes, and one for the centromere of one of those chromosomes. This way, each segregation mode gave a specific color combination, and we were able to evaluate the proportion of balanced (both with and without the translocation) and unbalanced spermatozoa (the adjacent 1, adjacent 2, 3:1, and 3:1 modes).
Analysis was performed with a fluorescent microscope (Olympus BX61), with a COHU 4912–5000 CCIR camera and a ×100 oil immersion objective. With this configuration, 12 pixels on the image correspond to 1 μm. Open-source software Fiji [14] along with the Shape Filter plug-in [15] were used for image analysis. For each spermatozoon, we made the following measurements: inter-telomeric distances for chromosome 1 and areas of the chromosomal territories of chromosomes 1 and 17 (Fig. 3). For each parameter, at least 50 spermatozoa, for each case and control, were analyzed, leading to a total of 250 cells per parameter. These measurements, in each case, were compared to the mean of the controls using a nonparametric one-way ANOVA by ranks test (Kruskal-Wallis test), and results with p < 0.05 were considered statistically significant (GraphPad, www.graphpad.com).
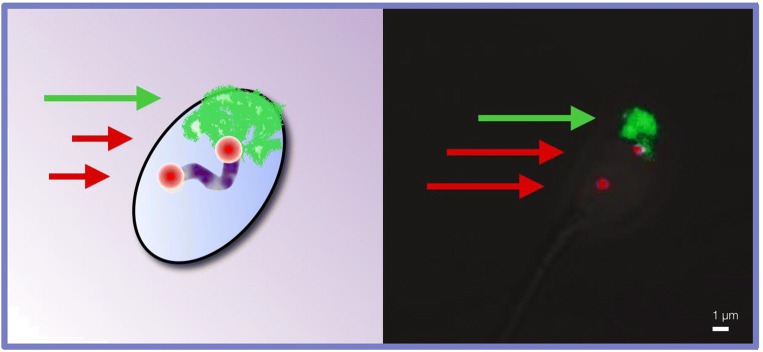
Fig. 3.
Fluorescent in situ hybridization (FISH) was used to evaluate the distance between the two telomeric ends of chromosome 1 (red arrows), and the surface area of the chromosomal territory of chromosomes 17 (green arrow) and 1 (not shown), in S1
Results
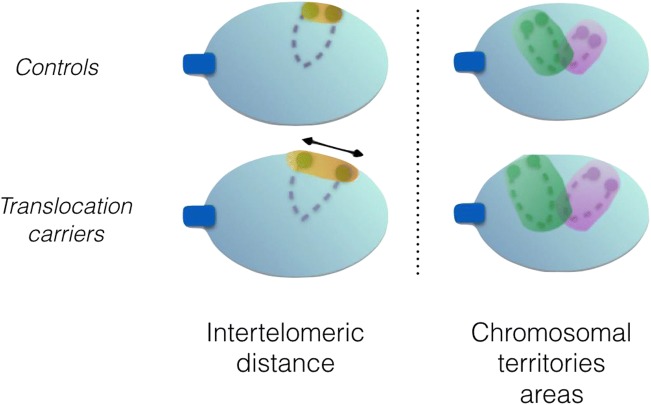
We first analyzed the inter-telomeric distance for chromosome 1 in all cases and controls. Cases exhibited a significantly larger inter-telomeric distance (708 nm; standard error of the mean, 50 nm) compared to controls (187 nm; SEM, 29 nm). Furthermore, a significant difference was observed for each case independently compared to the controls (Table 2, Fig. 4). Additionally, if one considers the resolution limit of 300 nm, which is inherent to the microscope, camera, and immersion oil (4 pixels), the proportion of spermatozoa with tightly bound telomeric ends (inter-telomeric distance < 300 nm) is significantly lower in patients compared to controls (47.6% vs. 82.8%, p < 0.001).
Table 2.
Inter-telomeric distance (distance between the telomeric end of the short arm and the telomeric end of the long arm) for chromosome 1, and chromosomal territories measured with whole chromosomal painting probes, for two chromosomes involved in none of the subject’s translocations (chromosomes 1 and 17). Expressed here are the ratios between the area of the chromosome and the total area of the sperm nucleus. Chromosomal territories are increased for all subjects for chromosome 1, and for all but S2 for chromosome 17. A Kruskal-Wallis one-way ANOVA test was used for statistical analysis, and results with p < 0.05 were considered to be statistically significant
| Inter-telomeric distance (chromosome 1) in nm | Inter-telomeric distance (chr 1), overhead length | Chromosome 1 territory area (over total nucleus area) | Chromosome 17 territory area (over total nucleus area) | |
|---|---|---|---|---|
| C1 | 238 nm | 5% | 13.5% | 5.5% |
| C2 | 164 nm | 3.6% | 13.1% | 5.2% |
| C3 | 129 nm | 2.7% | 8.3% | 4.3% |
| C4 | 270 nm | 5.5 | 7.9% | 2.7% |
| C5 | 133 nm | 2.5% | 8.2% | 2.9% |
| Controls |
187 SEM, 29 nm |
3.9% SEM, 0.6 pp |
10.5% SEM, 0.4 pp |
4.1% SEM, 0.1 pp |
| S1 | 425* (p < 0.05) | 8.9%* (p < 0.05) | 17.5%* (p < 0.00001) | 4.8%* (p < 0.05) |
| S2 | 767* (p < 0.00001) | 16.2%* (p < 0.00001) | 15.7%* (p < 0.00001) | 4.4% |
| S3 | 1150* (p < 0.00001) | 24.4%* (p < 0.00001) | 15.5%* (p < 0.00001) | 8.7%* (p < 0.00001) |
| S4 | 683* (p < 0.001) | 13.7%* (p < 0.001) | 12.2%* (p < 0.0001) | 5.2%* (p < 0.00001) |
| S5 | 533* (p < 0.01) | 10.1%* (p < 0.01) | 10.7* (p < 0.05) | 6%* (p < 0.00001) |
| All subjects |
708* (p < 0.00001) SEM, 50 nm |
14.6%* (p < 0.00001) SEM, 1.08 pp |
14.3%* (p < 0.00001) SEM, 0.3 pp |
5.8%* (p < 0.00001) SEM, 0.2 pp |
Fig. 4.
We evidence significant nuclear architecture differences between chromosomal rearrangement carriers and normal controls. First, we show that the inter-telomeric distance, for chromosome 1, is enlarged in translocation carriers compared to that in controls. Second, we found the chromosomal territories, for chromosomes 1 and 17, to be enlarged in carriers compared to controls
Experiments with whole-chromosome painting probes allowed for measurement of the surface area of chromosomal territories within the nucleus. We found an elevated area for the chromosomal territories in all cases for chromosome 1 and in all cases except S2 for chromosomes 17, both in absolute value (data not shown) as well as in its ratio to the nuclear area: 14.3% (SEM, 0.3 points of percentage) and 10.5% (SEM, 0.4) for chromosome 1, respectively for patients and controls, and 5.8% (SEM, 0.2) and 4.1% (SEM, 0.1) for chromosome 17, respectively for patients and controls.
We did not find any relationship between the percentage of sperm nuclei with abnormal architecture parameters and the rate of chromosomally unbalanced gametes for each patient.
Discussion
Genomic condensation within the nucleus is a paramount element of spermatogenesis. Indeed, while the volume of a lymphocyte nucleus is around 900 μm3, that of a spermatozoon is only about 16 μm3, albeit containing only one-half as much DNA. While this condensation allows for the protection of paternal DNA before fertilization, it also carries a functional significance. This extreme condensation is made possible by the replacement of somatic histones, first by transition proteins and then by protamines, leading to the very close coiling of DNA molecules following a doughnut model [5].
In this model, chromosomes have a specific three-dimensional conformation and pre-determined territories. We hypothesized that this architecture is fragile, and that the excess or lack of chromosome segments, as well as a shift in their position within the genome, could hinder it. To test this hypothesis, we used the model of chromosomal rearrangements carriers whose spermatozoa carry a number of different chromosomal combinations. Some of these spermatozoa carry an unbalanced karyotype, while some others have a balanced one carrying either normal or translocated chromosomes (Fig. 2). Indeed, it is noteworthy that, among balanced spermatozoa, a contingent exists which has a strictly normal chromosomal content, corresponding to 50% of the proportion of balanced gametes. During meiosis, the normal segregation pattern, ie., the alternate mode, leads to chromosomally balanced gametes half of which have balanced but translocated chromosomes. Therefore, among the spermatozoa that were analyzed, a certain proportion had a normal and non-translocated chromosomal content, similarly to the controls. It is possible that this had an influence on our results and could constitute a limitation factor. However, we did not find a correlation between the proportion of balanced spermatozoa and the rate of abnormalities in nuclear architecture.
In the present study, we focused on chromosomes that were not involved in the chromosomal rearrangements, in a way to study their effect on nuclear architecture as a whole. We did not study the implication of the involved chromosomes on nuclear architecture since their abnormal number in a significant proportion of cells would have made the interpretation of FISH signals more complex, if not impossible. Indeed, depending on the chromosomal segregation pattern, some of those chromosomal segments can exist in zero, one, two, or even three copies (in the 3:0 and 3:1 segregation modes), making the study of chromosomal architecture impossible.
We chose arbitrarily chromosomes 1 and 17. We first studied controls with a normal karyotype, and evaluated the inter-telomeric distances and chromosomal areas for those two chromosomes. For each of those parameters, the mean among controls was calculated in order to provide a picture of the “normal” nuclear architecture, to which those in translocation carriers were both individually and collectively compared.
We first studied the relative position of telomeric ends. Indeed, it has been evidenced in previous studies that the telomeric extremities of a given chromosome are bound to each other, thus keeping chromosomes in a hairpin conformation [5]. Increasing concentrations of NaOH have been shown to alter this bond, which has therefore been suggested to be of chemical covalent nature. We suggest here that the existence of a chromosomal imbalance in the sperm nucleus alters this bond, even on chromosomes that are not involved in the rearrangement. Thus, this imbalance alters the nuclear architecture as a whole. Whether telomeric distance could be used as a quality biomarker of fertility should be investigated in further studies.
Whole-chromosome painting studies allowed for the visualization of the actual chromosomal territories. We showed that the chromosomal surface areas, for two chromosomes not involved in the rearrangement, are significantly larger in cases as compared to controls.
We have previously shown that in chromosomal rearrangement carriers, unbalanced spermatozoa have a slightly larger nucleus (+ 11% overall, and more specifically + 24% for the 3:0 and 3:1 modes, which usually carry the most important imbalance) [11]. We suggested that abnormal chromosomal segments could prevent chromosomes from reaching their normal location within the sperm nucleus, thus hindering the normal nuclear condensation process.
One limitation of the present study is its two-dimensional character. Indeed, two loci that appear close to each other in two dimensions may actually be far apart on the vertical axis. This could have blurred differences between rearrangement carriers and controls. However, the fact that those differences were significant even in two dimensions suggests they are important. Further studies could use confocal microscopy as a method of obtaining a more accurate and three-dimensional portrait of nuclear architecture. Besides, utilizing more chromosomes, cases, and controls in the study could help to establish a better picture of the changes that occur in the nucleus in the presence of a chromosomal imbalance.
Furthermore, the use of two dimensional FISH paves the way for possibly using the procedure described here in andrology laboratories, with the aim of clinically assessing nuclear architecture and sperm quality in infertile patients. We kept the number of parameters evaluated low, in order to realistically reproduce this procedure in the clinical setting. One of our ongoing research projects actually shows a correlation between spermatic nuclear architecture, as evaluated here, and other sperm quality parameters.
Overall, we found the nuclear architecture to be significantly different between chromosomal rearrangement carriers and a population of controls with a normal karyotype. This highlights the fact that nuclear spatial organization is well-ordered and that the existence of abnormal chromosomal combinations can alter it. The fact that the chromosomal areas are larger in rearrangement carriers suggests that an abnormal karyotype prevents the normal nuclear condensation process, which is a key feature of spermatogenesis. This supports the hypothesis that extreme nuclear condensation is achieved through the precise ordering of chromosomes within the nucleus, and that any numerical chromosomal abnormality will prevent this condensation process from being fully accomplished. Furthermore, those findings could be used as a basis for new male fertility tests or pre-ICSI sperm selection procedures.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sehgal N, Fritz AJ, Vecerova J, Ding H, Chen Z, Stojkovic B, Bhattacharya S, Xu J, Berezney R. Large-scale probabilistic 3D organization of human chromosome territories. Hum Mol Genet. 2016;25:419–436. doi: 10.1093/hmg/ddv479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bickmore WA, Chubb JR. Dispatch. Chromosome position: now, where was I? Curr Biol. 2003;13:R357–R359. doi: 10.1016/S0960-9822(03)00276-8. [DOI] [PubMed] [Google Scholar]
- 3.Cremer T, Küpper K, Dietzel S, Fakan S. Higher order chromatin architecture in the cell nucleus: on the way from structure to function. Biol Cell. 2004;96:555–567. doi: 10.1016/j.biolcel.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Jost D, Carrivain P, Cavalli G, Vaillant C. Modeling epigenome folding: formation and dynamics of topologically associated chromatin domains. Nucleic Acids Res. 2014;42:9553–9561. doi: 10.1093/nar/gku698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zalensky A, Zalenskaya I. Organization of chromosomes in spermatozoa: an additional layer of epigenetic information? Biochem Soc Trans. 2007;35:609–611. doi: 10.1042/BST0350609. [DOI] [PubMed] [Google Scholar]
- 6.Zalenskaya IA, Zalensky AO. Non-random positioning of chromosomes in human sperm nuclei. Chromosom Res. 2004;12:163–173. doi: 10.1023/B:CHRO.0000013166.04629.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zalensky AO, Allen MJ, Kobayashi A, Zalenskaya IA, Balhórn R, Bradbury EM. Well-defined genome architecture in the human sperm nucleus. Chromosoma. 1995;103:577–590. doi: 10.1007/BF00357684. [DOI] [PubMed] [Google Scholar]
- 8.Meyer-Ficca M, Müller-Navia J, Scherthan H. Clustering of pericentromeres initiates in step 9 of spermiogenesis of the rat (Rattus norvegicus) and contributes to a well defined genome architecture in the sperm nucleus. J Cell Sci. 1998;111(Pt 10):1363–1370. doi: 10.1242/jcs.111.10.1363. [DOI] [PubMed] [Google Scholar]
- 9.Schmid M, Krone W. The relationship of a specific chromosomal region to the development of the acrosome. Chromosoma. 1976;56:327–347. doi: 10.1007/BF00292954. [DOI] [PubMed] [Google Scholar]
- 10.McKinlay S. Chromosome abnormalities and genetic counseling. 4. USA: Oxford University Press; 2011. [Google Scholar]
- 11.Rouen A, Lavillaureix A, Hyon C, Heide S, Clède S, Balet R, Kott E, Cassuto NG, Siffroi J-P. Nuclear volume differences between balanced and unbalanced spermatozoa in chromosomal translocation carriers. Reprod BioMed Online. 2015;30:290–295. doi: 10.1016/j.rbmo.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 12.Rouen A, Balet R, Dorna M, Hyon C, Pollet-Villard X, Chantot-Bastaraud S, Joyé N, Portnoï M-F, Cassuto NG, Siffroi J-P. Discontinuous gradient centrifugation (DGC) decreases the proportion of chromosomally unbalanced spermatozoa in chromosomal rearrangement carriers. Hum Reprod. 2013;28:2003–2009. doi: 10.1093/humrep/det121. [DOI] [PubMed] [Google Scholar]
- 13.Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HWG, Behre HM, Haugen TB, Kruger T, Wang C, Mbizvo MT, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–245. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 14.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagner T, Lipinski H. IJBlob: An ImageJ Library for Connected Component Analysis and Shape Analysis. J Open Res Softw. 2013;1(1):e6. doi: 10.5334/jors.ae. [DOI] [Google Scholar]