Abstract
Purpose
To establish parameters during mouse extended embryo culture that accurately predict fetal developmental potential of a blastocyst without performing embryo transfer.
Methods
Embryos of three varying qualities were produced: poor quality embryos produced from in vitro matured oocytes (IVM), intermediate quality embryos produced from in vivo matured oocytes followed by in vitro fertilization and embryo culture (IVF); high quality embryos developed in vivo (VIVO). Embryonic day (E) 3.5 embryos from each group with similar morphologies were used for surgical embryo transfer to assess implantation and fetal developmental potential, in addition to placing these embryos into extended culture until E 8.5 to examine outgrowth area, egg cylinder volume, epiblast cell number, and outgrowth morphologies by immunofluorescence and 3D confocal microscopy.
Results
The proportional differences in epiblast cell number are strikingly similar to fetal development following embryo transfer, suggesting that this parameter may be indicative of the potential of an embryo to successfully develop into a fetus.
Conclusion
Extended embryo culture provides more accurate information regarding developmental potential than blastocyst morphological assessment. Specifically, epiblast cell number is an accurate and valuable predictor of fetal developmental potential. This work sets the stage for routine evaluation of embryo quality past the time embryos would normally be transferred. The ability to determine post implantation potential without embryo transfer may greatly improve efforts to culture higher quality embryos in vitro for human IVF, as well as reducing animal use and eliminating confounding maternal factors associated with embryo transfer experiments in research.
Keywords: Assisted reproductive technology, Blastocyst, Implantation, Fetal development, Embryo transfer
Introduction
Identifying parameters indicative of a blastocyst’s developmental potential is highly desirable for human in vitro fertilization (IVF), agricultural production, and biomedical research. Human IVF requires the selection of the most viable embryo prior to transfer to improve clinical outcomes. Numerous approaches developed to assess embryo viability have had varying degrees of success in clinical application. These include morphological assessment by standard and novel optical methods, preimplantation genetic testing (PGT), morphokinetics, metabolomics, and proteomics profiling of embryo culture media, gene expression in the cumulus cells, and even the oxygen consumption of individual embryos (reviewed in [1–3]). For basic research, additional methods of assessment are available since embryo transfer and pregnancy is not the goal. These include analysis of blastocyst cell number, analysis of inner cell mass (ICM), and trophectoderm (TE) cell allocation, mitochondrial DNA content, mitochondrial activity, levels of reactive oxygen species, and examination of the expression of competence-related genes at the blastocyst stage. Some of these parameters, such as mtDNA, PGT, and blastocyst gene expression, can also be assessed clinically with an embryo biopsy. However, it is generally accepted that current methods for evaluating blastocyst quality for clinical use may not accurately reflect an embryo’s likelihood of producing a live birth [1]. Currently, morphological assessment remains the most frequently used method for selection of an embryo for transfer in the clinical setting. In the research setting, embryo transfer is, of course, the most direct and accurate approach to determine the ability of a blastocyst to implant and give rise to a fetus; however, this method is technically demanding, time consuming, and the results may be confounded with maternal effects. Furthermore, it is not possible to use embryo transfer to examine the developmental potential of a human embryo outside of a clinical treatment cycle.
More recently, an extended in vitro embryo culture system has been developed in both mouse and human that supports embryo self-organization and key developmental milestones beyond the blastocyst stage without any maternal communication [4–6]. This system allows us to monitor development during peri- and even post-implantation periods and offers an opportunity to find new parameters that may be useful in predicting embryo developmental potential more accurately. Thus, our aim was to establish parameters during mouse extended embryo culture that accurately predict fetal developmental potential of a blastocyst without the complications of performing ET.
During extended culture in vitro, the distal trophectoderm (TE) of the mouse blastocyst attaches to the bottom of the plate, the blastocoel collapses, and the TE begins to differentiate into trophoblast giant cells. The inner cell mass (ICM) and remaining TE form an emerging egg cylinder with a distinctive cluster of epiblast cells that express the pluripotency marker POU5F1. These epiblast cells become polarized into a rosette structure and upon the formation of a nascent lumen, create the pro-amniotic cavity lined by a cup-shaped layer of columnar epithelial epiblast cells [4, 5]. We hypothesized that an embryo with increased developmental potential would not only attach and form TE outgrowth, but would also form a more organized fetus proper and extra embryonic structures during this peri-implantation period.
Materials and methods
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise stated.
Animals
All procedures were approved by the Fertility Laboratories of Colorado Ethics in Research Committee and followed animal care and use guidelines, as described by the Guide for the Care and Use of Laboratory Animals [7]. Healthy female outbred mice (CF1; Envigo, Indianapolis, IN) were selected between 4 and 8 weeks of age for superovulation. Mice were kept in 14:10 light:dark group housing for females or individual housing for males (BDF1 strain; Envigo, Indianapolis, IN), and were offered food and water ad libitum.
Blastocyst production
Embryos of varying quality inferred by the duration of their time in vitro were produced. For the in vitro maturation (IVM) group, immature oocytes were collected from mouse ovaries in 3-(N-morpholino)-propanesulfonic acid (MOPS)-buffered medium with 5% (v/v) fetal calf serum (FCS) (Hyclone, Waltham, MA) 48 h post intraperitoneal injection of 5 I.U. pregnant mare serum gonadotropin (PMSG) (Calbiochem, Billerica, MA). Oocytes with even layers of unexpanded cumulus cells were matured in an in-house prepared maturation medium [8] for 18 h in 50 μl drops under mineral oil (OvOil, Vitrolife, Englewood, CO). For the in vitro fertilization (IVF) group, in vivo matured oocytes were collected from the ampulla in MOPS-buffered medium with 5% FCS 18 h post 5 I.U. human chorionic gonadotropin (hCG) (Calbiochem, Billerica, MA) administration and 66 h post PMSG stimulation. All IVM and IVF oocytes were moved to fertilization medium [8] and co-cultured with 1 × 106 spermatozoa/ml collected from a BDF1 male (10 oocytes/50 μl drop) for 6 h. Prospective zygotes with two pronuclei were selected and cultured in a sequential culture system for 84 h (D 3.5) before extended culture or embryo transfer, and embryos were cultured for 115 h (D 5) before fixation for blastocyst cell number. In vitro culture was performed under 37 °C in 6.5% O2 and 7.5% CO2 to compensate for the high altitude of our laboratory (equivalent to 5% O2 and 6% CO2 at sea level) and maintain a pH between 7.2 and 7.3.
For the in vivo produced (VIVO) group, embryonic day (E) 3.5 blastocysts were flushed directly from the uterine horns in MOPS-buffered medium approximately 138 h post PMSG and 90 h post hCG administration/mating. All females were checked for mating plugs 16 h post mating with an intact BDF1 male (> 10 weeks old).
Blastocyst cell number analysis
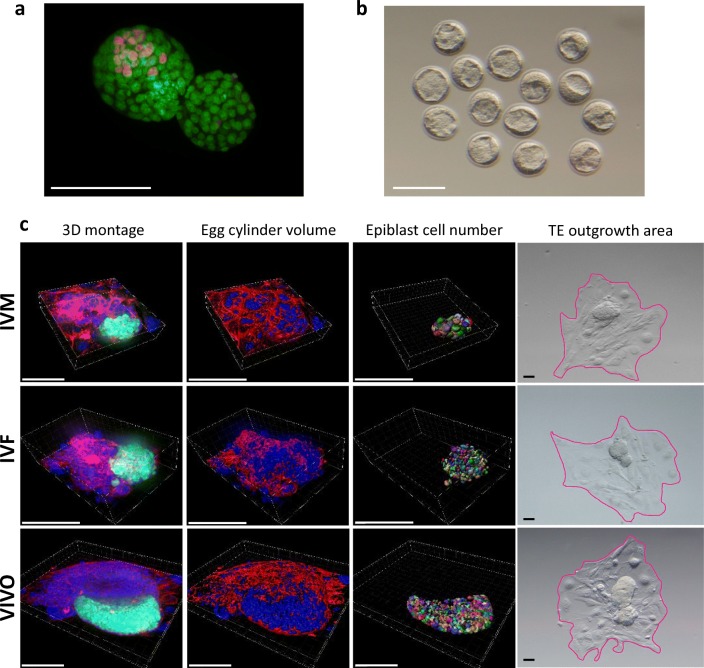
Hatching or hatched blastocysts from each group were fixed using 4% paraformaldehyde (PFA, Electron Microscopy Sciences, Hatfield, PA) and stained for antibodies against CDX2 (1:200; MU392A-UC, Biogenex, Freemont, CA) for TE and SOX2 (1:200; AN579, Biogenex, Freemont, CA) for ICM, as described previously [9]. Blastocysts were imaged under × 400 magnification on an Olympus BX52 microscope with the Photometrics CoolSnap HQ camera (Fig. 1a). Images were analyzed using the hand-count tool on Metamorph Advanced software version 7.7.0.0.
Fig. 1.
a A representative immunofluorescence image of blastocyst cell number and allocation. Embryos were stained with SOX2 (red) for ICM and CDX2 (green) for TE. Scale bar, 100 μM. b A representative image of E 3.5 expanded embryos of similar quality prior to zona removal and embryo extended culture. Scale bar, 100 μM. c Representative 3D confocal images, images for egg cylinder volume analyses, epiblast cell number analysis, and TE outgrowth area for IVM (top row), IVF (center row), and VIVO (bottom row) embryos. Embryos were stained with DAPI for nuclei (blue), rhodamine phalloidin for F-actin (red), and antibody against POU5F1 (green). Scale bars, 100 μM
Surgical embryo transfers and analysis
On D 3.5, expanded blastocysts from each treatment group (IVM, IVF, and VIVO) were surgically transferred as previously described [10] to pseudo-pregnant recipient Swiss Webster mice approximately 82 h post coitum. Pseudo-pregnancy was induced by mating females in estrus or proestrus with vasectomized CF1 males (Envigo, Indianapolis, IN) and was confirmed by the identification of a vaginal plug after mating [11]. Following ET, on E 17.5, females were euthanized and the number of fetuses and resorptions were noted. Implantation (sum of resorptions and viable fetuses) and fetal development were assessed, and the fetus and the placenta were weighed. Fetal crown-rump-lengths and placental diameters were measured using a Whitworth digital caliper.
Blastocyst extended culture
Blastocyst extended culture was performed as described earlier [4, 5] with minor modifications. The zona pellucida of E 3.5 expanded blastocysts was removed using acidic Tyrode’s solution and cultured in IVC1 medium (Cell Guidance Systems, Cambridge, UK) in μ-Slide 8 Well chambered coverslips (Ibidi, Martinsried, Germany) coated with 30 μg/cm2 fibronectin. After 72 h, 50% of the IVC1 medium was replaced by the same volume of IVC2 medium (Cell Guidance Systems, Cambridge UK) and embryos were cultured for an additional 48 h until E 8.5. All extended culture was carried out at 37 °C in 7.5% CO2 and atmospheric O2.
Extended culture immunofluorescence staining
Embryos were washed once with sterile PBS and fixed in 4% PFA in PBS for 30 min. Fixed embryos were permeabilized with 0.5% (v/v) Triton X-100 in PBS for 30 min and blocked in blocking buffer containing 10% (v/v) FCS (Hyclone), 3% BSA (MP Biomedicals, Solon, Ohio) and 0.1% Tween 20 (Fisher Scientific, Fair Lawn, NJ) in PBS overnight at 4 °C. Embryos were then stained with a primary antibody against POU5F1 (1:200; Santa Cruz Biotechnology, Dallas, TX) in the blocking buffer overnight in 4 °C. After three washes in 0.1% (v/v) Tween 20 in PBS (PBST), embryos were incubated with secondary antibody Alexa Fluor 488 (1:200; Invitrogen), NucBlue (Hoechst33342, R37605, Life Technologies, Carlsbad, CA) and rhodamine phalloidin (1:200; Cytoskeleton, Denver, CO) in the blocking buffer overnight in 4 °C. Embryos were washed three times in PBST and then coated in ProLong Gold Antifade with DAPI (Life Technologies) before confocal imaging.
3D confocal imaging and analysis
Confocal images were obtained using the 3i Marianas inverted spinning disk confocal microscope at the Advanced Light Microscopy Core Facility at the University of Colorado Anschutz Medical Campus (https://lightmicroscopy.ucdenver.edu/). Egg cylinders were captured in a 3D montage with 0.75 μM Z steps on Slidebook software version 6.0.16 (Fig.1c). The entire outgrowth area was not imaged with confocal microscopy. Egg cylinder volume included any embryonic tissues above a height of approximately 10 μM from the bottom of the well. Each image was deconvoluted using Slidebook before further quantitative and qualitative analysis.
Confocal images were analyzed using Imaris × 64 9.20. Using the surface tool, surfaces were created for F-actin, DAPI, and POU5F1 channels. Total surface volume for both actin and DAPI channels were summed to represent total egg cylinder volume (Fig. 1c). The POU5F1 channel surface was specified to identify objects approximately 5–10 μM in diameter within the positively stained surface for a total epiblast cell number (Fig. 1c).
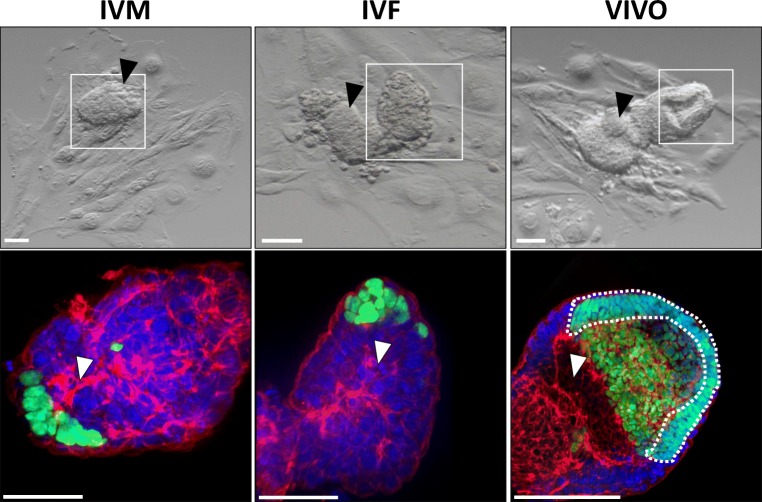
Egg cylinder morphology was qualitatively noted for each embryo upon visual confirmation of advanced structures including the ectoplacental cone, proamniotic cavity, and the morphological characterization of epiblast cells to a columnar shape surrounding the proamniotic cavity [5].
TE outgrowth area measurement
Before fixation on E 8.5, embryos were imaged using the Olympus DP22 camera on a stereomicroscope. ImageJ2 [12] was used to outline and quantify TE outgrowth area, as indicated in pink in Fig. 1c (right panel).
Statistical analysis
Results for ICM/TE cell number, epiblast cell number, egg cylinder volume, outgrowth area, and outgrowth morphologies were analyzed using one-way ANOVA by Graphpad Prism 8.2.0 and significance was noted at P ≤ 0.05. Statistical significance for surgical embryo transfers was determined using Pearson’s chi square analysis and significance was noted at P ≤ 0.05.
Results and discussion
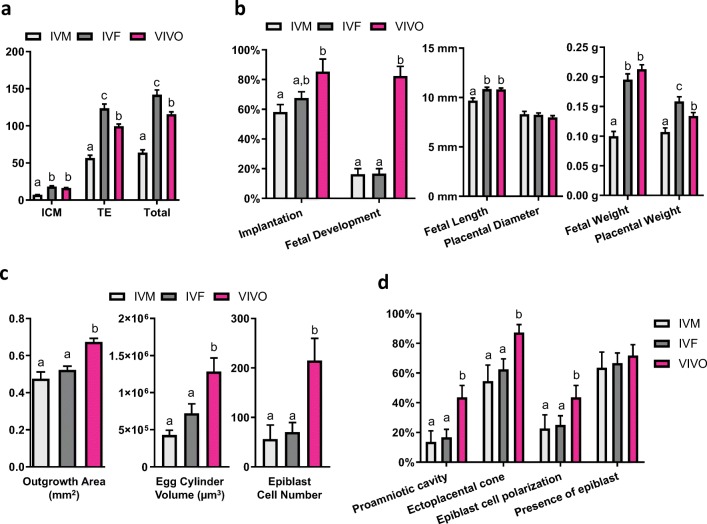
The amount of time spent in vivo during the preimplantation period directly correlates with embryo quality. Poor quality mouse blastocysts were produced from in vitro matured (IVM) oocytes followed by IVF and in vitro culture (IVC), and were defined as the “IVM” group in this study. Intermediate quality blastocysts were produced from in vivo matured oocytes followed by IVF/IVC, defined as the “IVF” group. High quality blastocysts developed in vivo were flushed directly from the uterine horns and defined as the “VIVO” group. At embryonic day (E) 3.5, embryos from these three experimental groups were morphologically similar (Fig. 1b). When fixed to examine ICM and TE cell distribution at E 5, IVM embryos had significantly less ICM and TE cells compared with the other groups, suggesting their compromised developmental potential. IVF embryos had similar numbers of ICM cells but significantly more TE cells compared with the VIVO embryos (Fig. 2a).
Fig. 2.
a ICM, TE, and total cell number in hatching or hatched blastocysts produced from in vitro matured oocytes (IVM) and in vivo matured oocytes (IVF) after 115 h in culture, and in vivo flushed blastocysts (VIVO) at E 4. This experiment was replicated three times with 116 embryos. b Implantation and fetal development after surgical embryo transfer (left), fetal and placental length and diameter, respectively (center), and fetal and placental weights for IVM, IVF, and VIVO fetuses. A total of 279 embryos and 31 recipient animals were used. c Outgrowth areas (left), egg cylinder volume (center), epiblast cell number (right), and d incidences of proamniotic cavity, ectoplacental cone formation, epiblast cell polarization, and presence of epiblast cells in IVM, IVF, and VIVO embryos during in vitro extended culture. Experiments in c and d were replicated three times with 109 embryos. Different letters (a, b, and c) indicate significant different (P < 0.05) value between treatment groups
At E 17.5 after embryo transfer, the IVM group had significantly reduced implantation compared with the VIVO group, and both IVM and IVF groups had significantly reduced fetal development compared with the VIVO group (Fig. 2b, left panel). For the embryos that developed into fetuses, the IVM embryos again had reduced fetal length, fetal weight, and placental weight, suggesting that the health of the fetuses resulting from IVM embryos may have also been compromised (Fig. 2b, middle and right panels). In humans, low fetal weight for gestational age, often defined as the intrauterine growth restriction, is associated with increased morbidity and mortality [13]. IVF embryos had normal fetal weight, but increased placenta weight (Fig. 2b, right panel). The overgrowth of placenta is a phenomenon that was observed in maternally aged mice and has been associated with developmental abnormalities [14]. It may be also correlated with the higher number of TE cells found in the IVF blastocysts. These results confirmed that mouse blastocysts from IVM, IVF, and VIVO groups had distinct implantation and fetal developmental potential, which cannot be accurately predicted by morphology alone. We then used the same model to study parameters during extended embryo culture that could be indicative of blastocyst developmental potential.
Embryos at E 3.5 from each group were placed into extended culture until E 8.5. The surface area covered by TE was obtained and defined as the outgrowth area (Fig. 1c) [15]. Embryos from the IVM and IVF groups had significantly smaller outgrowth area compared with those from the VIVO group (Fig. 2c, left panel). When we examined the average epiblast cell number, again, VIVO embryos had significantly more epiblast cells (Fig. 2c right panel). More interestingly, the proportional differences in epiblast cell number are strikingly similar to fetal development following embryo transfer, suggesting that this parameter may be indicative of the potential of an embryo to successfully develop into a fetus (Fig. 2b, left panel). The measurement of other advanced morphological structures (egg cylinder volume, formation of proamniotic cavity, ectoplacental cone, and epiblast cell polarization) also suggested that VIVO embryos had higher developmental potential than both IVM and IVF embryos (Fig. 2c, d, Fig. 3), although the proportional differences do not closely approximate fetal development.
Fig. 3.
Representative morphological (top) and confocal immunofluorescence (bottom) images for IVM (left), IVF (center), and VIVO (right) embryos. The white box on top panels delineates the area of the confocal image below. Black arrows indicate an emerging ectoplacental cone. White arrows indicate formation of a proamniotic cavity. The dashed white line in bottom right image indicates polarized epiblast cells. Embryos were stained with DAPI for nuclei (blue), rhodamine phalloidin for F-actin (red), and antibody against POU5F1 (green), and the maximum intensity projection of the 3D montage images are presented. Scale bars, 100 μM
Our results demonstrate that extended embryo culture provides more accurate information regarding developmental potential than blastocyst morphological assessment. Specifically, epiblast cell number is valuable in predicting fetal developmental potential. Outgrowth area, formation of the proamniotic cavity, ectoplacental cone, and epiblast cell polarization may also provide valuable information about embryo quality, although they are not as predictive of fetal developmental potential as is epiblast cell number. Both the presence of an organized egg cylinder with a proliferative epiblast cell population in conjunction with a proliferative TE during extended embryo culture reflects blastocyst viability. Thus, extended embryo culture offers a means to sensitively and accurately measure embryo viability without the use of embryo transfer.
Obviously, extended embryo culture cannot be used in clinical assisted reproductive technology (ART) `to predict implantation and fetal developmental potential prior to transfer to produce a pregnancy. However, this technique can still prove quite valuable for research assessment of human embryos and improvement of ART methodology. Either abnormally fertilized 3PN zygotes that would normally be discarded, or blastocysts donated for research, could be cultured in novel media or culture environments prior to extended embryo culture. Currently, after treatment, such embryos would be assessed morphologically and possibly for cell number and allocation or gene expression, as a means to decipher the effectiveness of the novel culture method. Using extended embryo culture, these embryos can be evaluated in a manner that accurately predicts embryo quality. Thus, decisions about new culture medium, supplements, or devices can be based on outcomes known to correlate with post transfer success. These new treatments can then be applied in clinical trials with a higher degree of confidence, less risk, and a better chance of success, leading to faster implementation of innovative culture techniques for human embryos and better clinical outcomes for ART patients.
Compliance with ethical standards
Ethical approval
All procedures were approved by the Fertility Laboratories of Colorado Ethics in Research Committee and followed animal care and use guidelines, as described by the Guide for the Care and Use of Laboratory Animals.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sallam HN, Sallam NH, Sallam SH. Non-invasive methods for embryo selection. Facts Views Vis Obgyn. 2016;8:87–100. [PMC free article] [PubMed] [Google Scholar]
- 2.Gardner DK, Meseguer M, Rubio C, Treff NR. Diagnosis of human preimplantation embryo viability. Hum Reprod Update. 2015;21:727–747. doi: 10.1093/humupd/dmu064. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez T, Seidler EA, Gardner DK, Needleman D, Sakkas D. Will noninvasive methods surpass invasive for assessing gametes and embryos? Fertil Steril. 2017;108:730–737. doi: 10.1016/j.fertnstert.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Bedzhov I, Leung CY, Bialecka M, Zernicka-Goetz M. In vitro culture of mouse blastocysts beyond the implantation stages. Nat Protoc. 2014;9:2732–2739. doi: 10.1038/nprot.2014.186. [DOI] [PubMed] [Google Scholar]
- 5.Bedzhov I, Zernicka-Goetz M. Self-organizing properties of mouse pluripotent cells initiate morphogenesis upon implantation. Cell. 2014;156:1032–1044. doi: 10.1016/j.cell.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shahbazi MN, Jedrusik A, Vuoristo S, Recher G, Hupalowska A, Bolton V, et al. Self-organization of the human embryo in the absence of maternal tissues. Nat Cell Biol. 2016:1–8. [DOI] [PMC free article] [PubMed]
- 7.National Research Council (US) Committee for the update of the guide for the care and use of laboratory animals. In: guide for the care and use of laboratory animals. Washington (DC): National Academies Press (US) National Academy of Sciences. 2011.
- 8.Herrick JR, Strauss KJ, Schneiderman A, Rawlins M, Stevens J, Schoolcraft WB, et al. The beneficial effects of reduced magnesium during the oocyte-to-embryo transition are conserved in mice, domestic cats, and humans. Reprod Fertil Dev. 2015;27:323–31. [DOI] [PubMed]
- 9.Bakhtari A, Ross PJ. DPPA3 prevents cytosine hydroxymethylation of the maternal pronucleus and is required for normal development in bovine embryos. Epigenetics. 2014;9:1271–1279. doi: 10.4161/epi.32087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagy A, Gertsenstein M, Vintersten K, Behringer R. Manipulating the mouse embryo: a laboratory manual. 3. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2003. [Google Scholar]
- 11.Byers SL, Wiles MV, Dunn SL, Taft RA. Mouse estrous cycle identification tool and images. PLoS One 2012; 7:9. [DOI] [PMC free article] [PubMed]
- 12.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garite TJ, Clark R, Thorp JA. Intrauterine growth restriction increases morbidity and mortality among premature neonates. Am J Obstet Gynecol. 2004;191:481–487. doi: 10.1016/j.ajog.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 14.Paczkowski M, Schoolcraft WB, Krisher RL. Dysregulation of methylation and expression of imprinted genes in oocytes and reproductive tissues in mice of advanced maternal age. J Assist Reprod Genet. 2015;32:713–723. doi: 10.1007/s10815-015-0463-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hannan NJ, Paiva P, Meehan KL, Rombauts LJ, Gardner DK, Salamonsen LA. Analysis of fertility-related soluble mediators in human uterine fluid identifies VEGF as a key regulator of embryo implantation. Endocrinology. 2011;152:4948–4956. doi: 10.1210/en.2011-1248. [DOI] [PubMed] [Google Scholar]