Abstract
Purpose
To correct a potentially damaging mutation in haploid human embryonic stem cells.
Methods
Exome sequencing was performed on DNA extracted from parthenogenetically derived embryonic stem cell line (pES12). An SLC10A2 gene mutation, which affects bile acid transport, was chosen as mutation of interest in this proof of concept study to attempt correction in human pluripotent haploid cells. Confirmation of the mutation was verified, and guide RNA and a correction template was designed in preparation of performing CRISPR. Haploid cells underwent serial fluorescence activated cell sorting (FACS) with Hoechst 33342 to create an increasingly haploid (1n) enriched culture. Nucleofection was performed on p. 37 and then cells were sorted for 1n DNA content with +GFP to identify the haploid cells that expressed Cas9 tagged with GFP.
Results
104,686 haploid GFP + cells were collected. Cells were cultured, individual colonies picked, and 48 clones were sent for Sanger sequencing. CRIPSR efficiency was 77.1%, with 7/48 (14.6%) clones resulting in a corrected SLC10A2 mutation. Confirmation of persistence of haploid cells was achieved with repeated FACS sorting and centromere quantification. Given the large number of passages and exposure to CRISPR, we also performed analysis of karyotypes and of off-target effects. Cells evaluated were karyotypically normal and there was no evident off target effects.
Conclusions
CRISPR/Cas9 can be effectively utilized to edit mutations in haploid human embryonic stem cells. Establishment and maintenance of a haploid cell culture provides a novel way to utilize CRISPR/Cas9 in gene editing, particularly in the study of recessive alleles.
Keywords: Haploid stem cells, CRISPR/Cas-9, Gene editing
Introduction
Most mammalian cells are diploid, containing two sets of chromosomes with inheritance of one set from each parent, with the exception of gametes, which are haploid. In contrast to many somatic diploid cell types, haploid human cells do not divide, and in women are present in limited numbers [1]. Fertilization of haploid gametes, each with a single set of chromosomes, results in a diploid zygote that gives rise to all the cells of the organism. The unfertilized oocyte contains a single genome of 23 chromosomes, and when artificially activated it can develop to the blastocyst stage and give rise to parthenogenetic human pluripotent stem cell lines [2]. Parthenogenetic blastocysts and the derived stem cell lines contain a combination of both haploid and diploid cells. However, upon stem cell derivation, haploid cells undergo spontaneous diploidization due to endoreduplication or missed cytokinesis [3]. Procuring primarily haploid mammalian cell cultures has remained elusive for a long time. Recent methodological progress has enabled the derivation of haploid stem cell lines from mammalian haploid embryos [4]. Recently, Sagi et al. have described the isolation of human haploid stem cells from parthenogenetically activated eggs [5].
Haploid stem cells are of particular interest for analysis of gene function. In diploid cells, heterozygous mutations often lead to small or no phenotypic changes because a functional allele on a second chromosome set can mask the effects of the disruption of the same allele on the first chromosome set. On the other hand, haploid cells contain only one copy of each chromosome, and disruption of one allele can therefore produce a loss-of-function phenotype [6]. These principles of haploid genetics have most successfully been used in yeast, and are now becoming available in human cells. Haploid stem cells contain the genetic equivalent of an oocyte, and therefore represent a proliferating germ cell. Because the number of oocytes in the female germ line is limited, with approximately 1–2 million oocytes at birth and steadily declines with age, there is a limited reproductive time span of women. Haploid parthenogenetic stem cells divide to unlimited numbers, and could potentially serve as a source of genomes for reproduction. In mice, haploid pluripotent stem cells have successfully been used to make mice by substituting for the oocyte genome [7]. In human cells, haploid androgenetic pluripotent stem cells can substitute for a sperm to form a diploid zygote able to develop to the blastocyst stage [8]. A significant difference to non-proliferating germ cells is that haploid stem cells allow expansion and thereby extensive genetic quality control. This is relevant in the context of gene editing of disease-causing alleles, which is being discussed for use in human reproduction.
Recently, it has become possible to edit the human genome with high efficiency utilizing the clustered regularly interspaced short palindromic repeats (CRISPR) system [3, 9–12]. This system uses the Cas9 protein alongside a small guide RNA (sgRNA) complementary to the target DNA sequence. Cas9 promotes genome editing by inducing a double stranded break (DSB) at a target genomic locus. Upon cleavage by Cas9, the target locus typically undergoes one of two major pathways for DNA damage repair: the error-prone non-homologous end joining (NHEJ) or the high-fidelity homology directed repair (HDR) pathway, the latter of which can be used to achieve a desired editing outcome [13]. HDR-mediated repair can be used to introduce specific point mutations or to insert desired sequences through recombination of the target locus with exogenously supplied DNA “donor templates” [14].
To date, CRISPR/Cas9 has been utilized to introduce genetic modifications into mammalian haploid cells from mice [4, 15] and rats [16]. However, to our knowledge, CRISPR has not been used to edit and correct a known mutation using HDR-mediated repair in haploid human pluripotent stem cells. In this study, the authors hypothesize that CRISPR/Cas9 could be utilized to correct a potentially damaging mutation in haploid human pluripotent stem cell line. Using exome sequencing, we identified damaging mutations in 65 genes in a haploid pluripotent stem cell line. An SLC10A2 gene mutation identified in this stem cell line, which affects bile acid transport, was chosen as the mutation of interest in this proof of concept study to attempt correction in human pluripotent haploid cell. This mutation was chosen because it was present in somatic cells of the oocyte donor, and because the gene has been implicated in a variety of gastrointestinal disorders. We demonstrate efficient correction of this mutation in haploid stem cells derived from parthenogenetically activated human oocyte.
Materials and methods
Haploid pluripotent stem cells
The derivation of pES12 was described previously by Sagi et al. [5]. In brief, oocyte donation for research was approved under the Columbia University IRB under protocol AAAI1347. Women who presented to donate oocytes for reproductive purposes at Columbia’s Center for Women’s Reproductive Care were also eligible to donate to research. There was no recruitment performed for woman to specifically donate for research purposes. Women were offered participation to donate to research if they met criteria for donation, and received identical compensation as donors who cycled for reproductive purposes. Donors were counseled and consented to cycle specifically for research, and all oocytes obtained from the cycle were utilized for research purposes. Donor assigned de-identified number 1160 underwent uncomplicated stimulation cycle and subsequent oocyte aspiration on July 26, 2013 (10 days of stimulation, Max E2 2048). A total of 26 oocytes were retrieved (22 MII, 3 MI, and 1 GV). Additionally, a skin biopsy was obtained at the time of oocyte aspiration. Six of the mature oocytes were activated by an electrical pulse, five developed to the blastocyst stage, resulting in four stem cell lines including two haploid stem cell lines pES10 and pES12.
Stem cell culturing was performed on growth arrested mouse embryonic fibroblast cells (MEFSs). Standard human embryonic stem cell medium containing knockout serum replacement was utilized. Cultures were sorted for DNA content every 3–4 passages to create an increasingly haploid enriched culture utilizing the protocol by I. Sagi et al. [17]. DNA-based fluorescence activated cell sorting (FACS) is performed by viably staining DNA with Hoechst 33342, followed by isolating the cells with a DNA content corresponding to less than two chromosomal copies, that is, haploid cells in G1 of the cell cycle.
Selection and confirmation of SLC10A2 mutation
DNA was extracted from pES12 and pES10 cells and sent for Agilent whole exome sequencing. Damaging mutations were classified according to combined annotation-dependent depletion (CADD) score. A CADD score of greater than 15 has been shown to be highly associated with a deleterious disease state [18].
Fibroblasts were cultured from Donor 1160 skin biopsy, and DNA was extracted and subsequently sent for Sanger sequencing to verify maternal origin of the mutation, as opposed to a de novo mutation.
Gene editing utilizing CRISPR/Cas9
In preparation for CRISPR, guide RNA (gRNA) and correction template were designed targeting the mutant SLC10A2 gene. gRNA was selected using software from cripsr.mit.edu. Guides were chosen with the highest index score in a region closest to the DNA region of interest. CRISPR gRNA and correction template was designed in order to induce homology-directed repair. The correction template incorporated a modification to the protospacer adjacent motif (PAM) recognized by the guide RNA to avoid cutting of the edited sequence by Cas9. Following nucleofection (Amaxa Cell Line Nucleofector Kit II, program A-23) of a plasmid encoding Cas9-GFP, a plasmid encoding the guide RNA, and the correction template, cells were plated, cultured for 2 additional days, stained with Hoechst 33342, and subsequently sorted via FACS for cells that had both haploid DNA content and GFP positivity. Single colonies were propagated and duplicates were made for cryopreservation and for DNA isolated for PCR and Sanger sequencing.
Results
Identification of damaging mutations in pES12
All human genomes contain a significant number of damaging mutations. It has been estimated that a “normal, healthy” individual is a heterozygous carrier of 40–100 highly penetrant deleterious variants that can potentially cause a Mendelian disease; many of these represent recessive carrier states [19]. To identify damaging mutations, we sequenced the exome of a haploid stem cell line pES12. A total of 65 mutations identified were thought to have potential for a damaging phenotype based on CADD scoring of greater than 15. Both cell lines, originating from the same oocyte donor, carried a mutation in the SLC10A2 gene. This gene, also known as ASBT, is involved in bile acid transportation, and has been implicated in disease states including cholelithasis, cholesterol homeostasis [20], hypertriglyceridemia [21], chronic ileitis, chronic diarrhea, and susceptibility to colon cancer [22].
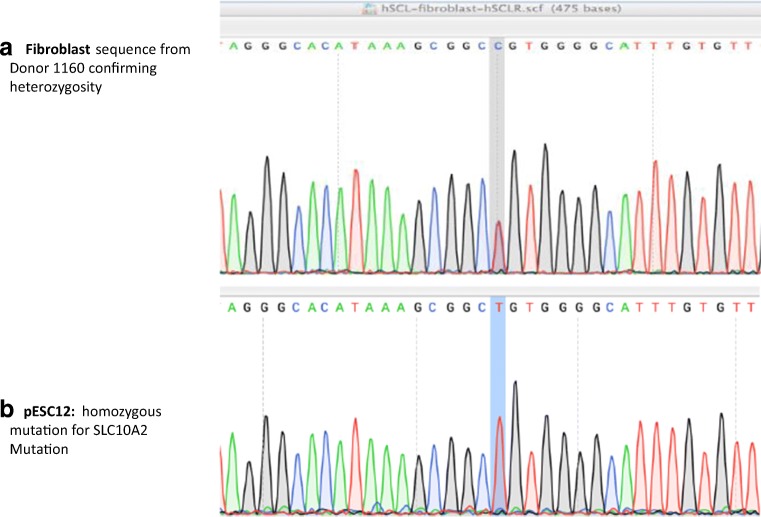
Following exome sequencing, confirmation of the mutation in both the donor and pESC 12 line was undertaken using Sanger sequencing. This mutation was confirmed in the fibroblast cells, which were heterozygous for the mutation, as well as the stem cell lines pES12, which was hemizygous for the mutation of interest (Fig. 1). This gene mutation, located on chromosome 13, is a non-synonymous single nucleotide variant resulting in a change of the amino acid proline to leucine at position 65 (rs112657170, P65L). According to the CADD score of 27.1, the mutation is predicted to be damaging; the amino acid sequence is highly conserved from lamprey to human. As skin fibroblasts, pES12 carried the identical mutation, the mutation was germ line, and not de novo.
Fig. 1.
Confirmation of the SLC10A2 mutation. a Donor is a heterozygous for SLC10A2 using DNA extracted from fibroblast cells. b pESC12 stem cell line homozygous for SLC10A2 mutation. Location of mutation in gray
Gene editing of SLC10A2
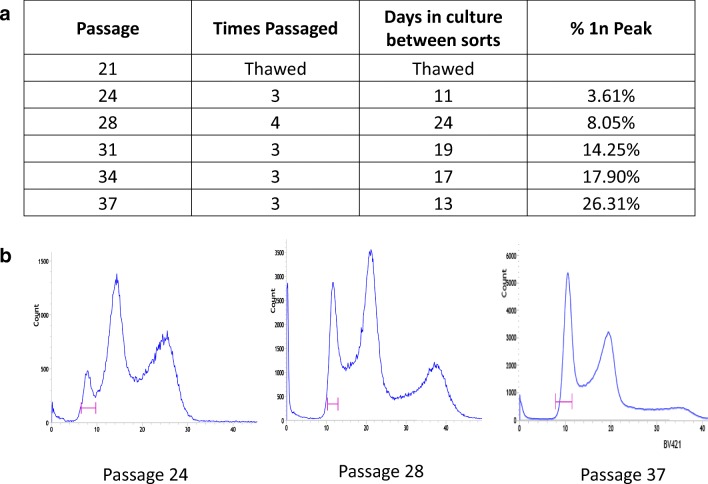
To correct the mutation in haploid cells, we thawed a frozen vial of pES12 at passage (p) 21, cultured, and performed sorting for haploid cells. Serial sorting was performed in order to create an increasingly purified population of cells. With each subsequent sort, the percentage of haploid cells in G1 was increased from 3.6 to 26.3% (Fig. 2). Because this number does not include haploid cells in S phase and in G2 and M phase of the cell cycle, and because in human stem cells only ~ 20% of the cells are in G1 while ~ 80% are in S/G2/M phase of the cell cycle [23], cells were almost uniformly haploid at the final sort prior to performing CRISPR. Cells sorted from p. 37 were cultured for one passage after sorting, to expand cells for plasmid transfection with CRISPR/Cas9.
Fig. 2.
FACS for haploid purification. a Percent of haploid cells in G1 with serial sorting. b FACS sorting peaks by passage number. Pink bar demarcating FACS gate created to capture cells with haploid DNA content. Thirty or more precent haploid in G1 are essentially pure haploid cultures
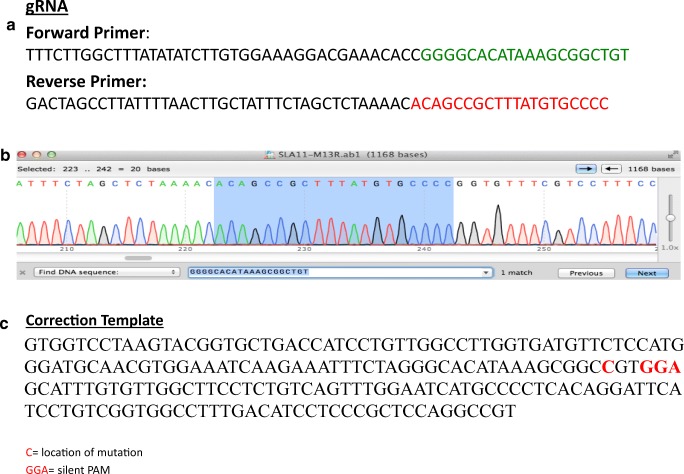
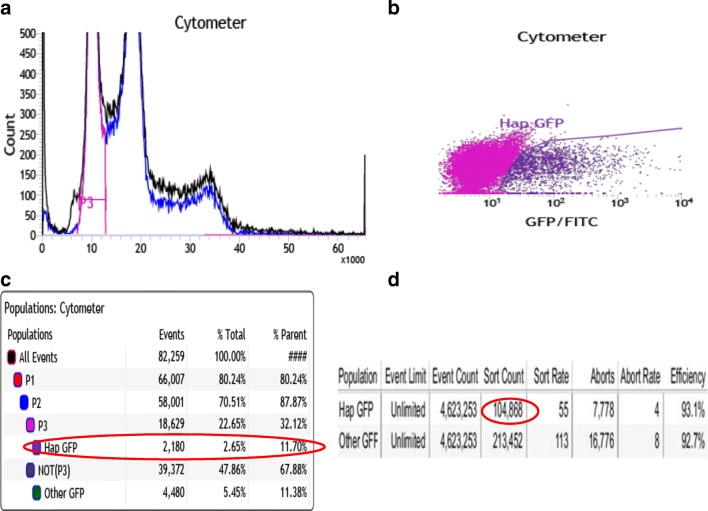
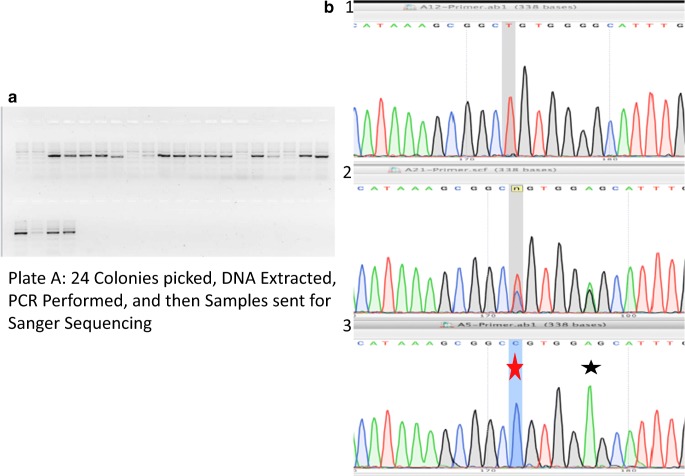
The gRNA, confirmation of bacterial transformation with gRNA, and correction template for SLC10A2 mutation utilized for gene editing can be seen in Fig. 3. Nucleofection was performed on 2 wells of a 6-well plate at 80% confluence. The cells were re-plated onto MEF’s and cultured for an additional 2 days. FACS sorting was performed to collect cells containing haploid DNA content and were also GFP+ (green florescent protein). This effectively collected the haploid cells that had taken up the plasmid vector (Cas9 tagged with GFP). There was a total of 32.12% haploids in G1, and 11.70% of the live cells were both G1 haploids and GFP+ (Fig. 4). A total of 104,686 haploid cells with GFP positivity were collected and plated onto 2 × 10 cm dishes (approximately 45,000 cells/10 cm dish) and 2 wells of 6-well plate (approximately 7000 cells/well). Cells were cultured for 8 days until cell colonies were large enough to be picked manually. A total of 168 colonies were picked and plated onto 7 × 24 well plates. Five to 6 days later, 120 colonies (5 × 24 well plates) were split to duplicate wells and cultured for an additional 5–6 days. At this point, one set of identical colonies of cells underwent DNA extraction, while the other set of cells remained in culture until there were sufficient cells to undergo freezing.
Fig. 3.
a SLC10A2 guide RNA. b Confirmation of bacterial transformation with plasmid incorporating gRNA sequence. c Correction template with silent PAM (protospacer adjacent motif)
Fig. 4.
Sorting for haploid + GFP+. a FACS sorting after CRISPR: P3 representing haploid peak. b Cells with haploid DNA content and expressing GFP. c When sorting for GFP + haploids, 11.7% of the live cells were isolated. d A total of 104,868 cells with haploid + GFP+ were isolated
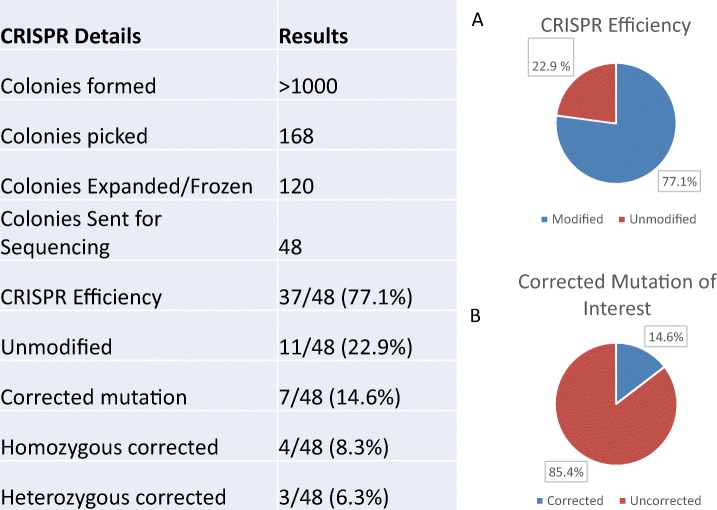
DNA was extracted from 120 colonies. PCR was performed and 2% gel agarose run in order to confirm sufficient DNA amplification to be sent for Sanger sequencing. Sequencing results from DNA extracted from uncorrected, heterozygous corrected, and homozygous corrected clones can be seen in Fig. 5. A total of 48 samples of DNA were sent for sequencing. Overall, 37/48 (77.1%) of the colonies showed evidence of CRISPR-mediated gene modification (Table 1, A). Seven of 48 (14.6%) cells resulting in correction of the mutation (Table 1, B), 4/7 (8/3%) homozygous corrected and 3/7 (6.3%) of the colonies contained cells with both corrected and mutant alleles, while 30/48 (62.5%) cells contained a deletion or insertion. Colonies containing more than one allele may be due to mosaicism from two mixed colonies or due to diploidization and were not further investigated.
Fig. 5.
Sanger sequencing of clones after CRISPR/Cas9. a Agarose gel. b Sequencing results. 1, uncorrected haploid clone; 2, heterozygous diploid corrected clone; 3, corrected haploid clone. Red star, corrected mutation. Black star, silent edit
Table 1.
Sequencing results
A shows the CRISPR efficiency; B, corrected clones for SLC10A2 mutation
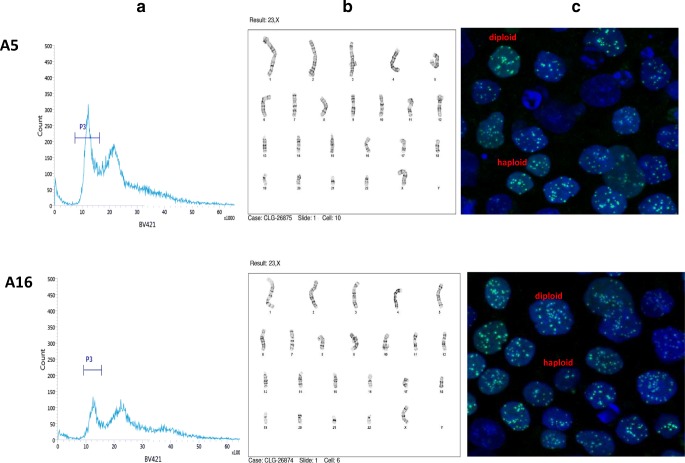
To determine whether corrected cells that maintained haploid status, 2 of the homozygous corrected lines, A5 and A16, continued to be cultured, repeat FACS sorting using Hoechst dye was performed, and A5 displayed 31.94% haploids and A16 with 20.77% haploids in G1 of the cell cycle (Fig. 6a). Additionally, cells from each line were plated onto glass bottom dishes and underwent centromere staining. This method can also distinguish between haploid and diploid pluripotent stem cells [5] by quantifying the number of centromeres seen, with haploids displaying ~ 23 centromeres and diploids with ~ 46 centromeres. Due to centromere clustering, the number of centromeres is not a precise measure of chromosome number, but allows to distinguish ploidy. Both corrected cell lines showed a high percentage of haploid cells using confocal microscopy (Fig. 6c). The two cell lines were also sent for karyotype analysis, with results for each displaying a mixture of normal haploid karyotype (23X) as well as normal female diploid karyotype (Fig. 6b). For A5 clone, there were 11/20 cells (55%) with normal haploid karyotype, and for A16, there were 8/20 cells (40%) with a normal haploid karyotype. The time from sorting of haploid cells to karyotyping was 15 days. Therefore, the conversion rate of haploid to diploid cells was low, or ~ 5% per day.
Fig. 6.
Confirmation of persistent haploid cells in corrected cell lines A5 and A16. a FACS sorting results: P3 representing haploid peak. b Karyotype: normal haploid female karyotype. c Centromere staining with demonstrating haploid and diploid cells
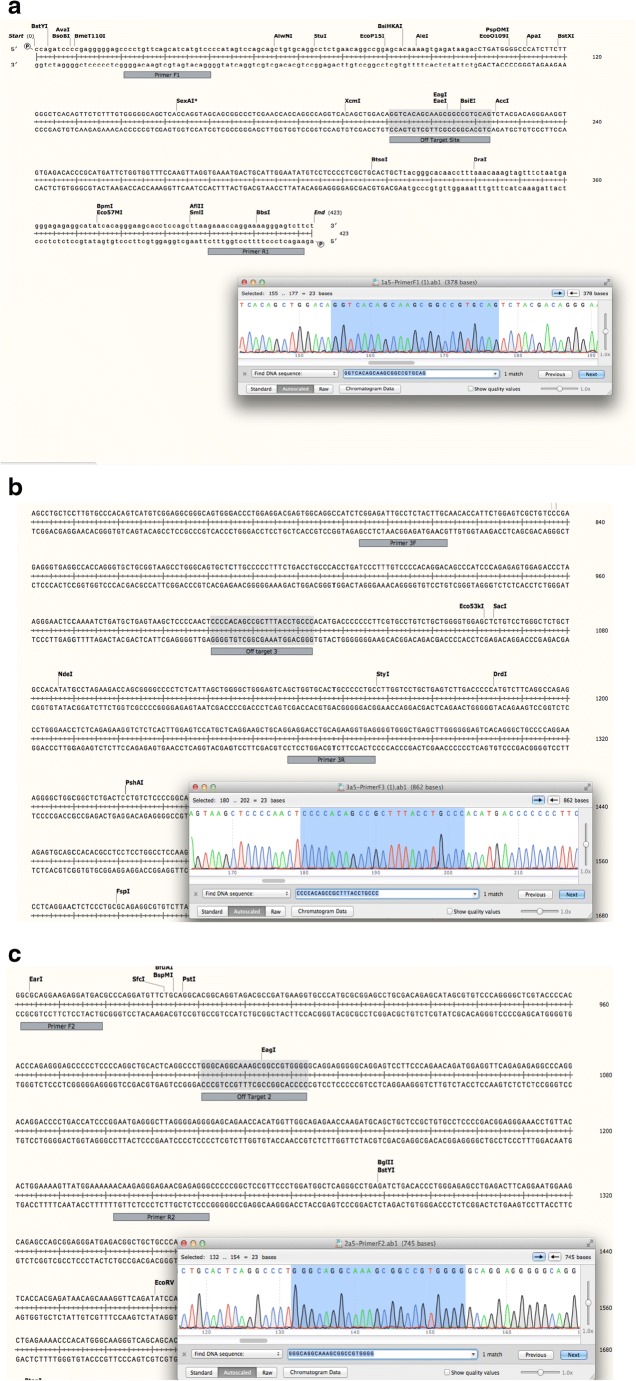
Because CRISPR can sometimes result in off-target effects [24], three regions with the most homology to the gRNA were examined for CRISPR cutting. PCR was performed on DNA extracted from seven corrected clones, and each site was sequenced using Sanger sequencing. We found that there was no off-target effect for any of the three regions in each of the seven corrected clones. For corrected clone A5, no off-target effect for the three regions of interested is exemplified in Fig. 7 a–c.
Fig. 7.
a–c Evaluation for off-target effects
Discussion
Here we show that the CRISPR/Cas9 can be used to effectively correct potentially damaging mutations in human haploid pluripotent cells. CRISPR efficiency in this study was 77.1%, with incorporation of correction template in 14.6% of the colonies sent for Sanger sequencing. Utilizing gene editing in haploid cells offers promising advancements in the way we study gene function. Because all of the genetic modifications result in a single allele, phenotypic effects are more conclusive. Our future experiments will focus on the phenotypic effects of the SLC10A2 mutation. To assess if there is a functional difference in bile acid transport following correction of SLC10A2 mutation, we plan to differentiate the pluripotent stem cells using the corrected pESC12 cells into gut cells, and compare them to uncorrected pESC12 cells, cells with resultant large deletions following CRISPR. This will allow for an analysis on bile acid transport and the functional evaluation of the naturally occurring variable of SLC10A2 mutation that is inherited in the human population.
The human genome contains numerous variants that may predispose to disease risk. The expansion of sequencing capabilities due to advances in next generation sequencing has led to the identification of more than 100 million SNPs [25]. The functional consequences of these SNPs are largely unknown, and few experimental tools are available to conclusively identify their function. Haploid stem cells provide a suitable research tool to gain such understanding, as the allelic complexity of the genome is reduced to homozygosity. Each SNP is present in homozygous form and, when edited, is also homozygous, likely providing greater amplitude in functional readouts. The utility of haploid cells in functional genetic screening has recently been demonstrated in HAP1 cells [26]. However, these cells represent only a single transformed cell type, while haploid pluripotent stem cells can be differentiated to any cell type of interest [5].
Beyond the study of gene function, haploid stem cells may offer new possibilities in human reproduction. Because the number of oocytes in the female germ line is limiting, haploid parthenogenetic stem cells could provide an additional source of genetic material for reproductive purposes. The haploid genome is genetically equivalent to the genome of the oocyte, and therefore is essentially a proliferating female gamete. Combined with gene editing as described here, haploid cells may provide a path to generate an embryo with an edited allele. The ability to replace the genome of the human oocyte by nuclear transfer has previously been demonstrated [27]. Furthermore, the oocyte is able to nucleate a functional spindle on transferred chromatin of cultured cells [28]. In mice, haploid parthenogenetic ESCs have been used as a substitute for the oocyte genome to create fertile mice [29]. However, it is not currently known whether human haploid stem cells can substitute for the oocyte genome and allow development to proceed.
Currently, inherited mutations that affect offspring can be screened for using pre-implantation genetic diagnosis of embryos, with the subsequent transfer of embryos free of a specific mutation. Pre-implantation genetic testing for monogenic/single gene disorders (PGT-M) does not involve any genetic manipulation and is effective for many single gene disorders, but has some significant limitations. PGT-M is most effective for exclusion of one disease-causing allele at a time, but less practical for the exclusion of multiple alleles associated with disease risk. The number of oocytes that can be obtained in a single egg donation is 5–20, with only half of the embryos developing to the blastocyst stage. The exclusion of embryos after genetic diagnosis will further reduce the number of embryos available for implantation. With each allele to be excluded, the number of embryos that are eligible for implantation will be reduced by 50%. While most heterozygous mutations do not usually cause severe disease, there is ample evidence for heterozygous mutations predisposing to risk. For instance, heterozygous mutations in genes involved in beta cell function can cause diabetes [30]. Pluripotent haploid stem cells potentially allow the modification of an unlimited number of such mutations prior to the generation of an embryo. Such extensive modifications could potentially reduce disease risk for most common human disorders.
The editing of human embryos by injection of CRISPR/Cas9 was recently reported [31, 32]. These embryos showed mosaicism, as well as insertions/deletions due to non-homologous end joining. Therefore, there is concern with regard to inadvertent genetic modifications in the germ line. The effect of such novel alleles is not known, but cannot be tested for safety in the setting of human reproduction. Inadvertent genetic modifications are incompatible with the principle of ensuring efficacy and safety prior to clinical translation. Comprehensive characterization to exclude the presence of novel and potentially damaging alleles in embryos is critical, but inferring the genotype of the embryo from one or a small number of cells may not be accurate, and the genetic characterization of all cells requires the destruction of the embryo. Furthermore, the genotyping from single cells or of a small number of cells is not trivial, due to the limited genetic material available. The challenges to accurately genotype human embryos and distinguish gene correction from other repair outcomes are apparent in a recent study on gene editing of a mutation causing heart disease [33–35]. The complexity of possible repair outcomes after induction of a double strand is a significant obstacle to clinical translation. An alternative to double strand break-induced gene editing is base editing; a technique that yielded promising results for the correction of a mutation causing Marfan syndrome [36]. This technique reduces the complexity of repair outcomes because no double strand break is induced, but is not applicable for the correction of deletions or insertions. Unlike gene editing of the embryo, the use of haploid stem cells as a gamete allows extensive and accurate quality controls because the genetic material is not limiting. Furthermore, an unlimited number of genes may be edited in succession, followed by quality controls. However, prior to potential use in reproduction, possible epigenetic consequences of using stem cells as a gamete need to be considered.
This proof of concept study to correct a mutation in haploid human pluripotent stem cells shows that this is a suitable technique to modify SNPs and generate isogenic stem cell lines with homozygous differences at a locus of interest. A number of important questions are needed to be addressed in future studies. The differentiation of stem cells and functional testing into gut cells, the cell type in which SLC10A2 is expressed. SLC10A2 is the primary bile acid transporter in gut cells, and we expect bile acid transport to be compromised in knockout cells and in cells with the P65L mutation. Furthermore, the maintenance of haploid cells currently requires FACS sorting approximately every month, because of spontaneous diploidization. Studies on the mechanisms of diploidization of haploid stem cells may provide insight on how to create more stable haploid cultures. And finally, the ability of a haploid genome to substitute the genome of an oocyte remains to be tested.
Conclusion
CRISPR/Cas9 can be effectively utilized to edit mutations in haploid human pluripotent stem cells. Correction of naturally occurring SNPs in haploid cells provides a novel way to utilize CRISPR/Cas9 in gene editing for the study of recessive alleles and of gene functionality. Gene editing combined with functional studies in different cell types, haploid pluripotent stem cells will provide an effective experimental system to the functional understanding of human genetic variants.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lauren Zakarin Safier, Email: lauren.safier@stonybrookmedicine.edu.
Dietrich Egli, Email: de2220@cumc.columbia.edu.
References
- 1.Baker TG. A quantitative and cytological study of germ cells in human ovaries. Proc R Soc Lond B. 1963;158(972):417–433. doi: 10.1098/rspb.1963.0055. [DOI] [PubMed] [Google Scholar]
- 2.Brevini TA, Pennarossa G, Antonini S, Gandolfi F. Parthenogenesis as an approach to pluripotency: advantages and limitations involved. Stem Cell Rev. 2008;4(3):127–135. doi: 10.1007/s12015-008-9027-z. [DOI] [PubMed] [Google Scholar]
- 3.Horii T, Hatada I. Genome editing using mammalian haploid cells. Int J Mol Sci. 2015;16(10):23604–23614. doi: 10.3390/ijms161023604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leeb M, Wutz A. Derivation of haploid embryonic stem cells from mouse embryos. Nature. 2011;479(7371):131. doi: 10.1038/nature10448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sagi I, Chia G, Golan-Lev T, Peretz M, Weissbein U, Sui L, Sauer MV, Yanuka O, Egli D, Benvenisty N. Derivation and differentiation of haploid human embryonic stem cells. Nature. 2016;532(7597):107–111. doi: 10.1038/nature17408. [DOI] [PubMed] [Google Scholar]
- 6.Wutz A. Haploid mouse embryonic stem cells: rapid genetic screening and germline transmission. Annu Rev Cell Dev Biol. 2014;30:705–722. doi: 10.1146/annurev-cellbio-100913-012920. [DOI] [PubMed] [Google Scholar]
- 7.Li W, Shuai L, Wan H, Dong M, Wang M, Sang L, Feng C, Luo GZ, Li T, Li X, Wang L. Androgenetic haploid embryonic stem cells produce live transgenic mice. Nature. 2012;490(7420):407–411. doi: 10.1038/nature11435. [DOI] [PubMed] [Google Scholar]
- 8.Zhang XM, Wu K, Zheng Y, Zhao H, Gao J, Hou Z, Zhang M, Liao J, Zhang J, Gao Y, Li Y, Li L, Tang F, Chen ZJ, Li J.. In vitro expansion of human sperm through nuclear transfer. 2019. 10.1038/s41422-019-0265-1. [DOI] [PMC free article] [PubMed]
- 9.Cho SW, Kim S, Kim JM, Kim JS. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat Biotechnol. 2013;31:230–232. doi: 10.1038/nbt.2507. [DOI] [PubMed] [Google Scholar]
- 10.Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, Lim WA. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell. 2013;152(5):1173–1183. doi: 10.1016/j.cell.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fogarty NM, McCarthy A, Snijders KE, Powell BE, Kubikova N, Blakeley P, Lea R, Elder K, Wamaitha SE, Kim D, Maciulyte V. Genome editing reveals a role for OCT4 in human embryogenesis. Nature. 2017;550(7674):67. doi: 10.1038/nature24033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paquet D, Kwart D, Chen A, Sproul A, Jacob S, Teo S, Olsen KM, Gregg A, Noggle S, Tessier-Lavigne M. Efficient introduction of specific homozygous and heterozygous mutations using CRISPR/Cas9. Nature. 2016;533(7601):125–129. doi: 10.1038/nature17664. [DOI] [PubMed] [Google Scholar]
- 14.Sander JD, Joung JK. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol. 2014;32(4):347–355. doi: 10.1038/nbt.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimura Y, Oda M, Nakatani T, Sekita Y, Monfort A, Wutz A, Mochizuki H, Nakano T. CRISPR/Cas9-mediated reporter knock-in in mouse haploid embryonic stem cells. Sci Rep. 2015;5:10710. doi: 10.1038/srep10710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li W, Li X, Li T, Jiang MG, Wan H, Luo GZ, Feng C, Cui X, Teng F, Yuan Y, Zhou Q. Genetic modification and screening in rat using haploid embryonic stem cells. Cell Stem Cell. 2014;14(3):404–414. doi: 10.1016/j.stem.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 17.Sagi I, Egli D, Benvenisty N. Identification and propagation of haploid human pluripotent stem cells. Nat Protoc. 2016;11(11):2274–2286. doi: 10.1038/nprot.2016.145. [DOI] [PubMed] [Google Scholar]
- 18.Itan Y, Shang L, Boisson B, Ciancanelli MJ, Markle JG, Martinez-Barricarte R, Scott E, Shah I, Stenson PD, Gleeson J, Cooper DN. The mutation significance cutoff: gene-level thresholds for variant predictions. Nat Methods. 2016;13(2):109–110. doi: 10.1038/nmeth.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzaga-Jauregui C, Lupski JR, Gibbs RA. Human genome sequencing in health and disease. Annu Rev Med. 2012;63:35–61. doi: 10.1146/annurev-med-051010-162644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao L, Pan G. An important intestinal transporter that regulates the enterohepatic circulation of bile acids and cholesterol homeostasis: the apical sodium-dependent bile acid transporter (SLC10A2/ASBT). Clin Res Hepatol Gastroenterol. 2017. [DOI] [PubMed]
- 21.Love MW, Craddock AL, Angelin B, Brunzell JD, Duane WC, Dawson PA. Analysis of the ileal bile acid transporter gene, SLC10A2, in subjects with familial hypertriglyceridemia. Arterioscler Thromb Vasc Biol. 2001;21(12):2039–2045. doi: 10.1161/hq1201.100262. [DOI] [PubMed] [Google Scholar]
- 22.Wang W, Xue S, Ingles SA, Chen Q, Diep AT, Frankl HD, Stolz A, Haile RW. An association between genetic polymorphisms in the ileal sodium-dependent bile acid transporter gene and the risk of colorectal adenomas. Cancer Epidemiol Prev Biomark. 2001;10(9):931–936. [PubMed] [Google Scholar]
- 23.Becker KA, Ghule PN, Therrien JA, Lian JB, Stein JL, Van Wijnen AJ, Stein GS. Self-renewal of human embryonic stem cells is supported by a shortened G1 cell cycle phase. J Cell Physiol. 2006;209(3):883–893. doi: 10.1002/jcp.20776. [DOI] [PubMed] [Google Scholar]
- 24.Fu Y, Foden JA, Khayter C, Maeder ML, Reyon D, Joung JK, Sander JD. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat Biotechnol. 2013;31(9):822–826. doi: 10.1038/nbt.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Library of Medicine (US). Genetics Home Reference [Internet]. Bethesda (MD): The Library; 2020. Available from: https://ghr.nlm.nih.gov/primer/genomicresearch/snp
- 26.Findlay GM, Daza RM, Martin B, Zhang MD, Leith AP, Gasperini M, et al. Accurate classification of BRCA1 variants with saturation genome editing. Nature. 2018:1. [DOI] [PMC free article] [PubMed]
- 27.Paull D, Emmanuele V, Weiss KA, Treff N, Stewart L, Hua H, Zimmer M, Kahler DJ, Goland RS, Noggle SA, Prosser R. Nuclear genome transfer in human oocytes eliminates mitochondrial DNA variants. Nature. 2013;493(7434):632–637. doi: 10.1038/nature11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chia G, Agudo J, Treff N, Sauer MV, Billing D, Brown BD, Baer R, Egli D. Genomic instability during reprogramming by nuclear transfer is DNA replication dependent. Nat Cell Biol. 2017;19(4):282. doi: 10.1038/ncb3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wan H, He Z, Dong M, Gu T, Luo GZ, Teng F, Xia B, Li W, Feng C, Li X, Li T. Parthenogenetic haploid embryonic stem cells produce fertile mice. Cell Res. 2013;23(11):1330–1333. doi: 10.1038/cr.2013.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thanabalasingham G, Owen KR. Diagnosis and management of maturity onset diabetes of the young (MODY) BMJ. 2011;343(7828):837–842. doi: 10.1136/bmj.d6044. [DOI] [PubMed] [Google Scholar]
- 31.Tang L, Zeng Y, Du H, Gong M, Peng J, Zhang B, et al. CRISPR/Cas9-mediated gene editing in human zygotes using Cas9 protein. Mol Gen Genomics. 2017:1–9. [DOI] [PubMed]
- 32.Liang P, Xu Y, Zhang X, Ding C, Huang R, Zhang Z, Lv J, Xie X, Chen Y, Li Y, Sun Y. CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes. Protein Cell. 2015;6(5):363–372. doi: 10.1007/s13238-015-0153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma H, Marti-Gutierrez N, Park SW, Wu J, Lee Y, Suzuki K, Koski A, Ji D, Hayama T, Ahmed R, Darby H. Correction of a pathogenic gene mutation in human embryos. Nature. 2017;548(7668):413–419. doi: 10.1038/nature23305. [DOI] [PubMed] [Google Scholar]
- 34.Egli D, Zuccaro MV, Kosicki M, Church GM, Bradley A, Jasin M. Inter-homologue repair in fertilized human eggs? Nature. 2018;560:E5–e7. doi: 10.1038/s41586-018-0379-5. [DOI] [PubMed] [Google Scholar]
- 35.Adikusuma F, Piltz S, Corbett MA, Turvey M, McColl SR, Helbig KJ, Beard MR, Hughes J, Pomerantz RT, Thomas PQ. Large deletions induced by Cas9 cleavage. Nature. 2018;560:E8–e9. doi: 10.1038/s41586-018-0380-z. [DOI] [PubMed] [Google Scholar]
- 36.Zeng Y, Li J, Li G, Huang S, Yu W, Zhang Y, Chen D, Chen J, Liu J, Huang X. Correction of the Marfan syndrome pathogenic FBN1 mutation by base editing in human cells and heterozygous embryos. Mol Ther. 2018;26(11):2631–2637. doi: 10.1016/j.ymthe.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]