Abstract
Parasite burdens are known to vary seasonally in wildlife, and rainfall is one key aspect of seasonality that has been linked to parasitism in a range of systems. Rainfall can have immediate effects on parasitism rates by affecting parasite survival and movement in the environment, or it can have delayed effects by affecting host susceptibility to parasites through changes in host body condition or immune function. In this study, we examined how helminth infection in a wild ungulate (Grant's gazelle, Nanger granti) is impacted by seasonal changes in rainfall. We looked at how the burdens of three helminth parasites varied in relation to current (immediate effect) and prior (delayed effect) rainfall by comparing parasite fecal egg and larval counts to rainfall 0, 1, and 2 months prior to parasite sampling. We found burdens of all three parasites to be negatively associated with rainfall, and that delayed effects were stronger than immediate effects. Our findings implicate rainfall as a driver of seasonal variation in infection and suggest one important mechanism may be through delayed effects on host susceptibility.
Keywords: Helminth, Nematode, Seasonality, Rainfall, Gazelle, Nanger granti
Graphical abstract
Highlights
-
•
Rainfall affects parasitism via delayed effects across three helminth taxa.
-
•
Rainfall is negatively associated with helminth parasite abundance and presence.
-
•
Host traits modify relationships between rainfall and parasitism.
1. Introduction
Seasonality is a well-known driver of variation in parasitism in host populations (Altizer et al., 2006; Turner and Getz, 2010). Seasonal changes in rainfall, temperature, and food availability can affect the intensity or prevalence of parasitism in a number of ways, including through changes in host susceptibility (Cattadori et al., 2005; Lass and Ebert, 2006), or changes in host exposure mediated by seasonal variation in host behavior (Nilssen, 1997; Petrić et al., 2011) or parasite development and survival (Farooq et al., 2012; Zamora-Vilchis et al., 2012). Interactions between hosts and helminth parasites, many of which spend a portion of their life cycle in the environment, are particularly interesting to study in the context of seasonality. For example, since both temperature and rainfall affect the development, behavior, and survival of helminths in the environment (Stromberg, 1997), seasonal variation in these factors can strongly influence between-host transmission (Morgan and van Dijk, 2012).
Rainfall is one aspect of seasonality that is predicted to have strong effects on helminth parasitism (Turner and Getz, 2010). These effects can come about in several ways. First, variation in rainfall can change host susceptibility by altering resource quantity and quality, driving changes in body condition (Marshal et al., 2008) and immunity (Fair and Whitaker, 2008), both of which can influence susceptibility to infection. Specifically, low rainfall and associated declines in food resources might lead to a gradual loss of body condition or immune function (Fair and Whitaker, 2008; Marshal et al., 2005, 2008). As a consequence, we might expect reduced rainfall to correlate with a relatively delayed increase in parasite loads. Second, rainfall could alter host exposure to parasites by driving changes in host behavior or the survival and density of free-living parasite stages in the environment. In the case of host behavior, high seasonal rainfall could result in hosts aggregating in areas with ephemeral patches of green vegetation (Bohrer et al., 2014; Fryxell, 1991), increasing host exposure to parasite transmission stages in resource hotspots. In the case of parasite development, high rainfall levels may improve the survival and development of parasites in the environment (O'Connor et al., 2007, 2008), also increasing host exposure to infectious parasite stages. However, in contrast to rainfall effects on host susceptibility, we might expect the parasite survival-related exposure effects of rainfall to be more immediate, and for higher rainfall to correlate with a relatively rapid increase in parasite exposure and associated burden (O'Connor et al., 2008). It is also possible that exposure-related effects of rainfall could be somewhat delayed and negative. This is because higher rainfall increases plant biomass, which may reduce the density of parasite transmission stages in the environment, lowering parasite-host encounter rates (Grenfell, 1992). However, there is very little support for this idea in tropical climates, where parasite densities in pasture tend to increase during rainy periods (Waruiru et al., 1998, 2001).
To better understand links between seasonality and parasite infection, in this study we examined the temporal relationship between rainfall and helminth parasitism in a free-ranging ungulate (Grant's gazelle, Nanger granti) inhabiting a seasonally variable environment. Specifically, we tested the relative importance of delayed versus more immediate effects of rainfall on parasitism rates in male gazelles by determining the timescale at which rainfall best predicted parasite infection. Parasitism could either correlate immediately with current rainfall or show a delayed response better predicted by prior rainfall. We expected that if rainfall effects on host susceptibility was the major seasonal driver of variation in parasitism, a delayed, negative association between rainfall and parasite burden would be the best supported model of parasitism. Conversely, if rainfall effects on host exposure was the major seasonal driver, a more immediate, positive association between rainfall and parasite burden would be the best supported model. We also expected that host traits might influence the magnitude of any seasonal response to parasitism. Male Grant's gazelles show pronounced variation in reproductive status, which is associated with helminth parasitism. Dominant males who defend territories tend to have higher helminth parasite burdens than subordinate bachelor males, likely due to differences in testosterone levels, energy expenditure and exposure rates (Ezenwa and Snider, 2016; Ezenwa et al., 2012). Given this, we hypothesized that differences between territorial and bachelor males in susceptibility and exposure to parasites would interact with rainfall resulting in a context-dependent seasonal response.
2. Materials and methods
2.1. Animal capture and longitudinal sampling
As part of a long-term study on reproductive behavior and parasitism, male Grant's gazelles (Nanger granti) were captured at the Mpala Research Center (MRC), Kenya (0°17′N, 37°52′ E) in 2009 and 2011 (Ezenwa and Snider, 2016; Ezenwa et al., 2012), and given unique color ear tags for identification. To track variation in helminth infection over time, fecal samples were collected from individually identified males for parasitological analysis. All samples were collected within 30 min of observing an individual defecate and stored on ice prior to transport to the laboratory for processing. Parasitological analyses were performed on the day of sample collection. Since half of the animals captured in 2011 were treated with an anthelmintic drug to clear their nematode infections (Ezenwa and Snider, 2016), these individuals were excluded from analysis. Overall, we used data on 499 fecal samples collected from 31 males (average no. samples/male = 16; range: 1–68) over a 45-month period.
2.2. Parasitological analysis
Gastrointestinal nematode egg output in host feces was quantified using a modification of the McMaster fecal egg counting technique (Ezenwa, 2003). In addition, lungworm larvae output was quantified using a beaker-modified Baermann technique (Forrester and Lankester, 1997). Immature stages of five distinct parasite types were distinguished, including strongyle nematode eggs, Trichuris spp. eggs, and first-stage larvae of three lungworm morphotypes (Ezenwa et al., 2012). For small ruminants, the time from host ingestion of parasite infective stages to the production of eggs or larvae by adult worms is typically 2–4 weeks for strongyles (Mehlhorn, 2016), 7–12 weeks for Trichuris (Baker, 2007; Kaufmann, 1996; Mehlhorn, 2016) and 4–5 weeks for Protostrongylid lungworms (Mehlhorn, 2016). We used fecal egg and larval counts as a proxy for the number, size, and fecundity of the adult worm population (i.e. burden) within a host (Gasbarre et al., 2001).
2.3. Statistical analyses
To test for associations between parasite burden and rainfall at the time of sampling or in the months prior to sampling, we compiled the following data for each fecal sample that was collected: parasite burden (strongyle egg count, Trichuris egg count, and total lungworm larvae count), male reproductive status (territorial or bachelor) and age at the time of sample collection, total rainfall (mm) for the month in which the sample was collected (Rt), total rainfall for the month prior to sample collection (Rt-1), and total rainfall two months prior to sample collection (Rt-2). The three different rainfall measures were used to test whether current (immediate effect) or prior (delayed effect) rainfall was more strongly associated with parasite burden. The 0–2 month rainfall time scales were selected to coincide with known time lags for the production of eggs or larvae following host ingestion of parasite infective stages (Mehlhorn, 2016). Annual rainfall in the study area ranged from ~320 to 1020 mm during the study period (2009–2012), with rainfall measures obtained from an archival MRC database (Caylor et al., 2017). Reproductive status was assigned as territorial (T), bachelor (B), or unknown (U), based on an assessment of male behavior, spatial location, and group composition as described in Ezenwa et al. (2012). Finally, male age in years, estimated using tooth wear (Ezenwa et al., 2012), was included in all analyses to account for any potential effects of male age on parasite infection.
Analyses were performed using linear (LMM) and generalized linear (GLMM) mixed models with measures of parasite burden as the response variables. Fixed predictor variables included one of the three measures of rainfall, male reproductive status, age, and the interaction between rainfall and reproductive status. Interaction effects between rainfall and reproductive status were included in the models to account for potential differences in the parasite response to rainfall of males with differing reproductive status. A random effect (individual ID) was also included in each model to account for repeated sampling of individuals over time. We used a model comparison approach to assess which hypothesis about parasite-rainfall relationships had the strongest support. Six distinct models were compared for each response variable, including models with (n = 3) and without (n = 3) rainfall-reproductive status interactions: Model 1, concurrent rainfall [X ~ Rt + RS + AGE + Rt × RS + (1|ID)]; Model 2, one-month delayed rainfall [X ~ Rt-1 + RS + AGE + Rt-1 × RS + (1|ID)]; Model 3, two-month delayed rainfall [X ~ Rt-2 + RS + AGE + Rt-2 × RS + (1|ID)]; Model 4, concurrent rainfall without interaction [X ~ Rt + RS + AGE + (1|ID)]; Model 5, one-month delayed rainfall without interaction [X ~ Rt-1 + RS + AGE + (1|ID)]; and Model 6, two-month delayed rainfall without interaction [X ~ Rt-2 + RS + AGE + (1|ID)]. Models were compared using Akaike's Information Criteria (AIC), and the model with the lowest AIC score and at least ≥ 2 AIC units lower than the next best model was selected as the model with greatest support (Burnham and Anderson, 2002).
Strongyle egg and lungworm larval counts were log-transformed for analysis. Model residuals were visually inspected to assess model validity (Zuur et al., 2009). Since Trichuris was present in only ~20% of samples, data for this parasite were converted into categorical (presence/absence) values for binomial analysis. All analyses were performed in R v. 3.5.1 (R Development Core Team, 2018) with the packages glmmTMB (Brooks et al., 2017) and bbmle (Bolker and R Development Core Team, 2017).
3. Results
Of the six models of strongyle egg count that we compared, the one including rainfall measured two months prior to parasite sampling (Rt-2) and an interaction between rainfall and reproductive status received the most support (Table 1; see Table S1 for details on all six strongyle models). Lagged rainfall was significantly and negatively associated with strongyle egg count (LMM: estimate ± standard error [se]: −0.0031 ± 0.0006, p < 0.0001; Fig. 1a). Furthermore, although reproductive status was not significantly associated with strongyle count (estimate ± se: 0.0184 ± 0.0569, p = 0.747), there was a significant interaction between rainfall and reproductive status whereby strongyle counts declined more steeply with increasing rainfall in bachelor males compared to territorial males (estimate ± se: 0.0015 ± 0.0007, p = 0.022; Fig. 1a). Age was not a significant predictor of strongyle count (estimate ± se: −0.0065 ± 0.0187, p = 0.729).
Table 1.
A comparison of models with different rainfall-related predictor variables (Rt: rainfall in the month of sample collection, Rt-1: rainfall 1 month prior to sample collection, Rt-2: rainfall 2 months prior to sample collection) explaining variation in helminth parasitism in Grant's gazelles. The model with the most support (lowest AIC score) for each parasite appears in bold. K refers to the number of parameters in each model. RS = reproductive status.
| Model | Formula | Shape | K | AIC | Δ AIC |
|---|---|---|---|---|---|
| Strongyles (N = 499) | log(x) ~ Rt-2+ RS + AGE + Rt-2× RS + (1|ID) | Linear | 6 | 310.6 | 0.0 |
| log(x) ~ Rt-2 + RS + AGE + (1|ID) | Linear | 5 | 313.8 | 3.2 | |
| log(x) ~ Rt-1 + RS + AGE + Rt-1 × RS + (1|ID) | Linear | 6 | 341.8 | 31.2 | |
| log(x) ~ Rt-1 + RS + AGE + (1|ID) | Linear | 5 | 352.0 | 41.4 | |
| log(x) ~ Rt + RS + AGE + (1|ID) | Linear | 5 | 358.5 | 47.9 | |
| log(x) ~ Rt + RS + AGE + Rt × RS + (1|ID) | Linear | 6 | 360.5 | 49.9 | |
| Lungworms (N = 451) | log(x+1) ~ Rt-2+ RS + AGE + Rt-2× RS + (1|ID) | Linear | 6 | 557.2 | 0.0 |
| log(x+1) ~ Rt-2+ RS + AGE + (1|ID) | Linear | 5 | 557.8 | 0.6 | |
| log(x+1) ~ Rt-1 + RS + AGE + (1|ID) | Linear | 5 | 563.4 | 6.3 | |
| log(x+1) ~ Rt + RS + AGE + (1|ID) | Linear | 5 | 563.4 | 6.3 | |
| log(x+1) ~ Rt-1 + RS + AGE + Rt-1 × RS + (1|ID) | Linear | 6 | 564.1 | 6.9 | |
| log(x+1) ~ Rt + RS + AGE + Rt × RS + (1|ID) | Linear | 6 | 565.4 | 8.3 | |
| Trichuris (N = 499) | x ~ Rt-2+ RS + AGE + (1|ID) | Binomial | 4 | 481.5 | 0.0 |
| x ~ Rt-2+ RS + AGE + Rt-2× RS + (1|ID) | Binomial | 5 | 481.7 | 0.2 | |
| x ~ Rt-1 + RS + AGE + (1|ID) | Binomial | 4 | 490.5 | 9.0 | |
| x ~ Rt + RS + AGE + Rt × RS + (1|ID) | Binomial | 5 | 490.5 | 9.1 | |
| x ~ Rt-1 + RS + AGE + Rt-1 × RS + (1|ID) | Binomial | 5 | 492.5 | 11 | |
| x ~ Rt + RS + AGE + (1|ID) | Binomial | 4 | 494.0 | 12.5 |
Fig. 1.
Relationship between rainfall measured two months prior to parasitological sampling (Rt-2) and (a) strongyle nematode egg and (b) lungworm larval counts. Dashed lines represent territorial males and solid lines represent bachelor males. Lines are predicted values (with 95% confidence intervals) from linear mixed models. Points represent raw data (triangles = territorial males, circles = bachelor males).
Similar to strongyles, models with rainfall measured two months prior to sampling (Rt-2) received the most support for explaining variation in lungworm larval counts (Table 1). Models with and without a rainfall-reproductive status interaction received equal support (Table 1). Here, we report the interaction model, which was the top model (see Table S2 for details on all six lungworm models). Lagged rainfall was significantly and negatively associated with lungworm count (LMM: estimate ± se: −0.0020 ± 0.0008, p = 0.0081; Fig. 1b). Territorial males had significantly lower lungworm counts than bachelor males (estimate ± se: −0.2318 ± 0.0329, p = 0.0042), and there was no interaction between rainfall and reproductive status (estimate ± se: 0.0014 ± 0.0009, p = 0.1059; Fig. 1b). Age was not a significant predictor of lungworm count (estimate ± se: 0.0361 ± 0.0329, p = 0.2722).
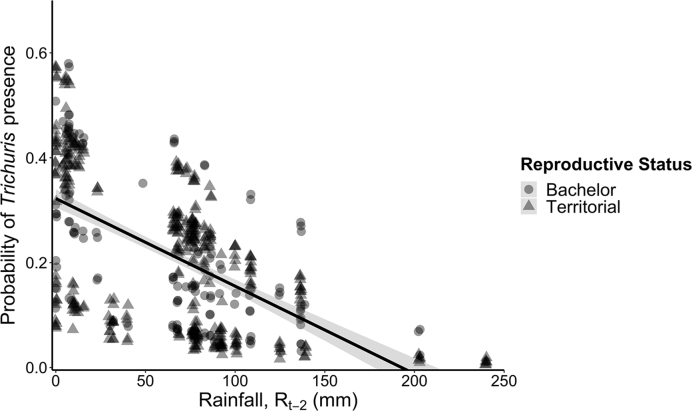
As we observed for both strongyles and lungworms, the model with rainfall measured two months prior to sampling (Rt-2) received the most support for explaining variation in Trichuris egg presence (Table 1). As with lungworms, models with and without a rainfall-reproductive status interaction received equal support (Table 1). Here, we report the non-interaction model, which was the top model (see Table S3 for details on all six Trichuris models). Lagged rainfall was significantly and negatively correlated with Trichuris egg presence (binomial GLMM: estimate ± se: −0.0105 ± 0.0029, p = 0.0003; Fig. 2). However, neither reproductive status (estimate ± se: −0.1612 ± 0.3150, p = 0.6089), nor age (estimate ± se: −0.0089 ± 0.1090, p = 0.9351) were significantly associated with Trichuris egg presence.
Fig. 2.
Relationship between rainfall measured two months prior to parasitological sampling (Rt-2) and the probability of Trichuris egg presence. Points (triangles = territorial males, circles = bachelor males) and the line (with 95% confidence intervals) are predicted values from a binomial generalized linear mixed model.
4. Discussion
Seasonality, and particularly rainfall, can be an important driver of variation in parasite exposure and susceptibility. We found that, in free-ranging Grant's gazelles, patterns of infection for three different helminth parasites were best predicted by prior, rather than concurrent, rainfall. Specifically, rainfall levels two months prior to parasite sampling were better predictors of variation in strongyle egg and lungworm larval counts and Trichuris egg presence than either concurrent rainfall or rainfall levels one month prior to sampling. Moreover, in all cases, the relationship between delayed rainfall and parasite measures was negative. These patterns suggest that in our system rainfall likely impacts parasite infection primarily through changes in host susceptibility not exposure. In addition, we also found that the strength of the association between rainfall and parasite burden differed by host reproductive status for at least one parasite type, suggesting that the magnitude of rainfall-associated changes in parasitism can vary depending on key host traits. Our results support the importance of delayed effects of rainfall as a driver of seasonal variation in parasite infection.
We found that helminth burdens were uniformly negatively associated with prior rainfall. This result suggests that rather than high rainfall increasing exposure to parasites, and therefore parasite burdens, either immediately or with some delay, that it is low rainfall that gradually increases host susceptibility increasing parasite burdens. The focal helminths examined in this study can develop from an ingested egg or larvae to egg-producing adult in as little as 2–7 weeks (Mehlhorn, 2016). Thus, relatively immediate effects of rainfall on host exposure would be expected to appear at the 0–1 month time lags. However, the uniformly strong support for a 2 month time lag suggests a more delayed change in helminth burden that is not due to immediate effects on parasite exposure. We propose that this effect may be caused by gradual changes in host susceptibility. Mechanistically, low rainfall reduces food availability and nutrient quantity and quality (Marshal et al., 2005), which likely leads to reductions in host body condition (Marshal et al., 2008) or immune function (Fair and Whitaker, 2008) and associated increases in host susceptibility to nematode infection (Coop and Kyriazakis, 1999). This reasoning is supported by Holmes (1993), who showed that sheep fed higher protein diets had significantly lower Haemonchus contortus burdens than those with low protein diets following repeated exposure to infection, and by evidence that rainfall is positively associated with crude protein levels in wild ungulate diets (Marshal et al., 2005). Furthermore, our results parallel those of Ezenwa (2004), who in the same system as our study, found that six out of nine wild ungulate species examined had higher strongyle nematode burdens during a period of sustained low rainfall (drought). Indeed, studies across a range of mammal host species have documented similar patterns, reporting that hosts had higher strongyle nematode loads during dry compared to wet seasons (e.g. western gorillas; Masi et al., 2012), or in dry versus wet years (e.g. elephants; Thurber et al., 2011). Overall, our findings implicate rainfall as a key driver of changes in host susceptibility to helminth infection. However, data on rainfall-associated changes in host body condition and immune function, and the effects of these changes on parasitism, are needed to clarify the mechanistic basis of the rainfall-parasitism association we describe.
An alternative explanation for the delayed negative effect of rainfall on parasitism could be a lagged decrease in parasite exposure. If rainfall-driven vegetation growth leads to decreased parasite density in the environment, hosts could encounter parasite transmission stages less frequently following periods of plant growth after rainfall (Grenfell, 1992). However, there is little empirical support for this idea, and several studies have found that the density of parasite transmission stages actually increases in the days (Amaradasa et al., 2010) and months (Waruiru et al., 2001) following higher rainfall. For this reason, we think that a delayed reduction in parasite exposure following rainfall is an unlikely explanation for the patterns we observed. Nevertheless, more work is needed to fully understand the mechanisms underlying the patterns we describe in this study.
Interestingly, a number of studies have reported the opposite rainfall-parasite pattern to what we describe here, where rainfall is positively associated with parasite measures with some delay. Thus, a delayed positive effect of rainfall on parasite exposure seems plausible under some circumstances. For example, Turner and Getz (2010) found that the intensity and presence of strongyle nematode infections were greater during wet periods, 1–2 months after rainfall peaked, for four different ungulate species in Etosha National Park, Namibia. This opposite rainfall-parasite pattern may be explained by the effects of extreme environmental conditions on the relative importance of exposure versus susceptibility effects of rainfall on parasitism. For example, under extreme environmental conditions such as in arid regions like Etosha National Park (mean annual rainfall: ~200–500 mm; Turner and Getz, 2010), limited rainfall may strongly suppress dry season parasite transmission (Jacquiet et al., 1995), increasing the relative importance of rainfall in enabling host exposure via effects on parasite development, survival, and movement in the environment (Amaradasa et al., 2010; Waruiru et al., 1998, 2001).
Finally, we also found that the relationship between rainfall and parasite burden varied by male reproductive status for one parasite, strongyles. Specifically, strongyle egg counts in territorial males were less affected by rainfall when compared to bachelor males. A likely mechanism by which rainfall reduces parasite load is through increasing host diet quality and decreasing susceptibility. For bachelors, when rainfall increases, greater access to high quality food might have a direct protective effect against parasites. But for territorial males, increases in rainfall may have less of an effect on susceptibility for several reasons. For example, physiological factors linked to immunosuppression, such as high testosterone levels (Ezenwa et al., 2012; Foo et al., 2017), the energy involved in territory defense (Ezenwa and Snider, 2016), or increased stress hormone levels (Berger et al., 2005) may explain why territorial males benefit less than bachelor males from increases in rainfall in terms of parasite loads. Improved forage quality may not be sufficient to compensate for these costs of reproduction in territorial males, or territorial males may spend too much time on territory defense to take full advantage of higher quality forage. This differential parasite response of territorial and bachelor males to rainfall suggests that host life-history plays a key role in modifying how the environment influences parasitism.
Overall, our study supports the importance of rainfall as a driver of seasonal variation in parasitism. While the mechanisms by which rainfall can influence parasite infection in free-ranging animals are diverse, our results suggest that one key pathway by which rainfall may affect parasite infection is via the delayed effects of low rainfall on host susceptibility. We also show that host life history can modify relationships between infection and environmental conditions. Further research is needed to better characterize how the various pathways linking rainfall and parasitism interact and under which general conditions rainfall should have immediate vs. delayed or positive vs. negative effects on parasitism.
Acknowledgements
Animal captures were conducted by Frontier Helicopters, NZ and the Kenya Wildlife Service Game Capture Unit. The Kenya Ministry of Science, Education and Technology and the Kenya Wildlife Service gave permission to conduct this research in Kenya. Animal protocols used in this study were approved by the University of Montana (#023-09VEDBS-051509) and the University of Georgia (#A2010 10–188) Animal Care and Use Committees. We thank J. Ewoi, A. Durcik, S. Hauver, M. Snider, V. Zero, Z. Chillag, S. Ekernas, A. Hines, J. Hooge, J. Loroo, A. Mwachanje, M. Rajeev, W. Sarmento, A. Williams and K. Worsley-Tonks for assistance in the field, and the Mpala Research Center for logistical support. We also thank Andrew Park for providing comments on an earlier version of the manuscript. Funding for this work was provided by a United States National Science Foundation CAREER Award to VOE (IOS-1101836).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2020.04.004.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Altizer S., Dobson A., Hosseini P., Hudson P., Pascual M., Rohani P. Seasonality and the dynamics of infectious diseases. Ecol. Lett. 2006;9:467–484. doi: 10.1111/j.1461-0248.2005.00879.x. [DOI] [PubMed] [Google Scholar]
- Amaradasa B.S., Lane R.A., Manage A. Vertical migration of Haemonchus contortus infective larvae on Cynodon dactylon and Paspalum notatum pastures in response to climatic conditions. Vet. Parasitol. 2010;170:78–87. doi: 10.1016/j.vetpar.2010.01.026. [DOI] [PubMed] [Google Scholar]
- Baker D.G. Blackwell; Ames, Iowa, USA: 2007. Flynn’s Parasites of Laboratory Animals. [Google Scholar]
- Berger S., Martin L.B., Wikelski M., Romero L.M., Kalko E.K.V., Vitousek M.N., Rödl T. Corticosterone suppresses immune activity in territorial Galápagos marine iguanas during reproduction. Horm. Behav. 2005;47:419–429. doi: 10.1016/j.yhbeh.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Bohrer G., Beck P.S.A., Ngene S.M., Skidmore A.K., Douglas-Hamilton I. Elephant movement closely tracks precipitation-driven vegetation dynamics in a Kenyan forest-savanna landscape. Mov. Ecol. 2014;2:2. doi: 10.1186/2051-3933-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolker B., R Development Core Team . The R Project for Statistical Computing; Vienna, Austria: 2017. bbmle: Tools for General Maximum Likelihood Estimation. [Google Scholar]
- Brooks M.E., Kristensen K., van Benthem K.J., Magnusson A., Berg C.W., Nielsen A., Skaug H.J., Maechler M., Bolker B.M. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R Journal. 2017;9:378–400. [Google Scholar]
- Burnham K.P., Anderson D.R. second ed. Springer-Verlag; New York: 2002. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. [Google Scholar]
- Cattadori I.M., Boag B., Bjørnstad O.N., Cornell S.J., Hudson P.J. Peak shift and epidemiology in a seasonal host–nematode system. Proc. R. Soc. B. 2005;272:1163–1169. doi: 10.1098/rspb.2004.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caylor K.K., Gitonga J., Martins D.J. Laikipia; Kenya: 2017. Mpala Research Centre Meteorological and Hydrological Datase. [Google Scholar]
- Coop R.L., Kyriazakis I. Nutrition–parasite interaction. Vet. Parasitol. 1999;84:187–204. doi: 10.1016/s0304-4017(99)00070-9. [DOI] [PubMed] [Google Scholar]
- R Development Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2018. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Ezenwa V.O. Habitat overlap and gastrointestinal parasitism in sympatric African bovids. Parasitology. 2003;126:379–388. doi: 10.1017/s0031182002002913. [DOI] [PubMed] [Google Scholar]
- Ezenwa V.O. Interactions among host diet, nutritional status and gastrointestinal parasite infection in wild bovids. Int. J. Parasitol. 2004;34:535–542. doi: 10.1016/j.ijpara.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Ezenwa V.O., Snider M.H. Reciprocal relationships between behaviour and parasites suggest that negative feedback may drive flexibility in male reproductive behaviour. Proc. R. Soc. B. 2016;283:20160423. doi: 10.1098/rspb.2016.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezenwa V.O., Stefan Ekernas L., Creel S. Unravelling complex associations between testosterone and parasite infection in the wild. Funct. Ecol. 2012;26:123–133. [Google Scholar]
- Fair J.M., Whitaker S.J. Avian cell-mediated immune response to drought. Wilson J. Ornithol. 2008;120:813–819. [Google Scholar]
- Farooq Z., Mushtaq S., Iqbal Z., Akhtar S. Parasitic helminths of domesticated and wild ruminants in Cholistan desert of Pakistan. Int. J. Agric. Biol. 2012;14:63–68. [Google Scholar]
- Foo Y.Z., Nakagawa S., Rhodes G., Simmons L.W. The effects of sex hormones on immune function: a meta-analysis. Biol. Rev. 2017;92:551–571. doi: 10.1111/brv.12243. [DOI] [PubMed] [Google Scholar]
- Forrester S.G., Lankester M.W. Extracting protostrongylid nematode larvae from ungulate feces. J. Wildl. Dis. 1997;33:511–516. doi: 10.7589/0090-3558-33.3.511. [DOI] [PubMed] [Google Scholar]
- Fryxell J.M. Forage quality and aggregation by large herbivores. Am. Nat. 1991;138:478–498. [Google Scholar]
- Gasbarre L.C., Leighton E.A., Sonstegard T. Role of the bovine immune system and genome in resistance to gastrointestinal nematodes. Vet. Parasitol. 2001;98:51–64. doi: 10.1016/s0304-4017(01)00423-x. [DOI] [PubMed] [Google Scholar]
- Grenfell B.T. Parasitism and the dynamics of ungulate grazing systems. Am. Nat. 1992;139:907–929. [Google Scholar]
- Holmes P.H. Interactions between parasites and animal nutrition: the veterinary consequences. Proc. Nutr. Soc. 1993;52:113–120. doi: 10.1079/pns19930043. [DOI] [PubMed] [Google Scholar]
- Jacquiet P., Colas F., Cabaret J., Dia M.L., Cheikh D., Thiam A. Dry areas: an example of seasonal evolution of helminth infection of sheep and goats in southern Mauritania. Vet. Parasitol. 1995;56:137–148. doi: 10.1016/0304-4017(94)00672-y. [DOI] [PubMed] [Google Scholar]
- Kaufmann J. Parasitic Infections of Domestic Animals: A Diagnostic Manual. Birkhäuser Verlag; Berlin, Germany: 1996. [Google Scholar]
- Lass S., Ebert D. Apparent seasonality of parasite dynamics: analysis of cyclic prevalence patterns. Proc. R. Soc. B. 2006;273:199–206. doi: 10.1098/rspb.2005.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshal J.P., Krausman P.R., Bleich V.C. Rainfall, temperature, and forage dynamics affect nutritional quality of desert mule deer forage. Rangel. Ecol. Manag. 2005;58:360–365. [Google Scholar]
- Marshal J.P., Krausman P.R., Bleich V.C. Body condition of mule deer in the Sonoran Desert is related to rainfall. SW. Nat. 2008;53:311–318. [Google Scholar]
- Masi S., Chauffour S., Bain O., Todd A., Guillot J., Krief S. Seasonal effects on great ape health: a case study of wild chimpanzees and western gorillas. PloS One. 2012;7 doi: 10.1371/journal.pone.0049805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlhorn H. Springer; Cham, Switzerland: 2016. Animal Parasites: Diagnosis, Treatment, Prevention. [Google Scholar]
- Morgan E.R., van Dijk J. Climate and the epidemiology of gastrointestinal nematode infections of sheep in Europe. Vet. Parasitol. 2012;189:8–14. doi: 10.1016/j.vetpar.2012.03.028. [DOI] [PubMed] [Google Scholar]
- Nilssen A.C. Effect of temperature on pupal development and eclosion dates in the reindeer oestrids Hypoderma tarandi and Cephenemyia trompe (Diptera: Oestridae) Environ. Entomol. 1997;26:296–306. [Google Scholar]
- O'Connor L.J., Kahn L.P., Walkden-Brown S.W. Moisture requirements for the free-living development of Haemonchus contortus: quantitative and temporal effects under conditions of low evaporation. Vet. Parasitol. 2007;150:128–138. doi: 10.1016/j.vetpar.2007.07.021. [DOI] [PubMed] [Google Scholar]
- O'Connor L.J., Kahn L.P., Walkden-Brown S.W. Interaction between the effects of evaporation rate and amount of simulated rainfall on development of the free-living stages of Haemonchus contortus. Vet. Parasitol. 2008;155:223–234. doi: 10.1016/j.vetpar.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Petrić M., Mladineo I., Šifner S.K. Insight into the short-finned squid Illex coindetii (Cephalopoda: Ommastrephidae) feeding ecology: is there a link between helminth parasites and food composition? J. Parasitol. 2011;97:55–62. doi: 10.1645/GE-2562.1. [DOI] [PubMed] [Google Scholar]
- Stromberg B.E. Environmental factors influencing transmission. Vet. Parasitol. 1997;72:247–264. doi: 10.1016/s0304-4017(97)00100-3. [DOI] [PubMed] [Google Scholar]
- Thurber M.I., O'Connell-Rodwell C.E., Turner W.C., Nambandi K., Kinzley C., Rodwell T.C., Faulkner C.T., Felt S.A., Bouley D.M. Effects of rainfall, host demography, and musth on strongyle fecal egg counts in African elephants (Loxodonta africana) in Namibia. J. Wildl. Dis. 2011;47:172–181. doi: 10.7589/0090-3558-47.1.172. [DOI] [PubMed] [Google Scholar]
- Turner W.C., Getz W.M. Seasonal and demographic factors influencing gastrointestinal parasitism in ungulates of Etosha National Park. J. Wildl. Dis. 2010;46:1108–1119. doi: 10.7589/0090-3558-46.4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waruiru R., Munyua W., Thamsborg S., Nansen P., Bøgh H., Gathuma J. Development and survival of infective larvae of gastrointestinal nematodes of cattle on pasture in central Kenya. Vet. Res. Commun. 1998;22:315–323. doi: 10.1023/a:1006112802459. [DOI] [PubMed] [Google Scholar]
- Waruiru R., Thamsborg S., Nansen P., Kyvsgaard N., Bogh H., Munyua W., Gathuma J. The epidemiology of gastrointestinal nematodes of dairy cattle in central Kenya. Trop. Anim. Health Prod. 2001;33:173–187. doi: 10.1023/a:1010322703790. [DOI] [PubMed] [Google Scholar]
- Zamora-Vilchis I., Williams S.E., Johnson C.N. Environmental temperature affects prevalence of blood parasites of birds on an elevation gradient: implications for disease in a warming climate. PloS One. 2012;7 doi: 10.1371/journal.pone.0039208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuur A., Ieno E.N., Walker N., Saveliev A.A., Smith G.M. Springer Science & Business Media; Berlin, Germany: 2009. Mixed Effects Models and Extensions in Ecology with R. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.