Abstract
Studies on the consequences of urbanization often examine the effects of light, noise, and heat pollution independently on isolated species providing a limited understanding of how these combined stressors affect species interactions. Here, we investigate how these factors interact to affect parasitic frog-biting midges (Corethrella spp.) and their túngara frog (Engystomops pustulosus) hosts. A survey of túngara frog calling sites revealed that frog abundance was not significantly correlated with urbanization, light, noise, or temperature. In contrast, frog-biting midges were sensitive to light pollution and noise pollution. Increased light intensity significantly reduced midge abundance at low noise levels. At high noise intensity, there were no midges regardless of light level. Two field experiments controlling light and noise levels to examine attraction of the midges to their host and their feeding behavior confirmed the causality of these field patterns. These findings demonstrate that both light and noise pollution disrupt this host-parasite interaction and highlight the importance of considering interactions among species and types of pollutants to accurately assess the impacts of urbanization on ecological communities.
Keywords: Corethrella, Túngara frogs, eavesdropper, ectoparasite, Physalaemus pustulosus, urbanization, anthropogenic pollution
Introduction
Humans have modified most of the earth’s surface, rapidly increasing the rate and scale of urbanization (Grimm et al. 2008, Ellis 2011). This process is complex and has rapidly altered habitat structure by modifying the type and amount of vegetation present while also changing patterns of abiotic factors resulting in novel light, noise, and temperature landscapes (McKinney 2002). For example, cities are predictable islands of heat in a backdrop of otherwise cooler environments (Shochat et al. 2006, Gaston et al. 2012). Similarly, artificial nighttime lighting forms a grid that expands across the globe (Longcore and Rich 2004, Elvidge et al. 2014). Elevated levels of low frequency noise produced by traffic and industry are pervasive (Barber et al. 2011, Ortega 2012) and characterize human-dominated ecosystems (Warren et al. 2006).
Heat, light, and noise pollution are common in urban environments (Douglas 1983), and affect the abundance, behavior and distribution of many species (Hoelker et al. 2010, Gomes et al. 2016b). Urban heat islands, for instance, can facilitate colonization by warm-tolerant species, which can have adverse impacts on native species (Shochat et al. 2006). Similarly, anthropogenic light can affect species abundance and distribution. Some species avoid light-polluted areas while others may alter their activity patterns; for example some species of urban songbirds start singing earlier in the morning in light polluted areas (Dominoni et al. 2013). More subtle effects can also occur. Streetlights negatively affect moth defensive behaviors and disrupt moth flight, navigation, vision, and feeding (Acharya and Fenton 1999). While increased light levels often have negative fitness consequences for species, positive effects are also possible (see, Acharya and Fenton 1999).
For species that use acoustic signals, such as many insects, birds and frogs, anthropogenic noise may generate acoustic interference affecting their communication systems (Slabbekoorn et al. 2010, Halfwerk et al. 2011a, Brumm 2013, Haven 2015, Kleist et al. 2016) and ultimately alter their intraspecific interactions (Slabbekoorn and Ripmeester 2008, Francis et al. 2009b, Ortega 2012, Kleist et al. 2016). Artificial noise, light, and heat can select for signaling strategies that affect the behavior and physiology of organisms (Slabbekoorn and Peet 2003, Patricelli and Blickley 2006), modulate habitat preferences (Parris et al. 2009, Holker et al. 2010) and ultimately influence the fitness of individuals living in urban environments (Fernández-Juricic et al. 2005, Barber et al. 2009, Francis et al. 2009a, Francis et al. 2009b, Parris et al. 2009, Laiolo 2010, Halfwerk et al. 2011b). Consequently, anthropogenic heat, lighting, and noise represent novel and important evolutionary challenges to many organisms.
Although urbanization concurrently modifies temperature, light, and noise levels (Ellis 2011), studies focusing on urbanization often only consider the effect of one of these abiotic factors (Halfwerk and Slabbekoorn 2015). In cases where more than one of these stressors are studied simultaneously, they are often examined independently. If these factors interact antagonistically, synergistically, or additively, then studies considering them in isolation could misestimate their impacts on biodiversity and species interactions.
Much like focusing on the effects of increased light, noise, and temperature independently, studies of urbanization often investigate individual species, paying less attention to potential consequences on species interactions (see Gaston et al. 2013). The handful of studies that have considered the effects of urbanization on species interactions suggest that the effects can be profound and complicated. For example, light pollution extends the foraging times of some crepuscular organisms increasing their temporal overlap and thus competition with diurnal species (Hoelker et al. 2010, Francis et al. 2012, Rich and Longcore 2013). Additionally, anthropogenic noise reduces the amount of time male frogs spend chorusing and impacts mixed-species breeding aggregations (Kaiser et al. 2011) with potential consequences on reproductive success, including reduced access to females. To truly understand the impacts of urbanization on communities, we need to study how species interactions are altered by multiple ecologically relevant pollutants (Halfwerk and Slabbekoorn 2015).
Given that parasitism is a very common consumer strategies and can impact food web stability, energy flow, and the health of humans, wildlife, and ecosystems (Lafferty et al. 2008), we need to understand how urbanization impacts this important and often overlooked interaction in communities. The effects of heat and chemical pollution on host-parasite interactions have been examined extensively (McMahon et al. 2013, Raffel et al. 2013, Rohr et al. 2013, Raffel et al. 2015). However, the effects of urban light and noise pollution on host-parasite interactions have not been studied and thus are poorly understood (Bradley and Altizer 2007).
In an effort to obtain a more complete understanding of the intricate ways in which anthropogenic environments affect natural systems, we investigated the combined effects of urban heat, noise, and light pollution on the abundance and behavior of nocturnal, frog-biting midges (Corethrella spp.) and their nocturnal host, túngara frogs (Engystomops pustulosus). Corethrellid midges are attracted in great numbers to their hosts using the mating calls of the frogs (McKeever 1977, Bernal et al. 2006, Borkent 2008). The midges depend on the mating calls produced by the frogs to locate and successfully feed on their host (Bernal and Silva 2015). In túngara frogs, once they reach the calling male, the midges walk to the nostrils of the frog, where they obtain a blood meal (de Silva et al. 2014). As in other species of hematophagous insects, female midges use this blood meal for egg production and mating success (Borkent 2008) so finding a host frog is thus, a critical component of their mating success.
We first conducted a field study in urban and rural areas to test for associations among temperature, light, and noise on the abundance of both túngara frogs and midges. We then conducted two field experiments to partition the main and interactive effects of noise and light pollution on the abundance and frog-finding behaviors of the midges. Given the importance of nighttime conditions and the intricacies of frog calls to the mating success of both túngara frogs and frog-biting midges, we hypothesized that noise and light pollution would interfere with these communication networks reducing the abundance of both species. Because frog-biting midges are active under laboratory conditions only at low light intensities (de Silva and Bernal 2013), and not present during the day in the wild (Bernal and McMahon pers. obs), we predicted that light pollution would be detrimental to them. However, because the midges find the vicinity of their host using mostly the call of the frog, we predicted that there would be a noise-by-light interaction and that noise pollution might be even more detrimental than light pollution. Although both noise and light levels affect communication in túngara frogs (Rand et al. 1997, Baugh and Ryan 2010, Halfwerk et al. 2016), these frogs occur across a wide range of light conditions and thus we expected that they would be less susceptible to light pollution than frog-biting midges. In contrast to our predictions for light and noise pollution, because of the generally stable warm nocturnal temperature conditions in the lowland tropics where these two species occur, we did not predict a strong effect of urban heat pollution on the distribution of either species.
Material and Methods
Urban vs Rural Survey
To quantify the relationships among temperature, light, and noise levels and the abundance of túngara frogs (Engystomops pustulosus) and their midge (Corethrella spp.) parasites, we surveyed 49 túngara frog calling sites in urban and rural areas (surveyed between 9/17–10/3/2012; each night the survey started half an hour after sunset, was conducted before moonrise, and did not continue past midnight; during this survey period the moon was waning). The urban sites were found within a 10 km transect, which started at Ancon Hill (Smithsonian Tropical Research Institute (STRI), Tupper-Tivoli Complex; N 08°57.742’ W079°32.649’) in Panama City and continued along Omar Torrijos H. Avenue (urban sites were all within the city limits). The rural sites were found along Omar Torrijos H. Avenue near or within a 10 km transect of Gamboa (N 09°06.780’ W079°64.884’), which is a small town surrounded by the mature rainforest of the Soberanía National Park (rural sites were all outside of the city and surrounded by forested areas). We conducted an auditory and visual encounter survey for frog calling sites. When a calling site was detected, we searched for additional calling sites within 15 m in all directions. Most sites were identified and located auditorily. At each site, we recorded abundance of túngara frogs counting all frogs present at the site which included both male and female frogs. We also counted all frog-biting midges on or flying above the frogs, the number of túngara frog foam nests (egg masses), and the presence of other flying insects to rule out the potential effect of variation in insecticide concentrations across breeding sites as insecticides could impact abundance of insects in general. For each site, we calculated the average of three measurements of light and noise intensity and substrate temperature. All measuring instruments were held 1 m above the center of the calling site. Light intensity was measured using a digital light meter (Lux/FC, Sper Scientific), noise intensity was measured with a digital-display sound-level meter (RadioShack # 33–2055; measurements included natural and anthropogenic sounds), and temperature was measured with a hand-held non-contact infrared thermometer (MT6 Raytek MiniTemp). We conducted this survey over six nights at the same time each night, and used the date of data collection as a blocking factor in the analysis to account for temporal and environmental variation.
Midge Attraction Experiment
Our field survey indicated that temperature was not a significant factor driving the distribution of frogs or midges, and thus we focused on light and noise pollution in our experiments. To test whether light and noise levels affect the ability of frog-biting midges to locate their hosts, we applied three fully crossed levels of light (0.l, 0.1,5 and 0.30 lx) and noise pollution (45, 60, and 75 dB sound pressure level [SPL; re. 20 mPa at 1m]; referred to as frog call only, frog call and low city noise, and frog call and high city noise treatments, respectively) to sound traps. The sound traps used were modified versions of Center for Disease Control (CDC) miniature light traps (McKeever and Hartberg 1980). Each trap broadcast the same recording of a túngara frog call at 80 dB SPL (re. 20 mPa at 1m, a natural calling decibel) from a speaker and MP3 player (ISound 1603 and Sylvania SMP2200, respectively; the speaker simultaneously played the frog call and respective city noise treatment), representing the calling intensity characteristic of this species (Ryan 1985).
To mimic conditions equivalent to noise pollution to the traps, traffic noise was recorded from Panama City, Panama, and broadcast at a trap at 4, 60, or 75 dB SPL. These noise pollution levels were chosen to match the range of noise intensity that we found in Panama City while sampling frog populations (see Fig. 1), we used this range because it was the range frogs were experiencing in the urban setting. To achieve the three desired light intensity levels, we placed artificial white lights at each trap that were covered with an opaque plastic shield to partially obscure the light source. The light was placed 1 m away from each trap to avoid an increase in temperature at the traps due to lighting (we verified there was no change in temperature at the end of each sampling period).
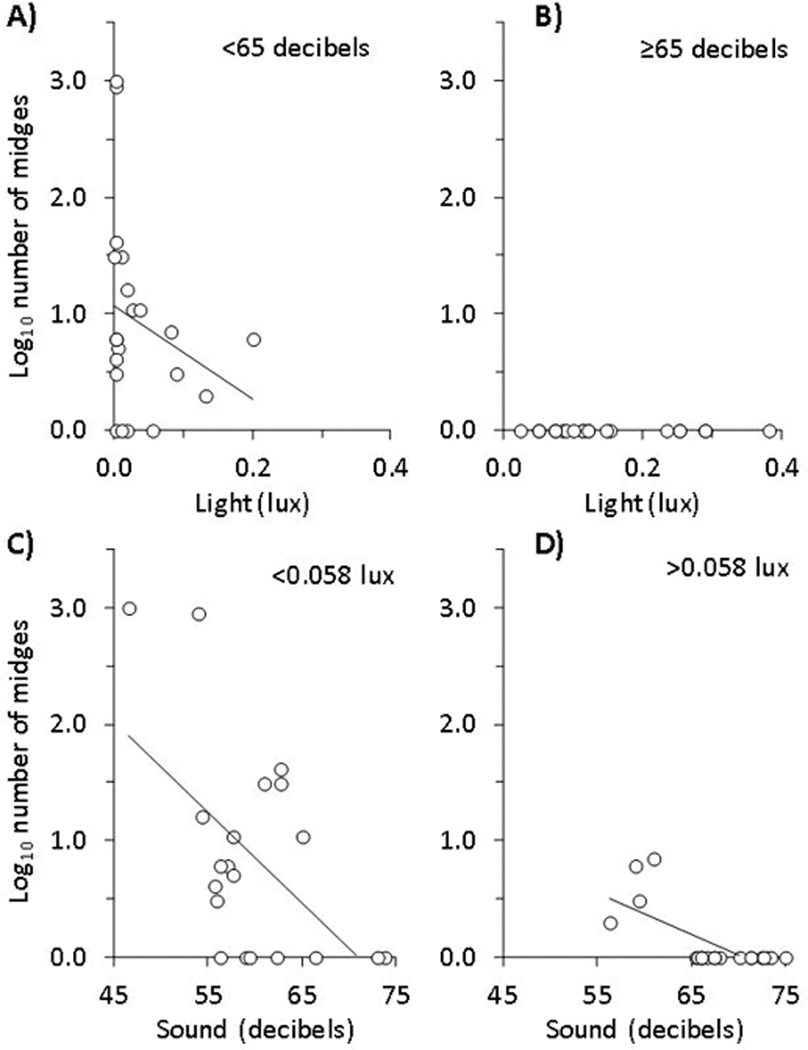
Fig. 1.
Interaction (χ21 = 3.30, P < 0.001) between light and noise levels on the number (log10 +1) of frog-biting midges (Corethrella spp.) counted per Engystomops pustulosus frog calling site at 49 breeding sites distributed from Panama City (urban) to Gamboa (rural) in the Republic of Panama. Panels A) and B) show the effects of light levels on midge captures above and below median sound levels (65 decibels), respectively, whereas panels C) and D) show the effects of sound levels on midge captures above and below the median light level (0.058 lux), respectively. Also shown are best-fit lines.
To conduct this experiment, we positioned three sound traps equidistant from a stream and forest edge in Gamboa, Panama (9°07.0’N, 79°41.9’W). The traps were separated by over 20m so the sound from the treatments was not detectable by a human standing at a different trap (McMahon, pers. obs.) and thus increased the chances that the treatments would be independent. Each trap was assigned one of the nine light-by-noise pollution treatments. We captured midges for 10 minutes at each trap and then the midges were euthanized and counted. There was a 20-minute break between trials, the traps were assigned a new treatment, and the midges were captured for 10 minutes and then counted. We conducted three trials per night; all nine treatments were tested each night in random order and location to reduce potential spatial and temporal biases. This procedure was repeated over 10 nights (surveyed between 9/19–9/29/2012) for a total of 10 replicates or temporal blocks of each of the nine treatments.
Manipulative Feeding Experiment
The Midge Attraction Experiment described above examined the ability of frog-biting midges to find their host under different levels of noise and light pollution, but because there were no frogs present, it could not evaluate whether midges that successfully found the host would have successfully fed. To address this question, we conducted another field experiment where we could observe the behavior of the midges in response to the túngara frogs, when exposed to the same light and noise treatments as in the Midge Attraction Experiment. Nine male túngara frogs were collected from Gamboa and placed individually inside nine circular containers (4 ×10 cm; height/diameter) covered with mesh with openings (2 mm) large enough for the midges to access the frogs. This container was placed on top of a speaker that broadcast the same noise treatments (frog call and city noise) used in the previous experiment. We did not want the frogs themselves to call in this experiment because frog-biting midges are sensitive to variation in túngara frog call properties (Bernal et al. 2006, Aihara et al. 2015). To prevent the frogs from calling, we did not provide standing water, which túngara frogs require to call. Both the speaker and frog container were placed inside a larger container (an opaque plastic container 13 × 24 × 35 cm, height/width/length), containing 20 frog-biting midges (collected with a hand-operated insect aspirator) that did not have a fresh blood meal (recognizable because midges have expanded red abdomens after blood ingestion). The container was open on top and was covered by fine white mesh that prevented the midges from escaping while allowing observations. The containers received the same light treatments used in the Midge Attraction Experiment and were placed outside in the field approximately 20 m apart from each other to reduce treatment interference given that the stimuli did not carry that far (McMahon, pers. obs.).
Over a two-hour period, six 1-minute observations were completed per treatment, recording number of animal movements (midge: walks or flights; frog: jumps). At the end of the 2-hour trial, we recorded the number of midges 1) on the container holding the frog, 2) on the frog, and 3) with a blood meal. These procedures were repeated across 10 nights (conducted between 9/17–9/29/2012; each time we started half an hour after sunset before moon rise) resulting in 10 replicates or temporal blocks of each treatment. The location of each treatment was randomized within and across the temporal blocks and 180 midges and nine frogs were collected and released each night so that different individual midges and frogs were used in each trial.
Statistical Analysis
Data were analyzed with R statistical software. Significance was attributed when P < 0.05. Frog-biting midges were considered a guild and thus their numbers were pooled together following previous studies interested in interspecific interactions between túngara frogs and frog-biting midges (Bernal et al. 2006, Trillo et al. 2016). We conducted multimodel inference analyses (package: glmer, function: glmer, family: poisson) considering all possible models using the dredge function in the MuMIn package. For these analyses we report model-averaged, weighted coefficients and associated p-values from models with delta AICc < 4 (see Appendix S1: Tables S1–4).
Urban vs Rural Survey:
A generalized linear model (package: glm, function: glm, family: Gaussian) was used to determine whether urbanization had an effect on temperature, light, and noise levels. For noise levels, the number of calling frogs was included as a covariate in the analysis. We used a generalized linear model (package: glm, function: glm, family: Poisson) to determine if there was an effect of rural or urban location on midge abundance. We used the same package and function to test whether light and noise levels correlated with one another across the sampling sites. We also used a negative binomial generalized linear model (package: glm, function: glm.nb) to determine if there was a difference in the number of frogs and frog foam egg nests in urban and rural sites; for the frog foam egg nests we used number of frogs at each site as a covariate. To determine the effects of temperature, light, noise, night, canopy cover (open/closed) and number of frogs on the abundance of frog-biting midges at each site, and to determine the effects of those same factors on the abundance of túngara frogs at each site, we conducted multimodel inference analyses (package: glmmADMB, function: glmmadmb, zeroinflated, family: nbinom) considering all possible models using the dredge function in the MuMIn package. For these analyses we report model-averaged, weighted coefficients and associated p-values from models with delta AICc < 4 (see Appendix S1: tables S1–4).
Midge Attraction Experiment:
We analyzed the effect of light and noise levels and their interaction on the number of frog-biting midges collected in acoustic traps using a general linear model (package: glmmADMB, function: glmmadmb, family: nbinom) with night and treatment order as blocking factors.
Manipulative Feeding Experiment:
We used a general linear mixed model (package: nlme, function: glmer, family: poisson) to test how light and noise levels, and their interaction affected the movement of the midges and frog, the proportion of midges on the frog container or the proportion of midges that obtained a blood meal, treating night as a blocking factor and the container as a random effect.
Results
Urban vs Rural Survey
Urban sites had significantly higher light, noise, and temperature than rural sites (F1,39 = 29.73, F1,39 = 30.51 and F1,39 = 140.70, respectively, and P < 0.0001 for all analyses; Table 1). Light and noise intensity were positively correlated with one another (χ21 = 19.73, P <0.0001). There was no significant difference in túngara frog abundance between rural and urban sites (Table 1; χ21 = 1.53 P = 0.13) but there were more foam nests in rural than urban sites (Table 1; χ21 = 2.06, P = 0.04). Neither, light, noise, temperature, nor the interaction between light and noise were significant predictors of frog abundance (light: χ21 = 1.56, P = 0.12; noise: χ21 = 1.27, P = 0.21; temperature: χ21 = 0.36, P = 0.72; light*noise: χ21 = 0.36, P = 0.72; Figs. S1 and S2; see Appendix S1: Table S1 for model selection information).
Table 1.
Abiotic factors related with anthropogenic changes, host (Engystomops pustulosus) and parasite (Corethrella spp) abundance at 49 frog breeding sites found between Panama City (urban) and Gamboa (rural) in the Republic of Panama. Mean ± SEM are shown.
| Factor | Urban | Rural |
|---|---|---|
| Light intensity | 0.16 ± 0.02 lx | 0.11 ± 0.02 lx |
| Noise intensity | 69.0 ± 0.80 dB | 59.2 ± 1.00 dB |
| Temperature | 27.6 ± 0.09°C | 25.9 ± 0.04°C |
| Túngara frog abundance | 6.09 ± 2.63 frogs/site | 4.05 ± 1.11 frogs/site |
| Foam nest abundance | 2.06 ± 0.74 nests/site | 0.24 ± 0.23 nests/site |
| Frog-biting midge abundance | 0.00 ± 0.00 midges/site | 67.75 ± 43.27 midges/site |
In contrast to the abundance of the frogs, there were more frog-biting midges per calling site in rural than urban sites, where there were no frog-biting midges found (Table 1; χ21 = 2214.9, P < 0.001). Average temperature did not significantly affect midge abundance (χ21 = 0.24, P = 0.08) and thus could not account for the absence of midges in the urban sites. However, midge abundance was associated positively with the number of frogs (χ21 = 5.88, P < 0.0001) and negatively with light (χ21 = 3.24, P = 0.001) and noise levels (χ21 = 2.94, P = 0.003). Additionally, there was an interaction between light and noise levels (χ21 = 3.30, P < 0.001); at low levels of noise, light intensity was negatively associated with midge abundance, but at high levels of noise, there were no midges regardless of light level (Fig. 1; see Appendix S1: Table S2 for model selection information). Flying insects other than frog-biting midges, such as mosquitos, were found at all of the sites.
To reduce the likelihood that a third variable that differed between urban and rural sites was the true factor causing the decline in midges across the rural to urban gradient, we tested whether variation in light and noise levels in the rural sites only was also associated negatively with midge abundance. Within rural sites, midge abundance was again not significantly associated with temperature (χ21= 0.82, P = 0.41), but was positively associated with frog abundance (χ21= 24.63, P = 0.001). Additionally, light and noise intensity remained the most important negative predictors of midge abundance (χ21= 8.75, P = 0.03 and χ21= 5.01, P = 0.02, respectively). At low noise levels, light intensity had a significant negative effect on midge abundance but at high noise levels, light did not matter because of the strong negative effects of high noise intensity (interaction: χ21= 66.96, P < 0.0001; Figs. S2 and S3).
Midge Attraction Experiment
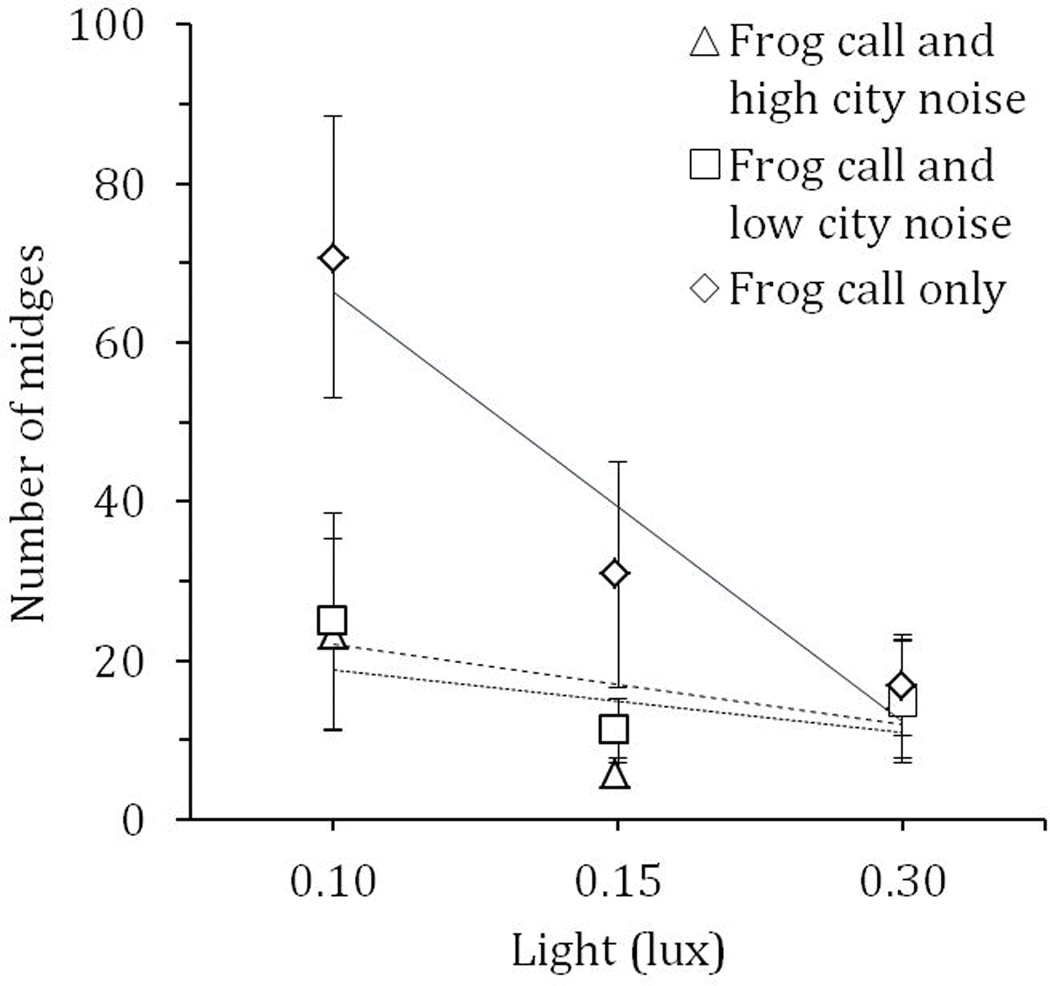
There was no significant effect of order of presentation of the treatments (χ21 = 0.09, P = 0.77), but there was a significant effect of sampling night on the number of midges collected (χ21 = 44.25, P < 0.0001). Despite fluctuations mediated by environmental conditions, we found a significant interaction between light and noise pollution (χ21 = 18.88, P = 0.0008; Fig. 2). As with the field survey, at low light intensity, the number of midges was significantly reduced by artificial noise, whereas at high light, few midges were collected regardless of noise level (See Figs. 1C and 1D for field survey).
Fig. 2.
Number of frog-biting midges (Corethrella spp.) collected in acoustic traps (Gamboa, Panama) using a fully crossed 3×3 design: no, low or high artificial light treatments (0.10, 0.15, 0.30 lux, respectively) crossed with Engystomops pustulosus frog call plus no (solid trendline), low (dashed trendline), or high (dotted trendline) city noise (45, 60, and 75 decibels, respectively). Shown are means ± 1 SE.
Manipulative Feeding Experiment
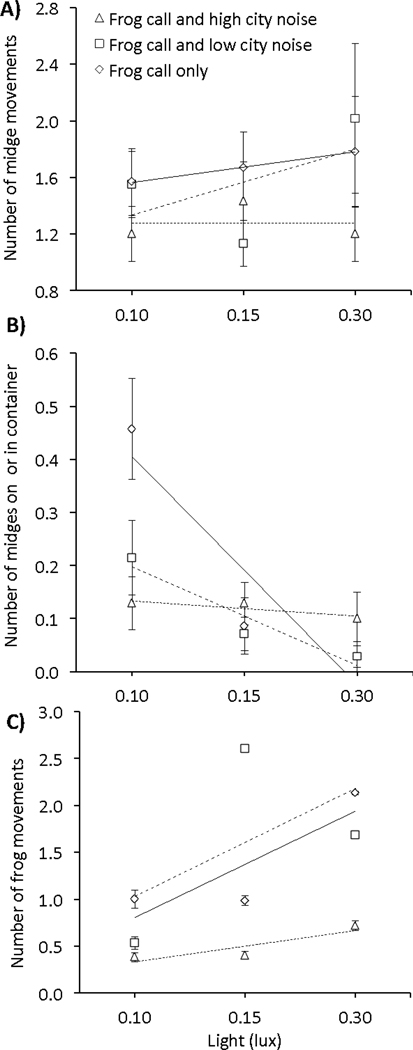
We examined the effect of light and noise pollution on midge behavior once they are in close proximity to their host and found that light and noise intensity reduced the ability of midges to find and feed on their frog hosts. There was an effect of light intensity on midge activity but no effect of noise intensity or an interaction between these factors (χ21 = 9.26, P = 0.002, χ21 = 3.09, P = 0.08; light and noise respectively; Fig. 3A, Appendix S1: Table S4 for model selection information). In terms of the number of midges that found the frog container, there was an interaction between light and noise intensity (χ21 = 14.4, P = 0.006). At low light levels, there was a negative effect of noise intensity on the number midges that reached the frog container, but at high light, few midges reached the frog container regardless of noise treatment (Fig. 3B). On the other hand, noise and light pollution significantly increased the movement of the frogs (noise: χ21 = 57.70, P < 0.001; light: χ21 = 57.60, P < 0.001; Fig. 3C), but there was no interaction between noise and light on frog activity (χ21 = 0.31, P = 0.57). Midges did not feed in treatments with artificial noise or light (0% had successful feeding events), whereas 40% of the replicates with no artificial noise or light had successful feeding events (noise and light: χ21 = 5.46, P = 0.03).
Fig. 3.
Effect of artificial light and noise on the number of A) frog-biting midge (Corethrella spp.) movements, B) midges that successfully located the container holding the frog (Engystomops pustulosus; please note, the data points for the frog call only and frog call plus low city noise at 0.3 lux, are the same values and therefore overlap one another) and C) frog movements within the container with a fully crossed 3×3 design with no, low or high artificial light treatments (0.10, 0.15, 0.30 lux, respectively) crossed with frog call plus no (solid trendline), low (dashed trendline), or high (dotted trendline) city noise (45, 60, and 75 decibels, respectively). Shown are means ± 1 SE.
Discussion
Results from our field survey and two field experiments demonstrate that anthropogenic noise and light pollution associated with urbanization disrupt the túngara frog and frog-biting midge, host-parasite, interaction. Most previous work on the effects of light and noise pollution on the responses of organisms has considered the effects of these abiotic factors independently (Halfwerk and Slabbekoorn 2015), often also investigating only one species at a time. Our results show that the effects of artificial light and noise depend on the level of the other factor and highlight that the interaction between light and noise pollution can have important consequences on species interactions that would be missed if these factors were studied in isolation.
Although the abundances of hosts and parasites are typically correlated within their range (Hudson and Dobson 1995), parasitic frog-biting midges are absent in urbanized areas where their túngara frog hosts are present. Given that other flying insects, such as mosquitos, are found throughout all areas surveyed, the lack of frog-biting midges at the frogs’ breeding sites in the city is unlikely to be caused by higher insecticide use in urban than rural populations. Additionally, although urban sites had slightly warmer conditions, midge abundance was not affected by temperature in the range documented in this study.
Variation in noise and light pollution, both across the urban and rural sites and within the rural sites, were the main predictors of midge abundance. In general, higher levels of artificial noise and light resulted in decreased abundance, and even absence, of frog-biting midges. This pattern suggests that even low levels of light or noise (tested here: 0.15 lux, 60 dB) can affect midge abundance and disrupt this host-parasite interaction. Our two experiments demonstrated that these field patterns were indeed causal. Noise intensity was an important negative predictor of midge abundance in the field survey and artificial noise and light levels decreased the ability of the midges to find the frogs. In fact, in both experiments we found that noise over approximately 60 dB affected parasite behaviors, such as the ability of the midges to localize their host. This reduced performance is probably caused by acoustic interference that results from the presence of anthropogenic noise. Given the frequency overlap of anthropogenic noise and the calls of túngara frogs, this novel source of noise could reduce the ability of the midges to detect and localize the frogs based on their calls. Some species of frogs, for example, increase the frequency of their mating calls in traffic noise, which may alter both inter- and intra-specific interactions (Parris et al. 2009). In addition to using acoustic signals to locate hosts for blood meals, frog-biting midges also use acoustic signals for intraspecific communication. Specifically, the midges use the wingbeats of conspecifics for courting purposes and to deter rival males (de Silva et al. 2015). The frequency bandwidth of the mating signal of the midges also overlaps with the spectral properties of anthropogenic noise and efficient communication during mating is thus also likely reduced with noise pollution. Therefore, acoustic interference elicited by anthropogenic noise could compromise midge fitness by affecting reproductive potential in two ways, 1) interfering with host detection and location and thus blood meal acquisition that will, in turn, reduce egg production, and 2) diminishing the chances of mating. These factors combined may have reduced the ability of the midges to have sustainable populations in urban areas where anthropogenic noise is high.
While it is clear that frog-biting midges depend on the acoustic signals produced by their hosts to acquire blood meals, little is known about how these midges hear. It is possible that frog-biting midges rely on antennal hearing to detect and respond to the calls of their anuran host. Frog-biting midges, however, respond to frog calls at distances greater than those expected to be within the range of antennal hearing suggesting a more elaborate, tympanic organ may be involved in hearing (Page et al. 2014). Current research is investigating the hearing mechanisms in this midge; regardless of the specific organ(s) involved, given the spectral overlap of traffic noise and the calls of túngara frogs, a reduction in the ability of the midges to hear and respond to the frog calls is to be expected.
Our results also revealed an effect of anthropogenic light on midge abundance and their ability to successfully locate and bite their host. In the Midge Attraction Experiment, increased light intensity reduced midge abundance, and in the Manipulative Feeding Experiment, midges reduced their movement when exposed to any level of artificial light, indicating that light is also an important stressor affecting the behavior of the midges. The reduced activity of the midges under high light levels made them less likely to find a frog host and is consistent with their lack of activity during the day in captivity and the wild. While it is expected that their host seeking behavior is timed with those hours in which the frogs are actively calling at night, the midges concentrate all their activities to the night hours becoming almost immobile when light intensity increases (de Silva & Bernal 2013). Ultimately, reduced activity at high light levels might be a strategy to minimize predation risk. In fact, when túngara frogs were presented with frog-biting midges in daylight hours, frogs readily consumed them (McMahon and Bernal, pers. obs.). Additionally, artificial light and noise increased the movements of the frogs, which could result in increased risk for the midges. Host movement and defensive behaviors can be a significant source of mortality for hematophagous insects (Walker and Edman 1985, Edman and Scott. 1987), so augmented frog activity in high light conditions could further deter approaches by midges.
We found that túngara frogs in urban habitats were free of their frog-biting midge parasites. At this point, we do not have a good understanding of how the absence of this parasite may affect the fitness of urban túngara frogs. Given that the midges can take substantial amounts of blood from túngara frogs (~10% of blood volume in a night of active calling, Bernal unpub data), losing this parasite may result in higher reproductive success. Male frogs in areas that are not attacked by midges may be able to invest more energy into their signaling strategy, such as, increasing call rate, hours of activity each night or chorus attendance which would result in increased attractiveness to female frogs. The absence of this host-parasite relationship in urban areas could also potentially influence other organisms and processes in the community. For example, túngara frogs are preyed upon by frog-eating bats who also depend on the mating call of the frogs to detect, localize them and finally eat them (Tuttle and Ryan 1981). Given that these bats may be able to compensate for the effect of noise through acoustic interference by using other cues (Gomes et al. 2016a), frogs in midge-free breeding areas may encounter yet another source of antagonistic selection limiting their signaling strategies. Urban frogs, however, by being in areas with light and noise pollution may have also escaped a different natural enemy. Frog-biting midges can transmit blood parasites, such as Trypanosoma tungarae, to túngara frogs (Bernal and Pinto 2016), so the absence of midges could have impacts on infectious disease dynamics in this community.
We found that both light and noise pollution disrupt host-parasite interactions. These findings highlight that more research is needed on the effects of urbanization on species interactions, parasitism in particular, and the importance of considering interactions among types of pollutants. This work is necessary to accurately assess the impacts of urbanization and altered interactions on ecological communities and ecosystems.
Supplementary Material
Acknowledgements.
We thank Dr. Roberto Ibañez for all of his time, equipment, and knowledge; he greatly contributed to this work. We would also like to thank the anonymous reviewers for comments. This work was supported by grants from the Smithsonian Tropical Research Institute (STRI Short-Term Fellowship) and the University of Tampa (Faculty Development Dana and Delo Grants) to T.A.M. and grants from the National Science Foundation (EF-1241889 and DEB-1518681), NIH (R01GM109499 and R01TW010286-01), US Department of Agriculture (2009-35102-0543), and US Environmental Protection Agency (CAREER 83518801) to J.R.R., and a grant from the National Science Foundation (IOS-1258039/1433990) to X.E.B.
TAM and JRR conceived and designed the experiments. TAM performed the experiments. XEB provided equipment and XEB provided expertise on Corethrella work. TAM analyzed the data and wrote the manuscript and all authors provided editorial advice and critical intellectual contributions. JRR and XEB share equal final author position. Data enquiries can be made through an email request to the corresponding author.
Literature cited
- Acharya L, and Fenton MB. 1999. Bat attacks and moth defensive behaviour around street lights. Canadian Journal of Zoology 77:27–33. [Google Scholar]
- Aihara I, Silva P. d., and Bernal XE. 2015. Acoustic preference of frog-biting midges (Corethrella spp) attacking Tungara frogs in their natural habitat. Ethology 122:105–113. [Google Scholar]
- Barber JR, Burdett CL, Reed SE, Warner KA, Formichella C, Crooks KR, Theobald DM, and Fristrup KM. 2011. Anthropogenic noise exposure in protected natural areas: estimating the scale of ecological consequences. Landscape Ecology 26:1281–1295. [Google Scholar]
- Barber JR, Crooks KR, and Fristrup KM. 2009. The costs of chronic noise exposure for terrestrial organisms. Trends in ecology and evolution 25. [DOI] [PubMed] [Google Scholar]
- Baugh AT, and Ryan MJ. 2010. Ambient light alters temporal updating behaviour during mate choice in a Neotropical frog.. Canadian Journal of Zoology 88:448–453. [Google Scholar]
- Bernal XE, and Pinto CM. 2016. Sexual differences in prevalence of a new species of trypanosome infecting túngara frogs. Internatinal Journal of parasitology: Parasites and Wildlife:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal XE, Rand AS, and Ryan MJ. 2006. Acoustic preferences and localization performance of blood-sucking flies (Corethrella coquillett) to tungara frog calls. Behavioral Ecology. [Google Scholar]
- Bernal XE, and Silva P. d.. 2015. Cues used in host-seeking behavior by frog-biting midges (Corethrella spp Coquillet). Journal of Vector Ecology 40:122–128. [DOI] [PubMed] [Google Scholar]
- Borkent A 2008. The frog-biting midges of the world (Corethrellidae:Diptera). Zootaxa:1–456. [Google Scholar]
- Bradley CA, and Altizer S. 2007. Urbanization and the ecology of wildlife diseases. Trends in ecology and evolution 22:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumm H 2013. Animal Communication and Noise. Springer, New York NY. [Google Scholar]
- de Silva P, and Bernal XE. 2013. First report of the mating behavior of a species of frog-biting midge (Diptera: Corethrellidae). Florida Entomologist 96:1522–1529. [Google Scholar]
- de Silva P, Jaramillo C, and Bernal XE. 2014. Selection of biting sites on anuran hosts by Corethrella coquillett species. Journal of Insect Behavior 27:302–316. [Google Scholar]
- de Silva P, Nutter B, and Bernal XE. 2015. Use of acoustic mating signals in an eavesdropping frog-biting midge. Animal Behaviour 103:45–51. [Google Scholar]
- Dominoni D, Carmona-Wagner EO, Hofmann M, Kranstauber B, and Partecke J. 2013. Individual-based measurements of light intensity provide new insights into the effects of artificial light at night on daily rhythms of urban-dwelling songbirds. Journal of Animal Ecology 83:681–692. [DOI] [PubMed] [Google Scholar]
- Douglas I 1983. The urban environment. Edward Arnold, London. [Google Scholar]
- Edman JD, and Scott TW. 1987. Host defensive behavior and the feeding success of mosquitoes. Insect Science and its Application 8:617–622. [Google Scholar]
- Ellis EC 2011. Anthropogenic transformation of the terrestrial biosphere. Philosophical Transactions of the Royal Society A Mathamatical, physical and engineering sciences 369. [DOI] [PubMed] [Google Scholar]
- Elvidge CD, Hsu FC, Baugh K, and Ghosh T. 2014. National trends in satellite observed lighting: 1992–2012 Global Urban Monitoring and Assessment Through Earth Observation. CRC Press Boca Raton, FL. [Google Scholar]
- Fernández-Juricic E, Poston R, Collibus KD, Morgan T, Bastain B, Martin C, JOnes K, and Treminio R. 2005. Micro-habitat selection and singing behavior patterns of male house finches (Carpodacus mexicanus) in urban parks in a heavily urbanized landscape in the Western U.S. Urban Habitats 3. [Google Scholar]
- Francis CD, Kleist n. J., Ortega CP, and Cruz A. 2012. Noise pollution alters ecological services: enhanced pollination and disrupted seed dispersal. Proceedings of the Royal Society B-Biological Sciences 279:2727–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis CD, Ortega CP, and Cruz A. 2009a. Cumulative consequences of noise pollution: noise changes avian communities and species interactions.. Current biology 19:1415–1419. [DOI] [PubMed] [Google Scholar]
- Francis CD, Ortega CP, and Cruz A. 2009b. Noise pollution changes avian communities and species interactions. Current biology 19:1415–1419. [DOI] [PubMed] [Google Scholar]
- Gaston KJ, Bennie J, Davies TW, and Hopkins J. 2013. The ecological impacts of nighttime light pollution: a mechanistic appraisal. Biological Reviews 88:912–927. [DOI] [PubMed] [Google Scholar]
- Gaston KJ, Davies TW, Bennie J, and Hopkins J. 2012. Reducing the ecological consequences of night-time light pollution: options and developments. Journal of Applied Ecology 49:1256–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes DGE, Page RA, Geipel I, Taylor RC, Ryan MJ, and Halfwerk W. 2016a. Bats perceptually weight prey cues across sensory systems when hunting in noise. Science 353. [DOI] [PubMed] [Google Scholar]
- Gomes DGE, Page RA, Geipel I, Taylor RC, Ryan MJ, and Halfwerk W. 2016b. Bats perceptually weight prey cues across sensory systems when hunting in noise. Science 353:1277–1280. [DOI] [PubMed] [Google Scholar]
- Grimm NB, Faeth SH, Golubiewski NE, Redman CL, Wu J, Bai X, and Briggs JM. 2008. Global change and the ecology of cities. Science 319:756–760. [DOI] [PubMed] [Google Scholar]
- Habib L, Bayne EM, and Boutin S. 2007. Chronic industrial noise affects pairing success and age structure of ovenbirds Seiurus aurocapilla. Journal of Applied Ecology 44:176–184. [Google Scholar]
- Halfwerk W, Bot S, Buikx J, Velde M. v. d., Komdeur J, Cate C. t., and Slabbekoorn H. 2011a. Low-frequency songs lose their potency in noisy urban conditions. Proceedings of the National Academy of Science 108:14549–14554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfwerk W, Holleman LJM, Lessells CKM, and Slabbekoorn H. 2011b. Negative impact of traffic noise on avian reproductive success. Journal of Applied Ecology 48:210–219. [Google Scholar]
- Halfwerk W, Lea AM, Guerra MA, Page RA, and Ryan MJ. 2016. Vocal responses to noise reveal the presence of the Lombard effect in a frog. Behavioral Ecology. [Google Scholar]
- Halfwerk W, and Slabbekoorn H. 2015. Pollution going multimodal: the complex impact of the human-altered sensory environment on animal perception and performance. Biology Letters 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haven WR 2015. Noise Matters: The Evolution Of Communication. Harvard University Press, Cambridge, Massachusetts and London England. [Google Scholar]
- Hoelker F, Moss T, Griefahn B, Kloas W, Voigt CC, Henckel D, Haenel A, Kappeler PM, Voelker S, Schwope A, Franke S, Uhrlandt D, Fischer J, Klenke R, Wolter C, and Tockner K. 2010. The Dark Side of Light: A Transdisciplinary Research Agenda for Light Pollution Policy. Ecology and Society 15. [Google Scholar]
- Holker F, Wolter C, Perkin EK, and Tockner K. 2010. Light pollution as a biodiversity threat. Trends in ecology and evolution 25. [DOI] [PubMed] [Google Scholar]
- Hudson PJ, and Dobson AP. 1995. Macroparasites: Observed patterns in naturally fluctuating animal populations. Cambridge University Press, The Pitt Building, Trumpington Street, Cambridge CB2 1RP, England; Cambridge University Press, 40 W. 20th Street, New York, New York 10011–4211, USA. [Google Scholar]
- Kaiser K, Scofield DG, Alloush M, Jones RM, Marczak S, Martineau K, Oliva MA, and Narins PM. 2011. When sounds collide: the effect of anthropogenic noise on a breeding assemblage of frogs in Belize, Central America. Behaviour 148:215–232. [Google Scholar]
- Kleist NJ, Guralnick RP, Cruz A, and Francis CD. 2016. Anthropogenic noise weakens territorial response to intruder’s songs. Ecosphere 7. [Google Scholar]
- Lafferty KD, Allesina S, Arim M, Briggs CJ, Leo GD, Dobson AP, Dunne JA, Johnson PTJ, Kuris AM, Marcogliese DJ, Martinez ND, Memmott J, Marquet PA, McLaughlin JP, Mordecai EA, Pascual M, Poulin R, and Thieltges DW. 2008. Parasites in food webs: the ultimate missing links. Ecology Letters 11:533–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiolo P 2010. The emerging significance of bioacoustics in animal species conservation. Biological Conservation 143:1635–1645. [Google Scholar]
- Longcore T, and Rich C. 2004. Ecological light pollution. Frontiers of Ecology and Environment 2:191–198. [Google Scholar]
- McKeever S 1977. Observations of Corethrella feeding on tree frogs (Hyla). Mosquito News 37:522–523. [Google Scholar]
- McKeever S, and Hartberg W. 1980. An effective method for trapping adult female Corethrella (Diptera: Chaoboridae). Mosquito News 40:111–112. [Google Scholar]
- McKinney M, L. 2002. Urbanization, Biodiversity, and Conservation. Bioscience 52:883–890. [Google Scholar]
- McMahon TA, Romansic JM, and Rohr JR. 2013. Non-monotonic and monotonic effects of pesticides on the pathogenic fungus Batrachochytrium dendrobatidis in culture and on tadpoles. Environmental Science and Technology 47:7958–7964. [DOI] [PubMed] [Google Scholar]
- Ortega CP 2012. Chapter 2: Effects of noise pollution on birds: A brief review of our knowledge - Efectos de la Polución Sonora en Aves: Una Breve Revisión de Nuestro Conocimiento.. Ornithological Monographs 74:6–22. [Google Scholar]
- Page RA, Ryan MJ, and Bernal XE. 2014. Be Loved, Be Preyed, Be Eatenin Yasakawa K, editor. Animal Behavior. BC-CLIO Editorial, Santa Barbara, CA. [Google Scholar]
- Parris KM, Velik-Lord M, and North JMA. 2009. Frogs call at a higher pitch in traffic noise. Ecology and Society 14:25. [Google Scholar]
- Patricelli GL, and Blickley JL. 2006. Avian communication in urban noise: causes and consequences of vocal adjustment. The Auk 123:639–649. [Google Scholar]
- Raffel TR, Halstead NT, McMahon TA, Davis AK, and Rohr JR. 2015. Temperature variability and moisture synergistically interact to exacerbate an epizootic disease. Proceedings of the Royal Society B-Biological Sciences 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffel TR, Romansic JM, Halstead NT, McMahon TA, Venesky MD, and Rohr JR. 2013. Disease and thermal acclimation in a more variable and unpredictable climate. Nature Climate Change 3:146–151. [Google Scholar]
- Rand AS, Bridarolli ME, Dries L, and Ryan MJ. 1997. Light levels influence female choice in Tungara frogs: predation risk assessment? Copeia 447–450:447–450. [Google Scholar]
- Rich C, and Longcore T. 2013. Ecological consequences of artificial night lighting. Island Press. [Google Scholar]
- Rohr JR, Raffel TR, Halstead NT, McMahon TA, Johnson SA, Boughton RK, and Martin LB. 2013. Early-life exposure to a herbicide has enduring effects on pathogen-induced mortality. Proceedings of the Royal Society B-Biological Sciences 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MJ 1985. Energetic efficiency of vocalization by the frog Physalaemus pustulosus. Journal of Experimental Biology 116:47–52. [Google Scholar]
- Shochat E, Warren PS, Faeth SH, McIntyre NE, and Hope D. 2006. From patterns to emerging processes in mechanistic urban ecology. Trends in ecology and evolution 21:186–191. [DOI] [PubMed] [Google Scholar]
- Slabbekoorn H, Bouton N, Opzeeland I. v., coers A, Cate C. t., and Popper AN. 2010. A noisy spring: the impact of globally rising underwater sound levels on fish. Trends in ecology and evolution 25:419–427. [DOI] [PubMed] [Google Scholar]
- Slabbekoorn H, and Peet M. 2003. Birds sing at a higher pitch in urban noise. Nature 424. [DOI] [PubMed] [Google Scholar]
- Slabbekoorn H, and Ripmeester EAP. 2008. Birdsong and anthropogenic noise: implications and applications for conservation. Molecular Ecology 17:72–83. [DOI] [PubMed] [Google Scholar]
- Trillo P, Bernal X, Caldwell M, Halfwerk W, Wessel M, and Page R. 2016. Collateral damage or a shadow of safety? The effects of signalling eterospecific neighbours on the risks of parasitism and predation. Proceedings of Biological Science B 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle MD, and Ryan MJ. 1981. Bat predation and the evolution of frog vocalizations in the Neotropics. Science 214:677–678. [DOI] [PubMed] [Google Scholar]
- Walker ED, and Edman JD. 1985. The influence of host defensive behavior on mosquito (Diptera: Culicidae) biting persistence.. Journal of Medical Entomology 22:370–372. [DOI] [PubMed] [Google Scholar]
- Warren PS, Katti M, Ermann M, and Brazel A. 2006. Urban bioacoustics: it’s not just noise. Animal Behaviour 71:491–502 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.