Significance
Many stroke patients exhibit long-term disability. Transplantation of stem cells has been proposed as a novel therapeutic strategy to promote recovery. However, little is known about whether these cells can form functionally integrated connections with neurons in the stroke-injured recipient’s brain. Here we show extensive morphological and functional integration of axonal projections from intracortically transplanted human iPS cell–derived cortical neurons in brains of rats with ischemic lesions in the cerebral cortex. We also demonstrate that neuronal activity in these grafts is involved in the regulation of the stroke-affected animals’ motor behavior. Our findings raise the possibility that injured neural circuitry might be restored by stem cells also in humans affected by stroke, which would have major clinical implications.
Keywords: stroke, iPS cells, cerebral cortex, transplantation, optogenetics
Abstract
Stem cell transplantation can improve behavioral recovery after stroke in animal models but whether stem cell–derived neurons become functionally integrated into stroke-injured brain circuitry is poorly understood. Here we show that intracortically grafted human induced pluripotent stem (iPS) cell–derived cortical neurons send widespread axonal projections to both hemispheres of rats with ischemic lesions in the cerebral cortex. Using rabies virus–based transsynaptic tracing, we find that at 6 mo after transplantation, host neurons in the contralateral somatosensory cortex receive monosynaptic inputs from grafted neurons. Immunoelectron microscopy demonstrates myelination of the graft-derived axons in the corpus callosum and that their terminals form excitatory, glutamatergic synapses on host cortical neurons. We show that the stroke-induced asymmetry in a sensorimotor (cylinder) test is reversed by transplantation. Light-induced inhibition of halorhodopsin-expressing, grafted neurons does not recreate the impairment, indicating that its reversal is not due to neuronal activity in the graft. However, we find bilateral decrease of motor performance in the cylinder test after light-induced inhibition of either grafted or endogenous halorhodopsin-expressing cortical neurons, located in the same area, and after inhibition of endogenous halorhodopsin-expressing cortical neurons by exposure of their axons to light on the contralateral side. Our data indicate that activity in the grafted neurons, probably mediated through transcallosal connections to the contralateral hemisphere, is involved in maintaining normal motor function. This is an example of functional integration of efferent projections from grafted neurons into the stroke-affected brain’s neural circuitry, which raises the possibility that such repair might be achievable also in humans affected by stroke.
Based on findings in experimental models, stem cell transplantation has been proposed as a potential novel treatment for stroke (1). Clinical trials have been initiated using intracerebral delivery of, e.g., human bone marrow–derived mesenchymal stem cells (2) or multipotent adult progenitor cells (3) and a human neural stem cell line (4). These studies have not aimed at replacing dead neurons but at other mechanisms of improvement such as modulation of inflammation, trophic action, and stimulation of plasticity (see, e.g., ref. 5). Solid evidence for functional integration and reconstruction of neural circuitry by stem cells in the stroke-injured brain has been lacking.
In many stroke patients, loss of function is caused by the ischemic lesion extending into the cerebral cortex (6). For recovery by neuronal replacement, implantation of cortical neurons would, therefore, be required. We have previously shown that transplantation of human cortical neuronal progenitors, derived from induced pluripotent stem (iPS) cells, into the cerebral cortex adjacent to an ischemic lesion induced by distal middle cerebral artery occlusion (dMCAO) leads to improvement of sensorimotor deficits (7). Grafted neurons receive synaptic inputs from thalamocortical afferents in the stroke-affected host brain (8) and alter their activity in response to physiological sensory stimuli. These findings indicate that transplanted human cortical neurons can establish functional afferent connections with host neurons.
Neurons derived from human embryonic stem (ES) and iPS cells, implanted into neonatal or adult, intact rodents, send axonal projections to widespread brain areas, including the contralateral cortex (9, 10). Similarly, following stroke in rats, axonal projections from intracortically transplanted human ES (11) and iPS cell–derived (7) cortical neurons extend to both ipsilateral and contralateral hemispheres. Whether these efferent connections become incorporated into stroke-injured host neural circuitry and whether activity in the grafted neurons influences sensorimotor function are unknown.
Here we have addressed two fundamental questions regarding the potential for reconstruction of the stroke-injured brain by transplantation of human stem cell–derived cortical neurons: first, whether the projections from intracortical grafts of iPS cell–derived cortical neurons can become myelinated and form functional synaptic contacts with host neurons in the contralateral cortex and, second, whether the grafted neurons can influence motor function and reverse stroke-induced deficits and, if this is the case, whether the observed effects are dependent on neuronal activity in the transplants.
Results
Widespread Projections from Grafts in a Stroke-Injured Brain.
We subjected rats to dMCAO, and 48 h later, cortically fated human iPS cell–derived long-term self-renewing neuroepithelial-like stem (lt-NES) cells (7, 8) were implanted close to the injury. At 6 mo, the ischemic lesion was restricted to the cortex, mostly somatosensory cortex (S1FL and S1BF) and partially including motor area (M1), sparing subcortical structures. Lesion volume did not differ between nontransplanted (43.8 ± 7.5 mm3) and transplanted (48.0 ± 7.8 mm3) animals. The majority of grafted cells, identified by the human nuclear marker STEM101, were located adjacent to the lesion in the somatosensory cortex and part of the M1 motor cortex. Some grafted cells had migrated to the peri-infarct cortex, a substantial number were found in the corpus callosum, and a few cells had reached the internal capsule and caudate–putamen bilaterally (SI Appendix, Fig. S1).
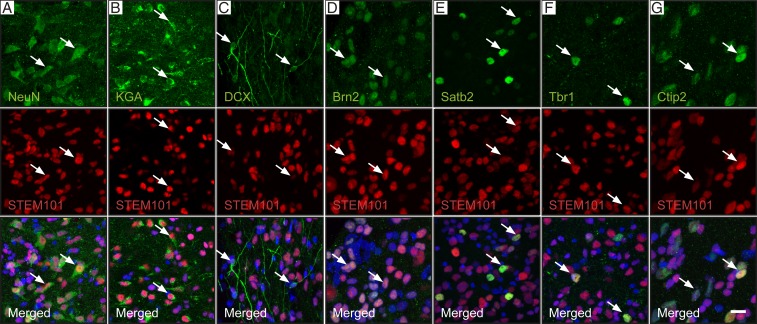
At 6 mo, 41% of STEM101+ cells in the transplant core expressed the mature neuronal marker NeuN, and 3% expressed the neuronal progenitor marker DCX. The vast majority of grafted neurons were excitatory, expressing KGA, and only a few individual cells exhibited the GABAergic (GAD65/67+) inhibitory phenotype (Fig. 1 A–C and SI Appendix, Fig. S2A). No STEM101+ cells expressed the proliferation marker Ki67 (SI Appendix, Fig. S2B). About 40% of the STEM101+ cells expressed the oligodendrocyte marker Sox10, and a low number of human astrocytes were also found (SI Appendix, Fig. S3) (12). The human cells expressed neuronal markers of upper cortical layers, e.g., Brn2 (layers II and III) and Satb2 (layers II to V, callosal projection neurons), and deep cortical layers such as Ctip2 (layers V and VI, corticospinal projection neurons) and Tbr1 (layers V and VI) (Fig. 1 D–G) (13, 14). A low number of cells expressed the corticothalamic projection neuron marker Fog2 (15). Grafted cells in other areas expressed no neuronal markers (NeuN and DCX) but coexpressed markers of glial cells, mostly Sox10 (oligodendrocytes).
Fig. 1.
Cellular composition of cortically fated human lt-NES cell–derived grafts in stroke-injured rat somatosensory cortex. (A–C) Confocal immunohistochemical images showing mature neurons (A, NeuN), glutamatergic neurons (B, KGA), and neuronal progenitors (C, DCX) in the transplant core (human cells are STEM101+). (D–G) Expression of markers characteristic of neurons in upper (D, Brn2 and E, Satb2) and deep (F, Tbr1 and G, Ctip2) cortical layers in the human cells. Arrows indicate colocalization. (Scale bar, 20 μm.)
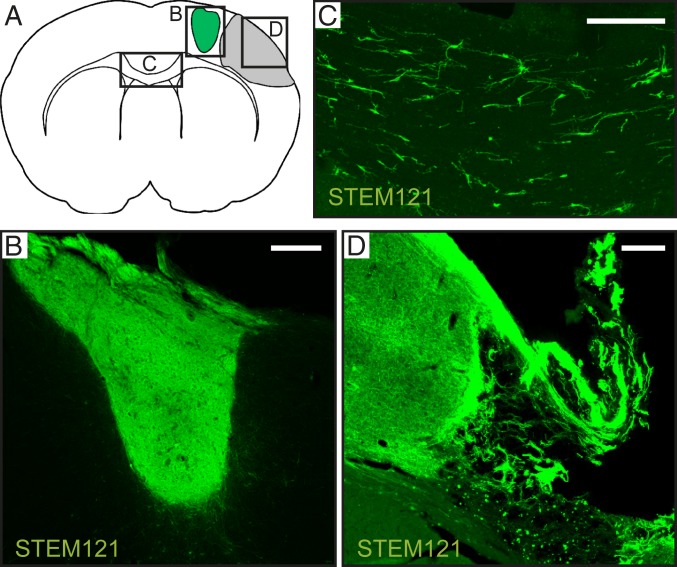
Massive numbers of graft-derived fibers reached the peri-infarct cortex, and medium to high fiber density was detected bilaterally in frontal and somatosensory cortices (Table 1). There was a rich fiber projection also to the corpus callosum (Fig. 2). Only a few fibers were found in the caudate–putamen, septum, and hippocampus (SI Appendix, Fig. S4).
Table 1.
Relative density of fibers originating from intracortical transplants of human-derived cortically fated lt-NES cells in various areas of stroke-injured rat brain
| Area/nucleus | Ipsilateral | Contralateral |
| Peri-infarct cortex | +++ | |
| Frontal cortex | +++ | ++ |
| Somatosensory cortex | +++ | +++ |
| Claustrum | ++ | + |
| Corpus callosum | ++ | |
| Ventral posterior thalamic nucleus | 0 | 0 |
| Ventral anterior thalamic nucleus | + | + |
| Internal capsule | + | + |
| Septum | + | |
| Fimbria | + | + |
| Hippocampus (CA1) | + | + |
| Basolateral amygdaloid nucleus | 0 | 0 |
| Caudate–putamen | + | 0 |
Semiquantitative representation of the density of graft-derived STEM121+ fibers: 0, no fibers; +, low density, 1 to 5 fibers in counting area; ++, medium density, 6 to 50 fibers; and +++, high density, more than 50 fibers.
Fig. 2.
Intracortical grafts of human lt-NES cell–derived cortical neurons project extensively in stroke-injured rat brain. (A) Location of illustrated areas. (B–D) Fibers immunoreactive for human cytoplasmic marker STEM121 in the transplant core (B), corpus callosum (C), and peri-infarct area (D). (Scale bar, 200 μm.)
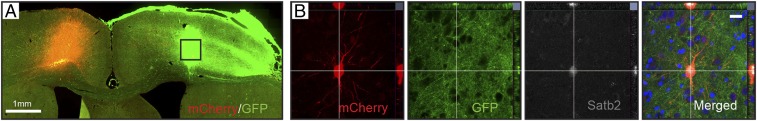
We analyzed further whether grafted neurons could form connections with host neurons in contralateral cortex using a retrograde transsynaptic tracing method with a modified rabies virus (ΔG-rabies). The lentiviral tracing vector and ΔG-rabies virus carrying the mCherry reporter were injected into the contralateral somatosensory cortex at 6 mo after transplantation (Fig. 3A). One week later, mCherry+ grafted cells were located in the transplant core, showing that they had established monosynaptic connections with contralateral host cortical neurons. In the brains of four rats, we found in total 14 mCherry+/GFP+ cells within the graft after rabies virus injection in the contralateral hemisphere. Most labeled cells were pyramidal shaped (SI Appendix, Fig. S5), and interestingly, we detected coexpression with Satb2, a marker of corticocallosal projection neurons (Fig. 3B).
Fig. 3.
Grafted human lt-NES cell–derived cortical neurons establish monosynaptic connections with host neurons in the contralateral cortex of stroke-injured rats. (A) Brain slice showing GFP+ grafted neurons in ipsilateral and mCherry+ host neurons transduced by rabies virus in the contralateral somatosensory cortex. (B) Confocal images of mCherry+, GFP+ grafted cell expressing the corticocallosal projection neuron marker Satb2 (gray) in the transplant core. Nuclear staining (Hoechst, blue) is included in the merged panel. (Scale bar in B, 20 μm.)
Axonal Myelination and Functional Synapses on Host Cortical Neurons.
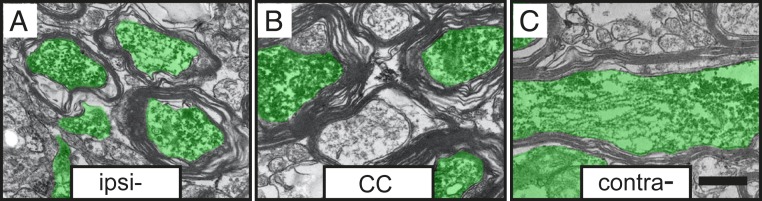
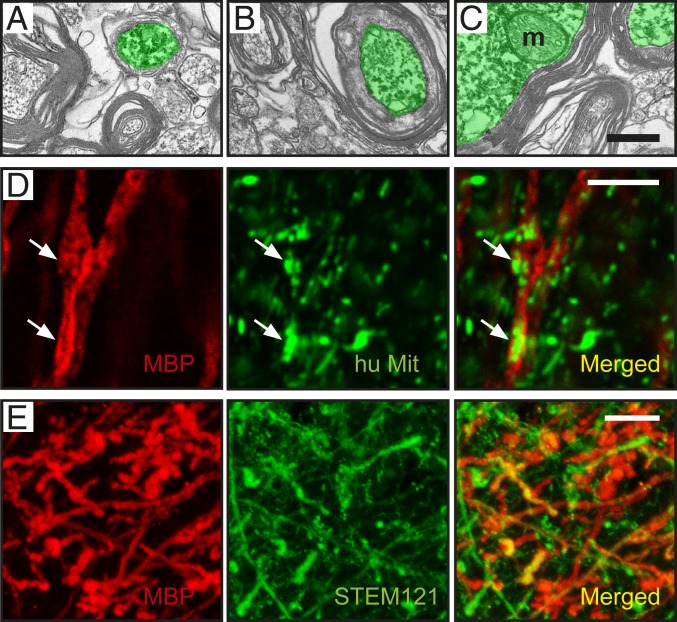
At 6 mo after transplantation, immunoelectron microscopy (iEM) combined with anti-GFP antibodies showed that graft-derived GFP+ axons in the corpus callosum exhibited ultrastructural features similar to those of host axons, such as mitochondria, the agranular endoplasmic reticulum, microtubules, and neurofilaments. Microtubules were unevenly distributed throughout the cross section of the axon, whereas neurofilaments often appeared uniformly dispersed throughout the GFP+ axoplasm (Fig. 4 A–C). The GFP+ axons were myelinated, supporting their full integration in the host brain. We observed GFP+ axons at initial (Fig. 5A) and intermediate (Fig. 5B) stages of myelination, as well as axons enclosed by compact myelin sheaths (Fig. 5C).
Fig. 4.
Axons originating in grafted human lt-NES cell–derived cortical neurons become myelinated in stroke-injured rat brain. Graft-derived axons (marked in green) ensheathed by myelin in the (A) ipsilateral somatosensory cortex, (B) corpus callosum (CC), and (C) contralateral somatosensory cortex. (Scale bar, 0.2 μm.)
Fig. 5.
Axon myelination occurs at different stages at 6 mo posttransplantation, and myelin partly originates from human-derived oligodendrocytes. (A–C) Graft-derived axons in the contralateral somatosensory cortex. (A) Initial and (B) intermediate stages of myelination. (C) Axons with compact myelin sheaths. m: mitochondria. (Scale bar, 0.2 μm.) (D) Confocal image of an axon ensheathed by human myelin close to the transplant, as evidenced by colocalization of MBP (red) and human mitochondrial marker (green). (Scale bar, 5 μm.) (E) Confocal image of graft-derived fibers expressing the human cytoplasmic marker STEM121 (green) colocalizing with MBP (red). (Scale bar, 10 μm.)
To explore whether the myelin was derived from the graft, we used antibodies recognizing myelin basic protein (MBP) and human mitochondria. Due to the high density of MBP+ fibers in the corpus callosum, it was impossible to unequivocally distinguish colocalization of both markers in individual myelinated fibers in this area. In contrast, many individual MBP+ fibers positively stained for human mitochondria or the human cytoplasmic marker STEM121 (16) were detected close to the graft (Fig. 5 D and E). This finding indicates that the graft-derived oligodendrocytes were functional and able to produce myelin. However, whether the ensheathed axons were of human or rat origin needs further studies.
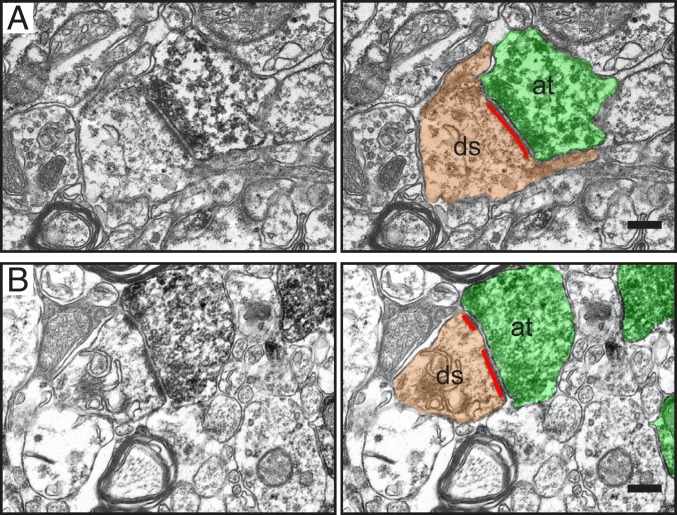
The GFP+ axon terminals had established characteristic synapses with host neurons in the contralateral somatosensory cortex: clustering of synaptic vesicles close to the presynaptic membrane, a synaptic cleft containing an intermediate layer of dense material, and a postsynaptic membrane with postsynaptic densities (Fig. 6). The vast majority (94.2%) of synaptic contacts were axodendritic and contacted host neuron dendritic spines, identified on the basis of shape, size, and the presence of a spiny apparatus. All analyzed GFP+ axodendritic synaptic contacts were asymmetric, with characteristics of excitatory/glutamatergic synapses, e.g., prominent postsynaptic density, a wide synaptic cleft, and spherical synaptic vesicles. Most postsynaptic densities (87.4%) were continuous nonperforated (simple) (Fig. 6A), and only 12.6% were perforated (Fig. 6B). The GFP+ axon terminals displayed abundant synaptic and docked vesicles at the presynaptic membrane (Fig. 6 A and B), indicating the presence of a readily releasable pool of synaptic vesicles and functional activity of synapses (17).
Fig. 6.
Grafted human lt-NES cell–derived cortical neurons establish asymmetric synapses with host neurons in the contralateral somatosensory cortex of stroke-injured rats. Synapses with continuous (A) and perforated (B) postsynaptic densities (red lines) between host dendritic spines (ds, brown) and grafted GFP+/DAB+ presynaptic axon terminals (at, green). (Scale bar, 0.2 μm.)
Graft-Induced Reversal of Stroke-Induced Sensorimotor Deficit Independent of Neuronal Activity.
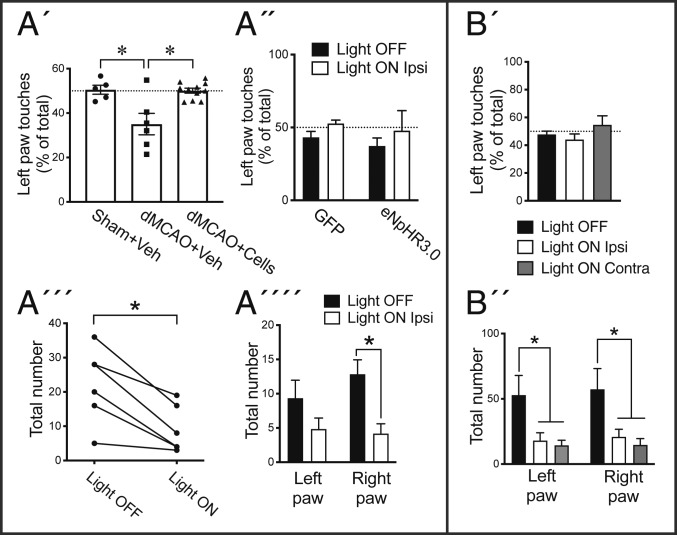
We assessed sensorimotor function using a cylinder test at 6 mo posttransplantation in three groups of animals: sham surgery + vehicle injection, dMCAO + vehicle injection, and dMCAO + transplantation of cortically fated lt-NES cells (Fig. 7A). The forelimb contralateral to the ischemic lesion was impaired in the dMCAO + vehicle group compared to the sham group. Cell transplantation completely mitigated this behavioral asymmetry (Fig. 7A′). Arguing against a neuroprotective action by the transplant, as mentioned above, we found no differences in lesion volume between grafted and vehicle-injected animals.
Fig. 7.
Activity in grafts of human lt-NES cell–derived cortical neurons is not responsible for reversal of stroke-induced sensorimotor deficit in the cylinder test but regulates motor function bilaterally similar to endogenous cortical neurons. (A′) Percentage the left limb touches in sham surgery + vehicle injection (sham+Veh; n = 5), dMCAO+Veh (n = 6), and dMCAO + transplantation of cortically fated lt-NES cells (dMCAO+Cells) (n = 11) groups in the cylinder test (*P < 0.05 vs. dMCAO+Veh). (A´´) Percentage the left limb touches in rats transplanted with GFP (n = 5) or eNpHR3.0-YFP lt-NES (n = 6) cells with and without light stimulation (P > 0.05). (A´´´) Total number of touches and (A´´´´) number of touches of individual limbs in animals with eNpHR3.0 lt-NES cell transplants with and without light stimulation (*P < 0.05 light on vs. light off). (B′) Percentage the left limb touches (P > 0.05 vs. light off) and (B´´) total number of touches of individual limbs with and without light stimulation in the ipsilateral or contralateral hemisphere (*P < 0.05 vs. light off; n = 6) in intact animals injected with eNpHR3.0 virus in the somatosensory cortex. Means ± SEM.
We then explored whether the reversal of the impairment was due to neuronal activity in the graft. All rats in the transplantation group had been implanted with cells transduced either to express the inhibitory chloride pump halorhodopsin, expressed under a syn1 promotor and with YFP reporter (eNpHR3.0 group), or to constitutively express GFP (control group). We first validated that the cells responded to light in vitro using whole-cell patch-clamp recordings of the eNpHR3.0-YFP cell line under the cortical differentiation protocol at 52 d. The generated neurons expressing halorhodopsin were inhibited by orange light-emitting diode (LED) light (SI Appendix, Fig. S6).
We implanted an optical fiber at the same coordinates as the ones used for cell transplantation 5 mo earlier, and 1 mo later, all grafted animals were assessed in the cylinder test. Neither animals with grafted neurons expressing halorhodopsin nor those with only GFP showed asymmetry in the cylinder test in response to illumination (Fig. 7A″). Thus, the reversal of the stroke-induced impairment in this sensorimotor test was not due to neuronal activity in the grafts.
Regulation of Motor Function Bilaterally by Neuronal Activity in Grafted and Endogenous Cortical Neurons.
We found that light stimulation of the halorhodopsin-expressing, but not the GFP-only-expressing, grafted neurons in vivo gave rise to bilateral reduction of forelimb touches in the cylinder test (Fig. 7 A″′ and A″″). In order to validate whether this effect was really due to light-induced inhibition of activity in grafted eNpHR3.0-expressing neurons, we performed whole-cell patch-clamp recordings on acute brain slices through the transplant (SI Appendix, Fig. S7). In support, light illumination activated eNpHR3.0, as seen by upward deflecting current, and hyperpolarized grafted neurons (SI Appendix, Fig. S7 H–J), thus inhibiting action potential generation (SI Appendix, Fig. S7 K and L).
We finally explored whether the effects on motor behavior induced by inhibiting activity in grafted neurons mimicked those caused by inhibition of endogenous cortical neurons located in the same area. Intact rats were injected with eNpHR3.0-expressing lentivirus unilaterally into somatosensory and adjacent motor cortex (Fig. 7B). One week after virus injection, we illuminated either the ipsilateral side, close to the transduced area, or the corresponding area on the contralateral side. Both approaches gave rise to similar bilateral reductions of the number of forelimb touches in the cylinder test without any asymmetry (Fig. 7 B′ and B″). We confirmed the extension of the transduced area by YFP staining. No unspecific effects such as epileptiform activity were observed in the optogenetics studies.
Discussion
Here we show extensive morphological and functional integration of axonal projections from intracortically grafted human iPS cell–derived cortical neurons in brains of rats with ischemic cortical stroke. We also demonstrate that neuronal activity in these grafts is involved in the regulation of the stroke-affected animals’ motor behavior. At 6 mo after stroke, the transplant, located in the somatosensory and part of M1 motor cortices, contained about 40% mature neurons expressing markers of both upper and deep cortical layers. In agreement with previous findings after transplantation of human ES cell–derived neurons into the intact (9, 10) or stroke-injured (11) brain, the grafted human iPS cell–derived cortical neurons extended projections to ipsilateral and contralateral hemispheres. The axonal outgrowth from the transplants closely resembled the previously reported projections from intrinsic cortical neurons located in the same area (18, 19). One notable exception was the projection to the ipsilateral thalamic ventral posterior nucleus (VPN). Whereas this nucleus receives numerous afferent fibers from the somatosensory cortex in the intact brain (20), we found no fibers in the VPN originating in the transplant in the stroke-injured brain. This finding was consistent with the occurrence of few cells expressing the corticothalamic projection neuron marker Fog2 in the transplant. It remains to be clarified whether the lack of projection to the VPN was due to low yield of this subtype of cortical neuron or it reflects degeneration of target neurons in the VPN due to secondary damage after the ischemic insult.
We report several findings regarding the projection from the intracortical graft to the contralateral somatosensory cortex: First, in agreement with the large number of graft-derived axons traversing the midline in the corpus callosum, Satb2+ human callosal projection neurons were present in the transplant. Second, the contralateral cortex received monosynaptic inputs from the grafted neurons, their axon terminals forming excitatory, glutamatergic synapses on host neurons. Third, the human graft-derived axons in the corpus callosum were myelinated, which is of particular interest since demyelination and subsequent axonal damage are major problems in stroke (21). Current knowledge about myelination of axons from human stem cell–derived transplants is scarce. Striatopallidal axons originating from rat embryonic striatal grafts implanted into neurotoxin-injured adult rat striatum have been reported to become myelinated (22). Also, Espuny-Camacho and coworkers (23) observed myelination of axons extending from human ES cell–derived neurons within the thalamus after transplantation into neonatal mouse brain. We detected different stages of myelination of the human axons in the corpus callosum of adult rats, suggesting that this process is still ongoing at 6 mo after transplantation. Our findings using immunohistochemical colocalization of MBP and human mitochondria provide strong evidence that at least some of the myelin in the transplant core and in different areas, including the corpus callosum, was produced by graft-derived oligodendrocytes. However, whether the myelin ensheathing the human axons is host or graft derived remains to be determined.
We assessed the involvement of neuronal activity in the grafted cells for their effect on behavior using optogenetics. A previous study on stroke-affected animals showed that 4 wk of daily exposure to light of human neural stem cell grafts expressing the excitatory channel channelrhodopsin gave rise to improved sensorimotor performance (24). Recently, Yu et al. (25) transplanted mouse iPS cell–derived neural progenitors expressing an optochemogenetics fusion protein, which consisted of a bioluminescent luciferase and channelrhodopsin, close to a cortical stroke in mice. Daily stimulation of the grafted cells for 2 wk after transplantation promoted behavioral recovery. However, none of these studies determined the acute effect of changes in neuronal activity in stem cell grafts on motor function in stroke-affected animals.
Here we exposed intracortically grafted human cortical neurons, transduced to express the inhibitory chloride pump halorhodopsin, to light. A similar approach was used by Steinbeck et al. (26) to demonstrate the involvement of neuronal activity in intrastriatal transplants of stem cell–derived dopaminergic neurons for recovery in a mouse model of Parkinson's disease. We chose to assess the behavioral effects of neuronal activity in the grafts at 6 mo after transplantation because our previous studies (7, 8, 27) have shown that the process of differentiation and integration of the grafted neurons requires at least 4 to 5 mo. The cylinder test was selected for behavioral analysis because it can reveal impairments of both motor and sensory forelimb function (28). We found that at 6 mo, the stroke-induced asymmetry in the cylinder test observed in the nongrafted group had been completely reversed by the grafts. However, illumination of the halorhodopsin-expressing, grafted neurons, leading to their inhibition as confirmed in the subsequent patch-clamp analysis of the transplants in acute brain slices, did not recreate this impairment. Thus, the reversal of the stroke-induced deficit in the cylinder test was not due to neuronal activity in the graft. Our finding is in accordance with the bulk of experimental evidence indicating that intracerebral transplantation of stem cells can lead to behavioral improvements by nonneuronal mechanisms operating early after stroke (1, 29, 30).
In fact, we previously showed (7) that intracortical transplants of cortically fated lt-NES cells, as used here, had given rise to recovery in a behavioral task already at 2 mo after stroke, arguing against neuronal replacement as the major underlying mechanism. Interestingly, we found bilateral decrease of forelimb touches in the cylinder test after inhibition of either grafted or endogenous halorhodopsin-expressing cortical neurons, located in the same area, by exposure to light in stroke-injured and intact animals, respectively. A similar change in motor performance was observed when inhibiting the endogenous halorhodopsin-expressing cortical neurons by exposing their axons in the contralateral hemisphere to light. Taken together, these data provide evidence that activity in intracortically grafted cortical neurons, through transcallosal connections to the corresponding cortical area in the contralateral hemisphere, is involved in maintaining motor function. This is an example of functional integration of grafted neurons in the stroke-affected brain’s neural circuitry and their direct involvement in motor control. It is interesting to note that several studies (31–33) have indicated that transcallosal connections are importantly involved in spontaneous interhemispheric structural reorganization and behavioral improvement after cortical stroke. Conceivably, the grafted neurons’ transcallosal connections take part in this reorganization.
The present data together with those of Tornero et al. (8), obtained using intracortical transplantation of cortically fated lt-NES cells, show that both afferent and efferent morphological and functional connections can be established between grafted human iPS cell–derived cortical neurons and rat host neurons following stroke. Several recent studies in other experimental models support the idea, raised by our findings, that reconstruction of cortical neural circuitry is possible using cell transplantation in the injured adult brain. Falkner et al. (34) demonstrated that mouse embryonic cortical neurons transplanted into the photolytically injured visual cortex of adult mice receive specific inputs from host neurons. The grafted neurons grow axons, reaching proper targets, and exhibit functional properties indistinguishable from those of the original cells in the visual cortex (34). Similarly, Michelsen et al. (35) found that visual cortical neurons, generated from mouse ES cells and transplanted into the ibotenic acid-injured adult mouse visual cortex, reestablished reciprocal synaptic connections with targets of damaged cortex. Some of the grafted neurons responded to visual stimuli. Also, human ES cell–derived visual cortical neurons implanted in the same model are able to send axonal projections matching the normal ones and may receive functional synaptic input from the host brain (36). Our findings here provide evidence of a previously unknown, high level of functional integration of human stem cell transplants in ischemic stroke, i.e., in a clinically relevant model of a common human disease.
Restoration of injured neural circuitry will most likely be necessary for stem cells to induce major symptomatic recovery after stroke. Our findings raise the possibility that this might be achievable also in humans, which would have major clinical implications. For a successful outcome not only in a xenograft situation, as used here, but also in the clinical setting, the transplanted human-derived neurons must be able to establish afferent and efferent synaptic connections with host neurons in the adult human brain. Recent studies with transplantation of human-derived neurons onto long-term organotypic cultures of adult human cortex provide supportive evidence that this could be possible (37). It will now be necessary, e.g., to explore further the involvement of neuronal activity in well-integrated grafts for recovery following stroke using a broader repertoire of behavioral tasks. Thus, although the perspective is exciting, much work in animal models remains before intracerebral transplantation of stem cell–derived neurons with the aim to repair the stroke-affected patient’s brain can even be considered.
Materials and Methods
For detailed information and protocols, see SI Appendix, Materials and Methods.
Cell Line and Cortical Priming.
Human iPS cell–derived lt-NES cells were produced from skin fibroblasts and primed toward a cortical neuronal phenotype (7). Briefly, growth factors (fibroblast growth factor, epidermal growth factor) and B27 were omitted, and cells were cultured at low density in differentiation-defined medium in the presence of BMP4 (10 ng/mL), Wnt3A (10 ng/mL), and cyclopamine (1 μM) for 4 d. Neuronal progenitors were then dissociated and resuspended at 100,000 cells/μL for transplantation.
Distal Middle Cerebral Artery Occlusion and Cell Transplantation.
dMCAO was performed in adult male athymic, nude rats. Intracortical transplantation of a total of 200,000 lt-NES cells carrying either the GFP (control group and for iEM) or eNpHR3.0-YFP (for optogenetic experiments) gene was performed stereotaxically 48 h later at two sites close to the ischemic injury. All procedures were conducted in accordance with European Union Directive 2010/63/EU and approved by the ethical committee for the use of laboratory animals at Lund University and the Swedish Department of Agriculture.
Immunohistochemistry.
Animals were perfused 6 mo after transplantation, and tissue was processed. A list of antibodies and detailed protocols for immunohistochemistry and quantification of cell numbers and infarct volume are provided in SI Appendix, Materials and Methods.
Rabies Virus Retrograde Tracing.
Six months after cell transplantation, 1 μL of tracing vector was injected stereotaxically into the contralateral cortex. One week later, ΔG-rabies virus injection was performed at the same coordinates. Animals were perfused 1 wk after the last injection.
Immunoelectron Microscopy.
Coronal 150 μm whole-brain sections were cut on a vibratome, and GFP signal amplification was performed as described in SI Appendix, Materials and Methods. Ultrathin sections were cut and mounted on grids, examined, and photographed using a JEM-100CX transmission electron microscope (JEOL).
Optogenetics.
The lt-NES cells were stably transduced with pLenti-hSyn-eNpHR3.0-EYFP and transplanted as described above. In a separate experiment, AAV-CaMKII-eNpHR3.0-EYFP was injected at the same coordinates as those used for cell transplantation. At 5 mo after cell transplantation or concomitantly with virus injection, an optical cannula was implanted at the location of the graft/virus injection. In some animals subjected to virus injection, the optical cannula was implanted in the contralateral hemisphere. The cannula was connected by an optical fiber to an orange-LED light source; light was applied during the duration of the behavioral test.
Electrophysiology.
For electrophysiological studies on cell culture, cortically fated eNpHR3.0-YFP-transfected lt-NES cells were grown on coverslips for 52 d. The same cells differentiated for 4 d were transplanted into the cortex 2 d after stroke, and 6 mo later, after in vivo experiments had been carried out, the rats were killed, and acute brain slices were prepared (27). Whole-cell patch-clamp recordings were performed from grafted cells stimulated by orange light, generated by an LED light source and applied through a water immersion objective with light power ranging from 0.25 to 1.75 µW. Light-induced responses were elicited either by pulses lasting 2 to 5 ms or by constant illumination for a maximum of 5 s. The detailed protocol for electrophysiological recordings is summarized in SI Appendix, Materials and Methods.
Behavioral Test.
The cylinder test was performed 6 mo after cell transplantation or 1 wk after eNpHR3.0-virus injection. Data are expressed as the percentage use of impaired forelimb or number of forelimb touches (total or with individual forelimbs).
Statistical Analysis.
Statistical analysis was performed using Prism 8 software (GraphPad). An unpaired t test was used when data were normally distributed, whereas a Mann–Whitney U test was used when data did not pass the normality test. Significance was set at P < 0.05. Data are mean ± SEM.
Data Availability Statement.
All data are available in the main text and SI Appendix.
Supplementary Material
Acknowledgments
We thank Linda Jansson and Johan Strömblad for technical assistance. This work was supported by grants from the Swedish Research Council, Swedish Brain Foundation, Torsten Söderberg Foundation, Swedish Institute, Region Skåne, Sparbanksstiftelsen Färs & Frosta, and Swedish Government Initiative for Strategic Research Areas (StemTherapy).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2000690117/-/DCSupplemental.
References
- 1.Baker E. W., Kinder H. A., West F. D., Neural stem cell therapy for stroke: A multimechanistic approach to restoring neurological function. Brain Behav. 9, e01214 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinberg G. K., et al. , Clinical outcomes of transplanted modified bone marrow-derived mesenchymal stem cells in stroke: A phase 1/2a study. Stroke 47, 1817–1824 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hess D. C., et al. , Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 16, 360–368 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Kalladka D., et al. , Human neural stem cells in patients with chronic ischaemic stroke (PISCES): A phase 1, first-in-man study. Lancet 388, 787–796 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Kokaia Z., Martino G., Schwartz M., Lindvall O., Cross-talk between neural stem cells and immune cells: The key to better brain repair? Nat. Neurosci. 15, 1078–1087 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Delavaran H., et al. , Proximity of brain infarcts to regions of endogenous neurogenesis and involvement of striatum in ischaemic stroke. Eur. J. Neurol. 20, 473–479 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Tornero D., et al. , Human induced pluripotent stem cell-derived cortical neurons integrate in stroke-injured cortex and improve functional recovery. Brain 136, 3561–3577 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Tornero D., et al. , Synaptic inputs from stroke-injured brain to grafted human stem cell-derived neurons activated by sensory stimuli. Brain 140, 692–706 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Steinbeck J. A., Koch P., Derouiche A., Brüstle O., Human embryonic stem cell-derived neurons establish region-specific, long-range projections in the adult brain. Cell. Mol. Life Sci. 69, 461–470 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denham M., et al. , Neurons derived from human embryonic stem cells extend long-distance axonal projections through growth along host white matter tracts after intra-cerebral transplantation. Front. Cell. Neurosci. 6, 11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Somaa F. A., et al. , Peptide-based scaffolds support human cortical progenitor graft integration to reduce atrophy and promote functional repair in a model of stroke. Cell Rep. 20, 1964–1977 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Zheng W., et al. , Differentiation of glial cells from hiPSCs: Potential applications in neurological diseases and cell replacement therapy. Front. Cell. Neurosci. 12, 239 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molyneaux B. J., Arlotta P., Menezes J. R., Macklis J. D., Neuronal subtype specification in the cerebral cortex. Nat. Rev. Neurosci. 8, 427–437 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Leone D. P., Srinivasan K., Chen B., Alcamo E., McConnell S. K., The determination of projection neuron identity in the developing cerebral cortex. Curr. Opin. Neurobiol. 18, 28–35 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galazo M. J., Emsley J. G., Macklis J. D., Corticothalamic projection neuron development beyond subtype specification: Fog2 and intersectional controls regulate intraclass neuronal diversity. Neuron 91, 90–106 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawabata S., et al. , Grafted human iPS cell-derived oligodendrocyte precursor cells contribute to robust remyelination of demyelinated axons after spinal cord injury. Stem Cell Reports 6, 1–8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneggenburger R., Sakaba T., Neher E., Vesicle pools and short-term synaptic depression: Lessons from a large synapse. Trends Neurosci. 25, 206–212 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Zakiewicz I. M., Bjaalie J. G., Leergaard T. B., Brain-wide map of efferent projections from rat barrel cortex. Front. Neuroinform. 8, 5 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeong M., et al. , Comparative three-dimensional connectome map of motor cortical projections in the mouse brain. Sci. Rep. 6, 20072 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landisman C. E., Connors B. W., VPM and PoM nuclei of the rat somatosensory thalamus: Intrinsic neuronal properties and corticothalamic feedback. Cereb. Cortex 17, 2853–2865 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Rosenzweig S., Carmichael S. T., The axon-glia unit in white matter stroke: Mechanisms of damage and recovery. Brain Res. 1623, 123–134 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wictorin K., et al. , Extensive efferent projections of intra-striatally transplanted striatal neurons as revealed by a species-specific neurofilament marker and anterograde axonal tracing. Prog. Brain Res. 82, 391–399 (1990). [DOI] [PubMed] [Google Scholar]
- 23.Espuny-Camacho I., et al. , Pyramidal neurons derived from human pluripotent stem cells integrate efficiently into mouse brain circuits in vivo. Neuron 77, 440–456 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Daadi M. M., et al. , Optogenetic stimulation of neural grafts enhances neurotransmission and downregulates the inflammatory response in experimental stroke model. Cell Transplant. 25, 1371–1380 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu S. P., et al. , Optochemogenetic stimulation of transplanted iPS-NPCs enhances neuronal repair and functional recovery after ischemic stroke. J. Neurosci. 39, 6571–6594 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinbeck J. A., et al. , Optogenetics enables functional analysis of human embryonic stem cell-derived grafts in a Parkinson’s disease model. Nat. Biotechnol. 33, 204–209 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oki K., et al. , Human-induced pluripotent stem cells form functional neurons and improve recovery after grafting in stroke-damaged brain. Stem Cells 30, 1120–1133 (2012). [DOI] [PubMed] [Google Scholar]
- 28.Schallert T., Fleming S. M., Leasure J. L., Tillerson J. L., Bland S. T., CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology 39, 777–787 (2000). [DOI] [PubMed] [Google Scholar]
- 29.Green C., et al. , Sensorimotor functional and structural networks after intracerebral stem cell grafts in the ischemic mouse brain. J. Neurosci. 38, 1648–1661 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Green C., et al. , Persistent quantitative vitality of stem cell graft is necessary for stabilization of functional brain networks after stroke. Front. Neurol. 10, 335 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Z., et al. , Bone marrow stromal cells enhance inter- and intracortical axonal connections after ischemic stroke in adult rats. J. Cereb. Blood Flow Metab. 30, 1288–1295 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carmichael S. T., Chesselet M. F., Synchronous neuronal activity is a signal for axonal sprouting after cortical lesions in the adult. J. Neurosci. 22, 6062–6070 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Napieralski J. A., Butler A. K., Chesselet M. F., Anatomical and functional evidence for lesion-specific sprouting of corticostriatal input in the adult rat. J. Comp. Neurol. 373, 484–497 (1996). [DOI] [PubMed] [Google Scholar]
- 34.Falkner S., et al. , Transplanted embryonic neurons integrate into adult neocortical circuits. Nature 539, 248–253 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Michelsen K. A., et al. , Area-specific reestablishment of damaged circuits in the adult cerebral cortex by cortical neurons derived from mouse embryonic stem cells. Neuron 85, 982–997 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Espuny-Camacho I., et al. , Human pluripotent stem-cell-derived cortical neurons integrate functionally into the lesioned adult murine visual cortex in an area-specific way. Cell Rep. 23, 2732–2743 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miskinyte G., et al. , Transcription factor programming of human ES cells generates functional neurons expressing both upper and deep layer cortical markers. PLoS One 13, e0204688 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the main text and SI Appendix.