Significance
iNKT cells can both provide help and inhibit B cell responses. Our data show that when iNKT cells are activated with the glycolipid agonist αGalCer together with the inflammatory cytokine IL-18, they switch from regulating autoreactive B cells to promoting their expansion. As a consequence, autoreactive B cell responses remain unchecked by iNKT cells. The glycolipid αGalCer has been shown to have promising effects when administered as an adjuvant to achieve a better response to vaccines, as an antitumor agent, as well as in the regulation of autoimmunity. Our results highlight a facet of αGalCer-mediated iNKT cell activation in the context of inflammation and have broad implications for understanding the regulation of autoimmunity and use of αGalCer in therapy.
Keywords: NKT cells, B cells, autoimmunity, neutrophils, inflammation
Abstract
Invariant natural killer T (iNKT) cells serve as early rapid responders in the innate immune response to self-derived autoantigens and pathogen-derived danger signals and antigens. iNKT cells can serve both as helpers for effector B cells and negatively regulate autoreactive B cells. Specifically, iNKT cells drive B cell proliferation, class switch, and antibody production to induce primary antigen-specific immune responses. On the other hand, inflammasome-mediated activation drives accumulation of neutrophils, which license iNKT cells to negatively regulate autoreactive B cells via Fas ligand (FasL). This positions iNKT cells at an apex to support or inhibit B cell responses in inflammation. However, it is unknown which effector mechanism dominates in the face of cognate glycolipid activation during chronic inflammation, as might result from glycolipid vaccination or infection during chronic autoimmune disease. We stimulated iNKT cells by cognate glycolipid antigen α-galactosylceramide (αGalCer) and measured B cell activation during interleukin 18 (IL-18)-induced chronic inflammation. Moreover, glycolipid-activated iNKT cells increased the serum concentration of autoantibodies, frequency of germinal center (GC) B cells, and antigen-specific plasma cells induced during chronic IL-18–mediated inflammation, as compared with IL-18 alone. Further, activation of iNKT cells via cognate glycolipid during IL-18–mediated inflammation overrides the licensing function of neutrophils, instead inducing iNKT follicular helper (iNKTfh) cells that in turn promote autoimmunity. Thus, our data demonstrate that glycolipids which engage iNKT cells support antigen-specific B cell help during inflammasome-mediated inflammation.
CD1d-restricted invariant natural killer T (iNKT) cells express a limited T cell receptor (TCR) repertoire composed of an invariant Vα14-Jα18 combined with Vβ8.2, 7, or 2 in mice that recognizes lipid antigens (1). The glycolipid alpha-galactosylceramide (αGalCer), originally isolated from the marine sponge Agelas mauritianus, is a prototypic iNKT cell agonist capable of inducing robust cytokine production and proliferation in both mouse and human iNKT cells (2). In steady-state conditions, αGalCer is primarily taken up by CD8+, DEC205+ dendritic cells (DCs) and presented in the context of CD1d (3). Administration of αGalCer has been shown to be both beneficial and disease-exacerbating in mouse models of autoimmunity. Repeated injections of αGalCer were reported to be effective in preventing autoimmune diseases such as type 1 diabetes and rheumatoid arthritis but disease-exacerbating in atherosclerosis, asthma, and allergy. Such varying results have been attributed in part to timing and duration of αGalCer treatment and age and sex of the mice (4).
Among the many functions of iNKT cells in the innate and adaptive immune response, interaction with B cells and the outcome thereafter are context-dependent. iNKT cells can provide both cognate and noncognate help to B cells. αGalCer enhances immunoglobulin G (IgG) titers in mice immunized with bacterial or viral proteins and promotes humoral memory responses (5). iNKT cells provide cognate help to B cells and injection of haptenated αGalCer (NP-αGalCer) results in increased serum titers of IgM, IgG2c, and IgG3 but does not lead to long-lived memory responses (6). We and others have demonstrated that this response is dependent on iNKT cell-derived interleukin 21 (IL-21). Furthermore, lipid agonist-conjugated antigens elicited plasmablasts and early germinal center (GC) B cells and required PD-1–, CXCR5-, and BCL-6–expressing iNKT follicular helper (iNKTfh) cells. Interestingly, such cognate help from iNKT cells did not result in long-lived humoral and memory responses, unlike the response driven via DCs (7, 8). Cognate iNKT cell help also results in expansion of marginal zone B (MZB) cells and IL-10–expressing regulatory B cells (9). Apart from providing B cell help, iNKT cells can take on a regulatory role, especially during inflammatory responses. In atopic eczema patients, we previously observed a reduction in a CD4+ subset of iNKT cells and this coincided with increased levels of serum IgE in an IL-18–dependent manner (10). High serum IL-18 correlates with disease onset and/or severity in autoimmune and inflammatory diseases including systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) (11). In SLE it has been shown that peripheral blood iNKT cells induce autologous B cell autoantibody production in vitro in a CD40- and TCR-dependent manner (12). However, several reports also show decreased numbers of iNKT cells in SLE patients, which would suggest a protective role and thus potential dual role of these cells in disease (13, 14).
In line with this, using two mouse models of inflammation where injection of syngeneic apoptotic cells or the cytokine IL-18 breaks tolerance and results in self-reactivity, we demonstrated that iNKT cells play a regulatory role by forming a pre-GC checkpoint to limit autoreactive B cells. NKT cell-deficient mice, when injected with IL-18, had significantly more autoreactive antibodies (15, 16). Further, we recently reported that CD1d expression by neutrophils was needed to license iNKT cells to limit autoreactive B cells (17). In short, iNKT cells have been found to play two distinct roles in their interactions with B cells—as helpers of B cell humoral response, or as regulators of autoreactive B cells. Here we investigated which one of these two roles dominates when cognate activation (via αGalCer) is provided during sterile inflammation induced by the proinflammatory cytokine IL-18. We demonstrate that cognate activation of iNKT cells by αGalCer in the context of inflammation drives them to acquire an iNKTfh phenotype and, in the process, enhances autoreactive B cell responses. Our study identifies the B cell helper function of iNKT cells as dominant, suggesting that iNKT cells may respond as B cell helpers during infection with inflammation-inducing pathogens (or autoimmune conditions) containing cognate glycolipid antigens compared to the default function of iNKT cells as regulators of B cell responses to an inflammatory pathogen or autoimmune stimulus without cognate glycolipid antigens. This further implicates cognate glycolipids and inflammatory stimuli as coadjuvants for a robust humoral immune response assisted by iNKT cells.
Results
αGalCer-Mediated iNKT Cell Activation Enhances Self-Reactive Innate B Cell Responses in Chronic Inflammation.
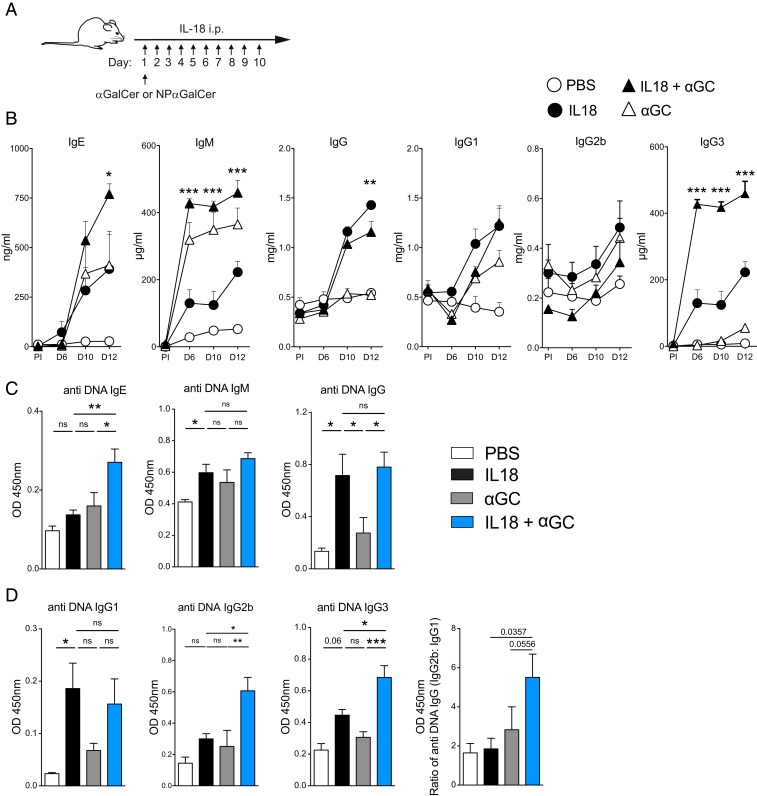
Injection of the inflammatory cytokine IL-18 for 10 consecutive days results in self-reactive B cell responses, which are regulated by iNKT cells (16, 17). On the other hand, we have also shown that (αGalCer) glycolipid-dependent activation of iNKT cells drives B cell proliferation and class-switched antibody production (7). To identify whether iNKT cells regulate or promote B cell responses when inflammation- and glycolipid-mediated activation occur simultaneously, we employed a model of chronic inflammation by injecting IL-18 for 10 consecutive days. We coadministered αGalCer on day 1 to consider which would be the dominant response (Fig. 1A). Total Ig levels for IgE, IgG3, and IgM were significantly higher in the IL-18 + αGalCer group compared with the IL-18–, αGalCer-, and vehicle-injected groups. However, total IgG was marginally lower in the IL-18 + αGalCer group compared with the IL-18 group and IgG1 and IgG2 levels were similar in both groups (Fig. 1B). We next measured anti-DNA reactive antibodies in the sera of these mice. Interestingly, we detected a significant increase in double-stranded DNA reactive IgE, IgG2b, and IgG3 antibodies (Fig. 1 C and D) but not for anti-DNA IgG, IgG1, and IgM. However, there was a shift in the composition of subclasses, with relatively more proinflammatory IgG2b antibodies compared with IgG1 (Fig. 1D). These data demonstrate that activation of iNKT cells during inflammation results in an increase in B cell responses and particularly amplifies the self-reactive antibody response toward DNA, and the balance of IgG subclasses is altered toward those that preferentially bind activating Fc receptors (18).
Fig. 1.
αGalCer-mediated iNKT cell activation enhances self-reactive innate B cell responses in chronic inflammation. (A) Experimental scheme. Mice were immunized i.p. with 5 μg α-galactosylceramide (αGC) on day 1 and i.p. 2 μg IL-18 for days 1 to 10. To follow NP-specific B cell responses, 0.5 μg of NP-αGC was injected intravenously. (B) ELISA was used to measure total Ig concentrations from serum at the indicated time points (the statistical difference between IL-18 and IL-18 + αGalCer is shown). (C) OD of anti-DNA IgE, IgM, and IgG on day 12. (D) Anti-DNA IgG1, IgG2b, and IgG3 and the ratio of IgG2b vs. IgG1 on day 12. Two-way (B) or one-way ANOVA (C and D) was employed to assess statistical differences. *P < 0.05, **P < 0.001, ***P < 0.0001; ns, not significant. Data are representative of two to four experiments with three to five mice per group. Graphs display mean ± SEM.
αGalCer Promotes GC Formation during Chronic Inflammation.
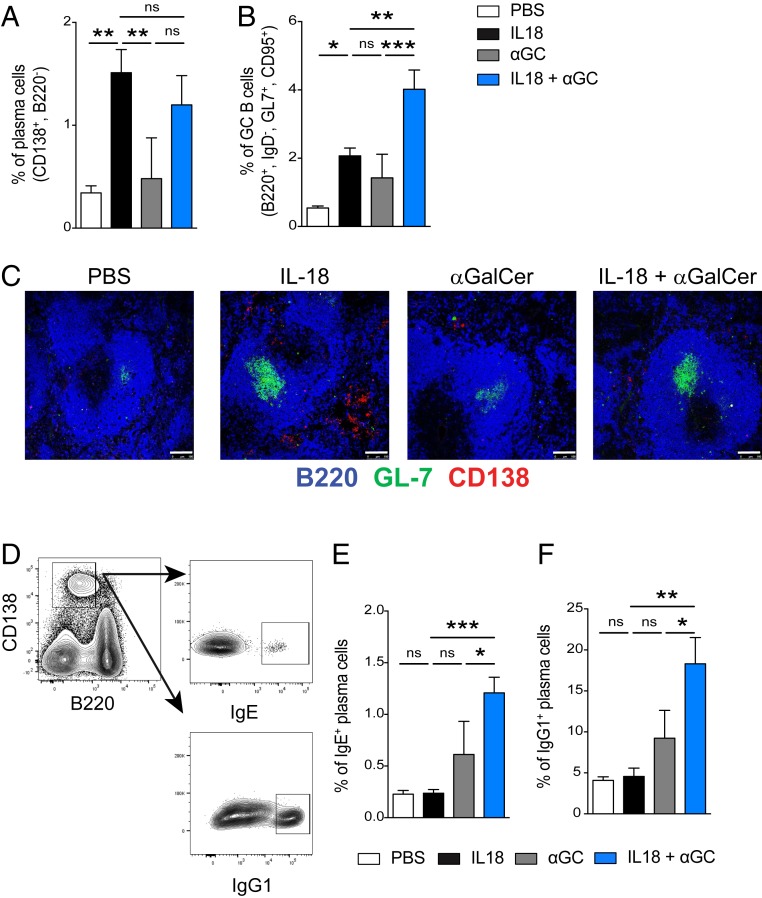
The presence of iNKT cells during B cell stimulation with glycolipids results in the formation of extrafollicular foci and immature GCs, while iNKT cell absence allows up-regulation of the gene activation-induced cytidine deaminase (AID) and mature GC formation (16). This suggests that iNKT regulation happens before GC formation. Given the effect of glycolipid-mediated iNKT cell activation, we wanted to assess plasma cell and GC B cell compartments when αGalCer is combined with chronic inflammation induced by IL-18. We performed analyses to identify changes in the size of the plasma cell (CD138+, B220−) and GC B cell (IgD−, B220+, GL-7+, CD95+) compartments in the spleen following IL-18–induced inflammation paired with or without αGalCer stimulation. We found that the frequency of plasma cells in the spleen was similar in both IL-18– and IL-18 + αGalCer–injected groups (Fig. 2A). However, coadministration of αGalCer in addition to IL-18 induced more GC B cells compared with IL-18 or αGalCer alone (Fig. 2B). We next used immunofluorescence to confirm the development of extrafollicular foci outside the B cell follicle in spleens from IL-18–injected mice but interestingly not in spleens from mice injected with the IL-18 + αGalCer combination (Fig. 2C). This shows that αGalCer shifts the IL-18 response toward a more robust GC response. To assess the effect of isotype switch in the pool of plasma cells now primarily coming from the GC, we measured the percent of class-switched plasma cells by intracellular staining for Ig subclasses (Fig. 2D). We observed a three- to fourfold increase in the percentage of plasma cells expressing intracellular IgE and IgG1 in spleens of mice immunized with both IL-18 and αGalCer compared with the groups injected with IL-18 alone (Fig. 2 E and F). These data are in line with the increased GC B cell population and anti-DNA responses we observed when αGalCer is combined with chronic inflammation and indicate that both GC B cell formation and isotype switch are favored when iNKT cells are activated by cognate lipid antigen in combination with IL-18.
Fig. 2.
αGalCer promotes germinal center formation during chronic inflammation. (A and B) Percent of (A) plasma cells (CD138+, B220−) and (B) germinal center B cells (IgD−, B220+, GL-7+, CD95+) in the spleen 12 d after in vivo stimulation with the indicated treatments. (C) Immunofluorescence microscopy of the spleen (day 12; mice are treated as indicated) stained with B220 (blue), GL-7 (green), and CD138 (red). (Scale bars, 100 μm.) (D) Representative plots of intracellular IgE and IgG1 staining of splenic plasma cells on day 12. (E and F) Percent of (E) IgE+ and (F) IgG1+ CD138+ B220− plasma cells in the spleen of mice on day 12 of treatment as identified in the key. Representative plots are from two to four experiments, n = 3 to 5 mice per group. One-way ANOVA was employed to assess statistical differences. *P < 0.05, **P < 0.005, ***P < 0.0001. Graphs display group mean ± SEM.
Licensing of iNKT Cells by Neutrophils Is Abrogated by αGalCer during IL-18–Mediated Chronic Inflammation.
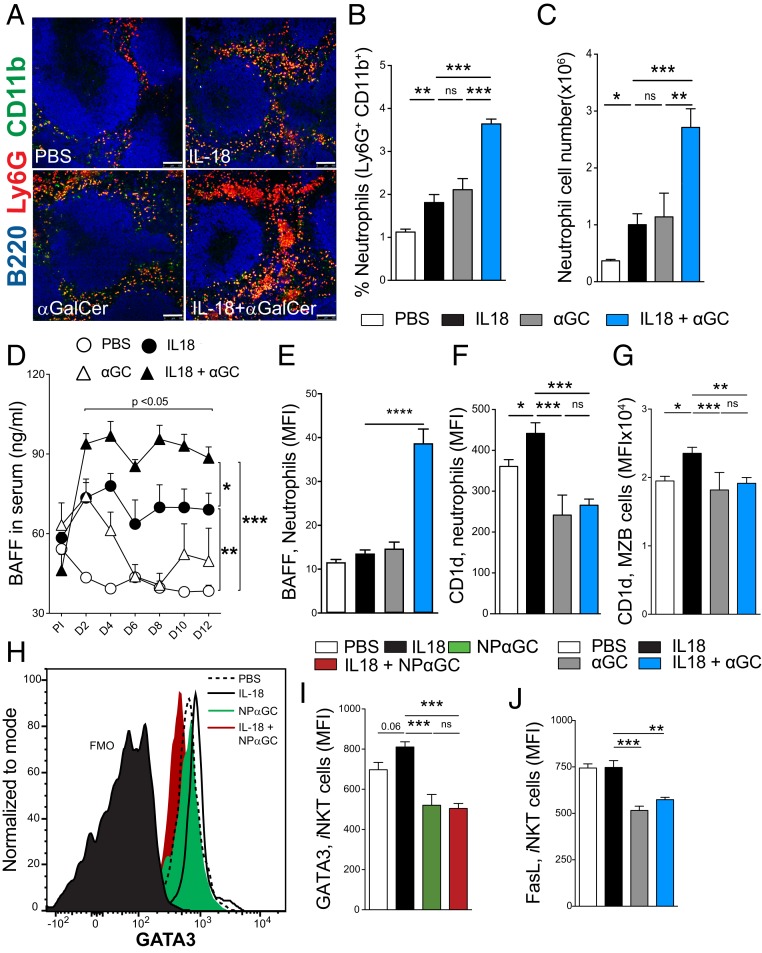
We recently demonstrated a key role played by neutrophils in licensing iNKT cells to regulate B cell responses during sterile inflammation (17). This regulation is mediated by CD1d on neutrophils interacting with the iNKT TCR. The requirement for CD1d suggests the possible involvement of a self-lipid antigen(s). Here, we tested the effect of αGalCer on neutrophil licensing of iNKT cells. We performed immunofluorescence staining of spleen sections to identify neutrophil infiltrates (Fig. 3). As previously reported, we observed an increase in neutrophil infiltrates in the spleen on day 12 in IL-18–injected mice compared with the vehicle group (Fig. 3A). However, αGalCer treatment also appeared to enhance the neutrophil infiltration, and the combination of IL-18 and αGalCer resulted in a significantly higher number of neutrophils in the spleen compared with all of the other groups (Fig. 3A). To further verify this neutrophil increase, we performed flow cytometry analysis of total spleen cells and IL-18 + αGalCer immunization induced a significantly higher frequency (Fig. 3B) and numbers (Fig. 3C) of neutrophils in the spleen compared with IL-18 or αGalCer treatment. We next investigated induction of B cell-activating factor (BAFF), a cytokine needed for B cell survival and homeostasis (19). Neutrophils are major BAFF producers in the spleen and have been shown to induce Ig production by activating MZB cells (20). As we observed a significant neutrophil infiltration in the spleens of mice injected with IL-18 + αGalCer, we asked whether this infiltration correlated with an increase in serum BAFF concentration. As expected, IL-18–treated mice had higher serum BAFF concentration compared with phosphate-buffered saline (PBS)-injected controls. Interestingly, mice injected with IL-18 + αGalCer had a significantly higher concentration of serum BAFF compared with all other groups and this increase was evident as early as day 2 (Fig. 3D). This increase in BAFF corresponds to the observed increase in neutrophils and is consistent with neutrophils being a dominant source of BAFF in the spleen of IL-18 + αGalCer–injected mice.
Fig. 3.
Neutrophil licensing of iNKT cells is inhibited by αGalCer during IL-18–mediated chronic inflammation. (A) Immunofluorescence microscopy of the spleen (day 12) with B220 (blue), CD11b (green), and Ly6G (red). (Scale bars, 100 μm.) (B and C) Percent (B) and total cell number (C) of neutrophils (Ly6G+, CD11b+) in the spleen. (D) Serum BAFF measured by ELISA. (E–G) Median fluorescence intensity (MFI) of (E) BAFF and (F) CD1d expression on neutrophils and (G) CD1d on MZB cells. (H–J) iNKT cell (TCRb+, CD1d TET [PBS-57]+) GATA3 (H) representative flow cytometry plot and (I) summary of GATA3 (MFI) and (J) FasL expression. One-way (B, C, E–G, I, and J) or two-way ANOVA with Tukey’s correction for multiple comparisons (D). *P < 0.05, **P < 0.005, ***P < 0.001, ****P < 0.0001. Graphs display group mean ± SEM. Pooled data from four experiments are shown in B (PBS [n = 13], IL-18 [n = 14], αGC [n = 10], and IL-18 + αGC [n = 15]). C, D, F, and I show representative data from three experiments (n = 3 to 5 per group). E shows data from one experiment (n = 6 per group).
Interaction of CD1d-expressing neutrophils with iNKT cells results in the activation of iNKT cells and increases iNKT expression of the transcription factor GATA3 (17). We therefore measured CD1d expression on splenic neutrophils following injections of IL-18 alone or in combination with αGalCer. Surprisingly, we observed that neutrophils from IL-18 + αGalCer–treated mice expressed high levels of surface-bound BAFF but had less CD1d on their cell surface compared with IL-18 alone (Fig. 3 E and F). We also detected a reduction in surface levels of CD1d on MZB cells (Fig. 3G), which are known to express very high levels of CD1d under steady-state conditions. This decrease in surface CD1d suggests that αGalCer alters the inflammatory milieu and may prevent IL-18–driven CD1d up-regulation.
Neutrophil-licensed iNKT cells were previously found to express higher levels of the transcription factor GATA3, corresponding to development of a regulatory phenotype (17). Given the increases we detected in total Ig following coadministration of IL-18 and αGalCer, we next wanted to dissect the changes that αGalCer induced in antigen-specific B cell responses during IL-18–induced inflammation. To do this, we immunized IL-18–injected mice with the haptenated form of αGalCer, NP-αGalCer. We found that mice immunized with NP-αGalCer or IL-18 + NP-αGalCer had significantly lower levels of GATA3 in the iNKT cell compartment compared with the IL-18 group (Fig. 3 H and I). This shows that during antigen-specific B cell activation, αGalCer prevents up-regulation of GATA3 in iNKT cells. Induction of GATA3 in iNKT cells correlates with increased surface expression of Fas ligand (FasL). Given that the gene encoding FasL contains GATA3-binding motifs (21), this could be a direct effect of GATA3-mediated transcription. Thus, we next investigated whether FasL expression by iNKT cells correlated with GATA3 down-regulation in IL-18 + αGalCer– or αGalCer-treated mice. Activation of iNKT cells with αGalCer in addition to IL-18 stimulation led to reduced surface expression of FasL (Fig. 3J). Overall, these data confirm that chronic inflammation induced by IL-18 alone drives up-regulation of CD1d on neutrophils, which in turn license iNKT cells to regulate B cell responses. However, IL-18, in combination with αGalCer or NP-αGalCer, reduces the levels of CD1d expressed on the neutrophil cell surface and results in a failure to license iNKT cells.
Activation of iNKT Cells by the Combination of αGalCer and IL-18 Induces an iNKT Follicular Helper Phenotype.
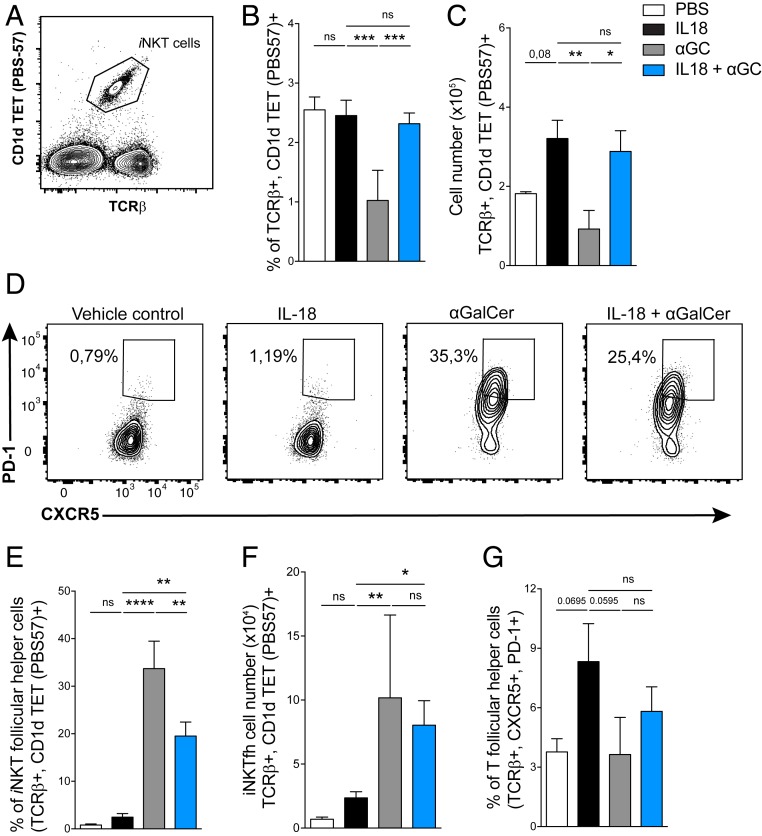
We next investigated how αGalCer alone or in combination with IL-18 altered the splenic iNKT cell phenotype. As previously shown (22), αGalCer-treated mice had a significant reduction in their proportion of splenic iNKT cells on day 12. However, in mice that received IL-18 + αGalCer, the percentage and numbers of splenic iNKT cells were similar to those of IL-18–injected mice (Fig. 4 A–C). As IL-18 + αGalCer induced more GC B cells compared with IL-18 or αGalCer alone, we next asked if iNKT cells took on a follicular T helper function. Mice injected with αGalCer had the highest percentage of PD-1+, CXCR5+ iNKTfh cells followed by those injected with IL-18 + αGalCer, whereas both PBS and IL-18 alone groups had very few iNKTfh cells. When we quantified cell counts, both αGalCer- and IL-18 + αGalCer–treated mice had similar numbers of iNKTfh cells (Fig. 4 D–F). IL-18 + αGalCer–induced iNKTfh cells expressed BCL-6 and CD40L and had higher expression of the activation marker CD86 compared with iNKTfh cells in the IL-18 alone group (SI Appendix, Fig. S1 A–C). iNKT cells down-regulate their TCR upon stimulation with high doses of αGalCer but at the dose given here we could not see this, and in addition iNKTfh cells accumulated as early as day 7 (SI Appendix, Fig. S1 D and E). There was no significant difference in conventional CD4+ Tfh cells across all experimental groups (Fig. 4G). These data suggest that iNKT cells provide B cell help when cognate lipid activation coincides with chronic inflammation.
Fig. 4.
Activation of iNKT cells with IL-18 + αGalCer induces an iNKT follicular helper phenotype. (A) Representative flow cytometry plot for iNKT cells stained with TCR and CD1d tetramer (PBS-57). (B and C) iNKT cell (B) percent and (C) total cell count on day 12 in the spleen. (D–F) Analysis of iNKTfh cells (TCR+ CD1d TET+, PD-1hi, CXCR5hi). (D) Representative flow cytometry plots, (E) percent, and (F) total cell number. (G) Percent of conventional, CD4+ T follicular helper cells. Data are representative of four experiments (n = 4 or 5 mice per group). One-way ANOVA; *P < 0.05, **P < 0.005, ***P < 0.001, ****P < 0.0001. Graphs display group mean ± SEM.
iNKTfh Cells Activated by IL-18 and αGalCer Provide B Cell Help.
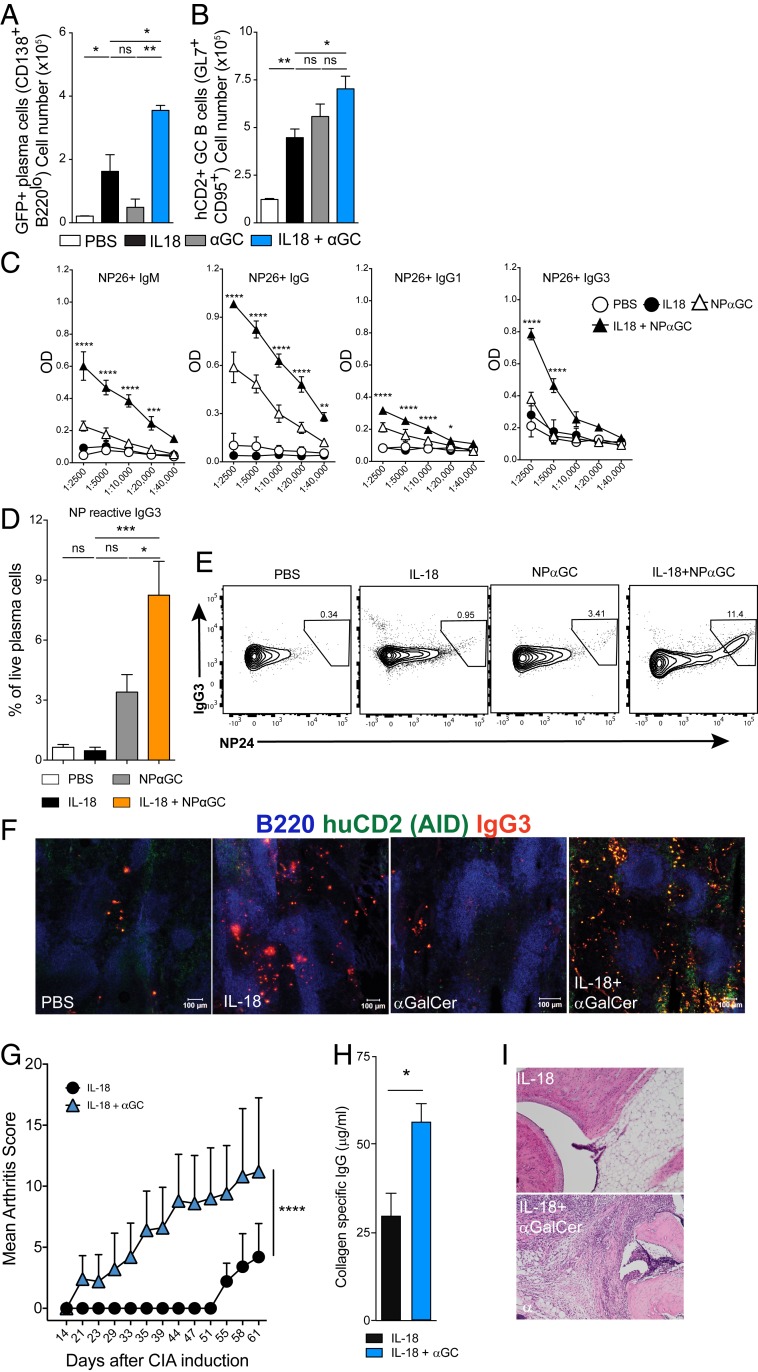
Increased induction of serum antibody titers and induction of iNKTfh cells by the combination of IL-18 + αGalCer prompted us to study the B cell response in greater detail. Tfh cells are important in GC formation and determine the length and efficiency of an adaptive B cell response (23). To investigate the effect of GC formation, we next used Aicda Cre GT Rosaflox/flox mice, in which AID expression in the GC turns on green fluorescent protein (GFP) expression for the duration of the cells’ remaining life, thereby serving as a reporter for AID expression and GC experience of B cells including plasmablasts. The Aicda Cre GT Rosaflox/flox mice are also engineered to express human CD2 (hCD2) on the cell surface in those that actively express AID (24, 25). In these mice, we observed a robust induction of GFP+ plasma cells in the IL-18 + αGalCer–treated mice, close to twice as many as those detected in the IL-18–injected group (Fig. 5A). We also observed that more GC B cells in the IL-18 + αGalCer–treated mice expressed hCD2 compared with the IL-18 or αGalCer alone groups (Fig. 5B). Next, we addressed if IL-18 + αGalCer activation induced class switch using NP-αGalCer. Serum titers of NP-reactive IgM, IgG, IgG1, and IgG3 antibodies in mice treated with IL-18 + NP-αGalCer were significantly higher compared with mice injected with IL-18 or NP-αGalCer alone (Fig. 5C). The switch to the IgG3 subclass has previously been shown to be induced by NP-αGalCer alone (6). Here, we had the opportunity to also investigate if this IgG3 was derived from a GC reaction. We found that a significant portion of GC-experienced plasma cells expressed intracellular IgG3 that was specific for NP24 (Fig. 5 D and E). This was also evident from immunofluorescence microscopy performed on spleens from Aicda Cre GT Rosaflox/flox mice, where we found IgG3-positive cells that expressed hCD2 and were negative for B220 in the IL-18 + αGalCer group but not others (Fig. 5F). When compared with other groups, a proportion of the GC B cells from the IL-18 + αGalCer–treated group expressed hCD2, indicating that these cells had recently turned on AID expression. This suggests that the antigen-specific IgG3 response resulting from the combined stimulation of glycolipid (αGalCer or NP-αGalCer) and IL-18 is derived from the GC. This is especially interesting in light of evidence that MZB cells have high CD1d expression and have been shown to be required for normal IgG3 production (26). However, if MZB cells have the capacity to enter the GC reaction in this situation is still an unresolved question (27, 28).
Fig. 5.
Cognate activation of iNKT cells during chronic inflammation provides B cell help and IL-18 + αGalCer exacerbates collagen-induced arthritis. (A and B) Percent of (A) GFP+ plasma cells and (B) hCD2+ germinal center B cells from spleens of Aicda Cre GT Rosa Flox mice treated as indicated. Data are representative of three experiments (n = 3 mice per group). (C) OD (450 nm) of NP-reactive IgM, IgG, IgG1, and IgG3 measured by ELISA. Sera were diluted as shown on the x axis. Data are representative of two experiments (n = 4 or 5 mice per group). (D) Percent of intracellular, NP24-reactive IgG3+ cells among plasma cells (CD138+, B220−). (E) Representative flow cytometry plots for intracellular staining. (F) Representative immunofluorescence microscopy of the spleen (day 12) showing B220 (blue), hCD2 (green), and IgG3 (red) antibody-forming cells. (Scale bars, 100 μm.) Data are representative of two experiments (n = 4 or 5 mice per group). One-way (A, B, D) or two-way (C) ANOVA, Student's t test (H). *P<0.05, **P<0.005, ***P<0.001, ****P<0.0001. Graphs display group mean ± SEM. (G) Arthritis score among B10.Q mice immunized with CII and 1 d later treated i.p. with IL-18 (2 μg) or IL-18 (2 μg) + αGalCer (5 μg); two-way ANOVA. (H) Comparison of collagen type II-specific IgG titers. (I) Examples of histological analysis of hind limbs using H&E staining.
αGalCer is one of the strongest iNKT cell glycolipid agonists. To test whether glycolipids other than αGalCer can induce the B cell helper iNKT cell phenotype during chronic inflammation, we tested two glycolipids, GSL-1 and OCH, known to predominantly induce Th1 and Th2 responses, respectively, when injected alone (29). Interestingly, we did not detect an increase in GC B cells or plasma cells in the spleens of mice treated with IL-18 in combination with either GSL-1 or OCH (SI Appendix, Fig. S2 A and B). Upon further investigation, we also found no significant increase in the percentage of infiltrating splenic neutrophils or their CD1d expression (SI Appendix, Fig. S2 C and D) compared with the IL-18 alone group. Analysis of the iNKT cell phenotype revealed that IL-18 + GSL-1–injected mice had a similar percentage of iNKTfh cells compared with the IL-18 group. However, in mice injected with IL-18 + OCH, we observed an increase in the percentage of iNKTfh cells compared with the IL-18 group, but this was significantly less when compared with the IL-18 + αGalCer group (SI Appendix, Fig. S2E). These data strongly suggest that activation of iNKT cells by αGalCer, but not by GSL-1 or OCH, in the face of chronic inflammation induces a robust GC B cell response. This is likely due to differences in cytokine skewing induced by these ligands. Finally, to investigate if the combination of IL-18 + αGalCer could support autoimmunity, we injected IL-18 alone or in combination with αGalCer in the collagen-induced arthritis (CIA) model of RA (30). We selected a protocol that has been shown to induce mild autoimmune arthritis (31) and found that the combination of IL-18 + αGalCer clearly enhanced the development of arthritis (Fig. 5G). Further, the combination of IL-18 + αGalCer resulted in higher anti-collagen IgG antibodies in sera from the mice compared with IL-18–injected mice (Fig. 5H). Histological analysis of hind limbs using hematoxylin and eosin (H&E) staining showed infiltration of immune cells in mice treated with IL-18 + αGalCer (Fig. 5I). Together, these data show that iNKT cells lose their regulatory role induced by IL-18 alone when combined with αGalCer. Instead, they now aggravate autoimmunity and autoimmune disease by providing help for autoreactive B cells.
Discussion
Τhe glycolipid αGalCer is a very potent iNKT cell antigen and has been tested as an adjuvant candidate to improve immune responses to vaccines or as an antitumor therapeutic. It is therefore important to understand the effect of αGalCer on iNKT cells in the context of inflammation. Here we report that when stimulated by the proinflammatory cytokine IL-18 in combination with αGalCer, iNKT cells are activated to help B cells and become iNKTfh cells. This is in contrast to stimulation with IL-18 alone, where we previously showed that iNKT cells negatively regulate an autoreactive B cell response (SI Appendix, Fig. S3) (17). This negative regulation was dependent on neutrophils that up-regulated CD1d to instruct iNKT cells and we find here that this interaction is absent. In an adaptive immune response, B cells require T cell help to mount efficient, long-lasting humoral responses. Conventional CD4+ T cells provide such help for protein antigens in the GC reaction, where they are limiting for selection during affinity maturation (23). iNKT cells, on the other hand, have the capacity to participate in both innate and adaptive immune response. It is known that cognate activation of iNKT cells with hapten-conjugated αGalCer provides help and supports GC reactions (9). However, this response is not sustained and resulted primarily in IL-10–producing B cells and no B cell memory even when PD-1 was selectively absent on iNKT cells to boost the T cell activation through deletion of negative costimulation. In contrast, using nonhaptenated αGalCer, Oleinika et al. (32) recently showed that IL-10–producing B cells could also activate iNKT cells to secrete interferon γ and in turn block experimental arthritis. Thus, in a potent adaptive immune response driving systemic autoimmunity, iNKT and B cell interactions can suppress the response by influencing T helper cell profiles. These different roles reflect the capacity of iNKT cells to modify the B cell responses in a context-dependent fashion and drive them in separate directions. The timing of the iNKT interaction with B cells is important as is the type of B cell response that iNKT cells are activated to regulate. In this study, αGalCer was given at the earliest phase of an IL-18–induced B cell response, which normally results in negative regulation of iNKT cells. We hypothesized that one possible outcome would be that the regulatory phenotype would be enhanced and that the iNKT cells would impose an even more potent negative regulation of B cells. In contrast to our hypothesis, we find that iNKT cells support the GC reaction when IL-18 is combined with αGalCer. This is in line with the publication by Gaya et al. (33), where they report that iNKT cells provide an early boost of IL-4 in the lymph node, which was important to support a potent antiviral immune response. It has been shown that when mice are injected with αGalCer alone, iNKT cells down-regulate their TCR as a result of their activation (22). Here, we confirmed this phenotype in αGalCer-injected mice; however, mice that received the combination of IL-18 and αGalCer had similar percentages and numbers of iNKT cells, suggesting that chronic inflammation induced by IL-18 prevented down-regulation of their TCR. Instead, upon further investigation, we observed an increase in CXCR5+, PD-1+ iNKTfh cells following injection of IL-18 in combination with αGalCer. The increase in GC B cells was solely dependent on iNKTfh cells and not conventional Tfh, as we did not observe an increase in CD4+ Tfh cells after injection of IL-18 and αGalCer in combination. This change in the response was also specific for αGalCer, because when we investigated mice injected with IL-18 in combination with the GSL-1 (Th1-inducing) and OCH (Th2-inducing) glycolipids, we did not observe an increase in the GC B cells. Compared with the IL-18–injected group, IL-18 + OCH–treated mice displayed a modest but significant increase in iNKTfh cells but no such increase was detected in the IL-18 + GSL-1 group, suggesting the iNKT helper response may also depend on the nature of the cytokines and/or the affinity of the glycolipid.
In many autoimmune and inflammatory diseases, such as SLE and RA, high serum titers of IL-18 correlate with onset and/or severity of disease (11). Also, IL-18 is a product of the NLRP3 inflammasome and numerous studies have connected activation of this inflammatory machinery with autoimmune disease as well as obesity-driven inflammation (34). However, in vivo administration of IL-18 alone in wild-type mice has been shown to induce IgG1 and IgE antibody production and this response depended on CD4+ T cells (35). This IL-18–induced response, like the one induced by NP-αGalCer, did not give a sustained GC response and this can be attributed to the fact that systemic inflammation allows for a boost of natural antibodies that is benign and could help to restore homeostasis. Here we find that the combination of IL-18 and αGalCer gives a more mature and sustained GC response with up-regulation of AID as shown in the reporter mice. Total serum Ig titers and anti-DNA IgE and IgG3 were also significantly higher in the IL-18 + αGalCer–injected group compared with control groups with either of these alone. One interesting finding is that we observe a shift toward the complement-fixing IgG3 subclass, suggesting that cells that normally would give a boost of natural IgG3 can now enter the GC and go through affinity maturation. In addition, we find a change in the IgG subclass ratio toward IgG2 that has been shown to drive autoimmunity by engagement of activating Fc receptors for IgG including FcγRIV (36). We also found that during CIA, IL-18 in combination with αGalCer induced an earlier onset and more severe disease as compared with IL-18 alone. This model has been shown to be dependent on the GC response (30) and our data show that iNKTfh activation supports an autoreactive B cell response beyond anti-DNA responses. It has also been shown that mice lacking iNKT cells have lower disease severity in the CIA models (37). In addition, in the K/BxN serum transfer model of RA, the arthritic score was lower in NKT cell-deficient mice (37). The data from these models together suggest a disease-promoting role for iNKT cells in RA but it remains to be determined if this is true for patients and if it is connected to the relative higher level of IL-18 found in serum.
iNKT cells are early responders to infection and can be activated by pathogen-associated molecular patterns (PAMPs), either directly or via antigen-presenting cells, to clear the pathogen (38). Apart from carrying PAMPs, a few pathogens are known to express certain glycolipids that can activate iNKT cells via CD1d (39). Based on the data presented here, it can be speculated that such infections could lead to potential autoreactivity which, if unchecked, can result in autoimmune disease. Evidence for this comes from studies of primary biliary cirrhosis, where iNKT cells react to cell-wall glycolipids from Novosphingobium aromaticivorans and initiate organ-specific autoimmunity (40). The data presented here clearly show that during inflammation induced by IL-18, αGalCer can activate iNKT cells to predominantly provide B cell help and this help extends to self-reactive B cells. We hypothesize that this mechanism could be part of breaking B cell tolerance, especially inducing the shift in IgG subclasses. We have previously reported that CD1d-expressing neutrophils infiltrate the spleen and are a major source of BAFF in the IL-18 model and the increase in serum BAFF levels observed here coincides with accumulation of B helper neutrophils in the spleen. Here we find that the combination of IL-18 and αGalCer recruits a significantly higher number of neutrophils to the spleen compared with IL-18 alone. However, IL-18 + αGalCer–stimulated neutrophils had significantly lower CD1d expression compared with mice injected with IL-18 alone. We also found that neutrophil-driven licensing of iNKT cells was absent in mice injected with IL-18 + αGalCer and we observed lower expression of FasL and the transcription factor GATA3. This suggests that B helper neutrophils can have a dual role: licensing iNKT cells to regulate B cells when stimulated by IL-18 alone or, on the other hand, supporting B cell activation without licensing of iNKT cells when stimulated with αGalCer + IL-18. In this study, we focused on the role of iNKT cells in the activation of naïve B cells. Further studies are warranted to understand whether iNKT cells regulate memory B cell responses. In mice lacking conventional CD4+ Tfh cells, Chen et al. (41) suggest that memory iNKTfh cells can recognize lipid antigens presented by memory B cells and induce recall responses. Our data highlight the role of strong cognate glycolipid activation combined with chronic inflammation in inducing a B cell-supporting iNKTfh phenotype and this could be of great interest if mechanisms described here can also support memory to self-antigens and drive autoimmune disease.
Materials and Methods
Mice.
C57BL/6 wild-type and Aicda Cre+ GT Rosaflox/flox mice (24, 25) were housed and bred under specific pathogen-free conditions at the animal facility of the Department of Microbiology, Tumor and Cell Biology, Karolinska Institutet. All experiments were approved by the local ethical committee (the North Stockholm District Court). Female or male age- and sex-matched mice between the ages of 8 and 14 wk were used. Mice were euthanized on day 12 to harvest spleen and serum.
Antigens and Immunization.
Mice were intraperitoneally (i.p.) immunized with 2 μg of carrier-free recombinant mouse IL-18 (Reprokine) in PBS daily for 10 d. αGalCer (KRN7000) was prepared in dimethyl sulfoxide (DMSO) and diluted in 0.5% bovine serum albumin (BSA) containing PBS with a final DMSO concentration of <0.05%. Five μg of αGalCer or 0.5 μg of NP-αGalCer (in 0.1% BSA/PBS, <0.05% DMSO) was injected i.p. on day 1. GSL-1 and OCH (NIH Tetramer Core Facility, Emory University) were dissolved in 0.5% BSA/PBS, <0.05% DMSO and administered i.p. on day 1. For the collagen-induced arthritis model, B10.Q mice were immunized intradermally at the base of the tail with 100 μg of rat collagen type II (CII) emulsified in complete Freund’s adjuvant (CFA; Difco). One day later, these mice received either 2 μg IL-18 or 2 μg IL-18 plus 5 μg αGalCer. They were boosted subcutaneously on day 35 with 50 μg of CII in incomplete Freund’s adjuvant (IFA; Difco). Control mice were immunized with 0.1 M acetic acid emulsified in CFA and boosted on day 35 with 0.1 M acetic acid in IFA. Arthritis development was evaluated by clinical scoring as described earlier (31); in brief, each peripheral paw joint was given 1 point, resulting in a maximum score of 60.
Flow Cytometry.
Red blood cell-lysed, single-cell suspensions of spleen cells were used for flow cytometry analysis. Cells were incubated with anti-mouse CD16/32 (clone 93) antibody and Live/Dead Fixable Aqua viability dye (Invitrogen) for 10 min at room temperature to reduce nonspecific binding and stain for dead cells. Further, staining for cell-surface markers was performed at 4 °C in the dark for 30 min using CD1d (1B1), CD4 (RM4-5), CD8a (53-6.7), CD11b (M1/70), CD21/35 (7E9), CD23(B3B4), CD95(Jo2), CD138 (281-2), Ly6G (1A8), PD-1 (CD279, 29F.1A12), FasL (CD198, MFL-3), CXCR5 (L138D7), IgM (RMM-1), IgD (11-26c.2a), TCRb (H57-597), CD40L (anti-CD154, MR1), CD86 (GL1), BAFF (BAFF/BLyS/TNFSF13B), and B220 (RA3-6B2) (BD Biosciences, R&D Systems, and BioLegend). PBS-57 loaded or empty control CD1d tetramers were used to detect iNKT cells (NIH Tetramer Core Facility, Emory University). Intracellular staining to detect IgM (RMM-1), IgE (BC4), IgG1 (RMG1-1), IgG3 (R40-82), BCL6 (K112-91), and GATA3 (L50-823) was performed using the Foxp3 Staining Kit (eBioscience) according to the manufacturer’s instructions. Samples were acquired on a BD LSRFortessa X-20 or BD LSRII (BD Biosciences) and data were analyzed using FlowJo software (TreeStar). The minimum numbers of cells acquired for extracellular and intracellular stained samples were 2.5 × 105 and 5.0 × 105, respectively.
Immunofluorescence and Histology.
Spleens were frozen in OCT (Sakura) and cut into 8-μm-thick sections using a cryostat microtome. The sections were dried overnight, blocked with 5% goat sera (Dako), and stained with the following anti-mouse antibodies: B220-APC (RA3-6B2; BD Biosciences), CD138-PE (281-2; BD Biosciences), GL-7-FITC (BD Biosciences), Ly6G-PE (1A8; BioLegend), hCD2-FITC (RPA-2.10, BioLegend), IgG3-APC (R40-82), and CD11b-FITC (M1/70; BD Biosciences). Images were acquired on a confocal laser scanning microscope (Leica; TCS SP5) and processed with Photoshop software (Adobe Systems). For histopathological evaluation of the joints, paws were removed from the mice at the end point (day 61) and fixed by immersion in 4% paraformaldehyde. Paws were then decalcified in 10% ethylenediaminetetraacetate for 2 to 3 wk at room temperature, followed by paraffin embedding and sectioning. Deparaffinized and rehydrated slides were stained with H&E and used to assess joint inflammation and bone degradation (seven slides per joint at 5- and 200-μm intervals between slides). Images were acquired on a Zeiss Axioplan microscope that was equipped with an Olympus SC30 digital camera.
ELISA.
Serum was isolated from blood samples collected from the tail vein at the indicated intervals and at the end point and stored at −20 °C until further analysis. BAFF enzyme-linked immunosorbent assays (ELISAs) were performed as described previously (42) using BAFF-specific capture (clone 5A8) and detection (clone 1C9, biotinylated) antibodies. Streptavidin-HRP (horseradish peroxidase) was used to bind BAFF-biotin and the assay was developed using TMB substrate (BioLegend). Total Ig was measured using an anti–H+L chain capture antibody for IgM, IgG, IgG1, and IgG3 (SouthernBiotech) and purified anti-mouse IgE (BD Pharmingen). Anti-DNA antibodies were detected as described previously (42). In short, methylated BSA-coated plates were coated with calf thymus DNA (Sigma-Aldrich) to capture anti-DNA antibodies of various subtypes. NP-specific antibodies were detected as follows: 96 well ELISA plates were coated with 10 μg/mL NP (24)-BSA (Biosearch Technologies) and blocked with 1% BSA/PBS. Fifty μL of serially diluted sera (in 1.5% BSA/3 mM EDTA/0,1% gelatine/PBS) were incubated for 2 h and antibodies were detected using HRP conjugated goat anti-mouse IgM, IgE, IgG, IgG1, and IgG3 (Southern Biotech). All ELISAs were developed with TMB substrate (BioLegend).
To measure collagen-specific antibodies in mouse serum from the CIA model, 96-well ELISA plates were coated with collagen type II at a final concentration of 10 µg/mL (in 50 µL) and incubated overnight at 4 °C. Coated plates were washed three times and blocked with 1% BSA-containing PBS for 1 h at room temperature. After washing four times, standards or serum (50 µL) was added to the plate and incubated for 2 h at room temperature. Detection antibodies conjugated with HRP (diluted at 1:4,000 in PBS; SouthernBiotech) were added after washing the plates four times and incubated for 1 h at room temperature. Plates were developed using ABTS buffer (Sigma-Aldrich) according to the manufacturer’s instructions and optical density (OD) was measured at 405 nm.
Statistics.
One-way or two-way ANOVA and two-tailed Student’s t test were used to assess statistical differences between experimental groups. A P value of <0.05 was considered statistically significant. Data analysis was performed using Prism 6 software.
Data Availability.
All data for this paper are included in the manuscript and SI Appendix.
Supplementary Material
Acknowledgments
We thank the US National Institutes of Health Tetramer Core Facility (Emory University, Atlanta, GA) for providing mouse CD1d tetramers, GSL-1, and OCH. This study was funded by the Swedish Research Council, Center for Allergy Research Karolinska Institutet, Swedish Medical Society, King Gustaf V’s 80-Year Foundation, Magnus Bergvall Foundation, Karolinska Institutet (M.C.I.K.), Swedish Research Council (D.P.L.; Grant 2013_08807), O. E. and Edla Johansson’s Foundation, Tore Nilsson’s Foundation, European Molecular Biology Organization Short-Term Fellowship, Karolinska Institutet (S.K.S.), Erik and Edith Fernström Foundation, Swedish Society for Medical Research, Centre for Allergy Research, Ellen, Walter and Lennart Hesselman Foundation (T.H.), National Institutes of Health (Grant AI104788-01A1; to E.A.L.), Trudeau Institute, and University of Texas Health Science Center at San Antonio (E.A.L.). We thank Emma Lindh for technical assistance and the animal facility staff at the Department of Microbiology, Tumor and Cell Biology for animal housing and care.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1920463117/-/DCSupplemental.
References
- 1.Kronenberg M., Toward an understanding of NKT cell biology: Progress and paradoxes. Annu. Rev. Immunol. 23, 877–900 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Kawano T., et al. , CD1d-restricted and TCR-mediated activation of Valpha14 NKT cells by glycosylceramides. Science 278, 1626–1629 (1997). [DOI] [PubMed] [Google Scholar]
- 3.Arora P., et al. , A single subset of dendritic cells controls the cytokine bias of natural killer T cell responses to diverse glycolipid antigens. Immunity 40, 105–116 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simoni Y., Diana J., Ghazarian L., Beaudoin L., Lehuen A., Therapeutic manipulation of natural killer (NK) T cells in autoimmunity: Are we close to reality? Clin. Exp. Immunol. 171, 8–19 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galli G., et al. , Invariant NKT cells sustain specific B cell responses and memory. Proc. Natl. Acad. Sci. U.S.A. 104, 3984–3989 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leadbetter E. A., et al. , NK T cells provide lipid antigen-specific cognate help for B cells. Proc. Natl. Acad. Sci. U.S.A. 105, 8339–8344 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King I. L., et al. , Invariant natural killer T cells direct B cell responses to cognate lipid antigen in an IL-21-dependent manner. Nat. Immunol. 13, 44–50 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang P.-P., et al. , Identification of Bcl-6-dependent follicular helper NKT cells that provide cognate help for B cell responses. Nat. Immunol. 13, 35–43 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Vomhof-DeKrey E. E., et al. , Cognate interaction with iNKT cells expands IL-10-producing B regulatory cells. Proc. Natl. Acad. Sci. U.S.A. 112, 12474–12479 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lind S. M., et al. , IL-18 skews the invariant NKT-cell population via autoreactive activation in atopic eczema. Eur. J. Immunol. 39, 2293–2301 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Sedimbi S. K., Hägglöf T., Karlsson M. C. I., IL-18 in inflammatory and autoimmune disease. Cell. Mol. Life Sci. 70, 4795–4808 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen L., et al. , Invariant natural killer T cells in lupus patients promote IgG and IgG autoantibody production. Eur. J. Immunol. 45, 612–623 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bosma A., Abdel-Gadir A., Isenberg D. A., Jury E. C., Mauri C., Lipid-antigen presentation by CD1d(+) B cells is essential for the maintenance of invariant natural killer T cells. Immunity 36, 477–490 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kojo S., Adachi Y., Keino H., Taniguchi M., Sumida T., Dysfunction of T cell receptor AV24AJ18+, BV11+ double-negative regulatory natural killer T cells in autoimmune diseases. Arthritis Rheum. 44, 1127–1138 (2001). [DOI] [PubMed] [Google Scholar]
- 15.Wermeling F., Lind S. M., Jordö E. D., Cardell S. L., Karlsson M. C. I., Invariant NKT cells limit activation of autoreactive CD1d-positive B cells. J. Exp. Med. 207, 943–952 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enoksson S. L., et al. , The inflammatory cytokine IL-18 induces self-reactive innate antibody responses regulated by natural killer T cells. Proc. Natl. Acad. Sci. U.S.A. 108, E1399–E1407 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hägglöf T., et al. , Neutrophils license iNKT cells to regulate self-reactive mouse B cell responses. Nat. Immunol. 17, 1407–1414 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Nimmerjahn F., Ravetch J. V., Divergent immunoglobulin G subclass activity through selective Fc receptor binding. Science 310, 1510–1512 (2005). [DOI] [PubMed] [Google Scholar]
- 19.Naradikian M. S., Perate A. R., Cancro M. P., BAFF receptors and ligands create independent homeostatic niches for B cell subsets. Curr. Opin. Immunol. 34, 126–129 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Puga I., et al. , B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat. Immunol. 13, 170–180 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei G., et al. , Genome-wide analyses of transcription factor GATA3-mediated gene regulation in distinct T cell types. Immunity 35, 299–311 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parekh V. V., et al. , Glycolipid antigen induces long-term natural killer T cell anergy in mice. J. Clin. Invest. 115, 2572–2583 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crotty S., T follicular helper cell differentiation, function, and roles in disease. Immunity 41, 529–542 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwon K., et al. , Instructive role of the transcription factor E2A in early B lymphopoiesis and germinal center B cell development. Immunity 28, 751–762 (2008). [DOI] [PubMed] [Google Scholar]
- 25.Qin H., et al. , Activation-induced cytidine deaminase expression in CD4+ T cells is associated with a unique IL-10-producing subset that increases with age. PLoS One 6, e29141-15 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guinamard R., Okigaki M., Schlessinger J., Ravetch J. V., Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nat. Immunol. 1, 31–36 (2000). [DOI] [PubMed] [Google Scholar]
- 27.Hendricks J., Bos N. A., Kroese F. G. M., Heterogeneity of memory marginal zone B cells. Crit. Rev. Immunol. 38, 145–158 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song H., Cerny J., Functional heterogeneity of marginal zone B cells revealed by their ability to generate both early antibody-forming cells and germinal centers with hypermutation and memory in response to a T-dependent antigen. J. Exp. Med. 198, 1923–1935 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long X., et al. , Synthesis and evaluation of stimulatory properties of Sphingomonadaceae glycolipids. Nat. Chem. Biol. 3, 559–564 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Dahdah A., et al. , Germinal center B cells are essential for collagen-induced arthritis. Arthritis Rheumatol. 70, 193–203 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Holmdahl R., et al. , “Genetic analysis of murine models for rheumatoid arthritis” in Human Genome Methods, Adolpho K. W., Ed. (New York CRC Press, 1998), pp. 215–238. [Google Scholar]
- 32.Oleinika K., et al. , CD1d-dependent immune suppression mediated by regulatory B cells through modulations of iNKT cells. Nat. Commun. 9, 684 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaya M., et al. , Initiation of antiviral B cell immunity relies on innate signals from spatially positioned NKT cells. Cell 172, 517–533.e20 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen H.-H., et al. , NLRP3: A promising therapeutic target for autoimmune diseases. Autoimmun. Rev. 17, 694–702 (2018). [DOI] [PubMed] [Google Scholar]
- 35.Hoshino T., Yagita H., Ortaldo J. R., Wiltrout R. H., Young H. A., In vivo administration of IL-18 can induce IgE production through Th2 cytokine induction and up-regulation of CD40 ligand (CD154) expression on CD4+ T cells. Eur. J. Immunol. 30, 1998–2006 (2000). [DOI] [PubMed] [Google Scholar]
- 36.Ehlers M., Fukuyama H., McGaha T. L., Aderem A., Ravetch J. V., TLR9/MyD88 signaling is required for class switching to pathogenic IgG2a and 2b autoantibodies in SLE. J. Exp. Med. 203, 553–561 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiba A., Kaieda S., Oki S., Yamamura T., Miyake S., The involvement of V(alpha)14 natural killer T cells in the pathogenesis of arthritis in murine models. Arthritis Rheum. 52, 1941–1948 (2005). [DOI] [PubMed] [Google Scholar]
- 38.Paget C., et al. , Activation of invariant NKT cells by Toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity 27, 597–609 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Mattner J., et al. , Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 434, 525–529 (2005). [DOI] [PubMed] [Google Scholar]
- 40.Mattner J., et al. , Liver autoimmunity triggered by microbial activation of natural killer T cells. Cell Host Microbe 3, 304–315 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Z., et al. , Memory follicular helper invariant NKT cells recognize lipid antigens on memory B cells and elicit antibody recall responses. J. Immunol. 200, 3117–3127 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Chen Y., et al. , A regulatory role for macrophage class A scavenger receptors in TLR4-mediated LPS responses. Eur. J. Immunol. 40, 1451–1460 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data for this paper are included in the manuscript and SI Appendix.