Abstract
Background
Overweight and obesity are major risk factors for gestational diabetes among U.S. women. Evidence suggests that longer duration of breastfeeding among women with a history of gestational diabetes is associated with lower incidence of developing type 2 diabetes after pregnancy. Women may potentially benefit from a lifestyle change program that includes breastfeeding education and support.
Purpose
To describe the design and justification of a combined breastfeeding, national Diabetes Prevention Program (DPP)-based feasibility randomized controlled trial, the electronic Monitoring Of Mom's Schedule (eMOMSTM) study. eMOMSTM compares the feasibility and efficacy of three interventions on six-month postpartum weight loss among women with a BMI ≥25.
Methods
The intervention is delivered via Facebook and includes three groups: DPP and breastfeeding (eMOMS1); DPP only (eMOMS2); and Usual Care (eMOMS3). Recruitment is ongoing at two clinical sites (rural and urban). A total of 72 women, 24 per group, will be randomly assigned to one of the three groups. It is anticipated that women in eMOMS1 will have greater weight loss and increased length of breastfeeding at three and six months postpartum compared to women in eMOMS2 and eMOMS3. Additional data will be collected on metabolic markers, anthropometrics, physical activity, nutrition, breastfeeding, and depression. Program cost will be compared to that of traditionally scheduled group meetings. Expected study completion date: October 2021.
Conclusions
This study has the potential to define a high impact, cost effective intervention that can improve public health by reducing negative health outcomes associated with gestational diabetes among an at-risk population.
Keywords: Weight loss, Breastfeeding, Diabetes, Reproductive age women, Social media, DPP
Highlights
-
•
Delivery of a combined breastfeeding and lifestyle change program via social media.
-
•
Comparison of 3 programs on 6-month postpartum weight loss among high-BMI women.
-
•
Comparison of 3 programs on length of breastfeeding among high-BMI women.
-
•
Programs: lifestyle change and breastfeeding, lifestyle change only, and usual care.
-
•
Cost analysis of online program delivery versus in-person meetings.
1. Introduction
1.1. Background and rationale
In the United States, gestational diabetes mellitus (GDM) affects 6%–20% of pregnant women [[1], [2], [3]]. Specifically, women with GDM are at increased risk for pregnancy and delivery complications — including preeclampsia [[4], [5], [6], [7], [8]], cesarean section [4,5,8,9], pregnancy-induced hypertension [6,9,10], preterm birth [5,6], shoulder dystocia [4,6,11], and macrosomia [[11], [12], [13]] — leading to increased maternal and neonatal morbidity and mortality. Additionally, women with a history of GDM have at least a seven-fold increased risk of developing type 2 diabetes in the future compared to women who did not have GDM [14,15].
Overweight and obesity are major risk factors for gestational diabetes and their prevalence rates are high among U.S. women [[16], [17], [18]]. About 27% of U.S. women are overweight and 41% of women are considered obese; these rates are even higher for Hispanic and non-Hispanic black women [19,20]. A high maternal body mass index (BMI) increases the risk of adverse health outcomes for mother [[21], [22], [23], [24]] and child [[25], [26], [27], [28], [29], [30]]. More importantly, complications of having a high BMI and gestational diabetes are further increased for rural women who often experience limited access to obstetrical healthcare services [[31], [32], [33], [34]], leading to poor birth outcomes including low birth weight infants and preterm delivery [[35], [36], [37]].
Overall risk factors for diabetes are well documented, but less is known about protective factors. Evidence suggests that longer duration of breastfeeding among women with a history of gestational diabetes is associated with lower incidence of developing type 2 diabetes up to two years after pregnancy [[38], [39], [40], [41]]. Breastfeeding has also been shown to lower maternal postpartum weight [[42], [43], [44]], facilitate the resetting of maternal metabolism after pregnancy [45], and protect against breast cancer [46], ovarian cancer [47,48], and type 2 diabetes [49,50]. These benefits are optimized when women exclusively breastfeed for six months [51,52].
Unfortunately, most women do not reach their breastfeeding goals. About 84% of U.S. women start breastfeeding, but only 25% exclusively breastfeed at six months [53]. Moreover, breastfeeding exclusivity rates at six months among Hispanic and non-Hispanic black women who also have increased obesity rates, are lower at 20% and 21% respectively, compared to their non-Hispanic white counterparts at 29% [53]. Compared to normal weight women, overweight and obese women are even less likely to start and continue breastfeeding [28,[54], [55], [56]].
As underrepresented minority women carry the largest burden of obesity and related adverse health outcomes, these women may especially benefit from a lifestyle change program during their reproductive years. One such program is the evidence-based national Diabetes Prevention Program (DPP) that is associated with a reduced risk of developing diabetes by 58% [57] and that is shown to be effective for preventing diabetes in women with a history of gestational diabetes [58]. To date, no studies have used a DPP-enhanced version that includes breastfeeding support to reduce postpartum weight thereby reducing progression to type 2 diabetes after pregnancy. This is where the current project narrows the knowledge gap as we seek to implement a combined breastfeeding, DPP-based intervention and determine its feasibility and efficacy, as well as its effect on metabolic outcomes among an at-risk population. The SPIRIT guidelines for the content of a clinical trial protocol and feasibility studies are adhered to in this paper [[59], [60], [61]].
1.2. Objectives
This study has three aims. The first aim is to test the efficacy of a combined breastfeeding, DPP-based intervention to improve 6-month postpartum weight loss among healthy women with a BMI ≥25. The second aim is to test the efficacy of this intervention to improve 6-month postpartum hemoglobin A1C and arterial blood pressure among women with a BMI ≥25. The third aim is to test the efficacy of this intervention to increase duration of any breastfeeding through six months postpartum among women with a BMI ≥25. The purpose of this study is to assess feasibility and acceptability of an approach to be used in a future larger scale study.
1.3. Trial design
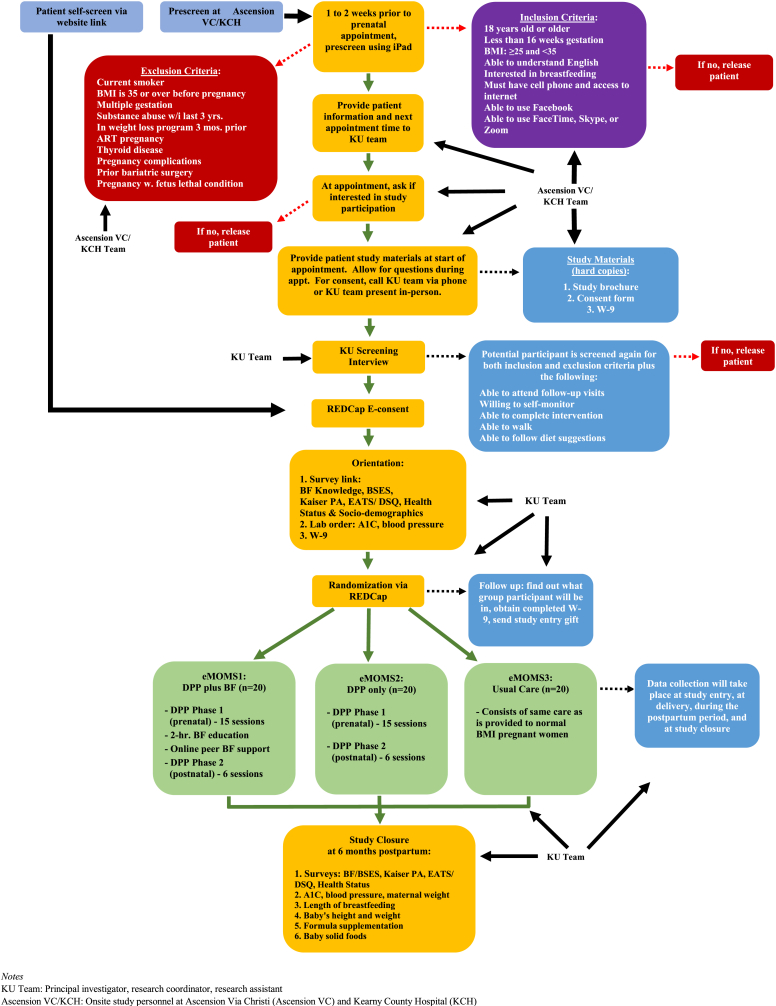
This study is an unmasked, parallel randomized controlled trial (RCT) with three study arms: DPP and breastfeeding (eMOMS1); DPP only (eMOMS2); and Usual Care (eMOMS3). Eligible pregnant women are randomized to one of the two treatment groups (eMOMS1 or eMOMS2) or usual care (eMOMS3). Maternal baseline data are obtained at study entry, followed by data collection at delivery and six months postpartum. It is anticipated that women in eMOMS1 will have greater weight loss postpartum, improved A1C and arterial blood pressure postpartum, and increased duration of breastfeeding compared to women in eMOMS2 and eMOMS3. Trial procedures are summarized in Fig. 1.
Fig. 1.
Study Flow Chart
Notes
KU Team: Principal investigator, research coordinator, research assistant
Ascension VC/KCH: Onsite study personnel at Ascension Via Christi (Ascension VC) and Kearny County Hospital (KCH).
2. Methods
2.1. Study setting
Eligible healthy women will be recruited from at least two study sites: an obstetrical clinic at Ascension Via Christi Hospitals Wichita, Inc. in Wichita, Kansas, and an obstetrical clinic at Kearny County Hospital in Lakin, Kansas. Kearny County Hospital is a full-service critical access hospital located in an extremely remote frontier rural region in Kansas. An obstetrical healthcare provider from Ascension Via Christi Hospitals Wichita, Inc. travels once a month to this region and sees at-risk pregnant women in-person. This same healthcare provider also practices in the Wichita metropolitan area and therefore eligible women located in the Wichita area are considered for recruitment as well. Nonetheless, Kearny County Hospital is the setting for the majority of births with a birth rate of 332 live births in 2018 (Benjamin Anderson, MBA, MHCDS, e-mail communication, October 4, 2019). Additional rural sites are considered for recruitment as well.
2.2. Eligibility criteria
Overall study eligibility criteria were intended to obtain a study population with a low likelihood of developing pregnancy complications. Inclusion criteria for a healthy pregnant woman to participate in the study are as follows:
-
•
Aged 18 or older
-
•
Less than 16 weeks gestation at recruitment
-
•
Body mass index (BMI) 3 months prior to pregnancy: ≥25 and < 35
-
•
Able to understand English
-
•
Interested in breastfeeding
-
•
Have a cell phone and internet access
-
•
Able to use Facebook
-
•
Able to use Facetime, Skype, or Zoom
Exclusion criteria that may increase the risk for mother and infant are as follows:
-
•
Current smoker
-
•
Multiple gestation
-
•
Substance abuse within last 3 years
-
•
In weight-loss program 3 months prior to pregnancy
-
•
IVF (In-Vitro Fertilization) pregnancy
-
•
Diagnosed with or treated for thyroid disease
-
•
Diagnosed with pregnancy complications that are potentially life-threatening as determined by the participant's healthcare provider
-
•
Prior bariatric surgery
-
•
Pregnancy complicated with a fetus diagnosed with lethal malformation/condition
-
•
Unwilling to participate in study procedures
-
•
Presence of any condition that limits walking
-
•
Presence of any condition that limits diet suggestions
2.3. Intervention
2.3.1. Conceptual model
Two theoretical models have been used to guide program development and selection of measures: Social Cognitive Theory (SCT) [62,63] and Self-Determination Theory (SDT) [64,65]. SCT stipulates that behavior modification results from the interaction between behavior, cognition (self-efficacy, perception of barriers to lifestyle changes), and the environment (support), while modeling and reinforcement serve to encourage change. SDT is a broad-based theory of human motivation [66] that explains how intrinsic motivation can lead to improved eating and exercise patterns [67]. According to SDT, a person's increased intrinsic motivation to improve eating and exercise patterns should positively relate to self-efficacy and the ability to overcome barriers and solicit support. eMOMS1 and eMOMS2 use assignments, individualized goal setting, and shared problem-solving to increase mastery and goal achievement in incremental steps to enhance self-regulatory skills.
2.3.2. Intervention
The intervention is up to 12 months long and consists of two phases. Phase one starts on or before week 18 of pregnancy and ends by week 32 of pregnancy. Phase two starts at delivery of the baby and ends at six months postpartum. The purpose of phase one is educational only and helps the participant: 1) Think of new ways to eat healthier foods and set attainable goals; 2) Think of inserting more movement into one's daily routine and set attainable goals; and 3) Access breastfeeding educational resources and set breastfeeding goals. The purpose of phase two is behavioral and helps the participant implement her goals outlined during phase one. Both phases consist of periodic one-on-one counseling sessions via telephone, use of private Facebook groups for delivery of educational content, email reminders, and answering of questions related to the topic of interest for that week. An 80% completion rate of each phase is considered successful completion of the intervention. There are no in-person visits pertaining to the intervention during and after pregnancy. All email reminders and answering of questions are administered using Research Electronic Data Capture software (i.e., REDCap).
The intervention is based on the national Diabetes Prevention Program (DPP) coupled with breastfeeding education and support. Phase one of the intervention comprises the following components:
-
•
Fifteen educational videos that are each 15 min long, pre-recorded and archived within a private, secure Facebook group to be accessed by study participants. The curriculum's content is based on the national DPP [57] and two sessions have been replaced with the Cooking Matters curriculum [68].
-
•
Four 30-min breastfeeding educational videos, pre-recorded and archived within a private, secure Facebook group to be accessed by study participants. The content is based on the Office on Women's Health Your Guide to Breastfeeding [69].
-
•
Two to three content-type scripted online questions after completion of each video to be emailed to study participants.
-
•
Weekly one-on-one counseling sessions via telephone by a certified DPP lifestyle coach.
-
•
Joining a designated online peer support group.
Upon completion of phase one and until delivery, all trial participants will receive periodic engagement emails, two to three scripted online questions to jog their memory of what they learned during phase one, and bi-weekly one-on-one counseling sessions via telephone by a certified DPP lifestyle coach.
Phase two starts at delivery of the baby and comprises the following components:
-
•
Six educational videos that are each 15 min long, pre-recorded and archived within a private, secure Facebook group to be accessed by study participants. The curriculum's content is based on the national DPP [57].
-
•
Periodic one-on-one counseling sessions via telephone by a certified DPP lifestyle coach.
-
•
Periodic scripted online questions on breastfeeding and infant feeding experience to be emailed to participants followed by periodic telephone calls to assess breastfeeding.
-
•
Active engagement in a designated online peer support group.
eMOMS1 (DPP and breastfeeding). Women in eMOMS1 participate in the full intervention as described above.
eMOMS2 (DPP only). Women in eMOMS2 do not have access to the educational breastfeeding videos but participate in all other components of the intervention: phase one of the national Diabetes Prevention Program during pregnancy; content-type scripted online questions after completion of each educational video; weekly one-on-one counseling sessions via telephone up to week 32 of pregnancy; after week 32 of pregnancy, periodic engagement emails and bi-weekly one-on-one counseling sessions via telephone prior to delivery of the baby; periodic scripted online questions on breastfeeding and infant feeding experiences after delivery of the baby followed by periodic telephone calls to assess breastfeeding; at six weeks postpartum, content-type scripted online questions after completion of each educational video of phase two of the national Diabetes Prevention Program; and periodic one-on-one counseling sessions via telephone through six months postpartum.
eMOMS3 (Usual Care). Women in eMOMS3 do not have access to the educational DPP and breastfeeding videos but participate in all other components of the intervention: weekly content-type scripted online questions; weekly one-on-one counseling sessions via telephone up to week 32 of pregnancy; after week 32 of pregnancy, periodic engagement emails and bi-weekly one-on-one counseling sessions via telephone prior to delivery of the baby; periodic scripted online questions on breastfeeding and infant feeding experiences after delivery of the baby followed by periodic telephone calls to assess breastfeeding; and periodic one-on-one counseling sessions via telephone after delivery of the baby through six months postpartum.
2.4. Outcomes
There is one primary outcome: maternal postpartum weight loss. Maternal weight is measured in kilograms and collected at the following time points: three months prior to pregnancy; delivery; days three and ten postpartum; weeks three, six, eight, ten, and twelve postpartum; and months four, five, and six postpartum. Time points for weight measurement at delivery and postpartum were selected because they are aligned with the national benchmark measurements for breastfeeding. These time points are also critical for breastfeeding as many women stop breastfeeding well before six months of exclusive breastfeeding as recommended by the American Academy of Pediatrics and the World Health Organization [51,52,[70], [71], [72]].
Secondary outcomes are listed below (data collection at baseline is described as data collected at study entry on or before week 16 of pregnancy):
-
•
Maternal hemoglobin A1C: assessed between week 10 and 16 of pregnancy and six months postpartum.
-
•
Maternal arterial blood pressure: assessed at baseline, delivery, and six months postpartum.
-
•
Breastfeeding status, assessed at the following time points: delivery; days three and ten postpartum; weeks three, six, eight, ten, and twelve postpartum; and months four, five, and six postpartum.
-
•
Maternal level of breastfeeding knowledge: assessed at baseline and six months postpartum.
-
•
Maternal breastfeeding self-efficacy: assessed at baseline and six months postpartum.
-
•
Maternal diet quality: assessed at baseline and six months postpartum.
-
•
Maternal physical activity levels: assessed at baseline and six months postpartum.
-
•
Maternal depression: assessed at weeks three, six, and twelve postpartum and six months postpartum.
2.5. Sample size
The main outcomes of interest are: [1] postpartum weight loss; [2] A1C and arterial blood pressure; and [3] breastfeeding duration. Because this is a feasibility study, the power analysis focuses on the primary outcome: mean postpartum weight loss. The number of participants to be included in the intervention was limited with a maximum of 72 women to be recruited, or 24 per group. A power analysis was conducted to determine the level of power we could expect, given a mean difference between groups of 10-pounds with a standard deviation set at 10. This mean difference represents a 5% weight loss for a woman weighing 200 pounds, and was considered a clinically meaningful difference [73]. An alpha level of 0.05, a 2-tailed, and a t-test were used for two independent samples with common variance. Results showed a sample size of 24 per group would have 92.4% power to detect a weight change of this magnitude (mean difference of 10, and sd = 10).
2.6. Recruitment
To optimize recruitment, one-page flyers and study brochures are posted in the waiting rooms and exam rooms at each study site. A website has also been created containing the same information as the brochures and flyers. Healthy pregnant women are recruited into the study by 1) screening themselves, and 2) screening by clinic personnel at each study site.
First, the website contains a link named “Do I qualify for eMOMS?” that interested women can use to screen themselves. If an interested participant meets eligibility, wants to participate, and electronically consents to study participation, then a study team member will contact her.
Second, each week, potential participants are screened electronically for eligibility by designated clinic personnel at each study site. If the potential participant meets eligibility, her contact information and appointment time are provided to a member of the study team. When the potential participant comes in for her prenatal appointment, clinic personnel acquaints her with the study. Immediately after her prenatal appointment, a study team member performs the same screening for eligibility in-person or via the telephone. During this process, the participant must have an electronic mailing address (i.e., email address) and if she does not, a study team member will help her create an email address. If the potential participant meets eligibility criteria, wants to participate in the study, and has all her questions answered, then electronic consent is obtained via use of an iPad.
Upon electronic consent, the participant receives an email welcoming her to the study and informing her of the next step: completion of baseline survey instruments. Upon survey completion, the participant is randomized to one of three groups: eMOMS1 (DPP and breastfeeding), eMOMS2 (DPP only), or eMOMS3 (Usual Care) (see previous section titled “Intervention” for additional detail). After randomization, the participant is instructed to go to her Facebook account and request to be added to her assigned group, each with private security settings. Lastly, the participant is scheduled on the calendar, which lets her know when weekly activities are to be completed and lets the study team know when to contact her for weekly follow-up.
Participation in the trial is enhanced in several ways. All women in the trial receive program incentives starting with a pack of diapers at e-consent, a $50 Amazon gift card upon survey completion, a scale and pedometer at completion of phase one, a $10 engagement gift card before delivery, and a $75 Amazon gift card at study closure. If a trial participant withdraws prior to completing the study, then she will only receive incentives for the program components that she has completed.
2.7. Randomization
Upon completion of baseline survey instruments, an electronic data capture system - Research Electronic Data Capture (REDCap) - will randomize the participant to one of three groups: eMOMS1 (DPP and breastfeeding), eMOMS2 (DPP only), or eMOMS3 (Usual Care). Randomization is stratified by race and ethnicity so that each group has equal representation. Randomization is revealed to the participant and the study team immediately afterwards. Due to the nature of the intervention (use of Facebook for program delivery, weekly one-on-one coaching sessions, weighing scale, tracking of foods and physical activity), no masking is involved in the study.
2.8. Data collection methods
2.8.1. Time of data collection
Clinical data (i.e., anthropometric data and metabolic markers) and non-clinical data (i.e., data from self-administered surveys) are collected at designated time periods (see Table 1). On or before week 16 of pregnancy (i.e., study entry) and at six months postpartum (i.e., study closure), self-administered survey data are collected on physical activity, dietary habits, breastfeeding knowledge, breastfeeding self-efficacy, and health status. Socio-demographic characteristics, maternal pre-pregnancy weight and height are also measured at study entry. Maternal A1C and arterial blood pressure are measured between weeks ten and 16 of pregnancy, and at six months postpartum. Fifty gram oral glucose tolerance test results are obtained as standard of care between 24 and 28 weeks of gestation. At delivery, maternal weight and arterial blood pressure are measured along with baby's weight, height, breastfeeding initiation, and formula supplementation. Maternal weight, baby's weight, baby's height, any breastfeeding (i.e., breast milk and supplementation), exclusive breastfeeding (i.e., breast milk only), formula supplementation, and solid foods are measured at the following time points postnatally: day three and day 10; week three, six, eight, 10, and 12; and month four, five, and six. Self-administered survey data on maternal depression are collected at week three, six, and 12 postpartum and at six months postpartum.
Table 1.
Data collection for all study participants.
| Approximate day of study |
0 |
14 |
119 |
169 |
172 |
|---|---|---|---|---|---|
| Prenatal |
Postnatal |
||||
| Section I |
Section II |
Section III |
Section IV |
Section V |
|
| Approximate prenatal and postnatal timea | Week 16 | Week 18–32 | Week 33–40 | Delivery | Day 3 - Month 6 |
| Recruitment | x | ||||
| Prescreen | x | ||||
| Consent and orientation | x | ||||
| Incentive1 | x | ||||
| Surveys | x | x | |||
| Incentive2 | x | ||||
| Randomize and set investigator scheduling | x | ||||
| Maternal labs | x | x | |||
| Join Facebook groups | x | ||||
| Intervention | x | x | x | x | |
| Breastfeeding education (eMOMS1 only) | x | x | x | x | |
| Glucose tolerance (standard of care) | x | ||||
| Telephone calls to participant | x | x | x | x | |
| Incentive3 | x | ||||
| Delivery announcement email | x | ||||
| Incentive4 | x | ||||
| Maternal and infant measures | x | x | |||
| Incentive5 | x | ||||
Applies to all groups (eMOMS1-3) unless otherwise noted.
2.8.2. Survey instrument data
Participants will access the self-administered surveys by a link sent to her via electronic mail immediately after obtaining consent. Except for the socio-demographics questionnaire, participants will receive another link to these surveys via electronic mail at six months postpartum.
Socio-demographics questionnaire: assesses race and ethnicity, age, level of education, health insurance status, WIC status, and household income.
Health status questionnaire: assesses parity, number of stillbirths, history of preterm delivery, if affirmative then reason for preterm delivery, level of risk, and smoking status.
Breastfeeding knowledge assessment questionnaire: assesses physiology of breastfeeding, positions to hold baby when breastfeeding, birthing experience, signs that breastfeeding goes well, breast milk supply and supplementation, common breastfeeding concerns, breastfeeding duration, and substance use when breastfeeding.
Breastfeeding Self-Efficacy Scale-Short Form (BSES-SF): a reliable and validated 14-item questionnaire to assess levels of breastfeeding self-efficacy in prenatal and postnatal women [[74], [75], [76], [77]].
Kaiser Physical Activity Survey (KPAS): a validated survey for pregnant and non-pregnant women; assesses multiple domains of physical activity (i.e., household/family care, occupational, active living habits, participation in sports/exercise) and total physical activity [78,79].
Fruit and Vegetable Intake Screener (Eating at America's Table Study: EATS): a reliable and validated 10-item questionnaire to assess the intake of fruits and vegetables [[80], [81], [82]].
Selected items from the Dietary Screener Questionnaire (DSQ): six items were selected from the Dietary Screener in the National Health and Nutrition and Examination Survey (NHANES 2009-10) and assess intake of fiber, added sugars, dairy, calcium and meat [83,84].
Edinburgh Postnatal Depression Scale (EPDS): a reliable and validated 10-item questionnaire to assess levels of depression during the postpartum period [85,86]. Participants with an EPDS score of 10 or higher will be referred to a mental healthcare professional for further psychological evaluation.
Throughout the study, all participants receive electronic mails with two to three questions asking about the content of the respective session for that week. Even though participants in eMOMS3 (Usual Care) do not watch any educational videos, the questions are phrased in such a way as to be answerable without having watched the videos. Examples of such questions are:
-
•
What are your goals for eating healthier and managing physical activity during pregnancy?
-
•
What are some ways you can incorporate your family into nutritional goals?
-
•
How comfortable do you feel reading food labels? Please tell us more.
-
•
What are some activities you enjoy doing that allow you to get extra movement in throughout the day?
-
•
Identify a barrier you experience when trying to complete your physical activity goals.
-
•
What are some ways you can add variety to your physical activity routine?
All questions are open-ended. There are two to three questions for each of the 15 sessions during phase one, two to three questions related to each of the four breastfeeding sessions, two to three questions during each of the four telephone follow-up calls between phase one completion and baby's delivery, and two to three questions for each of the six sessions during phase two after delivery. After these questions are answered, a follow-up telephone call is placed to the participant by a certified DPP lifestyle coach. These telephone calls constitute the one-on-one counseling session with the participant.
2.9. Data management
Data from survey instruments, anthropometric data, and other clinical and qualitative data are captured by the Research Electronic Data Capture software, REDCap. REDCap is a secure, web-based application designed to support data capture for research studies, providing 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources.
2.10. Statistical methods
Clinical data, socio-demographic characteristics, and postpartum measures for both mother and baby will be summarized using descriptive statistics. Means and standard deviations, (or medians and interquartile ranges), will be reported for continuous variables; counts and percentages will be reported for categorical variables. Missing observations attributed to either attrition or nonresponse will be evaluated for type of missingness. To avoid potential selection bias, values missing completely at random or at random will be imputed. For example, we may perform multiple imputation utilizing chained equations [87].
2.10.1. Proposed model building strategy for main outcomes of interest
Differences in postpartum weight (weight loss) between consecutive time points will be computed: birth and 3 months postpartum, birth and six months postpartum, and self-reported weight 3 months prior to pregnancy (pre-pregnancy weight) vs. six months postpartum. To assess maternal postpartum weight loss by intervention groups, eMOMS1 vs. eMOMS2 vs. eMOMS3, we will include a model building strategy that compares mean changes over time during the postpartum period. Models will also be developed for predicting differences in secondary outcomes of interest, including mean blood glucose (A1C), arterial blood pressure, breastfeeding duration (measured as a dichotomous variable during the same time points as maternal weight loss), and survey response differences. All models will include potential confounding variables such as BMI, race/ethnicity, level of education, annual household income, family history of diabetes, pre-diabetes status, parity and location as covariates. Development and reporting of these proposed models will follow the TRIPOD statement for multivariable prediction models for individual prognosis or diagnosis [88].
To predict maternal weight loss, a random coefficient model (subject-specific random intercepts and random slopes) will be developed using general linear mixed models (LMM). This approach will allow us to a) account for the individual fluctuations during the measurement time points, b) address questions of scientific interest about trajectories for individual units, either ones in the study or future units, and c) account for the within-individual and among-individual variations.
Similar models will be developed for both blood glucose (A1C) and arterial blood pressure, (e.g. subject-specific random intercepts and random slopes using LMM). Further, multiple linear regression or low-rank, thin-plate regression splines (splines with the degree of smoothness and number of spline knots) approach will be used to compare measures between eMOMS1 and eMOMS2, as well as eMOMS2 and eMOMS3 [89].
The risk of stopping breastfeeding prior to six months postpartum in eMOMS1 relative to eMOMS2 and eMOMS3 will be estimated by the random coefficients binary regression model with appropriate link function using generalized linear mixed model methodology. Time dependent repeated measurement proportional hazards regression model (PHREG) may also be used to compare the risk (hazard) of stopping breastfeeding across each study arm. To avoid the monotone likelihood problem, which occurs in small samples, Firth's bias correction method to Cox regression models may be conducted. Firth's penalized partial likelihood approach reduces asymptotic bias and addresses the monotone likelihood problem [90].
2.10.2. Comparison of scores on the breastfeeding knowledge assessment, BSES-SF, KPAS, EATS, selected items from the DSQ, and EPDS
The difference in scores for each survey instrument at study entry and at study closure across all three study arms will be assessed using the multiple linear regression approach. As before, these models will adjust for the effects of age, race/ethnicity, level of education, annual household income, family history of diabetes, pre-diabetes status, and parity. In addition, maternal depression over time will be evaluated using a random coefficient model with subject-specific random intercepts and random slopes from LMM procedure, similar to the one proposed for predicting maternal postnatal weight loss.
2.10.3. Proposed analysis of open-ended questions via electronic mail
Consistent with phenomenological procedures described by Gale and colleagues [91] and Creswell and colleagues [92,93], a content analysis will be conducted of all responses to the open-ended online questions provided by participants throughout each phase of the trial. Each question will be entered into an Excel spreadsheet designed to house the iterative coding process. The principal investigator and two study team members will highlight statements, sentences, or quotes that provide a textual description of participants’ experiences, followed by developing clusters of meaning and common themes. After each investigator will complete the first layer of coding, a meeting will be held to discuss and build consensus. If needed, a second layer of coding will be applied by each investigator, followed by another meeting to compare coding schemes and to come to consensus on underlying themes, patterns, and opinions.
2.11. Safety parameters
No serious adverse events are anticipated in this study and any such events will be reported to the Institutional Review Board of record. Throughout the study, issues related to the safety and wellbeing of the participant as well as privacy of data will be monitored. Participants with a score of 10 or higher on the Edinburgh Postnatal Depression Scale (EPDS) will be referred to a mental healthcare professional for further psychological evaluation. Women who develop gestational diabetes while in the study will remain in the study unless their condition is potentially life-threatening as determined by the participant's healthcare provider. A data and safety officer is in place throughout the course of the study. The principal investigator meets with the data and safety officer quarterly to review and discuss trial data. In the event of a potential adverse event, the principal investigator will contact the data and safety officer immediately.
2.12. Regulatory aspects and ethical considerations
The study has been approved by the Institutional Review Board (IRB) at Ascension Via Christi Hospitals Wichita, Inc. The Institutional Review Board with the University of Kansas School of Medicine-Wichita and Kearny County Hospital rely on Ascension Via Christi's IRB. The trial has been registered with ClinicalTrials.gov with the following identifier: NCT04021602. Participants who will be assigned to eMOMS3 (Usual Care) will be provided access to all educational videos upon completion of the trial. Research reported in this publication is supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number K01DK113048. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
3. Discussion
Overweight and obesity are major risk factors for gestational diabetes, and their prevalence rates are high among U.S. women [[16], [17], [18]]. Overweight and obesity rates are even higher among Hispanic (35% and 50% respectively) and non-Hispanic black women (28% and 47% respectively) compared to non-Hispanic white women (32% and 38% respectively) [19,20].
Being overweight or obese before and during pregnancy increases the risk of adverse health outcomes for both mother [[21], [22], [23], [24]] and child [[25], [26], [27], [28], [29], [30]], including excessive postpartum weight and an increased likelihood to develop type 2 diabetes [[94], [95], [96], [97]]. Moreover, rural pregnant women who experience limited access to obstetrical healthcare services are at increased risk of adverse outcomes [[31], [32], [33], [34]] and are therefore at additional risk for poor birth outcomes [[35], [36], [37]]. Compared to urban women, rural women also lead less healthy lives such as higher rates of smoking, lower rates of breastfeeding, and fewer options for healthy foods and physical activity [[98], [99], [100], [101], [102], [103], [104]].
Due to geographic barriers, higher rates of chronic disease, and limited availability of health promotion programs, it is important to administer a lifestyle change program that incorporates nutrition and physical activity counseling along with breastfeeding support during and after pregnancy. This period is particularly conducive to behavioral changes because: [1] women are more likely to modify their behavior to benefit their children; [2] behavior change interventions are most successful in the short term; and [3] effective interventions that start during pregnancy are more likely to be sustained after birth [105,106].
One such program is the national Diabetes Prevention Program (DPP) that is associated with a reduced risk of developing diabetes and postpartum weight loss [57,107,108]. Though, to date, the DPP has not been combined with breastfeeding education and support, which lowers the incidence of developing type 2 diabetes after pregnancy [[38], [39], [40], [41]]. This is where eMOMSTM fills the knowledge gap. eMOMSTM - electronic Monitoring Of Mom's Schedule - is a unique lifestyle change program delivered via Facebook that focuses on diet, exercise, and breastfeeding education and support using periodic one-on-one counseling sessions during and after pregnancy.
The design of this study has many strengths. First, the three-arm design allows for comparisons between weight loss with active breastfeeding education and support, weight loss without active breastfeeding education and support, and a contact control. Second, educational content for eMOMSTM is based on the evidence-based national DPP [57] and the evidence-based Your Guide to Breastfeeding [69]. Third, eMOMSTM is guided by Social Cognitive Theory and Self-Determination Theory that uses the principles of self-monitoring, planning, goal-setting, and feedback to improve self-regulation and to master self-efficacy to healthy eating, physical activity, and breastfeeding. Fourth, the delivery of eMOMSTM is done via social media – Facebook – so that participants can access program content on their own time from any location without having to attend scheduled face-to-face group meetings. Fifth, data are collected on weight loss, metabolic markers, breastfeeding, infant feeding practices, and baby's anthropometrics to measure program effectiveness. Finally, as eMOMSTM is delivered via social media, program cost will be compared to the cost of traditionally scheduled group meetings.
In summary, this randomized controlled trial will inform future intervention efforts with the aim of reducing diabetes risk factors among a high-risk hard-to-reach population. As such, this study has the potential to define a high impact, cost effective intervention that is easy to deliver and disseminate, and that can improve public health by reducing the effect of gestational diabetes and type 2 diabetes among a culturally diverse, at-risk population.
Acknowledgements
The authors would like to acknowledge the following individuals for their contributions to the project: Patricia M. Kluding, PT, PhD; Kelsey Lu, MS; Jolynn Dowling, MSN, APRN, NNP-BC, IBCLC; Lauren E. Haag, MD; and Michelle Vitztum, MPH. The authors would also like to thank the administration and staff at Ascension Via Christi Hospitals Wichita, Inc. and Kearny County Hospital. Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number K01DK113048. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Lisette T. Jacobson, Email: ljacobson@kumc.edu.
Tracie C. Collins, Email: tccollins@salud.unm.edu.
Meredith Lucas, Email: mlucas4@kumc.edu.
Rosey Zackula, Email: rzackula@kumc.edu.
Hayrettin Okut, Email: hokut@kumc.edu.
Niaman Nazir, Email: nnazir@kumc.edu.
David Robbins, Email: drobbins@kumc.edu.
Judy E. Stern, Email: judy.e.stern@dartmouth.edu.
Michael Wolfe, Email: michael.wolfe@ascension.org.
David A. Grainger, Email: dgrainger@kumc.edu.
References
- 1.Casagrande S.S., Linder B., Cowie C.C. Prevalence of gestational diabetes and subsequent Type 2 diabetes among U.S. women. Diabetes Res. Clin. Pract. 2018;141:200–208. doi: 10.1016/j.diabres.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 2.DeSisto C.L., Kim S.Y., Sharma A.J. Prevalence estimates of gestational diabetes mellitus in the United States, pregnancy risk assessment monitoring system (PRAMS), 2007-2010. Prev. Chronic Dis. 2014;11:E104. doi: 10.5888/pcd11.130415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metzger B.E., Gabbe S.G., Persson B., Buchanan T.A., Catalano P.M., Damm P. The diagnosis of gestational diabetes mellitus: new paradigms or status quo? J. Matern. Fetal Neonatal Med. 2012;25(12):2564–2569. doi: 10.3109/14767058.2012.718002. [DOI] [PubMed] [Google Scholar]
- 4.Ovesen P.G., Jensen D.M., Damm P., Rasmussen S., Kesmodel U.S. Maternal and neonatal outcomes in pregnancies complicated by gestational diabetes, a nation-wide study. J. Matern. Fetal Neonatal Med. 2015:1–5. doi: 10.3109/14767058.2014.966677. [DOI] [PubMed] [Google Scholar]
- 5.Saydah S.H., Chandra A., Eberhardt M.S. Pregnancy experience among women with and without gestational diabetes in the U.S., 1995 National Survey of Family Growth. Diabetes Care. 2005;28(5):1035–1040. doi: 10.2337/diacare.28.5.1035. [DOI] [PubMed] [Google Scholar]
- 6.Metzger B.E., Lowe L.P., Dyer A.R., Trimble E.R., Chaovarindr U., Coustan D.R. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008;358(19):1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 7.The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study Cooperative Research Group Hyperglycemia and adverse pregnancy outcome (HAPO) study: preeclampsia. Am. J. Obstet. Gynecol. 2010;202:255 e1–7. doi: 10.1016/j.ajog.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catalano P.M., McIntyre H.D., Cruickshank J.K., McCance D.R., Dyer A.R., Metzger B.E. The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes Care. 2012;35(4):780–786. doi: 10.2337/dc11-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srichumchit S., Luewan S., Tongsong T. Outcomes of pregnancy with gestational diabetes mellitus. Int. J. Gynaecol. Obstet. 2015;131(3):251–254. doi: 10.1016/j.ijgo.2015.05.033. [DOI] [PubMed] [Google Scholar]
- 10.Task Force on Hypertension in Pregnancy Hypertension in pregnancy. Obstet. Gynecol. 2013;122(5):1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 11.Young B.C., Ecker J.L. Fetal macrosomia and shoulder dystocia in women with gestational diabetes: risks amenable to treatment? Curr. Diabetes Rep. 2013;13(1):12–18. doi: 10.1007/s11892-012-0338-8. [DOI] [PubMed] [Google Scholar]
- 12.Mitanchez D., Yzydorczyk C., Siddeek B., Boubred F., Benahmed M., Simeoni U. The offspring of the diabetic mother - short- and long-term implications. Best Pract. Res. Clin. Obstet. Gynaecol. 2015;29(2):256–269. doi: 10.1016/j.bpobgyn.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 13.The American College of Obstetricians and Gynecologists ACOG practice Bulletin No. 173: fetal macrosomia. Obstet. Gynecol. 2016;128(5):e195–e209. doi: 10.1097/AOG.0000000000001767. [DOI] [PubMed] [Google Scholar]
- 14.Bellamy L., Casas J.P., Hingorani A.D., Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373(9677):1773–1779. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 15.Lee A.J., Hiscock R.J., Wein P., Walker S.P., Permezel M. Gestational diabetes mellitus: clinical predictors and long-term risk of developing type 2 diabetes: a retrospective cohort study using survival analysis. Diabetes Care. 2007;30(4):878–883. doi: 10.2337/dc06-1816. [DOI] [PubMed] [Google Scholar]
- 16.Bottalico J.N. Recurrent gestational diabetes: risk factors, diagnosis, management, and implications. Semin. Perinatol. 2007;31(3):176–184. doi: 10.1053/j.semperi.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Chen P., Wang S., Ji J., Ge A., Chen C., Zhu Y. Risk factors and management of gestational diabetes. Cell Biochem. Biophys. 2015;71(2):689–694. doi: 10.1007/s12013-014-0248-2. [DOI] [PubMed] [Google Scholar]
- 18.Mao L., Ge X., Xu Y., Huang K., Pan W., Zhou S. Pregestational body mass index, weight gain during first half of pregnancy and gestational diabetes mellitus: a prospective cohort study] Zhonghua Liuxingbingxue Zazhi. 2015;36(5):416–420. [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance System-United States. Age-Adjusted Percentage with Overweight and Obesity by Gender and BMI for 2015-2016. https://nccd.cdc.gov/ckd/detail.aspx?Qnum=Q145&Strat=Gender%2c+BMI#refreshPosition National Health and Nutrition Examination Survey.
- 20.Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance System-United States. Age-Adjusted Percentage with Overweight and Obesity by Race/Ethnicity and BMI for 2015-2016. https://nccd.cdc.gov/ckd/detail.aspx?Qnum=Q145&Strat=Race%2fEthnicity%2c+BMI#refreshPosition National Health and Nutrition Examination Survey.
- 21.Athukorala C., Rumbold A.R., Willson K.J., Crowther C.A. The risk of adverse pregnancy outcomes in women who are overweight or obese. BMC Pregnancy Childbirth. 2010;10:56. doi: 10.1186/1471-2393-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burstein E., Levy A., Mazor M., Wiznitzer A., Sheiner E. Pregnancy outcome among obese women: a prospective study. Am. J. Perinatol. 2008;25(9):561–566. doi: 10.1055/s-0028-1085623. [DOI] [PubMed] [Google Scholar]
- 23.Denison F.C., Norwood P., Bhattacharya S., Duffy A., Mahmood T., Morris C. Association between maternal body mass index during pregnancy, short-term morbidity, and increased health service costs: a population-based study. BJOG An Int. J. Obstet. Gynaecol. 2014;121(1):72–81. doi: 10.1111/1471-0528.12443. [DOI] [PubMed] [Google Scholar]
- 24.Gaillard R., Durmus B., Hofman A., Mackenbach J.P., Steegers E.A., Jaddoe V.W. Risk factors and outcomes of maternal obesity and excessive weight gain during pregnancy. Obesity. 2013;21(5):1046–1055. doi: 10.1002/oby.20088. [DOI] [PubMed] [Google Scholar]
- 25.Gaillard R., Steegers E.A., Duijts L., Felix J.F., Hofman A., Franco O.H. Childhood cardiometabolic outcomes of maternal obesity during pregnancy: the Generation R Study. Hypertension. 2014;63(4):683–691. doi: 10.1161/HYPERTENSIONAHA.113.02671. [DOI] [PubMed] [Google Scholar]
- 26.Harpsoe M.C., Basit S., Bager P., Wohlfahrt J., Benn C.S., Nohr E.A. Maternal obesity, gestational weight gain, and risk of asthma and atopic disease in offspring: a study within the Danish National Birth Cohort. J. Allergy Clin. Immunol. 2013;131(4):1033–1040. doi: 10.1016/j.jaci.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Hinkle S.N., Sharma A.J., Swan D.W., Schieve L.A., Ramakrishnan U., Stein A.D. Excess gestational weight gain is associated with child adiposity among mothers with normal and overweight prepregnancy weight status. J. Nutr. 2012;142(10):1851–1858. doi: 10.3945/jn.112.161158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masho S.W., Cha S., Morris M.R. Prepregnancy obesity and breastfeeding noninitiation in the United States: an examination of racial and ethnic differences. Breastfeed. Med. 2015;10(5):253–262. doi: 10.1089/bfm.2015.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reynolds R.M., Allan K.M., Raja E.A., Bhattacharya S., McNeill G., Hannaford P.C. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: follow-up of 1 323 275 person years. BMJ. 2013;347:f4539. doi: 10.1136/bmj.f4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stothard K.J., Tennant P.W., Bell R., Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. J. Am. Med. Assoc. 2009;301(6):636–650. doi: 10.1001/jama.2009.113. [DOI] [PubMed] [Google Scholar]
- 31.The American College of Obstetricians and Gynecologists ACOG Committee Opinion No. 586: health disparities in rural women. Obstet. Gynecol. 2014;123(2 Pt 1):384–388. doi: 10.1097/01.AOG.0000443278.06393.d6. [DOI] [PubMed] [Google Scholar]
- 32.Chandler D. Late entry into prenatal care in a rural setting. J. Midwifery Wom. Health. 2002;47(1):28–34. doi: 10.1016/s1526-9523(01)00214-8. [DOI] [PubMed] [Google Scholar]
- 33.Gallagher A., Liu J., Probst J.C., Martin A.B., Hall J.W. Maternal obesity and gestational weight gain in rural versus urban dwelling women in South Carolina. J. Rural Health. 2013;29(1):1–11. doi: 10.1111/j.1748-0361.2012.00421.x. [DOI] [PubMed] [Google Scholar]
- 34.Rayburn W.F., Richards M.E., Elwell E.C. Drive times to hospitals with perinatal care in the United States. Obstet. Gynecol. 2012;119(3):611–616. doi: 10.1097/AOG.0b013e318242b4cb. [DOI] [PubMed] [Google Scholar]
- 35.Blumenshine P., Egerter S., Barclay C.J., Cubbin C., Braveman P.A. Socioeconomic disparities in adverse birth outcomes: a systematic review. Am. J. Prev. Med. 2010;39(3):263–272. doi: 10.1016/j.amepre.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 36.McElroy J.A., Bloom T., Moore K., Geden B., Everett K., Bullock L.F. Perinatal mortality and adverse pregnancy outcomes in a low-income rural population of women who smoke. Birth Defects Res. Part A Clin. Mol. Teratol. 2012;94(4):223–229. doi: 10.1002/bdra.22891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strutz K.L., Dozier A.M., van Wijngaarden E., Glantz J.C. Birth outcomes across three rural-urban typologies in the Finger Lakes region of New York. J. Rural Health. 2012;28(2):162–173. doi: 10.1111/j.1748-0361.2011.00392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gunderson E.P., Hurston S.R., Ning X., Lo J.C., Crites Y., Walton D. Lactation and progression to type 2 diabetes mellitus after gestational diabetes mellitus: a prospective cohort study. Ann. Intern. Med. 2015;163(12):889–898. doi: 10.7326/M15-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gunderson E.P., Hedderson M.M., Chiang V., Crites Y., Walton D., Azevedo R.A. Lactation intensity and postpartum maternal glucose tolerance and insulin resistance in women with recent GDM: the SWIFT cohort. Diabetes Care. 2012;35(1):50–56. doi: 10.2337/dc11-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chouinard-Castonguay S., Weisnagel S.J., Tchernof A., Robitaille J. Relationship between lactation duration and insulin and glucose response among women with prior gestational diabetes. Eur. J. Endocrinol. 2013;168(4):515–523. doi: 10.1530/EJE-12-0939. [DOI] [PubMed] [Google Scholar]
- 41.O'Reilly M., Avalos G., Dennedy M.C., O'Sullivan E.P., Dunne F.P. Breast-feeding is associated with reduced postpartum maternal glucose intolerance after gestational diabetes. Ir. Med. J. 2012;105(5 Suppl):31–36. [PubMed] [Google Scholar]
- 42.Binns C., Lee M., Low W.Y. The long-term public health benefits of breastfeeding. Asia Pac. J. Publ. Health. 2016;28(1):7–14. doi: 10.1177/1010539515624964. [DOI] [PubMed] [Google Scholar]
- 43.Kirkegaard H., Stovring H., Rasmussen K.M., Abrams B., Sorensen T.I., Nohr E.A. How do pregnancy-related weight changes and breastfeeding relate to maternal weight and BMI-adjusted waist circumference 7 y after delivery? Results from a path analysis. Am. J. Clin. Nutr. 2014;99(2):312–319. doi: 10.3945/ajcn.113.067405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin J., MacDonald-Wicks L., Hure A., Smith R., Collins C.E. Reducing postpartum weight retention and improving breastfeeding outcomes in overweight women: a pilot randomised controlled trial. Nutrients. 2015;7(3):1464–1479. doi: 10.3390/nu7031464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stuebe A.M., Rich-Edwards J.W. The reset hypothesis: lactation and maternal metabolism. Am. J. Perinatol. 2009;26(1):81–88. doi: 10.1055/s-0028-1103034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bernier M.O., Plu-Bureau G., Bossard N., Ayzac L., Thalabard J.C. Breastfeeding and risk of breast cancer: a meta-analysis of published studies. Hum. Reprod. Update. 2000;6(4):374–386. doi: 10.1093/humupd/6.4.374. [DOI] [PubMed] [Google Scholar]
- 47.Danforth K.N., Tworoger S.S., Hecht J.L., Rosner B.A., Colditz G.A., Hankinson S.E. Breastfeeding and risk of ovarian cancer in two prospective cohorts. Cancer Causes Control. 2007;18(5):517–523. doi: 10.1007/s10552-007-0130-2. [DOI] [PubMed] [Google Scholar]
- 48.Jordan S.J., Cushing-Haugen K.L., Wicklund K.G., Doherty J.A., Rossing M.A. Breast-feeding and risk of epithelial ovarian cancer. Cancer Causes Control. 2012;23(6):919–927. doi: 10.1007/s10552-012-9963-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stuebe A.M., Rich-Edwards J.W., Willett W.C., Manson J.E., Michels K.B. Duration of lactation and incidence of type 2 diabetes. J. Am. Med. Assoc. 2005;294(20):2601–2610. doi: 10.1001/jama.294.20.2601. [DOI] [PubMed] [Google Scholar]
- 50.Schwarz E.B., Brown J.S., Creasman J.M., Stuebe A., McClure C.K., Van Den Eeden S.K. Lactation and maternal risk of type 2 diabetes: a population-based study. Am. J. Med. 2010;123(9):863 e1–6. doi: 10.1016/j.amjmed.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eidelman A.I. Breastfeeding and the use of human milk: an analysis of the American Academy of Pediatrics 2012 breastfeeding policy statement. Breastfeed. Med. 2012;7(5):323–324. doi: 10.1089/bfm.2012.0067. [DOI] [PubMed] [Google Scholar]
- 52.Saadeh M.R. A new global strategy for infant and young child feeding. Forum Nutr. 2003;56:236–238. [PubMed] [Google Scholar]
- 53.Centers for Disease Control and Prevention Breastfeeding among U.S. Children Born 2009-2016, CDC national immunization survey. 2019. https://www.cdc.gov/breastfeeding/data/nis_data/results.html
- 54.Winkvist A., Brantsaeter A.L., Brandhagen M., Haugen M., Meltzer H.M., Lissner L. Maternal prepregnant body mass index and gestational weight gain are associated with initiation and duration of breastfeeding among Norwegian mothers. J. Nutr. 2015;145(6):1263–1270. doi: 10.3945/jn.114.202507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verret-Chalifour J., Giguere Y., Forest J.C., Croteau J., Zhang P., Marc I. Breastfeeding initiation: impact of obesity in a large Canadian perinatal cohort study. PloS One. 2015;10(2) doi: 10.1371/journal.pone.0117512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thompson L.A., Zhang S., Black E., Das R., Ryngaert M., Sullivan S. The association of maternal pre-pregnancy body mass index with breastfeeding initiation. Matern. Child Health J. 2013;17(10):1842–1851. doi: 10.1007/s10995-012-1204-7. [DOI] [PubMed] [Google Scholar]
- 57.Knowler W.C., Barrett-Connor E., Fowler S.E., Hamman R.F., Lachin J.M., Walker E.A. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aroda V.R., Christophi C.A., Edelstein S.L., Zhang P., Herman W.H., Barrett-Connor E. The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: the Diabetes Prevention Program outcomes study 10-year follow-up. J. Clin. Endocrinol. Metab. 2015;100(4):1646–1653. doi: 10.1210/jc.2014-3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chan A.W., Tetzlaff J.M., Altman D.G., Laupacis A., Gotzsche P.C., Krleza-Jeric K. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann. Intern. Med. 2013;158(3):200–207. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chan A.W., Tetzlaff J.M., Gotzsche P.C., Altman D.G., Mann H., Berlin J.A. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346 doi: 10.1136/bmj.e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Whitehead A.L., Sully B.G., Campbell M.J. Pilot and feasibility studies: is there a difference from each other and from a randomised controlled trial? Contemp. Clin. Trials. 2014;38(1):130–133. doi: 10.1016/j.cct.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 62.Bandura A. Human agency in social cognitive theory. Am. Psychol. 1989;44(9):1175–1184. doi: 10.1037/0003-066x.44.9.1175. [DOI] [PubMed] [Google Scholar]
- 63.Bandura A. Self-efficacy mechanism in human agency. Am. Psychol. 1982;37(2):122–147. [Google Scholar]
- 64.Deci E.L., Ryan R.M. In: The Empirical Exploration of Intrinsic Motivational Processes. B L., editor. Academic Press; New York, NY: 1980. [Google Scholar]
- 65.Deci E.L., Ryan R.M. A motivational approach to self: integration in personality. In: Dienstbier R., editor. Nebraska Symposium on Motivation: Volume 38 Perspectives on Motivation. University of Nebraska Press; Lincoln, NE: 1991. [PubMed] [Google Scholar]
- 66.Ryan R.M., Deci E.L. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Am. Psychol. 2000;55(1):68–78. doi: 10.1037//0003-066x.55.1.68. [DOI] [PubMed] [Google Scholar]
- 67.Deci E.L., Ryan R.M. Plenum; New York, NY: 1985. Intrinsic Motivation and Self-Determination in Human Behavior. [Google Scholar]
- 68.Share Our Strength . Share Our Strength; Washington DC: 2013. Cooking Matters for Parents, Instructor Guide. [Google Scholar]
- 69.U.S. Department of Health and Human Services Office on women's health. Your guide to breastfeeding. https://www.womenshealth.gov/patient-materials/health-topic/breastfeeding
- 70.Gallo S., Kogan K., Kitsantas P. Racial and ethnic differences in reasons for breastfeeding cessation among women participating in the Special Supplemental Nutrition Program for Women, Infants, and Children. J. Midwifery Wom. Health. 2019 doi: 10.1111/jmwh.13031. [DOI] [PubMed] [Google Scholar]
- 71.Hedberg I.C. Barriers to breastfeeding in the WIC population. MCN Am. J. Matern./Child Nurs. 2013;38(4):244–249. doi: 10.1097/NMC.0b013e3182836ca2. [DOI] [PubMed] [Google Scholar]
- 72.Sriraman N.K., Kellams A. Breastfeeding: what are the barriers? Why women struggle to achieve their goals. J. Womens Health. 2002;25(7):714–722. doi: 10.1089/jwh.2014.5059. 2016. [DOI] [PubMed] [Google Scholar]
- 73.Ackermann R.T., Finch E.A., Brizendine E., Zhou H., Marrero D.G. Translating the diabetes prevention program into the community. The DEPLOY pilot study. Am. J. Prev. Med. 2008;35(4):357–363. doi: 10.1016/j.amepre.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dennis C.L. The breastfeeding self-efficacy scale: psychometric assessment of the short form. J. Obstet. Gynecol. Neonatal Nurs. 2003;32(6):734–744. doi: 10.1177/0884217503258459. [DOI] [PubMed] [Google Scholar]
- 75.Tuthill E.L., McGrath J.M., Graber M., Cusson R.M., Young S.L. Breastfeeding self-efficacy: a critical review of available instruments. J. Hum. Lactation. 2016;32(1):35–45. doi: 10.1177/0890334415599533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Creedy D.K., Dennis C.L., Blyth R., Moyle W., Pratt J., De Vries S.M. Psychometric characteristics of the breastfeeding self-efficacy scale: data from an Australian sample. Res. Nurs. Health. 2003;26(2):143–152. doi: 10.1002/nur.10073. [DOI] [PubMed] [Google Scholar]
- 77.Otsuka K., Taguri M., Dennis C.L., Wakutani K., Awano M., Yamaguchi T. Effectiveness of a breastfeeding self-efficacy intervention: do hospital practices make a difference? Matern. Child Health J. 2014;18(1):296–306. doi: 10.1007/s10995-013-1265-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ainsworth B.E., Sternfeld B., Richardson M.T., Jackson K. Evaluation of the kaiser physical activity survey in women. Med. Sci. Sports Exerc. 2000;32(7):1327–1338. doi: 10.1097/00005768-200007000-00022. [DOI] [PubMed] [Google Scholar]
- 79.Schmidt M.D., Freedson P.S., Pekow P., Roberts D., Sternfeld B., Chasan-Taber L. Validation of the kaiser physical activity survey in pregnant women. Med. Sci. Sports Exerc. 2006;38(1):42–50. doi: 10.1249/01.mss.0000181301.07516.d6. [DOI] [PubMed] [Google Scholar]
- 80.Thompson F.E., Subar A.F., Smith A.F., Midthune D., Radimer K.L., Kahle L.L. Fruit and vegetable assessment: performance of 2 new short instruments and a food frequency questionnaire. J. Am. Diet Assoc. 2002;102(12):1764–1772. doi: 10.1016/s0002-8223(02)90379-2. [DOI] [PubMed] [Google Scholar]
- 81.Yaroch A.L., Tooze J., Thompson F.E., Blanck H.M., Thompson O.M., Colon-Ramos U. Evaluation of three short dietary instruments to assess fruit and vegetable intake: the National Cancer Institute's food attitudes and behaviors survey. J. Acad. Nutr. Diet. 2012;112(10):1570–1577. doi: 10.1016/j.jand.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.George S.M., Thompson F.E., Midthune D., Subar A.F., Berrigan D., Schatzkin A. Strength of the relationships between three self-reported dietary intake instruments and serum carotenoids: the Observing Energy and Protein Nutrition (OPEN) Study. Publ. Health Nutr. 2012;15(6):1000–1007. doi: 10.1017/S1368980011003272. [DOI] [PubMed] [Google Scholar]
- 83.Dietary screener questionnaire in the NHANES 2009-10: background. http://epi.grants.cancer.gov/nhanes/dietscreen/
- 84.Dietary screener questionnaire (DSQ) in the NHANES 2009-10: dietary factors, food items asked, and testing status for DSQ. http://epi.grants.cancer.gov/nhanes/dietscreen/evaluation.html#pub
- 85.Cox J.L., Holden J.M., Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh postnatal depression scale. Br. J. Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 86.Gibson J., McKenzie-McHarg K., Shakespeare J., Price J., Gray R. A systematic review of studies validating the Edinburgh Postnatal Depression Scale in antepartum and postpartum women. Acta Psychiatr. Scand. 2009;119(5):350–364. doi: 10.1111/j.1600-0447.2009.01363.x. [DOI] [PubMed] [Google Scholar]
- 87.Leurent B., Gomes M., Faria R., Morris S., Grieve R., Carpenter J.R. Sensitivity analysis for not-at-random missing data in trial-based cost-effectiveness analysis: a tutorial. Pharmacoeconomics. 2018;36(8):889–901. doi: 10.1007/s40273-018-0650-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moons K.G., Altman D.G., Reitsma J.B., Ioannidis J.P., Macaskill P., Steyerberg E.W. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann. Intern. Med. 2015;162(1):W1–W73. doi: 10.7326/M14-0698. [DOI] [PubMed] [Google Scholar]
- 89.Kelly M.G., Winkler S.S., Lentz S.S., Berliner S.H., Swain M.F., Skinner H.G. Serum calcium and serum albumin are biomarkers that can discriminate malignant from benign pelvic masses. Cancer Epidemiol. Biomark. Prev. 2015;24(10):1593–1598. doi: 10.1158/1055-9965.EPI-15-0443. [DOI] [PubMed] [Google Scholar]
- 90.Nagashima K., Sato Y. Information criteria for Firth's penalized partial likelihood approach in Cox regression models. Stat. Med. 2017;36(21):3422–3436. doi: 10.1002/sim.7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gale N.K., Heath G., Cameron E., Rashid S., Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med. Res. Methodol. 2013;13:117. doi: 10.1186/1471-2288-13-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Creswell J.W. third ed. SAGE Publications, Inc.; Thousand Oaks, CA: 2013. Qualitative Inquiry and Research Design. [Google Scholar]
- 93.Creswell J.W., Plano Clark V.L. second ed. SAGE Publications, Inc.; Thousand Oaks, CA: 2011. Designing and Conducting Mixed Methods Research. [Google Scholar]
- 94.Gunderson E.P., Abrams B. Epidemiology of gestational weight gain and body weight changes after pregnancy. Epidemiol. Rev. 2000;22(2):261–274. doi: 10.1093/oxfordjournals.epirev.a018038. [DOI] [PubMed] [Google Scholar]
- 95.Rooney B.L., Schauberger C.W. Excess pregnancy weight gain and long-term obesity: one decade later. Obstet. Gynecol. 2002;100(2):245–252. doi: 10.1016/s0029-7844(02)02125-7. [DOI] [PubMed] [Google Scholar]
- 96.Gilmore L.A., Klempel-Donchenko M., Redman L.M. Pregnancy as a window to future health: excessive gestational weight gain and obesity. Semin. Perinatol. 2015;39(4):296–303. doi: 10.1053/j.semperi.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Norman J.E., Reynolds R.M. The consequences of obesity and excess weight gain in pregnancy. Proc. Nutr. Soc. 2011;70(4):450–456. doi: 10.1017/S0029665111003077. [DOI] [PubMed] [Google Scholar]
- 98.Matthews K.A., Croft J.B., Liu Y., Lu H., Kanny D., Wheaton A.G. Health-related behaviors by urban-rural county classification - United States, 2013. MMWR Surveillance Summ. 2017;66(5):1–8. doi: 10.15585/mmwr.ss6605a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hardin-Fanning F., Rayens M.K. Food cost disparities in rural communities. Health Promot. Pract. 2015;16(3):383–391. doi: 10.1177/1524839914554454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Taber D.R., Chriqui J.F., Quinn C.M., Rimkus L.M., Chaloupka F.J. Cross-sector analysis of socioeconomic, racial/ethnic, and urban/rural disparities in food policy enactment in the United States. Health Place. 2016;42:47–53. doi: 10.1016/j.healthplace.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lutfiyya M.N., Chang L.F., Lipsky M.S. A cross-sectional study of US rural adults' consumption of fruits and vegetables: do they consume at least five servings daily? BMC Publ. Health. 2012;12:280. doi: 10.1186/1471-2458-12-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Larson N.I., Story M.T., Nelson M.C. Neighborhood environments: disparities in access to healthy foods in the U.S. Am. J. Prev. Med. 2009;36(1):74–81. doi: 10.1016/j.amepre.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 103.Jacobson L.T., Dong F., Scheuermann T.S., Redmond M.L., Collins T.C. Smoking behaviors among urban and rural pregnant women enrolled in the Kansas WIC program. J. Community Health. 2015;40(5):1037–1046. doi: 10.1007/s10900-015-0029-x. [DOI] [PubMed] [Google Scholar]
- 104.Jacobson L.T., Twumasi-Ankrah P., Redmond M.L., Ablah E., Hines R.B., Johnston J. Characteristics associated with breastfeeding behaviors among urban versus rural women enrolled in the Kansas WIC program. Matern. Child Health J. 2015;19(4):828–839. doi: 10.1007/s10995-014-1580-2. [DOI] [PubMed] [Google Scholar]
- 105.Gillman M.W., Ludwig D.S. How early should obesity prevention start? N. Engl. J. Med. 2013;369(23):2173–2175. doi: 10.1056/NEJMp1310577. [DOI] [PubMed] [Google Scholar]
- 106.Horan M.K., McGowan C.A., Gibney E.R., Donnelly J.M., McAuliffe F.M. Maternal diet and weight at 3 months postpartum following a pregnancy intervention with a low glycaemic index diet: results from the ROLO randomised control trial. Nutrients. 2014;6(7):2946–2955. doi: 10.3390/nu6072946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ferrara A., Hedderson M.M., Albright C.L., Ehrlich S.F., Quesenberry C.P., Jr., Peng T. A pregnancy and postpartum lifestyle intervention in women with gestational diabetes mellitus reduces diabetes risk factors: a feasibility randomized control trial. Diabetes Care. 2011;34(7):1519–1525. doi: 10.2337/dc10-2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nicklas J.M., Zera C.A., England L.J., Rosner B.A., Horton E., Levkoff S.E. A web-based lifestyle intervention for women with recent gestational diabetes mellitus: a randomized controlled trial. Obstet. Gynecol. 2014;124(3):563–570. doi: 10.1097/AOG.0000000000000420. [DOI] [PMC free article] [PubMed] [Google Scholar]