Highlights
-
•
Males reported more recent happy occasions with class/teammates than females.
-
•
Males activated fusiform gyrus more than females while viewing unfamiliar peers.
-
•
Striatum functional connectivity mediated gender differences in social behavior.
Keywords: Adolescence, Gender differences, Ecological momentary assessment, Functional magnetic resonance imaging, Face processing, Social reward
Abstract
Peers become increasingly important during adolescence, with emerging gender differences in peer relationships associated with distinct behavioral and emotional outcomes. Males tend to socialize in larger peer groups with competitive interactions, whereas females engage in longer bouts of dyadic interaction with more intimacy. To examine gender differences in neural response to ecologically valid displays of positive affect and future social interactions, 52 adolescents (14–18 years old; female = 30) completed a social reward functional magnetic resonance imaging (fMRI) task with videos of a same-gender best friend (BF) or unfamiliar peer (UP) expressing positive (versus neutral) affect. Participants completed ecological momentary assessment of social experiences for two 5-day intervals. Compared with females, males more often reported that their happiest experience in the past hour occurred with class/teammates. Females and males displayed greater fusiform gyrus (FG) activation during BF and UP conditions, respectively (pvoxel<0.0001, pcluster<0.05, family-wise error). Compared with males, females exhibited greater nucleus accumbens (NAcc)-precuneus functional connectivity to BF Positive> UP Positive. An exploratory analysis indicated that the association of male gender with a greater proportion of positive experiences with class/teammates was statistically mediated by greater NAcc-precuneus functional connectivity. Gender differences in positive social experiences may be associated with reward and social cognition networks.
1. Introduction
Adolescence is a time for exploration and learning that is facilitated by heightened motivation for reward. In addition to possessing greater motivation for typical rewards like money, adolescents are more motivated by social rewards than are adults (Wang et al., 2017). Peer relationships gain significant importance in adolescence (Nelson et al., 2005), and social rewards associated with these relationships are highly motivating and salient. In fact, peer influence can affect adolescent behavior in diverse and meaningful ways, including increasing risk-taking (Chein et al., 2011) and prosocial (Van Hoorn et al., 2016) behaviors. Examining the associations between neural response to social rewards and real-world positive experiences in social contexts may help identify neural factors relevant to social functioning.
Important gender differences in social behavior and experiences emerge during adolescence (Rose and Rudolph, 2006). For instance, adolescent females report greater investment in peer relationships and place greater value on friendship intimacy (Li and Wright, 2014), whereas males report more competition with peers (Deaner et al., 2012) and more often engage with larger peer groups (Flynn et al., 2017) than vice versa. These gender-linked peer relationship processes contribute to the emotional and behavioral development of male and female youth. Specifically, the peer socialization model (Rose and Rudolph, 2006) posits that female-linked relationship processes may promote the development of intimate relationships and inhibit antisocial behavior, but may increase vulnerability to emotional difficulties. By contrast, male-linked processes may promote group relationships and protect against emotional difficulties but also contribute to externalizing behavior problems (Rose and Rudolph, 2006). Although this model does not address brain development, it is possible that gender differences in the neural correlates of social reward during peer interactions are a mechanism for the contributions of social processes to gender differences in experiences and behavior. Thus, we propose that gender differences will be apparent in the function of adolescents’ affective and social neural circuitry, as well as in their affective experiences in peer social contexts. EMA has previously been used to study gender differences in adolescent mood and behavior (Weinstein and Mermelstein, 2013). By repeatedly sampling behavior in the real world, EMA reduces recall bias and response error variance, with the added benefit of obtaining measurements in natural environments (Shiffman et al., 2008).
Developmental changes in adolescent reward processing reflect the underlying maturation of related neural circuitry. Several studies have reported an inverted U-shaped relationship between ventral striatal activation and age, such that neural responsiveness to reward reaches a peak in adolescence, compared to childhood and adulthood (Casey et al., 2008). Although adolescents report greater motivation for social versus monetary reward than adults (Wang et al., 2017), the functioning of neural circuitry during social reward specifically has not been investigated as a mechanism of social behavior during adolescence. Recent research suggests that adolescent males display greater behavioral (Wang et al., 2017) and neural (Alarcon et al., 2017) sensitivity to monetary rewards than do females. Conversely, relative to males, adolescent females demonstrate greater activation of ventral striatum during social reward (Guyer et al., 2009), which has been shown to be associated with greater self-reported positive feelings of wellbeing (Guyer et al., 2014). Similarly, in adolescent females, but not males, activation of brain regions that comprise the social brain network (e.g., fusiform gyrus, superior temporal gyrus) during social reward is positively associated with age (Guyer et al., 2012). Although not generally conceptualized as social reward tasks, face processing tasks engage ventral striatum when the target face belongs to a loved one (Sugiura, 2014) or a member of an in-group (Chen et al., 2015), but not when viewing familiar faces lacking personal familiarity (e.g., learned faces, celebrity faces) (Kosaka et al., 2003), at least in adulthood. Similarly, viewing faces of novel peers as a form of social reward has been shown to elicit activation in ventral striatum (Guyer et al., 2009; Guyer et al., 2012) and dorsal striatum (Gunther Moor et al., 2010) in adolescence. Thus, viewing the happy face of a personally familiar individual like a best friend (BF) may engage the striatum even further during adolescence, particularly in females versus males.
Processing familiar faces engages two visual neural systems – the core and extended systems (Gobbini and Haxby, 2007). The core system encodes visual appearance through lateral fusiform gyrus (FG) and posterior superior temporal gyrus (pSTS) activation. The extended system encodes 1) person knowledge via anterior paracingulate gyrus, pSTS/temporoparietal junction (TPJ), anterior temporal cortex and precuneus/posterior cingulate cortex (PCC) and 2) emotional representations via amygdala, insula and striatum. A few studies have compared familiar versus unfamiliar face processing in adolescence (Ambrosia et al., 2018; Whittle et al., 2012; Saxbe et al., 2015); however, only one has compared activation to familiar and unfamiliar peer faces (Ambrosia et al., 2018). This study found that viewing a BF express positive affect elicits more activation in ventrolateral prefrontal cortex, superior temporal gyrus and inferior frontal gyrus than viewing an unfamiliar peer (Ambrosia et al., 2018). Thus, adolescents may be particularly sensitive to positive emotional expression of familiar peer faces. Moreover, the neural systems processing emotional faces continue to develop across adolescence. Although adolescents do not differ from adults when viewing neutral faces (Scherf et al., 2007), when viewing emotional novel faces, they display greater activation than adults in prefrontal cortex, caudate nucleus and parahippocampal gyrus (Passarotti et al., 2009), despite exhibiting equivalent facial emotion labeling abilities to adults (Wiggins et al., 2016). Among adolescents, findings of gender differences in neural reactivity to novel faces expressing negative (anger, fear) or neutral emotion have been mixed (Guyer et al., 2008; Schneider et al., 2011; Tahmasebi et al., 2012); however, gender differences in the neural response to novel faces expressing positive emotion has not been investigated. Notably, adolescent females show higher sensitivity in processing emotional facial expressions of novel peers than males, as reflected by faster and more sensitive perception of facial emotion in a forced-choice emotion discrimination task (Lee et al., 2013). Thus, behavioral and neural reactivity to positive affect expressed by personally familiar peers is likely to differ by gender during adolescence.
The current study examined adolescent gender differences in neural activity and ventral striatum (i.e., nucleus accumbens [NAcc]) functional connectivity while watching and listening to a BF (versus an unfamiliar peer [UP]) express positive affect. NAcc functional connectivity was examined because 1) the NAcc plays a critical role in social reward in adolescence (Guyer et al., 2009); 2) NAcc functional connectivity with social cognition brain regions (anterior and posterior cingulate cortices and medial prefrontal cortex) is associated with social anhedonia (Healey et al., 2014; Wang et al., 2016) and parent-reported social problems (Fareri et al., 2017) in adolescence and young adulthood; and 3) NAcc functional connectivity with social cognition brain regions (i.e., medial prefrontal cortex) is related to social interactions that depend on familiarity (i.e., playing with a friend, stranger or computer) in young adulthood (Fareri and Delgado, 2014). Moreover, this study examined whether these neural correlates were associated with real-world positive experiences in social contexts, measured with ecological momentary assessment (EMA).
1.1. Hypotheses
-
1)
As supported by the peer socialization model (Rose and Rudolph, 2006), we hypothesized that in real-life, unconstrained contexts, females would indicate that a greater proportion of their positive social experiences would be those in which a single friend was present, while males would report having a greater proportion of positive experiences when with several friends or class/teammates (gender-by-relationship).
-
2)Based on research showing adolescent females display higher sensitivity to emotional faces than males (Lee et al., 2013) and studies showing facial familiarity elicits activation of a face processing network (Gobbini and Haxby, 2007; Ambrosia et al., 2018), we hypothesized:
-
afemales and males would show comparable neural activation during face processing, collapsing across stimuli familiarity and valence (main effect of gender);
-
bcompared to males, females would display greater activation of the face processing network and the ventral striatum when processing the face of a BF, collapsing stimuli valence (gender-by-familiarity);
-
ccompared to males, females would display greater activation of the face processing network and the ventral striatum when processing positive facial expressions, collapsing stimuli familiarity (gender-by-valence); and
-
dcompared to males, females would display greater activation of the face processing network and the ventral striatum when processing a BF expressing positive affect (gender-by-valence-by-familiarity).
-
a
-
3)
Based on research showing that adolescent females may be more sensitive to social reward than males (Guyer et al., 2009; Guyer et al., 2014; Guyer et al., 2012), and that social reward is processed by ventral striatum and social cognition brain regions – e.g., (Healey et al., 2014; Davey et al., 2010) – we hypothesized that females would show greater functional connectivity than males between ventral striatum and social cognition brain areas (e.g., pSTS, medial prefrontal cortex) while processing their BF express positive affect.
Finally, an exploratory analysis was planned to test the hypothesis that gender differences in positive experiences in social contexts (as identified in Hypothesis 1) would be mediated by gender differences in functional connectivity between ventral striatum and social cognition brain regions.
2. Materials and methods
2.1. Participants
Fifty-two typically developing adolescents between the ages of 14 and 18 years old (female = 30) completed a novel, naturalistic functional magnetic resonance imaging (fMRI) task measuring personally relevant social reward in which they watched and listened to a BF express positive affect. Adolescents were recruited from the Pittsburgh, Pennsylvania metropolitan area using fliers in community settings and ads on Craigslist and other internet sources. Participants were generally healthy, free of current or past neuropsychiatric disorders and not taking psychotropic medication. Additional exclusionary criteria included MRI contraindications: claustrophobia, obesity, dental braces, metallic implants or other forms of metal in the body and pregnancy. Of 70 participants originally enrolled in the study, 7 did not complete MRI scanning due to ineligibility (i.e., recent concussion, n = 3; claustrophobia, n = 2; history of mental illness, n = 2). Three participants could not be contacted after the initial visit and two refused to be scanned. Of the remaining 58 participants, 3 did not complete the fMRI task because of technical difficulties and 3 others had missing behavioral data (i.e., could not confirm attention/alertness during the task) because of a coding error.
A parent or legal guardian and the participant provided informed consent and assent, respectively, at the time of laboratory assessment. Participants identified a same-sex, similar-aged best friend who could be invited to participate in the study with them. Participants’ friends were included if they had no current or past neuropsychiatric disorders and no current psychotropic medication. If eligible, friends and their parent or legal guardian provided verbal consent (over the telephone) to participate. All procedures were approved by the University of Pittsburgh Institutional Review Board.
Males and females did not differ in age (males = 16.10 ± 1.51 years; females = 16.23 ± 1.41 years; t49 = −0.33, p = 0.74), race (χ2(2) = 2.52, p = 0.11) or parent education (χ2(5) = 257.50, p = 0.25). The racial distribution of the sample was white (69 %), black (22 %) and biracial/multiracial (10 %). The parents of most of the sample had either a master/doctoral degree (35 %) or college degree (31 %); smaller percentages of parents only completed high school/GED (18 %), technical school (8%), some college (6%) or primary school (2%).
2.2. Procedure
Participants completed a 3-part protocol: 1) a laboratory assessment, including questionnaires and a peer interaction task, 2) an fMRI assessment of neural response to social reward and 3) ecological monetary assessment (EMA) of social experiences for two 5-day intervals.
2.2.1. Laboratory assessment
Participants completed a peer interaction task in the laboratory where they discussed fun and exciting times shared with a best friend who accompanied them to the laboratory. The task, which was developed based on previous work (Sheeber et al., 2007), involved a 10-minute interaction with the participant’s friend that was video recorded. Participants and their friends were instructed to talk about “the most fun you’ve ever had together” (past conversation) for 5 min and “a fun or exciting event you’d like to plan together” (future conversation) for the remaining 5 min. Topics were selected based on participants’ responses to a list of pleasant events (e.g., amusement park visit, soccer camp).
2.2.2. fMRI assessment: best friend task
This innovative paradigm is an adaptation of a family affect fMRI paradigm (Morgan et al., 2015; Whittle et al., 2009) that includes peers rather than family and assesses neural response to positive and neutral affect expressed by a BF and UP. Stimuli were created from video clips of the peer interaction task that included the participants’ friend (BF condition) or an actor (UP condition) and audio from both adolescents. Video clips from dyadic interactions of adolescent actors from Eugene, Oregon were used as UP stimuli to minimize the possibility that participants would recognize the UP. Actors in the video clips were also friends. The same procedures were used to create BF and UP stimuli. All stimuli included the entire head and upper torso, with gaze directed off-camera towards the target participant. Efforts were made to ensure video clips had equivalent lighting, camera angle, zoom and intensity of affect. Videos were coded in 5-s epochs by a team of trained observers using a modified AFFEX coding system (Ambrosia et al., 2018; Izard et al., 1983). Positive affect was coded with scores of 0–2 based on presence and intensity, whereas neutral affect was coded 0–1 based on whether or not it was present. From these videos, 20-s segments were selected based on the predominance of positive or neutral affect. Both BF and UP positive affect video segments depicted higher levels of positive affect than neutral affect videos. Conversely, both BF and UP neutral affect video segments depicted higher levels of neutral affect than the positive affect videos (Ambrosia et al., 2018). Moreover, positive affect clips tended to be from the past conversation (66 %), while neutral clips tended to be from the future conversation (60 %) (Ambrosia et al., 2018). Approximately 25 % of the videotapes were also coded by an extensively trained master coder for reliability (positive affect mean interclass correlation coefficient [ICC] = 0.93, range = 0.86−0.96; neutral affect mean ICC = 0.92; range = 0.89−0.94).
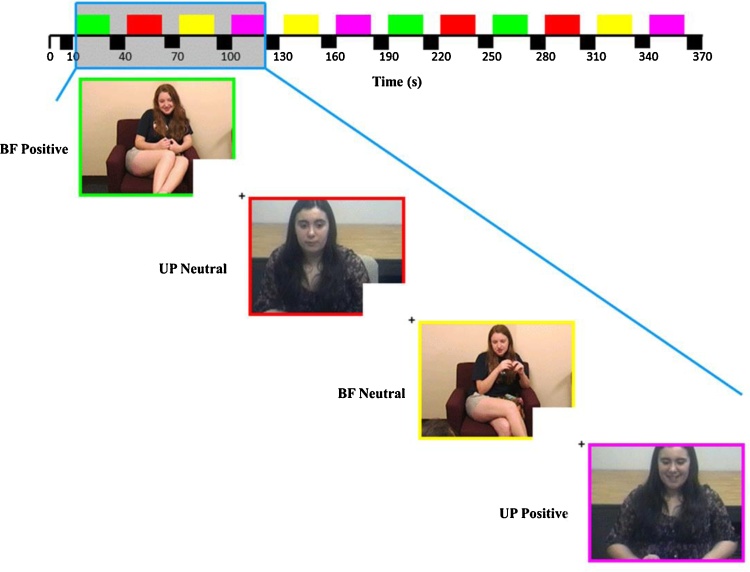
Stimuli were presented in a block design with six blocks of each affect (i.e., positive and neutral) alternating between BF and UP (i.e., familiarity) conditions, such that each possible familiarity-affect combination (i.e., BF-Positive, BF-Neutral, UP-Positive, UP-Neutral) was presented three times. The order of block presentation was fixed so that each of the four familiarity-affect combinations was presented before showing a combination for a second and third time. A total of twelve 20-s blocks separated by 10-s inter-block fixation intervals were presented (6 min total). Participants were asked to make a button press to acknowledge the start of each clip to confirm attention was directed toward the stimuli and to try remembering what they were talking about during the clip (Fig. 1).
Fig. 1.
The Best Friend Task. Colors denote type of stimuli presented during each block (20 s). BF = best friend; UP = unfamiliar peer.
A published study from our laboratory using the same fMRI task and an overlapping sample of participants (n = 50) compared neural activation during BF-Positive > BF-Neutral and found significant activation in ventrolateral/dorsomedial prefrontal cortex, middle/superior temporal gyrus, anterior insula and fusiform gyrus (Ambrosia et al., 2018). No differences were detected for the UP-Positive > UP-Neutral contrast; however, the BF-Positive > UP-Positive contrast elicited significant activation in the ventrolateral prefrontal cortex, superior temporal gyrus and inferior frontal gyrus (Ambrosia et al., 2018).
2.2.3. Ecological momentary assessment (EMA)
The EMA procedure was based on an established EMA protocol (Axelson et al., 2003; Silk et al., 2003). Participants were called during two 5-day intervals that included the weekend (Thursday-Monday); they received 14 calls at specified times over each of the 5-day periods (2 calls on Thursdays, Fridays and Mondays; 4 calls on Saturdays and Sundays). Each call lasted approximately 4 min and did not take place during school hours. Participants answered scripted questions about their mood, current activities and companions; responses were coded by callers trained to reliability (κ≥0.80). Due to the aims of the present study, the analysis of positive social experiences, as reported with EMA, was focused on the participants’ responses to the following questions: 1) “Think about the most enjoyable or happy time in the past hour. Was anyone with you?” (coded as 0=no or 1=yes) and 2) “If yes, who was with you?” (coded as 1=non-romantic friend, 2=romantic friend, 3=several friends, 4=classmates/teammates, 5=mother/step-mother, 6=father/step-father, 7=siblings/step-siblings, 8=grandparents, 9=child relative, 10=adult relative, 11=family (general), 12=acquaintance, 13=family member’s friend, 14=co-worker, 15=counselor or 16=unclear). This variable captured adolescent enjoyment of various social configurations.
2.3. Imaging data acquisition and preprocessing
Structural and functional images were acquired with a Siemens 3 T Tim Trio scanner at the University of Pittsburgh Magnetic Resonance Research Center. Functional blood oxygen level dependent (BOLD) images were acquired during the Best Friend task in the axial plane using a gradient echo planar imaging sequence oriented along the anterior commissure-posterior commissure line (TR/TE = 2000 ms/30 ms, FOV = 205 × 205, matrix = 64 × 64, slices = 39, slice thickness = 3.1 mm, repetitions = 240). Structural images were acquired for co-registration with individual subjects’ functional images using a T1-weighted high-resolution MPRAGE sequence (TR/TE/flip angle = 2300 ms/2.98 ms/9°; FOV = 256 × 240; matrix = 256 × 240; slices = 160; slice thickness = 1.2 mm).
Image preprocessing was conducted with Statistical Parametric Mapping 12 (SPM12; http://www.fil.ion.ucl.ac.uk/spm). Briefly, preprocessing included realignment (six-parameter rigid body motion correction), co-registration of each subject’s own structural image to functional data, segmentation of structural image by tissue type (e.g., white matter, grey matter), spatial normalization into Montreal Neurological Institute (MNI) template space using the deformations resulting from segmentation, smoothing with a 6-mm full-width half-maximum Gaussian kernel and high-pass temporal filtering (128 s). Motion across functional volumes was corrected with Artifact Detection Tools (ART; http://www.nitrc.org/projects/artifact_detect); participants with high motion (>25 % of functional volumes had >2 mm of movement from adjacent volume) were excluded from analyses.
Subject-level functional data were modeled with the general linear model (GLM) using a canonical hemodynamic response function. Separate regressors for each condition (i.e., BF-Positive, BF-Neutral, UP-Positive, UP-Neutral), including baseline fixation, were included in the GLM (condition onset and duration) to estimate BOLD signal for each condition. The four condition contrasts were then submitted to a group-level analysis (2.4.3 Functional activation).
2.4. Statistical analysis
2.4.1. Demographics
Analyses used SPSS 24 (IBM Corp.), with log transform for variables with skew and kurtosis (>2.0). Independent samples t-tests or chi-square analysis tested gender effects.
2.4.2. EMA
Six individuals (males = 5, females = 1) opted not to complete EMA procedures, which reduced the sample to 45 youth (males = 16, females = 29). Of those who responded, the number of total calls completed varied from 4 to 27 (average = 17.11, median = 18, mode = 19) out of 28 total possible calls across participants. Youth primarily reported being with a mother or step-mother (116 occasions), followed by a non-romantic friend (98 occasions), a sibling/step-sibling (62 occasions), family in general (62 occasions), several friends (56 occasions), a romantic friend (24 occasions), a father/step-father (23 occasions), a child relative (17 occasions), classmates/teammates (14 occasions), grandparents (9 occasions), an adult relative (7 occasions), an acquaintance (4 occasions), a co-worker (4 occasions), a counselor/therapist (2 occasions), unclear (2 occasions) and a family member’s friend (1 occasion), for a total of 501 occasions. Youth also reported being alone when most happy in the previous hour on 255 occasions.
To account for variation in response rates, for each participant, the proportion of positive experiences during social contexts was calculated for each relationship type (e.g., occasions with non-romantic friend/total occasions with someone). To further account for variation in response rates for each relationship type and increase data normality, only variables with a continuous range of values for both males and females were included in analyses: 1) non-romantic friend, 2) several friends, 3) class/teammates, 4) mother/step-mother, 5) father/step-father, 6)_sibling/step-sibling and 7) family in general. Proportions of positive experiences with each relationship type are reported by gender and age in Table 1. Gender differences in the proportion of occasions participants were with someone when most happy in the previous hour was examined for each relationship type (covarying for age) with a multivariate analysis of covariance (MANCOVA). Greenhouse-Geisser correction was applied when sphericity assumptions were violated.
Table 1.
Mean (Standard Deviation) Proportion of Self-Reported Most Positive Experiences in the Past Hour by Gender and Age.
| Gender |
Age |
||||||
|---|---|---|---|---|---|---|---|
| Male | Female | 14 | 15 | 16 | 17 | 18 | |
| Friend, non-romantica | 0.23 (0.29) | 0.35 (0.38) | 0.25 (0.18) | 0.11 (0.15) | 0.31 (0.37) | 0.30 (0.20) | 0.49 (0.53) |
| Friend, romantic | 0.03 (0.08) | 0.08 (0.21) | 0 | 0 | 0.08 (0.26) | 0.06 (0.15) | 0.13 (0.20) |
| Several friendsa | 0.22 (0.11) | 0.15 (0.20) | 0.19 (0.20) | 0.07 (0.13) | 0.13 (0.24) | 0.11 (0.09) | 0.32 (0.35) |
| Class/teammatesa | 0.11 (0.17) | 0.02 (0.06) | 0.07 (0.09) | 0.14 (0.22) | 0.01 (0.04) | 0.04 (0.08) | 0.01 (0.04) |
| Mother/step-mothera | 0.41 (0.36) | 0.39 (0.37) | 0.61 (0.27) | 0.60 (0.36) | 0.36 (0.94) | 0.25 (0.22) | 0.23 (0.35) |
| Father/step-fathera | 0.28 (0.75) | 0.07 (0.13) | 0.16 (0.22) | 0.04 (0.12) | 0.35 (0.94) | 0.07 (0.15) | 0.06 (0.14) |
| Sibling/step-siblinga | 0.21 (0.25) | 0.23 (0.27) | 0.28 (0.23) | 0.25 (0.24) | 0.28 (0.35) | 0.15 (0.11) | 0.15 (0.27) |
| Grandparent | 0.08 (0.22) | 0.02 (0.06) | 0.14 (0.26) | 0 | 0.08 (0.17) | 0 | 0 |
| Relative, child | 0 | 0.08 (0.17) | 0.03 (0.07) | 0.08 (0.15) | 0.06 (0.20) | 0.07 (0.19) | 0.03 (0.08) |
| Relative, adult | 0.01 (0.05) | 0.03 (0.07) | 0 | 0.05 (0.10) | 0.04 (0.10) | 0.01 (0.04) | 0 |
| Family (general)a | 0.24 (0.29) | 0.29 (0.45) | 0.11 (0.14) | 0.26 (0.28) | 0.31 (0.41) | 0.35 (0.32) | 0.31 (0.60) |
| Acquaintance | 0 | 0.01 (0.04) | 0.03 (0.07) | 0 | 0.01 (0.03) | 0 | 0 |
| Family friend | 0 | 0.005 (0.03) | 0 | 0 | 0 | 0.02 (0.05) | 0 |
| Co-worker | 0.005 (0.02) | 0.01 (0.06) | 0 | 0.01 (0.03) | 0.03 (0.09) | 0.01 (0.04) | 0 |
| Counselor | 0.006 (0.02) | 0.003 (0.02) | 0.01 (0.03) | 0 | 0 | 0 | 0.008 (0.03) |
| Unclear | 0.005 (0.02) | 0.003 (0.02) | 0.01 (0.03) | 0 | 0 | 0 | 0.007 (0.02) |
Used in primary analyses due to sufficient distribution of values needed for parametric analysis.
Happiness during those occasions when most happy in the previous hour was quantified by asking youth, “At the best point, how happy did you feel?” on a 5-point Likert scale (1 = not at all; 5 = extremely). Average happiness was calculated for occasions when youth were alone, with specific types of relationships – non-romantic friend, several friends, class/teammates, mother/step-mother, father/step-father, sibling/step-sibling and family in general – and with someone (any relationship type).
2.4.3. Functional activation
Neural response to positive affect expressed by a BF was examined with a full factorial group-level analysis with one between-subject factor – gender (male and female) – two within-subject factors – familiarity (BF and UP) and valence (positive and neutral) – and a covariate of no interest (age) in SPM12. Whole-brain results were thresholded at a voxel-height p < 0.0001 (uncorrected) and cluster-extent threshold of p < 0.05 with family-wise error (FWE) correction for multiple comparisons.
2.4.4. Functional connectivity
A psychophysiological interaction (PPI) analysis was employed to test condition effects on task-dependent ventral striatal functional coupling. The ventral striatal seed region was defined as bilateral NAcc using the WFU_PickAtlas (Maldjian et al., 2003). For each participant, BOLD signal time courses from the conditions of interest were extracted from the bilateral NAcc and convolved with the contrast of interest – BF-Positive > UP-Positive – resulting in PPI activation maps. A whole-brain multiple regression with NAcc functional connectivity was conducted with the following regressors: age, gender, EMA values and gender-by-EMA values. Only EMA values that differed significantly by gender (i.e., proportion of positive experiences with class/teammates; see 3.1 EMA (Hypothesis 1)) were included in this multiple regression analysis. Whole-brain results were thresholded at a voxel-height of p < 0.0001 (uncorrected) and cluster-extent threshold of p < 0.05 with FWE correction for multiple comparisons.
2.4.5. Exploratory mediation analysis
As an exploratory analysis, a statistical mediation analysis was pursued to test whether gender differences in EMA values were mediated by NAcc functional connectivity during BF-Positive > UP-Positive. Using the PROCESS macro version 3 (Hayes, 2013) in SPSS, statistical significance was determined using 95th percentile bootstrapping confidence intervals (5000 samples).
3. Results
On average, when asked to report the happiest they felt in the past hour, youth reported being with someone (0.63) versus alone (0.37) proportional to the number of total times reported (t44 = 4.68, p < 0.001). Youth also reported being happier with someone (4.06 ± 0.49) than when alone (3.50 ± 0.62; t41 = 5.91, p < 0.001).
3.1. EMA (Hypothesis 1)
Consistent with our first hypothesis, a significant multivariate effect of gender emerged (F(7, 35) = 2.35, p = 0.04, partial η2 = 0.32), such that females reported a smaller proportion of positive experiences with class/teammates than males (F(1, 41) = 9.03, p = 0.005, partial η2 = 0.18; Table 1).
Furthermore, we observed a significant multivariate effect of age (F(7, 35) = 2.81, p = 0.02, partial η2 = 0.36), such that the proportion of positive experiences reported with a non-romantic friend (F(1, 41) = 4.06, p = 0.05, partial η2 = 0.09; Table 1) increased with age, whereas the proportion of positive experiences reported with a mother/step-mother decreased with age (F(1, 41) = 8.90, p = 0.005, partial η2 = 0.18; Table 1).
Compared to being alone (3.50 ± 0.62), youth reported feeling happier when with a non-romantic friend (4.30 ± 0.55; t31 = 6.40, p < 0.001, Bonferroni correction), family (general) (4.10 ± 0.70; t23 = 3.84, p = 0.001, Bonferroni correction) and several friends (4.25 ± 0.50; t21 = 4.62, p < 0.001, Bonferroni correction). Youth also reported feeling happier being with several friends (4.36 ± 0.44) compared to siblings/step-siblings (3.86 ± 0.52; t13 = 4.18, p = 0.001, Bonferroni correction). All other comparisons were not significantly different. Males and females did not differ significantly in their reports of happiness when alone or with someone (overall or examined by relationship type): all F ≤ 4.17, p ≥ 0.05 (Bonferroni correction).
3.2. Functional activation
Data were excluded from one (male) subject for excessive motion (>25 % of volumes with motion exceeding 2 mm) during the Best Friend task. Results that did not directly involve gender differences (i.e., main effect of familiarity [Figure S1; Table S1], main effect of valence [Figure S2; Table S1] and familiarity-by-valence interaction; Table S1) are reported in Supplemental Information.
3.2.1. Main effects of gender (Hypothesis 2a)
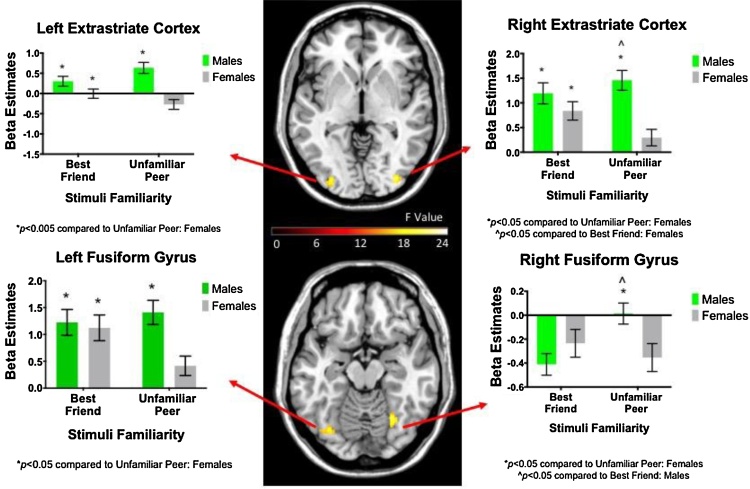
Inconsistent with our hypothesis of comparable neural reactivity in overall face processing between males and females, bilateral fusiform gyrus (FG; extending to inferior occipital gyrus) activation was significantly more robust for males than females, across stimuli familiarity and valence (Table 2).
Table 2.
Gender-Related Task Activation Results.
| Peak Brain Region | Peak MNI Coordinate (X, Y, Z) | Cluster Size (Voxels) | Peak | Direction of Effects |
|---|---|---|---|---|
| F(1, 194) Value | ||||
| Main Effect of Gender (Hypothesis 2a) | ||||
| Fusiform Gyrus (L) | −40, −80, −14 | 264 | 43.13 | M > F |
| Fusiform Gyrus (R) | 32, −90, −8 | 370 | 31.97 | M > F |
| Gender-by-Familiarity (Hypothesis 2b) | ||||
| Fusiform Gyrus (L) | −42, −72, −18 | 95 | 24.57 | UP: M > F |
| BF: M = F | ||||
| Fusiform Gyrus (R) | 28, −64, −10 | 80 | 22.36 | UP: M > F |
| BF: M = F | ||||
| Extrastriate Cortex (R) | 34, −86, 0 | 58 | 21.96 | UP: M > F |
| BF: M = F | ||||
| Extrastriate Cortex (L) | −32, −94, −6 | 74 | 21.47 | UP: M > F |
| BF: M = F | ||||
MNI = Montreal Neurological Institute; L = left; R = right; M = Male; F = Female; BF = Best Friend; UP = Unfamiliar Peer.
3.2.2. Gender-by-familiarity (Hypothesis 2b)
Significant interactions between gender and stimuli familiarity were found in bilateral FG and extrastriate cortices (part of general face processing network (Haxby et al., 2000)). In all cases, males showed greater activation than females while processing a UP, whereas males and females showed comparable activation during BF conditions (Table 2; Fig. 2). Notably, females displayed the expected neural activation pattern (BF > UP; left fusiform gyrus and bilateral extrastriate cortex), whereas males did not differentiate between conditions (with the exception of right fusiform gyrus activation, in which males showed the reverse pattern), providing partial support for our hypothesis that females would display greater activation of the face processing network than males when processing the face of a BF versus UP (Table 2; Fig. 2).
Fig. 2.
Gender-by-Familiarity Effect. Males displayed greater activation of bilateral fusiform gyrus and extrastriate cortex than females when viewing an unfamiliar peer (UP), regardless of stimuli valence. Females displayed greater activation these brain regions during Best Friend (BF) versus UP conditions, whereas males did not differentiate between conditions, with the exception of right fusiform gyrus in the opposite direction (UP > BF).
3.2.3. Gender-by-valence (Hypothesis 2c)
Contrary to our hypothesis that females would display greater activation of the face processing network and the ventral striatum than males when processing positive facial expressions, the gender-by-valence interaction did not reach statistical significance.
3.2.4. Gender-by-familiarity-by-valence (Hypothesis 2d)
The interaction of gender, familiarity and valence did not reach statistical significance, which stands in contrast with our hypothesis that females would display greater activation of the face processing network and the ventral striatum than males when processing a BF express positive affect.
3.3. Functional connectivity (Hypothesis 3)
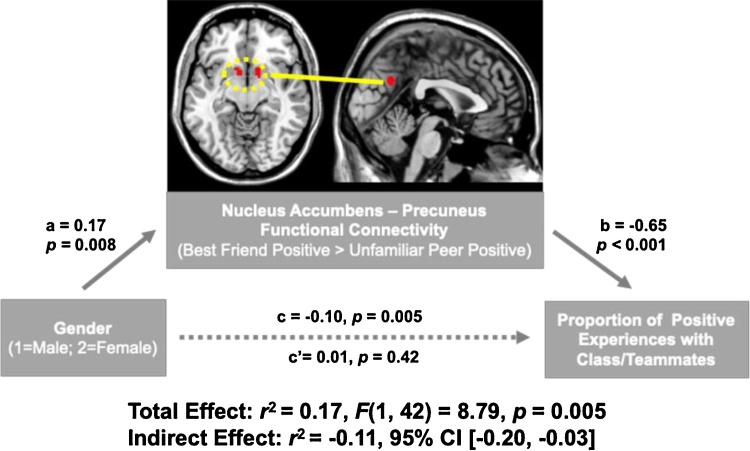
A whole-brain multiple regression predicting NAcc functional connectivity during BF-Positive > UP-Positive conditions included age, gender, EMA values (i.e., proportion of positive experiences with class/teammates) and gender-by-EMA values as regressors. In support of our hypothesis, NAcc functional connectivity with precuneus (MNI coordinates [x, y, z]: 0, -62, 30, k = 36, t37 = 5.00) was significantly higher for females than males. The other regressors – age, EMA values and the interaction of gender and EMA values – were not associated with NAcc functional connectivity.
3.4. Exploratory mediation analysis (Hypothesis 4)
A statistical mediation analysis was pursued to test if gender differences in NAcc functional connectivity with precuneus statistically mediated gender differences in the proportion of positive experiences with class/teammates. The direct effect of gender on the proportion of positive experiences with class/teammates (ß = −0.10, p = 0.005) became non-significant when NAcc-precuneus functional connectivity was introduced as a mediator (ß = 0.01, p = 0.42). The indirect effect of NAcc-precuneus functional connectivity was significant (ß = −0.11, 95 % CI [−0.20, −0.03]), indicating that connectivity between these brain regions explained the higher the proportion of positive experiences with class/teammates in males compared to females (Fig. 3).
Fig. 3.
Mediation Analysis. Nucleus accumbens functional connectivity with precuneus during Best Friend-Positive > Unfamiliar Peer-Positive conditions, which was significantly higher in females than males, mediated gender differences in the proportion of positive experiences with class/teammates.
4. Discussion
Results of this investigation indicate adolescent gender differences in real-world positive experiences in social contexts and neural processing of dynamic stimuli of a BF and UP. Furthermore, gender differences in functional connectivity of NAcc with precuneus statistically mediated gender differences in real-world positive experiences in a social context, such that girls’ greater functional connectivity explained the smaller proportion of positive experiences reported with class/teammates, relative to boys. Below we discuss how these findings may suggest that positive experiences with peers may be supported through the interaction of reward and social cognition systems.
Hypothesis 1 was partially substantiated, as males reported a higher proportion of positive experiences with class/teammates compared to females. Conversely, females did not report a greater proportion of positive experiences with a single friend. The former finding is anchored by a large body of research showing distinct friendship styles in adolescent males and females that begin in childhood (Rose and Rudolph, 2006). Females tend to engage in longer bouts of dyadic interactions, whereas males are more likely to engage with larger friend groups (Flynn et al., 2017) in competitive settings (Deaner et al., 2012), such as in sports teams and classrooms. Although the proportion of positive experiences reported with class/teammates was only 2.8 % of total social experiences, the effect size for this finding ranged from medium to large (partial η2 = 0.18). Moreover, reported happiness with class/teammates was rated highly on average (4.25; 1 = not at all happy - 5 = extremely happy), indicating that engagement with this peer group was largely positive.
Although not hypothesized (Hypothesis 2a), main effects of gender indicated that males activated brain regions of the visual processing network – bilateral fusiform gyrus – to a larger extent than females, regardless of stimuli familiarity or valence. This finding may have been driven by a greater than anticipated neural reactivity to UP by males (Hypothesis 2b). Indeed, although females displayed the expected response pattern in the visual processing network (i.e., bilateral fusiform gyrus and extrastriate cortex) – greater reactivity to BF versus UP stimuli – males did not clearly differentiate between stimuli and when they did, was in the opposite direction (UP > BF; right fusiform gyrus). Notably, gender differences in neural activation did not depend on stimuli valence (Hypothesis 2c) or interactions with stimuli familiarity and valence (Hypothesis 2d), standing in contrast to our hypotheses and suggesting that the emotional valence of peer faces, even when considering familiarity with peers, did not play a large role in differentiating neural reactivity of males and females.
The network of brain regions that support face processing, including fusiform gyrus and extrastriate cortex, overlap extensively with regions supporting social information processing more broadly, such that the familiar face processing network (Gobbini and Haxby, 2007) has been described as a part of the social information processing network (SIPN) (Nelson et al., 2005); for a review, see (Scherf et al., 2012). The SIPN is postulated to include three processing nodes – detection (FG, STS, extrastriate cortex, anterior temporal pole), affective (amygdala, hypothalamus, NAcc) and cognitive regulation (dorsomedial PFC, ventrolateral PFC) – supporting a developmental social re-orientation towards peers during adolescence. Thus, detection of unfamiliar males may be more salient to adolescent males than a male BF, whereas female BFs may be more affectively salient to adolescent females than unfamiliar females, regardless of valence. This gender difference may be due to distinct social motivational aspects of facial and/or vocal familiarity in males and females.
From an evolutionary perspective, detection of unfamiliar versus familiar males may be more adaptive in males, as they may be perceived as a threat, regardless of facial expression (Neuberg and Schaller, 2015). In adults, research shows that evolutionary threatening stimuli (e.g., predators) evoke greater activation in FG than modern threatening stimuli (e.g., car accident), even though a higher perceived threat is reported for modern threatening stimuli. This suggests that neural reactivity to threat is partially driven by the evolutionary relevance of the stimulus (Dhum et al., 2017). In contrast with our findings, FG activation while viewing faces of learned in-group versus out-group members increases with age across adolescence (Guassi Moreira et al., 2017), and adults also display greater FG activation while viewing (racial) in-group versus out-group members (Van Bavel et al., 2008). However, these studies included facial stimuli from both sexes and did not examine gender differences directly. Thus, FG reactivity to facial stimuli may be more nuanced than the distinction between in- and out-group or familiar and unfamiliar stimuli and requires the additional consideration of same- versus other-sex stimuli to fully understand the social motivational underpinnings of FG reactivity in adolescence.
Our third hypothesis was supported by the finding that females showed greater NAcc-precuneus functional connectivity while processing a BF versus UP express positive affect. The precuneus is implicated in autobiographical memory and social cognition, specifically as it pertains to self-processing and perspective taking (Cavanna and Trimble, 2006). The precuneus does not share projections with NAcc, however, it shares structural connections with dorsal striatum (Cavanna and Trimble, 2006), which shifts attentional resources toward memory retrieval of motivationally salient stimuli such as a BF expressing happiness. Therefore, the rewarding properties of viewing/listening to a BF display positive affect may be related, in part, to successful retrieval of memories encoded during the initial interaction with the BF. Additionally, the reward processing of a BF expressing positive affect could reflect the degree to which adolescents, specifically females, experience high self-other resonance with their BF, as ventral tegmental area (primary mesolimbic projection site to NAcc) and precuneus co-activation is associated with self-other resonance (Cacioppo et al., 2017). Self-other resonance has been shown to influence behavior in competitive settings (Wittmann et al., 2016); thus, it is possible that weaker NAcc-precuneus functional connectivity, reflecting weaker self-other resonance, facilitated (or is linked to) male interactions with larger peer groups where competition was more likely (i.e., classroom or sports arena). Indeed, results from our exploratory analysis showing that the larger proportion of positive experiences with class/teammates reported by males was mediated by weaker NAcc-precuneus functional connectivity – provide some support for this interpretation. Although sometimes associated with negative outcomes (Randall et al., 2019), competition in adolescent peer relationships is not necessarily negative and may reflect positive individual characteristics (e.g., leadership, generosity) among athletes (Agans et al., 2017) or greater subjective wellbeing in school (Tian et al., 2017).
Utilization of naturalistic and personalized stimuli during brain imaging and EMA to measure real-world social experiences greatly enhanced the ecological validity of the current study. However, there are some limitations and caveats to be mindful of when interpreting the outcomes of this investigation. First, we could not account for variation in degree of closeness or friendship quality between the participants and their BF, as these measures were not collected. Moreover, we did not have information about the participants’ degree of closeness with the individual(s) they reported being with when they were most happy in the previous hour. Second, our coding system for the peer interaction task yielded a general construct for positive affect but did not differentiate more subtle aspects of positive affect, such as excitement or affection, which may be related to distinct aspects of social interactions with peers. Thirdly, although our EMA variables appear to reflect positive social experiences (as characterized by subject self-report), we cannot make assumptions about the participants’ degree of agency since some youth have less freedom in selecting social experiences (e.g., family commitments). Fourthly, the findings of the mediation analysis should be considered tentative and interpreted with caution since EMA response rates were limited for this particular variable, the effect size of the mediation (indirect) effect was small (r2 = −0.11) and competitive behavior was not assessed directly. Other interpretations should also be considered (e.g., autobiographical memory retrieval). Finally, the distinction between BF and UP conditions of the task may be confounded by episodic memory given that BF task stimuli were based on a shared experience between the participant and BF, whereas the UP stimuli were completely novel to the participant.
5. Conclusions
These findings suggest that adolescent gender differences in real-world positive experiences with peers may be associated with differential patterns of neural functional connectivity in reward and social-cognitive circuitry. Specifically, the interaction between reward and social cognition systems, as reflected by NAcc-precuneus functional connectivity, may contribute to gender differences in social experiences (i.e., engaging with larger friend groups in competitive settings). Gender differences in peer relationship processes may affect emotional and behavioral development and may therefore elucidate some of the basic mechanisms supporting social behaviors during adolescence and into adulthood. This study especially highlights the importance of examining brain-behavior interactions when investigating gender differences in social development.
Funding
This research was supported by funding from the National Institutes of Health (grant numbers DA033612, MH018951). Funding sources had no role in study design, data collection, analysis and interpretation, writing this report or the decision to publish this manuscript.
Declaration of Competing Interest
None.
Acknowledgement
Members of the Affective Neuroscience & Developmental Psychopathology Lab are thanked for their efforts with data collection and preprocessing.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.dcn.2020.100779.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Agans J.P., Johnson S.K., Lerner R.M. Adolescent athletic participation patterns and self-perceived competence: associations with later participation, depressive symptoms, and health. J. Res. Adolesc. 2017;27(3):594–610. doi: 10.1111/jora.12301. [DOI] [PubMed] [Google Scholar]
- Alarcon G., Cservenka A., Nagel B.J. Adolescent neural response to reward is related to participant sex and task motivation. Brain Cogn. 2017;111:51–62. doi: 10.1016/j.bandc.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosia M. Temptations of friends: adolescents’ neural and behavioral response to best friends predicts risky behavior. Soc. Cogn. Affect. Dev. 2018 doi: 10.1093/scan/nsy028. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelson D.A. Measuring mood and complex behavior in natural environments: use of ecological momentary assessment in pediatric affective disorders. J. Child Adolesc. Psychopharmacol. 2003;13(3):253–266. doi: 10.1089/104454603322572589. [DOI] [PubMed] [Google Scholar]
- Cacioppo S., Juan E., Monteleone G. Predicting intentions of a familiar significant other beyond the mirror neuron system. Front. Behav. Neurosci. 2017;11:155. doi: 10.3389/fnbeh.2017.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Getz S., Galván A. The adolescent brain. Dev. Rev. 2008;28(1):62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna A.E., Trimble M.R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chein J. Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Dev. Sci. 2011;14(2):F1–10. doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P.A. Brain reward activity to masked in-group smiling faces predicts friendship development. Soc. Psychol. Personal. Sci. 2015;8(4):415–421. doi: 10.1177/1948550614566093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey C.G. Being liked activates primary reward and midline self-related brain regions. Hum. Brain Mapp. 2010;31(4):660–668. doi: 10.1002/hbm.20895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaner R.O. A sex difference in the predisposition for physical competition: males play sports much more than females even in the contemporary U.S. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0049168. p. e49168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhum M. Evolutionary and modern image content differentially influence the processing of emotional pictures. Front. Hum. Neurosci. 2017;11:415. doi: 10.3389/fnhum.2017.00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri D.S., Delgado M.R. Differential reward responses during competition against in- and out-of-network others. Soc. Cogn. Affect. Neurosci. 2014;9(4):412–420. doi: 10.1093/scan/nst006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri D.S. Altered ventral striatal-medial prefrontal cortex resting-state connectivity mediates adolescent social problems after early institutional care. Dev. Psychopathol. 2017;29(5):1865–1876. doi: 10.1017/S0954579417001456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn H.K., Felmlee D.H., Conger R.D. The social context of adolescent friendships: parents, peers, and romantic partners. Youth Soc. 2017;49(5):679–705. [Google Scholar]
- Gobbini M.I., Haxby J.V. Neural systems for recognition of familiar faces. Neuropsychologia. 2007;45(1):32–41. doi: 10.1016/j.neuropsychologia.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Guassi Moreira J.F., Van Bavel J.J., Telzer E.H. The neural development of’ us and them’. Soc. Cogn. Affect. Neurosci. 2017;12(2):184–196. doi: 10.1093/scan/nsw134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther Moor B. Do you like me? Neural correlates of social evaluation and developmental trajectories. Soc. Neurosci. 2010;5(5–6):461–482. doi: 10.1080/17470910903526155. [DOI] [PubMed] [Google Scholar]
- Guyer A.E. Probing the neural correlates of anticipated peer evaluation in adolescence. Child Dev. 2009;80(4):1000–1015. doi: 10.1111/j.1467-8624.2009.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A.E. Will they like me? Adolescents’ emotional responses to peer evaluation. Int. J. Behav. Dev. 2014;38(2):155–163. doi: 10.1177/0165025413515627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A.E. Neural circuitry underlying affective response to peer feedback in adolescence. Soc. Cogn. Affect. Neurosci. 2012;7(1):81–92. doi: 10.1093/scan/nsr043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A.E. A developmental examination of amygdala response to facial expressions. J. Cogn. Neurosci. 2008;20(9):1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby J.V., Hoffman E.A., Gobbini M.I. The distributed human neural system for face perception. Trends Cogn. Sci. 2000;4(6):223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Hayes A.F. Methodology in the Social Sciences. 1 ed. The Guilford Press; New York: 2013. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. [Google Scholar]
- Healey K.L. Social anhedonia and medial prefrontal response to mutual liking in late adolescents. Brain Cogn. 2014;89:39–50. doi: 10.1016/j.bandc.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izard C.E., Marcus M., Campbell S.B. University of Delaware: Instructional Resources Center; Newark: 1983. A System for Identifying Affect Expressions by Holistic Judgments (Affex) [Google Scholar]
- Kosaka H. Neural substrates participating in acquisition of facial familiarity: an fMRI study. Neuroimage. 2003;20(3):1734–1742. doi: 10.1016/s1053-8119(03)00447-6. [DOI] [PubMed] [Google Scholar]
- Lee N.C. Do you see what I see? Sex differences in the discrimination of facial emotions during adolescence. Emotion. 2013;13(6):1030–1040. doi: 10.1037/a0033560. [DOI] [PubMed] [Google Scholar]
- Li Y., Wright M.F. Adolescents’ social status goals: relationships to social status insecurity, aggression, and prosocial behavior. J. Youth Adolesc. 2014;43(1):146–160. doi: 10.1007/s10964-013-9939-z. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Morgan J.K. Maternal response to child affect: role of maternal depression and relationship quality. J. Affect. Disord. 2015;187:106–113. doi: 10.1016/j.jad.2015.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E.E. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol. Med. 2005;35(2):163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Neuberg S.L., Schaller M. In: Mikulincer M., editor. Vol. 1. American Psychological Association; Washington, D.C: 2015. (Evolutionary Social Cognition. APA Handbooks in Psychology. APA Handbook of Personality and Social Psychology). [Google Scholar]
- Passarotti A.M., Sweeney J.A., Pavuluri M.N. Neural correlates of incidental and directed facial emotion processing in adolescents and adults. Soc. Cogn. Affect. Neurosci. 2009;4(4):387–398. doi: 10.1093/scan/nsp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall E.T. Under pressure to perform: impact of academic goal orientation, school motivational climate, and school engagement on pain and somatic symptoms in adolescents. Clin. J. Pain. 2019;35(12):967–974. doi: 10.1097/AJP.0000000000000765. [DOI] [PubMed] [Google Scholar]
- Rose A.J., Rudolph K.D. A review of sex differences in peer relationship processes: potential trade-offs for the emotional and behavioral development of girls and boys. Psychol. Bull. 2006;132(1):98–131. doi: 10.1037/0033-2909.132.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxbe D. Neural correlates of adolescents’ viewing of parents’ and peers’ emotions: associations with risk-taking behavior and risky peer affiliations. Soc. Neurosci. 2015;10(6):592–604. doi: 10.1080/17470919.2015.1022216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf K.S. Visual category-selectivity for faces, places and objects emerges along different developmental trajectories. Dev. Sci. 2007;10(4):F15–30. doi: 10.1111/j.1467-7687.2007.00595.x. [DOI] [PubMed] [Google Scholar]
- Scherf K.S., Behrmann M., Dahl R.E. Facing changes and changing faces in adolescence: a new model for investigating adolescent-specific interactions between pubertal, brain and behavioral development. Dev. Cogn. Neurosci. 2012;2(2):199–219. doi: 10.1016/j.dcn.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S. Boys do it the right way: sex-dependent amygdala lateralization during face processing in adolescents. Neuroimage. 2011;56(3):1847–1853. doi: 10.1016/j.neuroimage.2011.02.019. [DOI] [PubMed] [Google Scholar]
- Sheeber L.B. Adolescents’ relationships with their mothers and fathers: associations with depressive disorder and subdiagnostic symptomatology. J. Abnorm. Psychol. 2007;116(1):144–154. doi: 10.1037/0021-843X.116.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S., Stone A.A., Hufford M.R. Ecological momentary assessment. Annu. Rev. Clin. Psychol. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- Silk J.S., Steinberg L., Morris A.S. Adolescents’ emotion regulation in daily life: links to depressive symptoms and problem behavior. Child Dev. 2003;74(6):1869–1880. doi: 10.1046/j.1467-8624.2003.00643.x. [DOI] [PubMed] [Google Scholar]
- Sugiura M. Neuroimaging studies on recognition of personally familiar people. Front. Biosci. (Landmark Ed) 2014;19:672–686. doi: 10.2741/4235. [DOI] [PubMed] [Google Scholar]
- Tahmasebi A.M. Creating probabilistic maps of the face network in the adolescent brain: a multicentre functional MRI study. Hum. Brain Mapp. 2012;33(4):938–957. doi: 10.1002/hbm.21261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L., Yu T., Huebner E.S. Achievement goal orientations and adolescents’ subjective well-being in school: the mediating roles of academic social comparison directions. Front. Psychol. 2017;8:37. doi: 10.3389/fpsyg.2017.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bavel J.J., Packer D.J., Cunningham W.A. The neural substrates of in-group bias: a functional magnetic resonance imaging investigation. Psychol. Sci. 2008;19(11):1131–1139. doi: 10.1111/j.1467-9280.2008.02214.x. [DOI] [PubMed] [Google Scholar]
- Van Hoorn J. Neural correlates of prosocial peer influence on public goods game donations during adolescence. Soc. Cogn. Affect. Neurosci. 2016;11(6):923–933. doi: 10.1093/scan/nsw013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Liu T., Shi J. Development of monetary and social reward processes. Sci. Rep. 2017;7(1):11128. doi: 10.1038/s41598-017-11558-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. Altered corticostriatal functional connectivity in individuals with high social anhedonia. Psychol. Med. 2016;46(1):125–135. doi: 10.1017/S0033291715001592. [DOI] [PubMed] [Google Scholar]
- Weinstein S.M., Mermelstein R.J. Influences of mood variability, negative moods, and depression on adolescent cigarette smoking. Psychol. Addict. Behav. 2013;27(4):1068–1078. doi: 10.1037/a0031488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S. Adolescents’ depressive symptoms moderate neural responses to their mothers’ positive behavior. Soc. Cogn. Affect. Neurosci. 2012;7(1):23–34. doi: 10.1093/scan/nsr049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S. Maternal responses to adolescent positive affect are associated with adolescents’ reward neuroanatomy. Soc. Cogn. Affect. Neurosci. 2009;4(3):247–256. doi: 10.1093/scan/nsp012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins J.L. Developmental differences in the neural mechanisms of facial emotion labeling. Soc. Cogn. Affect. Neurosci. 2016;11(1):172–181. doi: 10.1093/scan/nsv101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M.K. Self-other mergence in the frontal cortex during cooperation and competition. Neuron. 2016;91(2):482–493. doi: 10.1016/j.neuron.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.