Abstract
Development of resistance to chemo- and radiotherapy in patients suffering from advanced cervical cancer narrows the therapeutic window for conventional therapies. Previously we reported that a combination of the selective BCL-2 family inhibitors ABT-263 and A-1210477 decreased cell proliferation in C33A, SiHa and CaSki human cervical cancer cell lines. As ABT-263 binds to both BCL-2 and BCL-XL with high affinity, it was unclear whether the synergism of the drug combination was driven either by singly inhibiting BCL-2 or BCL-XL, or inhibition of both. In this present study, we used the BCL-2 selective inhibitor ABT-199 and the BCL-XL selective inhibitor A1331852 to resolve the individual antitumor activities of ABT-263 into BCL-2 and BCL-XL dependent mechanisms. A-1210477 was substituted for the orally bioavailable S63845. Four cervical cancer cell lines were treated with the selective BCL-2 family inhibitors ABT-199, A1331852 and S63845 alone and in combination using 2-dimensional (2D) and 3-dimensional (3D) cell culture models. The SiHa, C33A and CaSki cell lines were resistant to single agent treatment of all three drugs, suggesting that none of the BCL-2 family of proteins mediate survival of the cells in isolation. HeLa cells were resistant to single agent treatment of ABT-199 and A1331852 but were sensitive to S63845 indicating that they depend on MCL-1 for survival. Co-inhibition of BCL-2 and MCL-1 with ABT-199 and S63845, inhibited cell proliferation of all cancer cell lines, except SiHa. However, the effect of the combination was not as pronounced as combination of A1331852 and S63845. Co-inhibition of BCL-XL and MCL-1 with A1331852 and S63845 significantly inhibited cell proliferation of all four cell lines. Similar data were obtained with 3-dimensional spheroid cell culture models generated from two cervical cancer cell lines in vitro. Treatment with a combination of A1331852 and S63845 resulted in inhibition of growth and invasion of the 3D spheroids. Collectively, our data demonstrate that the combination of MCL-1-selective inhibitors with either selective inhibitors of either BCL-XL or BCL-2 may be potentially useful as treatment strategies for the management of cervical cancer.
Keywords: Cervical cancer, Selective BCL-2 inhibitors, A1331852, ABT-199, S63845, Apoptosis
Highlights
-
•
Co-inhibition of BCL-XL and MCL-1 inhibited cervical cancer cell proliferation.
-
•
Co-inhibition of BCL-XL and MCL-1 inhibited growth and invasion of 3D spheroids.
-
•
MCL-1-BCL-XL selective inhibitors are potential treatment strategies.
1. Introduction
The BCL-2 family of proteins are crucial regulators of the intrinsic apoptosis pathway and can be divided into pro-apoptotic and anti-apoptotic proteins and have one to four BCL-2 homology motifs (BH1–BH4). The anti-apoptotic multidomain (BH1–BH4) members namely BCL-2, BCL-XL, BCL-w, BFL-1/A1 and MCL-1 function to counteract the pore-forming activity of the pro-apoptotic multidomain proteins (BH1–BH4), BAX and BAK which permeabilize the mitochondria outer membrane. Following various stress signals, the BH3-only proteins either neutralize the anti-apoptotic proteins or directly activate effector proteins BAX and BAK which will eventually lead to apoptosis in cells [1,2].
One strategy that cancer cells employ to evade apoptosis, triggered by oncogenesis or drug treatment is via overexpressing the BCL-2 anti-apoptotic proteins [3]. Hence, treatment that is effective in activating pro-death signaling either by upregulating the pro-apoptotic protein BIM or effector proteins BAX or BAK are inefficient, as cancer cells can survive this cytotoxic insult by sequestering pro-apoptotic proteins with anti-apoptotic proteins [4]. Cellular anti-apoptotic mechanisms can also be suppressed by selective BCL-2 family inhibitors [4], which mimic the action of certain BH3-only proteins. For example, ABT-263 (Navitoclax) mimics the BH3-only protein BAD which selectively inhibits BCL-2, BCL-XL and BCL-w [5]. ABT-263 has also demonstrated anti-tumor activity in lymphoid malignancies in clinical studies, but induced dose-dependent thrombocytopenia as a consequence of inhibiting BCL-XL [6,7]. This toxicity prompted the development of the BCL-2 selective inhibitor ABT-199/venetoclax [8]. Venetoclax was approved by the FDA for the treatment of chronic lymphocytic leukemia (CLL) [9] but has shown activity in other cancers such as acute myeloid leukemia (AML) [10] and T-cell acute lymphoblastic leukemia (T-ALL) in combination with the MCL-1 selective inhibitor S63845 [11]. In order to determine the contribution of BCL-XL for survival of cancer cells, a number of specific BCL-XL inhibitors such as WEHI-539 [12], A1331852 and A1155463 [13] have been developed.
Previously we evaluated the sensitivity of a number of cervical cancer cell lines to a combination of ABT-263 and the MCL-1 selective inhibitor A-1210477 [14]. This drug combination exhibited synergistic anti-proliferative effects on the cervical cancer cell lines tested. However, given that ABT-263 exhibits high affinity towards BCL-2 and BCL-XL, it was unclear whether the effect of ABT-263 was driven by BCL-2, BCL-XL, or both proteins. We hypothesized that an improved treatment strategy may be implemented if the contributions of BCL-XL and BCL-2 inhibition could be delineated, as this treatment strategy could result in reduced toxicity and maximize antitumor activity in specific cancers.
In this present study, ABT-199 and A1331852 [13] were used experimentally to investigate the contributions of BCL-2 and BCL-XL in mediating cervical cancer cell survival. In order to study the role of MCL-1 for cell survival S63845 (a small molecule inhibitor of MCL-1) was used. S63845 was reported to demonstrate higher affinity towards MCL-1 (Ki < 1.2 nM) compared to A-1210477 (Ki = 28 nM). In addition, S63845 was 1000-fold more potent in killing (MCL-1 dependent) H929 cells compared to A-1210477 [15], and its use therefore would be more appropriate in helping delineate its role in cervical cancer cell survival.
Four cervical cancer cell lines C33A, SiHa, HeLa and CaSki were subjected to single agent treatment with ABT-199, A1331852 and S63845. These four different cancer cell lines were also tested with combinations of A1331852/S63845 and ABT-199/S63845 in monolayer (2D) culture and in 3-dimensional (3D) spheroids, which provide a microenvironment closer to tumours in vivo [16].
2. Material and methods
2.1. Drugs and cell lines
ABT-199, A1331852 and S63845 (MedChemExpress, NJ, USA) were dissolved in dimethyl sulfoxide (DMSO) at a stock concentration of 10 mM. All four cell lines were purchased from the American Type Culture Collection (Manassas, VA, USA), and maintained in culture as described previously [14].
2.2. Drug sensitivity assay
Drug sensitivity assays were performed as described previously [17]. Cells were treated with ABT-199, A1331852 or S63845 diluted in two-fold steps (0.25, 0.5, 1, 2, 4, 8, 16 and 32 μM) either alone or in combination for 72 h. ABT-199 is a selective BCL-2 inhibitor, A1331852 is a BCL-XL selective inhibitor and S63845 is a selective inhibitor of MCL-1. Sensitivity of cells to drug combinations was measured by testing a fixed concentration of S63845 with increasing concentrations of either A1331852 or ABT-199. Cell proliferation was quantified by fluorescence using SYBR Green as described previously [14]. All drug sensitivity assays were conducted four times (n = 4) and average IC50 values were calculated from the experimental data. In the plots, the y-axis represents cell proliferation, with cell proliferation of the untreated controls representing 100%. The x-axis was formatted to have a base 10 logarithmic scale but the drug concentrations used were not log-transformed prior to plotting of the graphs.
2.3. Three-dimensional spheroids
Approximately 5000 cells (2.5 × 104 cells/ml) cells were seeded in an Ultra-Low Attachment (ULA) 96-well U bottom-plate (Corning, NY, USA). Plates containing the cells were centrifuged at 210 Gav for 2 min to pellet the cells. Plates were incubated at 37 °C, 95% O2, 5% CO2 for 72 h. After 72 h, 3D spheroids were embedded into collagen mix [18]. Spheroids were treated with A1331852, ABT-199 and S63845, alone and in combination for 72 h. Spheroid growth and invasion were photographed every 24 h using a Nikon C2+ inverted confocal microscope. Upon termination of the assay, live-dead staining of spheroids was conducted as described previously [19]. Images were taken using a Nikon-300 inverted fluorescence microscope. Growth of spheroids were analyzed using ImageJ (v1.51s, NIH) and statistical analysis (2-sided paired Student t tests) were performed using Microsoft® Excel. Using ImageJ (v1.51s, NIH), an outline was drawn around each spheroid in a focal Z plane which showed the maximum size and area and mean fluorescence was measured, along with adjacent background readings for control spheroids, spheroids treated with either S63845 or A1331852 and spheroids treated with combination of the two drugs. The total corrected red fluorescence (TCRF) = integrated density – (area of selected cell × mean fluorescence of background readings).
3. Results
3.1. Selective BCL-2 family inhibitors resolve the individual contributions of anti-apoptotic proteins BCL-2, BCL-XL and MCL-1 in cervical cancer cell lines survival
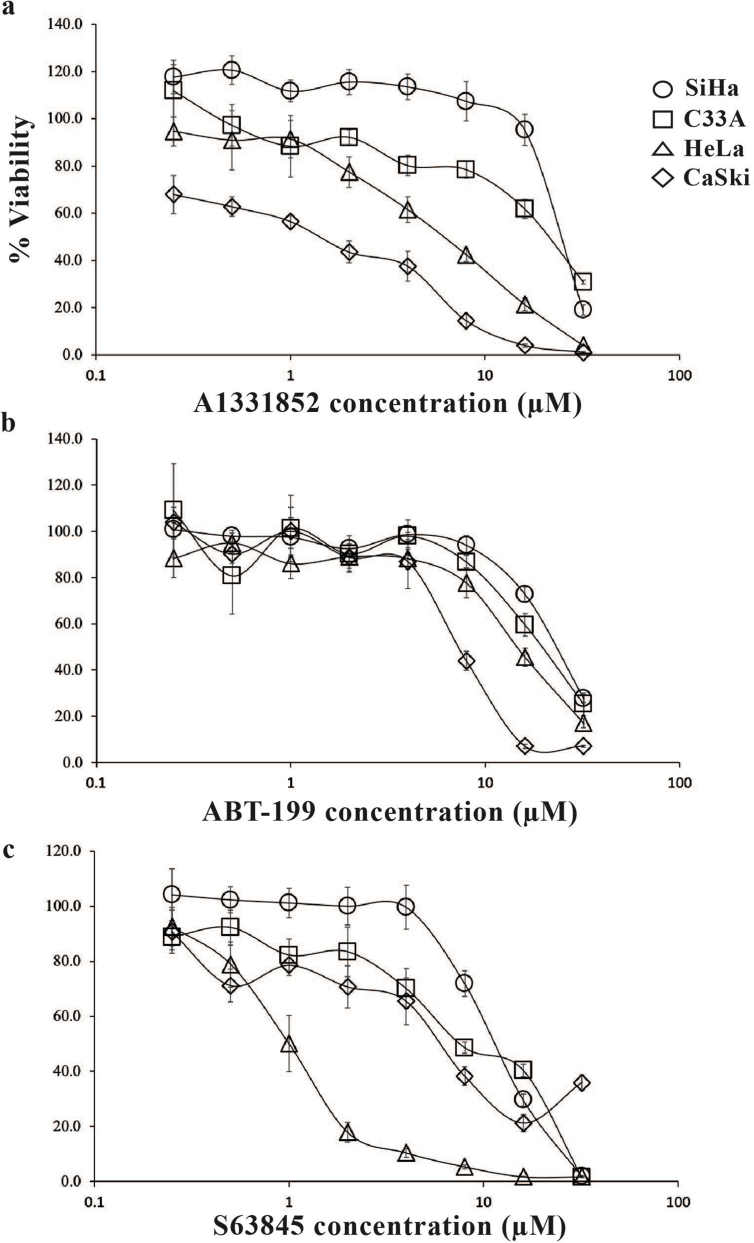
Four cervical cancer cell lines were subjected to single agent treatment of ABT-199, A1331852 and S63845 (which inhibit BCL-2, BCL-X and MCL-1 respectively), either alone or in combination (Table S1). HeLa cells were resistant to single agent treatment with A1331852 (Fig. 1a & Table S2) and ABT-199 (Fig. 1b & Table S2) but sensitive to single agent treatment with S63845 (Fig. 1c & Table S2). C33A (Fig. 1a–c & Table S2), and SiHa (Fig. 1a–c & Table S2) cells were resistant to single agent treatment with all three selective BCL-2 family inhibitors. CaSki cells were slightly sensitive to A1331852 (Fig. 1a & Table S2), but were resistant to single agent ABT-199 (Fig. 1b & Tables S2) and S63845 (Fig. 1c & Table S2). Although slightly sensitive to A1331852, it appears that single inhibitor targeting is not very effective in inhibiting cell proliferation of CaSki cells.
Fig. 1.
Sensitivity of the cervical cancer cell lines to single agent treatment of ABT-199, A1331852 and S63845. (a) HeLa, C33A and SiHa were resistant to single agent treatment of A1331852. CaSki cells were slightly sensitive to A1331852; (b) All four cell lines were resistant to single agent treatment of ABT-199. (c) Except for HeLa, all other cervical cancer cell lines were insensitive to single agent treatment of S63845. Points represent mean ± SEM of four experiments.
Collectively, these data suggest that insensitivity of HeLa cells to single agent treatment of ABT-199 and A1331852 shows that they depend on MCL-1 for survival, as the cells were susceptible to single agent treatment of S63845. Insensitivity of the other cell lines to all three selective BCL-2 family drugs used as monotherapy suggest that the cells are resistant to apoptosis due to the need to target multiple pro-survival proteins rather than just one. These data also suggest that other molecular pathways may be responsible for apoptotic death mechanisms in these cells. For example, it has been demonstrated that there are non-caspase dependent cell death mechanisms that are dependent on the cathepsins [20].
3.2. Sensitivity of the cervical cancer cell lines to co-inhibition of BCL-2 and MCL-1 was variable
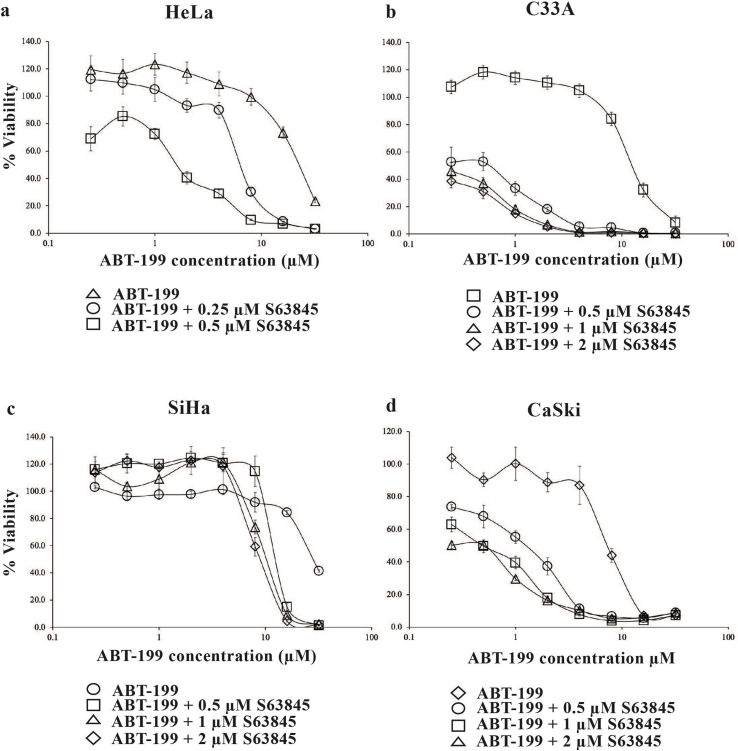
In HeLa cells, 0.25 μM S63845 shifted the concentration-response curve to the left (Fig. 2a) sensitizing the cells to ABT-199 by 6-fold (Table S3). An increase in concentration of S63845 to 0.5 μM, resulted in a significant shift of the concentration-response curve to the left (Fig. 2a) and the cells were sensitized to ABT-199by 13-fold (Table S3).
Fig. 2.
Co-inhibition of BCL-2 and MCL-1 using BCL-2 selective inhibitors ABT-199 and S63845. Cervical cancer cell lines (a) HeLa; (b) C33A; (c) SiHa and (d) CaSki cells were treated with increasing concentrations of ABT-199 (0–32 μM) in the presence and absence of S63845. Points represent mean ± SEM of four experiments.
The drug interaction analyses demonstrated that combination of ABT-199 with 0.25 μM of S63845 could not be determined. At the concentrations tested, the poor efficacy of the combination treatment, meant that we were unable to conduct drug interaction analyses (Table S4). Concentrations of ABT-199 > 1 μM combined with 0.25 μM S63845 were antagonistic (Table S4). The combination of S63845 with ABT-199 only resulted in synergism at 0.5 μM S63845 with concentrations of ABT-199 > 1 μM (Table S4). The words “antagonism”, and “synergism” refer to the overall effect on cell proliferation and are not in any way meant to infer the properties of a classical pharmacological ligand that is an antagonist in relation for example to a cell surface receptor and agonists/antagonists.
At 0.5 μM S63845, C33A cells were sensitized to ABT-199 b y 22-fold (Fig. 2b & Table S3). The sensitization increased to >40-fold at a concentration of 1 μM S63845 and 2 μM of S63845 (Fig. 2b & Table S3) and drug interaction analyses demonstrated strong synergism at multiple concentrations of S63845 and ABT-199 (Table S4).
In SiHa cells, S63845 at 0.5 μM (Fig. 2c & Table S3) and 1 μM (Fig. 2c) only sensitized SiHa cells to ABT-199 b y 2-fold (Table S3). This sensitization only increased to 3-fold (Table S3) when the concentration of S63845 was increased to 2 μM (Fig. 2c).
Combination with 0.5 μM of S63845 sensitized the CaSki cells to ABT-199 b y 6-fold (Fig. 2d & Table S3). The sensitization increased to 14 - fold when the concentration of S63845 was increased to 1 μM and 2 μM (Fig. 2d & Table S3). Drug interaction analyses indicated that the drug combinations demonstrated strong synergism at several concentrations of S63845 and ABT-199 (Table S4). Collectively, the findings demonstrate that inhibition of either BCL-2 or BCL-XL alone is not adequate to kill the CaSki cells, and co-inhibition of MCL-1 with either BCL-XL or BCL-2 appears to be essential to kill these particular cancer cells.
We next tested a combination of ABT-199/S63845 in 3D spheroids generated from HeLa cells. In monolayer culture, S63845 only modestly sensitized HeLa cells to ABT-199 in (Fig. 2a). The combination (ABT-199/S63845) however, had minimal effect on the growth and invasion of the 3D HeLa spheroids even at the highest combination concentration used, indicating higher combination concentrations may be required to inhibit growth and invasion of the spheroids (Figs. S1a and S1b). There was a slight increase in red fluorescence intensity at the highest combination concentration which is expected but generally there were no obvious increase in red fluorescence for the other combination concentrations tested (Fig. S1c).
3.3. Substantial inhibition of cell proliferation driven by co-inhibition of BCL-XL and MCL-1
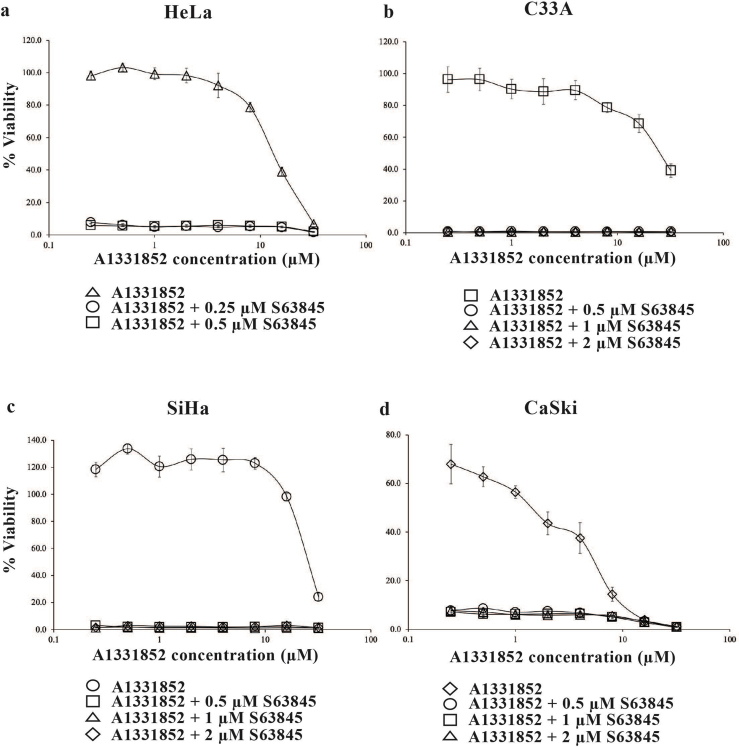
As HeLa cells were sensitive to single agent S63845 (Fig. 1c & Table S2), we tested the sensitivity of the HeLa cell line to fixed concentrations of S6835 (below 1 μM) with increasing concentrations of either A1331852 or ABT-199.
HeLa cells were treated with either a fixed concentration of 0.25 μM or 0.5 μM S63845 and increasing concentrations of A1331852 (0–32 μM). At a concentration of 0.25 μM S63845, there was complete 100% cell killing resulting in 0% cell viability (Fig. 3a). 0.25 μM S63845 sensitized HeLa cells to A1331852 > 44-fold (Table S5) indicating maximum cell death can be achieved with lower drug concentrations, hence the fold-change in sensitivity could be higher. Similar data were obtained when the concentration of S63845 was increased to 0.5 μM (Fig. 3a & Table S5). In C33A cells, the presence of 0.5 μM S63845, resulted in the complete loss of a concentration-dependent curve resulting in 0% cell viability (Fig. 3b) and sensitized the cells to A1331852 close to 100-fold (Table S5). Addition of 1 μM and 2 μM S63845 (Fig. 3b & Table S5) resulted in similar data. Comparably, in the presence of 0.5 μM S63845 (Fig. 3c), SiHa cells were sensitized to A1331852 > 100-fold (Table S5). Similar data were obtained in SiHa cells when the concentration of S63845 was increased to 1 μM and 2 μM (Fig. 3c & Table S5). In CaSki cells, combination with S63845 sensitized the cells to A1331852 for all concentrations tested (Fig. 3d & Table S5) indicating that co-inhibition with MCL-1, enhances cell killing compared to inhibition of BCL-XL alone. The CI values obtained for combination of A1331852 and S63845 exhibited synergism at several concentrations for all four cervical cancer cell lines (Table S6).
Fig. 3.
Co-inhibition of BCL-XL and MCL-1 using BCL-2 selective inhibitors A1331852 and S63845. Cervical cancer cell lines (a) HeLa; (b) C33A; (c) SiHa and (d) CaSki cells were treated with increasing concentrations of A1331852 (0–32 μM) in the presence and absence of S63845. Points represent mean ± SEM of four experiments.
3.4. S63845 sensitized 3-dimensional (3D) spheroids generated from cervical cancer cell lines to A1331852
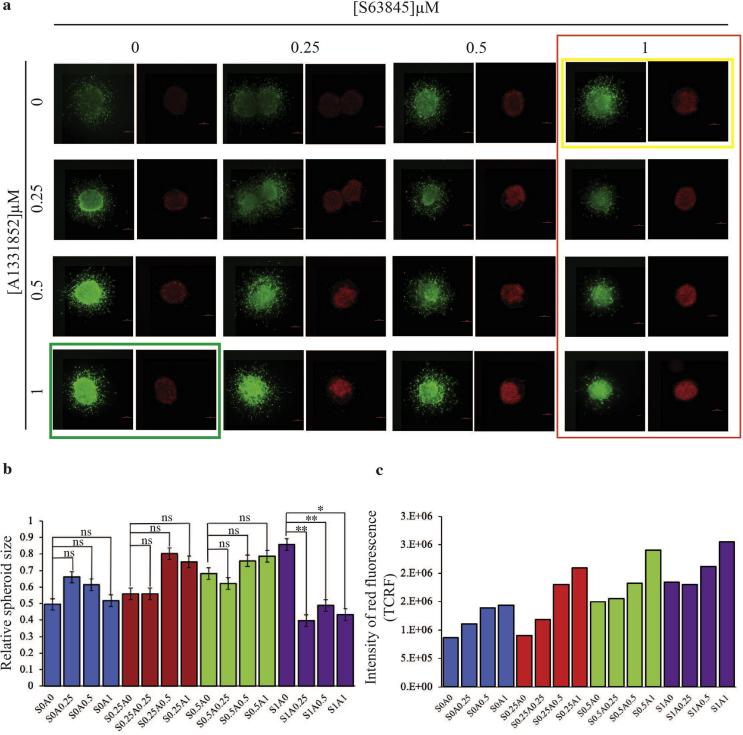
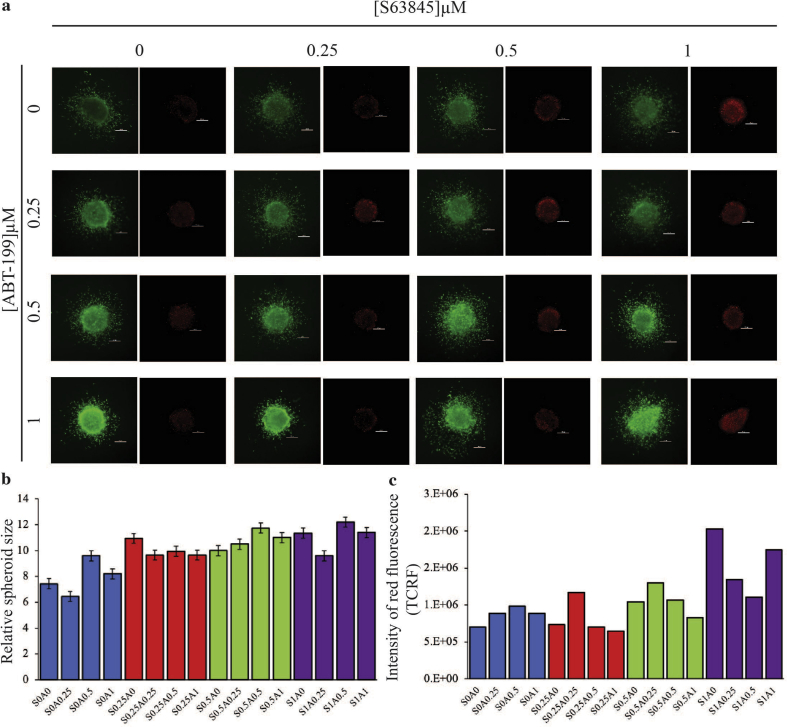
S63845 and A1331852 used as single agents had less effect on the growth and invasion of the HeLa spheroids except at 0.5 μM and 1 μM S63845 (Fig. 4a – see yellow box) and 0.5 μM and 1 μM of A1331852 (Fig. 4a – see green box), there was a noticeable decrease in viability in the periphery of the spheroids and an increase in the intensity of red fluorescence when compared to the untreated spheroid (Fig. 4c). In the presence of 1 μM of S63845, there was obvious sensitization of the spheroids to A1331852, which manifested as reduced spheroid growth and invasion (Fig. 4a – see the column in red). There was a significant decrease in spheroid size in the presence of 1 μM of S63845 with increasing concentrations of A1331852 compared to spheroids only treated with S63845 (Fig. 4b – see purple bars). Moreover, the same drug combinations resulted in the increase of red fluorescence intensity indicating more dead cells at these combination concentrations (Fig. 4c – see purple bars). Taken together, the synergistic effect of the drug combination on growth and invasion of the spheroids was similar to the cytotoxicity curves obtained for the 2D cultures (Fig. 3a). Similar data were obtained when the drug combination was tested on 3D spheroids generated from SiHa cells. S63845 at 2 μM was able to sensitize the spheroids to A1331852, reflected in concentration-dependent inhibition of spheroid growth and invasion. Similarly, A1331852 at 2 μM was able to sensitize the spheroids to S63845 (Fig. S2). Taken together, the effect of combination of A1331852/S63845 observed in the 3D spheroid model was consistent with the 2D monolayer culture data, suggesting that this drug combination may be effective in vivo.
Fig. 4.
The effect of combination of S63845 and A1331852 on the growth and invasion of 3D HeLa spheroids over three days. (a) The spheroids were treated with single agents S63845 and A1331852 and combination of both over three days at the indicated concentrations, n = 2–3 spheroids per combination. Cell viability was determined using the live/dead assay (Viable cells: stained green by Calcein-AM; Dead cells: stained red by Ethidium-homodimer I). Size bar: 200 μm. (b) Graphs show corresponding quantification of spheroid growth for each drug combination tested, n = 2–3 spheroids per combination. Error bars indicate standard errors. Statistically significant differences of the relative growth of the combination treated spheroids are shown as **p < 0.01 or *p < 0.05 determined by two-tailed paired T-test. (c) The intensity of red fluorescence was measured for each drug combination and presented as TCRF, n = 2 spheroids per combination. TCRF quantification were performed as described previously [30]. TCRF: Total Corrected Red Fluorescence; “S” denotes S63845 and “A” denotes A1331852. The numbers next to “S” and “A” indicate the concentrations of the drug combinations”. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Collectively our data demonstrate that there was a greater response to co-inhibition of MCL-1 and BCL-XL. Cells responded to combination of S63845 and A1331852 more rapidly at low concentrations. In contrast, the response to co-inhibition of MCL-1 and BCL-2 was variable suggesting that other cell death mechanisms that do not rely on MCL-1 and BCL-XL may be involved.
4. Discussion
Our data suggest that in the four cervical cancer cell lines tested co-inhibition of MCL-1 is important and necessary to induce cell death, as none of the cell lines responded to ABT-263 used singly. However, ABT-263 is reported to cause thrombocytopenia due to BCL-XL inhibition [6,7]. Hence, it is important to investigate whether selective inhibition of BCL-XL or BCL-2 would minimize toxicity and serve as a substitute for ABT-263. Hence, we employed the selective BCL-2 family inhibitors ABT-199, A1331852 and S63845 to define the contributions of these anti-apoptotic proteins in maintaining survival of the cervical cancer cells.
All four cervical cancer cell lines tested were resistant to single agent treatment of ABT-199 and A1331852. None of the cell lines, except HeLa responded to S63845, when used singly, indicating that they were not solely MCL-1-dependent. However, although HeLa cells responded to single agent treatment of S63845, treatment with a combination of ABT-199 or A1331852 with concentrations of S63845 of <1 μM resulted in synergy, indicating that inhibition of either BCL-XL or BCL-2 is still required to achieve cell killing at lower concentrations of S63845. These data demonstrate that survival of the cervical cancer cell lines is maintained by more than one anti-apoptotic protein and selectively inhibiting them in combination kills the cells more effectively.
Several studies have also shown that survival of cancer cells is dependent on the expression of several different anti-apoptotic proteins. For example, chronic lymphocytic leukemia (CLL) cells are killed when either BCL-2 and BCL-XL or BCL-2 and MCL-1 were inhibited, indicating that these cells depend on more than one anti-apoptotic protein for survival [21]. Acute myeloid leukemia (AML) cells developed resistance to inhibition of BCL-2 by upregulating BCL-XL and MCL-1. Therefore, inhibiting both BCL-XL and MCL-1 resensitized AML cells to ABT-199, which inhibits BCL-2 [22]. Furthermore, co-inhibition of MCL-1 and BCL-2 killed T-ALL cells in vitro and in vivo [11]. These present data show that all four cervical cancer cell lines used were sensitive to combinations of A1331852 and S63845 at lower combination concentrations, indicating that they depend on both BCL-XL and MCL-1 for survival, and co-inhibition of these molecules are sufficient to cause cell death. Moreover, our data show that BCL-XL is the key target of ABT-263, for inducing the synergy observed previously with A-1210477 [14].
C33A cells were sensitive to combinations of ABT-199 and S63845. Given that the C33A cells also responded effectively to a combination of A1331852 and S63845, it appears that co-inhibition of either BCL-2 or BCL-XL with MCL-1 is sufficient to cause cell death in C33A cells. SiHa cells responded poorly to combination of ABT-199 and S63845 but the cells were sensitive to combination of A1331852 and S63845. Therefore, SiHa cells may be dependent on BCL-XL and MCL-1 for survival rather than BCL-2. Therefore, it is possible that co-inhibition of BCL-2 and MCL-1 may have led to overexpression of BCL-XL as a compensatory survival adaptation which has been reported in other cancer cell lines. CLL cells developed resistance to ABT-737 (which selectively inhibits BCL-2 and BCL-XL) treatment due to concurrent upregulation of BCL-XL and BFL-1/A1 [23] and upregulation of MCL-1 and BFL-1/A1 resulted in acquired resistance in a number of cancer cells to ABT-737 [19,24,25].
All four cervical cancer cell lines used were more responsive to lower concentrations of combinations of S63845 and A1331852 compared to combinations of S63845 and ABT-199, indicating that BCL-XL and MCL-1 are better targets for inducing the death of these particular cervical cancer cell lines. The sensitization obtained in the 2D monolayer culture was analogous to the data obtained with the 3D spheroid studies. The 3D HeLa spheroids were sensitized to A1331852 by S63845 but sensitization was only obvious following treatment with 1 μM of S63845, indicating that higher concentrations of S63845 are required to sensitize spheroids to A1331852 compared to the concentration of S63845 required to cause the same sensitization effect in monolayer culture. One explanation for the need of higher drug combination concentrations in the spheroids, could be attributed to the 3D orientation of the tumor cells which is likely to limit diffusion of drugs to the cells in the center of the spheroid.
Other studies have also demonstrated that inhibition of BCL-XL rather than BCL-2 has resulted in sensitization of solid tumor cancer cell lines to other drugs. For example, the BCL-XL inhibitor WEHI-539 (but not BCL-2 inhibitors) sensitized osteosarcoma cell lines to doxorubicin [26]. Breast cancer, non-small cell lung cancer ovarian cancer cell lines were also sensitized to docetaxel by ABT-263 and BCL-XL selective inhibitors but not to BCL-2 inhibitors [13]. Furthermore, chondrosarcoma cell lines were reported to be sensitized to doxorubicin or cisplatin by BCL-XL inhibitors and not BCL-2 inhibitors both in vitro and in vivo [27]. More recently, drug combinations targeting BCL-XL and MCL-1, and to a lesser extent BCL-2 were reported to synergistically kill melanoma cells in 2D and 3D cell culture models [28]. Collectively, solid tumours may be more susceptible to inhibition of BCL-XL and MCL-1. However, co-targeting BCL-XL and MCL-1 may pose an issue in the clinic, as inhibition of BCL-XL may result in thrombocytopenia [6,7]. At present neither A1331852 nor S63845 are useful in the clinic, due to toxicity issues and co-targeting of BCL-XL and MCL-1 can cause fatal hepatotoxicity [29]. However, our present data suggest that selective, less toxic BCL-XL inhibitors may be useful in combination with conventional chemotherapy and/or the use of selective pro-apoptotic agents that directly activate type 2 mitochondrial pathways [2]. Another strategy would be to co-inhibit BCL-2 and MCL-1.
Testing the drug combinations used here in rodent models is necessary for determining safety and efficacy profiles. The data presented here strongly suggest that the combination of selective inhibitors of BCL-XL plus MCL-1 and BCL-2 plus MCL-1 may be important new chemotherapeutic strategies in the management of cervical cancer.
CRediT authorship contribution statement
Siti Fairus Abdul Rahman: Investigation, Formal analysis. Kalaivani Muniandy: Investigation. Yong Kit Soo: Investigation. Elvin Yu Huai Tiew: Investigation. Ke Xin Tan: Investigation. Timothy E. Bates: Writing - review & editing. Nethia Mohana-Kumaran: Conceptualization, Methodology, Validation, Formal analysis, Writing - original draft, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgments
This work was supported by the Fundamental Research Grant Scheme, Ministry of Education Malaysia, Malaysia (203/PBIOLOGI/6711541), L'Oréal -UNESCO National Fellowship for Women in Science, Malaysia (304/PBIOLOGI/650853/L117) and Universiti Sains Malaysia RU grant, Universiti Sains Malaysia, Malaysia (1001/PBIOLOGI/8012268).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2020.100756.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
References
- 1.Campbell K.J., Tait S.W.G. Targeting BCL-2 regulated apoptosis in cancer. Open Biol. 2018;8 doi: 10.1098/rsob.180002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quayle L.A., Pereira M.G., Scheper G., Wiltshire T., Peake R.E., Hussain I., Rea C.A., Bates T.E. Anti-angiogenic drugs: direct anti-cancer agents with mitochondrial mechanisms of action. Oncotarget. 2017;8:88670–88688. doi: 10.18632/oncotarget.20858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Montero J., Letai A. Why do BCL-2 inhibitors work and where should we use them in the clinic? Cell Death Differ. 2018;25:56–64. doi: 10.1038/cdd.2017.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tse C., Shoemaker A.R., Adickes J., Anderson M.G., Chen J., Jin S., Johnson E.F., Marsh K.C., Mitten M.J., Nimmer P., Roberts L., Tahir S.K., Xiao Y., Yang X., Zhang H., Fesik S., Rosenberg S.H., Elmore S.W. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Canc. Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 6.Mason K.D., Carpinelli M.R., Fletcher J.I., Collinge J.E., Hilton A.A., Ellis S., Kelly P.N., Ekert P.G., Metcalf D., Roberts A.W., Huang D.C., Kile B.T. Programmed anuclear cell death delimits platelet life span. Cell. 2007;128:1173–1186. doi: 10.1016/j.cell.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 7.Zhang H., Nimmer P.M., Tahir S.K., Chen J., Fryer R.M., Hahn K.R., Iciek L.A., Morgan S.J., Nasarre M.C., Nelson R., Preusser L.C., Reinhart G.A., Smith M.L., Rosenberg S.H., Elmore S.W., Tse C. Bcl-2 family proteins are essential for platelet survival. Cell Death Differ. 2007;14:943–951. doi: 10.1038/sj.cdd.4402081. [DOI] [PubMed] [Google Scholar]
- 8.Souers A.J., Leverson J.D., Boghaert E.R., Ackler S.L., Catron N.D., Chen J., Dayton B.D., Ding H., Enschede S.H., Fairbrother W.J., Huang D.C., Hymowitz S.G., Jin S., Khaw S.L., Kovar P.J., Lam L.T., Lee J., Maecker H.L., Marsh K.C., Mason K.D., Mitten M.J., Nimmer P.M., Oleksijew A., Park C.H., Park C.M., Phillips D.C., Roberts A.W., Sampath D., Seymour J.F., Smith M.L., Sullivan G.M., Tahir S.K., Tse C., Wendt M.D., Xiao Y., Xue J.C., Zhang H., Humerickhouse R.A., Rosenberg S.H., Elmore S.W. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013;19:202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 9.Delbridge A.R., Grabow S., Strasser A., Vaux D.L. Thirty years of BCL-2: translating cell death discoveries into novel cancer therapies. Nat. Rev. Canc. 2016;16:99–109. doi: 10.1038/nrc.2015.17. [DOI] [PubMed] [Google Scholar]
- 10.Pan R., Hogdal L.J., Benito J.M., Bucci D., Han L., Borthakur G., Cortes J., DeAngelo D.J., Debose L., Mu H., Dohner H., Gaidzik V.I., Galinsky I., Golfman L.S., Haferlach T., Harutyunyan K.G., Hu J., Leverson J.D., Marcucci G., Muschen M., Newman R., Park E., Ruvolo P.P., Ruvolo V., Ryan J., Schindela S., Zweidler-McKay P., Stone R.M., Kantarjian H., Andreeff M., Konopleva M., Letai A.G. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Canc. Discov. 2014;4:362–375. doi: 10.1158/2159-8290.CD-13-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Z., He S., Look A.T. The MCL1-specific inhibitor S63845 acts synergistically with venetoclax/ABT-199 to induce apoptosis in T-cell acute lymphoblastic leukemia cells. Leukemia. 2019;33:262–266. doi: 10.1038/s41375-018-0201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lessene G., Czabotar P.E., Sleebs B.E., Zobel K., Lowes K.N., Adams J.M., Baell J.B., Colman P.M., Deshayes K., Fairbrother W.J., Flygare J.A., Gibbons P., Kersten W.J., Kulasegaram S., Moss R.M., Parisot J.P., Smith B.J., Street I.P., Yang H., Huang D.C., Watson K.G. Structure-guided design of a selective BCL-X(L) inhibitor. Nat. Chem. Biol. 2013;9:390–397. doi: 10.1038/nchembio.1246. [DOI] [PubMed] [Google Scholar]
- 13.Leverson J.D., Phillips D.C., Mitten M.J., Boghaert E.R., Diaz D., Tahir S.K., Belmont L.D., Nimmer P., Xiao Y., Ma X.M., Lowes K.N., Kovar P., Chen J., Jin S., Smith M., Xue J., Zhang H., Oleksijew A., Magoc T.J., Vaidya K.S., Albert D.H., Tarrant J.M., La N., Wang L., Tao Z.F., Wendt M.D., Sampath D., Rosenberg S.H., Tse C., Huang D.C., Fairbrother W.J., Elmore S.W., Souers A.J. Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci. Transl. Med. 2015;7 doi: 10.1126/scitranslmed.aaa4642. 279ra240. [DOI] [PubMed] [Google Scholar]
- 14.Lian B.S.X., Yek A.E.H., Shuvas H., Abdul Rahman S.F., Muniandy K., Mohana-Kumaran N. Synergistic anti-proliferative effects of combination of ABT-263 and MCL-1 selective inhibitor A-1210477 on cervical cancer cell lines. BMC Res. Notes. 2018;11:197. doi: 10.1186/s13104-018-3302-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotschy A., Szlavik Z., Murray J., Davidson J., Maragno A.L., Le Toumelin-Braizat G., Chanrion M., Kelly G.L., Gong J.N., Moujalled D.M., Bruno A., Csekei M., Paczal A., Szabo Z.B., Sipos S., Radics G., Proszenyak A., Balint B., Ondi L., Blasko G., Robertson A., Surgenor A., Dokurno P., Chen I., Matassova N., Smith J., Pedder C., Graham C., Studeny A., Lysiak-Auvity G., Girard A.M., Grave F., Segal D., Riffkin C.D., Pomilio G., Galbraith L.C., Aubrey B.J., Brennan M.S., Herold M.J., Chang C., Guasconi G., Cauquil N., Melchiore F., Guigal-Stephan N., Lockhart B., Colland F., Hickman J.A., Roberts A.W., Huang D.C., Wei A.H., Strasser A., Lessene G., Geneste O. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature. 2016;538:477–482. doi: 10.1038/nature19830. [DOI] [PubMed] [Google Scholar]
- 16.Siva Sankar P., Che Mat M.F., Muniandy K., Lian B.S.X., Phang S.L., Hoe S.L.L., Khoo A.S., Mohana-Kumaran N. Modeling nasopharyngeal carcinoma in three dimensions (Review) Oncology Letters. 2017;13:2034–2044. doi: 10.3892/ol.2017.5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen J.D., Jackson S.C., Schinkel A.H. A mutation hot spot in the Bcrp1 (Abcg2) multidrug transporter in mouse cell lines selected for Doxorubicin resistance. Canc. Res. 2002;62:2294–2299. [PubMed] [Google Scholar]
- 18.Smalley K.S., Lioni M., Noma K., Haass N.K., Herlyn M. In vitro three-dimensional tumor microenvironment models for anticancer drug discovery. Expet Opin. Drug Discov. 2008;3:1–10. doi: 10.1517/17460441.3.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Lucas K.M., Mohana-Kumaran N., Lau D., Zhang X.D., Hersey P., Huang D.C., Weninger W., Haass N.K., Allen J.D. Modulation of NOXA and MCL-1 as a strategy for sensitizing melanoma cells to the BH3-mimetic ABT-737. Clin. Canc. Res. : Off. J. Am. Assoc. Canc. Res. 2012;18:783–795. doi: 10.1158/1078-0432.CCR-11-1166. [DOI] [PubMed] [Google Scholar]
- 20.Levicar N., Dewey R.A., Daley E., Bates T.E., Davies D., Kos J., Pilkington G.J., Lah T.T. Selective suppression of cathepsin L by antisense cDNA impairs human brain tumor cell invasion in vitro and promotes apoptosis. Canc. Gene Ther. 2003;10:141–151. doi: 10.1038/sj.cgt.7700546. [DOI] [PubMed] [Google Scholar]
- 21.Peperzak V., Slinger E., Ter Burg J., Eldering E. Functional disparities among BCL-2 members in tonsillar and leukemic B-cell subsets assessed by BH3-mimetic profiling. Cell Death Differ. 2017;24:111–119. doi: 10.1038/cdd.2016.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin K.H., Winter P.S., Xie A., Roth C., Martz C.A., Stein E.M., Anderson G.R., Tingley J.P., Wood K.C. Targeting MCL-1/BCL-XL forestalls the acquisition of resistance to ABT-199 in acute myeloid leukemia. Sci. Rep. 2016;6:27696. doi: 10.1038/srep27696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vogler M., Butterworth M., Majid A., Walewska R.J., Sun X.M., Dyer M.J., Cohen G.M. Concurrent up-regulation of BCL-XL and BCL2A1 induces approximately 1000-fold resistance to ABT-737 in chronic lymphocytic leukemia. Blood. 2009;113:4403–4413. doi: 10.1182/blood-2008-08-173310. [DOI] [PubMed] [Google Scholar]
- 24.Morales A.A., Kurtoglu M., Matulis S.M., Liu J., Siefker D., Gutman D.M., Kaufman J.L., Lee K.P., Lonial S., Boise L.H. Distribution of Bim determines Mcl-1 dependence or codependence with Bcl-xL/Bcl-2 in Mcl-1-expressing myeloma cells. Blood. 2011;118:1329–1339. doi: 10.1182/blood-2011-01-327197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yecies D., Carlson N.E., Deng J., Letai A. Acquired resistance to ABT-737 in lymphoma cells that up-regulate MCL-1 and BFL-1. Blood. 2010;115:3304–3313. doi: 10.1182/blood-2009-07-233304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baranski Z., de Jong Y., Ilkova T., Peterse E.F., Cleton-Jansen A.M., van de Water B., Hogendoorn P.C., Bovee J.V., Danen E.H. Pharmacological inhibition of Bcl-xL sensitizes osteosarcoma to doxorubicin. Oncotarget. 2015;6:36113–36125. doi: 10.18632/oncotarget.5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Jong Y., van Maldegem A.M., Marino-Enriquez A., de Jong D., Suijker J., Briaire-de Bruijn I.H., Kruisselbrink A.B., Cleton-Jansen A.M., Szuhai K., Gelderblom H., Fletcher J.A., Bovee J.V. Inhibition of Bcl-2 family members sensitizes mesenchymal chondrosarcoma to conventional chemotherapy: report on a novel mesenchymal chondrosarcoma cell line. Lab. Invest. 2016;96:1128–1137. doi: 10.1038/labinvest.2016.91. [DOI] [PubMed] [Google Scholar]
- 28.Lee E.F., Harris T.J., Tran S., Evangelista M., Arulananda S., John T., Ramnac C., Hobbs C., Zhu H., Gunasingh G., Segal D., Behren A., Cebon J., Dobrovic A., Mariadason J.M., Strasser A., Rohrbeck L., Haass N.K., Herold M.J., Fairlie W.D. BCL-XL and MCL-1 are the key BCL-2 family proteins in melanoma cell survival. Cell Death Dis. 2019;10:342. doi: 10.1038/s41419-019-1568-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weeden C.E., Ah-Cann C., Holik A.Z., Pasquet J., Garnier J.M., Merino D., Lessene G., Asselin-Labat M.L. Dual inhibition of BCL-XL and MCL-1 is required to induce tumour regression in lung squamous cell carcinomas sensitive to FGFR inhibition. Oncogene. 2018;37:4475–4488. doi: 10.1038/s41388-018-0268-2. [DOI] [PubMed] [Google Scholar]
- 30.McCloy R.A., Rogers S., Caldon C.E., Lorca T., Castro A., Burgess A. Partial inhibition of Cdk1 in G2 phase overrides the SAC and decouples mitotic events. Cell Cycle. 2014;13:1400–1412. doi: 10.4161/cc.28401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.