Significance
“How can we stop aging?” is still a largely unanswered question. Understanding the possible mechanisms that lead to the gradual deterioration of the organism over time is key to answer this question and finding possible antidotes. A central tenet of the evolutionary theory of aging is the possible trade-off between the maintenance of the immortal germ line and the disposable soma. Male vertebrates continue somatic and germline proliferation throughout life, offering an ideal opportunity to study this hypothesis. We show that in male zebrafish exposed to stressful conditions, the experimental removal of the germ line improves somatic recovery. Our results provide direct evidence for the cost of the germ line in a vertebrate.
Keywords: aging, somatic maintenance, germ line
Abstract
The disposable soma theory is a central tenet of the biology of aging where germline immortality comes at the cost of an aging soma [T. B. L. Kirkwood, Nature 270, 301–304 (1977); T. B. L. Kirkwood, Proc. R. Soc. Lond. B Biol. Sci. 205, 531–546 (1979); T. B. L. Kirkwood, S. N. Austad, Nature 408, 233–238 (2000)]. Limited resources and a possible trade-off between the repair and maintenance of the germ cells and growth and maintenance of the soma may explain the deterioration of the soma over time. Here we show that germline removal allows accelerated somatic healing under stress. We tested “the expensive germ line” hypothesis by generating germline-free zebrafish Danio rerio and testing the effect of the presence and absence of the germ line on somatic repair under benign and stressful conditions. We exposed male fish to sublethal low-dose ionizing radiation, a genotoxic stress affecting the soma and the germ line, and tested how fast the soma recovered following partial fin ablation. We found that somatic recovery from ablation occurred substantially faster in irradiated germline-free fish than in the control germline-carrying fish where somatic recovery was stunned. The germ line did show signs of postirradiation recovery in germline-carrying fish in several traits related to offspring number and fitness. These results support the theoretical conjecture that germline maintenance is costly and directly trades off with somatic maintenance.
Aging is a physiological deterioration of an organism manifested in a reduced reproductive performance and an increased probability of death with advancing age (1). Cellular mechanisms of repair and maintenance have evolved (2, 3); yet in multicellular organisms, only a few specific cell lineages, such as the germ line, seem to exploit these to their full potential (4). Why are somatic repair and maintenance mechanisms not running at the same level as in the germ line? The disposable soma theory stipulates that growth and the maintenance of the cellular and genomic integrity in tissues is costly (5–8) and under the assumption of resource limitation, somatic maintenance may suffer due to resource reallocation toward key fitness functions in the organism such as reproduction. Because reproduction is a more direct proxy to fitness and because even intrinsically immortal organisms will eventually fall victim to extrinsic mortality, investing into error-proof somatic maintenance does not pay off. Limiting error-proof maintenance to the germ line and producing a disposable soma offers the opportunity to optimize resource allocation between lifespan and reproduction (9, 10, 11).
This apparent link between lifespan and reproduction has led to the widely accepted idea of a direct energy trade-off between reproduction and survival (12–17). However, more recent results seem to challenge the idea of a direct energy trade-off between reproduction and lifespan and, more importantly, its intricate role in the evolution of aging as the empirical evidence is often inconclusive, and sometimes even contradictory (16, 17, 18–24). In fact, recent discoveries undermine this simple association and a more complex interaction between growth and development time early in life and the maintenance of the soma and the germ line has been proposed (16, 25, 26, 27). The negative association between growth rate early in life and lifespan experimentally demonstrated in the house mouse Mus musculus (7) and Tasmanian snow skink Niveoscinctus microlepidotus (8), as well as comparative studies in birds and mammals showing a negative association between increased growth rate during embryo development and lifespan (28) support this notion. However, studies in Caenorhabditis elegans and Drosophila melanogaster generally have not found consistent phenotypic or genetic correlations between developmental rate, body size, and lifespan (10, 15, 17, 18–21, 29). In fact, one study looking at the association between lifespan and body size in nematode worms showed that germline ablation may result in an increase in lifespan, in body size or in both (29). In our study, we therefore set out to test an alternative trade-off, namely the one between the costs of the maintenance of the germ line and the soma—the expensive germ line hypothesis.
At the heart of the complex interaction between the soma and the germ line lies the tight linkage of mitotic growth and DNA repair and maintenance mechanisms and the apparent mutual influence on the regulation of these mechanisms in soma and germ line. The germ line has traditionally been perceived as a cell lineage that has no important role for “the survival or immediate physiological function of the individual, but rather, [is] endowed with the ability to contribute to the next generation” (22). However, recent developments in aging biology suggest otherwise and report key regulatory functions of the germ line for metabolic pathways and the immune condition of an organism (23, 24). The elimination of germ cells increases lifespan and modulates the nutrient sensing insulin/insulin-like (IIS) growth factor signaling in C. elegans (26, 27) and D. melanogaster (24) implying that the germ line controls somatic lifespan via the IIS signaling pathway. Furthermore, exogenous and endogenous DNA damage induced in germ cells in C. elegans evokes increased somatic resilience to heat and oxidative stress mediated by the ERK MAP kinase MPK-1 in germ cells triggering the induction of presumed secreted peptides associated with innate immunity (23). In turn, somatic signals sent to the germ line during stress-intense periods may result in an increased investment into somatic maintenance at the cost of reduced germline maintenance.
The mutual influence of the regulation of maintenance and repair in the soma and the germ line offer an ideal opportunity to test the expensive germ line hypothesis. Under the assumption of limited energy availability for tissue maintenance, we expect a reallocation of energy toward the maintenance of the germ line, particularly so during stressful situations requiring additional resources. The role of the germ line–soma interaction for the process of aging has mainly been tested in invertebrates and in C. elegans and D. melanogaster more specifically (23, 24). The soma of one of the key study species in aging research, C. elegans, is mitotically inactive (30). While this trait renders C. elegans an ideal study system for many aspects of aging biology, it does not reflect the processes occurring in most vertebrates and in vertebrate males in particular. Male vertebrates not only maintain a germ line with continued mitotic and meiotic divisions at generally higher rates than in female vertebrates, but also a mitotically dividing soma throughout life, and therefore offer an ideal opportunity to study the potential costs of germline maintenance. In this study we tested the expensive germ line hypothesis in a vertebrate, the zebrafish Danio rerio by testing for a possible trade-off between the maintenance of soma and germ line under stressful conditions.
Results and Discussion
Here, we tested for the potential costs of germline maintenance in male zebrafish D. rerio. We combined experimental removal of the germ line and induced DNA damage with assessments of soma and testes, DNA integrity, and cellular growth rate. In order to create germline-free fish, we collected clutches from female zebrafish immediately after spawning to perform microinjections. Each clutch was split into three groups (subclutches), and each subclutch was exposed to one of three treatments: 1) In the “germline-free” treatment (GLF), we injected antisense morpholino oligonucleotides (MOs) to knock down the germline-specific gene dead end (dnd). Knocking down dnd causes an interruption in the migration of the primordial germ cells and prohibits the formation of a germ line (31, 32) without observable side effects on somatic development. Fish in this treatment invariably developed into males with somatic testes but lacking the germ cells within (see SI Appendix for detailed tests of the efficiency of the knockdown treatment). 2) In the “injected control” treatment (IC), we performed microinjections but only injected standard control morpholino that had small phenotypic effects on zebrafish (Materials and Methods) to control for potential side effects of the injection procedure and morpholino on later life stages. 3) In the “noninjected control” treatment (NIC), we left the eggs untouched. Offspring in all three treatments were reared to sexual maturity (>6 mo old), and males were divided into two groups for two subtreatments within each treatment. One set of groups served as controls (“nonirradiated” subtreatment [NIR]) and the other set of groups was exposed to a sublethal dosage (20 Gy) of gamma-irradiation (33) using a 137Cesium source irradiator to induce DNA damage in the germ line and the soma (“irradiated” subtreatment [IR]). This resulted in a total of six experimental treatment:subtreatment combinations from three morpholino treatments and two irradiation protocols (Materials and Methods and SI Appendix, Fig. S1).
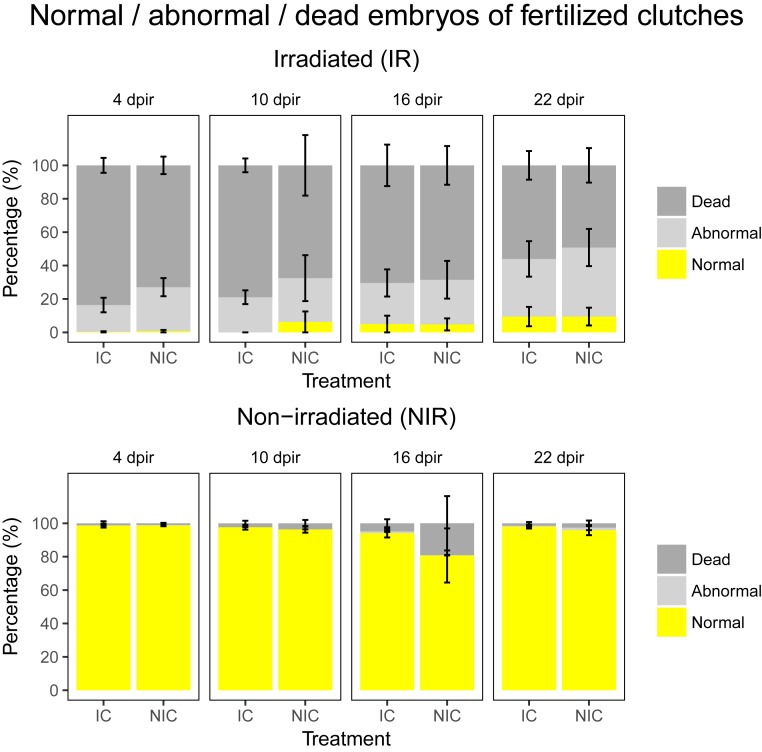
In a first step, we investigated whether our irradiation protocol had any effects on the germ cells at all. We set up the nonirradiated and irradiated males from the NIC and IC treatments with wild-type females for natural spawning starting at 4 d postirradiation (4 dpir) and repeated the setup every 6 d until 22 dpir (n = 8 for NIC:IR, IC:NIR, and NIC:NIR; n = 9 for NIC:IR; see SI Appendix, Fig. S4). We monitored the number of females that spawned, the number of eggs laid by the spawning females, and the number of fertilized and unfertilized eggs as well as the number of abnormal embryos occurring in each clutch (34). This setup served to confirm that irradiation negatively affects the germ line and that males invest in the repair of the germ line after irradiation. Supporting our expectations, irradiated males suffered reduced spawning success with a marked low in relative number of spawning events 10 dpir compared to nonirradiated males after which they recovered slowly (irradiation: χ21 = 10.519; P = 0.001, treatment: χ21 = 0.883, P = 0.347, treatment*irradiation: χ21 = 0.004, P = 0.948; see SI Appendix, Table S1 for full model; see SI Appendix, Fig. S4). Similarly, females paired with irradiated males produced smaller clutches than females paired with nonirradiated males, and the difference was particularly pronounced at 10 and 26 dpir (irradiation: χ21 = 655.614, P < 0.001, treatment: χ21 = 62.815, P < 0.001, treatment*irradiation: χ21 = 0.795, P = 0.373; see SI Appendix, Table S2 for full model; see SI Appendix, Fig. S5). The rate of fertilization success was significantly lower in clutches sired by irradiated males than clutches sired by nonirradiated males and was particularly low at 10 and 16 dpir (irradiation: χ21 = 213.280, P < 0.001; treatment: χ21 = 1.875, P = 0.171, irradiation*dpir: χ23 = 72.977, P < 0.001; see SI Appendix, Table S3 for full model; see SI Appendix, Figs. S6 and S7). Most importantly, embryos sired by irradiated males showed significantly higher levels of abnormal development and death but males started to recover 16 and 22 dpir (see Fig. 1 and SI Appendix, Fig. S8). Abnormal and dead embryos are likely a result of deleterious mutations due to DNA damage and incorrect repair caused by irradiation. These results suggest that irradiation caused an initial reduction in the numbers of intact germ cells and gametes, which were replaced over time as implied by the gradual increase in numbers of fertilized eggs and normal embryos over time postirradiation.
Fig. 1.
Percentage of normal, abnormal, and dead embryos in the clutches laid by males in two different treatments of germline-carrying fish (IC and NIC) and subtreatments (IR, NIR) from 4 to 22 dpir. (Upper) Results for irradiated fish with a clear detrimental effect of irradiation on embryo survival and development. (Lower) Males of both treatments bred at the same time points without irradiation. Values displayed represent mean ± SE. The amount of normal embryos differed significantly between treatments (irradiation: χ21 = 525.285, P < 0.001; treatment: χ21 = 73.209, P < 0.001; irradiation*dpir: χ23 = 26.407, P < 0.001; see SI Appendix, Table S4 for full model).
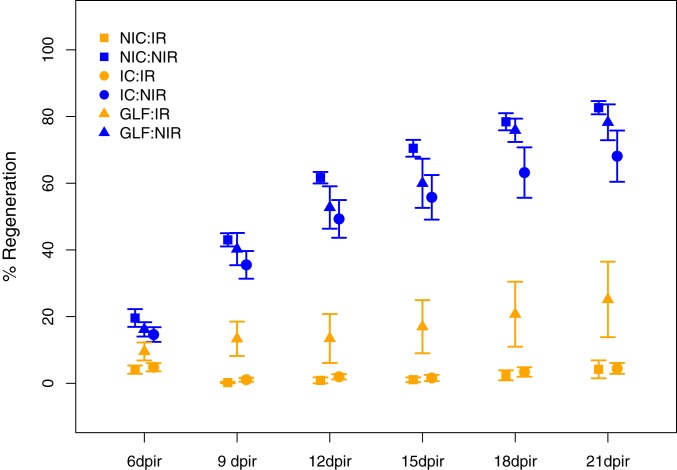
In a next step, we monitored somatic regeneration and compared this phenotype across the six experimental groups. Irradiation is known to strongly affect the regeneration time in wild-type fish (33, 35), and we employed a previously developed protocol to assess caudal fin regrowth in irradiated and nonirradiated GLF, IC, and NIC males (n = 10 for all groups). At 3 dpir, we anesthetized the males and removed 50% of the caudal fin in one clean cut. We then measured the area of the fin to monitor regrowth starting at 6 dpir and repeated this procedure every 3 d up to 21 dpir (34). As expected, irradiation had a significant effect on regeneration as all nonirradiated fish exhibited an 80% regrowth by 21 dpir, while irradiated fish showed a maximum of 40% regeneration by that time (Fig. 2). More importantly, however, irradiated GLF males showed a much higher regeneration rate already at 9 dpir where they had regrown about 16% of their caudal fin, while irradiated IC and NIC males showed hardly any regrowth at this stage. By 21 dpir, irradiated GLF males had regrown an average of about 21% while irradiated IC and NIC fish still only had about 5% regrowth by that time (Fig. 2). These striking results suggest that GLF males are capable of regenerating their soma at a higher rate, potentially because they had no need to perform repair in the germ line. This interpretation is further supported by our results that irradiated IC and NIC males only start to show recovery from the effects of irradiation 22 dpir as indicated by the increase in the number of fertilized eggs and normal embryos after a maximum dip in these numbers at 16 dpir (Fig. 1 and SI Appendix, Fig. S6).
Fig. 2.
Regrowth of caudal fin after dissection to test for somatic regeneration in males of all three treatments (GLF, IC, and NIC) with (IR) and without (NIR) irradiation measured from 6 to 21 dpir. Values displayed represent mean ± SE.
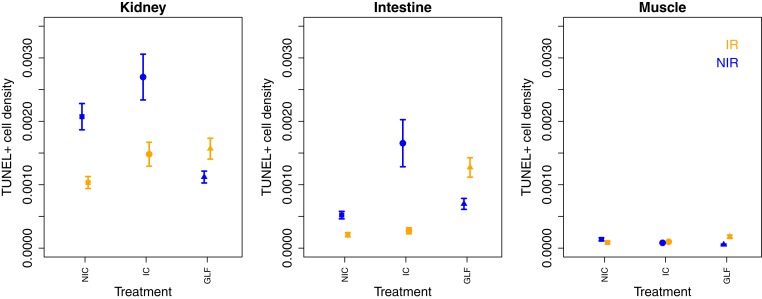
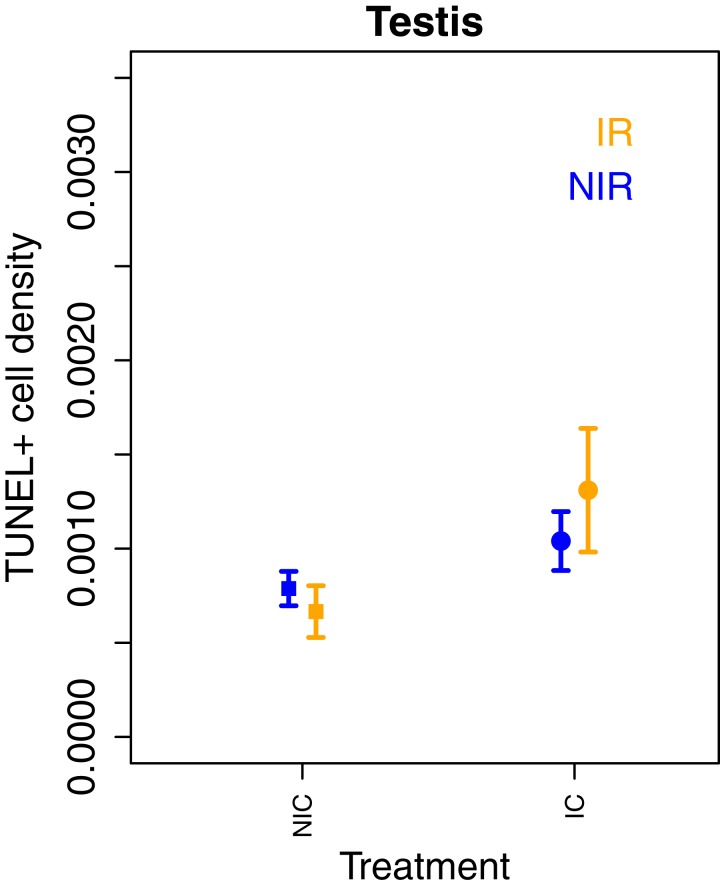
Finally, we assessed the effects of irradiation on cells in the soma and the testes (where possible). Irradiation is known to cause severe DNA damage in all tissues and particularly affects fast proliferating tissues. The most severe damages resulting from irradiation are double-strand breaks (DSBs) that may manifest themselves in premature cell apoptosis. Tissues exposed to irradiation are expected to exhibit a quick cell replacement shortly after exposure followed by a slower cell division rate in this new generation of cells. The TUNEL assay (short for terminal deoxynucleotidyl transferase dUTP nick-end labeling) allows identifying cells exhibiting DNA DSBs typical of apoptotic cells during tissue growth. We measured TUNEL+ cell density in three somatic tissues (kidney, intestine, and muscle) from four to six males per experimental groups (n = 4 in NIC:IR; n = 5 in IC:IR, IC:NIR, NIC:NIR, and GLF:NIR, n = 6 in GLF:IR) and testes (only from IC and NIC males; SI Appendix, Fig. S9) (34). We found a significant effect of treatment and irradiation on the density of TUNEL+ cells but the effects differed between tissues as indicated by significant interaction terms (Fig. 3). Overall, we observed a lower number of cells exhibiting DSBs (TUNEL+) in two of the three somatic tissues (intestines and kidney) of irradiated IC and NIC males compared to tissues of nonirradiated males, but the pattern was the exact opposite in GLF males, where irradiated males showed lower levels of TUNEL+ cells than nonirradiated males. Natural cell death through apoptosis is a sign of active tissue growth and proliferation, and a reduction may indicate the cessation of growth activities. Alternatively, irradiation may have caused increased cell deaths shortly after irradiation, and dead cells were replaced by young cells reducing the occurrence of natural cell death during normal somatic growth and maintenance. The TUNEL assay was performed only in the testes of germline-carrying fish (IC:IR, IC:NIR, NIC:IR, and NIC:NIR). Neither irradiation nor treatment affected the density of TUNEL+ cells in the testes (treatment: χ22 = 1.60, P = 0.21; irradiation: χ22 = 2.30, P = 0.13; treatment*irradiation: χ22 = 0.528, P = 0.468; Fig. 4). These results suggest an important role of the germ line in regulating somatic growth and maintenance for three reasons: First, irradiation seems to have little effect on somatic tissues in germline-free fish, whereas it causes a drastic reduction in germline-carrying fish (IC and NIC). Second, without irradiation, germline-free fish show significantly lower densities of TUNEL+ cells in somatic tissues than males from both germline-carrying treatments, which may indicate a higher maintenance and less cell death in the soma of germline-free fish under normal conditions. Third, irradiation seems to have comparatively little effect on apoptosis in both germline-carrying fish, which may indicate an up-regulation of repair mechanisms in the germ line after irradiation.
Fig. 3.
Density of TUNEL+ cells in three somatic tissues, two fast proliferating tissues (kidney and intestine) and one slow proliferating tissue (muscle) of males from three treatments (GLF, IC, and NIC) with (IR) and without (NIR) irradiation. Values displayed represent mean ± SE. Tunel+ density differed significantly across treatments and tissues (irradiation: χ21 = 693.22, P < 0.001; treatment: χ22 = 26.65, P < 0.001; dpir: χ25 = 207.32, P = 0.001, treatment*irradiation: χ22 = 50.17, P < 0.001, irradiation*dpir: χ25 = 26.34, P < 0.001, treatment*dpir: χ21 = 26.34, P < 0.001; see SI Appendix, Table S5 for complete model).
Fig. 4.
Density of TUNEL+ cells in the testes of males from two treatments (IC and NIC) with (IR) and without (NIR) irradiation. Values displayed represent mean ± SE. We found no significant differences between treatments (irradiation: χ21 = 12.42, P = 0.0004; treatment: χ22 = 6.18, P = 0.046; tissue: χ22 = 75.08, P < 0.0001; treatment*irradiation: χ22= 8.55, P = 0.013; treatment*tissue: χ24 = 23.75, P < 0.0001; irradiation*tissue: χ22 = 46.56, P < 0.0001; irradiation*treatment*tissue: χ24 = 49.10, P < 0.0001).
Overall, our results provide compelling support for the hypothesis that germline maintenance is costly and trades off with somatic maintenance. Under irradiation, germline-carrying males appear to invest much of their resources into the repair of the germ line while germline-free fish have no such costs and continue repair and maintenance of the soma and restore somatic growth capacity at a faster rate (Fig. 2). The reversed pattern of the amount of DSBs in tissues of germline-carrying and germline-free fish with and without irradiation further supports the idea that putative germline signals play a key role in the maintenance of the soma. The lower amount of apoptosis in irradiated germline-carrying fish may indicate a signal from the germ line to increase repair, resulting in reduced growth and apoptosis. Fast-proliferating tissues such as the kidney and intestines were more affected by irradiation than muscle tissue, which further supports the idea that natural apoptosis reflects active tissue growth based on natural cell death.
The essential immortality of the germ line requires a highly efficient mechanism to maintain the integrity of germ cells during mitotic and meiotic divisions and in the face of continuous damage of the proteome and the genome. Indirect evidence for the cost of germline maintenance comes from several different lines of evidence. Drosophila lines exposed to X-ray irradiation, for example, evolved increased resistance to DNA damage but this resistance was lost after cessation of exposure to X-ray, further supporting the idea that increased DNA maintenance is costly (36). The cost of germline maintenance can also be inferred from the fact that many mammals and birds reduce the size of their germ line to a minimum outside the breeding season (37, 38) and that germline ablation in C. elegans nematodes and male D. melanogaster fruitflies results in an increase in lifespan (29, 39). Further indirect evidence comes from studies showing that castration in males results in a significant increase in lifespan in rats (40) and humans (41). However, a direct link between the repair and maintenance of soma and germ line has to date not been demonstrated in any vertebrate. Our study provides a step in that direction but further investigations are needed to understand the underlying signaling pathways.
Mechanisms involved in the protection of the germline genome from DNA damage include active DNA maintenance and repair through checkpoints. Besides the DNA repair mechanisms, germ cells contain molecular chaperones and different proteolytic systems, in particular the ubiquitin–proteasome system (UPS) and autophagy that detect and repair damaged proteins, or fully degrade the ones that are beyond repair in order to maintain high quality cells (42). These maintenance mechanisms are vital for the extended proliferation of a cell lineage but they are likely to come at the cost of a slowed down rate of proliferation. Evidence for the cost of DNA replication comes from studies in microorganisms where elevated stress levels generate increased genetic variation, but may also reflect the limited resources available for high-fidelity replication under suboptimal conditions (43). Moreover, high replication fidelity in the T4 bacteriophage has been shown to impose time and energy costs (44). A recent study in cell cultures of house mice showed that the replication timing in germ cells is positively correlated with mutation rate, suggesting again a trade-off between in-cell proliferation and replication fidelity (45). Comparing somatic and germ line mutation rates in germline-free and germline-carrying organisms will be an interesting next step.
Conclusions
Our findings support the idea of a cost in male germline maintenance in a vertebrate. The positive effect of germline ablation on somatic growth described here suggests that resources allocated to germline repair in germline-carrying fish are available to restore growth in the soma of germline-free fish. The density of cells showing signs of apoptotic DNA DSBs after irradiation seems to be unaffected by irradiation in germline-free fish, which further supports the idea that germline and somatic maintenance are tightly linked in vertebrates. Understanding the underlying mechanisms is now a necessary next step.
Materials and Methods
General Fish Maintenance.
Wild-type AB zebrafish, D. rerio, were purchased from the Zebrafish International Resource Center, University of Oregon, Eugene, OR and maintained under standard laboratory conditions in the SciLifeLab facility at Evolutionary Biology Center at Uppsala University, Sweden (https://www.scilifelab.se/facilities/genomeengineeringzebrafish/). Descendants of the original fish were used in this study and were bred following a strict outbreeding regime to preserve the genetic variation. The fish were kept in 3-L tanks a 12:12 h dark:light cycle at 28 °C and fed with live Artemia (brine shrimp cysts; ZM System) and dried flake food (Zeigler adult zebrafish diet; Aquatic Habitats) three (for adult fish) to five (for juvenile fish) times a day.
Split-Clutch Design.
We employed a split-clutch design to minimize potential genotype-by-treatment effects (SI Appendix, Fig. S1). Briefly, we bred AB zebrafish in pairs (i.e., one male and one female) and collected the clutch that they produced. We split each clutch and randomly assigned embryos to one of the six different treatments so that offspring from each family were present in all six treatments.
MO and the Production of Germline-Free Zebrafish.
We employed antisense MOs, a tool commonly used to study gene function through targeted knockdown in zebrafish (46), to target a germline-specific gene, dead end (dnd). In zebrafish, dead end (dnd) RNA is a key component of germ plasm formation and is expressed specifically in primordial germ cells (PGCs) throughout embryogenesis (31). In dnd-MO-injected zebrafish embryos, dnd RNA translation is inhibited, which causes defected PGC migration and eventually the death of PGCs (31). PGC-depletion induced by dnd-MO is dosage dependent, and a dosage above 200 pg results in complete PGC loss (31). PGC survival is crucial for sex determination in zebrafish and their removal precludes the development of female fish (47, 48). Importantly, while the germ plasm is fully ablated in dnd-MO-injected, PGC-depleted embryos, somatic development of the gonads remains unaffected, and the embryos develop into germline-free, sterile males (31, 47, 48). As a result, dnd-MO-injected males have somatic testes (albeit smaller) but no germ cells and exhibit a male phenotype (body size and color) and display normal male sexual behavior toward females. They are also able to induce female spawning but fail to fertilize the eggs.
The evening before the day of microinjection, pairs of one male and one female were transferred into breeding tanks with a divider separating the fish from each other. We removed the dividers in the morning to allow spawning and resulting clutches of eggs were collected. The collected eggs were rinsed and kept in E3 medium for later use. We split each clutch into three equal parts and each part was randomly assigned to one of three treatments: NIC, MO-injected (IC), or dnd-MO–injected (GLF).
To produce germline-free zebrafish, we delivered dnd-MO (5′- GCTGGGCATCCATGTCTCCGACCAT-3′) to zebrafish embryos by microinjection. To control for the effect of microinjection on embryos (IC), we used a standard control MO, which targets a human beta-globin intronic mutation and has little phenotypic effect on zebrafish. Both MOs were purchased from Genetools and prepared in Danieau buffer according to the manufacturer’s instructions. To ensure complete germline ablation, 400 pg of dnd-MO was injected into zebrafish embryos assigned to dnd-MO-injected treatment. The same quantity of control-MO was injected into embryos of control-MO-injected treatment.
Eggs assigned to the two MO-injected treatments were aligned on a nylon mesh in a 9-cm Petri dish filled with E3 medium. Microinjection of MO was performed before the two-cell stage as previously described (31, 49). After microinjection, eggs were placed in an incubator at 28.5 °C. The day after injections, all clutches were monitored for dead or malformed embryos and these were removed. Normally developing embryos were cleaned and raised to sexual maturity and then maintained at a 1:1 sex ratio until use with random females.
To assure the successful removal of germ cells, we performed several tests on the adult males used for the different parts of the experiment: 1) we collected ejaculates from males in all three treatments and looked for the presence of sperm; 2) we set up males from all treatments for natural spawning with wild-type AB females and assessed the percentage of successful spawning and the successful development of any embryos in a clutch; and 3) we performed reverse transcription PCRs to test for the expression of the germline-specific gene piwil1 in the testes of males from all treatments (see SI Appendix for details on all tests).
Visible Implant Elastomer Tagging and Whole-Body Irradiation.
Sexually mature (approximately 6 mo old) zebrafish of NIC, IC, or GLF treatments were randomly assigned to either IR or NIR subtreatments, resulting in six treatment:subtreatment regimes: NIC:IR, NIC:NIR, IC:IR, IC:NIR, GLF:IR, and GLF:NIR. To facilitate individual identification, fish were tagged using a visible implant elastomer (VIE) tagging kit (purchased from Northwest Marine Technology, Inc.) according to the manufacturer’s instructions. VIE-tagged fish were allowed to recover for at least 2 wk, and fish assigned to IR subtreatment (NIC:IR, IC:IR, and GLF:IR) were exposed to a sublethal dosage (20 Gy) of gamma-irradiation using a 137Cesium source irradiator at Stockholm University. For irradiation, fish were placed in standard six-well plates (volume 16.8 mL per cell) half-filled with system water (one to two fish per well) without being anesthetized. Plates were then tape sealed and placed in the irradiator until desired dosage of irradiation was reached (approximately 25 min).
Reproduction Assay.
We used germline-carrying fish (NIC and IC) for testing the effect of irradiation on male reproductive success by pairing them with wild-type females for spawning. We monitored spawning success (i.e., spawned or did not spawn), number of eggs per clutch, fertilization success rate (2 h postfertilization [hpf]), and embryo morphological normality and survival (24 hpf).
One to two males from each of the four treatment:subtreatment regimes (NIC:IR, NIC:NIR, IC:IR, and IC:NIR) were kept in groups of 8 to 10 fish (four to five males, and an equal number of wild-type females of similar age) in 3-L tanks. Reproduction was measured at 4, 10, 16, and 22 dpir, with 6 d between each time point to allow for the completion of one spermatogenetic cycle (50). Fish were set up for breeding as described above with the only modification that males were given 2 h to breed with a first wild-type female, and if this attempt failed, the wild-type females were replaced by a second wild-type female. Males that successfully reproduced with the first wild-type female were not set up with a second one. We monitored all spawning events and noted if spawning occurred or not (i.e., if eggs were laid or not; SI Appendix, Fig. S4).
The clutches obtained from these spawning events were assessed for number of eggs (SI Appendix, Fig. S4), fertilization success, and the ratio of normal, abnormal, and dead embryos in each laid clutch (SI Appendix, Figs. S6–S8). To assess embryo normality, we followed the descriptions and figures in Westerfield (51).
Regeneration Assay.
Caudal fin clipping was performed 3 dpir. For fin clipping, fish were anesthetized using AquaCalm (metomidate hydrochloride; purchased from Syndel Laboratories Ltd.) and placed on their sides in a 6-cm Petri dish half filled with fresh system water. Using a scalpel, ∼50% of the caudal fin was removed in one clean cut. Pictures were taken using a Nikon DS-2MV camera mounted on a Nikon SMZ18 dissection microscope before and after clipping for later reference. After clipping, the fish were placed in a separate tank for approximately 1 h to allow recovery and when fully recovered, they were placed back into the system. To measure regeneration, we assessed the area of regrown fin every 3 d until 21 dpir (i.e., on 6, 9, 12, 15, 18, and 21 dpir). To do so, we anesthetized fish as described above to take pictures of the caudal fin. For each time point, three pictures were taken for each fish, and the mean fin area was calculated and used for analysis. Fin area was measured using FIJI software (https://fiji.sc/; ref. 52), and regeneration was calculated as new tissue area/original area clipped as described before (53).
TUNEL Staining.
We assessed DNA DSBs in irradiated and nonirradiated fish in somatic tissues (all treatment groups) and in the germline (NIC and IC). We performed a TUNEL assay, which allows the labeling and visualization of the blunt ends of double-strand DNA breaks by the TdT enzyme resulting in cells being TUNEL-positive (TUNEL+).
Seven days postirradiation, fish were killed, fish torsos were dissected (head and caudal area were discarded), the body cavity was cut open to allow the fixative to penetrate all tissues, and the torso fixed in Bouin’s fixative at room temperature overnight on a rotator. Samples were then washed in 70% ethanol and dehydrated in an ethanol/xylene series. Following stepwise dehydration (SI Appendix), the body was embedded in paraffin (SI Appendix) and sliced into 5-μm sections using a Leica RM 2155 microtome. TUNEL staining was performed using a DeadEnd Fluorometric TUNEL System kit (Promega, G3250) following the manufacturer’s instructions. For logistic reasons, TUNEL staining was done in four independent blocks. Fluorescent images of kidney, intestine, muscle (in all fish), and testis (in NIC and IC fish) were taken using a Zeiss LSM710 SIM confocal microscope at BioVis, Uppsala University. We used five consecutive sections for each of four to six males per treatment from the central region of the body cavity (see SI Appendix, Fig. S9 for examples). In each section, TUNEL+ cells were counted for the entire specific tissue area available (measured using a brightfield image) and tissue area was measured using FIJI software. TUNEL+ cell density was derived by dividing TUNEL+ cell number by tissue area measured following the process described in ref. 54.
Statistical Analysis.
All analyses were performed using packages lme4 (55) and car (56) R version 3.3.3 (57) and R Studio version 1.1.414. Data on cell density from the TUNEL assay were transformed by square root to obtain a normal error structure. We first tested how somatic tissues responded to irradiation in both germline-carrying fish and germline-free fish. We fitted TUNEL+ cell density to a linear mixed-effect model, with treatment (NIC, IC, or GLF), irradiation (IR or NIR), and tissue (kidney, intestine, or muscle) fitted as fixed effects and block and male identification (ID) included as a random effects. To test how the germline DNA was affected by irradiation in germline-carrying fish (IC and NIC), we included TUNEL+ cell density as response variable and treatment and irradiation (IR or NIR) as fixed effects and block as a random effect in a linear mixed-effect model.
Spawning success was analyzed using a generalized linear model, with treatment (NIC or IC), irradiation (IR or NIR), days postirradiation, and their interactions fitted as predictors. Clutch size, fertilization success, and embryo development were analyzed in generalized linear mixed-effects models with binomial error structure and logit link function with treatment (NIC or IC), irradiation (IR or NIR), days postirradiation, and their interactions fitted as fixed effects, and tank was included as random effects.
Regeneration was analyzed using a generalized linear model with binomial error structure and logit link function where treatment (NIC, IC, or GLF), irradiation (IR or NIR), days postirradiation, and their interactions were included as fixed effects. We included family ID and male ID as random factors where needed.
Ethics.
All experimentation in this study was in accordance with the guidelines of the Swedish Board of Agriculture and approval was also provided by the board (permit no. C28/16).
Data Availability.
All data are available on Dryad (34).
Supplementary Material
Acknowledgments
We thank Alexei Maklakov and two anonymous reviewers for comments on earlier drafts of this manuscript. This study was funded by a Wallenberg Academy Fellowship and by grants from the European Research Council (ERCStG HapSelA-336633) and the Human Frontier Science Program (HFSP R0025/2015) to S.I.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: All data are available on Dryad (DOI: 10.5061/dryad.mkkwh70w8).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1918205117/-/DCSupplemental.
References
- 1.Partridge L., Barton N. H., Optimality, mutation and the evolution of ageing. Nature 362, 305–311 (1993). [DOI] [PubMed] [Google Scholar]
- 2.Vilchez D., Saez I., Dillin A., The role of protein clearance mechanisms in organismal ageing and age-related diseases. Nat. Commun. 5, 5659 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Schumacher B., Garinis G. A., Hoeijmakers J. H. J., Age to survive: DNA damage and aging. Trends Genet. 24, 77–85 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Weismann A., Das Keimplasma: Eine Theorie der Vererbung (Gustav Fisher Verlag, Jena, Germany, 1892). [Google Scholar]
- 5.Kirkwood T. B. L., Evolution of ageing. Nature 270, 301–304 (1977). [DOI] [PubMed] [Google Scholar]
- 6.Kirkwood T. B. L., Holliday R., The evolution of ageing and longevity. Proc. R. Soc. Lond. B Biol. Sci. 205, 531–546 (1979). [DOI] [PubMed] [Google Scholar]
- 7.Eklund J., Bradford G. E., Longevity and lifetime body weight in mice selected for rapid growth. Nature 265, 48–49 (1977). [DOI] [PubMed] [Google Scholar]
- 8.Olsson M., Shine R., Growth to death in lizards. Evolution 56, 1867–1870 (2002). [DOI] [PubMed] [Google Scholar]
- 9.Kirkwood T. B. L., Austad S. N., Why do we age? Nature 408, 233–238 (2000). [DOI] [PubMed] [Google Scholar]
- 10.Gerisch B., Antebi A., “Molecular basis of life history regulation in C. elegans and other organisms” in Mechanisms of Life-History Evolution, Flatt T., Heyland A., Eds. (Oxford University Press, Oxford, UK, 2011). [Google Scholar]
- 11.Maklakov A. A., Immler S., The expensive germline and the evolution of ageing. Curr. Biol. 26, R577–R586 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Jenkins N. L., McColl G., Lithgow G. J., Fitness cost of extended lifespan in Caenorhabditis elegans. Proc. Biol. Sci. 271, 2523–2526 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker D. W., McColl G., Jenkins N. L., Harris J., Lithgow G. J., Natural selection–Evolution of lifespan in C. elegans. Nature 405, 296–297 (2000). [DOI] [PubMed] [Google Scholar]
- 14.Briga M., Verhulst S., What can long-lived mutants tell us about mechanisms causing aging and lifespan variation in natural environments? Exp. Gerontol. 71, 21–26 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Kenyon C., The plasticity of aging: Insights from long-lived mutants. Cell 120, 449–460 (2005). [DOI] [PubMed] [Google Scholar]
- 16.Leroi A. M., et al. , What evidence is there for the existence of individual genes with antagonistic pleiotropic effects? Mech. Ageing Dev. 126, 421–429 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Flatt T., Survival costs of reproduction in Drosophila. Exp. Gerontol. 46, 369–375 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Zwaan B. J., Bijlsma R., Hoekstra R. F., On the developmental theory of ageing. I. starvation resistance and longevity in Drosophila melanogaster in relation to pre-adult breeding conditions. Heredity 66, 29–39 (1991). [DOI] [PubMed] [Google Scholar]
- 19.Zwaan B., Bijlsma R., Hoekstra R. F., Direct selection on life span in Drosophila melanogaster. Evolution 49, 649–659 (1995). [DOI] [PubMed] [Google Scholar]
- 20.Partridge L., Prowse N., Pignatelli P., Another set of responses and correlated responses to selection on age at reproduction in Drosophila melanogaster. Proc. Biol. Sci. 266, 255–261 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCulloch D., Gems D., Evolution of male longevity bias in nematodes. Aging Cell 2, 165–173 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Lehmann R., Germline stem cells: Origin and destiny. Cell Stem Cell 10, 729–739 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ermolaeva M. A., et al. , DNA damage in germ cells induces an innate immune response that triggers systemic stress resistance. Nature 501, 416–420 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flatt T., et al. , Drosophila germ-line modulation of insulin signaling and lifespan. Proc. Natl. Acad. Sci. U.S.A. 105, 6368–6373 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansen M., Hsu A. L., Dillin A., Kenyon C., New genes tied to endocrine, metabolic, and dietary regulation of lifespan from a Caenorhabditis elegans genomic RNAi screen. PLoS Genet. 1, 119–128 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsin H., Kenyon C., Signals from the reproductive system regulate the lifespan of C. elegans. Nature 399, 362–366 (1999). [DOI] [PubMed] [Google Scholar]
- 27.Arantes-Oliveira N., Apfeld J., Dillin A., Kenyon C., Regulation of life-span by germ-line stem cells in Caenorhabditis elegans. Science 295, 502–505 (2002). [DOI] [PubMed] [Google Scholar]
- 28.Ricklefs R. E., Embryo growth rates in birds and mammals. Funct. Ecol. 24, 588–596 (2010). [Google Scholar]
- 29.Patel M. N., Knight C. G., Karageorgi C., Leroi A. M., Evolution of germ-line signals that regulate growth and aging in nematodes. Proc. Natl. Acad. Sci. U.S.A. 99, 769–774 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimble J. E., White J. G., On the control of germ cell development in Caenorhabditis elegans. Dev. Biol. 81, 208–219 (1981). [DOI] [PubMed] [Google Scholar]
- 31.Weidinger G., et al. , Dead end, a novel vertebrate germ plasm component, is required for zebrafish primordial germ cell migration and survival. Curr. Biol. 13, 1429–1434 (2003). [DOI] [PubMed] [Google Scholar]
- 32.Slanchev K., Stebler J., de la Cueva-Méndez G., Raz E., Development without germ cells: The role of the germ line in zebrafish sex differentiation. Proc. Natl. Acad. Sci. U.S.A. 102, 4074–4079 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Traver D., et al. , Effects of lethal irradiation in zebrafish and rescue by hematopoietic cell transplantation. Blood 104, 1298–1305 (2004). [DOI] [PubMed] [Google Scholar]
- 34.Immler S., Chen H.-y., Bublys K., Jolly C., Marcu D., Data from: Trade-off between somatic and germline repair in a vertebrate supports the expensive germ line hypothesis. Dryad. 10.5061/dryad.mkkwh70w8. Deposited 26 March 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai S. B., et al. , Differential effects of genotoxic stress on both concurrent body growth and gradual senescence in the adult zebrafish. Aging Cell 6, 209–224 (2007). [DOI] [PubMed] [Google Scholar]
- 36.Nöthel H., Adaptation of Drosophila melanogaster populations to high mutation pressure: Evolutionary adjustment of mutation rates. Proc. Natl. Acad. Sci. U.S.A. 84, 1045–1049 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malecki I. A., et al. , Endocrine and testicular changes in a short-day seasonally breeding bird, the emu (Dromaius novaehollandiae), in southwestern Australia. Anim. Reprod. Sci. 53, 143–155 (1998). [DOI] [PubMed] [Google Scholar]
- 38.Jiménez R., Burgos M., Barrionuevo F. J., Circannual testis changes in seasonally breeding mammals. Sex Dev. 9, 205–215 (2015). [DOI] [PubMed] [Google Scholar]
- 39.Barnes A. I., Boone J. M., Jacobson J., Partridge L., Chapman T., No extension of lifespan by ablation of germ line in Drosophila. Proc. Biol. Sci. 273, 939–947 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drori D., Folman Y., Environmental effects on longevity in the male rat: Exercise, mating, castration and restricted feeding. Exp. Gerontol. 11, 25–32 (1976). [DOI] [PubMed] [Google Scholar]
- 41.Min K.-J., Lee C.-K., Park H.-N., The lifespan of Korean eunuchs. Curr. Biol. 22, R792–R793 (2012). [DOI] [PubMed] [Google Scholar]
- 42.Koga H., Kaushik S., Cuervo A. M., Protein homeostasis and aging: The importance of exquisite quality control. Ageing Res. Rev. 10, 205–215 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sniegowski P. D., Gerrish P. J., Johnson T., Shaver A., The evolution of mutation rates: Separating causes from consequences. BioEssays 22, 1057–1066 (2000). [DOI] [PubMed] [Google Scholar]
- 44.Bessman M. J., Muzyczka N., Goodman M. F., Schnaar R. L., Studies on the biochemical basis of spontaneous mutation. II. The incorporation of a base and its analogue into DNA by wild-type, mutator and antimutator DNA polymerases. J. Mol. Biol. 88, 409–421 (1974). [DOI] [PubMed] [Google Scholar]
- 45.Yehuda Y., et al. , Germline DNA replication timing shapes mammalian genome composition. Nucleic Acids Res. 46, 8299–8310 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bill B. R., Petzold A. M., Clark K. J., Schimmenti L. A., Ekker S. C., A primer for morpholino use in zebrafish. Zebrafish 6, 69–77 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tzung K.-W., et al. , Early depletion of primordial germ cells in zebrafish promotes testis formation. Stem Cell Reports 4, 61–73 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siegfried K. R., Nüsslein-Volhard C., Germ line control of female sex determination in zebrafish. Dev. Biol. 324, 277–287 (2008). [DOI] [PubMed] [Google Scholar]
- 49.Rosen J. N., Sweeney M. F., Mably J. D., Microinjection of zebrafish embryos to analyze gene function. J. Vis. Exp. 25, 1115 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leal M. C., et al. , Histological and stereological evaluation of zebrafish (Danio rerio) spermatogenesis with an emphasis on spermatogonial generations. Biol. Reprod. 81, 177–187 (2009). [DOI] [PubMed] [Google Scholar]
- 51.Westerfield M., The Zebrafish Book , A Guide for the Laboratory Use of Zebrafish (Danio rerio) (University of Oregon Press, ed. 4, 2000). [Google Scholar]
- 52.Schindelin J., et al. , Fiji: An open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petrie T. A., Strand N. S., Yang C. T., Rabinowitz J. S., Moon R. T., Macrophages modulate adult zebrafish tail fin regeneration. Development 141, 2581–2591 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zilberberg J., McElhaugh D., Gichuru L. N., Korngold R., Friedman T. M., Inter-strain tissue-infiltrating T cell responses to minor histocompatibility antigens involved in graft-versus-host disease as determined by Vbeta spectratype analysis. J. Immunol. 180, 5352–5359 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bates D., Maechler M., Bolker B., lme4: Linear mixed-effects models using S4 classes. R package Version 1.1-21. https://cran.r-project.org/web/packages/lme4/index.html. Accessed 3 May 2019.
- 56.Fox J., Weisberg S., An {R} Companion to Applied Regression (SAGE Publications, Thousand Oaks, ed. 3, 2019). [Google Scholar]
- 57.R Core Team , R: A language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria, 2019). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available on Dryad (34).