Abstract
Background
Diet and physical activity are the most commonly recommended strategies for preventing and treating metabolic syndrome (MetS). This randomized trial aims to examine the effectiveness of a weight reduction intervention based on caloric restriction, low-impact aerobics (LIA), and a resistance-training program in improving body composition, metabolic parameters and cardiovascular disease (CVD) risk factors among obese students diagnosed with MetS.
Methods
In all, 23 male participants, aged 19–24 years, were randomly introduced to a dieting program (the diet group, or DG = 09) or to dieting associated with a supervised physical training program (the diet plus training group, or DTG = 14). Before and after the intervention, the participants’ anthropometric measures and cardiovascular disease risk factors were assessed.
Results
Following the diet-based intervention, significant improvements were noted in BMI (p = 0.39), PBF (p = 0.022) and LDL-c (p = 0.024). However, in response to the diet plus aerobic and resistance exercise intervention, obese participants had significant reductions in body weight (p = 0.018), WC (p = 0.042), BMI (p = 0.001), BFP (p < 0.001), DBP (p = 0.013), SBP (p = 0.016), TG level (p = 0.026), TC (p = 0.016), LDL-c (p = 0.001) and VLDL-c (p = 0.026). Notable differences were also observed between groups in terms of changes in WC (p = 0.003), BFP (p = 0.05), WHR (p = 0.029), FBG level (p = 0.022), TG level (p = 0.001), TC (p = 0.006), LDL-c (p = 0.014) and VLDL-c (p < 0.001).
Conclusion
Diet-based intervention could be an effective tool in reducing body composition and some MetS components. However, adding three weekly aerobic and resistance-training sessions to the dieting program may deliver better outcomes, particularly in terms of reducing WC, BFP, WHR, FBG level, TG level, TC, LDL-c, and VLDL-c.
Keywords: Obesity, Metabolic syndrome, Diet, Low-impact aerobics, Resistance training
Introduction
Metabolic syndrome (MetS) is a cluster of interconnected factors that directly increase the risk of heart disease, stroke, and type 2 diabetes.1 The National Cholesterol Education Program’s Adult Treatment Panel III (ATP III)2 define MetS as the co-occurrence of any three of the five following abnormalities: central obesity (WC > 102 cm in men and >88 in women), high blood pressure (>130/85 mm Hg), high fasting blood glucose (>110 mg.dL−1), hypertriglyceridemia (>150 mg.dL−1), and low level of high-density lipoprotein cholesterol (>40 mg.dL−1 in men and >50 mg.dL in women; HDL-C). In a recent study, Kaur3 reported that the prevalence of MetS varied from 10% to 84% worldwide depending on the age, ethnicity and gender. Moreover, the International Diabetes Federation affirmed that around 25% of the world population has MetS, and ∼80% of them are at risk of death from cardiovascular diseases.4
The underlying causes of MetS include controllable factors e.g. weight excess and physical inactivity, and uncontrollable factors e.g. genetic factors and aging. Throughout a 10-year longitudinal study, Hwang et al.5 noted a strong association between high body mass indices and the incidence of metabolic disorders related to CVDs. An excess of fat mass can facilitate a greater release of metabolites and pro-inflammatory biomarkers that may increase the relative risk of diabetes development by three-to five-fold and CVD by two-fold.6 Consequently, weight loss interventions are used as important healthcare measures for reducing the harmful effects of obesity, MetS, and other diseases.7 Several epidemiological studies have shown that dyslipidemia, insulin resistance, and risk of CVD reduce after approximately 5% of a patient’s initial body weight is shed.8 Stewart et al.9 noted that these changes depend largely on the reduction in abdominal fat more than that in body weight.
Diet and/or physical activity are the strategies most commonly prescribed by studies and health organizations to improve glucose metabolism, skeletal muscle function, bone stability, psychological well-being, and other organ functions.10 Prior observational research has shown that consuming foods rich in dairy, fish, fruits, vegetables and whole grains is an efficient strategy to metabolic abnormalities and heart diseases, whereas, a high intake of saturated fat, trans fats, refined grains and fried foods may increase the risk to develop MetS or its components.11,12 Schulze and Hu13 indicated in addition that lowering total fat, mainly by consuming unsaturated fats from natural foods, is an efficient strategy to counteract MetS and prevent coronary heart disease.
Evidence noted also that healthy diet associated with sufficient physical activity might have greater effects than diet alone or physical exercises alone. Physical activity presents significant interactions with the naturally representative dietary patterns in individuals with MetS.14,15 In a systematic review of 24 studies, Vasconcellos et al.10 noted that interventions that include physical activity programs are very likely to induce important changes in BFP, WC, systolic blood pressure (SBP), insulin resistance, LDL-c, and TC as well as noticeable but statistically insignificant changes in diastolic blood pressure (DBP), FBG level, and HDL-c. The authors noted that only four of the reviewed studies used resistance-training programs, while the others used cyclic or acyclic aerobic exercises. None applied aerobic plus resistance training programs. These studies affirmed in addition that the use of aerobic exercises coupled with caloric restriction plays an essential role in the prevention and treatment of MetS.16 Resistance training can also produce significant effects on some MetS components and could be used as an effective tool to manage the syndrome.17 Sorace et al.16 indicated that resistance training performed at least twice weekly may reduce the risk and prevalence of MetS and its components in adults. Lee et al.18 have shown that resistance training associated with caloric restriction has an important role in managing central adiposity, insulin sensitivity, and FBG in adolescent boys. Significant reductions in TC, LDL-c, TG level, and hypertension were also noted in participants following a resistance-training program.19
Previous intervention studies/systematic reviews have looked at the impact of diet plus aerobic and resistance training programs among metabolically healthy obese individuals, nevertheless, none of them has studied these effects among patients with MetS.17,20 In addition, Sorace et al.16 affirmed that practitioners should be careful when recommending aerobic exercise combined with resistance training in attempts to prevent or treat obesity and MetS risk factors. This may be due to the smaller effects observed after resistance training alone on body compositions,20 and the association between a higher muscle mass and some metabolic disturbances in obese individuals.21 Thus, in the current study, we entirely focus on the effects of a weight reduction intervention based on caloric restriction and low-impact aerobics (LIA) combined with a resistance-training program on body composition, metabolic parameters and cardiovascular disease risk factors among obese students with metabolic syndrome. We hypothesized that a daily caloric restriction program could be an effective tool in improving body weight, body composition, and several MetS components. However, the introduction of three weekly aerobic and resistance-training sessions to the caloric restriction program may deliver better outcomes.
Materials and methods
Participants
In all, 299 male students from four colleges of King Faisal University (KFU), aged 19–24 years, were evaluated for potential enrollment. The inclusion criteria were a sedentary lifestyle (walking less than 1.5 miles daily), a BMI between 30 and 40 kg m−2, a lack of any contraindications regarding physical exercises according to the American College of Sports Medicine guidelines,22 and presenting the characteristics of the MetS as defined by the ATP III.2 Participants who met the first three inclusion criteria (N = 64) were screened for MetS, and those diagnosed with this disease (N = 23) were randomly introduced to a dieting program (the dieting group, or DG: N = 9) or to dieting associated with a supervised physical training program (the diet and training group, or DTG: N = 14) (Fig. 1). The randomization scheme consists of an arbitrary introduction of all students from the same college to one of the two intervention groups. Each group acts separately from the other one, and a prospective single-blinded was adopted for this study. All participants were informed about the possible risks and benefits of their intervention and signed a consent form in advance.
Fig. 1.
The flow of participant selection throughout the study.
Study design and ethics
This study was conducted between November 2017 and April 2018 in compliance with the Declaration of Helsinki reporting to ethical principles for medical research involving human subjects.23 Ethical approval was obtained from the Ethical Committee of the Deanship of Scientific Research of KFU (project number 186020, Saudi Arabia).
The study spanned 16 weeks, the first four of which were dedicated to establishing changes in the participants’ dietary behaviors (Run-in phase = 04 weeks). Following this stage, the participants were randomly distributed into two groups. The first group, the DG, continued with the dieting program without any further changes, while supervised aerobic and resistance-training sessions were added to the next 12 weeks of the program of the second group, the DTG (Training phase = 12 weeks). In addition, one day prior the intervention and after each 4 weeks, all participants were scheduled for lifestyle counseling sessions conducted by lifestyle coaches and aimed to motivate them and adjust their programs, if necessary. Before and after intervention, body compositions and CVD risk factors were assessed on alternate days in both groups by the same investigators.
Outcomes and assessments
Anthropometric measurements
Anthropometric measurements were performed with a multi-frequency, whole body and segmental body composition analyzer ACCUNIQ BC360 (SELVAS Healthcare Inc., Daejeon, Korea), which measures the changes in the fat and the muscles in the body using bioelectrical impedance analysis technology. Measurements were conducted in standing position and using eight electrodes placed on both hands and feet. Height was measured with an Ultrasonic Height meter connected to the device and body weight, WC, BMI, PBF, and waist-to-hip ratio (WHR) were automatically determined. The participants’ palms and soles should be cleaned before each measurement and participants were prohibited from eating or drinking any beverage 4 h before testing.24
Blood sample analysis
Following a fast of 12 h or more and a 10-min relaxation period in the laboratory, participant blood pressures (BPs) were measured in duplicate using the right arm, and using a wireless BP monitor (iHealth Labs Inc., Canada). In addition, approximately 10 ml of venous blood were collected from each patient into sterile Vacutainer-SST II advance tubes (Becton Dickinson, Plymouth, UK). Blood was centrifuged at 3000 rpm for 15 min at 4 °C and the serum was withdrawn and stored until analysis. FBG, TC, TG and HDL-c concentrations were performed on Vitros 250 Chemistry Analyzer (Ortho-Clinical Diagnostics, Inc. Rochester, NY, USA) using specific VITROS Chemistry Products. FBG was measured using an enzymatic colorimetric method with glucose oxidase (VITROS GLU Slide method). The intra- and inter-assay coefficients of variations (CVs) for the Vitros GLU were 1.5–1.7%. TC was assessed with cholesterol ester hydrolase and cholesterol oxidase using an enzymatic method (VITROS CHOL Slide method). Intra- and inter-assay CVs were 1.5–1.8% for both. For measurement of TGs, an enzymatic method with lipase and glycerol kinase was used. Inter- and intra-assay CVs were 3.17% and 1.34%, respectively. HDL-c level was determined by a non-HDL precipitation method followed by an enzymatic detection using phosphotungstic acid and magnesium chloride. Intra- and inter-assay CVs varied between 1.4% and 2.2%. LDL-c concentrations was determined by direct LDL Cholesterol method on Beckman Coulter AU analyzers (Beckman Coulter, Inc., CA, USA). The within run precision is less than 3% CV and the total precision is less than 5% CV. VLDL-c was estimated by dividing the triglyceride value by five. All measurements were performed at the KFU Medical Center Laboratory.
Interventions
Enhanced lifestyle counseling
A description of enhanced lifestyle counseling is given in Wadden et al.25 In the present study, lifestyle coaches supervised all counseling sessions to reinforce motivation and support the weight loss program by reinforcing the patient’s desire to lose weight, helping them cultivate an identity as a successful weight loser, eliciting autonomous motivation from them, and creating an array of non-food.
Dietary protocol
A skilled dietitian established a balanced and personalized dietary restriction program for each participant based on an initial dietary behavior assessment using a four day weighed dietary record during the week preceding the intervention. Each participant was requested to record dietary intake over two weekdays and two weekend days in a diary.26 Scales weighing to the nearest gram were provided to record the weight of all food and beverages consumed. Foods category, cooking methods and ingredients were also recorded. All food lists were analyzed using a software for food coaching (S.C.D.A. Nutrisoft, Le Hallier 37390 Cerelles, France) and the published food tables by Saudi Ministry of Health. Each individual’s diet was designed according to their dietary habits and other selected foods with low glycemic indices, which were mainly fruit, vegetables, and whole grains. Fast foods, sugar-sweetened drinks, energy drinks, French fries, potato chips, cake, donuts, and sweets were to be avoided. Participants’ targeted daily caloric intake deficit was approximately 500 kcal/day, and diets were composed of approximately 15–20% proteins and 25–30% lipids, with carbohydrates representing the remainder of the caloric intake. The dietitian provided monthly assistance to all participants to promote healthy eating, and a second energy input calculation will be done where needed.
Physical activity
The program includes three sessions per week over a 12-week period of Low-impact aerobics, and resistance exercises performed on machines or with free weights. Each session involved a 5- to 10-min warmup including walking and stretching exercises, an exercise session, and a 5- to 10-min cooldown involving breathing and stretching exercises. The exercise session was divided into two sections (section I: LIA; section II: resistance exercises). The LIA section lasted between 30 and 40 min and involved rhythmic, non-jumping exercises, to elevate the heart rate and improve circulation without jarring knees or worsening back pain: walking on treadmill and/or moving on elliptical and/or TAEBO workout. The training load was set at 60–65% of the subject’s HRmax (220 - age in years) for 30 min at the first four weeks, at 65–70% for 35 min at the second four weeks, and at 70–75% for 40 min at the last four weeks. The resistance training section involved a circuit of selected six exercises performed on machines or with free weights involving most muscle groups: Abdominal muscles (abdominal curl, sit-ups, and abdominal crunch machine), chest muscles (bench press, pec-deck fly, and incline dumbbell press), shoulder muscles (shoulder press, barbell upright row, and cable raise), arm muscles (lateral pulldown, triceps extension, and biceps curl), back and neck muscles (low seated row, back extension, and pull-over machine), and leg muscles (leg extension, leg flexion, and leg-press). Each session involved one exercise for each muscles group, and each exercise was performed for three sets of 8–12 repetitions each with a 1-min rest between sets and a 3-min rest between exercises. The initial training load was set at 60% of the one-repetition maximum (1-RM) and was increased every four weeks by 5–10%. The 1-RM values were predicted from the 4-6-RM tests performed at the first workout of the first week. Professional trainers supervised all training sessions, and a polarity analyzer (Polar Electro Oy, Finland) was used to control heart rates throughout the session.
Sample size
The power of the sample size was determined with the software G∗Power version 3.1.3, and using differences between pre and post measurements. Considering the final sample sizes and a significance level of 5%, the calculated power analyses (1-β) values were between 0.8 and 1.00 for all variables excepting the WHR (1-β = 0.62). Therefore, the data regarding this parameter should be carefully interpreted mainly when comparing the between groups changes (1-β = 0.44). However, the effect size of the correlation between WHR and SBP was large and the sample power was strong (1-β = 0.95).27
Statistical analysis
Changes expressed in percentage (X2 – X1)∗100/X1 were calculated and all results are presented as mean ± standard deviation (SD). Data distributions were checked for normality by the Shapiro-Wilk test. A two-way (group × time) repeated-measures ANOVA was used to identify significant within group differences in all variables, when the ANOVA P value was significant, a Tukey’s post hoc comparison test was performed to determine the differences between groups. Unpaired t-test was used to compare the between groups changes and a Pearson’s correlation coefficient (r) was performed to assess the linear relationships that exist between the studied variables. Differences were considered significant at p ≤ 0.05 and SPSS software version 16.0 (IBM Inc., Armonk, NY, USA) was used.
Results
Of 299 participants who were evaluated for a potential participation in our study, 64 individuals met the first three inclusion criteria and were screened for MetS. Individuals diagnosed with this disease (N = 23; 35.94%) were randomly introduced to a dieting program (N = 9) or to dieting associated with a supervised physical training program (N = 14). At four months, all participants completed the post-intervention measures.
Anthropometric parameters
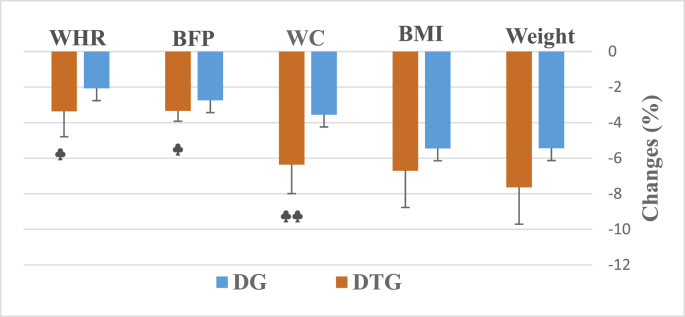
The study’s results relative to the participants’ anthropometric parameters are presented in Table 1. At the end of the diet intervention, significant improvements were noted only in BMI (p = 0.39) and PBF (p = 0.022). However, in response to the exercise and diet intervention, obese participants had significant reductions in body weight (p = 0.018), WC (p = 0.042), BMI (p = 0.001) and BFP (p < 0.001). Notable differences were observed between groups (Fig. 2) in terms of changes in WC (p = 0.003), BFP (p = 0.05) and WHR (p = 0.029).
Table 1.
Body weight and body composition levels before and after 16-week of diet (DG) or diet plus aerobic and resistance training (DTG) intervention.
| Body Weight (kg) |
Waist Circumference (cm) |
Body Mass Index (kg.m−2) |
Body Fat Percentage (%) |
Waist-to-Hip Ratio |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | Before | After | |
| DG (N = 9) | 113.44 (7.95) | 107.22 (6.87) | 115.67 (6.26) | 111.56 (6.11) | 36.29 (1.97) | 34.31 (1.75) ∗ |
32 (2.24) | 29.26 (2.03) ∗ |
0.961 (0.043) | 0.941 (0.042) |
| DTG (N = 14) | 121.79 (6.5) | 113.64 (6.9) ∗ |
119.93 (6.78) | 112.29 (6.28) ∗ |
38.31 (1.59) | 35.74 (1.59) ∗∗ |
34.22 (1.9) | 30.89 (1.71) ∗∗∗ |
0.949 (0.041) | 0.917 (0.033) |
Values are presented as mean (SD). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 compared to before the intervention.
Fig. 2.
Changes in body weight and body composition after 16-week of diet (DG) or diet plus aerobic and resistance training (DTG) intervention. Values are presented as mean ± SD. ♣ p < 0.05, ♣♣ p < 0.01 compared to DG.
In all participants, the pre-study body weight was strongly related to the WC (r = 0.706, p < 0.001) and the PBF (r = 0.768, p < 0.001). A positive correlation was also recorded at the end of the intervention between changes in weight and changes in WC among DTG (r = 0.746, p = 0.002).
Cardiovascular risk (CVR) factor biomarkers
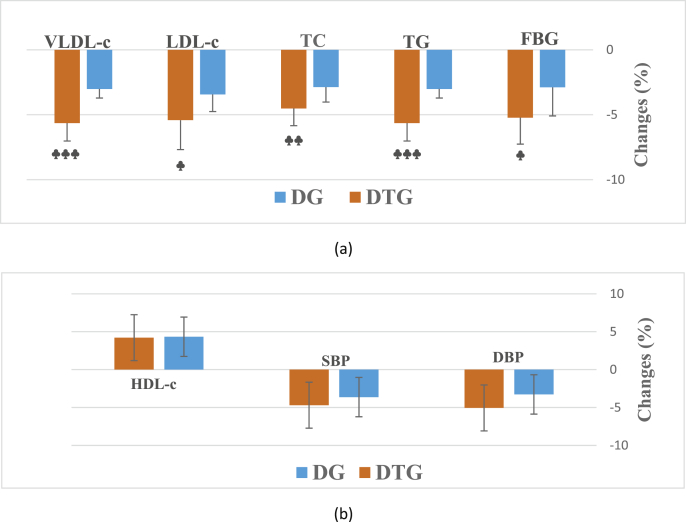
As shown in Table 2, in response to the diet-based intervention, obese participants had significant reductions only in the LDL-c concentration (p = 0.024). However, in response to the diet plus aerobic and resistance exercise, significant improvements were noted in DBP (p = 0.013), SBP (p = 0.016), TG level (p = 0.026), TC (p = 0.016), LDL-c (p = 0.001) and VLDL-c (p = 0.026). Significant differences were also observed between groups in terms of changes in FBG level (p = 0.022), TG level (p = 0.001), TC (p = 0.006), LDL-c (p = 0.014) and VLDL-c (p < 0.001) (Fig. 3).
Table 2.
CVR factor biomarkers concentrations at baseline and after 16-week of diet (DG) or diet plus aerobic and resistance training (DTG) intervention.
| FBG (mg/dL) |
DBP (mmHg) |
SBP (mmHg) |
TG (mg/dL) |
TC (mg/dL) |
HDL-c (mg/dL) |
LDL-c (mg/dL) |
VLDL-c (mg/dL) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | |
| DG (N = 9) | 112.78 (9.69) | 109.44 (8.93) | 87.56 (5.62) | 84.67 (5.59) | 132.11 (7.04) | 127.33 (7.35) | 145.89 (19.26) | 141.11 (18.29) | 197.11 (24.09) | 191.44 (23.57) | 42.6 (3.75) | 44.39 (3.3) | 151.87 (7.91) | 146.72 (9.02) ∗ |
29.18 (3.85) | 28.29 (3.66) |
| DTG (N = 14) | 116.36 (10.1) | 109.79 (10.93) | 92.71 (7.88) | 88.07 (8.39) ∗ |
136.43 (9.42) | 130 (8.99) ∗ |
154.21 (7.78) | 145.5 (7.13) ∗ |
210.36 (12.39) | 200.86 (11.96) ∗ |
42.66 (4.09) | 44.42 (3.8) | 152.31 (9.13) | 143.89 (5.78) ∗∗ |
30.84 (1.56) | 29.1 (1.43) ∗ |
Values are presented as mean (SD). ∗p < 0.05, ∗∗p < 0.01 compared to before the intervention.
Fig. 3.
Significant (a) and non-significant (b) changes in CVR factor biomarkers concentrations after 16-week of diet (DG) or diet plus aerobic and resistance training (DTG) intervention. Values are presented as mean ± SD. ♣ p < 0.05, ♣♣ p < 0.01, ♣♣♣ p < 0.001 compared to DG.
In all participants, the correlation analysis showed that the baseline FBG level (r = 0.574, p = 0.004), TG level (r = 0.549, p = 0.007) and HDL-c (r = −0.407, p = 0.05) were significantly correlated with the initial body weight. The initial SBP also had a significant correlation with the initial WHR (r = 0.541, p = 0.008) and WC (r = 0.505, p = 0.014). Following the interventions, a positive correlation was noted between changes in TG level and changes in body weight (r = 0.433, p = 0.039). The changes in WC also had a positive correlation with the changes in DBP (r = 0.416, p = 0.048) and SBP (r = 0.536, p = 0.008).
Discussion
To our knowledge, this is the first randomized trial studying the effects of a caloric restriction associated with combined aerobic and resistance training among patients with MetS. The primary findings were that caloric restriction alone induced significant modifications in BMI, BFP and LDL-c concentration among young adults with MetS. Adding a weekly three sessions of combined aerobic and resistance training to this approach delivers better outcomes than does dieting alone, particularly in terms of reducing WC, BFP, WHR, FBG level, TG level, TC, LDL-c, and VLDL-c. Data reported also that the prevalence of MetS among KFU students with obesity is around 35.94%. This is consistent with the findings of Gosadi,28 whose review study found an overall MetS prevalence of approximately 39.8% (between 13.6% and 57%) among Saudi adults aged 20 years and older. This variation in prevalence is mainly due to age, gender, and the MetS definition used. Recently, Al-Rubeaan et al.29 reported an overall MetS prevalence of 39.8% (34.4% in men and 29.2% in women) among a cohort of 12,126 Saudi adults randomly recruited from the 13 administrative regions of Saudi Arabia. The authors also noted that the proportion of individuals with at least two risk factors increased by about two-fold to three-fold from the lowest to the highest WC and BMI categories, respectively. Our findings are also in line with those of Ahmadi et al.30 who reported a MetS prevalence between 40.3% and 55% among Iranian high school students with obesity.
The most common indicators observed in this study were increased WC (95.65%), impaired FBG (86.95%), altered TG level (73.91%), increased BP (65.22%), TC (65.22%), and reduced HDL-c (30.44%). These findings are in line with several epidemiologic studies indicating that central obesity is the most significant contributor to MetS prevalence among youth.31 Ervin32 noted, in the Third National Health and Nutrition Examination Survey (NHANES) 2003–2006, that the most frequently observed risk factors were central obesity (53%), elevated BP (40%), and hyperglycemia (39%). In this way, Ribeiroa et al.33 suggested that a large waist size results from a high concentration of visceral adipose tissue that increases the efflux of fatty acids from the adipocytes. High levels of fatty acids lead to the accelerated synthesis of TGs, cholesterol, and apolipoproteins by the liver. This causes elevated VLDL-c and LDL-c concentrations in the blood, resulting in the accumulation of the small and dense low-density lipoprotein (LDL) cells characteristic of adiposopathy.
Several epidemiologic studies have suggested that insulin resistance and HDL-c are the most significant contributors to MetS prevalence. Li and colleagues34 reported that elevated FBG levels followed by low HDL-c were the most commonly observed metabolic components among overweight and obese male Chinese students. Khalid et al.,35 using the International Diabetes Federation criteria, noted that the prevalence of the MetS components among obese Saudi adults was increased by raised FBG level (72.7), reduced HDL-c (54.8), raised BP (45.2), and high TG level (33.6%). Abdominal or visceral fat has a central role in the establishment of a pro-inflammatory state that is considered to be a precursor of MetS development.36 In fact, there is evidence that the relationship between obesity, insulin resistance, and MetS is mainly a result of the insulin-responsive tissues partitioning pattern, which is more closely related to the metabolic phenotype of obese young individuals than to their degree of obesity. The accumulation of ectopic fat in tissue surrounding the viscera has been associated with an abundant secretion of pro-inflammatory biomarkers, such as cytokines, leptin, tumor necrosis factor alpha, prostaglandins, adiponectin, and anti-atherosclerotic adipokine.37,38 These pro-inflammatory mediators contribute to the development of diabetes mellitus type 2, hyperlipidemia, and CVD.36
The pathogenesis of MetS is complex and incompletely understood; however, most epidemiologic studies have affirmed that the interaction of genetic determinants with several modifiable factors (such obesity, physical inactivity, diet, and large amounts of sedentary screen time) greatly contributes to its development.39 Stress, especially occupational stress, could also be an important risk factor that increases the prevalence of MetS components.39 Gosadi,28 in his review article, noted that unhealthy eating habits associated with low levels of physical activity were the most significant causes of the increased prevalence of MetS in Saudis. Therefore, lifestyle interventions based on healthy, balanced diets and physical activity programs are becoming increasingly recommended as a first-line strategy to prevent and combat MetS.14 A notable relationship was observed between dietary and physical activity behaviors and risk factors for MetS.40 Our findings supported these suggestions and demonstrated that dieting associated with a combination of aerobic and resistance exercises may reduce body weight and body compositions and decrease the prevalence of MetS. Results also revealed that a 12-week program involving caloric restriction and 3 weekly sessions of aerobic and resistance training reduces WC, BFP, WHR, FBG level, TG level, TC, LDL-c, and VLDL-c, significantly more than does caloric restriction alone. No significant between-group differences were noted in weight, BMI, BP and HDL-c.
Several epidemiological studies and clinical trials found significant improvements in both body composition and MetS components after caloric restriction interventions alone.41 Diet-based MetS treatment programs have resulted in significant changes in body weight, WC, lean body mass, TG level, TC, and HDL-c and LDL-c concentrations.42,43 Aerobic exercise also facilitated widespread improvements to MetS components, particularly in terms of abdominal fat, intrahepatic lipid,18 TG level, TC, HDL-c, BP, body mass, and resting heart rate.39,44 Resistance exercise alone also provides significant improvement to some MetS risk factors, but is typically recommended as an adjunct to caloric restriction or aerobic exercise.45 In a randomized, controlled trial designed to examine the effects of aerobic versus resistance exercise without caloric restriction, Lee et al.18 reported that both aerobic and resistance exercises performed for 180 min weekly improved cardiorespiratory fitness and reduced total fat, visceral adiposity, WC, and intrahepatic lipid in adolescent boys with obesity. Furthermore, resistance training on its own was effective in increasing skeletal muscle mass and muscular strength and improving insulin sensitivity. Nevertheless, Lemes et al.46 noted that resistance-training interventions have been found to reduce SBP but not FBG levels, HDL-c, TG levels, DBP, or WC. Resistance exercise may improve body compositions and the metabolic capacity of skeletal muscle that influences resting metabolic rate and the use of FBG and TGs. An inverse relationship between muscular strength and MetS prevalence was identified.47 Therefore, an American College of Sports Medicine Position Stand22 recommended that a small amount of resistance exercise, around 1 h over two weekly sessions, should be added to aerobic training to allow for superior MetS and future CVD management. The combination of meeting these resistance exercise guidelines, dieting, and undergoing aerobic exercises produces better outcomes than does dieting alone or aerobic training alone.39
The present study’s findings should be interpreted with an awareness of certain research limits. First, the population investigated was limited to young male students at King Faisal University, located in the Eastern Province of Saudi Arabia. This region is characterized by a rapidly growing economy, several changes in the population’s nutritional habits, and a harsh desert climate not conducive to the playing of sports or physical activity for a long period each year. Therefore, the results of this study cannot be extrapolated to other settings (e.g. to female patients). Second, the input energy was based on self-reported food consumption data; no objective measures were carried out during the study to assist in participant diet adherence, so the possibility of bias resulting from poor food consumption recollections or unawareness about specific food items and the amounts that were consumed cannot be eliminated.48 Finally, this study is based on preexisting data, so causal relationships cannot be determined.49 Despite these limitations, the present study has meaningful implications for Saudi youth with obesity diagnosed with MetS facing climatic conditions, social norms, and cultural attitudes and beliefs that prevent their engagement in physical outdoor activities.28 In conclusion, our findings demonstrated that a slight daily caloric restriction (500 kcal) could be an effective tool in combating MetS and that the addition of three weekly sessions of aerobic and resistance training in a gymnasium to this approach delivers better outcomes than does dieting alone, particularly in terms of reducing WC, BFP, WHR, FBG level, TG level, TC, LDL-c, and VLDL-c.
CRediT authorship contribution statement
Mohamed Ahmed Said: Conceptualization, Methodology, Investigation, Writing - review & editing. Mohamed Abdelmoneem: Investigation, Data curation, Resources. Mohamed Chaab Alibrahim: Project administration, Investigation, Funding acquisition. Moustafa Ahmed Elsebee: Software, Formal analysis, Data curation. Ahmed Abdel Hamed Kotb: Investigation, Visualization, Supervision, Validation, Writing - review & editing.
Declaration of competing interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be considered as a potential conflict of interest.
Acknowledgements
The Deanship of Scientific Research at King Faisal University, Al-Hassa, Saudi Arabia financed this study (project number 186020). Authors wish to thank all individuals who participated in the achievement of this study. A special thanks to Mr. Ammari Hsen for the help.
References
- 1.Kassi E., Pervanidou P., Kaltsas G. Metabolic syndrome: definitions and controversies. BMC Med. 2011;9:48–61. doi: 10.1186/1741-7015-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The National Cholesterol Education Program (NCEP) Third Report of the National Cholesterol Education Program (NCEP) Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 3.Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014 doi: 10.1155/2014/943162. 943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.International Diabetes Federation (IDF) The IDF consensus worldwide definition of the metabolic syndrome. Int Diabetes Fed. 2006 http://www.idf.org/metabolic-syndrome IDF Web site. Accessed Jan 5, 2020. [Google Scholar]
- 5.Hwang L.C., Tsai C.H., Chen T.H.H. Overweight and obesity-related metabolic disorders in hospital employees. J Formos Med Assoc. 2006;105(1):56–63. doi: 10.1016/S0929-6646(09)60109-1. [DOI] [PubMed] [Google Scholar]
- 6.Bluher M. Adipose tissue inflammation: a cause or consequence of obesity-related insulin resistance? Clin Sci. 2016;130:1603–1614. doi: 10.1042/CS20160005. [DOI] [PubMed] [Google Scholar]
- 7.Singh N., Scmhrd Metabolic syndrome: practice essentials, background and pathophysiology. J Heart Stroke. 2018;3(1):1044. [Google Scholar]
- 8.Millstein R.A. Measuring outcomes in adult weight loss studies that include diet and physical activity: a systematic review. J Nutr Metabol. 2014;2014 doi: 10.1155/2014/421423. Article ID 421423, 13 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart K.J., Bacher A.C., Turner K. Exercise and risk factors associated with metabolic syndrome in older adults. Am J Prev Med. 2005;28(1):9–18. doi: 10.1016/j.amepre.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Vasconcellos F., Seabra A., Katzmarzyk P.T. Physical activity in overweight and obese adolescents: systematic review of the effects on physical fitness components and cardiovascular risk factors. Sports Med. 2014;44:1139–1152. doi: 10.1007/s40279-014-0193-7. [DOI] [PubMed] [Google Scholar]
- 11.Choi J.H., Woo H.D., Lee J.H. Dietary patterns and risk for metabolic syndrome in Korean women: a cross-sectional study. Medicine (Baltim) 2015;94(34) doi: 10.1097/MD.0000000000001424. e1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gadgil M.D., Anderson C.A.M., Kandula N.R. Dietary patterns are associated with metabolic risk factors in South Asians living in the United States. J Nutr. 2015;145(6):1211–1217. doi: 10.3945/jn.114.207753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schulze M.B., Hu F.B. Dietary approaches to prevent the metabolic syndrome. Quality versus quantity of carbohydrates. Diabetes Care. 2004;27(2):613–614. doi: 10.2337/diacare.27.2.613. [DOI] [PubMed] [Google Scholar]
- 14.Kim Y.J., Hwang J.Y., Hyesook K. Diet quality, physical activity, and their association with metabolic syndrome in Korean adults. Nutrition. 2019;59:138–144. doi: 10.1016/j.nut.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 15.He Y., Li Y., Lai J. Dietary patterns as compared with physical activity in relation to metabolic syndrome among Chinese adults. Nutr Metabol Cardiovasc Dis. 2013;23:920–928. doi: 10.1016/j.numecd.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Sorace P., Ronai P., Churilla J.R. Resistance training and metabolic syndrome. ACSM’s Health & Fit J. 2014;18(6):24–29. [Google Scholar]
- 17.Wewege M.A., Thom J.M., Rye K.A. Aerobic, resistance or combined training: a systematic review and meta-analysis of exercise to reduce cardiovascular risk in adults with metabolic syndrome. Atherosclerosis. 2018;274:162–171. doi: 10.1016/j.atherosclerosis.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Lee S.J., Bacha F., Hannon T. Effects of aerobic versus resistance exercise without caloric restriction on abdominal fat, intrahepatic lipid, and insulin sensitivity in obese adolescent boys. Diabetes. 2012;61:2787–2795. doi: 10.2337/db12-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelley G.A., Kelley K.S. Progressive resistance exercise and resting blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2000;35:838–843. doi: 10.1161/01.hyp.35.3.838. [DOI] [PubMed] [Google Scholar]
- 20.Sigal R.J., Alberga A.S., Goldfield G.S. Effects of aerobic training, resistance training, or both on percentage body fat and cardiometabolic risk markers in obese adolescents. JAMA Pediatr. 2014;168(11):1006–1014. doi: 10.1001/jamapediatrics.2014.1392. [DOI] [PubMed] [Google Scholar]
- 21.Ostman C., Smart N.A., Morcos D. The effect of exercise training on clinical outcomes in patients with the metabolic syndrome: a systematic review and meta-analysis. Cardiovasc Diabetol. 2017;16:110. doi: 10.1186/s12933-017-0590-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American College of Sports Medicine (ACSM) ACSM position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41(3):687–708. doi: 10.1249/MSS.0b013e3181915670. [DOI] [PubMed] [Google Scholar]
- 23.World Medical Association (WMA) Declaration of Helsinki: ethical principles for medical research involving human subjects, as amended by the 64th WMA general assembly, Fortaleza, Brazil. October 2013. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ Available from: Accessed Jan 5, 2020.
- 24.Yang S.W., Kim T.H., Choi H.M. The reproducibility and validity verification for body composition measuring devices using bioelectrical impedance analysis in Korean adults. J Exerc Rehabil. 2018;14(4):621–627. doi: 10.12965/jer.1836284.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wadden T.A., Volger S., Sarwer D.B. A two-year randomized trial of obesity treatment in primary care practice. N Engl J Med. 2011;365:1969–1979. doi: 10.1056/NEJMoa1109220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill R.J., Davies P.S.W. The validity of a four day weighed food record for measuring energy intake in female classical ballet dancers. Eur J Clin Nutr. 1999;53:752–754. doi: 10.1038/sj.ejcn.1600836. [DOI] [PubMed] [Google Scholar]
- 27.Tibana R.A., Nascimento Dda C., de Sousa N.M. Enhancing of women functional status with metabolic syndrome by cardioprotective and anti-inflammatory effects of combined aerobic and resistance training. PloS One. 2014;9(11) doi: 10.1371/journal.pone.0110160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gosadi I.M. Assessment of the environmental and genetic factors influencing prevalence of metabolic syndrome in Saudi Arabia. Saudi Med J. 2016;37(1):12–20. doi: 10.15537/smj.2016.1.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Rubeaan K., Bawazeer N., Al Farsi Y. Prevalence of metabolic syndrome in Saudi Arabia - a cross sectional study. BMC Endocr Disord. 2018;18:16–25. doi: 10.1186/s12902-018-0244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmadi A., Gharipour M., Nouri F. Association between adolescence obesity and metabolic syndrome: evidence from Isfahan healthy heart program. Indian J Endocr Metab. 2014;18:569–573. doi: 10.4103/2230-8210.137523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perona J.S., Schmidt-RioValle J., Rueda-Medina B. Waist circumference shows the highest predictive value for metabolic syndrome, and waist-to-hip ratio for its components, in Spanish adolescents. Nutr Res. 2017;45:38–45. doi: 10.1016/j.nutres.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Ervin R.B. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States. Nat Health Stat. 2009;5(13):1–7. [PubMed] [Google Scholar]
- 33.Ribeiroa A.S., Seixasa R., Gálveza J.M. Cardiovascular risk factors: is the metabolic syndrome related to aging? Epidemiology in a Portuguese population. Diabetes Metab Syndrome: Clin Res Rev. 2018;12:885–891. doi: 10.1016/j.dsx.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 34.Li Y., Zhao L., Yu D. Metabolic syndrome prevalence and its risk factors among adults in China: a nationally representative cross-sectional study. PloS One. 2018;13(6) doi: 10.1371/journal.pone.0199293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khalid S.A., Samia A.B., Muneera A.A. Prevalence of metabolic syndrome in obese Saudi population. Interv Obes Diabetes. 2018;2(1):1–7. [Google Scholar]
- 36.Paley C.A., Johnson M.I. Abdominal obesity and metabolic syndrome: exercise as medicine? BMC Sports Sci Med Rehabil. 2018;10:7. doi: 10.1186/s13102-018-0097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellulu M.S., Khaza’ai H., Rahmat A. Obesity can predict and promote systemic inflammation in healthy adults. Int J Cardiol. 2016;215:318–324. doi: 10.1016/j.ijcard.2016.04.089. [DOI] [PubMed] [Google Scholar]
- 38.Golbidi S., Mesdaghinia A., Laher I. Exercise in the metabolic syndrome. Oxid Med Cell Longev. 2012 doi: 10.1155/2012/349710. ID 349710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niazi E., Saraei M., Aminian O. Frequency of metabolic syndrome and its associated factors in health care workers. Diabetes Metab Syndr. 2019;13(1):338–342. doi: 10.1016/j.dsx.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 40.Pitsavos C., Panagiotakos D., Weinem M. Diet, exercise and the metabolic syndrome. Rev Diabet Stud. 2006;3:118–126. doi: 10.1900/RDS.2006.3.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gomez-Huelgas R., Jansen-Chaparro S., Baca-Osorio A.J. Effects of a long-term lifestyle intervention program with Mediterranean diet and exercise for the management of patients with metabolic syndrome in a primary care setting. Eur J Intern Med. 2015;26:317–323. doi: 10.1016/j.ejim.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 42.Romieu I., Dossus L., Barquera S. Energy balance and obesity: what are the main drivers? Cancer Causes Control. 2017;28:247–258. doi: 10.1007/s10552-017-0869-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu M., Wang S., Wang D. Combined moderate and high intensity exercise with dietary restriction improves cardiac autonomic function associated with a reduction in central and systemic arterial stiffness in obese adults: a clinical trial. Peer J. 2017;5 doi: 10.7717/peerj.3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ostman C., Smart N.A., Morcos D. The effect of exercise training on clinical outcomes in patients with the metabolic syndrome: a systematic review and meta-analysis. Cardiovasc Diabetol. 2017;16:110. doi: 10.1186/s12933-017-0590-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Normandin E., Chmelo E., Lyles M.F. Effect of resistance training and caloric restriction on the metabolic syndrome. Med Sci Sports Exerc. 2017;49(3):413–419. doi: 10.1249/MSS.0000000000001122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lemes Í.R., Ferreira P.H., Linares S.N. Resistance training reduces systolic blood pressure in metabolic syndrome: a systematic review and meta-analysis of randomized controlled trials. Br J Sports Med. 2016;50(23) doi: 10.1136/bjsports-2015-094715. 1438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bakker E.A., Lee D.C., Sui X. Association of resistance exercise, independent of and combined with aerobic exercise, with the incidence of metabolic syndrome. Mayo Clin Proc. 2017;92(8) doi: 10.1016/j.mayocp.2017.02.018. 1214-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karasu S.R. An Overview of the complexities in obesity: limitations and challenges. Am J Lifestyle Med. 2013;7(3):192–205. [Google Scholar]
- 49.Kim S., Kim D.I. Association of regular walking and body mass index on metabolic syndrome among an elderly Korean population. Exp Gerontol. 2018;106:178–182. doi: 10.1016/j.exger.2018.03.004. [DOI] [PubMed] [Google Scholar]