Abstract
Background/Objective
Muscle soreness and damage occurs after completing a full marathon. Here we refer to muscle soreness induced by prolonged running as early-onset muscle soreness (EOMS) because muscle soreness and damage markers induced after prolonged running are different from delayed-onset muscle soreness (DOMS) and muscle damage markers induced after eccentric contraction, such as resistance exercise. The dynamics and relationship between muscle damage markers and EOMS are unclear; therefore, in this study, we aimed to elucidate the relationship between EOMS and indirect muscle damage markers, and their dynamics after a full marathon.
Methods
The following measurements were performed in 19 subjects who completed a full marathon: perceived muscle soreness (using a numeric rating scale), thigh circumference (CIR), hip joint range of motion (ROM), jump height (JH) and muscle damage marker activities in the blood (CK, AST, LDH, ALD) before (Pre), after (Post) and every day for 4 days after a full marathon (D1−4).
Results
EOMS was induced, as determined by the numeric rating scale score peaking immediately after a full marathon. ROM and JH significantly decreased and all muscle damage markers significantly increased after a full marathon. Serum CK and AST peaked at D1. Serum LDH and ALD peaked at Post and D3. Each marker showed different dynamics. CIR significantly decreased after a full marathon.
Conclusion
Muscle soreness peaked and muscle damage markers in the blood showed different dynamics after a full marathon. In other words, this is different from DOMS.
Keywords: Running, Circumference, Lactate dehydrogenase
Background
There has been an increase in the number of amateur runners competing in full marathons who started jogging or running to maintain health and because they enjoyed it. Completing a full marathon (42.195 km) gives a sense of well-being and achievement; however, long-distance running is also associated with severe stress to the body. Therefore, muscle damage occurs after a full marathon1, 2, 3, 4, 5, 6, 7, 8, 9 and reduced the performance or motivation towards subsequent exercise.
Mechanical stress by eccentric contraction is the main factor for muscle damage,10 and structural gastrocnemius muscle damage has been reported after a full marathon, that is, repeated eccentric exercise which induced muscle damage.1,2 Moreover, several studies have shown that muscle damage markers, such as creatine kinase and lactate dehydrogenase, in the blood increased after a full marathon.3, 4, 5, 6, 7, 8 Decreased performance, during a counter movement jump (CMJ), after long-term exercise is related to increased serum muscle damage markers.11 Thus, completing a full marathon clearly caused muscle damage and was related to decreased performance. Indirect muscle damage markers such as maximal isometric contraction, range of motion (ROM), and circumference (CIR) have been used as estimation indices of induced muscle damage by eccentric contraction of upper flexed limbs.12,13
Muscle soreness is often reported after a full marathon.14, 15, 16 Here we refer to muscle soreness after completing long-term exercise as immediate-onset muscle soreness (IOMS).16 This was distinguished from delayed-onset muscle soreness (DOMS) that occurred some hours after exercise and peaked at 24–72 h.10,17,18 The characteristics of IOMS include muscle soreness immediately after exercise. In the present study, we also refer to early-onset muscle soreness (EOMS) as soreness considered to occur during long-distance running. In addition, it has been reported that the time-course of muscle soreness in a full marathon is different from that of DOMS after resistance exercise that a main factor was mechanical stress.1,14
The dynamics of indirect muscle damage markers with respect to DOMS are clear12,13,17; muscle damage markers in the blood and circumference of the exercised muscle increased and ROM decreased. In particular, muscle damage markers in the blood and muscle circumference started to increase for several hours and peaked 3–4 days after eccentric exercise induced DOMS. Unexpectedly, these markers showed slower changes than DOMS.17 To the best of our knowledge, although muscle damage markers in the blood and ROM were measured after a full marathon,3, 4, 5, 6, 7, 8, 9 no study has yet described the relationship between EOMS and indirect muscle damage markers.
This study aimed to elucidate the relationship between EOMS and the dynamics of muscle damage markers after prolonged exercise. We analysed the characteristics and dynamics of indirect markers on EOMS before and after a full marathon and during the recovery period.
Patients and methods
Subjects
Nineteen healthy individuals who regularly exercised to complete a full marathon were recruited (17 men; 2 women) from a class in University of Tsukuba. There is not a difference on our results by gender. The subjects’ age, height, weight and body fat are shown in Table 1. Subjects were recruited from the University of Tsukuba and they participated in the 34th Tsukuba marathon race. They performed a warm-up and drank water freely on the day of the full marathon. In addition, they were instructed to sleep well and refrain from drinking and eating too much not to get out of shape during the experimental period. The study was approved by the Ethical Committee of the Faculty of Health and Sport Science in the University of Tsukuba. The subjects received a complete explanation in advance on the purpose of the experiment, its contents and safety issues, and informed consent was obtained.
Table 1.
Characteristics of subjects.
| Age (year) | Height (cm) | Weight (kg) | Body fat (%) |
|---|---|---|---|
| 22 ± 0.3 | 167.2 ± 1.5 | 60.9 ± 1.9 | 17.4 ± 1.2 |
Values are means ± SE (n = 19).
Methods
Experimental protocol
Measurements were made before (Pre), after (Post) and every day for 4 days after completion of the full marathon (D1−4). Except for Post, measurements were made early in the morning. Pre measurements were made the day before the full marathon. During early morning measurements, a numeric rating scale (NRS) was completed to evaluate muscle soreness and blood samples were collected from a vein. Afterwards, thigh CIR, hip joint ROM and jump height (JH) using CMJ, were measured. The same measurements were conducted after completing the full marathon (Post). The full marathon was completed at a temperature of 16.3 °C and with a humidity level of 66.3%.
Measurements
Body weight was measured by body composition meter (body composition inner scan, BC-600-WH, TANITA Co., Japan) before and after a full-marathon.
Muscle soreness using NRS
Subjective muscle soreness was measured based on NRS, i.e. 11 points from 0: No pain to 10: Extremely painful.19 NRS were completed for the back, lumbar, buttock, front and back thigh and front and back lower leg.
Indirect muscle damage markers
Swelling of the thigh was calculated using the average of two measurements of CIR at the midpoint from the anterior superior iliac spine to the patella of the right thigh using a tape.20 A mark was made at the measured point during the experimental period. The same examiner measured their CIR throughout the experimental period. The interclass correlations in the one-way analysis change model were Pre, 0.995; Post, 0.997; D1, 1.000; D2, 0.997; D3, 0.999; D4, 1.000. Hip joint ROM was measured as an indirect muscle damage marker using a large protractor. ROM was adjusted for the anterior superior iliac spine with the outer top of the thigh bone based on a greater trochanter. ROM was measured twice via maximal flexion with a dorsal position angle, and maximal extension with a prone position angle. The ROM value was subtracted from the dorsal or prone position angle from each maximal moved angle. The same examiner measured ROM during the experimental period. The interclass correlation in the one-way analysis change model was Pre, 0.956 and 0.980; Post, 0.935 and 0.977; D1, 0.974 and 0.976; D2, 0.955 and 0.986; D3, 0.990 and 0.968 and D4, 0.942 and 0.985 for maximal extension and flexion, respectively. CMJ were performed, and JH was measured to determine muscle power. A mat switch calculated JH from the duration of a flight after CMJ. Jumps were performed with the hands on the waist on the mat switch.11 Measurement of JH was conducted twice and with a 1-min interval. Subjects were asked to jump as high as possible without bending their knees during the jump.
Biochemical markers
Blood samples were collected from an antecubital vein and serum was obtained after centrifugation (3000 rpm) for 15 min. Samples were aliquoted and stored in a freezer at −30 °C until analysed. Blood sampling before the full-marathon (Pre) and from D1 to D4 after the full marathon was conducted before breakfast after an overnight fast.
Serum activities of creatine kinase (CK), lactate dehydrogenase (LDH), aldolase (ALD) and aspartate aminotransferase (AST) were analysed as indices of muscle damage using the standardised method of the Japanese Society of Clinical Chemistry.
Statistical analysis
Data are shown as mean ± standard error. The area under the curve (AUC) in each EOMS was calculated for each time-point. Statistical analyses were conducted using the Steel−Dwass method for multiple comparisons in non-parametric tests to reject homoscedasticities and normalisations. In addition, the interclass correlation coefficient in one-way analysis of variance was calculated. Significant differences for all tests were set at p < 0.05.
Results
Body weight significantly was decreased after the full-marathon (Pre, 60.9 ± 1.9 vs Post, 58.7 ± 1.8; p < 0.01). Also, their goal time were 262.3 ± 12.0 min.
Muscle soreness based on NRS
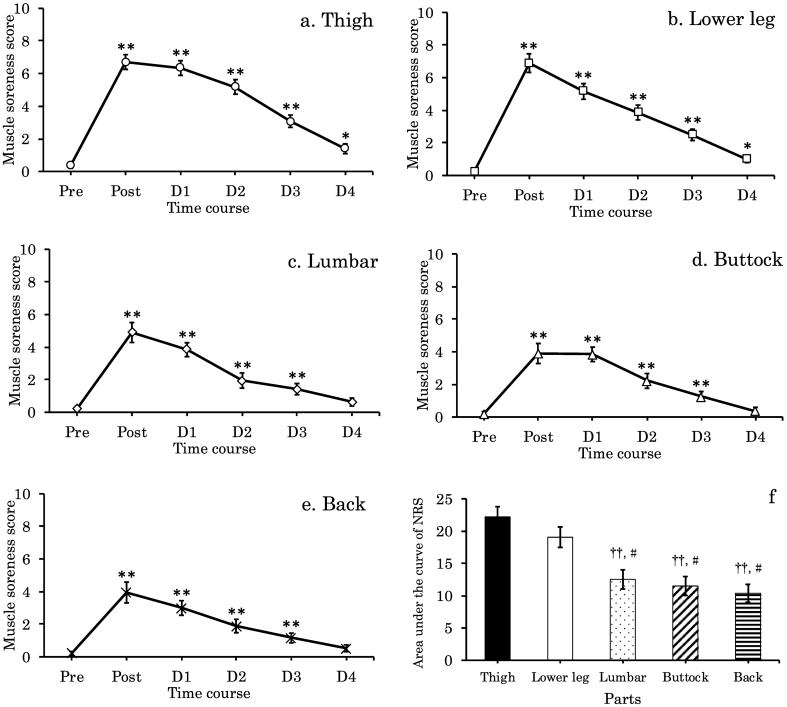
Changes in NRS, and AUC before and from D1 to D4 after the full marathon are shown in Fig. 1. All measurements were significantly high at Post compared with those at Pre and peaked at Post (p < 0.01). The thigh and lower leg showed higher values for muscle soreness until D4 compared with those at Pre (p < 0.05 and p < 0.01, respectively), although all measurements gradually decreased from D1. Lumbar and buttock muscle soreness recovered by D4. For AUC measurements, thigh and lower leg showed significantly higher values than back, lumbar and buttock, respectively (p < 0.01 and p < 0.05).
Fig. 1.
Changes in numeric rating scale (NRS) score of the full-marathon for muscle soreness in the thigh (a), lower leg (b), lumbar (c), buttock (d), back (e) and area under the curve of NRS(f) from Pre to day 4 (D4) (n = 19). Values are means ± SE. Single asterisk indicates significant difference versus Pre (p < 0.05). Double asterisks indicate significant difference versus Pre (p < 0.01). Double daggers indicate significant difference versus Thigh (p < 0.01). Single hash sign indicates significant difference versus Lower leg (p < 0.05).
NOTE: Pre; one day before the full-marathon, Post; immediately following the full-marathon, D1; one day after the full-marathon, D2; two day after the full-marathon, D3; three day after the full-marathon, D4; four day after the full-marathon.
Indirect muscle damage markers
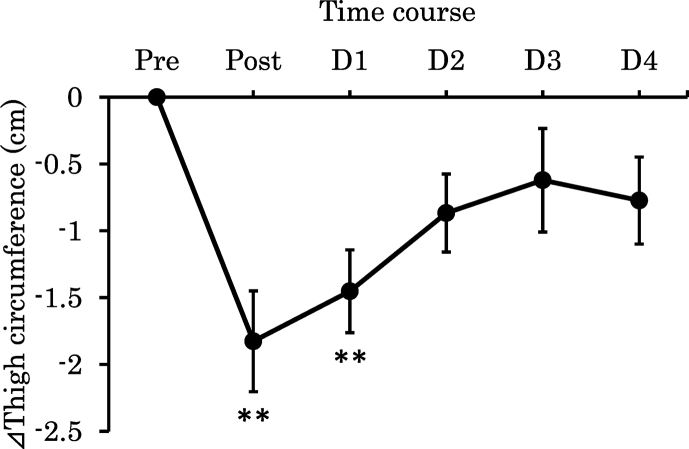
Normalised changes in CIR from Pre value to Post and D1−D4 after the full marathon are shown in Fig. 2. CIR significantly decreased at Post compared with that at Pre (p < 0.01). Furthermore, the decrease was significant until D1. Normalised changes in hip joint ROM and JH based on CMJ from the Pre value, after Post and D1−D4 after the full marathon are showed in Table 2. ROM on extension significantly decreased at Post and D1 compared with that at Pre (p < 0.05). On the other hand, ROM on the flexion did not change. JH significantly decreased at Post compared with that Pre (p < 0.01). In addition, a significant decrease was maintained until D2 (p < 0.01).
Fig. 2.
Normalised changes in thigh circumference from Pre value, after Post and D1 - D4 after the full-marathon (n = 19). Values are means ± SE. Double asterisks indicate significant difference versus Pre (p < 0.01).
NOTE: Pre; one day before the full-marathon, Post; immediately following the full-marathon, D1; one day after the full-marathon, D2; two day after the full-marathon, D3; three day after the full-marathon, D4; four day after the full-marathon.
Table 2.
Normalised changes in indirect markers from the Pre to Post and D1–D4 values after the full-marathon.
| Pre | Post | Day 1 | Day 2 | Day 3 | Day 4 | |
|---|---|---|---|---|---|---|
| ⊿ROM in extension (°) | 0 | −12.4 ± 3.9∗ | −10.6 ± 5.3∗ | −9.8 ± 3.8 | −5.4 ± 4.3 | −2.4 ± 4.7 |
| ⊿ROM in flexion (°) | 0 | 1.0 ± 1.3 | −0.4 ± 1.1 | 0.5 ± 1.0 | −0.3 ± 1.0 | 0.0 ± 1.3 |
| ⊿JH (cm) | 0 | −26.1 ± 7.7∗∗ | −27.7 ± 6.3∗∗ | −17.0 ± 6.6∗∗ | −4.1 ± 6.8 | 0.8 ± 7.4 |
Values are means ± SE (n = 19). Single asterisk indicates significant difference versus the values at Pre (p < 0.05). Double asterisks indicate significant difference versus the values at Pre (p < 0.01).
NOTE: Pre; 1 day before the full-marathon, Post; immediately following the full-marathon.
Muscle damage markers in the blood
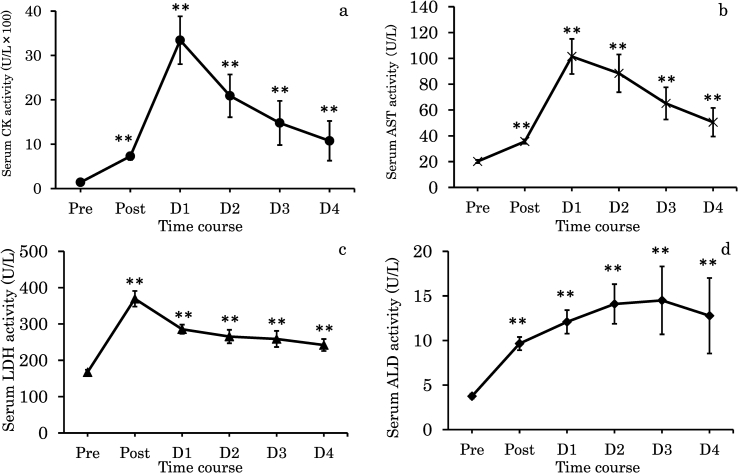
Fig. 3 shows the changes in skeletal muscle damage markers in the blood before and Post and D1−D4 after the full marathon. CK showed significantly higher values at Post than at Pre and peaked at D1 (p < 0.01), showing high values until D4 compared with those at Pre (p < 0.01); however, all values gradually decreased from D1. In addition, AST showed the same time course as CK (p < 0.01). LDH showed significantly higher values and peaked at Post compared with those at Pre (p < 0.01). LDH showed high values until D4 compared with Pre (p < 0.01), although all results decreased from Post. ALD showed significantly high values at Post compared with those at Pre and peaked at D3 (p < 0.01). ALD showed high values until D4 compared with those at Pre (p < 0.01), although there were lower than those at D3.
Fig. 3.
Changes in serum creatine kinase (CK - a), aspartate aminotransferase (AST - b), lactate dehydrogenase (LDH - c), aldrase (ALD - d) activities of the full-marathon from Pre to D4 (n = 19). Values are means ± SE. Double asterisks indicate significant difference versus Pre (p < 0.01).
NOTE: Pre; one day before the full-marathon, Post; immediately following the full-marathon, D1; one day after the full-marathon, D2; two day after the full-marathon, D3; three day after the full-marathon, D4; four day after the full-marathon.
Correlation
There were significant negative correlations between normalised JH and each muscle damage marker in the blood from Pre to Post (p < 0.05 and p < 0.01, respectively) (Table 3).
Table 3.
Correlation between normalised changes in jump height and muscle damage markers in the blood from Pre to Post.
| ⊿Serum CK activity | ⊿Serum AST activity | ⊿Serum LDH activity | ⊿Serum ALD activity | |
|---|---|---|---|---|
| ⊿Jump height | −0.528∗ | −0.521∗ | −0.560∗ | −0.644∗∗ |
Discussion
In this study, indirect muscle damage markers involving muscle power were measured using an evaluation model of muscle damage induced by DOMS. In this study, hip joint ROM for flexion did not change after a full marathon. This finding are in line with a decreased hip joint in previous study.21 Moreover, the decreased ROM for extension was maintained until D1 and it recovered afterwards. In addition, JH, as a muscle power index, significantly decreased from Post to D2 compared with that at Pre. This result was similar to changes in maximal muscle force observed in a DOMS study.17 Moreover, decreased isometric force after eccentric contraction was reported to best reflect muscle damage.22 There was a significant correlation between normalised changes in hip joint ROM for extension and JH in this study. Therefore, it might be possible to evaluate hip joint ROM for extension as well as JH.
CIR showed a significant decrease at Post and D1 compared with that at Pre. Although CIR showed increased swelling in a DOMS study, CIR decreased in this study. Knechtle et al. (2012)23 reported a correlation between decreased weight and body water before and after a 100-km ultramarathon. Body water may have decreased because of the decreased weight before and after a full marathon in this study. The decreased weight possibly resulted in decreased CIR. However, CIR significantly increased one day after a half-marathon.24 Therefore, CIR would need to be measured in combination with body water in future studies. We suggested that CIR should not be used as an indirect marker for a full marathon, although it has traditionally been used in many studies as an indirect marker of DOMS.12,17,25
Serum CK, LDH, AST and ALD activities significantly increased at Post. However, their dynamics differed; CK and AST peaked at D1, LDH peaked at Post and high values were maintained compared with those at Pre, though they gradually decreased until D4 and ALD peaked at D3 and showed a delayed increment compared with the other markers. Muscle damage markers in the blood have been shown to increase after a full marathon.3, 4, 5, 6, 7, 8 Kim et al. (2009)3 reported that CK activity showed significantly high values until 4 days after compared with those before a full marathon.
Muscle damage markers in the blood for DOMS peaked more slowly than DOMS.17 These markers showed almost the same dynamics for DOMS, peaking at 3 or 4 days after exercise.12,13,18,25 However, in this study these markers showed different dynamics. LDH activity peaked at Post and showed the same dynamics as EOMS. This suggested that muscle soreness and damage were caused at the same time-points after a full marathon. Serum LDH increased earlier than the other markers because serum LDH is abundant in everyone.26 Rose et al. (1970)27 reported that increased LDH activity after a full marathon originated from LDH isoenzyme 3–5. Although these isoenzymes mostly account for LDH originating from skeletal muscle, other organs, such as liver or lung, may be involved. Therefore, LDH might be increased even though the skeletal muscle was not damaged.
Muscle soreness showed peaked values at Post on all parts. This study confirmed that EOMS was induced immediately after prolonged running.16 In particular, the thigh and lower leg showed high values for EOMS until 4 days after compared with those before a full marathon. In addition, it appeared that loads on the thigh and lower leg were more because AUC in these NRS showed higher values than those for the other areas of the body. EOMS showed similar dynamics as indirect muscle damage markers using ROM for hip extension and JH.
After eccentric exercise, EOMS occurred earlier than DOMS. However, the precise mechanisms governing the same remained unclear. Damages associated with ROS and inflammation have been often attributed as the causes of DOMS.10,28 Additionally, it has been reported that mechanical stress during eccentric contraction contributes to muscle fiber damage, followed by inflammation and oxidative stress which manifest as secondary events. Nonetheless, there have been limited studies elucidating the relationship between muscle soreness and its causative factors. Radák et al. (1999) discussed that elevated levels of 8-hydroxy-2’ -deoxyguanosine is related to muscle soreness, 24 h after eccentric contraction.29 Moreover, oxidative stress and inflammation have also been shown to occur after a full marathon.30,31 Therefore, there might be a close association between oxidative stress and EOMS upon prolonged exercise in individuals. A possible reason contributing to the difference between EOMS and DOMS is metabolic stress. Muscle contraction might be affected by a lack of energy during protracted periods of running. There could also be a malfunction in the muscle contraction system because of fatigue in the skeletal muscle, consequent to glycogen depletion.32 Thus, increased eccentric contraction might occur when the muscle contraction system is damaged. This, in turn, would lead to greater deterioration of the skeletal muscle fiber during exercise.
Moreover, pain-producing substances, such as bradykinin (BK) and prostaglandin E2 (PGE2), are directly related to muscle soreness.33 Uchida et al. (2010) have reported that there was a significant correlation between peak DOMS and peak PGE2 concentration.34 On the other hand, Santos et al. (2004) have demonstrated that plasma PGE2 concentrations significantly increased after a 30-km race.35 Nerve growth factor (NGF) and glial cell line-derived neurotrophic factor (GDNF), which are produced by muscle, are related to DOMS.36 EOMS might involve factors induced by DOMS because it immediately occurred after exercise and persisted for several days. Future studies are warranted to measure the effect of pain-producing substances, such as BK, PGE2, NGF, and GDNF, on exercise in EOMS or DOMS.
Muscle damage markers in the blood showed significant high values compared with Pre. Moreover, the dynamics of these markers showed three patterns. These markers were characteristic of EOMS but differed from markers for DOMS that showed similar dynamics. Also, JH significantly decreased at Post compared with that at Pre. Moreover, muscle damage was induced after a full marathon because ROM for hip extension was similar to the result of JH after a full-marathon.
Conclusions
In conclusion, we showed differences between EOMS and DOMS (Table 4) on muscle damage markers. It was clear that EOMS occurred after a full marathon because of the perceived muscle soreness determined using the NRS score. All muscle damage markers showed significantly high values until D4. Most characteristics did not alter thigh swelling. This study is the first to show the characteristics of the relationships between EOMS and muscle damage induced after a full marathon.
Table 4.
Different points between DOMS (Ra et al., 2015; Aboodarda et al., 2011) and EOMS (This study).
| Post exercise | Day 1 | Day 2 | Day 3 | Day 4 | ||
|---|---|---|---|---|---|---|
| Muscle soreness | DOMS | – | ↑ | ↑↑ | ↑ | ↑ |
| EOMS | ↑↑ | ↑ | ↑ | ↑ | ↑ | |
| ROM | DOMS | ↓↓ | ↓ | ↓ | ↓ | ↓ |
| EOMS | ↓↓ | ↓ | ↓ | ↓ | ↓ | |
| CIR | DOMS | ↑↑ | ↑ | ↑ | ↑↑ | ↑ |
| EOMS | ↓↓ | ↓ | ↓ | ↓ | ↓ | |
| Muscle power | DOMS | ↓↓ | ↓ | ↓ | ↓ | ↓ |
| EOMS | ↓↓ | ↓ | ↓ | ↓ | – | |
| Muscle damage makers | DOMS | The same dynamics in all makers and peaked at Day 3 or 4 | ||||
| EOMS | Different dynamics in each marker and peaked values earlier those of DOMS (Day 1–3) | |||||
NOTE: Up arrows indicate an increase and down arrows indicate a decrease.
Future studies are required to determine whether swelling is different between EOMS and DOMS. In addition, the relationship between muscle damage markers in the blood and the other markers should be examined to identify an index of muscle damage markers for prolonged exercise after a full marathon.
Declaration of competing interest
The author(s) have no conflicts of interest relevant to this article.
Acknowledgments
We thank all the subjects who participated and Tsuyoshi Ariizumi who belonged to Terumo Co. and provided us with blood-collection tubes and winged needles. In addition, We are grateful to Messrs. Yudai Kawaguchi, Takanobu Kuroiwa, Takaki Mizushima and Daisuke Yamamoto for helping us with measurements in this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jesf.2020.03.001.
NOTE: Values are correlation coefficients (n = 19). Single asterisk indicates significant difference versus values at Pre (p < 0.05). Double asterisks indicate significant difference versus values at Pre (p < 0.01). CK: creatine kinase, AST: aminotransferase, LDH: lactate dehydrogenase, ALD: aldolase.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Hikida R.S., Staron R.S., Hagerman F.C., Sherman W.M., Costill D.L. Muscle fiber necrosis associated with human marathon runners. J Neurol Sci. 1983;59(2):185–203. doi: 10.1016/0022-510x(83)90037-0. [DOI] [PubMed] [Google Scholar]
- 2.Warhol M.J., Siegel A.J., Evans W.J., Silverman L.M. Skeletal muscle injury and repair in marathon runners after competition. Am J Pathol. 1985;118(2):331–339. [PMC free article] [PubMed] [Google Scholar]
- 3.Kim H.J., Lee Y.H., Kim C.K. Changes in serum cartilage oligomeric matrix protein (COMP), plasma CPK and plasma hs-CRP in relation to running distance in a marathon (42.195km) and an ultra-marathon (200km) race. Eur J Appl Physiol. 2009;105:765–770. doi: 10.1007/s00421-008-0961-x. [DOI] [PubMed] [Google Scholar]
- 4.Del Coso J.D., Fernandez D., Abian-Vicen J. Running pace decrease during a marathon is positively related to blood markers of muscle damage. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0057602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryu J.H., Paik I.Y., Woo J.H., Shin K.O., Cho S.Y., Roh H.T. Impact of different running distance on muscle and lymphocyte DNA damage in amateur marathon runners. J Phys Ther Sci. 2016;28:450–455. doi: 10.1589/jpts.28.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tojima M., Noma K., Torii S. Changes in serum creatine kinase, leg muscle tightness, and delayed onset muscle soreness after a full marathon race. J Sports Med Phys Fit. 2016;56(6):782–788. [PubMed] [Google Scholar]
- 7.Clifford T., Allerton D.M., Brown M.A. Minimal muscle damage after a marathon and no influence of beetroot juice on inflammation and recovery. Appl Physiol Nutr Metabol. 2017;42(3):263–270. doi: 10.1139/apnm-2016-0525. [DOI] [PubMed] [Google Scholar]
- 8.Wilson L.J., Cockburn E., Paice K. Recovery following a marathon: a comparison of cold water immersion, whole body cryotherapy and a placebo control. Eur J Appl Physiol. 2018;118(1):153–163. doi: 10.1007/s00421-017-3757-z. [DOI] [PubMed] [Google Scholar]
- 9.Nakagawa K., Inami T., Yonezu T. Unstable rocker shoes promote recovery from marathon-induced muscle damage in novice runners. Scand J Med Sci Sports. 2017:1–9. doi: 10.1111/sms.12911. 00. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong R.B., Warren G.L., Warren J.A. Mechanisms of exercise-induced muscle fiber injury. Sports Med. 1991;12(3):184–207. doi: 10.2165/00007256-199112030-00004. [DOI] [PubMed] [Google Scholar]
- 11.Del Coso J.D., Millan C.G., Salinero J.J. Muscle damage and its relationship with muscle fatigue during a half-iron triathlon. PLoS One. 2012;7(8) doi: 10.1371/journal.pone.0043280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nosaka K., Newton M., Sacco P. Delayed-onset muscle soreness does not reflect the magnitude of eccentric exercise-induced muscle damage. Scand J Med Sci Sports. 2002;12:337–346. doi: 10.1034/j.1600-0838.2002.10178.x. [DOI] [PubMed] [Google Scholar]
- 13.Nosaka K., Newton M., Sacco P. Muscle damage and soreness after endurance exercise of the elbow flexors. Med Sci Sports Exerc. 2002;34(6):920–927. doi: 10.1097/00005768-200206000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Vickers A.J. Time course of muscle soreness following different types of exercise. BMC Muscoskel Disord. 2001;2:5. doi: 10.1186/1471-2474-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howatson G., McHugh, Hill J.A. Influence of tart cherry juice on indices of recovery following marathon running. Scand J Med Sci Sports. 2010;20(6):843–852. doi: 10.1111/j.1600-0838.2009.01005.x. [DOI] [PubMed] [Google Scholar]
- 16.Ishikura K., Miyazaki T., Song-Gyu R., Ohmori H. The ameliorating effect of branched-chain amino acids ingestion on different types of muscle soreness after swimming and full-marathon running. Adv Exerc Sports Physiol. 2014;20(1):9–17. [Google Scholar]
- 17.Clarkson P.M., Nosaka K., Braun B. Muscle function after exercise-induced muscle damage and rapid adaptation. Med Sci Sports Exerc. 1992;24(5):512–520. [PubMed] [Google Scholar]
- 18.Aboodarda S.J., George J., Mokhtar A.H., Thompson M. Muscle strength and damage following two modes of variable resistance training. J Sports Sci Med. 2011;10(4):635–642. [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson D., Nicholas C.W., Williams C. Muscular soreness following prolonged intermittent high-intensity shuttle running. J Sports Sci. 1999;17:387–395. doi: 10.1080/026404199365902. [DOI] [PubMed] [Google Scholar]
- 20.Crystal N.J., Townson D.H., Cook S.B., LaRoche D.P. Effect of cryotherapy on muscle recovery and inflammation following a bout of damaging exercise. Eur J Appl Physiol. 2013;113:2577–2586. doi: 10.1007/s00421-013-2693-9. [DOI] [PubMed] [Google Scholar]
- 21.Chan-Roper M., Hunter I., Myrer J.W., Eggett D.L., Seeley M.K. Kinematic changes during a marathon for fast and slow runners. J Sports Sci Med. 2012;11(1):77–82. [PMC free article] [PubMed] [Google Scholar]
- 22.Warren G.L., Lowe D.A., Armstrong R.B. Measurement tools used in the study of eccentric contraction-induced injury. Sports Med. 1999;27(1):43–59. doi: 10.2165/00007256-199927010-00004. [DOI] [PubMed] [Google Scholar]
- 23.Knechtle B., Knechtle P., Wirth A., Rust C.A., Rosemann T. A faster running speed is associated with a greater body weight loss in 100-km ultra-marathoners. J Sports Sci. 2012;30(11):1131–1140. doi: 10.1080/02640414.2012.692479. [DOI] [PubMed] [Google Scholar]
- 24.Dawson L.G., Dawson K.A., Tiidus P.M. Evaluating the influence of massage on leg strength, swelling, and pain following a half-marathon. J Sports Sci Med. 2004;3:37–43. YISI 1. [PMC free article] [PubMed] [Google Scholar]
- 25.Ra S.G., Miyazaki T., Ishikura K. Combined effect of branched-chain amino acids and taurine supplementation on delayed onset muscle soreness and muscle damage in high-intensity eccentric exercise. J Int Soc Sports Nutr. 2013;10(1):51. doi: 10.1186/1550-2783-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maekawa M. Lactate dehydrogenase isoenzymes. J Chromatogr. 1988;429:373–398. doi: 10.1016/s0378-4347(00)83879-7. [DOI] [PubMed] [Google Scholar]
- 27.Rose L.I., Bousser J.E., Cooper K.H. Serum enzymes after marathon running. J Appl Physiol. 1970;29(3):355–357. doi: 10.1152/jappl.1970.29.3.355. [DOI] [PubMed] [Google Scholar]
- 28.Pyne D.B. Exercise-induced muscle damage and inflammation: a review. Aust J Sci Med Sport. 1994;26(3-4) [PubMed] [Google Scholar]
- 29.Radák Z., Pucsok J., Mecseki S., Csont T., Ferdinandy P. Muscle soreness-induced reduction in force generation is accompanied by increased nitric oxide content and DNA damage in human skeletal muscle. Free Radic Biol Med. 1999;26(7-8):1059–1063. doi: 10.1016/s0891-5849(98)00309-8. [DOI] [PubMed] [Google Scholar]
- 30.Hessel E., Haberland A., Müller M., Lerche D., Schimke I. Oxygen radical generation of neutrophils: a reason for oxidative stress during marathon running? Clin Chim Acta. 2000;298(1-2):145–156. doi: 10.1016/s0009-8981(00)00295-3. [DOI] [PubMed] [Google Scholar]
- 31.Tsai K., Hsu T.G., Hsu K.M. Oxidative DNA damage in human peripheral leukocytes induced by massive aerobic exercise. Free Radic Biol Med. 2001;31(11):1465–1472. doi: 10.1016/s0891-5849(01)00729-8. [DOI] [PubMed] [Google Scholar]
- 32.Ørtenblad N., Westerblad H., Nielsen J. Muscle glycogen stores and fatigue. J Physiol. 2013;591:4405–4413. doi: 10.1113/jphysiol.2013.251629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mastaglia F.L. The relationship between muscle pain and fatigue. Neuromuscul Disord. 2012;22:S178–S180. doi: 10.1016/j.nmd.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Uchida M.C., Nosaka K., Ugrinowitsch C. Effect of bench press exercise intensity on muscle soreness and inflammatory mediators. J Sports Sci. 2009;27:499–507. doi: 10.1080/02640410802632144. [DOI] [PubMed] [Google Scholar]
- 35.Santos R.V.T., Bassit R.A., Caperuto E.C., Costa Rosa L.F.B.P. The effect of creatine supplementation upon inflammatory and muscle soreness markers after a 30km race. Life Sci. 2004;75:1917–1924. doi: 10.1016/j.lfs.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 36.Mizumura K., Taguchi T. Delayed onset muscle soreness: involvement of neurotrophic factors. J Physiol Sci. 2016;66:43–52. doi: 10.1007/s12576-015-0397-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.