Significance
The clinical presentation of Plasmodium falciparum malaria as severe versus uncomplicated is influenced by host genetic factors. Recently, a gain-of-function mutation in the mechanosensitive ion channel PIEZO1 was shown to protect mice against cerebral malaria, but its role in human malaria is unknown. Here we investigated a common PIEZO1 variant that encodes a mild gain-of-function mutant in human erythrocytes, and show that it is associated with malaria protection in Gabonese children. We found an epistatic relationship between PIEZO1 E756del and the sickle cell trait. While parasite growth and cell hydration were normal, surface expression of PfEMP-1 was reduced in the mutant cells. Understanding the link between common PIEZO1 variants and malaria susceptibility may yield new therapies for malaria.
Keywords: malaria, PIEZO1, genetic association study
Abstract
Malaria caused by the apicomplexan parasite Plasmodium falciparum has served as a strong evolutionary force throughout human history, selecting for red blood cell polymorphisms that confer innate protection against severe disease. Recently, gain-of-function mutations in the mechanosensitive ion channel PIEZO1 were shown to ameliorate Plasmodium parasite growth, blood–brain barrier dysfunction, and mortality in a mouse model of malaria. In humans, the gain-of-function allele PIEZO1 E756del is highly prevalent and enriched in Africans, raising the possibility that it is under positive selection due to malaria. Here we used a case-control study design to test for an association between PIEZO1 E756del and malaria severity among children in Gabon. We found that the E756del variant is strongly associated with protection against severe malaria in heterozygotes. In subjects with sickle cell trait, heterozygosity for PIEZO1 E756del did not confer additive protection and homozygosity was associated with an elevated risk of severe disease, suggesting an epistatic relationship between hemoglobin S and PIEZO1 E756del. Using donor blood samples, we show that red cells heterozygous for PIEZO1 E756del are not dehydrated and can support the intracellular growth of P. falciparum similar to wild-type cells. However, surface expression of the P. falciparum virulence protein PfEMP-1 was significantly reduced in infected cells heterozygous for PIEZO1 756del, a phenomenon that has been observed with other protective polymorphisms, such as hemoglobin C. Our findings demonstrate that PIEZO1 is an important innate determinant of malaria susceptibility in humans and suggest that the mechanism of protection may be related to impaired export of P. falciparum virulence proteins.
Malaria infection due to Plasmodium falciparum is a major cause of childhood morbidity and mortality in endemic countries. The symptoms of the disease start when the parasite invades and replicates in red blood cells. Upon infection, there are three possible clinical outcomes that are influenced by the host, parasite, and environmental factors: uncomplicated malaria, severe malaria, or asymptomatic parasitemia. The development of asymptomatic parasitemia is largely influenced by adaptive immunity that results from repeated exposures, whereas it is estimated that ∼25% of the clinical variation in malaria severity can be explained by innate genetic factors that act additively (1). A variety of studies have shown that one of the strongest protective factors is heterozygosity for the hemoglobin S allele (HbAS), which leads to impaired parasite proliferation at low oxygen tension and defects in the trafficking system for parasite-encoded exported proteins (2–6). Many other candidate susceptibility loci reside in genes encoding membrane or structural proteins of the red blood cell (7). However, associations between established candidate loci and malaria severity explain only a fraction of the variance in clinical outcome, suggesting additional susceptibility factors have yet to be discovered (8).
The mechanosensitive ion channel PIEZO1 acts as a nonselective cation channel in a variety of tissues and has established roles in sensing blood flow through the vasculature, cell migration and differentiation, and red blood cell volume control (9–13). Rare gain-of-function (GOF) mutations in PIEZO1 underlie hereditary xerocytosis, a disorder characterized by red blood cell dehydration, reduced osmotic fragility, and mild hemolytic anemia (14, 15). Previous work has shown that P. falciparum parasites replicate poorly in severely dehydrated red blood cells, including those from patients with hereditary xerocytosis (16). In a mouse model of human hereditary xerocytosis, a PIEZO1 GOF allele was recently shown to protect mice from cerebral malaria, potentially through the action of PIEZO1 in red cells and T cells (17).
A subsequent comparative genomics approach identified a common PIEZO1 polymorphism (present in approximately one third of African individuals) that may act as a gain-of-function allele in human red cells (17). The mutation, E756del (rs572934641), is a 3-nt deletion in a coding region of the gene that includes ∼60 bp of short tandem repeats. PIEZO1 E756del was classified as a gain-of-function mutant based on experiments in HEK cells, where it displayed a prolonged inactivation time constant after mechanical stimulation as compared to wild-type PIEZO1 (17). In vitro experiments using RBCs from human donors suggested an association between the presence of the E756del allele, RBC dehydration, and inhibition of P. falciparum growth (17). These findings raise the intriguing possibility that the PIEZO1 E756del allele may be protective against severe malaria in humans, perhaps explaining its high frequency in African populations.
Here we screened a case-control study group from Gabon for the PIEZO1 E756del mutation and assessed its impact on the risk of severe malaria. We found that E756del was associated with protection from severe malaria in heterozygotes and has an epistatic interaction with hemoglobin S. Using in vitro assays, we show that P. falciparum replicates well in both wild-type and E756del-carrying red cells and that the two genotypes have similar hydration states. We conclude that natural variation in PIEZO1 is an innate determinant of malaria susceptibility in humans and propose that the mechanism of protection may be related to impaired export of a major P. falciparum virulence protein.
Methods
Genetic Association Study Design and Participants.
Using a case-control approach, we assembled 542 samples from unique children aged 4 to 140 mo that had been obtained in three previously published studies conducted in Lambaréné and Libreville, Gabon (18–20). Written informed consent for each child was provided by the parents/guardians before enrollment. Samples were deidentified and coded with a unique identifier prior to study use. Their use in this study was approved by the local ethics committee of the International Foundation of the Albert Schweitzer Hospital, Gabon. After excluding samples for missing parameters, the analytic cohort consisted of 446 samples, of which 193 were controls with mild malaria and 253 were cases with severe malaria. All cases presented with microscopically confirmed P. falciparum parasitemia, signs and symptoms of severe malaria, and no evidence of other severe diseases. Severe malaria was defined as severe anemia (hemoglobin <50 g/L) and/or hyperparasitemia (>250,000 parasites/μL, corresponding to >10% infected erythrocytes), a Blantyre coma score ≤2, and/or other facultative signs of severe malaria such as cerebral malaria, convulsions, hypoglycemia, and respiratory distress. The control group was those with mild malaria coming from the same geographical area as the cases. Mild malaria was defined as parasitemia 1,000 to 50,000/μL on admission, no schizontemia, circulating leukocytes containing malarial pigment <50/μL, not homozygous for hemoglobin S, hemoglobin >80 g/L, platelets >50/nL, leukocytes <12/nL, lactate <3 mmol/L, and blood glucose >50 mg/dL
Mutation Screening.
PIEZO1 exon 17 was amplified by PCR using the following primers: 5′-CAGGCAGGATGCAGTGAGTG-3′ (forward) and 5′-GGACATGGCACAGCAGACTG-3′ (reverse). Amplification reactions were performed in one batch in the same laboratory. The thermal conditions were an initial denaturation (95 °C, 3 min) followed by 35 cycles of 95 °C for 30 s, 65 °C for 30 s, and 72 °C for 1 min. The PCR was completed with a final extension step of 72 °C for 5 min. PCR products were visualized through electrophoresis on a 1.2% agarose gel stained with SYBR Safe DNA Gel Stain (Thermo Fisher Scientific). Subsequently, the PCR products were purified with QIAquick PCR purification kit (Qiagen) and directly used as templates for DNA sequencing (Quintara Biosciences). Mutations were identified by aligning the sequences with the PIEZO1 reference sequence (NG_042229.1) using the Geneious 10.2.3 software and visually reconfirmed from their electropherograms. All samples were also genotyped for the HbS polymorphism (rs334) in the HBB gene (NG_059281.1). Briefly, we amplified exon 1 of the HBB gene using the primer pairs 5′-AGTCAGGGCAGAGCCATCTA-3′ (forward) and 5′-GTCTCCACATGCCCAGTTTC-3′ (reverse). The PCR conditions were initial denaturation (95 °C, 3 min) followed by 35 cycles of 95 °C for 30 s, 64 °C for 30 s, and 72 °C for 1 min. The amplification was completed with a final extension step of 72 °C for 5 min. The sequencing and variant detection were performed as described earlier.
Statistical Analysis.
Participants with HbSS or HbSC hemoglobin genotypes were excluded from all analyses due to the severity of their underlying hematologic diseases. Additionally, all primary analyses were conducted only on participants without missing observations. Deviation from Hardy–Weinberg equilibrium was assessed using χ2 analysis. Descriptive statistics were generated to evaluate the distributions of key characteristics by malaria severity status. The Wilcoxon rank-sum test and Fisher’s exact test were used to determine if there were significant differences in malaria severity status in continuous and categorical characteristics, respectively.
The primary outcome was an indicator for whether a patient had mild or severe malaria. A main effects logistic regression model was fit to malaria severity status as a function of PIEZO1 E756del genotype (normal [WT/WT = reference level], heterozygous [WT/DEL], and homozygous [DEL/DEL]), hemoglobin S genotype (normal HbAA, sickle cell trait HbAS), age in months, sex, and the study from which the participant information was extracted. The inclusion of study location as a covariate served as a proxy for fine-scale population structure, given the absence of genome-wide data for PC-based methods. Odds ratios (ORs) and 95% confidence intervals (CIs) were extracted from the logistic regression model. The estimated probability of severe malaria was extracted from the model for each participant, and pairwise comparisons between the PIEZO1 E756del variants and between hemoglobin S genotypes were presented. P values for the pairwise differences in PIEZO1 E756del were calculated from Tukey’s HSD (honestly significant difference) post hoc test. The Hosmer–Lemeshow goodness of fit was used to evaluate model fit using 10 bins.
In order to assess whether HbAS confounds the association between PIEZO1 E756del and malaria severity status, an additional model was fit to malaria severity status as a function of PIEZO1 E756del, HbAS, age, sex, study, and an interaction term between HbAS and PIEZO1 E756del. Due to convergence issues based on small sample size, a Bayesian logistic regression model with a noninformative prior was used by implementing the “bayesglm” function in the “arm” R package (21). This is a simple alteration of the standard logistic regression model that uses a modified expectation-maximization (EM) algorithm to update the coefficients at each step. The Student t prior distribution is used for the coefficients, and the prior distribution for the intercept is set so it applies to the value when all covariates are at their means. As the “bayesglm” function inherits from the “glm” function, we are able to obtain similar outputs to those used in the main effects model described earlier. Unadjusted logistic models were also fit to assess the association between severe malaria and seven additional PIEZO1 polymorphisms near E756del.
To assess whether population stratification may confound the association between PIEZO1 E756del and malaria severity, we first assembled genomic information for PIEZO1 E756del (rs572934641) from the 1000 Genomes database to determine the global geographic distribution of allele frequency for this mutation. Next, using self-reported ethnicity data from a subset of study subjects for whom such information was available, we assembled an “ethnicity subset” (n = 182) and compared the demographics to those of the original cohort. We applied the logistic regression model that was fit earlier (severe malaria = E756del + Hb + age + male) to the ethnicity subset, additionally adjusting for ethnic group.
Classification trees (CART) were implemented to determine whether a synergistic effect of any PIEZO1 polymorphisms existed in order to predict malaria severity status. CART is a tree-based learning technique for classifying observations, which yields a decision rule by partitioning the data into subsets based on variables entered into the algorithm through the minimization of an error function. Two trees were grown to a maximum depth of three levels and minimal node size of 4 using the ctree function in the “party” package in R (22), and included the following features, respectively: 1) all identified PIEZO1 mutations, HbAS, age, and sex; and 2) all identified PIEZO1 mutations excluding PIEZO1 E756del, HbAS, age, and sex. To ensure the validity of these models, we conducted a five-times-repeated 10-fold cross-validation (CV) for both CARTs. The CV area under the curve (AUC) and 95% CIs were reported for each tree (23). Spearman’s rank correlation test was also used in order to assess the genetic association (linkage) between PIEZO1 E756del and the other PIEZO1 mutations.
As a sensitivity analysis, missing values in age and sex were imputed by chained equations with predictive mean matching in order to assess the robustness of the two PIEZO1 E756del logistic regression results (24).
A P value < 0.05 was considered statistically significant. All analyses were conducted using R software v3.5.2 (25).
Blood Sample Collection and Genotyping for In Vitro Parasite Growth Assays.
Subjects who self-identified as African or African-American were recruited to donate blood at the Stanford Clinical Translational Research Unit in Stanford, CA. All participants gave informed consent according to a protocol approved by the Stanford University IRB (no. 40479). Whole blood samples were drawn into CPDA tubes, coded with a unique identifier, stored at ambient temperature, and processed within 24 h to remove serum and separate the buffy coat and red blood cells. Red blood cells were washed in RPMI-1640 medium (Sigma) supplemented with 25 mM Hepes, 50 mg/L hypoxanthine, 2.42 mM sodium bicarbonate media and used immediately for in vitro growth assays. Remaining cells were stored at 4 °C for up to 48 h and then cryopreserved in human AB+ serum and glycerol at −80 °C.
Genomic DNA was isolated from buffy coats using a DNeasy Blood and Tissue Kit (Qiagen). PIEZO1 exon 17 was amplified by PCR of genomic DNA using the primers described earlier and Q5 polymerase in the following reaction conditions: 98 °C for 30 s initial denaturation followed by 35 cycles of 98 °C for 10 s, 70 °C for 20 s, and 72 °C for 20 s, followed by a 2-min final extension at 72 °C. PCR products were visualized on an agarose gel and sent for Sanger sequencing at Elim Bio. The PIEZO1 E756 genotypes were manually determined from chromatograms examined using FinchTV software (Geospiza). All genotypes were assessed by two independent researchers.
P. falciparum Culture and Growth Assays.
Laboratory-adapted P. falciparum strains 3D7 and W2mef were obtained from MR4 and routinely cultured in human erythrocytes obtained from the Stanford Blood Center at 2% hematocrit in RPMI-1640 supplemented with 25 mM Hepes, 50 mg/L hypoxanthine, 2.42 mM sodium bicarbonate, and 4.31 mg/mL Albumax (Invitrogen) at 37 °C in 5% CO2 and 1% O2. 3D7 is a sialic acid-independent strain derived from NF54 by limiting dilution (26), and W2mef (from which strain Dd2 was cloned) is a sialic acid-dependent strain derived from the Indochina III isolate (27). Parasite growth assays were performed using fresh erythrocytes stored for <24 h at ambient temperature and then repeated after cryopreservation and thawing. Schizont-stage parasites were isolated using a MACS magnet (Miltenyi) and added at ∼0.5% initial parasitemia to wild-type or E756del heterozygous erythrocytes that had been washed and resuspended in complete RPMI with bicarbonate and Albumax as described earlier. Assays were performed at 0.5% hematocrit in a volume of 100 μL per well in 96-well plates in triplicate for each strain. Parasitemias were determined on day 0, day 1 (24 h), and day 3 (72 h) by staining with SYBR Green 1 nucleic acid stain (Invitrogen, ThermoFisher Scientific) at 1:2,000 dilution in PBS/0.3% BSA for 20 min, followed by flow cytometry analysis on a MACSQuant flow cytometer (Miltenyi). The parasite multiplication rate (PMR) in each genetic background was calculated by dividing the reinvasion parasitemia by the parasitemia in the previous cycle. To control for batch effects, PMRs were normalized relative to the PMR of each parasite strain in a consistent control sample.
PfEMP1 Export Assays.
Schizont-stage parasites of strain NF54CSA were isolated by MACS purification and added to wild-type or E756del heterozygous RBCs at 1% parasitemia. NF54CSA is a laboratory-adapted strain expressing VAR2CSA and was obtained from Benoit Gamain, INSERM, Paris, France. After ∼30 h, cells were harvested and stained with primary antibody rabbit anti-var2CSA (var11p) at 1:100, followed by secondary antibody goat anti-rabbit Alexa Fluor 647 (Invitrogen, A21244) at 1:1,000. After washing, cells were stained with SYBR Green I at 1:2,000 to detect parasite DNA and run on a MACSQuant flow cytometer to quantify the amount of PfEMP1 on the erythrocyte plasma membrane in infected cells. Data were analyzed by FlowJo version 10.6.1, and the geometric mean fluorescence attributable to PfEMP1 was calculated for each sample. The experiment was performed twice independently, and the normalized mean fluorescence intensity for each replicate was calculated relative to a sample from a control Caucasian donor run on the same day.
Osmotic Fragility Assays.
Osmotic fragility assays were performed using fresh blood samples stored for <24 h at ambient temperature, and then repeated after overnight storage at 4 °C. Fifteen different concentrations of NaCl (sodium chloride; EMD Millipore) were prepared in water (308, 245.5, 210, 196, 182, 168, 154, 147, 140, 133, 126, 112, 105, 91, and 84 mOsmol). One half milliliter of each saline solution was aliquoted into 1.5-mL centrifuge tubes, and 20 µL of whole blood sample was added and mixed well. The mixture was incubated at room temperature for 5 min, followed by centrifugation at 1,000 × g for 5 min. One hundred microliters of the supernatant was transferred to a 96-well flat-bottom plate in duplicate. Next, 100 µL of Drabkin’s reagent (Ricca Chemical) was added to the sample supernatants. Two blanks were also prepared by mixing 100 µL of water with 100 µL of Drabkin’s reagent. The samples were mixed and the absorbance read at 540 nm using a Synergy H1 Hybrid Reader spectrophotometer (BioTek). The data were analyzed using SSlogis, a self-starting four-parameter logistic model in the nls package in R, to estimate the sigmoid function. One hundred percent hemolysis was defined as the maximum value of hemoglobin at 91 or 84 mOsmol, whichever was greater.
Data Availability Statement.
All materials, data, and protocols associated with this manuscript are available to readers upon request from the corresponding author. All data are contained within the manuscript and SI Appendix.
Results
The PIEZO1 E756del Variant Is Associated with Protection from Severe Malaria.
To determine if the PIEZO1 E756del allele influences malaria susceptibility, we collected 542 DNA samples from three well-characterized malaria study cohorts from Gabon (18–20). Out of the 542 samples, 8 were excluded for HbSS or HbSC genotypes to minimize confounding effects of severe hematologic disease. A further 88 were excluded due to missing values in sex (n = 78) or failure to amplify PIEZO1 E756del (n = 10). Therefore, our analytic cohort consisted of 446 samples. All samples were from Gabonese children between the ages of 4 and 140 mo; 193 (43%) participants had mild malaria (controls) and 253 (57%) had severe malaria (cases). Cases of severe malaria had severe anemia, hyperparasitemia, signs of cerebral malaria, hypoglycemia, and/or respiratory distress in addition to microscopically confirmed parasitemia. Controls with mild malaria had parasitemia and fever with absence of severe signs. Their baseline demographics are summarized in Table 1. While cases and controls were similar in terms of sex (45% vs. 46% male, respectively), those with severe malaria on average were younger and had higher parasite densities. Differences by study are also summarized in SI Appendix, Table S1.
Table 1.
Baseline demographics
| Baseline characteristic | Overall (n = 446) | Malaria status | P value | |
| Mild (n = 193) | Severe (n = 253) | |||
| Sex, n (%) | ||||
| Male | 201 (45%) | 88 (46%) | 113 (45%) | 0.92 |
| Female | 245 (55%) | 105 (54%) | 140 (55%) | |
| Median age, mo (range) | 35 (4-140) | 43 (8-140) | 29 (4-133) | <0.001 |
| Median parasite density, parasite/µL1 (range) | 35,000 (20-1,544,880) | 15,000 (588-434,074) | 108,518 (20-1,544,880) | <0.001 |
| Study, n (%) | ||||
| Kremsner et al. | 195 (44%) | 49 (25%) | 146 (58%) | <0.001 |
| Kun et al. | 195 (44%) | 98 (51%) | 97 (38%) | |
| Kalmbach et al. | 56 (12%) | 46 (24%) | 10 (4%) | |
Continuous and categorical variables were compared across malaria status using the Wilcoxon rank-sum test and Fisher’s exact test, respectively. 1n = 44 excluded due to zero values.
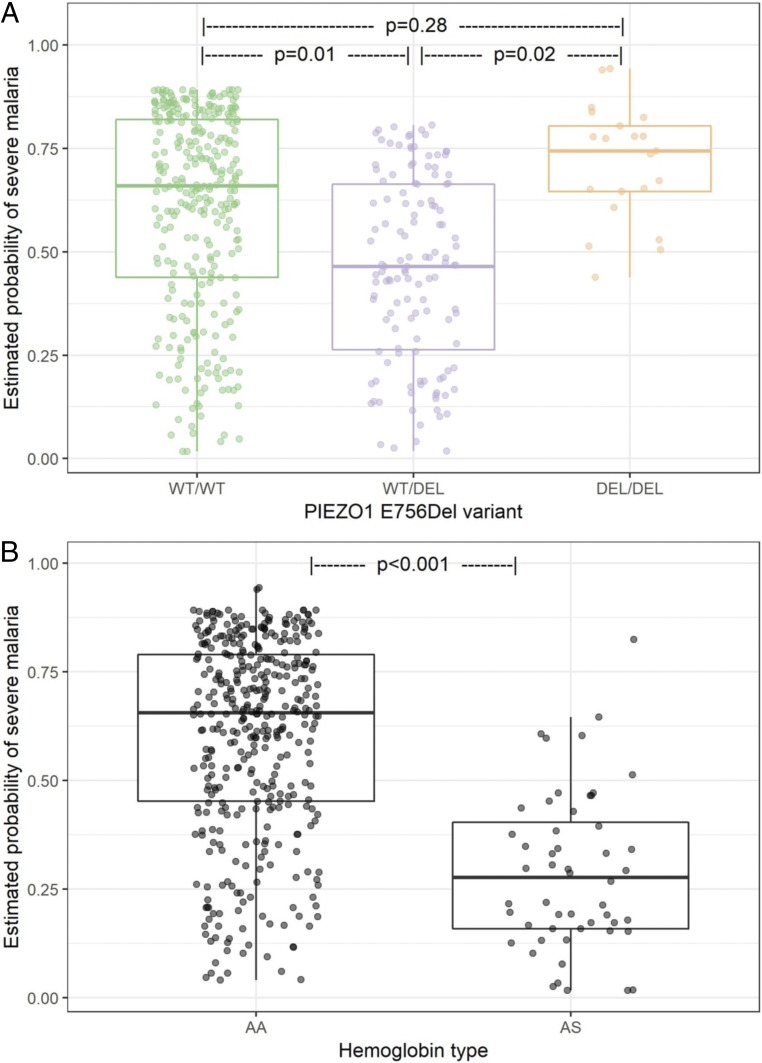
The PIEZO1 E756del variant was common among the study population, with a heterozygote prevalence of 36% in controls and 23% in cases (minor allele frequency = 0.19). The variant was in Hardy–Weinberg equilibrium in the controls but deviated from equilibrium in the cases, suggesting a possible disease association (SI Appendix, Table S2). We used a logistic regression model to predict the probability of severe malaria as a function of PIEZO1 genotype, HbAS status, age, sex, and study for each participant. The odds of severe malaria in those with the heterozygous E756del genotype (WT/DEL) were half the odds of those with the homozygous wild-type genotype (OR 0.50, 95% CI 0.31–0.81; P = 0.005), suggesting that having one copy of PIEZO1 E756del is protective against severe disease (Table 2 and SI Appendix, Table S3). Homozygous wild-type subjects at E756 (WT/WT) had a significantly higher predicted probability of severe malaria than E756del heterozygous (WT/DEL) individuals (median 67% vs. 46%, P = 0.01; Fig. 1A). These results suggest that the E756del variant confers protection for heterozygotes even as they age and develop increased adaptive immunity to malaria.
Table 2.
Associations with malaria severity
| Characteristic | Malaria status | Odds ratio (95% CI) | |
| Mild (n = 193) | Severe (n = 253) | ||
| PIEZO1 E756Del | |||
| WT/WT | 118 (40%) | 180 (60%) | Reference |
| WT/DEL | 69 (54%) | 58 (46%) | 0.50** (0.31, 0.81) |
| DEL/DEL | 6 (29%) | 15 (71%) | 2.26 (0.82, 6.94) |
| Hemoglobin type | |||
| AA | 156 (40%) | 238 (60%) | Reference |
| AS | 37 (71%) | 15 (29%) | 0.27*** (0.13, 0.52) |
| Age | 0.98*** (0.97, 0.99) | ||
| Male sex | 88 (44%) | 113 (56%) | 0.70 (0.45, 1.08) |
Percentages are out of the row totals. Model also adjusted for study in which the data were collected. The Hosmer–Lemeshow goodness of fit test suggested the model fit was appropriate (Χ2 = 11.91, P value = 0.16).
**P < 0.01, ***P < 0.001.
Fig. 1.
Association of malaria severity with PIEZO1 and hemoglobin genotypes. Estimated probability of severe malaria extracted from the model presented in Table 2 by (A) PIEZO1 E756 genotype and (B) hemoglobin beta genotype. P values for pairwise differences in PIEZO1 were calculated using Tukey’s HSD post hoc test. Model adjusted for age, sex, and study.
Participants homozygous mutant for PIEZO1 E756del (DEL/DEL) had a high probability of severe malaria compared to heterozygotes (P = 0.02), suggesting that harboring two copies of this mutant allele negates the protective effect observed in carriers (Fig. 1A). Homozygous mutant subjects (DEL/DEL) also had an elevated risk of severe malaria compared to wild-type subjects (WT/WT), but this association was not statistically significant, likely due to the low overall frequency of the DEL/DEL genotype (OR 2.26, 95% CI 0.82 to 6.94, P = 0.28; Table 2 and Fig. 1A). The different risk probabilities observed in heterozygous versus homozygous subjects may be due to differential effects on the ionic milieu of red cells or other PIEZO1-expressing tissues relevant to the development of severe malaria, depending on gene dosage.
Association between HbAS and Protection from Severe Malaria.
As expected, the sickle cell trait polymorphism (HbAS) was significantly associated with mild malaria in our study population (HbAS vs. HbAA, OR 0.27, 95% CI 0.13 to 0.52; P < 0.001; Table 2). Children with HbAS had a significantly lower predicted probability of severe malaria compared to children with normal hemoglobin (HbAA; 28% vs. 66%; P < 0.001; Fig. 1B), an effect approximately twice as strong as that of PIEZO1 E756del in our study population. This result is consistent with published literature on the protective effect of the sickle cell trait for severe malaria (28, 29).
Interplay between PIEZO1 E756del and HbS and Malaria Severity.
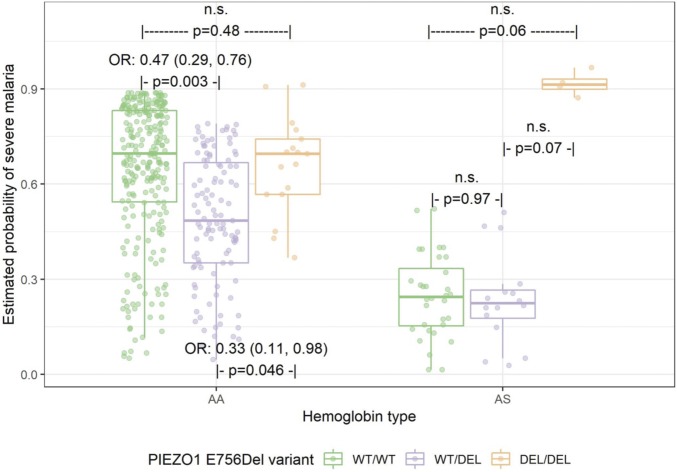
Because of the high frequencies of both the PIEZO1 E756del variant and HbAS in the study population, we sought to assess whether HbAS confounds the association found for E756del heterozygotes and malaria susceptibility by refitting the main effects model with the addition of an interaction term for HbAS and E756del. For subjects with HbAA, the E756 wild-type genotype (WT/WT) was associated with a significantly higher probability of severe malaria compared to the E756del heterozygous genotype (WT/DEL; OR 0.47, 95% CI 0.19 to 0.76; P = 0.003, Fig. 2), suggesting that PIEZO1 E756del is independently associated with protection against severe malaria. In contrast, in subjects with HbAS, heterozygosity for PIEZO1 E756del did not alter susceptibility to severe malaria compared to E756 wild-type (P = 0.97; Fig. 2 and SI Appendix, Table S4). These results suggest that the heterozygous PIEZO1 E756del genotype reduces the risk of severe malaria in individuals with normal hemoglobin, but that effect is masked by the strong protective effect of HbAS in those with sickle cell trait.
Fig. 2.
Association between PIEZO1 and malaria severity by hemoglobin type. n.s., not statistically significant. A Bayesian logistic regression model was fit to malaria severity status as a function of hemoglobin type, PIEZO1, and their interaction. Overall interaction effect is P = 0.08. P values for pairwise differences between PIEZO1 within each hemoglobin type were calculated using Tukey’s HSD post hoc test. Model also adjusted for age, sex, and study. The Hosmer–Lemeshow goodness-of-fit test suggested the model fit was appropriate (X2 = 6.03, P value = 0.64). n.s., not significant.
Notably, the homozygous mutant E756del genotype (DEL/DEL) was not associated with protection in either HbAA or HbAS subjects. The odds of severe malaria in HbAA subjects homozygous for E756del (DEL/DEL) was similar to wild-type subjects and approximately three times higher than for E756del heterozygotes (WT/DEL; OR 0.33, 95% CI 0.11 to 0.98; P = 0.046; Fig. 2). For subjects with HbAS, homozygosity for E756del (DEL/DEL) was associated with an elevated risk of severe malaria compared to those with E756 WT/WT or WT/DEL, though this failed to reach statistical significance (P = 0.06 and 0.07, respectively). These results suggest that the homozygous mutant E756del genotype may alter host physiology in a way that abolishes the protective effect of HbAS on malaria susceptibility, and are consistent with an epistatic relationship between HbAS and PIEZO1 E756del.
Analysis of Other Proximal PIEZO1 Polymorphisms.
In addition to E756del, we identified seven other single-nucleotide polymorphisms and insertion-deletions within the ∼160-bp region we sequenced, which ranged in frequency from 0.4 to 7.9% (SI Appendix, Table S5). Even though these mutations are in close proximity to E756del, none of them were significantly associated with severe malaria individually. To determine whether any of these PIEZO1 variants act synergistically with hemoglobin type, sex, or age to influence malaria susceptibility in the absence of E756del, we used classification trees (CART; SI Appendix, Fig. S1). For subjects with HbAA, when E756del was excluded, heterozygosity for E750Q appeared protective in younger children, whereas heterozygosity for Q749del was associated with an increased probability of severe malaria in older children. However, correlation testing showed E750Q and Q749del were significantly correlated with E756del, suggesting that these two variants may be serving as a proxy for E756del in predicting malaria severity. This hypothesis is further strengthened when the E756del polymorphism is included in the analysis, as no other PIEZO1 variants were predictive of disease severity in the presence of E756del (SI Appendix, Fig. S1). Together, these results suggest that E756del is the causal variant in this region.
Adjusting for Population Structure.
To assess whether the association between PIEZO1 E756del and malaria severity could be explained by population stratification, we first assembled genomic data from the 1000 Genomes database (30) to determine the geographic distribution of the polymorphism. The E756del heterozygous genotype was at high prevalence in all four west African populations surveyed (0.306 to 0.345) and closely resembled the prevalence in our study cohort (0.36; SI Appendix, Table S6), suggesting that the frequency of E756del is relatively stable throughout west Africa. Next, we used a subset of samples for which we had detailed ethnicity data (n = 182; SI Appendix, Table S7) to fit a logistic regression model to determine whether the association between PIEZO1 E756del and malaria severity is modified after adjusting for ethnic group (SI Appendix, Fig. S2). We observed that there was no association between ethnic group and malaria severity (SI Appendix, Table S8), and that adjusting for ethnic group did not modify the association between PIEZO1 E756del and mild malaria (SI Appendix, Table S9). Collectively, these results strongly suggest that the association between E756del and malaria susceptibility cannot be explained by population stratification.
Effect of PIEZO1 E756del on In Vitro Growth of P. falciparum.
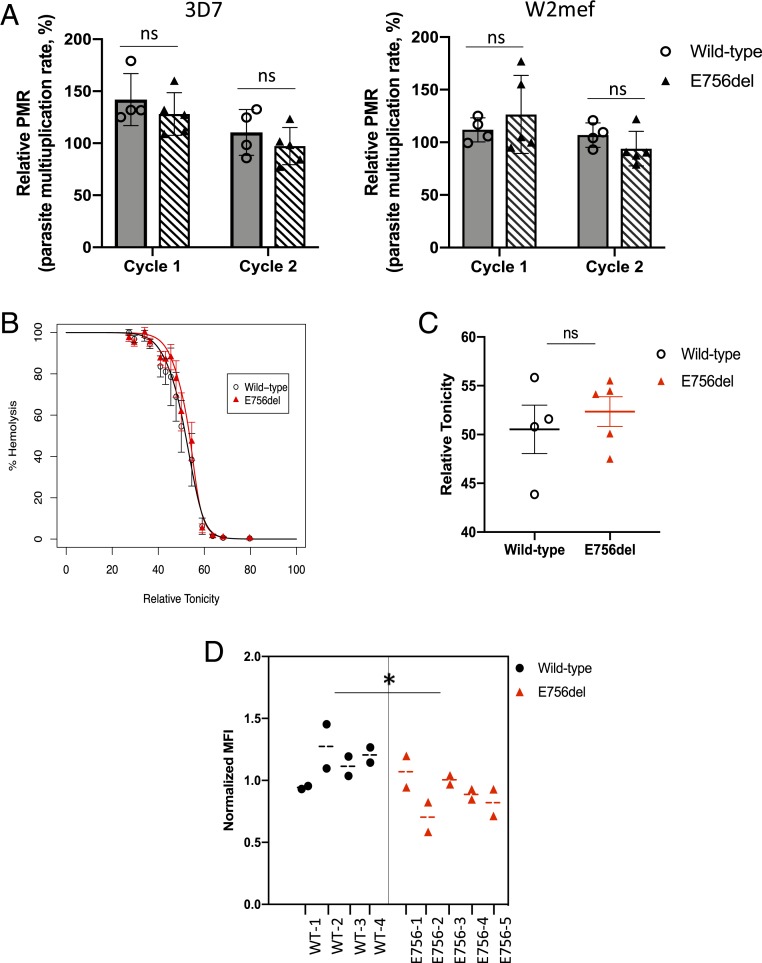
To investigate whether impaired intracellular growth could be a mechanism by which PIEZO1 E756del protects against severe malaria in humans, we identified local donors of African descent who are wild-type or heterozygous at PIEZO1 E756del and used their red blood cells in growth assays with two P. falciparum strains, 3D7 and W2mef. We found that P. falciparum replicated robustly in both wild-type and E756del heterozygous red cells over two cycles, with similar results observed for both strains (Fig. 3A). Repeating the assays after cryopreservation and thawing the red cells yielded similar findings (SI Appendix, Fig. S3). These results suggest that, while the E756del mutation may confer gain-of-function kinetics (17), at least in heterozygous RBCs, the phenotype is not strong enough to impair intracellular growth of P. falciparum in vitro.
Fig. 3.
Characterization of PIEZO1 E756del RBCs for P. falciparum and dehydration phenotypes. (A) Growth of P. falciparum strain 3D7 or W2mef in wild-type (n = 4) or E756del heterozygous (n = 5) RBCs over two cycles of reinvasion. PMR, parasite multiplication rate relative to sample from Caucasian donor, presented as mean ± SEM. (B) Osmotic fragility curves for PIEZO1 E756del RBCs (n = 5) versus wild-type (n = 4) at ambient temperature. (C) Quantification of osmotic fragility tests showing mean relative tonicity at which there was 50% hemolysis ± SEM. ns, not significant. (D) Quantification of VAR2CSA expression on the surface of wild-type (n = 4) versus PIEZO1 E756del (n = 5) RBCs infected with P. falciparum strain NF54CSA, as measured by flow cytometry. Normalized MFI, mean fluorescence intensity relative to a control sample measured on the same day (*P = 0.045, nested ANOVA). n.s., not significant.
Since gain-of-function mutations in PIEZO1 have been associated with red cell dehydration (15, 31), we sought to study the impact of the E756del mutation on red cell hydration using osmotic fragility assays. In these assays, cells are exposed to a range of salt concentrations and the concentration at which 50% of cells hemolyze is correlated with hydration status, with dehydrated cells being more resistant to lysis. Using fresh blood samples from wild-type or E756del heterozygous donors, we observed no significant difference in osmotic fragility between the two genetic backgrounds, indicating that the E756del cells are not dehydrated (Fig. 3 B and C). Since a recent report suggested that mechanoactivation of PIEZO1 ion channels is temperature-dependent (32), we also conducted the osmotic fragility assays after storing the blood overnight at 4 °C. Similar results were obtained at the lower temperature, with no significant difference in osmotic fragility observed between the PIEZO1 E756del heterozygous and wild-type samples (SI Appendix, Fig. S4).
Severe malaria is characterized by adherence of P. falciparum-infected red cells to the vascular endothelium, uninfected cells, and other host tissues. Cytoadherence is largely mediated by PfEMP-1, a family of P. falciparum virulence proteins exported onto the surface of the host red cell membrane. Abnormal display of PfEMP-1 in red cells carrying hemoglobin S or C, leading to altered cytoadherence, has been proposed as a mechanism of protection against severe malaria in these hemoglobinopathies (33–35). To determine whether abnormal display of PfEMP-1 might explain the protection from severe malaria associated with PIEZO1 E756del, we infected wild-type or E756del heterozygous red cells with P. falciparum strain NF54CSA, allowed the parasites to develop to the schizont stage, and measured the amount of PfEMP-1 on the red cell surface via flow cytometry using a strain-specific antibody. We observed significantly less PfEMP-1 on the surface of E756del heterozygous red cells as compared to wild-type cells (Fig. 3D), raising the possibility that the protective effect of the E756del heterozygous genotype may be due to a reduction in the efficiency of PfEMP-1 export.
Discussion
In this work, we sought to determine if there is a genetic association between malaria susceptibility and the human PIEZO1 mutation E756del, which is at high allelic frequency in populations of African descent in comparison to non-Africans (17). Using samples from Gabonese patients with severe or uncomplicated malaria, we found that PIEZO1 E756del is significantly associated with protection against severe disease. Children heterozygous for E756del had a significantly lower predicted probability of severe malaria compared to those with wild-type PIEZO1, even when controlling for age, sex, ethnicity, and HbAS status. As E756del is present at high frequencies in African populations (17), these results suggest that it may be a major determinant of innate resistance to severe malaria. Whether this advantage is extended to all severe malaria subphenotypes remains to be investigated in the context of a larger study.
Given the strong protective effect of PIEZO1 E756del on severe malaria in our study, its absence from GWAS candidate lists is particularly notable. The sensitivity of GWAS studies for malaria in Africa are limited by several factors, including weak linkage disequilibrium and population stratification (2), as well as technical limitations in assessing complex or repetitive regions via microarrays or deep sequencing. The PIEZO1 E756del mutation is a 3-nt deletion within a region of short tandem repeats, making it difficult to detect by high-throughput methods. In our study, accurate genotyping of this locus required Sanger sequencing and manual alignments. These studies on PIEZO1 highlight the benefit of using a combination of sequencing methods for the identification of host factors that may influence susceptibility to malaria.
Although we cannot rule out the possibility that the apparent effects of E756del are due to its linkage with another, truly casual allele, this possibility is less likely because our candidate-gene approach was inspired by functional data on channel activity (17). PIEZO1 E756del had a prolonged inactivation time constant after mechanical stimulation and showed increased responsiveness to a small molecule agonist, consistent with a gain-of-function phenotype (17). The observation that only E756del and one other low-frequency polymorphism had a detectable channel phenotype (out of 21 evaluated) (17) provides further rationale for testing this locus, and minimizes the chance that the observed association could be explained by linkage. While a true assessment of population structure would require genome-wide data, our logistic regression analysis showing that ethnicity does not modify the association between E756del and malaria severity supports the conclusion that the association results are unlikely to be due to population stratification. In the future, replication of the association in an independent cohort could provide important additional evidence that the results we observed are not due to differences in population structure.
The finding that E756del is protective against severe malaria only in heterozygotes is reminiscent of the protective effect of HbS, where heterozygotes with sickle cell trait are protected from malaria-related mortality but homozygotes with sickle cell disease are not, due to their underlying severe hematologic condition (36–38). Although GOF mutations in PIEZO1 are a recognized cause of hereditary xerocytosis (15), to our knowledge, the E756del allele has not been implicated in any hematologic disease (17). Our data indicate that PIEZO1 E756del is in Hardy–Weinberg equilibrium overall, suggesting there is no reproductive disadvantage for those carrying the homozygous mutant genotype. Given the high allele frequency of E756del in our Gabonese study population (19%), understanding how homozygosity for this mutation affects PIEZO1 channel function, RBC hydration status, and in vitro susceptibility to P. falciparum are important open questions.
We did not observe any influence of E756del heterozygosity on malaria susceptibility in an HbAS background, suggesting that having one E756del allele does not contribute any additional effect to the already powerful protection conferred by HbAS. In contrast, subjects with HbAS who are homozygous mutant for E756del had an extremely high predicted probability of severe malaria. While this association was based on few data points and did not reach statistical significance, it highlights the need for further research on the interplay between these genes, as it suggests that the protective effect of HbAS on malaria may be subverted by structural or permeability abnormalities associated with the homozygous E756del genotype. Future studies aimed at characterizing the hematologic and parasite phenotypes of E756del homozygous RBC samples +/− HbAS may shed additional light on this intriguing finding. A recent analysis of associations between E756del and clinical parameters in sickle cell disease (SCD) patients showed that the PIEZO1 E756del variant was associated with increased RBC density and dehydration in HbSS cells, providing an additional example of a potential phenotypic interplay between these genes in red cells (39). Alternatively, homozygosity for PIEZO1 E756del may alter the physiology of the vascular endothelium or other PIEZO1-expressing tissues relevant to the pathogenesis of severe malaria in addition to its role in red cells.
The hypothesis that PIEZO1 may influence malaria susceptibility arose from the observation that P. falciparum parasites grow poorly in dehydrated red blood cells and the knowledge that hereditary xerocytosis, a disorder characterized by red blood cell dehydration, is often caused by GOF mutations in PIEZO1 (14, 16, 40). The scientific rationale for specifically testing for an association between PIEZO1 E756del and malaria susceptibility was based on the report that E756del is highly prevalent in individuals of African descent and is a derived allele, suggesting it is under selective pressure. Functionally, E756del was demonstrated to be a gain-of-function mutant using patch clamping and calcium imaging assays in 293T cells (17). Although our results show that PIEZO1 E756del is protective against severe malaria in heterozygotes, the mechanism of protection is not yet known. In the mouse model of hereditary xerocytosis, expression of a strong, disease-causing PIEZO1 GOF allele was protective against cerebral malaria and appeared to impair growth of Plasmodium berghei ANKA parasites, at least during the initial phase of infection (17). The same study suggested that human red cells heterozygous for PIEZO1 E756del were dehydrated and could not support normal growth of P. falciparum strain Dd2 (17). However, we found that human red cells heterozygous for PIEZO1 E756del had a normal hydration status as measured by osmotic fragility assays. Additionally, two different P. falciparum strains (3D7 and W2mef, the parent of clone Dd2) grew equally well in E756del-carrying RBCs as compared to wild-type in our experiments, regardless of whether the cells were fresh or had been previously cryopreserved. These results demonstrate that heterozygosity for PIEZO1 E756del does not inherently lead to cell dehydration or hinder P. falciparum invasion or growth in vitro, though it remains possible that an in vivo environment where RBCs must traverse small capillaries could reveal a parasite phenotype that cannot be recapitulated in vitro. While the absence of a measurable dehydration phenotype was unexpected, it is probable that erythrocytes have some functional redundancy with regard to PIEZO1 channel activity and hydration status because they harbor a network of pathways to regulate salt and water content (31).
Given that in vitro growth of P. falciparum in RBCs is unaffected by PIEZO1 E756del and the cells are not dehydrated, how might this common polymorphism protect from severe malaria? As with many life-threatening systemic infections, the symptoms of severe malaria in patients are in large part caused by the body’s extreme response to overwhelming infection by a microorganism. Additionally, the adhesive properties of P. falciparum-infected RBCs enable them to sequester in the deep microvasculature, exacerbating organ dysfunction. Our observation that the amount of surface-expressed PfEMP-1 was reduced in P. falciparum-infected PIEZO1 E756del heterozygous red cells as compared to wild-type suggests that E756del may alter P. falciparum virulence through decreased export of PfEMP-1. This type of mechanism has previously been proposed to explain the malaria-protective nature of hemoglobin C and hemoglobin S (33, 35), possibly through aberrant formation of an actin-based transport network for export of virulence proteins (34). In the case of PIEZO1 E756del, we hypothesize that an imbalanced intracellular ionic milieu may similarly adversely impact the actin transport network, as it is well-established that cations regulate actin polymerization, bending mechanics, and interactions with regulatory proteins (41). The finding that PfEMP1 display is altered in PIEZO1 E756del heterozygous cells should stimulate future investigations aimed at quantifying the cytoadherence properties of these infected cells relative to wild-type. Additionally, as PIEZO1 is expressed in many tissues, including the vascular endothelium and immune cells, it is possible that dysregulation of its channel activity has effects on vascular tone, cell permeability, and/or signaling during malaria infection that are independent of any effects on RBCs.
Our findings demonstrate a significant association between PIEZO1 E756del and protection from severe P. falciparum malaria. Together with the previous mechanistic work on mouse PIEZO1 GOF mutations and P. berghei ANKA, these data firmly establish PIEZO1 as an important host susceptibility factor for malaria. Though the list of innate susceptibility determinants for severe malaria includes a range of factors such as hemoglobin variants, enzymes, membrane proteins, and immune-related molecules (7, 42), PIEZO1 is unique because it is considered a druggable molecule (43). The discovery that the E756del allele is associated with protection from severe malaria in patients suggests that PIEZO1 has potential as a compelling target for a host-directed therapy for malaria.
Supplementary Material
Acknowledgments
We thank the staff of Albert Schweitzer Hospital in Lambaréné and the Centre Hospitalier de Libreville in Gabon for their assistance with samples and data collection, and the study patients and their parents for consenting to participate in the original studies. We thank Bertrand Lell, Benjamin Mordmueller, Dmitri Petrov, Frans Kuypers, Joshua Zimmerberg, Svetlana Glushakova, Jude Przyborski, Ardem Patapoutian, Elizabeth Winzeler, and members of the Boothroyd, Petrov, Yeh, and Egan labs for helpful discussions. We thank Carrie Lin, Nick Bondy, and Tuya Yokoyama for technical assistance and Benoit Gamain for NF54CSA and anti-var2CSA. Local blood samples were drawn at the Stanford Clinical and Translational Research Unit, which is supported by CTSA Grant UL1 TR001085. C.N.N. received support from the Stanford Maternal and Child Health Research Institute, and E.S.E. was funded through awards from the Doris Duke Charitable Foundation (no. 2016098), NIH (DP2HL13718601), and the Stanford Maternal Child Health Research Institute, and is a Tashia and John Morgridge Endowed Faculty Scholar in Pediatric Translational Medicine and a Baxter Faculty Scholar.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1919843117/-/DCSupplemental.
References
- 1.Mackinnon M. J., Mwangi T. W., Snow R. W., Marsh K., Williams T. N., Heritability of malaria in Africa. PLoS Med. 2, e340 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jallow M., et al. ; Wellcome Trust Case Control Consortium; Malaria Genomic Epidemiology Network , Genome-wide and fine-resolution association analysis of malaria in West Africa. Nat. Genet. 41, 657–665 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Timmann C., et al. , Genome-wide association study indicates two novel resistance loci for severe malaria. Nature 489, 443–446 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Band G., Rockett K. A., Spencer C. C., Kwiatkowski D. P.; Malaria Genomic Epidemiology Network , A novel locus of resistance to severe malaria in a region of ancient balancing selection. Nature 526, 253–257 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Archer N. M., et al. , Resistance to Plasmodium falciparum in sickle cell trait erythrocytes is driven by oxygen-dependent growth inhibition. Proc. Natl. Acad. Sci. U.S.A. 115, 7350–7355 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pasvol G., Weatherall D. J., Wilson R. J., Cellular mechanism for the protective effect of haemoglobin S against P. falciparum malaria. Nature 274, 701–703 (1978). [DOI] [PubMed] [Google Scholar]
- 7.Nguetse C. N., Egan E. S., “Host genetic predisposition to malaria” in Encyclopedia of Malaria, Kremsner P. G., Krishna S., Eds. (Springer New York, New York, NY, 2019), pp 1–25. [Google Scholar]
- 8.Ndila C. M., et al. ; MalariaGEN Consortium , Human candidate gene polymorphisms and risk of severe malaria in children in Kilifi, Kenya: A case-control association study. Lancet Haematol. 5, e333–e345 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ranade S. S., et al. , Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc. Natl. Acad. Sci. U.S.A. 111, 10347–10352 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J., et al. , Piezo1 integration of vascular architecture with physiological force. Nature 515, 279–282 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pathak M. M., et al. , Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc. Natl. Acad. Sci. U.S.A. 111, 16148–16153 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cahalan S. M., et al. , Piezo1 links mechanical forces to red blood cell volume. eLife 4, e07370 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang S., et al. , Endothelial cation channel PIEZO1 controls blood pressure by mediating flow-induced ATP release. J. Clin. Invest. 126, 4527–4536 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Archer N. M., et al. , Hereditary xerocytosis revisited. Am. J. Hematol. 89, 1142–1146 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glogowska E., et al. , Novel mechanisms of PIEZO1 dysfunction in hereditary xerocytosis. Blood 130, 1845–1856 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tiffert T., et al. , The hydration state of human red blood cells and their susceptibility to invasion by Plasmodium falciparum. Blood 105, 4853–4860 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma S., et al. , Common PIEZO1 allele in African populations causes RBC dehydration and attenuates plasmodium infection. Cell 173, 443–455.e12 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kun J. F., et al. , Merozoite surface antigen 1 and 2 genotypes and rosetting of Plasmodium falciparum in severe and mild malaria in Lambaréné, Gabon. Trans. R. Soc. Trop. Med. Hyg. 92, 110–114 (1998). [DOI] [PubMed] [Google Scholar]
- 19.Kalmbach Y., et al. , Increase in annexin V-positive B cells expressing LILRB1/ILT2/CD85j in malaria. Eur. Cytokine Netw. 17, 175–180 (2006). [PubMed] [Google Scholar]
- 20.Kremsner P. G., et al. , Prognostic value of circulating pigmented cells in African children with malaria. J. Infect. Dis. 199, 142–150 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gelman A., Hill J., Data Analysis Using Regression and Multilevel/Hierarchical Models (Cambridge University Press, Cambridge, United Kingdom, 2018). [Google Scholar]
- 22.Hothorn T., Hornik K., Zeileis A., Unbiased recursive partitioning: A conditional inference framework. J. Comput. Graph. Stat. 15, 651–674 (2006). [Google Scholar]
- 23.Kohavi R., “A study of cross-validation and bootstrap for accuracy estimation and model selection” in Proceedings of the 14th International Joint Conference on Artificial Intelligence (Morgan Kaufmann Publishers, Montreal, Quebec, Canada, 1995), pp 1137–1143. [Google Scholar]
- 24.van Buuren S., Groothuis-Oudshoorn K., mice: Multivariate imputation by chained equations in R. J. Stat. Softw. 45, 1–67 (2011). [Google Scholar]
- 25.R Development Core Team , R: A Language and Environment for Statistical Computing 2014, v3.5.2 (R Foundation for Statistical Computing, Vienna, Austria, 2013).
- 26.Walliker D., et al. , Genetic analysis of the human malaria parasite Plasmodium falciparum. Science 236, 1661–1666 (1987). [DOI] [PubMed] [Google Scholar]
- 27.Oduola A. M., Milhous W. K., Weatherly N. F., Bowdre J. H., Desjardins R. E., Plasmodium falciparum: Induction of resistance to mefloquine in cloned strains by continuous drug exposure in vitro. Exp. Parasitol. 67, 354–360 (1988). [DOI] [PubMed] [Google Scholar]
- 28.Mangano V. D., et al. ; MalariaGEN Consortium , Novel insights into the protective role of hemoglobin S and C against Plasmodium falciparum parasitemia. J. Infect. Dis. 212, 626–634 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uyoga S., et al. , The indirect health effects of malaria estimated from health advantages of the sickle cell trait. Nat. Commun. 10, 856 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cunningham F., et al. , Ensembl 2019. Nucleic Acids Res. 47, D745–D751 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gallagher P. G., Disorders of erythrocyte hydration. Blood 130, 2699–2708 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng W., Nikolaev Y. A., Gracheva E. O., Bagriantsev S. N., Piezo2 integrates mechanical and thermal cues in vertebrate mechanoreceptors. Proc. Natl. Acad. Sci. U.S.A. 116, 17547–17555 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fairhurst R. M., et al. , Abnormal display of PfEMP-1 on erythrocytes carrying haemoglobin C may protect against malaria. Nature 435, 1117–1121 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Cyrklaff M., et al. , Hemoglobins S and C interfere with actin remodeling in Plasmodium falciparum-infected erythrocytes. Science 334, 1283–1286 (2011). [DOI] [PubMed] [Google Scholar]
- 35.Cholera R., et al. , Impaired cytoadherence of Plasmodium falciparum-infected erythrocytes containing sickle hemoglobin. Proc. Natl. Acad. Sci. U.S.A. 105, 991–996 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ambe J. P., Fatunde J. O., Sodeinde O. O., Associated morbidities in children with sickle-cell anaemia presenting with severe anaemia in a malarious area. Trop. Doct. 31, 26–27 (2001). [DOI] [PubMed] [Google Scholar]
- 37.Aidoo M., et al. , Protective effects of the sickle cell gene against malaria morbidity and mortality. Lancet 359, 1311–1312 (2002). [DOI] [PubMed] [Google Scholar]
- 38.Komba A. N., et al. , Malaria as a cause of morbidity and mortality in children with homozygous sickle cell disease on the coast of Kenya. Clin. Infect. Dis. 49, 216–222 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ilboudo Y., et al. , A common functional PIEZO1 deletion allele associates with red blood cell density in sickle cell disease patients. Am. J. Hematol. 93, E362–E365 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Andolfo I., et al. , Multiple clinical forms of dehydrated hereditary stomatocytosis arise from mutations in PIEZO1. Blood 121, 3925–3935, S1–S12 (2013). [DOI] [PubMed] [Google Scholar]
- 41.Kang H., Bradley M. J., Elam W. A., De La Cruz E. M., Regulation of actin by ion-linked equilibria. Biophys. J. 105, 2621–2628 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Apinjoh T. O., et al. ; MalariaGEN Consortium , Association of cytokine and Toll-like receptor gene polymorphisms with severe malaria in three regions of Cameroon. PLoS One 8, e81071 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Syeda R., et al. , Chemical activation of the mechanotransduction channel Piezo1. eLife 4, e07369 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All materials, data, and protocols associated with this manuscript are available to readers upon request from the corresponding author. All data are contained within the manuscript and SI Appendix.