Abstract
The effects of epilepsy and its treatments have contributed significantly to language models. The setting of epilepsy surgery, which allows for careful pre- and postsurgical evaluation of patients with cognitive testing and neuroimaging, has produced a wealth of language findings. Moreover, a new wave of surgical interventions, including stereotactic laser ablation and radiofrequency ablation, have contributed new insights and corrections to language models as they can make extremely precise, focal lesions. This review covers the common language deficits observed in focal dyscognitive seizure syndromes. It also addresses the effects of surgical interventions on language, and highlights insights gained from unique epilepsy assessment methods (e.g., cortical stimulation mapping, Wada evaluation). Emergent findings are covered including a lack of involvement of the hippocampus in confrontational word retrieval, possible roles for key white matter tracts in language, and the often-overlooked basal temporal language area. The relationship between language and semantic memory networks is also explored, with brief consideration given to the prevailing models of semantic processing, including the amodal Hub and distributed, multi-modal processing models).
Keywords: epilepsy, language functions, surgical effects, development, neural substrates
1.1. Introduction.
Language functions have a long history of being studied in the context of epilepsy (Penfield & Roberts, 1959), as they are frequently affected by this neurological condition and its treatments, particularly in the setting of epilepsy surgery. Great efforts have gone into understanding the risk of language dysfunction secondary to epilepsy surgery, as well as improving our ability to avoid this potential procedural complication. This has led to an examination of risk factors for post-surgical decline, including age of seizure onset, and the effects of resection extent and location (Griffin & Tranel, 2007). In turn, much has been learned about the neural substrates of language and its reorganization in this context, although significant controversy and unresolved questions remain due to the complexity of this cognitive system (Duchowny et al., 1996; Hamberger & Cole, 2011). The epilepsy surgical setting has made use of a variety of tools to study language, both invasive and non-invasive, including active and resting state fMRI tasks (Binder et al., 1996), diffusion tensor imaging (DTI) (McDonald et al., 2008), the intracarotid amobarbital (Wada) procedure (Wada & Rasmussen, 1960), and various methods of language mapping using both stimulation (e.g., cortical stimulation mapping, stereo-EEG mapping)(Ojemann, Ojemann, Lettich, & Berger, 1989; Trebuchon & Chauvel, 2016) and passive paradigms (e.g., high gamma activity; Sinai et al., 2005). It is the intent of this review to briefly highlight what we have learned about the key language findings for the focal epilepsies and their treatment, point out some of the inadequacies of our current knowledge and assessment approaches, and to suggest areas for further exploration.
1.2. Knowledge of Language Gleaned From Epilepsy Surgery.
The drive to control seizures through the use of surgery while preserving cognitive function has contributed a substantial wealth of knowledge in our understanding of language processes and their relationship to underlying brain networks. Most of these studies have been conducted with patients experiencing temporal lobe epilepsy (TLE), as this is the most common type of focal seizure onset (Schoenberg, Werz, & Drane, 2011). However, even frontal lobe epilepsy (FLE) and the posterior cortical epilepsies have provided insight into these processes. Neuropsychologists have sought better ways to confirm the seizure onset zone by finding presurgical baseline relationships between cognitive functions and specific brain regions, which have benefitted our knowledge of language processes (Loring, 2010). When presurgical deficits have been observed, an effort has been made to understand the mechanisms and secondary factors that drive these impairments. Studies of potential risk factors have included age of seizure onset, duration of epilepsy, status of baseline brain imaging (e.g., presence of mesial temporal sclerosis), baseline performance, and the effects of antiepileptic drugs (AEDs) (Ojemann et al., 2001; Schoenberg et al., 2011). Additionally, when language decline has occurred in our surgical patients, we have often gained knowledge of essential regions and networks involved its production, and an ever-heightened determination to improve outcomes, for both language and memory, as well as seizure control, for the next patient seen by our services.
A primary schema for understanding our current knowledge of language in epilepsy derived from the surgical setting involves summarizing findings associated with each of the more common focal epilepsy syndromes and the effect of their surgical treatments. A few basic concepts cut across each of these conditions:
1.2.1. Language dominance:
First of all, most individuals, healthy or otherwise, are left-hemisphere dominant for language functions (Hamberger & Cole, 2011; Knecht et al., 2000). In the context of epilepsy, there are a higher percentage of patients with atypical language representation, which can include bilateral or right-hemisphere only language. This is usually determined by Wada evaluation or fMRI of language (Janecek et al., 2013; Szaflarski et al., 2017; Wada & Rasmussen, 1960). Loring and colleagues (1990) suggested that language may exist on more of a continuum on the basis of Wada data, with some degree of bilateral representation, and this has been further confirmed by fMRI studies (Knecht et al., 2000). Nevertheless, from a purely pragmatic standpoint, primary language functions have never been shown to decline following a non-dominant surgical procedure, with the possible exception of verbal generative fluency (Martin, Loring, Meador, & Lee, 1990). Even in the case of the latter language construct, most tasks assessing this function place significant demands on non-language abilities (e.g., semantic memory, lexical knowledge, executive function).
1.2.2. Breadth of Studied Language Functions:
Not all language functions have been adequately studied to date for any of the focal epilepsies, as pointed out in a recent review by a neurolinguist (Bartha-Doering & Trinka, 2014). As covered in this review, some classic language functions do not seem to be at risk with some syndromes and conditions (e.g., sentence repetition and auditory comprehension are almost never affected in TLE unless disease or surgery encroaches upon posterior regions). However, some language functions have rarely been studied, such as evaluating natural speech production/discourse and timing of verbal responses.
2.1. Language in Temporal Lobe Epilepsy.
We will start with the most common surgical syndrome of TLE, which likely represents 60–70% of all epilepsy surgical cases (Spencer & Spencer, 1985). As the temporal lobes contain structures and pathways that are clearly part of the classic language networks of Wernicke and Broca, and their interconnecting white matter fibers (e.g., arcuate fasciculus), the involvement of language functions in the symptomatology of this disorder is not surprising. At core, we know that language dominant (typically left hemisphere) TLE patients frequently exhibit baseline deficits in aspects of verbal generative fluency (both letter and category) and naming ability (Hermann, Davies, Foley, & Bell, 1999; Martin et al., 1990). They will occasionally exhibit limitations in reading ability as well (Camfield et al., 1984; Vanasse, Beland, Carmant, & Lassonde, 2005). However, to the extent that broader language functions have been studied, these patients have not reliably shown dysfunction in auditory or written comprehension or speech repetition, and are not classically aphasic unless more posterior brain regions are also involved in their disease or surgery (Saykin et al., 1995).
2.1.1. Naming in TLE:
No language function has been explored more than naming in TLE, yet there are still numerous unanswered questions even in this area. Most studies have examined visual confrontational naming ability (Hermann et al., 1999; Ives-Deliperi & Butler, 2012), primarily examining human-made objects, while a few studies have explored a wider-range of object type or used a ‘naming to description’ paradigm (Hamberger, Goodman, Perrine, & Tamny, 2001; Hamberger & Tamny, 1999). Patients with seizure onset in the language dominant cerebral hemisphere are more likely to experience baseline problems with naming of all types examined (Drane et al., 2015; Hermann et al., 1999; Hermann & Wyler, 1988; Langfit & Rausch, 1996), although non-dominant TLE patients will often perform worse than healthy controls and patients with extratemporal lobe seizure onset on these tasks as well. Some studies have found no difference between dominant and non-dominant TLE patients at baseline (Hermann, Wyler, Steenman, & Richey, 1988). However, patients may be impaired on these tasks for reasons other than seizure focus (e.g., patients with lower IQ show restricted scores, which likely result from their general level of impairment or problems learning the information unrelated to language processing). Finally, patients undergoing dominant TL resection often experience significant declines on naming tasks (Davies et al., 1998; Drane et al., 2015; Hermann et al., 1999), while those undergoing non-dominant temporal lobe (TL) resection typically do not (Drane et al., 2015; Saykin et al., 1995), highlighting the critical involvement of the dominant TL in naming. Busch et al. (2016) recently noted that over 40% of left TLE surgical patients experienced significant naming decline on a common measure of man-made objects after open surgical resection in a very large surgical series, while approximately 5% of right TLE patients experienced naming decline. They did not formally assess language lateralization in the majority of right TLE cases with either Wada and/or fMRI, and this percentage of patients is highly consistent with the occurrence of atypical language lateralization observed across prior epilepsy studies (Drane et al., 2012). Busch and colleagues also noted that a subset of patients had a greater than 75% chance of experiencing naming decline for man-made objects in their large sample, with this being any patient with seizure onset after the age of 18 that was either: 50 years of age or older at the time of testing, or 2) had a baseline score of 50 or more on the test (normal performance).
Most studies of naming in epilepsy have suggested that the mechanism of naming impairment observed in language dominant TLE surgical cases involves lexical retrieval (Drane et al., 2013; Miozzo & Hamberger, 2015) That is, such patients can typically recognize and describe the objects and faces with which they are presented, but cannot spontaneously retrieve the specific name for these entities. They do not exhibit a visual associative agnosia, as they remain capable of providing descriptions of these objects and faces. Such patients perform better with multiple choice recognition options, although the benefit gained from these cues is short-lived (e.g., once the cue is no longer present or remembered they often are again unable to come up with the specific word or name) (Drane et al., 2013). It is presumed that there is an underlying semantic lexicon of words/names that becomes difficult to assess after damage is sustained to the dominant anterior TL region (Szubko-Sitarek, 2015).
Recent research demonstrates that naming deficits of TLE patients may be more extensive than previously recognized. Some studies show that language dominant (typically left hemisphere) TLE patients are impaired at naming famous faces (Benke, Kuen, Schwarz, & Walser, 2013; Drane et al., 2008; Glosser, Salvucci, & Chiaravalloti, 2003; Seidenberg et al., 2002), and three of these studies suggest these deficits are worse following anterior temporal lobe resection. These findings also appear to extend to other visually complex item categories (e.g., famous landmarks, non-unique animals) even when performance is completely normal on standard naming measures (e.g., Boston Naming Test; Drane et al., 2009; Glosser et al., 2003). In fact, research suggests deficits occur more frequently in these proper noun categories following ATL damage than they do on the standardly assessed man-made object paradigms (Damasio et al., 2004; Drane et al., 2008). These findings are also supported by functional neuroimaging paradigms highlighting a critical role for the ATLs in naming certain object categories (Damasio et al., 1996; Griffith et al., 2006). Functional neuroimaging paradigms have demonstrated differing regions of activation, which highlight dissociations across a variety of stimulus parameters, such as the specific category of object (e.g., animals, famous persons; Martin, 2007; Martin, Wiggs, Ungerleider, & Haxby, 1996), broad semantic categories (e.g., living vs. non-living stimuli; Smith et al., 2001), and levels of processing. As an example of the latter, the same object can lead to different regional patterns of activation depending on whether the subject is instructed to make a general semantic decision about it (e.g., male vs. female) or think of the item or person’s specific name (Tyler et al., 2004). Nevertheless, findings in this area have been contradictory at times (Martin, 2007; Smith et al., 2001), and more research is needed to explore the dissociations that can occur in naming of various objects and faces, as well as their underlying neural substrates. Preliminary evidence, derived from both DTI tractography studies (Drane et al., 2014; McDonald et al., 2008) and subcortical stimulation mapping paradigms (Duffau et al., 2008), also suggests that certain objects may be more related to the function of specific white matter pathways traversing the TL region. Overall, the clinician and researcher alike need to recognize that confrontational naming cannot be treated as a homogenous, unitary phenomenon, but rather a complex, multistep process that involves multiple brain regions.
It is also important to note that dominant TL patients with naming deficits remain fully capable of recognizing famous faces and objects, a finding demonstrated by having them provide verbal descriptions of these items or through the use of a multiple choice recognition format (Drane et al., 2008). In contrast, nondominant TL dysfunction leads to recognition deficits rather than naming problems (Drane et al., 2013; Drane et al., 2008). These patients have much greater difficulty recognizing famous persons, landmarks, and/or animals. It is believed that this dysfunction results from damage to the nondominant ventral visual processing stream (i.e., the “what is it pathway?” Kravitz, Saleem, Baker, Ungerleider, & Mishkin, 2013), and that these deficits may in fact represent a form of visual agnosia.
Hamberger and colleagues have championed the use of an auditory naming test through both cortical stimulation paradigms and surgical outcome studies (Hamberger et al., 2001; Hamberger & Tamny, 1999). This task is more accurately described as a naming to description task, as it requires the patient to provide the name of an object after being read a brief descriptive phrase (e.g., “the thing you order from in a restaurant”). Other groups have used auditory naming tasks that involve providing the name of an object based on a recorded sound associated with it (e.g., naming a cow based on its characteristic sounds; Tranel et al., 2003). This is an important distinction, as Drane et al. (2008, 2013) have demonstrated that left TLE patients can almost always provide their own descriptive phrase for a visual object that they are able to recognize. Therefore, on one level, when TLE patients fail a visual naming task, they are essentially failing a self-generated naming to description task. It has not been empirically demonstrated that providing patients with an object description makes this task significantly different from their own internally generated descriptions. However, it does remove the object recognition component of a visual naming task, which may decrease demands for accessing semantic memory stores for successful completion. It also remains possible that when dissociations between visual naming and naming to description tasks are observed, these are resulting from some other feature of the tasks that differs (e.g., item complexity, categorical differences). As the auditory naming to description task is presented orally, it is possible that it would be more impaired if brain regions underlying auditory processing were dysfunctional in the individual patient as opposed to dysfunction in visual-linguistic or visual-semantic pathways..
Hamberger and colleagues have reported that performance can dissociate between naming to description and visual confrontational naming tasks. Based on results from cortical stimulation mapping of language, they report that auditory description naming sites are more frequently found in anterior temporal cortex, whereas visual naming sites were found to occur in the mid-posterior temporal region (Hamberger, Seidel, Mckhann, Perrine, & Goodman, 2005). Of note, however, not all studies have reported visual naming sites to conform to the mid-posterior region. In fact, in the seminal paper of Ojemann and colleagues (1989), which reported stimulation results using visual naming in 117 left TL patients, it was concluded that naming “sites were not found uniformly in any given (“assessed” – sic) cortical region.” They also noted that stimulation mapping in their study, as well as the original reports of Penfield (see Penfield & Flanigin, 1954), showed that sites where visual naming could be disrupted varied widely in location and often did not conform to classic models of language.
Finally, the work of Tranel and colleagues (Abel et al., 2015) has recently suggested that specific categories of objects may be represented in a heteromodal fashion, which would argue against a dissociation occurring between auditory and visual presentations. This group examined presurgical TLE patients using grid mapping and a matched-task of naming US Presidents based on either their picture or a voice clip. Consistent EEG patterns were observed for these individuals across modalities of presentation. This study and broader work from Tranel and colleagues suggests proper nouns are processed in the anterior TL region as compared to common nouns (Tranel, 2006; Yucus & Tranel, 2007).
2.1.2. Verbal Generative Fluency:
Presurgical TLE patients also commonly exhibit deficits on verbal generative fluency tests (i.e., category fluency, letter fluency; Troster et al., 1995). However, deficits in category fluency, which involves generating items from specific categories, are often present preoperatively in patients with either dominant or non-dominant TL seizure onset (Bartha, Benke, Bauer, & Trinka, 2005; Joanette & Goulet, 1986; Martin et al. 1990), and can be observed in patients with either dominant of nondominant FL seizure onset as well (Drane et al., 2006; Metternich, Buschmann, Wagner, Schulze-Bonhage, & Kriston, 2014). As these findings could be affected by use of antiepileptic drugs, some of which are specifically linked to deficits in these areas (Fritz et al., 2005), it is important to note that other types of lesions in these regions result in category fluency deficits in patients without epilepsy (Joanette & Goulet, 1986; Stuss, 1998). Category fluency tasks are complex in nature, requiring involvement of several neural systems for successful completion. Therefore, the presence of baseline category fluency deficits may not be of lateralizing or localizing value when viewed in isolation, but may be helpful in this regard if the component parts of this task are examined. For example, presurgical patients with frontal lobe (FL) onset can sometimes be distinguished from those with TL onset using a category fluency paradigm that contrasts cued and uncued performance (Drane et al., 2006). This builds upon the idea that category fluency requires executive functions mediated by FL regions (i.e., organization/retrieval problems making it difficult to search one’s own semantic memory stores or deficits involving initiation of action or self-monitoring; Sylvester & Shimamura, 2002) and a semantic memory component thought to be mediated by the temporal lobes (Martin, & Fedio, 1983).
While studies examining postoperative category fluency are limited in nature at present, there is evidence that performance in this domain (e.g., generating types of animals) declines following dominant TL resection, and perhaps following non-dominant TL resection as well (Martin et al., 1990), although the latter study had only a 1-week post-operative follow-up period. One additional study supports the idea that dominant and nondominant TL resections may both affect category fluency performance, but found surgical effects may vary greatly between hemispheres depending upon object category (Jokeit, Heger, Ebner, & Markowitsch, 1998). Category fluency deficits have been shown to occur in selective amygdalohippocampectomy and standard anterior temporal lobectomy (ATL) alike (Bartha et al., 2004).
Performance on letter fluency tasks (e.g., generating words that start with specific letters of the alphabet) can be impaired preoperatively due to both FL and TL impairment (Helmstaedter, Kemper, & Elger, 1996; Martin et al., 2000), although performance on these measures typically does not decline following TL surgery. Deficits observed on these tasks in TL patients have been attributed to disruption of widespread neural networks secondary to epileptiform activity, and improvement on these measures can be seen if the TL patient becomes completely seizure free following surgery (Helmstaedter et al., 1998). A lack of decline on letter fluency tasks following TL surgery provides further evidence that the anterior TL does not play a major role in the performance of this task. Action (verb) fluency measures appear to perform similarly to letter fluency tasks in other patient populations (Peran et al., 2003; Piatt, Fields, Paolo, & Troster, 1999), although they have not been extensively studied in epilepsy.
2.1.3. Broader Language Issues in TLE:
Despite the commonly reported deficits mentioned in naming ability and verbal generative fluency, presurgical TLE patients do not demonstrate a classic aphasia, unless it is caused by a separate neurological insult, such as a prior stroke. While their prevalent naming problems would be most reminiscent of an anomic aphasia, they typically lack the positive signs of aphasia in spontaneous speech (e.g., paraphasic errors). It is rare for TLE patients to exhibit problems with comprehension, speech fluency, or repetition of words or phrases. They also rarely make errors that are of a phonetic or syntactic nature on these tasks (Bartha et al., 2005). Damasio and colleagues have shown that even when extensive naming deficits are present in such patients, including both common and proper nouns, they typically retain knowledge of concepts for activities and states that are related by verbs and grammatical constructions (e.g., conjunctions, prepositions) and do not exhibit any impairment of syntax (Damasio & Damasio, 1992). Similarly, standard dominant hemisphere ATL does not typically lead to declines in these areas (Saykin et al., 1995).
A few small studies suggest narrative discourse may be worse in patients with TLE than controls, although results have not always been consistent and research has been plagued by small and idiosyncratic patient samples (Bell, 2003; Field, Saling, & Berkovic, 2000; Howell, Saling, Bradley, & Berkovic, 1994). Bell and colleagues (2003) demonstrated that a significant minority of presurgical TLE patients (30%) with early onset of seizures (prior to age 14) exhibited mild narrative discourse dysfunction (description of a series of events) although all were normal on a procedural discourse task (description of the steps necessary to complete a task). TLE patients took longer to describe a series of cartoon strips, made more non-communicative errors (e.g., saying “umm” or stopping in the middle of a word), and produced slightly fewer core story components as compared to healthy controls. There was no significant correlation with disease-related or demographic factors, although worse performance correlated with limitations in working memory. Once again, this could also reflect a general deficit resulting from disruption of any number of cognitive constructs, which may be broader than a focal disruption of language. This is suggested by the strong correlation with working memory and no significant relationship to standard language measures. These studies have also typically not addressed the possible confounding effects of antiepileptic drugs, some of which have been demonstrated to have deleterious but reversible effects on verbal generative fluency (Fritz et al., 2005; Ojemann et al., 2001).
Finally, measures that assess semantic storage capacity can be affected in patients with TLE. Good examples include several verbal subtests from the Wechsler Intelligence Scale (e.g., Vocabulary, Information). Performance on these tasks is often abnormal compared to healthy control subjects at baseline for both left and right TLE patients, but no evidence exists that these functions decline with standard surgical operations (Schoenberg et al., 2011).
2.1.4. Reorganization of Language in Epilepsy:
While interhemispheric reorganization of language function is thought to occur in patients with chronic epilepsy, especially when disease onset or insult is very early in life (before 5 years of age) (Woods, Dodrill, & Ojemann, 1988), several sources of evidence suggest that intrahemispheric reorganization may be less likely to occur (DeVos, Wyllie, Geckler, Kotagal, & Comair, 1995; Ojemann et al., 2003). Several studies suggest that patterns of language function in patients may be much more comparable to healthy development than once thought. These include studies of childhood tumor patients where language sites are found very close to the lesion in expected topographical regions without any evidence of reorganization (DeVos et al., 1995), and studies of children with congenital epilepsy where cortical stimulation mapping (CSM) paradigms revealed language in expected locations despite their proximity to seizure foci (Duchowny et al., 1996). Likewise, in adults undergoing CSM for post-lesional epilepsies, baseline (presurgical) language dysfunction was shown to be associated with apparent compromise of language sites (Lucas, Drane, Dodrill, & Ojemann, 2008), although when the insult occurred early in life there tended to be a greater recruitment of additional frontal lobe regions. Nevertheless, in contrast, some studies have found more anterior TL language sites in epilepsy patients with very early seizure onset (Krauss et al., 1996).
More recently, Corina and colleagues (2010) have noted that patterns of language sites discovered through the use of CSM do not differ greatly when comparing patients with early onset epilepsy, those with late onset epilepsy, and a third series with new onset, fast growing tumors. They argued that data from these groups reveal “a highly similar pattern of language organization, suggesting that this mosaic of language function spread across perisylvian regions is likely reflective of the general population and is not simply an artifact of language organization in a clinical epileptic population (p. 102).” Overall, the clinician assessing language in TLE patients must explore the possibility that language functions could have atypical neural substrates, while being aware that in many cases there is no substantial evidence of reorganization (particularly after early epochs of brain development have passed).
Ojemann and colleagues (2003) have also raised the possibility that there may be maturational effects in the development of essential language sites that can complicate and sometimes confound this literature. Using CSM of language, they showed that children with epilepsy were less likely to have perisylvian language sites than were adults. However, they suggested this might be due to maturational issues rather than reflecting reorganization of function. They presented a single case where sequential mapping was performed over time, and found that additional perisylvian regions for naming were eventually observed that were not originally present. Some case examples have been presented by the same group that demonstrate that repeat mapping in a patient requiring additional surgical procedures often shows unchanged findings (Ojemann et al., 1989). However, they have more recently suggested that one must be looking at patients at specific epochs of development in order to catch the maturational element of this process (i.e., the appearance of stability may occur if the mapping sessions are close together and not spanning maturational time points; Ojemann et al., 2003).
There is also evidence that broader cortical surface areas are involved in the representation of language (i.e., essential sites) when there is less language competence, such as a second language (Ojemann & Whitaker, 1978). Greater competence with language would lead to more compact areas of language representation. It is possible that this concept explains why children have been shown to exhibit broader regions of activation for some language tasks (e.g., verbal generative fluency) than adults using fMRI paradigms (Gaillard et al., 2000), again highlighting that language systems may undergo maturational changes. Finally, there also appear to be gender differences related to language reorganization, with females having a much smaller window for reorganization (possibly less than one year; Strauss, Wada, & Goldwater, 1992). There are still many holes in our knowledge of language reorganization and maturation/development, which will need further refinement over time.
2.2. Language in Frontal Lobe and Posterior Cortical Epilepsies.
As there is much less published work involving the less common focal epilepsies and language, these regions will be covered in one section. Focal seizures of parietal lobe (PL) onset account for only 5–6% of all the partial epilepsies (Salanova, Andermann, Rasmussen, Olivier, & Quesney, 1995), and are frequently associated with tumors (Gleissner, Kuczaty, Clusmann, Elger, & Helmstaedter, 2008).
Neurocognitive functioning in parietal lobe (PL) onset patients have rarely been studied either pre- or post-surgically. Penfield and colleagues noted that disturbances of speech sometimes occurred in patients where parietal association cortex was resected (Penfield & Erickson, 1941), although these cases do not seem to have been systematically examined. One of the few studies in this area involves 15 children studied both pre- and post-surgically (Gleissner et al., 2008). It required 20 years to obtain even this small series of patients, and these researchers were not specifically targeting parietal lobe function in their evaluations. Only verbal generative fluency and naming were examined in these patients, and no breakdown was provided of individual subtest findings. Slightly less than half of the PL patients exhibited language deficits regardless of language lateralization both pre- and post-surgically. These findings may relate to a general baseline limitation in language regardless of hemispheric specialization for this cognitive ability, yet one would need to rule-out the effects of language reorganization issues as well.
Frontal lobe epilepsy (FLE) and its surgical treatment have been associated with verbal generative fluency deficits (Drane et al., 2006; Risse, 2006; Sarkis et al., 2013), while classic symptoms of aphasia are rarely produced interictally. However, studies examining baseline performance in FLE are scarce and typically of very small sample size, and resective surgery outcome data is even less common in this population (Schoenberg et al., 2011). Therefore, conclusions drawn in this area remain somewhat speculative.
At least two studies have found that patients undergoing FLE resection declined on a letter fluency task (Milner, 1964; Sarkis et al., 2013), which was more likely to occur if seizure outcome was poor and the language dominant hemisphere was resected. One study found a trend towards a greater risk of decline in patients with higher baseline performance (Sarkis et al., 2013). While other studies have not found any significant decline following FLE resection (Helmstaedter et al., 1998; Suchy, 2003) trends have been observed in this data, and overall sample sizes were generally underpowered to recognize change if it occurred. One group noted these trends in their data and indicated an absence of practice effects in their left FLE group in a chronic stage of post-surgical recovery could also suggest a deficit (Suchy, 2003). Letter fluency deficits appear more likely to occur when the lateral FL region is involved in the resection zone. Finally, the possibility of verbal generative fluency deficits associated with FLE and its surgical treatment are also heralded by literature in other neurologic conditions (Baldo, Shimamura, Delis, Kramer, & Kaplan, 2001; Stuss, 1998), although again laterality effects are not always found. More specifically, non-dominant FL damage appears to contribute to decreased verbal generative fluency, which is likely resulting from a disruption of underlying executive function. One study of FLE resections reported a decline in auditory comprehension as assessed with a measure involving auditory instructions (i.e., token test) in a small subset of FLE patients (Helmstaedter et al., 1998). These patients were assessed only 3 months after surgery, and may have continued to exhibit improvement over time. Moreover, patients experiencing auditory comprehension deficits in this study had all been diagnosed with an expressive aphasia. Post-surgical FLE patients also exhibited significant declines on an auditory comprehension subtest of a German intelligence test. An independent group reported a similar decline after FLE resection, although there was no laterality effect in this latter study. It was thought that this decline reflected a more general problem with concept formation rather than genuine language dysfunction.
In summary, when considering the focal epilepsies beyond TLE, very little systematic study of language has occurred, as the majority of clinical epilepsy surgery programs focus their neurocognitive assessments on tests tapping the functions of the temporal lobes. However, even language assessment in TLE has suffered from a chronic focus on a very limited range of language functions. When non-TLE focal epilepsies are occasionally studied, the research tends to be retrospective in nature, pulling from data collected as part of clinical care. There has been little focus on language functions that extend beyond naming and verbal generative fluency.
3.1. Insights into the Neuroanatomical Underpinnings of Language Derived from Epilepsy.
Several models of language function have been recently proffered in this area (Friederici, 2012; Hagoort, 2014; Hickok & Poeppel, 2007), building upon the classic Wernicke-Lichtheim-Geschwind model (Lichteheim, 1885; Wernicke, 1874), the latter of which developed from natural lesions studies. It is likely that these models will continue to be refined for years to come, particularly through the use of newer methodological advances (e.g., fMRI, DTI, MEG, focal laser ablation, cortico-cortical evoked potential network mapping). In this light, we will concentrate on emerging data in the field of epilepsy and epilepsy surgery that suggests some further refinements of our language models.
3.1.1. Role of the hippocampus in naming:
One area that has generated a significant amount of debate during the past few years has been the possible role of the hippocampus in naming ability (Drane et al., 2015; Hamberger, 2015; Klooster & Duff, 2015). Several independent pieces of information have pointed to the possible role of the hippocampus in visual confrontational naming. These have included: a) an association between presurgical naming scores and hippocampal volumes (Bonelli et al., 2011), b) hippocampal activation observed during fMRI tasks involving object and face naming (Seidenberg, Geary, & Hermann, 2005), c) worse naming scores observed in TLE patients with MTS than in those without (Baxendale, 1998; Davies et al., 1998), and d) observations that naming deficits always occurred when the hippocampus was included in the resection despite patients undergoing cortical stimulation mapping (Hamberger, Seidel, McKhann, & Goodman, 2010).
For several years, Drane and colleagues (2008, 2013) have held the position that name retrieval was unlikely to be a result of hippocampal damage in epilepsy patients, based primarily on work emerging from the semantic dementia literature. In this disease, patients typically have severe naming deficits with relatively spared episodic memory functioning in the earlier stages of the disease (Snowden, Thompson, & Neary, 2004). However, the initial brain pathology of this condition involves the temporal poles and inferior temporal gyri bilaterally, with relative sparing of mesial TL structures (Mummery et al., 2000). Drane and colleagues (2015) recently provided strong evidence of this idea, by demonstrating that visual naming decline occurs in the vast majority of TLE patients undergoing open resection, but never occurred with an initial group of TLE patients undergoing a selective ablation of the amygdalar-hippocampal complex (i.e., stereotactic laser amygdalohippocampotomy: SLAH). This study included patients with and without hippocampal sclerosis, again highlighting that even in cases with a normal-appearing hippocampus, visual confrontational naming was not harmed by destruction of this structure.
It should be pointed out that selective open resection approaches have not been shown to be more efficacious in terms of cognitive outcome than standard ATL in most studies (Bartha et al., 2004; Kuang, Yang, Gu, Kong, & Cheng, 2014). The reason for the lack of difference between ATL and selective open resection approaches has been posited to result from the necessity to transect white matter in all of these open resection procedures, with most approaches disrupting the temporal stem (Gross, Willie, & Drane, 2016). In contrast, SLAH is a new procedure being employed for epilepsy surgery that uses thermal energy delivered through an optic fiber during continuous MRI. Ablation extent is determined in real-time from temperature-sensitive images of estimated thermal damage (For technical information regard laser ablation see (Gross et al., 2016; Willie et al., 2014)). Figure 1 includes a depiction of the laser fiber assembly, the ablation process, and an MRI scan of the resulting ablation zone. Figure 2 highlights the difference between tissue destruction that is involved in SLAH versus open resection procedures.
Figure 1.
Depiction of the Optical Fiber, the Ablation Process, and Pre- and Post-Ablation MRI Images in an Axial Plane.
Figure 2.
This figure depicts the difference in tissue destruction involved in the standard anterior temporal lobe (ATL) resection, a selective amygdalohippocampectomy (SAH), and a stereotactic laser amygdalohippocampotomy (SLAH). Unlike most open resection approaches to temporal lobe surgery, SLAH does not significantly disrupt white matter integrity and spares much more of the ATL.
3.1.2. Role of White Matter Pathways in Language:
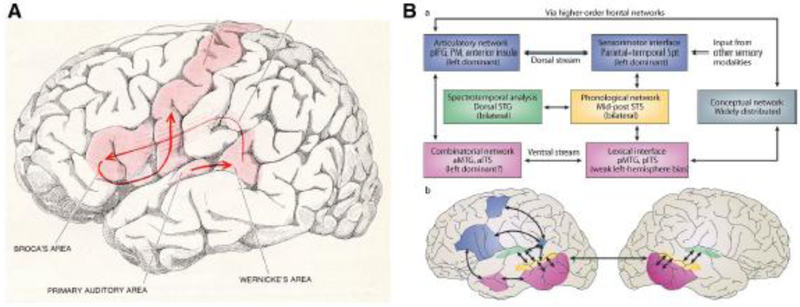
Recent language theories have put forward a “dual-stream” model of auditory language processing (Hickok & Poeppel, 2004; Poeppel, Emmorey, Hickok, & Pylkkanen, 2012; Rauschecker & Scott, 2009), which attempt to explain how sensory/phonological representations interface with both motor and conceptual/semantic systems. Such models reflect a growing appreciation for the broad number of regions involved in language processing and their numerous interconnections, yet significant disagreement prevails as to the precise white matter connections involved (Cloutman, Binney, Morris, Parker, & Lambon Ralph, 2013; Dick & Tremblay, 2012; Glasser & Rilling, 2008). Such models might be seen as similar to the dual stream model of visual processing (Ungerleider & Haxby, 1994). There is also substantial evidence for the importance of the ventral visual processing stream with regards to language functions requiring visual input and lexical and semantic processing (Duffau et al., 2008; Kravitz et al., 2013). Moreover, several groups have suggested subcortical connections involving the thalamus as critical components of the language network (Crosson, 1992; Hebb & Ojemann, 2013; Matsumoto et al., 2004). Thalamic lesions are often similar to lesions of the cortex to which the portion of the thalamus is reciprocally connected. The thalamic nuclei that are reciprocally connected to language areas include the pulvinar and mediodorsal nuclei, with the ventroanterior nucleus involved in articulation (Jones, 2007). The pulvinar is connected to lateral and basal temporal language regions and the mediodorsal nucleus to Broca’s area. Overall, it would appear that language functions are segmented into at least three general processing routes (and possibly more) that carry unique aspects of information necessary to subserve distinct language processes (Dick & Tremblay, 2012; Duffau et al., 2008; Glasser & Rilling, 2008; G. Hickok & D. Poeppel, 2004). An additional pathway, not covered in this discussion, involves analysis of speech prosody, which appears to be supported in the right cerebral hemisphere (George et al., 1996; Glasser & Rilling, 2008). While models of language will likely continue to be updated for years to come, technological advances allowing the study of networks and their interconnections are pushing their development, although these findings will need to be undergirded by neuroanatomical dissection studies in primates and human cadavers.
3.2.1.1. Dorsal Auditory Processing Stream and Phonological Processing:
Research suggests that there is a dorsal auditory processing stream (Poeppel & Hickok, 2004; Rauschecker & Scott, 2009), which is believed to connect regions important for the processing of speech sounds/phonemes (Heschl’s gyrus, and posterior unimodal regions that overlap with Wernicke’s area/posterior Brodmann’s area [BA] 22 and BA 40) with regions necessary for expressive production of phonemes in posterior Broca’s area (BA 44 and 6, then to primary motor area 4; Glasser & Rilling, 2008). This network is thought to allow humans to map sounds (phonological information) to motor articulation (Chang, Raygor, & Berger, 2015; Hickok & Poeppel, 2004). This pathway presumably begins in the posterior superior TL region and projects to inferior parietal and posterior FL regions (See Figure 1), and likely involves portions of the superior longitudinal fasciculus that are commonly referred to as arcuate fasciculus (SLF/AF; after Wernicke’s model; Saur et al., 2008; Yagmurlu, Middlebrooks, Tannover, & Rhoton, 2016). Glasser and Rilling (2008), using DTI probabilistic tractography, reported that there is a strong leftward asymmetry of the SLF/AF connecting the posterior superior temporal gyrus to the frontal lobe which they found to correspond well with fMRI peak activations from studies involving phonological processing in humans This idea that the dorsal pathway appears to be left hemisphere dominant is also bolstered by lesion data showing that speech repetition errors are isolated to the language dominant hemisphere (see Chang et al., 2015).
Research in epilepsy has contributed to models of the dorsal auditory processing pathway, and continues to refine our understanding of its functionality. For example, cortical stimulation mapping (CSM) studies in epilepsy demonstrate that stimulation in the region of the mid-posterior superior temporal gyrus can disrupt the processing of consonant sounds but not vowels (Boatman, Hall, Goldstein, Lesser, & Gordon, 1997; Boatman, Lesser, & Gordon, 1995). Similarly, recent work involving subcortical stimulation mapping of language preceding tumor surgery, has demonstrated that phonemic paraphasic errors result from stimulation of the AF of the language dominant hemisphere (Duffau et al., 2008). These component functions are likely critical for speech repetition, yet the proposed underlying network supporting them is not involved in a standard anterior TL resection, which is in keeping with the lack of dysfunction observed in this area in epilepsy surgery patients (Saykin et al., 1995).
3.2.2.2. Ventral Auditory Processing Stream and Auditory Comprehension:
This pathway is thought to link auditory input with conceptual meaning, which is represented across widely distributed regions (lexical-semantic system). Most feel that this presumed pathway is important for speech recognition and for managing the interface between lexical and semantic representations (Araki et al., 2015; Chang et al., 2015; Poeppel et al., 2012). Nevertheless, controversy still exists over the precise structural parameters of this pathway and its underlying functions, although there is agreement that it is represented bilaterally. However, even here, the work of Glasser and Rilling (2008) suggest that the left hemispheric SLF/AF pathway is structurally larger, and that the roles of the pathway differ by hemisphere, with the dominant hemisphere involved in lexical-semantics and the nondominant pathway being involved in analysis of prosody (George et al., 1996).
Hickok and Poeppel (2004) emphasized a posterior portion of this pathway, which they described as projecting from the posterior superior TL region (i.e., the dorsal to mid portion of the posterior superior temporal sulcus) ventrolaterally to the middle and inferior temporal gyri (See Figure 1). They felt that projections from these regions allow for processed auditory signal to be combined with lexical meaning, which they proposed to involve the posterior inferior temporal lobe region. Evidence cited for this possibility includes the basal temporal language area discovered through cortical stimulation mapping of epilepsy patients by Luders and colleagues (1991), which is located in the fusiform gyrus/inferior temporal gyrus (See section 3.2.3.4 of this manuscript), analysis of the patterns of the classic aphasias, and functional neuroimaging evidence of semantic processing (Binder, Desai, Graves, & Conant, 2009). This aspect of a presumed ventral pathway would not be involved in a standard anterior temporal lobectomy, suggesting that semantic content would not be at significant risk during such a procedure, if this is correct. This pathway also gains support from cortico-cortico stimulation studies showing short-latency connectivity between the presumed basal temporal language area and Wernicke’s area (Araki et al., 2015). In contrast, proponents of the hub model of semantic memory appear to take issue with this aspect of the model, as they believe that the center of semantic processing is more anterior (See section 3.3.1. on semantic memory). They believe there may be interconnections as proposed by Hickok and Poeppel (2004), but attribute different functions to this aspect of the temporal lobe and its interconnections.
There is also an aspect of the proposed ventral auditory processing pathway that runs anteriorly from the superior TL region or its interconnections to the middle temporal gyrus (Glasser & Rilling, 2008; Hickok & Poeppel, 2007), and on to FL regions (inferior frontal gyrus). Again, controversy remains as to the specific structural nature of the pathway(s) and its presumed role in language. Several researchers believe that this involves an inferior segment of the arcuate fasciculus (Glasser & Rilling, 2008; Rilling et al., 2012), while others believe that this part of the ventral auditory processing stream runs through the extreme capsule (Saur et al., 2008). As noted previously, these pathways are bilaterally represented, although they are structurally much greater in volume in the left hemisphere and generally absent from the left hemisphere of primates. Moreover, lexical-semantic processing also appears left lateralized in studies relating function to structure (Drane et al., 2008; Frost et al., 1999; Hickok & Poeppel, 2007; Koeda et al., 2006; Vigneau et al., 2011) (see section 3.3.1. for counterview).
3.2.2.3. Ventral Visual Processing Stream and Naming:
The ventral visual processing stream, which involves the inferior longitudinal fasciculus (ILF) and inferior frontal-occipital fasciculus (IFOF), is thought to relate visual information to lexical or semantic content. Subcortical stimulation of the IFOF for language mapping in the left hemisphere has demonstrated that semantic paraphasic errors can be reliably produced (Duffau et a l., 2008). This pathway has also been related to visual naming using diffusion tensor imaging (DTI) techniques (Drane et al., 2014). The original studies of Penfield and Roberts demonstrated visual naming deficits after resections of involving the inferior temporal gyrus (BA 20) and basal TL region (Penfield & Roberts, 1959). As covered thoroughly by the work of Luders and colleagues (1991), Penfield and Roberts related their findings to much earlier work that had postulated the posterior-inferior temporal gyrus to be a naming center or “language formulation area” (Mills & Martin, 1912; Nielsen, 1946). Of note, controversy remains regarding whether the IFOF is truly a unique pathway of simply part of the extreme capsule, an issue that arises when comparing neuroanatomical dissection studies in primates and humans with DTI and CSM paradigms (see ‘Dick & Tremblay, 2012’ for a thorough discussion).
3.3.1. Anterior Temporal Lobes as Hub of Semantic Memory versus Key Roles in Lexical and Object Recognition Processing:
Another recent area of debate has raged over the role of the anterior TL regions in semantic memory processing (Gainotti, 2011; Pulvermuller 2013; Simmons & Martin, 2009). Research in semantic dementia has led to a popular model of semantic memory, the hub model, which suggests that both anterior TL regions form a unitary, amodal hub that serves as a repository for conceptual information that can be accessed by multiple sensory and lexical routes (McClelland & Rogers, 2003; Patterson, Nestor, & Rogers, 2007). Over time, the hub model has emphasized that there are modality-specific “spokes” that feed into the hub region, allowing for some dissociation between component tasks. The most recent version of the model splits the hub region into “demi-hubs” that are separate for the two hemispheres (Lambon Ralph, Jefferies, Patterson, & Rogers, 2017). Nevertheless, this model suggests that impairments resulting from unilateral left and right ATL lesions are comparable, differing primarily in magnitude of impairment but not type. In contrast, other models view the ATLs as containing multiple processing regions that reactivate stored conceptual information residing in distributed neural networks (i.e., frequently presumed to be the primary sensory and motor areas where object perception occurs), and linking this information to the classic language network. This includes the modality-specific models of semantic memory, such as the convergence zone (CZ) model of Damasio, and Barsalou’s “embodied cognition” adaptations of the CZ model (Barsalou, Santos, Simmons, & Wilson, 2008; Barsalou, Simmons, Barbey, & Wilson, 2003; Antonio R. Damasio, 1989) These modality-specific models also tend to posit a complimentary differentiation of functions across the two ATL regions, with the right ATL more involved in object recognition processing and the left with lexical functions (i.e., linking names to more broadly stored semantic concepts). More posterior regions of the temporal lobe (e.g., posterior superior temporal gyrus, Heschl’s gyrus, Wernicke’s area, posterior half of the ventral inferior temporal gyrus) and broader brain regions (e.g., angular gyrus of parietal lobe) are involved in more complex language and semantic processing, but are not directly affected by standard ATL procedures.
Drane and colleagues have shown that epilepsy patients undergoing dominant TL resection clearly exhibit lexical deficits (i.e., cannot recall names in a variety of contexts), yet exhibit semantic knowledge for these objects and persons that appears unchanged from baseline (Drane et al., 2008; Drane et al., 2009; Drane, Ojemann, et al., 2013). Language dominant TLE patients can generate rich semantic information when given a name or a picture, and can accurately match semantic information to such stimuli. They still recognize the person, animal, or landmark, and can provide multiple specific details about them. This suggests that there is a one-way disruption in a presumed semantic-lexical pathway (i.e., information can still flow from lexical to semantic, but not the other way around). They believe that these pathways involve the ventral visual pathway and perhaps the arcuate fasciculus (i.e., the inferior segment of the arcuate fasciculus as postulated by Glasser and Rilling (2008) - discussed above). They feel that the posterior segment of the ventral visual pathway, which appears to reciprocally connect Wernicke’s area and the posterior inferior temporal gyrus, is likely sufficient for generating semantic information from lexical input but not for generating lexical information from semantic content. They conclude this based on their own findings in epilepsy surgery where the anterior portion of the TL region is routinely resected while this posterior portion remains intact. Naming deficits for visually presented stimuli may involve one category of object or may cut across several. Hamberger and colleagues (Hamberger et al., 2001; Hamberger et al., 2007) also report that performance on naming tasks is dissociable based on mode of sensory presentation.
In complimentary fashion, the nondominant (right hemisphere) ATL procedures frequently lead to deficits recognizing famous persons, landmarks, and even animals (although not man-made objects), but do not lead to deficits in language (Drane et al., 2015; Drane et al., 2008). For non-dominant surgical patients, famous persons, animals, or landmarks they could name and thoroughly describe prior to surgery, may now seem altogether unfamiliar or they will misrecognize them as someone of something else. For example, when presented with the picture of a flamingo they may respond that this is a parakeet. When asked to describe the image, they respond that it is a tiny bird that they kept in their home as a pet when they were a child. However, this misrecognition error does not hinder their ability to define words or recognize sound clips, even for the very same objects they cannot recognize to visual confrontation.
These nondominant TLE patients often exhibit object recognition deficits pre-surgically, and such deficits worsen following surgical intervention with surgery disturbing these regions (Benke et al., 2013; Drane et al., 2015; Drane et al., 2008). Such patients often exhibit “covert” recognition of previously known persons and places (i.e., they could often name and describe them prior to surgery) although, post-operatively, they typically lack “overt” awareness of having seen them before (Drane, Ojemann, et al., 2013). For example, even though they may misrecognize an individual or say they are unfamiliar, they will choose the correct multiple-choice option from a limited array of items even when they feel they are guessing. These patterns of deficit are similar in kind although not magnitude to the deficits observed in congenital prosopagnosia (Rivolta, Palermo, Schmalzl, & Coltheart, 2012). Similar deficits in congenital prosopagnosia have been related to DTI abnormalities involving the bilateral ventral visual processing stream (Thomas et al., 2009), although the strongest correlations involve the nondominant cerebral hemisphere. Additionally, human and animal studies have highlighted that the fusiform gyrus (BA 37) contains regions dedicated to highly specific aspects of visual image processing (Kanwisher, McDermott, & Chun, 1997; Kanwisher & Yovel, 2006; Tsao, Freiwald, Knutsen, Mandeville, & Tootell, 2003). Open resective epilepsy surgical procedures involving either temporal lobe routinely compromise portions of this visual processing stream and the fusiform gyrus. However, the object recognition deficits have only been observed in the nondominant cases (Drane et al., 2008, 2009, 2013).
Proponents of the hub model have suggested that patients undergoing right hemisphere resection, including those whose language is lateralized to the left hemisphere by Wada or fMRI paradigms, exhibit very subtle language deficits (e.g., response time slowing, poorer performance on tasks requiring rapid response) that are being missed by standard clinical practice (Lambon Ralph, Cipolloti, Manes, & Patterson, 2010; Lambon Ralph, Ehsan, Baker, & Rogers, 2012). However, this research remains inconclusive. Few studies have examined this idea, and those that do, have not examined change in a single set of patients undergoing surgery (i.e., they have compared different sets of pre- and post-surgical patients). Drane et al. (2013) demonstrated that the latter research approach can be misleading, as different cohorts may vary on verbal response time tasks due to a wide variety of variables (i.e., including antiepileptic drugs, interictal discharges, psychotropic medications; Drane et al., 2016; Ojemann et al., 2001). Additionally, Hamberger and colleagues (Miozzo & Hamberger, 2015), attempting to use a more sensitive set of semantic measures, found that pre-surgical TLE patients exhibited the usual naming deficits that are observed but no semantic dysfunction compared to healthy controls.
Another source of support for the hub model has included studies using repetitive transcranial magnetic stimulation (rTMS) to disrupt function in healthy controls (Pobric, Jefferies, & Lambon Ralph, 2007; Pobric, Jefferies, & Lambon Ralph, 2010). Pobric and colleagues found that healthy controls were slower to perform semantic tasks when rTMS was applied to either temporal pole while they were not slower at performing a control task. Hub proponents state that such disruption of function (e.g., slowed naming of objects) resulting from either temporal pole must indicate that each is performing this function. However, Drane and colleagues (Drane et al., 2013), Martin and Simmons (Martin, Simmons, Beauchamp, & Gotts, 2014; Simmons & Martin, 2009), and Gainnotti (Gainotti, 2011, 2015), have countered with a variety of objections, including that: a) Drane and Gainnotti’s alternative models propose that the end result (i.e., slowed naming) would be the same whether both temporal poles were engaged in semantic processing or if they were performing unique stages in the complex task of naming an object or face. For example, response slowing could reflect disrupted processing of sensory features in the right hemisphere and disrupted lexical processing in the left hemisphere. b) If the Hub model is correct that their results reflect disrupted semantic/linguistic processing in the right ATL as well as the left ATL, then one might expect greater equipoise in the deficits observed following left and right ATLs. As covered above, however, the qualitative nature of the deficits observed in left and right ATL patients is drastically different. c) rTMS can disrupt broader regions and pathways than the targeted area of stimulation. In keeping with the polymodal character of this region, a wide array of downstream cortical regions may be affected by this stimulation. In addition, interhemispheric connectivity is possible through the anterior commissure.
Hub model proponents also suggest that the lack of fMRI findings involving semantic processing and the ATL regions is likely due to difficulty imaging this region due to structural features of the skull (e.g., obscuration of signal by the temporal bone) (Visser, Embleton, Jefferies, Parker, & Lambon Ralph, 2010). Nevertheless, as pointed out by Martin and others (Martin et al., 2014; Simmons & Martin, 2009), fMRI signal is observed in these regions when the task design involves social processing rather than semantic. Some fMRI studies of semantic processing do show ATL activations (Visser et al., 2010), while many do not. Of note, even if activation occurs in this region, this would be correlative evidence for a role for these regions in semantic processing. Functional MRI studies (Bonelli et al., 2011), volumetric analyses (Seidenberg et al., 2005), and MRI spectroscopy (Sawrie et al., 2000) have long “implicated” the hippocampus of the language dominant cerebral hemisphere in naming on the basis of correlative methods, yet behavioral studies have not shown this to be an essential component of the naming network now that it can be removed in isolation (i.e., laser ablation of the hippocampus does not appear to affect visual confrontational naming) (Drane et al., 2015; Kang et al., 2016).
Drane and colleagues’ proposal that each cerebral hemisphere contributes uniquely to language and semantic memory processing (Drane et al., 2008; 2013), and their resulting data appears consistent with the framework of the dual processing model of language of Hickok and Poeppel (2007) with regards to the dorsal ventral stream and the posterior portion of the ventral stream. However, like the hub model proponents, Drane and colleagues do not believe that the anterior TL region is involved in “combinatorial semantics” (i.e., the ability to combine conceptual information in language and thought), as TLE patients have not been shown to experience deficits in this regard. Instead, recent research has suggested that combinatorial semantics may be a function of the angular gyrus [BA 39] (Price, Bonner, Peelle, & Grossman, 2015), again pointing to the breadth of the regions contributing to language, semantics, their reciprocal interface, and broader interactions with additional functional domains (e.g., episodic memory and learning). This is another area where significant disagreement remains, as Lambon Ralph and colleagues (2017) remain unconvinced of a role for the angular gyrus in this area, and raise objections that findings may be distorted by other task parameters.
The hub model of semantic memory claims that the bilateral ATL damage observed in semantic dementia obliterates a presumed abstracted, semantic store of information located in this region resulting in the characteristic features of this disease (i.e., decreased naming and semantic knowledge with relative sparing of episodic memory in early stages; Lambon Ralph et al., 2017). Instead, Drane and colleagues have proposed that bilateral ATL damage is simply leading to an inability to access more broadly distributed networks of semantic information by destroying the key zones for association necessary for object recognition and naming (Drane et al., 2008; 2009, 2013, 2015). Essentially, the semantic information would still presumably exist, but the systems required for its retrieval would be damaged. A similar theoretical position has also been proffered in the more recent works of Gainotti (2011) based upon a variety of neurological patient populations. A differential pattern of deficits resulting from left and right ATL lesions as described has even been described in semantic dementia patients experiencing pathology that is primarily restricted to one ATL more than the other (Snowden et al., 2004; Snowden, Thompson, & Neary, 2012). Likewise, Martin and colleagues (2014) point to studies showing that semantic deficits due not occur in other cases of bilateral temporal lobe damage, such as amnestic syndromes (Levy, Bayley, & Squire, 2004), and that action knowledge is spared after even after significant bilateral ATL damage (Damasio & Tranel, 1993).
Meta-analytic review of semantic memory and naming suggested a more critical role for the posterior, middle temporal gyrus (BA 21) (Binder et al., 2009). Likewise, a few studies conducted in stroke patients using voxel based lesion mapping (VBLM) to relate naming and semantic deficits to structural damage have found that lesions were required in the mid- to posterior TL to affect conceptual understanding (Baldo, Arevalo, Patterson, & Dronkers, 2013; DeLeon et al., 2007). More anterior lesions left comprehension intact for pictures and words alike and led to deficits in naming of objects only across modalities. These results relating a loss of semantic knowledge to more posterior lesions appear consistent with the existing epilepsy data (Drane et al., 2013; Hamberger, 2015).
Mesulam and colleagues have recently suggested on the basis of research involving primary progressive aphasia (PPA) that the dominant anterior temporal lobe (primarily the temporal pole) is critical for single word comprehension (i.e., recognition of the meaning of a word), and have suggested that language models need to be reworked to encompass this view (Mesulam, Thompson, Weintraub, & Rogalski, 2015). Like the hub model of semantic memory (Rogers et al., 2004), this idea is primarily being generated from patients with a disease that is typically bilateral and progressive. These findings seem at odds with the already cited work placing semantic function in more posterior TL regions, which has derived from functional neuroimaging (Binder et al., 2009; Simmons & Martin, 2009), stroke and epilepsy patients (Baldo et al., 2013; Drane et al., 2008; 2013), and cortical stimulation mapping (Luders et al., 1991). Moreover, Drane and colleagues have also recently shown that single word representation (i.e., the ability to define a word) was spared during ATL procedures that included the temporal pole (BA 38), but was affected if surgery involved significant portions of the superior temporal gyrus (BA 41), which likely reflects a problem with mapping speech sounds to motor output rather than an associational role between language and semantics (Ikanga et al., 2016). The greatest declines on a vocabulary test (i.e., defining words) occurred in patients with superior temporal lobe resections in the general region of Wernicke’s area (i.e., posterior superior temporal gyrus, angular gyrus).
Proponents of the hub model have contributed a wealth of data to this debate, with many outstanding studies that include evaluation of patients (primarily semantic dementia) (Lambon Ralph et al., 2007), disruption of function in healthy individuals with rTMS (Lambon Ralph, Pobric, & Jefferies, 2009; Pobric et al., 2007; Pobric et al., 2010), functional neuroimaging of lexical-semantic processes (Visser et al., 2010; Visser & Lambon Ralph, 2011), metaanalytic studies (Visser, Jefferies, & Lambon Ralph, 2009), and a variety of computational models (Lambon Ralph, Jefferies, Patterson, & Rogers, 2016; Rogers et al., 2004; Ueno, Saito, Rogers, & Lambon Ralph, 2011). They have also collaborated with others to gain data involving cortical stimulation mapping paradigms in neurosurgical patients and a single study of semantic/lexical function in post-surgical epilepsy patients (Lambon Ralph et al., 2012). They have also showed a willingness to adapt their model to address emerging evidence in other areas. The current version of the model involves a “demi-hub” and spoke framework, which provides greater flexibility to allow for modality specific regions of processing as well as a more central center(s) of transmodal processing that theoretically allows for integration of conceptual constructs (Lambon Ralph et al., 2017). Nevertheless, questions remain as to the structural boundaries of the proposed demi-hubs, the specific interconnections and their directionality, and the nature of the neural mechanism of action (Martin et al., 2014). It will likely be the case that the distributed multi-modal models and the hub model will find themselves working toward a shared middle ground, honing each other to a closer approximation of the brain’s underlying reality, which represents the best of the scientific endeavor. It is clear that models of language and semantic knowledge have grown more complex over time, involving broader regions than the original works of posterity (e.g., Wernicke, Broca, Geschwind).
3.2.3.4. Basal Temporal Language Area:
Lüders and colleagues discovered that application of electrical stimulation of the basal TL region (fusiform gyrus) of the dominant hemisphere at a high intensity produced a significant aphasia in a subset of patients involving disruption of both comprehension and speech output, and seizure onset in this region includes early speech-arrest (Luders et al., 1989; Luders, Lesser, Dinner, Morris, & Hahn, 1985; Luders et al., 1991). Of note, this region is not routinely assessed during clinical stimulation mapping studies. Stimulation of this region at lower intensities resulted in naming deficits only. This area was dubbed the basal temporal language area, and has been mentioned in several of the sections above.
While Lüders and colleagues initially reported no significant language deficits resulting from resection of this region (Luders et al., 1991), subsequent studies have indeed reported impairment (Bartha et al., 2005; Bartha et al., 2004; Pouratian, Bookheimer, Rubino, Martin, & Toga, 2003). The original work of Penfield also indicated that resection of this region could result in significant confrontational naming problems (Penfield & Roberts, 1959). Given that the electrical stimulation led to comprehension deficits as well as other language symptoms, this may be additional data suggesting a more posterior location of a region that is important for single word comprehension. One group has published a couple of case studies using subdural grid mapping of epilepsy patients which suggest that the posterior basal temporal language area may be most important for relating visual images to phonological content (Usui et al., 2003). Building upon these findings, a more recent electrophysiological study, which employed cortico-cortical evoked potentials to explore regional connectivity, suggests that there is direct connectivity between the basal temporal language area and a posterior language area (Wernicke’s area) (Araki et al., 2015). It is also possible that this reflects disruption of the ventral auditory processing pathway, perhaps when including basal temporal white matter (see coverage of both topics above).
4.1. Future Directions of Research and Clinical Practice in Language and Epilepsy.
In summary, presurgical evaluation of the epilepsy patient involving the use of various neuroimaging and electrophysiological techniques, as well as the assessment of performance changes following intervention (e.g., AEDs, neurosurgery), have contributed to substantial gains in knowledge about the structure-function relationship of language in the brain. Evidence is growing that broad regions beyond the traditional Wernicke’s and Broca’s areas are critical to language processing, and new models of language processing are emerging. While many structure-function issues remain controversial, significant evidence suggests more anterior TL regions are critical for lexical processing, while semantic processing may be managed by more posterior TL regions including both the middle and inferior temporal gyrus. Likewise, roles previously attributed to the hippocampus in language function are not being confirmed. Continuing to pull together results from neuroimaging studies (e.g., fMRI, DTI), patient outcome, and stimulation mapping paradigms should help refine our models. In particular, the rise of minimally invasive surgical procedures that can produce exquisitely focal lesions (e.g., stereotactic laser ablation, radiofrequency ablation; Drane et al., 2015; Gross, Mahmoudi, & Riley, 2015; Gross et al., 2016; Guenot et al., 2004) is allowing us to explore the brain in a manner never before possible in humans. Combining these more precise “lesional methods” with cortico-cortical evoked potentials, neuroanatomical dissection studies in primates and human cadavers, and available neuroimaging technologies should allow us to eventually establish a detailed neuroanatomical understanding of language and semantics.
It is also recognized that there are common interictal language deficits in epilepsy patients, which appear to often result directly from the effects of seizures on specific brain regions (e.g., naming and verbal generative fluency limitations in TLE). However, there appear to be more subtle aspects of language assessment that have been lacking (e.g., clinical assessment of narrative discourse, timing aspects of speech production, right-hemisphere processing of spectral characteristics, prosody, and other modulatory aspects of speech production and analysis), and should be considered in future research and clinical practice. Likewise, greater attention needs to be paid to semantic processing and its relationship with classic language functions, and greater efforts are required to incorporate these broader paradigms into models of clinical care in epilepsy. Overall, gaining a more complete understanding of the language system should allow us to improve our ability to manage epilepsy while optimizing cognitive function.
Figure 3.
A. Classic model of language. Originally published by Geschwind (1979),(Geschwind, 1979) and used with permission of Scientific American. B. Proposed dual processing stream model of language function of Hickok and Poeppel.(Hickok & Poeppel, 2007) Published with permission of Nature Publishing Group.
Significance.
This review summarizes key language-related findings from epilepsy and epilepsy surgery research, while highlighting novel theories relating to the neural organization of language resulting from this research.
Highlights.
Complex partial epilepsy and its treatment leads to circumscribed language deficits.
Minimally invasive surgery leads to new insights into neural substrates of language.
The hippocampus has no role in word retrieval.
White matter pathways are crucial to language and semantic networks.
Basal temporal language area is likely a critical hub in the language network.
Acknowledgements:
Dr. Drane receives grant support from the NIH/NINDS (R01 NS088748, K02 NS070960, L30 NS080215) and Medtronic, Inc. (A1225797). This review was partially supported by funds from Dr. Drane’s NIH/NINDS and industry awards. The authors would like to thank Dr. Amit Saindane, Chair of Neuroradiology at Emory University School of Medicine, for his assistance in the preparation of Figure 4 of the manuscript.
Figure 4.
A) The basal temporal language area is located in the fusiform gyrus of the temporal lobe. A) For reference, we have highlighted this region on a 3T MRI scan obtained from one of our epilepsy surgery patients. B) This figure, originally published in an article using stimulation mapping to study the basal temporal language area, depicts the placement of an intracranial strip electrode in the fusiform gyrus (Trebuchon-Da Fonseca et al., 2009).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel TJ, Rhone AE, Nourski KV, Kawasaki H, Oya H, Griffiths TD, … Tranel D(2015). Direct physiologic evidence of a heteromodal convergence region for proper naming in human left anterior temporal lobe. Journal of Neuroscience, 35(4), 1513–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki K, Terada K, Usui K, Usui N, Araki Y, Baba K, … Inoue Y (2015). Bidirectional neural connectivity between basal temporal and posterior language areas in humans. Clinical Neurophysiology, 126, 682–688. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Arevalo A, Patterson JP, & Dronkers NF (2013). Grey and white matter correlates of picture naming: Evidence from a voxel-based lesion analysis of the Boston Naming Test. Cortex, 49(3), 658–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo JV, Shimamura AP, Delis DC, Kramer J, Kaplan E (2001). Verbal and design fluency in patients with frontal lobe lesions. Journal of the International Neuropsychological Society, 7(5), 586–596. [DOI] [PubMed] [Google Scholar]
- Barsalou LW, Santos A, Simmons WK, & Wilson CD (2008). Language and simulation in conceptual processing In Vega MD, Glenberg AM & Graesser AC (Eds.), Symbols, embodiment, and meaning (pp. 245–283). Oxford: Oxford University. [Google Scholar]
- Barsalou LW, Simmons WK, Barbey AK, & Wilson CD (2003). Grounding conceptual knowledge in modality-specific systems. Trends in Cognitive Sciences, 7(2), 84–91. [DOI] [PubMed] [Google Scholar]
- Bartha L, Benke T, Bauer G, & Trinka E (2005). Interictal language functions in temporal lobe epilepsy Journal of Neurology Neurosurgery and Psychiatry, 76, 808–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartha L, Trinka E, Ortler M, Donnemiller E, Felber S, Bauer G, & Benke T (2004). Linguistic deficits following left selective amygdalohippocampectomy: A prospective study. Epilepsy and Behavior, 5(3), 348–357. [DOI] [PubMed] [Google Scholar]
- Bartha-Doering L, & Trinka E (2014). The interictal language profile in adult epilepsy. Epilepsia, 55(10), 1512–1525. [DOI] [PubMed] [Google Scholar]
- Baxendale SA, van Paesschen W, Thompson PJ, Connely A, Duncan JS, Harkness WF, & Shorvon SD (1998). The relationship between quantitative MRI and neuropsychological functioning in temporal lobe epilepsy. Epilepsia, 39(2), 158–166. [DOI] [PubMed] [Google Scholar]
- Bell B, Dow C, Watson R, Woodard A, Hermann B, Seidenberg M (2003). Narrative and procedural discourse in temporal lobe epilepsy. Journal of the International Neuropsychological Society, 9(5), 733–739. [DOI] [PubMed] [Google Scholar]
- Benke T, Kuen E, Schwarz M, & Walser G (2013). Proper name retrieval in temporal lobe epilepsy: Naming of famous faces and landmarks. Epilepsy and Behavior, 27, 371–377. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, & Conant LL (2009). Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex, 19, 2767–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Swanson SJ, Hammeke TA, Morris GL, Mueller WM, Fischer M, … Haughton VM (1996). Determination of language dominance using functional MRI: A comparison with the Wada test. Neurology, 46, 978–984. [DOI] [PubMed] [Google Scholar]
- Boatman D, Hall C, Goldstein MH, Lesser R, & Gordon B (1997). Neuroperceptual differences in consonant and vowel discrimination: As revealed by direct cortical electrical interference. Cortex, 33(1), 83–98. [DOI] [PubMed] [Google Scholar]
- Boatman D, Lesser RP, & Gordon B (1995). Auditory speech processing in the left temporal lobe: An electrical interference study. Brain and Language, 51(2), 269–290. [DOI] [PubMed] [Google Scholar]
- Bonelli SB, Powell R, Thompson PJ, Yogarajah M, Focke NK, Stretton J, … Koepp MJ (2011). Hippocampal activation correlates with visual confrontational naming: fMRI findings in controls and patients with temporal lobe epilepsy. Epilepsy Research, 95, 246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch RM, Floden DP, Prayson B, Chapin JS, Kim KH, Ferguson L, … Najm IM(2016). Estimating risk of word-finding problems in adults undergoing epilepsy surgery. Neurology, 87, 2363–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camfield PR, Gates R, Ronen G, Camfield C, Ferguson A, & Macdonald W (1984). Comparison of cognitive ability, personality profile, and school success in epileptic children with pure right versus left temporal lobe eeg foci. Annals of Neurology, 15(2), 122–126. [DOI] [PubMed] [Google Scholar]
- Chang EF, Raygor KP, & Berger MS (2015). Contemporary model of language organization: An overview for neurosurgeons. Journal of Neurosurgery, 122, 250–261. [DOI] [PubMed] [Google Scholar]
- Cloutman LI, Binney RJ, Morris DM, Parker GJM, & Lambon Ralph MA (2013). Using in vivo probabilistic tractography to reveal two segregated dorsal ‘language-cognitive’ pathways in the human brain Brain and Language, 127, 230–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corina DP, Loudermilk BC, Detwiler L, Martin RF, Brinkley JF, & Ojeman n G (2010). Analysis of naming errors during cortical stimulation mapping: Implications for models of language representation. Brain and Language, 115, 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson B (1992). Subcortical functions in language and memory. NY: Guilford Press. [Google Scholar]
- Damasio AR (1989). Time-locked multiregional retroactivation: A systems-level proposal for the neural substrates of recall and recognition. Cognition, 33(1–2), 25–62. [DOI] [PubMed] [Google Scholar]
- Damasio AR, & Damasio H (1992). Brain and language. Scientific American, 267(3), 88–95. [DOI] [PubMed] [Google Scholar]
- Damasio AR, & Tranel D (1993). Nouns and verbs are retrieved with differently distributed neural systems. Proceedings of the National Academy of Sciences of the United States of America, 90, 4957–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio H, Grabowski TJ, Tranel D, Hichwa RD, & Damasio A (1996). A neural basis for lexical retrieval. Nature, 380, 499–505. [DOI] [PubMed] [Google Scholar]
- Damasio H, Tranel D, Grabowski T, Adolphs R, & Damasio A (2004). Neural systems behind word and concept retrieval. Cognition, 92(1–2), 179–229. [DOI] [PubMed] [Google Scholar]
- Davies KG, Bell BD, Bush A, Hermann BP, Dohan FCJ, & Jaap AS (1998). Naming decline after left anterior temporal lobectomy correlates with pathological status of resected hippocampus. Epilepsia, 39(4), 407–419. [DOI] [PubMed] [Google Scholar]
- DeLeon J, Gottesman RF, Kleinman JT, Newhart M, Davis C, Heidler-Gary J, … Hillis AE (2007). Neural regions essential for distinct cognitive processes underlying picture naming. Brain, 130(Pt 5), 1408–1422. [DOI] [PubMed] [Google Scholar]
- DeVos K, Wyllie E, Geckler C, Kotagal P, & Comair Y (1995). Language dominance in patients with early childhood tumors near left hemisphere language areas. Neurology, 45(2), 349–356. [DOI] [PubMed] [Google Scholar]
- Dick AS, & Tremblay P (2012). Beyond the arcuate fasciculus: Consensus and controversy in the the connectional anatomy of language Brain, 135(12), 3529–3550. [DOI] [PubMed] [Google Scholar]
- Drane DL, Glasser MF, Voets NL, Ojemann JG, Saindane AM, Loring DW, … Gross RE (2014). Key pathways for visual naming and object recognition revealed by diffusion tensor imaging probabilistic tractography in epilepsy surgery patients. Neurology, 82(Meeting Abstracts), S43.008. [Google Scholar]
- Drane DL, Lee G,P, Cech H, Huthwaite JS, Ojemann GA, Ojemann JG, … Meador KJ (2006). Structured cueing on a semantic fluency task differentiates patients with temporal versus frontal lobe seizure onset. Epilepsy and Behavior, 9(2), 339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drane DL, Loring DW, Voets NL, Price M, Ojemann JG, Willie JT, … Gross RE (2015). Better object recognition and naming outcome with MRI-guided stereotactic laser amygdalohippocampotomy for temporal lobe epilepsy. Epilepsia, 56, 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drane DL, Ojemann GA, Aylward E, Ojemann JG, Johnson LC, Silbergeld DL, … Tranel D (2008). Category-specific naming and recognition deficits in temporal lobe epilepsy. Neuropsychologia, 46(5), 1242–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drane DL, Ojemann GA, Ojemann JG, Aylward EA, Silbergeld DL, Miller JW, & Tranel D (2009). Category-specific recognition and naming deficits following resection of a right anterior temporal lobe tumor in a patient with atypical language lateralization. Cortex, 45(5), 630–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drane DL, Ojemann JG, Kim M, Gross RE, Miller JW, Faught RE Jr., & Loring DW (2016). Interictal epileptiform discharge effects on neuropsychological assessment and epilepsy surgical planning. Epilepsy and Behavior, 56, 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drane DL, Ojemann JG, Phatak V, Loring DW, Gross RE, Hebb AO, … Tranel D (2013). Famous face identification in temporal lobe epilepsy: Support for a multimodal integration model of semantic memory. Cortex, 49(1648–1667). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drane DL, Roraback J, Hebb A, Hersonskey T, Lucas T, Ojemann GA, … Ojemann JG (2012). Cortical stimulation mapping and Wada results demonstrate a normal variant of right hemisphere language organization. Epilepsia, 53(10), 1790–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchowny M, Jayakar P, Harvey AS, Resnick T, Alvarez L, Dean P, & Levin B (1996). Language cortex representation: Effects of developmental versus acquired pathology. Annals of Neurology, 40, 31–38. [DOI] [PubMed] [Google Scholar]
- Duffau H, Gatignol P, Mandonnet E, Capelle L, & Taillandier L (2008). Intraoperative subcortical stimulation mapping of language pathways in a consecutive series of 115 patients with Grade II glioma in the left dominant hemisphere. Journal of Neurosurgery, 109(3), 461–471. [DOI] [PubMed] [Google Scholar]
- Field SJ, Saling MM, & Berkovic SF (2000). Interictal discource production in temporal lobe epilepsy. Brain and Language, 74, 213–222. [DOI] [PubMed] [Google Scholar]
- Friederici AD (2012). The cortical language circuit: from auditory perception to sentence comprehension. Trends in Cognitive Sciences, 16, 262–268. [DOI] [PubMed] [Google Scholar]
- Fritz N, Glogau S, Hoffmann J, Rademacher M, Elger CE, & Helmstaedter C (2005). Efficacy and cognitive side effects of tiagabine and topiramate in patients with epilepsy. Epilepsy & Behavior, 6(3), 373–381. [DOI] [PubMed] [Google Scholar]
- Frost JA, Binder JR, Springer JA, Hammeke TA, Bellgowan PSF, Rao SM, & Cox RW (1999). Langauge processing is strongly left lateralized in both sexes: Evidence from functional MRI. Brain, 122(2), 199–206. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Hertz-Pannier L, Mott SH, Barnett AS, LiBihan D, & Theodore WH (2000). Functional anatomy of cognitive development: fMRI of verbal fluency in children and adults. Neurology, 54(1), 180–185. [DOI] [PubMed] [Google Scholar]
- Gainotti G (2011). The organization and dissolution of semantic-conceptual knowledge: Is the ‘amodal hub’ the only plausible model? Brain and Cognition, 75, 299–309. [DOI] [PubMed] [Google Scholar]
- Gainotti G (2015). Is the difference between right and left ATLs due to the distinction between general and social cognition or between verbal and non-verbal representations? Neuroscience & Biobehavioral Reviews, 51, 296–312. [DOI] [PubMed] [Google Scholar]
- George MS, Parekh PI, Rosinsky N, Ketter TA, Kimbrell TA, & Heilman KM (1996). Understanding emotional prosody activates right hemisphere regions. Archives of Neurology, 53, 665–670. [DOI] [PubMed] [Google Scholar]
- Geschwind N (1979). The organization of language and the brain. Science, 170, 940–944. [DOI] [PubMed] [Google Scholar]
- Glasser MF, & Rilling JK (2008). DTI tractography of the human brain’s language pathways. Cerebral Cortex, 18(11), 2471–2482. [DOI] [PubMed] [Google Scholar]
- Gleissner U, Kuczaty S, Clusmann H, Elger CE, & Helmstaedter C (2008). Neuropsychological results in pediatric patients with epilepsy surgery in the parietal cortex. Epilepsia, 49(4), 700–704. [DOI] [PubMed] [Google Scholar]
- Glosser G, Salvucci AE, & Chiaravalloti ND (2003). Naming and recognizing famous faces in temporal lobe epilepsy. Neurology, 61, 81–86. [DOI] [PubMed] [Google Scholar]
- Griffin S, & Tranel D (2007). Age of seizure onset, functional reorganization, and neuropsychological outcome in temporal lobectomy. Journal of Clinical and Experimental Neuropsychology, 29(1), 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith HR, Richardson E, Pyzalski RW, Bell B, Dow C, Hermann BP, & Seidenberg M (2006). Memory for famous faces and the temporal pole: Functional imaging findings in temporal lobe epilepsy. Epilepsy and Behavior, 9(1), 173–180. [DOI] [PubMed] [Google Scholar]
- Gross RE, Mahmoudi B, & Riley JP (2015). Less is more: Novel less-invasive surgical techniques for mesial temporal lobe epilepsy that minimize cognitive impairment. Current Opinion in Neuorlogy, 28(2), 182–191. [DOI] [PubMed] [Google Scholar]
- Gross RE, Willie JT, & Drane DL (2016). The role of stereotactic laser amygdalohippocampotomy in mesial temporal lobe epilepsy. Neurosurgery Clinics of North America, 27(1), 37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenot M, Isnard J, Ryvlin P, Fischer C, Mauguiere F, & Sindou M (2004). S EEG-guided RF thermocoagulation of epileptic foci: Feasibility, safety, and preliminary results. Epilepsia, 45(11), 1368–1374. [DOI] [PubMed] [Google Scholar]
- Hagoort P (2014). Nodes and networks in the neural architecture for language: Broca’s region and beyond. Current Opinion in Neurobiology, 28, 136–141. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ (2015). Object naming in epilepsy and epilepsy surgery. Epilepsy and Behavior, 46, 27–33. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ, & Cole J (2011). Language organization and reorganization in epilepsy. Neuropsychology Review, 21(3), 240–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberger MJ, Goodman RR, Perrine K, & Tamny T (2001). Anatomic dissocation of auditory and visual naming in the lateral temporal cortex. Neurology, 56, 56–61. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ, McClelland S 3rd, McKhann GM 2nd, Williams AC, & Goodman RR (2007). Distribution of auditory and visual naming sites in nonlesional temporal lobe epilepsy patients and patients with space-occupying temporal lobe lesions. Epilepsia, 48(3), 531–538. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ, Seidel WT, Mckhann GM, Perrine K, & Goodman RR (2005). Brain stimulation reveals critical auditory naming cortex. Brain, 128, 2742–2749. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ, Seidel WT, McKhann GMN, & Goodman RR (2010). Hippocampal removal affects visual but not auditory naming. Neurology, 74(19), 1488–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberger MJ, & Tamny TR (1999). Auditory naming and temporal lobe epilepsy. Epilepsy Research, 35, 229–243. [DOI] [PubMed] [Google Scholar]
- Hebb AO, & Ojemann GA (2013). The thalamus and language revisited. Brain and Language, 126, 99–108. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C, Gleibner U, Zentner J, & Elger CE (1998). Neuropsychological consequences of epilepsy surgery in frontal lobe epilepsy. Neuropsychologia, 36(4), 333–341. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C, Kemper B, & Elger CE (1996). Neuropsychological aspects of frontal lobe epilepsy. Neuropsychologia, 34(5), 399–406. [DOI] [PubMed] [Google Scholar]
- Hermann B, Davies K, Foley K, & Bell B (1999). Visual confrontation naming outcome after standard left anterior temporal lobectomy with sparing versus resection of the superior temporal gyrus: A randomized prospective clinical trial. Epilepsia, 40(8), 1070–1076. [DOI] [PubMed] [Google Scholar]
- Hermann BP, & Wyler AR (1988). Effects of anterior temporal lobectomy on language function: A controlled study. Annals of Neurology, 23, 585–588. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Wyler AR, Steenman H, & Richey ET (1988). The interrelationship between language function and verbal learning/memory performance in patients with complex partial seizures. Cortex, 24(2), 245–253. [DOI] [PubMed] [Google Scholar]
- Hickok G, & Poeppel D (2004). Dorsal and ventral streams: A framework for understanding the functional neuroanatomy of language. Cognition, 92(1–2), 67–99. [DOI] [PubMed] [Google Scholar]
- Hickok G, & Poeppel D (2004). Towards a new functional anatomy of language. Cognition, 92(1–2), 1–12. [DOI] [PubMed] [Google Scholar]
- Hickok G, & Poeppel D (2007). The cortical organization of speech processing. Nat Rev Neurosci, 8, 393–402. [DOI] [PubMed] [Google Scholar]
- Howell RA, Saling MM, Bradley DC, & Berkovic SF (1994). Interictal language fluency in temporal lobe epilepsy. Cortex, 30(3), 469–478. [DOI] [PubMed] [Google Scholar]
- Ikanga J, Millis S, Wilson R, Collins K, Ivanisevic M, Kim M, … Drane D (2016). The left anterior temporal pole is critical for object naming but not single word comprehension Paper presented at the American Academy of Clinical Neuropsychology, Chicago, IL. [Google Scholar]
- Ives-Deliperi VL, & Butler JT (2012). Naming outcomes of anterior temporal lobectomy in epilepsy patients: A systematic review of the literature. Epilepsy and Behavior, 24, 194–198. [DOI] [PubMed] [Google Scholar]
- Janecek JK, Swanson SJ, Sabsevitz DS, Hammeke TA, Raghavan M, Rozman M, & Binder JR (2013). Language lateralization by fMRI and Wada testing in 229 epilepsy patients: Rates and predictors of discordance. Epilepsia, 54(2), 314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joanette Y, & Goulet P (1986). Criterion-specific reduction of verbal fluency in right brain-damaged right-handers. Neuropsychologia, 24, 875–879. [DOI] [PubMed] [Google Scholar]
- Jokeit H, Heger R, Ebner A, & Markowitsch HJ (1998). Hemispheric asymetries in category-specific word retrieval. NeuroReport, 9, 2371–2373. [DOI] [PubMed] [Google Scholar]
- Jones EG (2007). The thalamus (2 ed.). Cambridge, England: Cambridge University Press. [Google Scholar]
- Kang JY, Wu C, Tracy J, Lorenzo M, Evans J, Nei M, … Sperling MR (2016). Laser interstitial thermal therapy for medically intractable mesial temporal lobe epilepsy. Epilepsia, 57(2), 325–334. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, & Chun MM (1997). The fusiform face area: A module in human extrastriate cortex specialized for face perception. Journal of Neuroscience, 17, 4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, & Yovel G (2006). The fusiform face area: A cortical region specialized for the perception of faces. Phil. Trans. Royal Society of London B Biol Sci, 361 (1476), 2109–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klooster NB, & Duff MC (2015). Remote semantic memory is impoverished in hippocampal amnesia. Neuropsychologia, 79, 42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht S, Drager B, Deppe M, Bobe L, Lohmann H, Floel A, … Henningsen H (2000). Handedness and hemispheric language dominance in healthy humans. Brain, 123, 2512–2518. [DOI] [PubMed] [Google Scholar]
- Koeda M, takahashi H, Yahata N, Asai K, Okubo Y, & Tanaka H (2006). A functional MRI study: Cerebral laterality for lexical-semantic processing and human voice perception. American Journal of Neuroradiology(27), 7. [PMC free article] [PubMed] [Google Scholar]
- Krauss GL, Fisher R, Plate C, Hart J, Uematsu S, Gordon B, & Lesser RP (1996). Cognitive effects of resecting basal temporal language areas. Epilepsia, 37(5), 476–483. [DOI] [PubMed] [Google Scholar]
- Kravitz DJ, Saleem KS, Baker CI, Ungerleider LG, & Mishkin M (2013). The ventral visual pathway: An expanded neural framework for the processing of object quality. Trends in Cognitive Sciences, 17(1), 26–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang Y, Yang T, Gu J, Kong B, & Cheng L (2014). Comparison of therapeutic effects between selective amygdalohippocampectomy and anterior temporal lobectomy for the treatment of temporal lobe epilepsy: A meta-analysis. British Journal of Neurosurgery, 28(3), 374–377. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Cipolloti L, Manes F, & Patterson K (2010). Taking both sides: Do unilateral temporal lobe lesions disrupt semantic memory? Brain, 133, 3243–3255. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Ehsan S, Baker GA, & Rogers TT (2012). Semantic memory is impaired in patients with unilateral temporal lobe resection for temporal lobe epilepsy. Brain, 135, 242–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph MA, Jefferies E, Patterson K, & Rogers TT (2016). The neural and computational bases of semantic cognition. Nature Reviews Neuroscience, 18, 142–155. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Jefferies E, Patterson K, & Rogers TT (2017). The neural and computational bases of semantic cognition. Nature Reviews Neuroscience, 18, 42–55. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Lowe C, & Rogers TT (2007). Neural basis of category-specific semantic deficits for living things: Evidence from semantic dementia, HSVE, and a neural network model. Brain, 130, 1127–1137. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Pobric G, & Jefferies E (2009). Conceptual knowledge is underpinned by the temporal pole bilaterally: Convergent evidence from rTMS. Cerebral Cortex, 19, 832–838. [DOI] [PubMed] [Google Scholar]
- Langfit JT, & Rausch R (1996). Word-finding deficits persist after left anterotemporal lobectomy. Arch Neurol, 53(1), 72–76. [DOI] [PubMed] [Google Scholar]
- Levy DA, Bayley PJ, & Squire LR (2004). The anatomy of semantic knowledge: Medial vs. lateral temporal lobe. Proceedings of the National Academy of Sciences of the United States of America, 101, 6710–6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtheim L (1885). On aphasia. Brain, 7, 433–485. [Google Scholar]
- Loring DW (2010). History of neuropsychology through epilepsy eyes. Archives of Clinical Neuropsychology, 25(4), 259–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loring DW, Meador KJ, Lee GP, Murro AM, Flanigin HF, Gallagher BB, & King DW (1990). Cerebral language lateralization: Evidence from intracarotid amobarbital testing. Neuropsychologia, 28(8), 831–838. [DOI] [PubMed] [Google Scholar]
- Lucas TH 2nd, Drane DL, Dodrill CB, & Ojemann GA (2008). Language reorganization in aphasics: An electrical stimulation mapping investigation. Neurosurgery, 63(3), 487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders H, Hahn J, Lesser RP, Dinner DS, Morris r., H. H., Wyllie E, … Skipper G (1989). Basal temporal subdural electrodes in the evaluation of patients with intractable epilepsy. Epilepsia, 30(2), 131–142. [DOI] [PubMed] [Google Scholar]
- Luders H, Lesser RP, Dinner DS, Morris HH, & Hahn J (1985). Language disturbances produced by electrrical stimulation of the basal temporal region. Annals of Neurology, 18, 151. [Google Scholar]
- Luders J, Lesser RP, Hahn J, Dinner DS, Morris HH, Wyllie E, & Godoy J (1991). Basal temporal language area. Brain, 114, 743–754. [DOI] [PubMed] [Google Scholar]
- Martin A (2007). The representation of object concepts in the brain. Annual Review of Psychology, 58, 25–45. [DOI] [PubMed] [Google Scholar]
- Martin A, & Fedio P (1983). Word production and comprehension in Alzheimer’s disease: The breakdown of semantic knowledge. Brain and Language, 19, 124–141. [DOI] [PubMed] [Google Scholar]
- Martin A, Simmons WK, Beauchamp MS, & Gotts SJ (2014). Is a single ‘hub’, with lots of spokes, an accurate description of the neural architecture of action semantics? Physics of Life Reviews, 11(2), 261–262. [DOI] [PubMed] [Google Scholar]
- Martin A, Wiggs CL, Ungerleider LG, & Haxby JV (1996). Neural correlates of category-specific knowledge. Nature, 379(6566), 649–652. [DOI] [PubMed] [Google Scholar]
- Martin RC, Loring DW, Meador KJ, & Lee GP (1990). The effects of lateralized temporal lobe dysfunction on formal and semantic word fluency. Neuropsychologia, 28, 823–829. [DOI] [PubMed] [Google Scholar]
- Martin RC, Sawrie SM, Edwards R, Roth DL, Faught E, Kuzniecky RI, … Gilliam FG (2000). Investigation of Executive Function Change Following Anterior Temporal Lobectomy: Selective Normalization of Verbal Fluency. Neuropsychology, 14(2), 501–508. [DOI] [PubMed] [Google Scholar]
- Matsumoto R, Nair DR, LaPresto E, Najm I, Bingaman W, Shibasaki H, & Luders HO (2004). Functional connectivity in the human language system: A cortico-cortical evoked potential study. Brain, 127, 2316–2330. [DOI] [PubMed] [Google Scholar]
- McClelland JL, & Rogers TT (2003). The parallel distributed processing approach to semantic memory. Nature Reviews Neuroscience, 4(310–322). [DOI] [PubMed] [Google Scholar]
- McDonald CR, Ahmadi ME, Hagler DJ, Tecoma ES, Iragui VJ, Gharapetian L, Halgren E (2008). Diffusion tensor imaging correlates of memory and language impairments in temporal lobe epilepsy. Neurology, 71(23), 1869–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Thompson CK, Weintraub S, & Rogalski EJ (2015). The Wernick conundrum and the anatomy of language comprehension in primary progressive aphasia. Brain, 138, 2423–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metternich B, Buschmann F, Wagner K, Schulze-Bonhage A, & Kriston L (2014). Verbal fluency in focal epilepsy: A systematic review and meta-analysis. Neuropsychology Review, 24, 200–218. [DOI] [PubMed] [Google Scholar]
- Mills CK, & Martin E (1912). Aphasia and agraphia in some practical surgical relations. Journal of the American Medical Association, 59, 1513–1518. [Google Scholar]
- Milner B (1964). Some effects of frontal lobectomy in man In Warren JM & Akerts K (Eds.), The frontal granular cortex and behavior (pp. 313–334). New York: McGraw-Hill. [Google Scholar]
- Miozzo M, & Hamberger MJ (2015). Preserved meaning in the context of impaired naming in temporal lobe epilepsy. Neuropsychology, 29(2), 274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RS, & Hodges JR (2000). A voxel-based morphometry study of semantic dementia: Relationship between temporal lobe atrophy and semantic memory. Annals of Neurology, 47, 36–45. [PubMed] [Google Scholar]
- Nielsen JM (1946). Agnosia, apraxia, aphasia: Their value in cerebral localization New York: Paul B. Hoeber [Google Scholar]
- Ojemann G, Ojemann J, Lettich E, & Berger M (1989). Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. Journal of Neurosurgery, 71(3), 316–326. [DOI] [PubMed] [Google Scholar]
- Ojemann GA, & Whitaker HA (1978). The bilingual brain. Archives of Neurology, 35, 409–412. [DOI] [PubMed] [Google Scholar]
- Ojemann LM, Ojemann GA, Dodrill CB, Crawford CA, Holmes MD, & Dudley DL (2000). Language disturbances as side effects of topiramate and zonisamide therapy. Epilepsy and Behavior, 2, 579–584. [DOI] [PubMed] [Google Scholar]
- Ojemann SG, Berger MS, Lettich E, & Ojemann GA (2003). Localization of language function in children: Results of electrical stimulation mapping. Journal of Neurosurgery, 98, 465–470. [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, & Rogers TT (2007). Where do you know what you know? The representation of semantic knowledge in the human brain Natures Reviews Neuroscience, 8, 976–987. [DOI] [PubMed] [Google Scholar]
- Penfield W, & Erickson TC (1941). Epilepsy and cerebral localization. Baltimore, MD: Charles C. Thomas. [Google Scholar]
- Penfield W, & Flanigin H (1954). Epilepsy and the functional anatomy of the human brain Boston: Little, Brown, & Company. [Google Scholar]
- Penfield W, & Roberts L (1959). Speech and brain mechanisms. Princeton: Princeton University Press. [Google Scholar]
- Peran P, Rascol O, Demonet JF, Celsis P, Nespoulous JL, Dubois B, & Cardebat D (2003). Deficit of verb generation in nondemented patients with Parkinson’s disease. Movement Disorders, 18(2), 150–156. [DOI] [PubMed] [Google Scholar]
- Piatt AL, Fields JA, Paolo AM, & Troster AI (1999). Action (verb naming) fluency as an executive function measure: Convergent and divergent evidence of validity. Paper presented at the International Neuropsychological Society, Boston, Massachusetts. [DOI] [PubMed] [Google Scholar]
- Pobric G, Jefferies E, & Lambon Ralph MA (2007). Anterior temporal lobes mediate semantic representation: Mimicking semantic dementia by using rTMS in normal participants Proceedings of the National Academy of Sciences of the United States of America., 104, 20137–20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobric G, Jefferies E, & Lambon Ralph MA (2010). Category-specific versus category-general semantic impairment induced by transcranial magnetic stimulation. Current Biology, 20(10), 964–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeppel D, Emmorey K, Hickok G, & Pylkkanen L (2012). Towards a new neurobiology of learning. The Journal of Neuroscience, 32(41), 14125–14131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeppel D, & Hickok G (2004). Towards a new functional anatomy of language. Cognition, 92(1–2), 1–12. [DOI] [PubMed] [Google Scholar]
- Pouratian N, Bookheimer SY, Rubino G, Martin NA, & Toga AW (2003). Category-specific naming deficit identified by intraoperative stimulation mapping and p ostoperative neuropsychological testing. Case report. Journal of Neurosurgery, 99(1), 170–176. [DOI] [PubMed] [Google Scholar]
- Price AR, Bonner MF, Peelle JE, & Grossman M (2015). Converging evidence for the neuroanatomic basis of combinatorial semantics in the angular gyrus. The Journal of Neuroscience, 35(7), 3276–3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvermuller F (2013). Semantic embodiment, disemobidment or misembodiment? In search of meaning in modules and neuron circuits. Brain and Language, 127, 86–103. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, & Scott SK (2009). Maps and streams in the auditory cortex: Nonhuman primates illuminate human speech processing. Nature and Neuroscience, 12, 718–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, Glasser MF, Jbabdi S, Andersson J, & Preuss TM (2012). Continuity, divergence, and the evolution of brain language pathways. Frontiers in Evolutionary Neuroscience, 3(11), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, Glasser MF, Preuss TM, Ma X, Zhao T, Hu X, & Behrens TEJ (2008). The evoluation of the arcuate fasciculus revealed with comparative DTI. Nature Neuroscience, 11(4), 426–428. [DOI] [PubMed] [Google Scholar]
- Risse GL (2006). Cognitive outcomes in patients with frontal lobe epilepsy Epilepsia, 2, 87–89. [DOI] [PubMed] [Google Scholar]
- Rivolta D, Palermo R, Schmalzl L, & Coltheart M (2012). Covert face recognition in congenital prosopagnosia: A group study. Cortex, 48, 344–352. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Lambon Ralph MA, Garrard P, Bozeat S, McClelland JL, Hodges JR, & Patterson K (2004). Structure and deterioration of semantic memory: A neuropsychological and computational investigation. Psychological Review, 111(1), 205–235. [DOI] [PubMed] [Google Scholar]
- Salanova V, Andermann F, Rasmussen T, Olivier A, & Quesney LF (1995). Parietal lobe epilepsy: Clinical manifestations and outcomes in 82 patients treated surgically between 1929 and 1988. Brain, 118, 607–627. [DOI] [PubMed] [Google Scholar]
- Sarkis RA, Busch RM, Floden D, Chapin JS, Kaiman KC, Jehi L, … Najm I (2013). Predictors of decline in verbal fluency after frontlal lobe epilepsy surgery. Epilepsy and Behavior, 27(2), 326–329. [DOI] [PubMed] [Google Scholar]
- Saur D, Kreher BW, Schnell S, Kummerer D, Kellmeyer P, Vry MS, … Weiller C. Ventral and dorsal pathways for language. Proceedings of the National Academy of Sciences of the United States of America, 105(46), 18035–18040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawrie SM, Martin RC, Gilliam FG, Faught E, Maton B, Hugg JW, … Kuzniecky R (2000). Visual confrontation naming and hippocampal function A neural network study using quantitative 1H magnetic resonance spectroscopy. Brain, 123, 770–780. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Stafiniak P, Robinson LJ, Flannery KA, Gur RC, O’Connor MJ, & Sperling MR (1995). Language before and after temporal lobectomy: Specificity of acute changes and relation to early risk factors. Epilepsia, 33(11), 1071–1077. [DOI] [PubMed] [Google Scholar]
- Schoenberg MR, Werz MA, & Drane DL (2011). Epilepsy and seizures In Scott MRSJ (Ed.), Black book of neuropsychology (pp. 423–519). New York: Springer. [Google Scholar]
- Seidenberg M, Geary E, & Hermann B (2005). Investigating temporal lobe contribution to confrontational naming using MRI quantitative volumetrics. Journal of the International Neuropsychological Society, 11, 358–366. [PubMed] [Google Scholar]
- Seidenberg M, Griffith R, Sabsevitz D, Moran M, Haltiner A, Bell B, … Hermann B (2000). Recognition and identification of famous faces in patients with unilateral temporal lobe epilepsy. Neuropsychologia, 40(4), 446–456. [DOI] [PubMed] [Google Scholar]
- Simmons WK, & Martin A (2009). The anterior temporal lobes and the functional architecture of semantic memory. Journal of the International Neuropsychological Society, 15, 645–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinai A, Bowers CW, Crainiceanu CM, Boatman D, Gordon B, Lesser RP, … Crone NE (2005). Electrocorticographic high gamma activity versus electrical cortical stimulation mapping of naming. Brain, 128(7), 1556–1570. [DOI] [PubMed] [Google Scholar]
- Smith CD, Andersen AH, Kryscio RJ, Schmitt FA, Kindy MS, Blonder LX, & Avison MJ (2001). Differences in functional magnetic resonance imaging activation by category in a visual confrontation naming task Journal of Neuroimaging, 11(2), 165–170. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Thompson JC, & Neary D (2004). Knowledge of famous faces and names in semantic dementia. Brain, 127, 860–872. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Thompson JC, & Neary D (2012). Famous people knowledge and the right and left temporal lobes. Behavioral Neurology, 25(1), 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer DD, & Spencer SS (1985). Surgery for epilepsy. Neurologic Clinics, 3(2), 313–330. [PubMed] [Google Scholar]
- Strauss E, Wada J, & Goldwater B (1992). Sex differences in interhemispheric reorganization of speech. Neuropsychologia, 30(4), 353–359. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP, Hamer L, Palumbo C, Dempster R, Binns M, Levine B, & Izukawa D (1998). The effects of focal anterior and posterior brain lesions on verbal fluency. Journal of the International Neuropsychological Society, 4, 265–278. [PubMed] [Google Scholar]
- Suchy Y, Sands K, Chelune GJ (2003). Verbal and nonverbal fluency performance before and after seizure surgery. Journal of Clinical and Experimental Neuropsychology, 25(2), 190–200. [DOI] [PubMed] [Google Scholar]
- Sylvester C-YC, & Shimamura AP (2002). Evidence for intact semantic representations in patients with frontal lobe lesions. Neuropsychology, 16(2), 197–207. [PubMed] [Google Scholar]
- Szaflarski JP, Gloss D, Binder JR, Gaillard WD, Golby AJ, Holland SK, … Theodore WH (2017). Practice guideline summary: Use of fMRI in the presurgical evaluation of patients with epilepsy. Neurology, 88(4), 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szubko-Sitarek W (2015). Modelling the lexicon: Some general considerations. Berlin: Springer Berlin Heidelberg. [Google Scholar]
- Thomas C, Avidan G, Humphreys K, Jung K, Gao F, & Behrmann M (2009). Reduced structural connectivity in ventral visual cortex in congenital prosopagnosia. Nature and Neuroscience, 12, 29–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel D (2006). Impaired naming of unique landmarks is associated with left temporal polar damage. Neuropsychology, 20(1), 1–10. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Eichhorn GR, Grabowski T, Ponto LLB, & Hichwa RD (2003). Neural correlates of naming animals from their characteristic sounds. Neuropsychologia, 41(7), 847–854. [DOI] [PubMed] [Google Scholar]
- Trebuchon A, & Chauvel P (2016). Electrical stimulation for seizure induction and functional mapping in stereoelectroencephalography. Journal of Clinical Neurophysiology, 33(6), 511–521. [DOI] [PubMed] [Google Scholar]
- Trebuchon-Da Fonseca A, Benar CG, Bartolomei F, Regis J, Demonet JF, Chauvel P, & Liegeois-Chauvel C (2009). Electrophysiological study of the basal temporal language area: A convergence between language perception and production networks. Clinical Neurophysiology, 120, 539–550. [DOI] [PubMed] [Google Scholar]
- Troster AI, Warmflash V, Osorio I, Paolo AM, Alexander LJ, & Barr WB (1995). The roles of semantic networks and search efficiency in verbal fluency performance in intractable temporal lobe epilepsy. Epilepsy Research, 21(1), 19–26. [DOI] [PubMed] [Google Scholar]
- Tsao DY, Freiwald WA, Knutsen TA, Mandeville JB, & Tootell RB (2003). Faces and objects in macaque cerebral cortex. Nature and Neuroscience, 6, 989–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler L, Stamatakis E, Bright P, Acres K, Abdallah S, Rodd J, & Moss H (2004). Processing objects at different levels of specificity. Journal of Cognitive Neuroscience, 16, 351–362. [DOI] [PubMed] [Google Scholar]
- Ueno T, Saito S, Rogers TT, & Lambon Ralph MA (2011). Lichtheim 2: Synthesizing aphasia and the neural basis of language in a neurocomputational model of the dual dorsal-ventral language pathways. Neuron, 72, 385–396. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, & Haxby JV (1994). ‘What’ and ‘where’ in the human brain. Current Opinion in Neurobiology, 4(2), 157–165. [DOI] [PubMed] [Google Scholar]
- Usui K, Ikeda A, Takayama M, Matsuhashi M, Yamamoto J-I, Satoh T, … Shibasaki H (2003). Conversion of semantic information into phonological representation: a function in left posterior basal temoral area. Brain, 126, 632–641. [DOI] [PubMed] [Google Scholar]
- Vanasse CM, Beland R, Carmant L, & Lassonde M (2005). Impact of childhood epilepsy on reading and phonological processing abilities. Epilepsy and Behavior, 7(2), 288–296. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Jobard G, Petit L, Crivello F, … Tzourio-Mazoyer N (2011). What is right-hemisphere contribution to phonological, lexico-sematic, and sentence processing?: Insights from a meta-analysis. Neuroimage, 54(1), 577–593. [DOI] [PubMed] [Google Scholar]
- Visser M, Embleton KV, Jefferies E, Parker GJ, & Lambon Ralph MA (2010). The inferior, anterior temporal lobes and semantic memory clarified: Novel evidence from distortion-corrected fMRI. Neuropsychologia, 48(1689–1696). [DOI] [PubMed] [Google Scholar]
- Visser M, Jefferies E, & Lambon Ralph MA (2009). Semantic processing in the anterior temporal lobes: A meta-analysis of the functional neuroimaging literature. Journal of Cognitive Neuroscience, 22, 1083–1094. [DOI] [PubMed] [Google Scholar]
- Visser M, & Lambon Ralph MA (2011). Differential contributions of bilateral ventral anterior temporal lobe and left anterior superior temporal gyrus to semantic processes. Journal of Cognitive Neuroscience, 23(10), 3121–3131. [DOI] [PubMed] [Google Scholar]
- Wada J, & Rasmussen T (1960). Intracarotid injection of sodium amytal for the lateralization of cerebral speech dominance: Experimental and clinical observations. Journal of Neurosurgery, 17, 266–282. [DOI] [PubMed] [Google Scholar]
- Wernicke C (1874). The aphasic symptom-complex. A psychological study on an anatomical basis (Translated from German). Breslau, Germany: Cohn and Weigert. [Google Scholar]
- Willie JT, Laxpati NG, Drane DL, Gowda A, Appin C, Hao C, … Gross RE (2014). Real-time magnetic resonance-guided stereotactic laser amygdalophippocampotomy (SLAH) for mesial temporal lobe epilepsy. Neurosurgery, 74(6), 569–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods RP, Dodrill CB, & Ojemann GA (1988). Brain injury, handedness, and speech lateralization in a series of amobarbital studies. Annals of Neurology, 23, 510–518. [DOI] [PubMed] [Google Scholar]
- Yagmurlu K, Middlebrooks EH, Tannover N, & Rhoton J, A. I. (2016). Fiber tracts of the dorsal language stream in the human brain. Journal of Neurosurgery, 124(5), 1396–1405. [DOI] [PubMed] [Google Scholar]
- Yucus C, & Tranel D (2007). Preserved proper naming following left anterior temporal lobectomy is associated with early age of seizure onset. Epilepsia, 48(12), 2241–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]