Abstract
The native extracellular matrix (ECM) contains a host of matricellular proteins and bioactive factors that regulate cell behavior, and many ECM components have been leveraged to guide cell fate. However, the large size and chemical characteristics of these constituents complicate their incorporation into biomaterials without interfering with material properties, motivating the need for alternative approaches to regulate cellular responses. Mesenchymal stromal cells (MSCs) can promote osseous regeneration in vivo directly or indirectly through multiple means including (1) secretion of proangiogenic and mitogenic factors to initiate formation of a vascular template and recruit host cells into the tissue site or (2) direct differentiation into osteoblasts. As MSC behavior is influenced by the properties of engineered hydrogels, we hypothesized that the biochemical and biophysical properties of alginate could be manipulated to promote the dual contributions of encapsulated MSCs toward bone formation. We functionalized alginate with QK peptide to enhance proangiogenic factor secretion and RGD to promote adhesion, while biomechanical-mediated osteogenic cues were controlled by modulating viscoelastic properties of the alginate substrate. A 1:1 ratio of QK:RGD resulted in the highest levels of both proangiogenic factor secretion and mineralization in vitro. Viscoelastic alginate outperformed purely elastic gels in both categories, and this effect was enhanced by stiffness up to 20 kPa. Furthermore, viscoelastic constructs promoted vessel infiltration and bone regeneration in a rat calvarial defect over 12 weeks. These data suggest that modulating viscoelastic properties of biomaterials, in conjunction with dual peptide functionalization, can simultaneously enhance multiple aspects of MSC regenerative potential and improve neovascularization of engineered tissues.
Keywords: stress relaxation, alginate, angiogenesis, osteogenesis, mesenchymal stromal cells
INTRODUCTION
Marrow-derived mesenchymal stromal cells (MSCs) were first characterized for multipotency[1] with subsequent studies establishing a preference for osteogenic differentiation in vitro[2–4]. As a result, MSCs used in tissue engineering have historically been instructed to differentiate toward bone-forming osteoblasts. Based on limited evidence of transplanted MSCs differentiating to osteoblasts in situ, MSCs are more recently presumed to promote regeneration indirectly through secretion of trophic factors, such as vascular endothelial growth factor (VEGF) [5, 6]. Strategies involving prolonged in vitro cultivation with sophisticated perfusion bioreactors result in excellent bone formation[7–9], but osteogenic progenitor cell populations cannot differentiate down an endothelial lineage. When applied for concomitant bone and vessel formation, numerous challenges have limited the potential efficacy of co-cultures of mesenchymal progenitors and endothelial cells, owing to incompatibilities in the soluble medium formulations. For example, both β-glycerophosphate and dexamethasone, widely used as osteogenic supplements, inhibit endothelial cell growth[10]. Thus, there is a critical need for successful approaches to harness the osteogenic potency of MSCs while promoting their ability to secrete angiogenic factors.
MSC-secreted VEGF declines as osteogenic differentiation progresses[11]. This suggests that a dual-instructive strategy is necessary to promote the simultaneous upregulation of proangiogenic factors with osteogenic differentiation of this heterogeneous population. Our group observed sustained osteogenic potential with increasing proangiogenic potential when osteogenically-induced MSCs engaged a complex, cell-secreted, decellularized extracellular matrix (ECM)[12]. We demonstrated that the ECM complexity is key for enhancing cell response, suggesting that multiple signaling pathways can be activated simultaneously in a single MSC population. This ECM could be used as a coating on implantable substrates[13–16], but limited opportunities exist for fabricating 3-dimensional materials derived from cell-secreted ECM for injection. The use of bioactive peptides offers an attractive alternative. Peptides are smaller and are thus more easily presented from a material, such as through covalent binding to alginate, without changing biomaterial properties. They also recapitulate many of the functions of matricellular proteins and offer improved stability[3, 17, 18].
The biophysical properties of alginate are highly tunable. Alginate’s modulus is readily controlled by molar mass or crosslinker concentration[3, 19], making it well-suited for leveraging substrate stiffness to modulate cell fate[3, 20]. Recent studies have focused on the viscoelastic properties of biomaterials, which affect cellular proliferation, differentiation, and migration[21, 22]. The extent of viscoelastic versus elastic properties can be controlled by crosslinker type: divalent ions such as calcium form reversibly breakable bonds and a primarily viscoelastic material, whereas covalent crosslinking, achieved in various manners such as carbodiimide chemistry, results in a purely elastic gel. These features give alginate another layer of tunability[21, 22], which may facilitate the multi-modal signaling required to instruct an MSC population to build vascularized bone.
Here we investigate engineered alginate with two distinct instructive peptides and controlled biophysical properties to guide a population of human MSCs to (1) increase VEGF production and (2) simultaneously differentiate down the osteogenic lineage. As a corollary, we hypothesized that modulating the biophysical properties of the hydrogel would also influence the effect of the biochemical functionalization, underscoring the advantage of a single, tunable biomaterial engineered for multi-modal cell instruction.
MATERIALS AND METHODS
Peptide selection and synthesis
We selected three candidate peptides from the literature and tested their ability to promote network formation (1) when applied directly on human microvascular endothelial cells (hMVECs; Fig. 1a) and (2) when applied on MSCs and the conditioned medium used to promote hMVEC network formation (Fig. 1b). We sought to capture varying methods by which angiogenesis could be promoted: GHK (full sequence: KKGHK) is derived from osteonectin and has a wide array of functions in wound healing[18, 23], QK (full sequence: KLTWQELYQLKYKGI) is a mimic of the VEGF receptor-binding region[23], and HepIII (full sequence: GEFYFDLRLKGDKYG) is a fragment of collagen IV, a component of the basement membrane that regulates invasion and formation of blood vessels[24].
Figure 1: Schematic of experimental workflow.
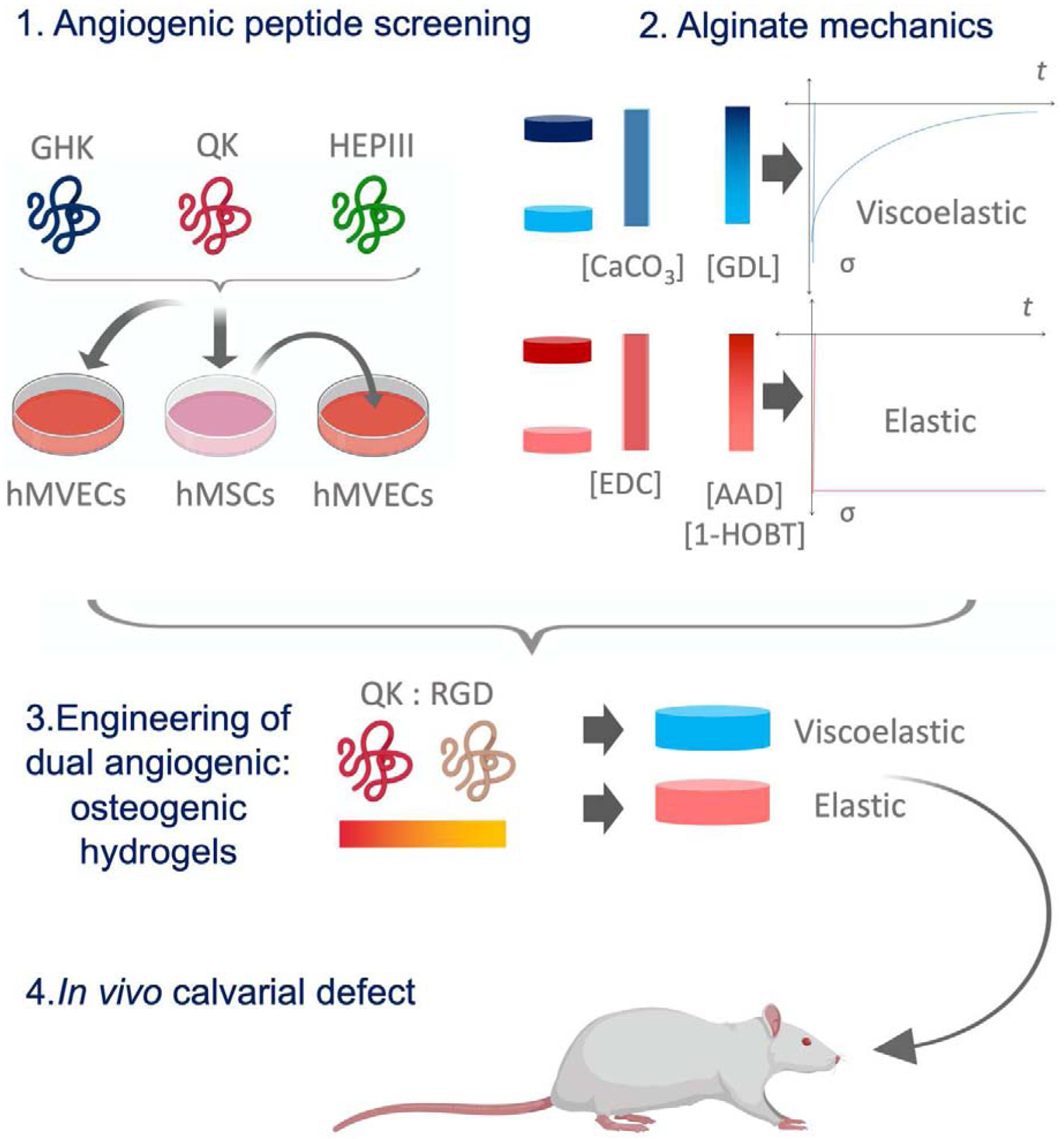
1) GHK, QK, and HepIII were applied in solution either directly to hMVECs or to MSCs to elicit production of conditioned medium, which was then applied to hMVECs. hMVEC network formation was assessed to select a peptide for further use. 2) In parallel, a library of viscoelastic or elastic alginate hydrogels were created with varying crosslinker concentrations to produce gels of varying moduli. 3) The effect of all combinations of peptide content, viscoelasticity, and stiffness on the ability of MSCs to promote hMVEC network formation and undergo osteogenic differentiation was assessed in a high-throughput experiment. 4) From this screen, one formulation was selected for in vivo studies.
Acetylated QK-amide (>95% purity) was custom synthesized by GenScript (Piscataway, NJ). Peptide-acids were synthesized on glycine-Wang resin using standard Fmoc solid phase peptide synthesis on a CEM Liberty Blue automated microwave peptide synthesizer.[23] Briefly, Fmoc-glycine-Wang resin was swollen with dimethylformamide (DMF), then deprotected with 20% piperidine in DMF for 1 min at 90°C before each amino acid coupling. Amino acids were coupled for 4 to 45 min each, depending on coupling optimization, at 90°C using 5 equivalents each of Fmoc-amino acids, N,N’-diisopropylcarbodiimide (DIC), and OxymaPure with 0.1 M diisopropylethylamine (DIPEA). Peptides were cleaved for 3 hrs with 88% trifluoroacetic acid (TFA), 5% phenol, 5% water, and 2% triisopropylsilane (TIPS) and precipitated with cold diethyl ether. Crude peptides were redissolved in 5% acetonitrile and purified to >90% purity through a C18 prep column against an acetonitrile gradient on an AKTApure 25 FPLC and confirmed by MALDI-TOF mass spectrometry.
Peptide screening
Synthesized peptides were dissolved at 30 μM in reduced growth factor endothelial growth medium (RGF), which consisted of EGM2-MV (Lonza) without VEGF, basic fibroblast growth factor (bFGF), and insulin-like growth factor (IGF). We confirmed previous observations[18] that omission of these three growth factors results in a medium that supports hMVEC viability but does not impart angiogenic signals (data not shown). hMVECs (Lonza, Walkersville, MD) were cultured on Matrigel (Corning, Corning, NY) in the RGF-peptide cocktail for 8 h prior to treatment with calcein (Invitrogen, Carlsbad, CA) for visualization of networks and fluorescent imaging. Fluorescent images were analyzed using AngioQuant[25] using threshold 15–255 and prune factor 25.
To assess the indirect effects of the peptides, we dissolved peptides at 30 μM in MSC expansion medium consisting of Dulbecco’s Modified Eagle Medium (DMEM) with 4.5 g/L glucose (Thermo Fisher, Waltham, MA) supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin (Cellgro, Manassas, VA), 10% v/v fetal bovine serum (FBS, Atlanta Biologicals, Flowery Branch, GA), and 2 mM L-glutamine (Thermo Fisher Scientific). Human MSCs (RoosterBio, Frederick, MD) were received at passage 2, and their trilineage potential was characterized by induction in lineage-specific media. MSCs at passage 4–5 were cultured in monolayer for 1 week in expansion medium with peptide. Medium with peptide was refreshed once during the culture duration[10]. At the end of the week, conditioned medium was collected and applied to hMVECs at a 1:3 ratio of conditioned medium to RGF. Culture, imaging, and analysis followed the same procedure as described above.
Peptide functionalization to alginate
We covalently modified VLVG alginate (50 kg/mol, G:M ratio > 1.5; Pronova, Sandvika, Norway) with QK, control QK peptide (CTRL QK; KVKFMDVYQRSYCHP; Genscript)[26], RGD (GGGGRGDSP; Peptide 2.0, Chantilly, VA), or RDG (GCRGYGRDGSPG; Genscript) as a scrambled peptide using carbodiimide chemistry as we reported[3, 18, 27] with a degree of substitution (DS) of 10. Peptide conjugation was confirmed by NMR and quantified using a ninhydrin assay[3]. We created alginate solutions of varying QK:RGD content by mixing solutions of QK-alginate and RGD-alginate in different volume ratios. The alginate was dissolved at 25 mg/mL in either 2-(N-morpholino)ethanesulfonic acid (MES) buffer or phosphate buffered saline (PBS). The two buffers were used to accommodate the different crosslinking methods as detailed below. Unmodified alginate served as a negative control.
Formulation of viscoelastic and elastic alginate hydrogels
Viscoelastic gels were formed following a modified internal calcium carbonate gelation protocol[28]. Alginate in PBS was mixed with (1) CaCO3 at 50 mg/mL and (2) glucono-δ-lactone (GDL; Sigma Aldrich, St. Louis, MO) at 150–200 mg/mL with a volume ratio of 8:1:1. The variation in GDL content controlled the amount of calcium ions liberated for crosslinking via lowering the pH, thereby varying the stiffness of the resulting gel. The mixture was pipetted into molds of 8 mm diameter and 1.5 mm height and allowed to gel at 37°C for 3 h.
Elastic gels underwent carbodiimide covalent crosslinking as described previously[21]. Alginate in MES buffer was mixed with (1) 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC; Sigma Aldrich) at 100 mg/mL and (2) a solution of adipic acid dihydrazide (AAD) and 1-hydroxybenzotriazole (1-HOBT; Sigma Aldrich), both at 25–75 mM. The mixture had a volume ratio of 8:1:1 alginate:EDC:AAD/1-HOBT. We controlled the number of crosslinks and resultant stiffness of the gel by variation in AAD content. The mixture was pipetted into identical molds as for viscoelastic hydrogels and allowed to gel under the same conditions. In both cases, the final concentration of alginate within the gels was 20 mg/mL.
Mechanical testing
Acellular and cell-containing gels were subjected to unconfined compression using an Instron 3345 (Instron, Norwood, MA) tester fitted with a 10 N load cell. For compressive stiffness, we applied a compressive deformation of 75 μm/s (5%/s) and determined the slope of the linear portion of the stress-strain curve. For stress relaxation tests, the ramp down was applied for 2 s and the deformation held constant for an additional 58 s. The stress versus time data from 2 s to 58 s was fit to a Maxwell stress relaxation model (Eqn. (1)):
| (1) |
where σ is normal stress along the axis of the gel disk, σ0 is the initial stress due to the deformation by 2 s, t is time, τ is the time constant of relaxation, and σe is the stress at equilibrium by the end of the test. The time constant τ and the percentage of stress relaxed (f, Eqn. (2)) were used to characterize the stress relaxation behavior of the gel.
| (2) |
Analysis of proangiogenic and osteogenic potential of MSCs entrapped in dual peptide alginate
We conducted a high-throughput study to test the ability of alginate gels of varying QK-RGD content, stiffness, and viscoelasticity to promote VEGF secretion and osteogenesis by entrapped MSCs. MSCs were encapsulated in all combinations of alginate formulations (Table 1) at 107 cells/mL, resulting in 48 groups with n = 3 per group. Constructs were cultured under expansion conditions for 1 week, when the conditioned medium was collected and applied to hMVECs on Matrigel. Network length, size, and number of junctions were quantified via AngioQuant and normalized to results from non-functionalized gels. Viability and metabolic activity of cells entrapped in viscoelastic and elastic gels were monitored by live/dead and alamarBlue assays, respectively.
Table 1:
List of all hydrogel compositions used in this study.
| QK:RGD ratio | Crosslinker concentration | Gelation mode |
|---|---|---|
| 0:1 | 150 mg/mL GDL or 25 mM AAD/1-HOBT | Ionic (CaCO3/GDL) |
| 1:3 | ||
| 167 mg/mL GDL or 42 mM AAD/1-HOBT | ||
| 1:1 | ||
| 3:1 | 183 mg/mL GDL or 58 mM AAD/1-HOBT | Covalent (AAD/1-HOBT/EDC) |
| 1:0 | ||
| 200 mg/mL GDL or 75 mM AAD/1-HOBT | ||
| Untreated |
We continued culture of the constructs in expansion medium supplemented with β-glycerophosphate (Sigma Aldrich) at 2 mM, a concentration that provides the MSCs with a phosphate source for mineralization but is insufficient for either dystrophic mineralization or a full osteoinductive signal[29]. This ensured that the biomaterial’s effect on mineralization would be more readily apparent. At the end of the culture period, constructs were digested in passive lysis buffer for calcium and DNA quantification. Results from non-functionalized gels were subtracted from all other results to account for any signal due to the calcium used for crosslinking as opposed to biomineralization. The formulation balancing the highest levels of calcium per cell with the most robust hMVEC network formation response was chosen for subsequent studies.
Loss-of-function studies
In light of QK-mediated angiogenic potential, we probed the mechanism of QK signaling on MSCs. QK is designed to bind VEGF receptors, which MSCs do not express[30], yet VEGF can signal MSCs through platelet-derived growth factor (PDGF) receptors (PDGFRs)[31]. We conducted a loss-of-function study designed to inhibit three nodes in the PDGF signaling pathway. Anti-PDGFRβ antibody (25 μg/mL, R&D Systems, Minneapolis, MN; inhibits the entire pathway), wortmannin (5 ng/mL, Sigma Aldrich; inhibits phosphoinositide 3-kinase (PI3K)), and Ruxolitinib (5 nM, Sigma Aldrich; inhibits Janus kinases (JAK) 1 and 2) were applied to viscoelastic constructs containing 1:1 QK-RGD and 107 cells/mL for 1 week. An untreated elastic group served as a negative control. We quantified VEGF in the conditioned media via enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems) and normalized VEGF concentrations to total cell number using the Quant-iT PicoGreen dsDNA Assay Kit (Thermo Fisher).
Rat calvarial defect studies
Treatment of experimental animals was in accordance with UC Davis animal care guidelines and all National Institutes of Health animal handling procedures. Viscoelastic and elastic hydrogels were equilibrated in growth medium overnight prior to implantation into a rat bilateral calvarial defect as previously described[32, 33]. Briefly, male 12-week-old nude rats (Taconic Biosciences, Germantown, NY) were anesthetized (3.0%) and maintained (1.5%) under an isoflurane/O2 mixture delivered through a nose cone at 6 L/min. As this study was designed to test the dual potential of MSCs, only male rats were used due to inferior vascularization observed with MSC-loaded constructs when implanted into female rats.[8] A mid-longitudinal 15 mm skin incision was made on the dorsal surface of the cranium. The periosteum was completely cleared from the surface of the cranial bone by scraping. A trephine bur was used to create one circular 3.5 mm diameter defect in the rat cranium on each side of the sagittal suture, and the full thickness (~1 mm) of the cranial bone was removed. 20 kPa viscoelastic and elastic gels functionalized with 1:1 QK:RGD and containing 107 cells/mL were generated with a final diameter of 3.5 mm and placed directly into the osteotomy site. Empty defects served as a negative control. Animals were euthanized at 2- or 12-weeks post-implantation, and calvariae were harvested, fixed in 4% w/v formalin and kept in 70% v/v ethanol until further processing.
Blood flow was measured on anesthetized animals using a Periscan PIM 3 laser Doppler perfusion imager (LDPI; Perimed, Stockholm, Sweden). The hair covering the surgical site was removed the day before scanning, and the skin was cleaned using alcohol wipes immediately prior to data acquisition. Perfusion measurements were obtained from a circular region of interest superimposed over the defect.
Microcomputed tomography (microCT) scans were performed using a high-resolution microCT specimen scanner (mCT 35; Scanco Medical, Brüttisellen, Switzerland) with a 70 kVp X-ray source at 114 μA and 300 ms integration time. Quantification was performed by setting a threshold of 256–3000 mg HA per cm3 to discriminate between mineralized and unmineralized tissue. After thresholding, the image noise was reduced using a low pass Gaussian filter (σ = 0.8, support = 1). Reconstructed 3D images were generated from the scans and used to visualize mineral distribution throughout the constructs. Bone volume fraction (BV/TV) was determined by dividing the number of pixels representing bone tissue (BV: bone volume) by the number of pixels in the cylindrical segment (TV: total volume).
After microCT analysis, the samples were demineralized in Calci-clear (National Diagnostics, Atlanta, GA) for 7 days, dehydrated in a graded series of ethanol baths, embedded in paraffin wax, sectioned at 10 μm, and affixed to microscope slides. The sections were stained with H&E and Masson’s trichrome to assess bone formation. To assess osteogenic differentiation and vascular invasion, sections were stained with a primary antibody against osteocalcin (1:1000, AB13420, Abcam, Cambridge, MA) and von Willebrand factor (vWF) (1:200, AB6994, Abcam). The presence of vascular structures was quantified by counting distinct areas of vWF staining by two blinded reviewers.
Statistical analysis
Data are presented as means ± standard deviation unless otherwise stated. Except in Fig. 4, statistical analysis utilized a one-way ANOVA with post-hoc Tukey test. p<0.05 was considered significant. In Fig. 4, we employed a three-way ANOVA with post-hoc Tukey test. In each graph, data points with different letters are significantly different from one another.
Figure 4: Viscoelastic gels with 1:1 QK:RGD content and high stiffness promote the most indirect angiogenesis and direct osteogenesis.
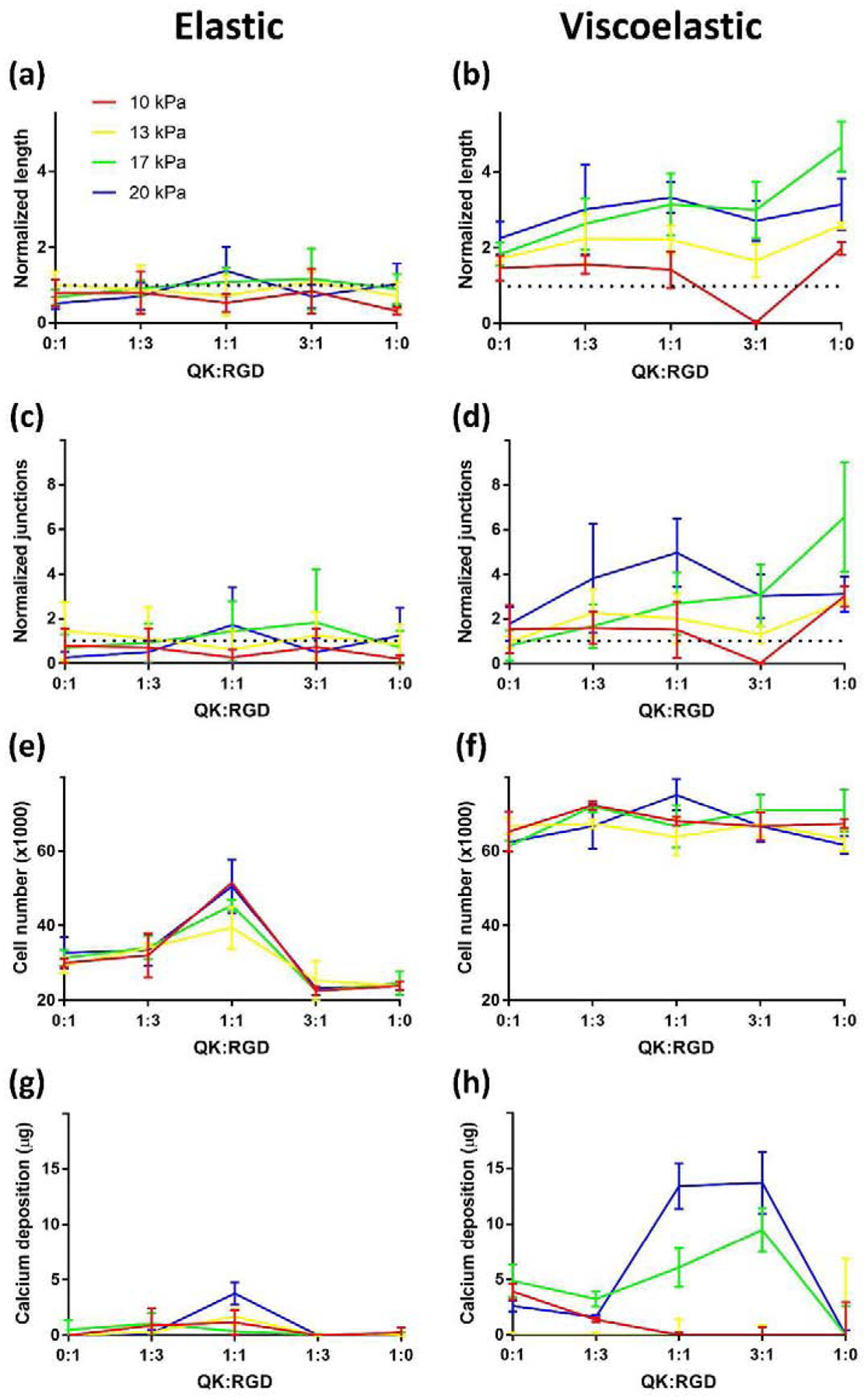
MSCs cultured in alginate gels of varying QK:RGD content (x-axes), stiffness (colored lines), and viscoelasticity (left versus right) exhibited differences in ability of conditioned medium to elicit hMVEC network formation, proliferation, and calcium deposition. Viscoelastic gels outperformed elastic gels in all outputs tested, with 17–20 kPa gels containing 1:1 or greater QK:RGD achieving the highest indirect network formation and direct proliferation/total calcium content (n=3). Representative images used to generate these data are shown in Supplementary Fig. S1b.
RESULTS
Peptide screen
When stimulating cells directly with peptides in the media, hMVECs exhibited greater average network length when exposed to GHK and QK (Fig. 2, left). In contrast, MSCs treated with QK and HepIII produced conditioned media that elicited the highest average hMVEC network length and size (Fig. 2, right). We detected no significant difference in the mean number of junctions within the hMVEC networks regardless of peptide or mode of stimulation (Supplementary Fig. 1a). Due to its ability to promote network formation in both direct and indirect modes, we selected QK for all subsequent studies.
Figure 2: Analysis of peptide stimulation of hMVEC network formation when applied directly to cells or when generating MSC conditioned medium for indirect hMVEC stimulation.
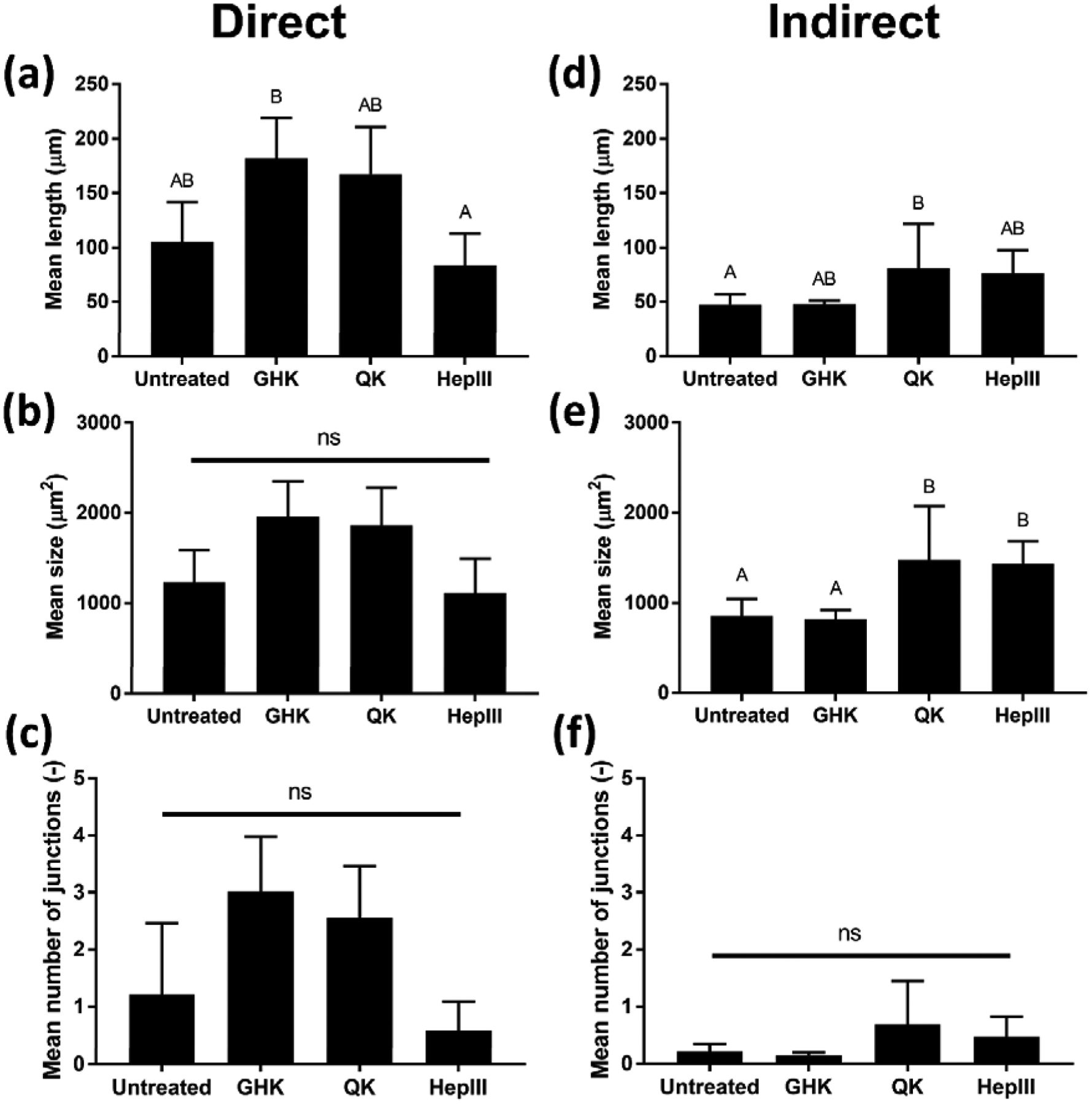
When applied directly to hMVECs, both QK and GHK achieved significant increases in network length compared to HepIII, which performed similarly to the negative control (a). When conditioned media from stimulated MSCs was applied to hMVECs (indirect), QK and HepIII outperformed GHK in hMVEC network length and area (d–e). We did not detect significant differences in network branching in either mode (c, f). Representative images used to generate these data are shown in Supplementary Fig. S1a. Due to superior performance in both direct and indirect modes of action (n=4), QK was chosen for all subsequent studies. Data points labeled with different letters are significantly different from one another at p<0.05.
Alginate mechanics
The different modes of crosslinking had a dramatic effect on the stress relaxation characteristics of the resulting gel (Fig. 3a). Viscoelastic gels produced by ionic crosslinking exhibited ~60% relaxation of the initial stress (Fig. 3d). Aside from an initial slight decrease in stress, which we attributed to an observable initial decrease in strain due to the transition between ramp-down and hold, elastic gels formed by covalent crosslinking exhibited constant stress at consistent strain. We could influence the average compressive modulus of hydrogels by changing the concentration of AAD/1-HOBT for elastic gels or GDL for viscoelastic gels. Specifically, 25–75 mM AAD/1-HOBT and 150–200 mg/mL GDL resulted in gels of comparable stiffness, possessing moduli of 10–20 kPa (Fig. 3b–c). Storage moduli between elastic and viscoelastic gels were similar as evidenced by a frequency sweep from 0.05–10 Hz (Fig. 3f), but strain energy dissipation only occurred in viscoelastic gels (Fig. 3g). Cell-laden viscoelastic gels (14.4 ± 1.0 s, n=3) exhibited a slight decrease in stress relaxation time compared to acellular viscoelastic gels (17.9 ± 1.7 s, n=4; p=0.023), suggesting that cells interfere with the crosslinking process. Interestingly, GDL concentration had no effect on the relaxation time constant of viscoelastic gels (Fig. 3e). We observed comparable efficiencies of peptide modification, yielding polymers with a degree of substitution of approximately 10 for both peptides.
Figure 3: Development of a mechanical library of alginate hydrogels.
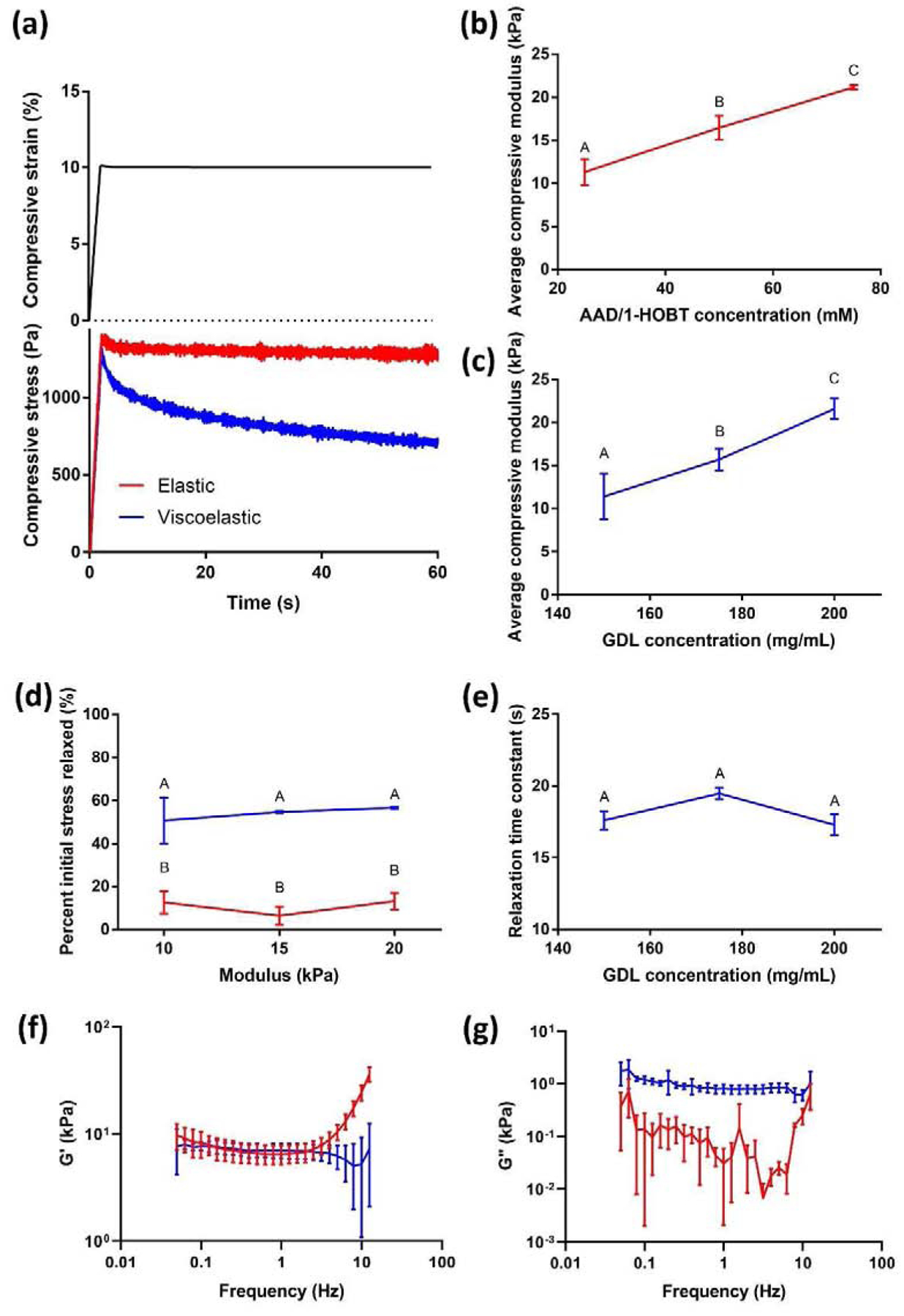
Representative strain and stress curves versus time of AAD/HOBT-crosslinked elastic gels (red) or GDL-crosslinked viscoelastic gels (blue) (a). Changing the concentration of either AAD and 1-HOBT for elastic gels (b) or GDL for viscoelastic gels (c) resulted in similar stiffness between 10 and 20 kPa. Ionically crosslinked gels exhibited significant stress relaxation (viscoelastic behavior), while the stress remained relatively constant for covalently crosslinked gels (d) consistent with elastic material behavior. The time constant of relaxation in the viscoelastic gels did not change with crosslinker concentration (e), (n=3). A frequency sweep of elastic and viscoelastic gels resulted in similar storage moduli (f), but a loss modulus was only detected in viscoelastic gels (g). Data points labeled with different letters are significantly different from one another at p<0.05.
Gel screen
We used Matrigel network formation induced by conditioned media, MSC proliferation, and MSC calcium deposition as outputs in the high-throughput experiment to determine the effect of relative peptide concentration and mechanics on MSC promotion of angiogenesis and MSC osteogenesis. In general, viscoelastic gels exhibited superior results compared to elastic gels in all outputs assessed (Fig. 4). MSCs in elastic gels, regardless of stiffness or peptide composition, did not produce conditioned medium significantly more angiogenic than non-functionalized controls. In contrast, MSCs in viscoelastic gels had noticeable differences, with increasing stiffness and QK-RGD ratio corresponding with increased network length and number of junctions (Supplementary Fig. 1b). Mean network area followed the same trend as did network length (data not shown).
At the end of the three-week culture, MSCs in elastic gels exhibited the greatest cell number at the 1:1 QK:RGD ratio (Fig. 4e). In contrast, we did not detect differences in MSC number within viscoelastic gels as a function of peptide ratio, yet MSC number was greater than in elastic gels. MSCs in viscoelastic gels also produced more calcium compared to cells in elastic gels except at (1) low stiffnesses and (2) high QK-RGD ratios. At 1:1 and 3:1 QK:RGD, both 17 and 20 kPa ionic gels induced markedly increased calcification. The 1:1 QK:RGD, 20 kPa, viscoelastic gels were the most significantly different from the other groups (Supplementary Table S1). Compared to viscoelastic gels, we observed a reduction in cell viability and metabolic activity after 1 day in elastic gels. However, DNA content was similar after 2 weeks in culture, suggesting that the cytotoxicity of chemicals used to crosslink covalent gels was transient. While 100% QK gels performed better in the angiogenesis assays, their lack of calcium deposition prompted the selection of 1:1 QK:RGD, 20 kPa for all subsequent studies.
To confirm that the observed effects on MSC behavior in the 1:1 QK:RGD 20 kPa gels were due to the specific peptides presented from the alginate backbone, we assessed the effects of unmodified and control peptide-functionalized elastic and viscoelastic hydrogels. The RDG peptide was chosen as scrambled peptide to RGD, while a control QK (CTRL QK) peptide, corresponding to the unmodified 14–28 region of QK which lacks secondary structure required for receptor interaction and does not bind to VEGFR[26], served as control for the QK peptide. 20 kPa elastic and viscoelastic unmodified alginate gels and peptide-functionalized gels containing a 1:1 ratio of either CTRL QK:RDG, CTRL QK:RGD, QK:RDG or QK:RGD were cultured in vitro for 7 days, when the conditioned media was collected and used to stimulate the network formation of HMVECs (Supplementary Fig. 2). Conditioned media from QK:RDG and QK:RGD viscoelastic gels induced greater HMVEC network formation, evidenced by endothelial networks with increased mean length (Supplementary Fig. 2a–b), size (Supplementary Fig. 2c–d) and number of junctions (Supplementary Fig. 2e–f). After collection of the conditioned media, hydrogels were further cultured to induce osteogenic differentiation of encapsulated MSCs. We observed increased calcium deposition in viscoelastic QK:RGD- and CTRL QK-RGD-functionalized gels compared to all other groups (Supplementary Fig. 3a–b), and these data were supported by histological analysis of calcium deposition (Supplementary Fig. 3c). H&E staining revealed a more spindle-like cellular morphology of cells in viscoelastic RGD-modified gels (Supplementary Fig. 3d), confirming the interaction of the encapsulated MSCs with the RGD peptide on the alginate backbone. In contrast, MSCs in RDG-modified gels and the covalent gels exhibited a rounded morphology.
Loss-of-function mechanistic studies
MSCs encapsulated in 1:1 QK:RGD, 20 kPa viscoelastic gels exhibited significant differences in both cell number and VEGF secretion per cell when exposed to various inhibitors of the PDGFRβ signaling cascade (Fig. 5a). Inhibition of the PDGF receptor and JAK1/2 had the greatest effect, increasing cell number and decreasing VEGF secretion (Fig. 5b, c). Notably, inhibition of the receptor decreased VEGF levels below those from elastic gels with the same peptide content and stiffness. Inhibition of PI3K had a smaller, but still significant, inhibitory effect on VEGF secretion.
Figure 5: QK exerts its effects through the PDGF receptor.
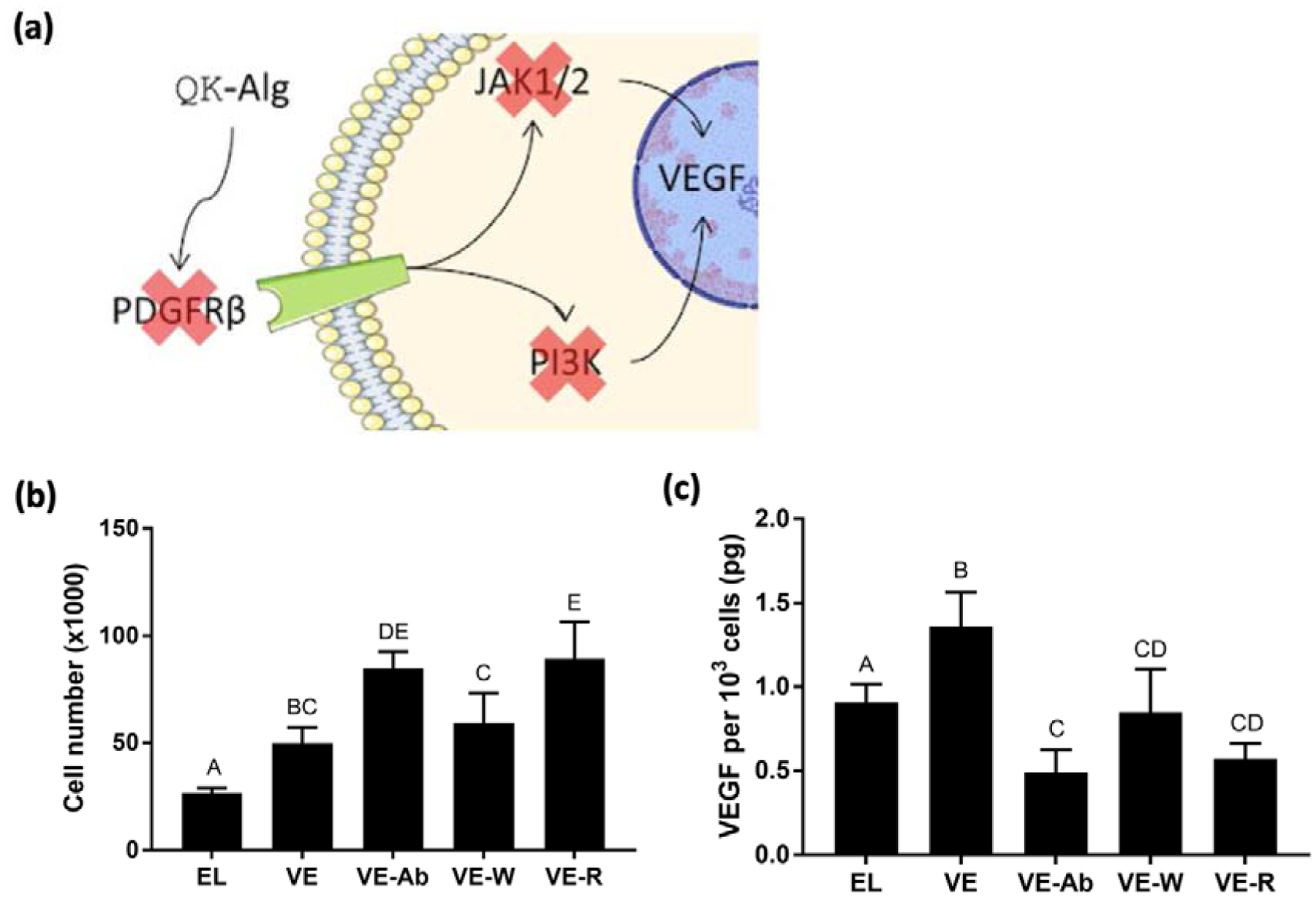
(a) Schematic of mechanistic studies to interrogate QK signaling pathways. Anti-PDGFRβ (inhibits PDGFRβ binding), wortmannin (inhibits PI3K), and Ruxolitinib (inhibits JAK1/2) were applied to MSCs in 20 kPa ionic gels with 1:1 QK:RGD (VE-Ab, VE-W, and VE-R, respectively) for 7 days. Untreated elastic (EL) and viscoelastic (VE) gels with the same stiffness and peptide content served as controls. (b) Cell number within 1:1 QK:RGD gels (n=4). (c) Quantification of VEGF secretion by entrapped MSCs in 20 kPa viscoelastic gels with 1:1 QK:RGD in presence of inhibitors (n=4). Data points labeled with different letters are significantly different from one another at p<0.05.
Angiogenesis and osteogenesis in vivo
Two weeks after implantation, we observed cell infiltration and vascularization in defects treated with MSC-containing QK:RGD elastic and viscoelastic hydrogels through macroscopic examination, H&E, and vWF staining (Fig. 6a–c). We quantified greater blood vessel density in defects treated with MSC-containing gels compared to the empty control (p=0.032 for elastic gels and p=0.019 for viscoelastic gels, respectively). H&E staining revealed the presence of alginate in both experimental groups (Fig. 6b), with elastic gels exhibiting a significantly thicker tissue inside the defect (Fig. 6f) compared to the empty control (p=0.042), suggesting faster alginate degradation in viscoelastic gels. The osteogenic potential of the implants was confirmed by osteocalcin immunostaining. Compared to elastic gels that exhibited limited cell infiltration and new tissue formation, we detected more intense osteocalcin staining in defects treated with viscoelastic gels, specifically located in the non-degraded alginate deposits (Fig. 6d). Perfusion within the defect site was noninvasively evaluated using laser Doppler perfusion imaging (Fig. 6g–h). Although treated defects exhibited similar perfusion through 2 weeks after implantation, in agreement with histological characterization of vascularization, perfusion within defects treated with viscoelastic gels continued to increase until week 4, while defects treated with elastic gel peaked at week 3 and began to decline.
Figure 6. Analysis of early vascularization and osteogenic potential in QK:RGD functionalized elastic and viscoelastic hydrogels after 2 weeks of in vivo implantation.
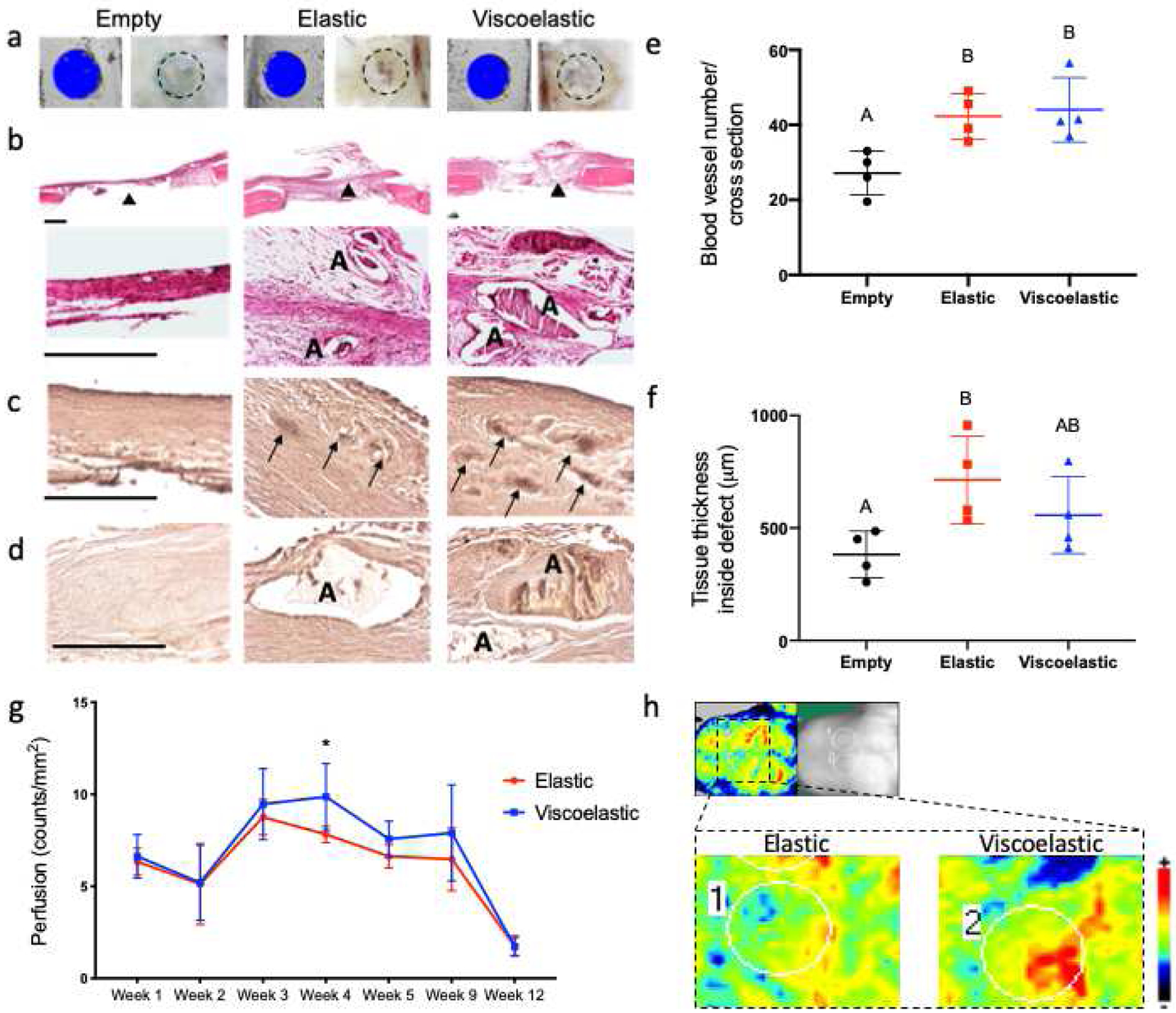
(a) microCT and macroscopic images of the control empty defects or defects filled with either viscoelastic or elastic hydrogels. Histologic sections stained with (b) H&E, (c) von Willebrand factor and (d) osteocalcin. Quantification of (e) blood vessel density (p=0.019 between the empty and the viscoelastic groups, p=0.032 between the empty and the elastic group and p=0.933 between the elastic and the viscoelastic groups) and (f) tissue thickness inside the defect (p=0.32 between the empty and the viscoelastic groups, p=0.042 between the empty and the elastic group and p=0.396 between the elastic and the viscoelastic groups) (n=4). (g) Quantification of blood perfusion at the defect area from laser Doppler perfusion imaging (LDPI). Statistical significance of p<0.05 between the groups at the same time point is denoted by one asterisk (n=5). (h) LDPI representative images of the covalent and ionic implantation areas at week 4. Black triangles denote the area where the 20x magnification picture was taken, “A” denotes the presence of residual alginate and the black arrows denote positively stained areas of blood vessel activity. Scale bars represent 500 μm for B and 250 μm for C and D.
MicroCT analysis of the defect area (Fig. 7a–b) revealed significant increases in bone volume fraction (p=0.022) (Fig. 7f) and average mineral density (p=0.0162) (Fig. 7g) in defects treated with viscoelastic gels compared to empty controls. Defects treated with elastic gels exhibited higher indices of bone formation than empty controls, on average, yet they were not significantly different (p=0.273 for bone volume fraction; p=0.2043 for average mineral density). H&E and Masson’s trichrome staining also demonstrated areas of new bone formation in defects treated with both elastic and viscoelastic gels (Fig. 7c–d), with osteoid regions rich in disorganized collagen apparent. We also observed the presence of alginate in defects treated with elastic gels but not viscoelastic gels (Fig.7c–d), suggesting slower degradation of elastic gels. We detected increased osteocalcin immunostaining within viscoelastic gels compared to elastic gels (Fig. 7e).
Figure 7. Analysis of bone formation in QK:RGD functionalized elastic and viscoelastic hydrogels after 12 weeks.
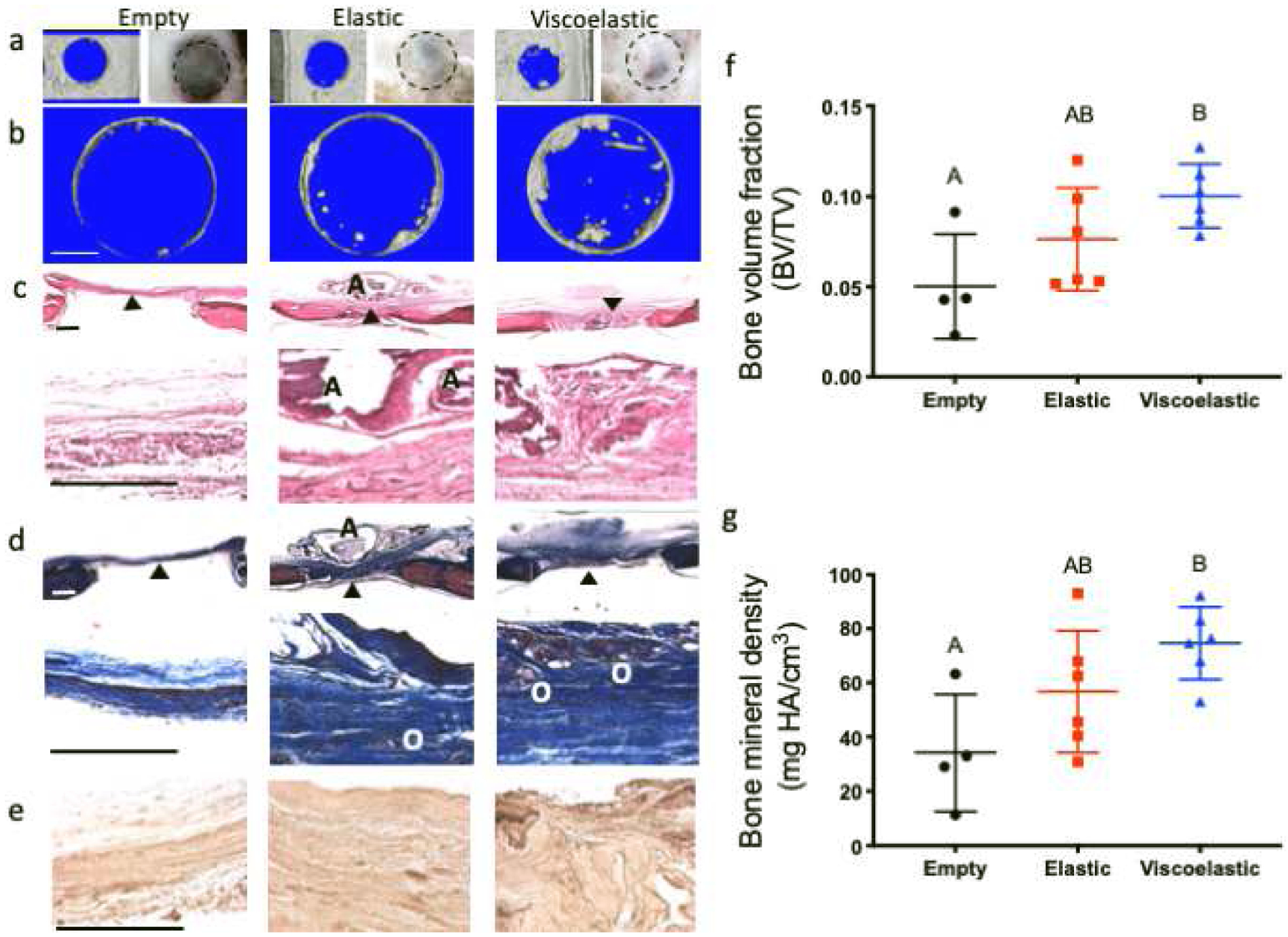
(a) microCT and macroscopic images of the control empty defects or defects transplanted with either elastic or viscoelastic hydrogels. (b) Area of microCT analysis to determine bone volume and mineral density in the defects. Histologic sections stained with (c) H&E, (d) Masson’s trichrome and (e) osteocalcin. Quantification of (f) bone volume fraction (p=0.022 between the empty and the viscoelastic groups, p=0.273 between the empty and the elastic group and p=0.261 between the elastic and the viscoelastic groups) and (g) average bone mineral density (p=0.016 between the empty and the viscoelastic groups, p=0.204 between the empty and the elastic group and p=0.276 between the elastic and the viscoelastic groups) (n=4 for the empty control, n=6 for the elastic and viscoelastic groups). Black triangles denote the area where the 20x magnification picture was taken, “A” denotes the presence of residual alginate and “O” denotes areas of osteoid formation. Scale bars represent 1 mm for (b), 500 μm for (c) and (d), and 250 μm for (e).
DISCUSSION
When used in a cell-based therapy, MSCs are commonly delivered by direct injection with no supporting biomaterial or differentiation-instructive signals, relying mostly on their regenerative secretome rather than on differentiation. In this study, we capitalized on the high tunability of alginate to direct two different modes of MSC action – paracrine promotion of angiogenesis via trophic factor secretion and direct osteogenic differentiation. A key finding in this work is the confirmation of our sub-hypothesis: variation in biophysical properties modulates the effect of peptide functionalization in vitro and in vivo. Both elastic and viscoelastic gels contained the same peptides at the same concentrations, yet MSCs were more responsive to these peptides when presented in viscoelastic gels as evidenced by the dramatic increases in both angiogenic and osteogenic potential in vitro and improved bone formation in vivo. As such, this study uniquely harnesses the synergy between biochemical and biomechanical functionalization of alginate to instruct MSCs.
As the method of peptide functionalization utilized here – carbodiimide chemistry – does not depend on the peptide itself, we conducted an initial screen to identify the best peptide for our objective. We examined the responsiveness of cells in monolayer to free peptides for simplicity, as our goal was to screen peptides rather than investigate their action in detail. While our previous work on GHK-modified hydrogels successfully signaled MSCs to upregulate VEGF production[18], the free GHK peptide was ineffective in indirectly stimulating angiogenesis in these studies. The discrepancy in presentation between substrate-bound and free peptides may account for this, as peptides bound to a substrate may exert enhanced effects[34], representing a potential limitation of our study. GHK may still be a viable candidate for indirect angiogenesis when substrate-bound, but its bioactivity appeared blunted in the free form. Interestingly, our data also showed that HepIII was more effective in indirect than direct angiogenesis. Regardless, QK performed well in both direct and indirect assays, supporting its use in the subsequent studies investigating direct and indirect stimulation of angiogenesis.
Although the focus of this study was on the ability of the peptide to promote angiogenesis indirectly by promoting trophic factor secretion by MSCs, we also assessed the direct effect of the peptides on hMVECs to explore a potential secondary function on resident endothelial cells migrating into the construct post-implantation. From this perspective, the observed angiogenic efficacy of QK on hMVECs was expected and supported by literature, as QK mimics the receptor-binding region of VEGF[23, 35]. While MSCs do not express VEGF receptors[30], they can respond to VEGF via activation of PDGFRs[31]. This upregulates VEGF secretion[36–38] via NF-κB translocation into the nucleus through the PI3k-Akt pathway[39] or STAT3 nuclear translocation via JAK1 activation[40]. Taken together, we hypothesized that QK was acting on MSCs by upregulating VEGF production via one or both of these pathways downstream of PDGFR activation in our system. We specifically focused on neutralizing PDGFRβ due to its higher affinity for PDGF-BB, a factor well-established to have an important role in mediating MSC support of angiogenesis[41]. As a result, QK could still influence MSCs via dimerized PDGFRα in our loss-of-function study. Despite this, antibody neutralization of PDGFRβ resulted in dramatic decreases in VEGF secretion per cell. We observed a similar drop with inhibition of the JAK-STAT pathway but not with inhibition of the PI3K pathway. Interestingly, in the groups with decreased VEGF production, proliferation was greatly increased. This suggests that QK may be exerting the mitogenic effects of PDGFR activation primarily through PDGFRα and proangiogenic effects, such as increased VEGF production, through PDGFRβ. As a detailed mechanistic study is outside the scope of the current study, these data will inform future studies on the biochemical events within MSCs when exposed to QK and on the secretome of MSCs in response to QK.
While we used QK to promote the proangiogenic function of MSCs, we used hydrogel mechanics to drive their osteogenic differentiation. The chemicals used to covalently crosslink the elastic gels have been reported as cytotoxic[21, 42]. Indeed, we observed a reduction in cell viability after 24 hours. However, DNA content was comparable across all groups after 2 weeks in culture, suggesting that the detrimental effect of these chemical was transient. This finding suggests alternative methods to prepare elastic gels are merited to further characterize the effect of gel mechanics on cell differentiation stimulated by multiple peptides. In this study, MSCs were never exposed to full osteogenic medium, and thus, they were dependent upon the material to differentiate. MSCs in elastic gels exhibited little to no osteogenic differentiation, even in groups with high concentrations of RGD and high stiffness, two factors that generally enhance osteogenesis[17, 20]. In contrast, MSCs in viscoelastic gels attained markedly increased calcification in higher stiffness gels. Interestingly, we observed low calcium content in 100% RGD gels. A 1:1 or 3:1 QK:RGD ratio promoted the most mineralization. This is unlikely due to any osteogenic effect of QK for a variety of reasons: (1) gels with 100% QK exhibited no osteogenesis regardless of stiffness; (2) gels with increased calcium content generally also showed increased proliferation, suggesting that MSCs were not being driven down the osteogenic lineage on a per-cell basis; and (3) PDGF signaling does not enhance MSC osteogenesis[43]. It is more likely that a viscoelastic substrate, coupled with sufficient RGD ligands for cell adhesion, along with the mitogenic effects of QK-PDGFR signaling, enhanced MSC proliferation and thereby increased total mineral content.
We characterized the stiffness of the viscoelastic gels in a manner similar to our characterization of elastic gels by using the slope of the stress-strain curve resulting from a linear ramp-down. This is a simplification of viscoelastic materials, which exhibit strain-rate dependency. Though this method has been used before to quantify stiffness of viscoelastic gels[22], it represents a limitation of the current work, resulting in difficulties comparing the defined 10–20 kPa elastic gels versus the viscoelastic gels. Nevertheless, characterization of storage moduli by frequency sweep rheometry revealed similar storage of strain energy between elastic and viscoelastic gels, supporting the comparison used in this study. A more detailed study focusing on the strain rates applied by resident cells in both elastic and viscoelastic substrates would better define the mechanical microenvironment experienced by the cells themselves, allowing for a more relevant quantification of modulus. In this work, the ability of viscoelastic gels to outperform elastic gels is clear, as is the enhancement of proangiogenic and osteogenic functions at higher stiffnesses in viscoelastic gels. Both of these observations support the initial hypothesis of the study.
The viscoelastic and elastic gels performed differently in vivo with respect to both angiogenesis and osteogenesis. Initial increases in vessel infiltration 2 weeks after implantation were similar in defects treated with MSC-containing gels but significantly higher than untreated defects. In addition, LDPI analysis of in vivo vascularization revealed similar perfusion levels between both groups until week 3, with vascularization continuing to increase in defects treated with only the viscoelastic gels. These data suggest the viscoelastic gels enhanced the proangiogenic activity of entrapped MSCs compared to their elastic counterparts despite identical peptide content, which was in agreement with our in vitro results. Furthermore, after 2 weeks of implantation, we observed more intense osteocalcin staining localized in the viscoelastic alginate deposits, suggesting a direct contribution of the encapsulated MSCs on de novo bone formation. At week 12, viscoelastic gels produced more mineralized tissue per unit volume, an observation supported by visible differences in staining for both collagenous matrix and osteocalcin. While stress relaxation was the main difference between the groups studied here, we also observed differences in the relative rate of degradation. We detected no residual alginate in defects treated with viscoelastic gels, yet defects treated with elastic gels contained remnants of the material at 12 weeks. This suggests that faster degradation of viscoelastic gels may represent an additional mechanism for increased angiogenesis and osteogenesis in vivo, thereby facilitating tissue infiltration and growth. These data are in agreement with other studies reporting that alginate gels undergoing faster stress relaxation resulted in accelerated bone formation, cell infiltration, extensive matrix remodeling, and hydrogel disappearance within a rat calvarial defect compared to slow-relaxing hydrogels[44]. Similarly, bone formation was markedly increased when MSCs were transplanted in alginate that had been oxidized to enable hydrolysis compared to non-oxidized gels.[45] The design of these studies prevents our determination of whether bone formation was due to degradation of the hydrogel or viscoelasticity, representing a limitation of these studies and an area of future investigation.
Importantly, the MSCs implanted into the rat calvarial defects were not preconditioned for any lineage. The orthotopic site contains osteoinductive factors, but these were insufficient for noticeable healing as evidenced by the low mineral and vessel content in the empty controls. The higher levels of regeneration achieved by the viscoelastic constructs by virtue of material properties alone is notable in this regard and reveals new possibilities for combination with other clinically relevant pro-regenerative strategies such as cellular aggregation into spheroids[46], lineage-specific preconditioning[3], or hypoxic preconditioning[47].
Supplementary Material
Supplementary Figure S1: Representative images of hMVEC networks visualized by calcein staining 8 hours after stimulation with peptide (a) or MSC conditioned medium (b). Calculated values from AngioQuant are listed below each image.
Supplementary Figure S2: Proangiogenic potential of conditioned media from MSCs cultured in elastic or viscoelastic unmodified and control peptide-modified alginate hydrogels. Mean network length (μm) of hMVEC cultured in conditioned media from elastic (a) or viscoelastic gels (b). Mean size (μm2) of hMVEC networks cultured in conditioned media from elastic (c) or viscoelastic gels (d). Mean number of junctions of hMVEC networks cultured in conditioned media from elastic (e) or viscoelastic gels (f). (g) Fluorescence imaging of hMVEC networks formed when cultured in conditioned media from MSCs encapsulated in ionic and covalent gels with different peptide combinations. Scale bar = 500 μm. n=3 per group. Data points labeled with different letters are significantly different from one another at p<0.05.
Supplementary Figure S3: Osteogenic differentiation of MSCs encapsulated in elastic or viscoelastic unmodified and control peptide-modified alginate hydrogels. Calcium content normalized to wet weight (calcium/ww) of elastic (a) and covalent (b) hydrogels. Representative Alizarin red (c) and H&E staining (b) to reveal calcium deposition and cell morphology, respectively. Images are at 40x magnification. Black arrows denote presence of elongated cells. Scale bar = 100 μm. n=5 per group. Data points labeled with different letters are significantly different from one another at p<0.05.
Supplementary Table S1: Number of output measures that are statistically significant among hydrogels that promote angiogenesis and osteogenesis as a function of peptide ratio and stiffness via 3-way ANOVA, with p<0.05 considered significant.
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institute of Dental and Craniofacial Research of the National Institutes of Health under award number R01 DE025899 to JKL. TGF was supported by an American Heart Association Postdoctoral Fellowship (19POST34460034). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in the decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DATA AVAILABILITY STATEMENT
The raw/processed data required to reproduce these findings will be made available on request.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- [1].Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR, Multilineage potential of adult human mesenchymal stem cells, Science 284 (1999) 143–147. [DOI] [PubMed] [Google Scholar]
- [2].Noel D, Caton D, Roche S, Bony C, Lehmann S, Casteilla L, Jorgensen C, Cousin B, Cell specific differences between human adipose-derived and mesenchymal-stromal cells despite similar differentiation potentials, Experimental Cell Research 314 (2008) 1575–1584. [DOI] [PubMed] [Google Scholar]
- [3].Hung BP, Harvestine JN, Saiz AM, Gonzalez-Fernandez T, Sahar DE, Weiss ML, Leach JK, Defining hydrogel properties to instruct lineage- and cell-specific mesenchymal differentiation, Biomaterials 189 (2019) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sakaguchi Y, Sekiya I, Yagishita K, Muneta T, Comparison of human stem cells derived from various mesenchymal tissues: Superiority of synovium as a cell source, Arthritis Rheum 52 (2005) 2521–2529. [DOI] [PubMed] [Google Scholar]
- [5].Caplan AI, Dennis JE, Mesenchymal stem cells as trophic mediators, J Cell Biochem 98 (2006) 1076–1084. [DOI] [PubMed] [Google Scholar]
- [6].Meirelles Lda S, Fontes AM, Covas DT, Caplan AI, Mechanisms involved in the therapeutic properties of mesenchymal stem cells, Cytokine Growth Factor Rev 20 (2009) 419–427. [DOI] [PubMed] [Google Scholar]
- [7].Bhumiratana S, Bernhard JC, Alfi DM, Yeager K, Eton RE, Bova J, Shah F, Gimble JM, Lopez MJ, Eisig SB, Vunjak-Novakovic G, Tissue-engineered autologous grafts for facial bone reconstruction, Sci Transl Med 8 (2016) 343ra83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mitra D, Whitehead J, Yasui OW, Leach JK, Bioreactor culture duration of engineered constructs influences bone formation by mesenchymal stem cells, Biomaterials 146 (2017) 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Harvestine JN, Gonzalez-Fernandez T, Sebastian A, Hum NR, Genetos DC, Loots GG, Leach JK, Osteogenic preconditioning in perfusion bioreactors improves vascularization and bone formation by human bone marrow aspirates, Sci Adv 6(7) (2020) eaay2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hutton DL, Moore EM, Gimble JM, Grayson WL, Platelet-derived growth factor and spatiotemporal cues induce development of vascularized bone tissue by adipose-derived stem cells, Tissue Eng Part A 19 (2013) 2076–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hoch AI, Binder BY, Genetos DC, Leach JK, Differentiation-dependent secretion of proangiogenic factors by mesenchymal stem cells, PLoS One 7(4) (2012) e35579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hoch AI, Mittal V, Mitra D, Vollmer N, Zikry CA, Leach JK, Cell-secreted matrices perpetuate the bone-forming phenotype of differentiated mesenchymal stem cells, Biomaterials 74 (2016) 178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bhat A, Hoch AI, Decaris ML, Leach JK, Alginate hydrogels containing cell-interactive beads for bone formation, FASEB J 27(12) (2013) 4844–4852. [DOI] [PubMed] [Google Scholar]
- [14].Decaris ML, Binder BY, Soicher MA, Bhat A, Leach JK, Cell-derived matrix coatings for polymeric scaffolds, Tissue Eng Part A 18(19–20) (2012) 2148–2157. [DOI] [PubMed] [Google Scholar]
- [15].Decaris ML, Mojadedi A, Bhat A, Leach JK, Transferable cell-secreted extracellular matrices enhance osteogenic differentiation, Acta Biomater 8(2) (2012) 744–752. [DOI] [PubMed] [Google Scholar]
- [16].Harvestine JN, Vollmer NL, Ho SS, Zikry CA, Lee MA, Leach JK, Extracellular matrix-coated composite scaffolds promote mesenchymal stem cell persistence and osteogenesis, Biomacromolecules 17(11) (2016) 3524–3531. [DOI] [PubMed] [Google Scholar]
- [17].Ho SS, Keown AT, Addison B, Leach JK, Cell migration and bone formation from mesenchymal stem cell spheroids in alginate hydrogels are regulated by adhesive ligand density, Biomacromolecules 18(12) (2017) 4331–4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jose S, Hughbanks ML, Binder BYK, Ingavle GC, Leach JK, Enhanced trophic factor secretion by mesenchymal stem/stromal cells with Glycine-Histidine-Lysine (GHK)-modified alginate hydrogels, Acta Biomater 10(5) (2014) 1955–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lee KY, Mooney DJ, Alginate: properties and biomedical applications, Prog Polym Sci 37 (2012) 106–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, Rivera-Feliciano J, Mooney DJ, Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate, Nat Mater 9 (2010) 518–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bauer A, Gu L, Kwee B, Li WA, Dellacherie M, Celiz AD, Mooney DJ, Hydrogel substrate stress-relaxation regulates the spreading and proliferation of mouse myoblasts, Acta Biomaterialia 62 (2017) 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC, Huebsch N, Lee HP, Lippens E, Duda GN, Mooney DJ, Hydrogels with tunable stress relaxation regulate stem cell fate and activity, Nat Mater 15 (2016) 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Van Hove AH, MJ GB, Benoit DS, Development and in vitro assessment of enzymatically-responsive poly(ethylene glycol) hydrogels for the delivery of therapeutic peptides, Biomaterials 35 (2014) 9719–9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mihardja SS, Gao D, Sievers RE, Fang Q, Feng J, Wang J, Vanbrocklin HF, Larrick JW, Huang M, Dae M, Lee RJ, Targeted in vivo extracellular matrix formation promotes neovascularization in a rodent model of myocardial infarction, PLoS ONE 5 (2010) e10384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Niemistö A, Dunmire V, Yli-Harja O, Zhang W, Shmulevich I, Robust quantification of in vitro angiogenesis through image analysis, IEEE Transactions on Medical Imaging 24 (2005) 549–553. [DOI] [PubMed] [Google Scholar]
- [26].Santulli G, Ciccarelli M, Palumbo G, Campanile A, Galasso G, Ziaco B, Altobelli GG, Cimini V, Piscione F, D’Andrea LD, Pedone C, Trimarco B, Iaccarino G, In vivo properties of the proangiogenic peptide QK, J Transl Med 7 (2009) 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ho SS, Murphy KC, Binder BYK, Vissers CB, Leach JK, Increased survival and function of mesenchymal stem cell spheroids entrapped in instructive alginate hydrogels, Stem Cells Transl Med 5(6) (2016) 773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Madrigal JL, Stilhano RS, Siltanen C, Tanaka K, Rezvani SN, Morgan RP, Revzin A, Han SW, Silva EA, Microfluidic generation of alginate microgels for the controlled delivery of lentivectors, J Mater Chem B 4 (2016) 6989–6999. [DOI] [PubMed] [Google Scholar]
- [29].Langenbach F, Handschel J, Effects of dexamethasone, ascorbic acid and beta-glycerophosphate on the osteogenic differentiation of stem cells in vitro, Stem Cell Res Ther 4 (2013) 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Delorme B, Ringe J, Gallay N, Vern YL, Kerboeuf D, Jorgensen C, Rosset P, Sensebé L, Layrolle P, Häpupl T, Charbord P, Specific plasma membrane protein phenotype of culture-amplified and native human bone marrow mesenchymal stem cells, Blood 111 (2008) 2631–2635. [DOI] [PubMed] [Google Scholar]
- [31].Ball SG, Shuttleworth CA, Kielty CM, Vascular endothelial growth factor can signal through platelet-derived growth factor receptors, J Cell Biol 177 (2007) 489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cheung WK, Working DM, Galuppo LD, Leach JK, Osteogenic comparison of expanded and uncultured adipose stromal cells, Cytotherapy 12(4) (2010) 554–562. [DOI] [PubMed] [Google Scholar]
- [33].Murphy KC, Hughbanks ML, Binder BYK, Vissers CB, Leach JK, Engineered fibrin gels for parallel stimulation of mesenchymal stem cell proangiogenic and osteogenic potential, Ann Biomed Eng 43(8) (2015) 2010–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Leslie-Barbick JE, Saik JE, Gould DJ, Dickinson ME, West JL, The promotion of microvasculature formation in poly(ethylene glycol) diacrylate hydrogels by an immobilized VEGF-mimetic peptide, Biomaterials 32 (2011) 5782–5789. [DOI] [PubMed] [Google Scholar]
- [35].Finetti F, Basile A, Capasso D, Di Gaetano S, Di Stasi R, Pascale M, Turco CM, Ziche M, Morbidelli L, D’Andrea LD, Functional and pharmacological characterization of a VEGF mimetic peptide on reparative angiogenesis, Biochem Pharmacol 84 (2012) 303–311. [DOI] [PubMed] [Google Scholar]
- [36].Caplan AI, Correa D, PDGF in bone formation and regeneration: new insights into a novel mechanism involving MSCs, J Orthop Res 29 (2011) 1795–1803. [DOI] [PubMed] [Google Scholar]
- [37].Hollinger JO, Hart CE, Hirsch SN, Lynch S, Friedlaender GE, Recombinant human platelet-derived growth factor: biology and clinical applications, J Bone Joint Surg Am 90 Suppl 1 (2008) 48–54. [DOI] [PubMed] [Google Scholar]
- [38].Sato N, Beitz JG, Kato J, Yamamoto M, Clark JW, Calabresi P, Raymond A, Frackelton a.R., Platelet-derived growth factor indirectly stimulates angiogenesis in vitro, Am J Pathol 142 (1993) 1119–1130. [PMC free article] [PubMed] [Google Scholar]
- [39].Karar J, Maity A, PI3K/AKT/mTOR pathway in angiogenesis, Front Mol Neurosci 4 (2011) 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Cheranov SY, Karpurapu M, Wang D, Zhang B, Venema RC, Rao GN, An essential role for SRC-activated STAT-3 in 14, 15-EET-induced VEGF expression and angiogenesis, Blood 111 (2008) 5581–5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lindahl P, Hellstrom M, Kalen M, Betsholtz C, Endothelial-perivascular cell signaling in vascular development: lessons from knockout mice, Curr Opin Lipidol 9 (1998) 407–411. [DOI] [PubMed] [Google Scholar]
- [42].Su WY, Chen YC, Lin FH, Injectable oxidized hyaluronic acid/adipic acid dihydrazide hydrogel for nucleus pulposus regeneration, Acta Biomater 6(8) (2010) 3044–55. [DOI] [PubMed] [Google Scholar]
- [43].Hung BP, Hutton DL, Kozielski KL, Bishop CJ, Naved BA, Green JJ, Caplan AI, Gimble JM, Dorafshar AH, Grayson WL, Platelet-derived growth factor BB enhances osteogenesis of adipose-derived but not bone marrow-derived mesenchymal stromal/stem cells, Stem Cells 33 (2015) 2773–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Darnell M, Young S, Gu L, Shah N, Lippens E, Weaver J, Duda G, Mooney D, Substrate stress-relaxation regulates scaffold remodeling and bone formation in vivo, Adv Healthc Mater 6 (2017) 1601185–1601192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kong HJ, Kaigler D, Kim K, Mooney DJ, Controlling rigidity and degradation of alginate hydrogels via molecular weight distribution, Biomacromolecules 5 (2004) 1720–1727. [DOI] [PubMed] [Google Scholar]
- [46].Murphy KC, Fang SY, Leach JK, Human mesenchymal stem cell spheroids in fibrin hydrogels exhibit improved cell survival and potential for bone healing, Cell Tissue Res 357(1) (2014) 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ho SS, Hung BP, Heyrani N, Lee MA, Leach JK, Hypoxic preconditioning of mesenchymal stem cells with subsequent spheroid formation accelerates repair of segmental bone defects, Stem Cells 36(9) (2018) 1393–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1: Representative images of hMVEC networks visualized by calcein staining 8 hours after stimulation with peptide (a) or MSC conditioned medium (b). Calculated values from AngioQuant are listed below each image.
Supplementary Figure S2: Proangiogenic potential of conditioned media from MSCs cultured in elastic or viscoelastic unmodified and control peptide-modified alginate hydrogels. Mean network length (μm) of hMVEC cultured in conditioned media from elastic (a) or viscoelastic gels (b). Mean size (μm2) of hMVEC networks cultured in conditioned media from elastic (c) or viscoelastic gels (d). Mean number of junctions of hMVEC networks cultured in conditioned media from elastic (e) or viscoelastic gels (f). (g) Fluorescence imaging of hMVEC networks formed when cultured in conditioned media from MSCs encapsulated in ionic and covalent gels with different peptide combinations. Scale bar = 500 μm. n=3 per group. Data points labeled with different letters are significantly different from one another at p<0.05.
Supplementary Figure S3: Osteogenic differentiation of MSCs encapsulated in elastic or viscoelastic unmodified and control peptide-modified alginate hydrogels. Calcium content normalized to wet weight (calcium/ww) of elastic (a) and covalent (b) hydrogels. Representative Alizarin red (c) and H&E staining (b) to reveal calcium deposition and cell morphology, respectively. Images are at 40x magnification. Black arrows denote presence of elongated cells. Scale bar = 100 μm. n=5 per group. Data points labeled with different letters are significantly different from one another at p<0.05.
Supplementary Table S1: Number of output measures that are statistically significant among hydrogels that promote angiogenesis and osteogenesis as a function of peptide ratio and stiffness via 3-way ANOVA, with p<0.05 considered significant.


