Summary
Background
Monitoring HIV treatment with laboratory testing introduces delays for providing appropriate care in resource-limited settings. The aim of our study was to determine whether point-of-care HIV viral load testing with task shifting changed treatment and care outcomes for adults on antiretroviral therapy (ART) when compared with standard laboratory viral load testing.
Methods
We did an open-label, non-inferiority, randomised controlled trial in a public clinic in Durban, South Africa. We enrolled HIV-positive adults (aged ≥18 years) who presented for their first routine HIV viral load test 6 months after ART initiation. Individuals were randomly assigned by a random number allocation sequence to receive either point-of-care viral load testing at enrolment and after 6 months with task shifting to enrolled nurses (intervention group), or laboratory viral load testing (standard-of-care group). The primary outcome was combined viral suppression (<200 copies per mL) and retention at 12 months after enrolment. A non-inferiority margin of 10% was used. Analysis was done by intention to treat. This study was registered with ClinicalTrials.gov, NCT03066128.
Findings
Between Feb 24, 2017, and Aug 23, 2017, we screened 657 participants, and 390 were enrolled and randomly assigned to either the intervention group (n=195) or standard-of-care group (n=195). 175 (90%) individuals in the intervention group and 148 (76%) individuals in the standard-of-care group had the primary outcome of retention with viral suppression, a difference of 13·9% (95% CI 6·4–21·2; p<0·00040). 182 participants (93%) in the intervention group had viral suppression compared with 162 (83%) in the standard-of-care group (difference 10·3%, 3·9–16·8; p=0·0025); 180 (92%) and 162 (85%) were retained in care (7·7%, 1·3–14·2; p=0·026). There were no adverse events related to point-of-care HIV viral load testing or task shifting.
Interpretation
Point-of-care viral load testing combined with task shifting significantly improved viral suppression and retention in HIV care. Point-of-care testing can simplify treatment and improve outcomes for HIV-positive adults receiving ART in resource-limited settings.
Funding
National Institute of Allergy and Infectious Diseases.
Introduction
Over half of the 37 million people living with HIV are accessing antiretroviral therapy (ART), which has substantially reduced AIDS-related deaths worldwide.1 The goal of ART is to maintain viral suppression, which restores immunological function, reduces transmission, and improves patient outcomes. UNAIDS has set an ambitious target for 90% of people receiving ART- to have viral suppression by 2020.2 Globally, less than half of all people living with HIV have viral suppression, and ART coverage and HIV treatment outcomes have large regional disparities, particularly in low-income and middle-income countries.1
WHO recommends monitoring ART effectiveness with routine HIV viral load testing.3 Patients with HIV viraemia should receive prompt adherence counselling and potential, whereas those with viral suppression might be candidates for differentiated care through task shifting to other health-care workers or community-based ART delivery programmes. These programmes aim to increase health-system efficiencies and improve patient care, and are facilitated by availability of viral load monitoring.4 WHO estimates the annual demand for viral load tests will increase from 10·7 million in 2016, to 28·5 million in 2021,5 but scaling up viral load testing has been limited by infrastructure and human resources.6–8 Even where laboratory viral load testing is available, such as in South Africa, some patients do not receive their results in a timely manner, which can lead to emergence of drug resistance, risk of ongoing transmission, and excess morbidity and mortality.9
Diagnostic point-of-care tests have rapidly emerged worldwide.10 The Xpert HIV-1 viral load assay (Cepheid, Sunnyvale, CA, US) requires 1 mL of plasma to provide a quantitative result (40–10 million copies per mL) in less than 2 h and has received European and WHO regulatory approvals.11,12 The assay is done on an automated molecular GeneXpert (Cepheid, Sunnyvale, CA USA) platform, which is widely used for the diagnosis of tuberculosis.12 We showed good accuracy of Xpert HIV-1 viral load when done in a South African clinic,13 but there have been no studies showing the effectiveness of point-of-care viral load testing to improve treatment outcomes. Furthermore, although ART is mainly provided by professional nurses in primary care clinics, there could be a role for task shifting of ART provision to health-care workers with less training, such as enrolled nurses
Therefore, we compared point-of-care Xpert HIV-1 viral load testing with same-day counselling and task shifting to enrolled nurses against standard-of-care laboratory viral load testing for achieving viral suppression and retention in care for HIV-positive adults receiving ART.
Methods
Study design and participants
We undertook the STREAM (Simplifying HIV TREAtment and Monitoring) study, a single site, open-label, two-arm, non-inferiority, randomised controlled trial. The study site was at the CAPRISA Ethekwini Clinical Research Site and the neighbouring Prince Cyril Zulu Clinic, which is a large urban public clinic that provides HIV and primary care services for a diverse and mobile population of more than 10 000 patients in Durban, South Africa. Details of the research protocol have been published previously.14
Eligible study participants were HIV-positive adults, aged 18 years or older, who presented for their first routine HIV viral load test 6 months after ART initiation. We enrolled participants best suited to receive differentiated HIV care by excluding those who were pregnant, had active tuberculosis, or required acute medical care by a physician. However, if participants became pregnant or were diagnosed with tuberculosis during follow-up, they were included in the final analysis.
All participants gave written informed consent. The study received ethical approval from the University of KwaZulu-Natal, Durban, South Africa (BFC296/16) and University of Washington, Seattle, WA, USA (STUDY00001466). This study is registered with ClinicalTrials.gov, NCT03066128.
Randomisation and masking
We randomly assigned participants (1:1) to receive either point-of-care viral load testing with same-day counselling and task shifted care by an enrolled nurse (registered nurse in the USA), or standard-of-care laboratory-based viral load testing and care by a professional nurse (nurse practitioner in the USA). The study statistician generated an allocation sequence with random numbers using SAS (version 9.4). Those who enrolled and allocated the participant were masked to the assignment.
Sequentially numbered, sealed, opaque envelopes containing a study group allocation and participant identification number were opened once an eligible, consenting participant was enrolled.
Procedures
At enrolment, we collected sociodemographic, medical information, and other potential risk factors for viral failure or loss to follow-up, which included the WHO Alcohol Use Disorder Identification Tool,15 WHO Violence Against Women instrument (female participants only),16 and Patient Health Questionnaire-2 to screen for depression.17 All participants- were followed up for 12 months and received care according to the South African HIV treatment guidelines, including testing for viral load and creatinine at enrolment, and viral load, creatinine, and CD4 cell count after 6 months.18 Any participant with a viral load of more than 1000 copies per mL received enhanced adherence counselling and repeat viral load testing after 2 months. If the repeat viral load was more than 1000 copies per mL, the participant was offered to switch to a standard second-line ART regimen. We did retrospective HIV genomic resistance testing using standard Sanger consensus sequencing at enrolment and at 12 months. Participants in both groups were reimbursed 100 South African rand per clinic visit, in accordance with South African research guidelines.19
In the intervention group, participants with viral suppression at enrolment and no comorbidities were seen by an enrolled nurse, rather than a professional nurse. In South Africa, enrolled nurses receive 2 years of training and are less highly qualified than professional nurses, who have 4 years of training and are the standard providers of HIV care. For this trial, enrolled nurses had received the standard South African Nurse Initiated and Managed ART training, which covers basic HIV management, ART side-effects, adherence counselling, and appropriate referral systems.20 At routine clinical visits, enrolled nurses noted vital signs, screened for tuberculosis, assessed adherence by asking individuals about missing one or more ART doses in past 4 days, and issued predispensed ART packages. If enrolled nurses detected clinical symptoms or abnormal vital signs, then the participant was referred up to a professional nurse for more comprehensive clinical management. All routine testing in the intervention group was done onsite using point-of-care Xpert HIV-1 viral load, Statensor Xpress-i (Nova Biomedical, Waltham, MA, USA) for creatinine, and Pima (Alere, Waltham, MA, USA) for CD4 cell count. Point-of-care viral load testing and associated clinical care was done by the research team.
Participants were asked to wait in the clinic for the point-of-care test results, and waiting times were minimised by initiating point-of-care testing at the start of the clinical visit. Therefore, the intention was to deliver results to the participants in the intervention group on the same day and during the same clinical visit.
In the standard-of-care group, participants were seen by a professional nurse, who had also received Nurse Initiated and Managed ART training. The nurse did the same procedures but sent all blood specimens to the routine National Health Laboratory Service for viral load testing using m2000 RealTime (Abbott, Chicago, IL, USA). In accordance with standard practice in the South African health-care system, participants were expected to receive their viral loads at their next clinical visit, which is scheduled at the clinician’s discretion and typically occurs after 4 weeks (28 days) or 8 weeks (56 days).
Based on the South African policy, the study clinic participated in a programme for clinically stable participants to collect ART at community pick-up points, such as private pharmacies and community-based organisations.21 Participants in both groups were eligible for referral into the programme if they were not pregnant, were clinically stable, both their enrolment and month 6 viral loads were less than 40 copies per mL, and CD4 count was more than 200 cells per µL. Once referred, participants collected ART every 2 months at community pick-up points and were reviewed at the clinic by a professional nurse after 6 months. All blood testing occurred at the clinic. Participants not eligible for community-based ART delivery continued with clinic visits every 2 months. Any participant more than 2 weeks late for a scheduled clinic or community pick-up point visit received one phone call from the clinical team and and was asked to resume ART collection at the clinic, per South African guidelines.14
Outcomes
The primary outcome was a composite measure of retention in care with viral suppression (<200 copies per mL) after 12 months. We used this composite because both components are necessary to have positive treatment outcomes for HIV-positive adults. Retained in care was defined as collecting ART at the study clinic or a community pick-up point between 44 weeks and 56 weeks after enrolment. Participants not retained in care at 56 weeks were tracked by the study team up to 60 weeks for viral load testing. All viral load testing for the primary outcome was done using a laboratory-based Cobas 6800/8800 machine (Roche, Basel, Switzerland).
A priori secondary outcomes detailed in the study protocol14 were viral suppression using thresholds of 1000 and 50 copies per mL, viral suppression and retention at any clinic, retention in care alone at any clinic, time to detection of virological failure and switch to second-line ART, and presence of HIV drug resistance mutations among participants with viraemia at study exit. We used laboratory and clinical records to assess the timing of viral load test results available in health-information systems, when test results were communicated to participants, and when participants were referred into the community-based ART delivery programme.
Statistical analysis
The study sample size was determined using a non-inferiority hypothesis for the intervention group compared with the control group, assuming that 80% of adults in the control group would be retained with viral suppression at 12 months. Using a non-inferiority margin of 10%, 390 participants were sufficient to provide 80% power to rule out a decrease of 10%, based on a one-sided 95% CI. If non-inferiority was successfully shown, we planned to assess superiority, as stated in the study protocol.14 We recorded all clinical data using standardised electronic case report forms in the iDataFax system (version 2014.1.1). All data entry underwent three stages of quality control, including immediate source document review, internal quality audits, and weekly quality reports.
The primary analysis was done in the intention-to-treat population enrolled in the study and compared the proportions who had the composite primary outcome by estimating the absolute risk difference between the intervention and control groups. The same population was used for all secondary outcomes, unless noted. For the outcomes assessing viral suppression among those who had a viral load done, we restricted the population to those with a viral load at study exit. Non-inferiority of the intervention group was defined as the one-sided 95% Newcombe-Wilson score CI for the difference in proportions to exclude a non-inferiority margin of 10%.22,23 Superiority was assessed with Fisher’s exact test, and two-sided 95% CIs were generated for the difference in proportions. We separately compared each component of the primary outcome by group, as well as secondary binary outcomes, using the same methods. For time-to-event outcomes, we compared groups using hazard ratios (HRs) generated from Cox proportional models, except when differences were so extreme that all events in the intervention group occurred before all events in the control group, resulting in perfect separation of events. In such cases, we could not compute a HR and report a log-rank test p value instead. We used Kaplan-Meier curves to estimate time to referral into the community-based ART delivery programme for each study group. We compared health-care utilisation variables using Fisher’s exact test and t tests when appropriate. We did all analyses using SAS (version 9.4). We did not have a data monitoring committee because no investigational product was used.
Role of the funding source
Cepheid loaned the GeneXpert instruments for this study at no cost. Cepheid and the funder of the study had no role in the study design, data collection, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
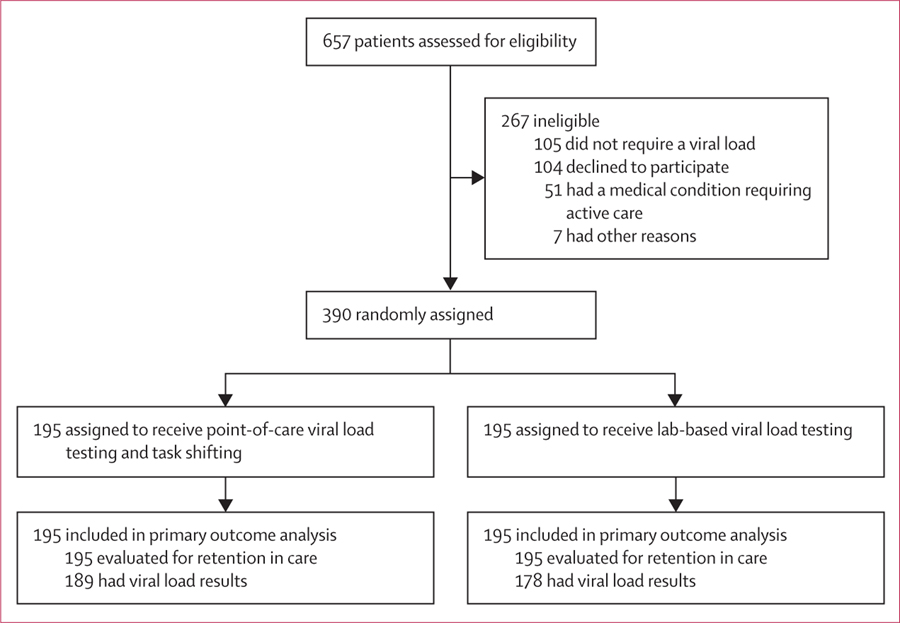
Between Feb 24, 2017, and Aug 23, 2017, we screened 657 patients, of whom 267 were ineligible or chose not to enroll, and 390 were enrolled, randomly assigned, and evaluated for the primary outcome (figure 1, appendix p 1). The median patient age was 32 years (IQR 27–38) and 235 (60%) were female (table 1). Most participants earned less than 4000 South African rand (approximately USD$280) per month, took public transportation to the clinic, and estimated their travel distance to be more than 5000 m and travel time less than 60 min.
Figure 1:
Trial profile
Table 1:
Baseline characteristics
| Intervention group (n=195) | Standard-of-care group (n=195) | |
|---|---|---|
| Median (IQR) age (years) | 31 (27–38) | 32 (27–38) |
| Sex | ||
| Female | 119 (61%) | 116 (60%) |
| Male | 76 (39%) | 79 (40%) |
| Ethnicity | ||
| Black | 193 (99%) | 193 (99%) |
| Other | 2 (1%) | 2 (1%) |
| Highest level of education | ||
| None or primary school | 10 (5%) | 18 (9%) |
| Did not pass secondary school | 90 (46%) | 75 (39%) |
| Passed secondary school | 64 (33%) | 69 (35%) |
| Tertiary education | 31 (16%) | 33 (17%) |
| Monthly income | ||
| R <1000 (~US$70) | 83 (43%) | 79 (41%) |
| R 1000–4000 (~$70–280) | 80 (41%) | 63 (32%) |
| R >4000 (~$280) | 29 (15%) | 46 (24%) |
| Did not disclose | 3 (2%) | 7 (4%) |
| Has a regular or stable partner | 155 (80%) | 156 (80%) |
| Children | ||
| None | 41 (21%) | 33 (17%) |
| ≥1 child | 154 (79%) | 162 (83%) |
| Primary method of travel to clinic | ||
| Walking | 15 (8%) | 16 (8%) |
| Public transportation | 173 (89%) | 172 (88%) |
| Private transportation | 7 (4%) | 7 (4%) |
| Distance travelled to clinic | ||
| ≤5000 m | 40 (21%) | 46 (24) |
| ≤60 min | 179 (92%) | 180 (92%) |
| Psychosocial and health behaviours | ||
| Positive depression screen* | 19 (10%) | 18 (9%) |
| Intimate partner violence in past year (women only) | 20/119 (17%) | 20/116 (17%) |
| AUDIT C score ≥4 (men only) | 33/76 (43%) | 24/79 (30%) |
| AUDIT C score ≥3 (women only) | 22/119 (19%) | 17/116 (15%) |
| Current smoker | 32 (16%) | 32 (16%) |
| Recreational drug use in past 6 months | 15 (8%) | 13 (7%) |
| Medical history, HIV care, and self-reported ART adherence | ||
| >12 months since HIV diagnosis | 62 (32%) | 66 (34%) |
| ART exposure before initiation of current regimen | 8 (4%) | 10 (5%) |
| Mean (SD) time from ART initiation to study enrolment (days) | 183·8 (17·3) | 183·1 (17·1) |
| ART regimen with tenofovir disoproxil fumarate, emtricitabine, and efavirenz | 192 (99%) | 195 (100%) |
| Received previous treatment for tuberculosis | 26 (13%) | 34 (17%) |
| Self-report of one or more doses missed in past 4 days | 43 (22%) | 31 (16%) |
| Clinical and laboratory parameters | ||
| WHO clinical stage | ||
| Stage I | 188 (96·4) | 193 (99·0) |
| Stage II | 7 (4%) | 2 (1%) |
| Mean (SD) BMI (kg/m²) | 25·6 (6·0) | 26·0 (6·0) |
| Mean (SD) haemoglobin (g/L) | 128·0 (17·0) | 130·0 (15·0) |
| Mean (SD) creatinine clearance (mL/min†) | 99·3 (29·0) | 100·0 (27·2) |
| Hepatitis B surface antigen positive | 12 (6·2) | 11 (5·6) |
| Median (IQR) CD4 count at ART initiation (cells per L) | 361 (171–546) | 371 (230–551) |
| Median (IQR) CD4 count at enrolment (cells per µL‡) | 462 (275–658) | 473 (340–680) |
| HIV viral load <200 copies per mL at enrolment§ | 179 (92%) | 184 (94%) |
| HIV viral load >1000 copies per mL at enrolment§ | 10 (5%) | 8 (4%) |
| Presence of any HIV drug resistance at enrolment | 7 (4%) | 7 (3%) |
Data are n (%), unless otherwise stated. AUDIT-C=Alcohol Use Disorder Identification Tool. ART=antiretroviral therapy. BMI=body-mass index. R=South African rand.
Defined as Patient Health Questionnaire-2 score of 2 or greater.
Missing five observations for creatinine clearance (intervention group).
Missing one observation for CD4 cell count at enrolment (standard-of-care group).
Viral loads at enrolment measured per protocol by study group.
128 (33%) of 390 participants had been diagnosed HIV positive at least 12 months earlier, and 18 (5%) reported receiving ART before initiation of the current regimen. The median CD4 cell count was 366 cells per µL (IQR 204–546) at ART initiation, and 468 cells per µL (309–666) at study enrolment. Of the 18 (5%) participants with unsuppressed viral load (>1000 copies per mL) at enrolment, 14 (78%) had evidence of any HIV drug resistance mutations. Sociodemographic, psychosocial, and clinical measures were similar between the two study groups (table 1).
After 12 months of clinical follow-up, 175 (90%) of 195 participants in the intervention group and 148 (76%) of 195 participants in the standard-of-care group were retained in care with viral suppression, a difference of 13·9% (95% CI 6·4–21·2; p<0·00040; table 2). Each component of the primary outcome was higher in the intervention group than the standard-of-care group: 10·3% higher rate of viral suppression and a 7·7% higher rate of retention (table 2). There were no adverse events related to point-of-care HIV viral load testing or task shifting care.
Table 2:
Primary and secondary outcomes
| Intervention group (n=195) | Standard-of-care group (n=195) | Absolute risk difference, % (95% CI) | p value | |
|---|---|---|---|---|
| Composite primary outcome | 175 (90%) | 148 (76%) | 13·9% (6·4 to 21·2) | 0·00040 |
| Components of primary outcome | ||||
| Viral suppression (<200 copies per mL) | 182 (93%) | 162 (83%) | 10·3% (3·9 to 16·8) | 0·0025 |
| Retention in care at study clinic | 180 (92%) | 165 (85%) | 7·7% (1·3 to 14·2) | 0·026 |
| Other secondary outcomes | ||||
| Viral suppression (<200 copies per mL) and retention at any clinic | 177 (91%) | 153 (79%) | 12·3% (5·2 to 19·4) | 0·0011 |
| Viral load <1000 copies per mL and retention at study clinic | 176 (90%) | 152 (78%) | 12·3% (5·1 to 19·5) | 0·0013 |
| Viral load <50 copies per mL and retention at study clinic | 167 (86%) | 139 (71%) | 14·4% (6·2 to 22·3) | 0·0080 |
| Viral load <1000 copies per mL | 184 (94%) | 166 (85%) | 9·2% (3·2 to 15·4) | 0·0041 |
| Viral load <50 copies per mL | 172 (88%) | 152 (78%) | 10·3% (2·8 to 17·6) | 0·0099 |
| Retention in care at any clinic at 12 months | 182 (93%) | 170 (87%) | 6·2% (0·2 to 12·2) | 0·059 |
| Viral suppression among those with a result at study exit | ||||
| Viral load <1000 copies per ml | 184/189 (97%) | 166/178 (93%) | 4·1% (–0·3 to 9·0) | 0·082 |
| Viral load <200 copies per ml | 182/189 (96%) | 162/178 (91%) | 5·3% (0·2 to 10·7) | 0·051 |
| Viral load <50 copies per ml | 172/189 (91%) | 152/178 (85%) | 5·6% (–1·0 to 12·4) | 0·11 |
| Communication of viral load result to participant | ||||
| Enrolment viral load communicated to participant | 195/195 (100%) | 171/192 (89%) | 10·9% (6·8 to 16·1) | <0·0001 |
| Mid-study viral load communicated to participant | 191/192 (>99%) | 127/170 (75%) | 24·8% (18·4 to 31·8) | <0·0001 |
| All viral load results communicated to participant | 397/398 (>99%) | 304/373 (82%) | 18·3% (14·5 to 22·5) | <0·0001 |
| Referral into community-based ART delivery programme | 116 (60%) | 52 (27%) | 32·8% (23·2 to 41·6) | <0·0001 |
Data are n (%) or n/N (%), unless otherwise stated. ART=antiretroviral therapy.
We evaluated several secondary study endpoints. All secondary outcomes, using higher and lower viral load thresholds, and including retention in any clinic, showed improvements for point-of-care viral load testing with task shifting versus standard-of-care, although not all were significant (table 2). When the composite outcome included retention in care at any clinic (ie, not just the Prince Cyril Zulu Clinic), which had less certainty, point-of-care viral load testing increased retention and viral suppression by 12·3% (p=0·0011; table 2). When evaluating the composite outcome with the WHO viral load threshold of less than 1000 copies per mL, or a more strict threshold of less than 50 copies per mL, the intervention group had 12% and 14% improvements in retention with viral suppression, respectively (table 2). When comparing each individual measure of HIV viral load of less than 1000 copies per mL or viral load of less than 50 copies per mL, the intervention point-of-care viral load testing group had significantly higher rates of viral suppression than did the standard-of-care group (table 2). When restricted to those with a viral load result at study exit, the proportion of patients with viral suppression (<200 copies per mL) was 5·3% higher in the intervention group than in the standard-of-care group (p=0·051). Reasons for not being retained in care are presented in the appendix (p 2).
All 398 point-of-care viral load results except for one (no reason noted) were entered into the health information system on the same day, whereas laboratory-based results were entered into the record and available to clinicians a median of 2 days (IQR 1–4) after blood draw (table 3). Overall, 397 of 398 point-of-care viral load results were communicated to participants by their provider, compared to 304 of 373 (82%) in the standard-of-care group, an improvement of 18·3% (p<0·0001; table 2). Almost all (99%) point-of-care viral load results were communicated to participants on the same day; three participants received their result the next day and one received a result 7 days later. Laboratory-based viral load results were communicated at participants’ next clinical visits, which were a median of 28 days (IQR 28–54) after the blood draw.
Table 3:
Time to event results for secondary outcomes
| Intervention group |
Standard-of-care group |
Hazard ratio (95% CI) | p value | |||
|---|---|---|---|---|---|---|
| n/N (%) | Median, days (IQR) | n/N (%) | Median, days (IQR) | |||
| Time to entry of viral load result into health-information system* | ||||||
| Enrolment blood draw | 195/195 (100%) | 0 (0–0) | 192/192 (100%) | 3 (2–4) | 96·0 (23·8–386·4) | <0·0001 |
| Mid-study blood draw | 192/192 (100%) | 0 (0–0) | 170/170 (100%) | 2 (1–3) | 11·6 (7·8–17·3) | <0·0001 |
| Any blood draw | 398/398 (100%) | 0 (0–0) | 373/373 (100%) | 2 (1–4) | 11·7 (8·9–15·3) | <0·0001 |
| Time to communication of viral load result to participant | ||||||
| Enrolment blood draw | 195/195 (100%) | 0 (0–0) | 171/192 (89%) | 28 (28–30) | ··† | <0·0001† |
| Mid-study blood draw | 191/192 (>99%) | 0 (0–0) | 127/170 (75%) | 41 (28–69) | 12·7 (8·5–19·1) | <0·0001 |
| Any blood draw | 397/398 (>99%) | 0 (0–0) | 304/373 (34%) | 28 (28–54) | 17·7 (13·0–24·2) | <0·0001 |
| Follow-up HIV treatment and care | ||||||
| Enrolment to detection of viral failure | 7/195 (4%) | 55 (55–57) | 9/195 (5%) | 123 (98–162) | 0·8 (0·3–2·2) | 0·69 |
| Appropriate switch to second-line ART after viral failure‡ | 6/6 (100%) | 0 (0–7) | 4/9 (44%) | 76 (20–134) | 10·9 (2·1–57·5) | 0·0050 |
| Enrolment to referral into community-based ART delivery program | 116/195 (59%) | 168 (168–175) | 52/195 (27%) | 261 (231–281) | 3·5 (2·5–4·8) | <0·0001 |
ART=antiretroviral therapy.
During the study, 2·9% of point-of-care viral load tests had invalid results, and all were repeated on the same day, whereas 1·1% of laboratory-based viral load tests had invalid results and were repeated.
Data excludes people evaluated for viral load after the 12-month study exit visit.
Hazard ratio could not be calculated for time to communication of viral load results to participant for the enrolment blood draw because the events are separated by group: in the intervention group, all events were on day 0 or within 1 day of the blood draw, whereas for control participant events the earliest event was on day 12. The p value from a log rank test is provided instead.
During the study, seven (4%) participants in the intervention group and nine (5%) in the standard-of-care group developed viral failure, defined as two consecutive viral loads of more than 1000 copies per mL. Among these, the intervention improved the median time to detection of viral failure from 123 days (IQR 98–162) to 55 days (55–57). Of those with viral failure, six (100%) participants in the intervention group were appropriately switched to second-line ART; three participants were switched on the same day, and two participants were switched within 1 week. Among nine participants with viral failure in the standard-of-care group, four (44%) were appropriately switched to second-line ART, a median of 76 days (IQR 20–134) after the second confirmatory viral load blood draw.
At study end, five (2·6%) participants in the intervention group and seven (3·6%) participants in the standard-of-care group had presence of any HIV drug resistance mutations (data not shown). Overall, during the course of the study participants in the intervention group had fewer total clinic visits and more visits with an enrolled nurse (p<0·0001; appendix p 3).
Patients were eligible for referral into the community ART delivery programme if they met the criteria, which included a suppressed viral load after 6 months of study enrolment, which was 12 months after ART initiation. Overall, 116 (56%) participants in the intervention group and 52 (27%) in the standard-of-care group were referred into the community-based ART delivery programme, an increase of 32·8% (95% CI 23·2–41·6; table 2). Among those referred into the community-based ART delivery programme, the time to referral for the intervention group was 168 days (IQR 168–175) and for the standard-of-care group was 261 days (231–28; table 3). Overall, participants in the intervention group had a 3·5-fold (95% CI 2·5–4·8) higher probability of referral into the community-based ART delivery programme than did participants in the standard-of-care group (figure 2).
Figure 2:
Kaplan-Meier estimates of time from study enrolment to referral into a community-based ART delivery programme by intervention group and standard-of-care group
ART=antiretroviral therapy.
Discussion
In this randomised controlled trial that followed the South African guidelines for HIV treatment and monitoring, point-of-care HIV viral load testing combined with task shifting to enrolled nurses improved viral suppression and retention in care for HIV-positive adults receiving ART. These improvements were achieved in part by ensuring rapid turnaround of viral load results, which facilitated adherence counselling the same day and led to a faster identification of viral failure and a subsequent switch to second-line ART. Viral failure was identified significantly earlier in the intervention group due to the cumulative delays for acting upon both the original and confirmatory laboratory viral load tests in the standard-of-care group. Point-of-care HIV viral load testing and task shifting allowed earlier identification of participants with viral suppression on the same day that their viral load was taken. This same-day testing meant that patients could be rapidly referred into differentiated care through the community-based ART delivery programme. In interviews and focus groups, participants and health-care workers from our study reported that point-of-care testing and taskshifting was acceptable (data not shown). In settings where patient follow-up and HIV treatment outcomes remain suboptimal, use of point-of-care viral load testing with task shifting could accelerate reaching the global UNAIDS target of 90% of people on ART achieving viral suppression by 2020.
Additional clinical trials are evaluating point-of-care HIV viral load testing, but have not yet reported results. Studies in Haiti (NCT03288246) and Uganda (NCT03553693) are evaluating HIV testing and outcomes in children, adolescents, and adults. Another South African study (NCT03187964) is comparing point-of-care to laboratory-based testing for the difference in the time to adherence counselling and resistance testing. A clinical trial (NCT03533868) of HIV-positive adults in Nigeria is evaluating on-site Xpert HIV-1 viral load testing for reaching viral suppression (<1000 copies per mL) at 1 year after ART initiation. Results from most of these trials are expected later this year.
We used the Cepheid Xpert HIV-1 viral load test in our study, which has high diagnostic accuracy,24 and there are other point-of-care HIV viral load platforms.25,26 The SAMBA I/II semi-Q (Diagnostics for the Real World, Cambridge, UK) can be done in 90 min on plasma or whole blood and has a lower limit of quantification of 1000 copies per mL.27 The m-PIMA, formerly Alere Q NAT, (Abbott, Chicago, IL, USA) can complete testing in 70 min on plasma, and is currently the only point-of-care viral load test for both HIV-1 and HIV-2.28
Our study had several strengths and limitations. Point-of-care viral load testing and associated clinical care was done by the research team, which might not always reflect implementation in routine clinical services. Study reimbursements could have increased retention in care, but we provided the same incentives for participants in both study groups to minimise differences. The study was designed as an open-label clinical trial, and research implementers were blinded to data by study groups. Study enrolment occurred 6 months after ART initiation to coincide with the first viral load test in the routine South African ART monitoring schedule. Therefore, our results might not be generalisable to all patients initiating ART because they do not account for people who default early in their HIV care. These people will require additional interventions to ensure retention until they are due their first viral load test. Because our intervention contained two components, we cannot be certain to what extent point-of-care testing or task shifting to enrolled nurses was responsible for the improved clinical outcomes. Finally, the study was done at a single clinical site serving an urban population of HIV-positive adults in South Africa, and additional studies might be necessary to determine achievable outcomes in other clinics or countries. Further assessment of the effect on quality of life, wider health-systems costs, and cost-effectiveness is also warranted.
In conclusion, point-of-care HIV viral load testing combined with task shifting improved treatment outcomes in an urban clinic in South Africa. To accommodate the rapid expansion of ART, efficient methods to monitor HIV-positive people on ART will be required in resource-limited settings. These innovative models of HIV care might be sustainable only if they do not increase the existing burden on HIV care providers, laboratories, and health systems. By improving and expediting delivery of viral load results to patients and their care providers, HIV treatment programmes could use point-of-care testing for reducing barriers to achieve viral suppression.
Supplementary Material
Research in context.
Evidence before this study
On May 30, 2019, we searched PubMed for studies assessing the outcomes of point-of-care HIV viral load testing in people living with HIV and receiving antiretroviral therapy (ART). We used the search terms “point-of-care,” “viral load,” and “HIV,” without language restrictions, and did not find any randomised controlled trials or implementation science studies. Therefore, we are not aware of any published data to evaluate the clinical effectiveness or effect of point-of-care HIV viral load testing, when compared with standard laboratory viral load testing, for improving treatment monitoring for HIV-positive adults on ART.
Added value of this study
To our knowledge, this is the first randomised controlled trial to evaluate the effect of point-of-care HIV viral load monitoring for people living with HIV receiving ART. Overall, implementation of point-of-care HIV viral load testing, following the recommended schedule for testing in South Africa, significantly increased the outcome of retained in care with viral suppression. Additionally, the intervention substantially accelerated switching to second-line ART for those with treatment failure (sustained viral load >1000 copies per mL), as well as referral into a community-based ART delivery programme.
Implications of all the available evidence
Our results suggest that ensuring rapid receipt of viral load results to patients and their providers can improve HIV viral suppression and retention in care, lead to a faster switch to second-line ART for those with viral failure, and improve referral into community-based ART delivery programmes in resource-limited settings.
Acknowledgments
We thank all the people who participated in this study. This research was supported by the National Institute of Allergy and Infectious Diseases (grant R21AI124719), the Center for AIDS Research at the University of Washington (grant P30 AI027757), and the South African National Health Laboratory Service Research Trust (grant 2018–1DEV-PMO01).
PKD reports receiving consulting and speaking fees from Gilead Science; and research support from the National Institute of Health, Centers for Disease Control and Prevention, Gilead Sciences, and the Bill & Melinda Gates Foundation, during the conduct of the study. KN and SSAK report grants from the National Institute of Health during the conduct of the study. DD reports grants from USAID, outside of the conduct of this study; and grants from the National Institute of Health during the conduct of the study.
Footnotes
Declaration of interests
All other authors declare no competing interests.
Data sharing
We will share individual participant data that underlie the results reported in this article, after de-identification (text, tables, figures, and appendices). The study protocol will be available. Patient data will be available beginning 9 months and ending 36 months after article publication. The data will be made available by the investigators whose proposed use of the data has been approved by an independent review committee (“learned intermediary”) identified for this purpose. Proposals can be submitted up to 36 months following article publication. After 36 months the data will be available in our university’s data warehouse but without investigator support other than deposited metadata.
See Online for appendix
Contributor Information
Paul K Drain, Department of Global Health, School of Public Health and School of Medicine, Department of Medicine, School of Medicine, Department of Epidemiology, School of Public Health, University of Washington, Seattle, WA, USA.
Jienchi Dorward, Nuffield Department of Primary Care Health Sciences, University of Oxford, Oxford, UK, Centre for the AIDS Programme of Research in South Africa, University of KwaZulu-Natal, Durban, South Africa.
Lauren R Violette, Department of Medicine, School of Medicine, University of Washington, Seattle, WA, USA.
Justice Quame-Amaglo, Department of Global Health, School of Public Health and School of Medicine, University of Washington, Seattle, WA, USA.
Katherine K Thomas, Department of Global Health, School of Public Health and School of Medicine, University of Washington, Seattle, WA, USA.
Natasha Samsunder, Centre for the AIDS Programme of Research in South Africa, University of KwaZulu-Natal, Durban,South Africa.
Hope Ngobese, Prince Cyril Zulu Communicable Disease Clinic, Durban Municipality, Durban, South Africa, National Health Laboratory Service, Durban, South Africa.
Koleka Mlisana, School of Laboratory Medicine and Medical Sciences, University of KwaZulu-Natal, Durban,South Africa.
Pravikrishnen Moodley, School of Laboratory Medicine and Medical Sciences, University of KwaZulu-Natal, Durban,South Africa, Department of Virology, Inkosi Albert Luthuli Central Hospital, Cato Manor, South Africa.
Deborah Donnell, Department of Global Health, School of Public Health and School of Medicine, University of Washington, Seattle, WA, USA.
Ruanne V Barnabas, Department of Global Health, School of Public Health and School of Medicine, Department of Medicine, School of Medicine, University of Washington, Seattle, WA, USA.
Kogieleum Naidoo, Centre for the AIDS Programme of Research in South Africa, School of Nursing and Public Health, CAPRISA-MRC HIV-TB Pathogenesis and Treatment Research Unit, and Doris Duke Medical Research Institute, University of KwaZulu-Natal, Durban,South Africa.
Salim S Abdool Karim, Centre for the AIDS Programme of Research in South Africa, School of Nursing and Public Health, University of KwaZulu-Natal, Durban,South Africa, Department of Epidemiology, Columbia University, New York, NY, USA.
Connie Celum, Department of Global Health, School of Public Health and School of Medicine, Department of Medicine, School of Medicine, University of Washington, Seattle, WA, USA.
Nigel Garrett, Centre for the AIDS Programme of Research in South Africa, School of Nursing and Public Health, University of KwaZulu-Natal, Durban,South Africa.
References
- 1.Joint United Nations Programme on HIV/AIDS. Miles to go: closing gaps, breaking barriers, righting injustices. 2018. http://www.unaids.org/sites/default/files/media_asset/miles-to-go_en.pdf (accessed May 30, 2019).
- 2.Joint United Nations Programme on HIV/AIDS. 90–90–90. An ambitious treatment target to help end the AIDS epidemic. 2014. http://www.unaids.org/sites/default/files/media_asset/90–90–90_en.pdf (accessed May 30, 2019).
- 3.WHO. Interim technical update: technical and operational considerations for implementing HIV viral load testing. July 2014 https://www.who.int/hiv/pub/arv/viral-load-testing-technical-update/en/ (accessed Jan 29, 2020).
- 4.Grimsrud A, Barnabas RV, Ehrenkranz P, Ford N. Evidence for scale up: the differentiated care research agenda. J Int AIDS Soc 2017; 20: 22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Habiyambere V, Dongmo Nguimfack B, Vojnov L, et al. Forecasting the global demand for HIV monitoring and diagnostic tests: a 2016–2021 analysis. PLoS One 2018; 13: e0201341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.United States Department of Health and Human Services. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. Department of Health and Human Services, 2019. 2019. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf (accessed Jan 29, 2020).
- 7.Peter T, Zeh C, Katz Z, et al. Scaling up HIV viral load—lessons from the large-scale implementation of HIV early infant diagnosis and CD4 testing J Int AIDS Soc 2017; 20: 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lecher S, Ellenberger D, Kim AA, et al. Scale-up of HIV viral load monitoring—seven sub-Saharan African countries. MMWR Morb Mortal Wkly Rep 2015; 64: 1287–90. [DOI] [PubMed] [Google Scholar]
- 9.Murphy RA, Court R, Maartens G, Sunpath H. Second-line antiretroviral therapy in sub-Saharan Africa: it’s time to mind the gaps. AIDS Res Hum Retroviruses 2017; 33: 1181–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drain P, Hyle E, Noubary F, et al. Evaluating diagnostic point-of-care tests in resource-limited settings. Lancet Infect Dis 2014; 14: 239–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cepheid. Monitoring for quality of life, Xpert HIV-1 viral load. 2015. http://www.cepheid.com/administrator/components/com_productcatalog/library-files/fadd5b909331644ab1415fe39ec94dd5-Xpert-HIV-1-Viral-Load-Brochure-CEIVD-3039–03.pdf (accessed May 30, 2019).
- 12.epheid. Cepheid and FIND announce European approval of Xpert HIV-1 Viral Load. 2014. http://ir.cepheid.com/releasedetail.cfm?releaseid=888964 (accessed May 30, 2019).
- 13.Garrett NJ, Drain PK, Werner L, Samsunder N, Abdool Karim SS. Diagnostic accuracy of the point-of-care Xpert HIV-1 viral load assay in a South African HIV clinic. J Acquir Immune Defic Syndr 2016; 72: e45–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorward J, Garrett N, Quame-Amaglo J, et al. Protocol for a randomised controlled implementation trial of point-of-care viral load testing and task-shifting: the Simplifying HIV TREAtment and Monitoring (STREAM) study. BMJ Open 2017; 7: e017507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peltzer K, Simbayi L, Kalichman S, et al. Alcohol use in three different inner cities in South Africa: AUDIT-C and CAGE. J Psychol Africa 2007; 17: 99–104. [Google Scholar]
- 16.Bernstein M, Phillips T, Zerbe A, et al. Intimate partner violence experienced by HIV-infected pregnant women in South Africa: a cross-sectional study. BMJ Open 2016; 6: e011999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhana A, Rathod SD, Selohilwe O, et al. The validity of the patient health questionnaire for screening depression in chronic care patients in primary health care in South Africa. BMC Psychiatry 2015; 15: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.South Africa Department of Health. National consolidated guidelines for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents, and adults. 2015. https://sahivsoc.org/Files/HIV%20clinicians%20ADULT%20GUIDELINES%202017%20-%20David%20Stead.pdf (accessed May 30, 2019).
- 19.South African National Health Research Ethics Council. Payment of trial participants in South Africa: ethical considerations for Research Ethics Committees (RECs). 2012. www.nhrec.org.za/index.php/grids-preview?download=11:guidelines-for-payment (accessed May 30, 2019).
- 20.Georgeu D, Colvin CJ, Lewin S, et al. Implementing nurse-initiated and managed antiretroviral treatment (NIMART) in South Africa: a qualitative process evaluation of the STRETCH trial. Implement Sci 2012; 7: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts P. Centralised chronic medicine dispensing and distribution: a public/private partnership to increase access to HIV/chronic medication. 2018. http://files.icap.columbia.edu/files/uploads/ICAP_Grand_Rounds_Project_Last_Mile_Slides.pdf (accessed Jan 29, 2020).
- 22.Wilson EB. Probable Inference, the Law of succession, and statistical inference. JASA 1927; 22: 209–12. [Google Scholar]
- 23.Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods. Statistics in Medicine 1998; 17: 873–90. [DOI] [PubMed] [Google Scholar]
- 24.Nash M, Huddart S, Badar S, Baliga S, Saravu K, Pai M. Performance of the Xpert HIV-1 viral load assay: a systematic review and meta-analysis. J Clin Microbiol 2018; 56: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joint United Nations Programme on HIV/AIDS. HIV/AIDS diagnostics technology landscape; semi-annual update 2015. 2015. https://aidsfree.usaid.gov/sites/default/files/hivaids_diag_tech.pdf (accessed Jan 29, 2020).
- 26.Drain PK, Dorward J, Garrett N, et al. Point-of-care HIV viral load testing: An essential tool for a sustainable global HIV/AIDS response. Clin Microbiol Rev 2019; 32: e00097–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goel N, Ritchie AV, Mtapuri-Zinyowera S, et al. Performance of the SAMBA I and II HIV-1 Semi-Q Tests for viral load monitoring at the point-of-care. J Virol Methods 2017; 244: 39–45. [DOI] [PubMed] [Google Scholar]
- 28.Jani IV, Meggi B, Vubil A, et al. Evaluation of the whole-blood Alere Q-nat point-of-care RNA assay for HIV-1 viral load monitoring in a primary health care setting in Mozambique. J Clin Microbiol 2016; 54: 2104–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.