Abstract
Background
Diabetes mellitus (DM) is associated with poor TB treatment outcome. Previous studies examining the effect of DM on TB drug concentrations yielded conflicting results. No studies have been conducted to date in an African population.
Objectives
To compare exposure to TB drugs in Tanzanian TB patients with and without DM.
Patients and methods
A prospective pharmacokinetic study was performed among 20 diabetic and 20 non-diabetic Tanzanian TB patients during the intensive phase of TB treatment. Plasma pharmacokinetic parameters of isoniazid, rifampicin, pyrazinamide and ethambutol were compared using an independent-sample t-test on log-transformed data. Multiple linear regression analysis was performed to assess the effects of DM, gender, age, weight, HIV status and acetylator status on exposure to TB drugs.
Results
A trend was shown for 25% lower total exposure (AUC0–24) to rifampicin among diabetics versus non-diabetics (29.9 versus 39.9 mg·h/L, P=0.052). The AUC0–24 and peak concentration (Cmax) of isoniazid were also lower in diabetic TB patients (5.4 versus 10.6 mg·h/L, P=0.015 and 1.6 versus 2.8 mg/L, P=0.013). Pyrazinamide AUC0–24 and Cmax values were non-significantly lower among diabetics (P=0.08 and 0.09). In multivariate analyses, DM remained an independent predictor of exposure to isoniazid and rifampicin, next to acetylator status for isoniazid.
Conclusions
There is a need for individualized dosing of isoniazid and rifampicin based on plasma concentration measurements (therapeutic drug monitoring) and for clinical trials on higher doses of these TB drugs in patients with TB and DM.
Introduction
Diabetes mellitus (DM) was a well-known risk factor for TB in the past,1 but this was largely forgotten during the second half of the 20th century, with the advent of widely available treatment for both DM and TB. The association between the two diseases has now re-emerged as a result of the global increase in cases of type 2 DM.2–4 The greatest increase in type 2 DM occurs in developing countries, where TB is highly endemic.2–4 As noticed before, patients with DM have a higher risk of developing TB,5 probably caused by impaired immunity.6 Moreover, TB is more difficult to treat in diabetic patients, as shown by higher TB treatment failure, relapse and death rates.7
It has been shown that patients with DM have lower plasma concentrations of various drugs.8,9 If this also applies to TB drugs, this may at least partly explain the suboptimal response to TB treatment in patients with DM, considering that lower plasma concentrations of TB drugs have been associated with clinical failure and acquired drug resistance.10–17
Only few studies, some with limitations in design and sample size, have evaluated the effect of DM on the exposure to TB drugs. In a first study in Indonesia, TB patients with DM had 53% lower rifampicin concentrations than TB patients without DM,18 which was attributed to DM per se as well as to the higher body weight of diabetic patients, associated with a lower dose of TB drugs on a mg/kg base. In a follow-up study, Indonesian TB patients with and without DM were matched for weight and, in contrast to previous observations, no differences in drug exposure for all four first-line TB drugs (isoniazid, rifampicin, pyrazinamide and ethambutol) were found between the two patient groups.19 Another study found decreases by ∼50% in isoniazid and rifampicin, but not pyrazinamide and ethambutol, plasma concentrations among Turkish diabetic TB patients.20 Rifampicin concentrations were also predicted to be lower in Korean patients with TB and DM.21 After adjustment for other factors, only pyrazinamide concentrations were lower in Indian patients with both diseases.22 A study in Peruvian patients, however, did not find a difference in rifampicin peak concentrations between diabetic and non-diabetic TB patients.23 Some of the above-mentioned studies were hampered by measurement of just a single or a few pharmacokinetic samples.
Clearly, these findings need more research. It needs to be properly studied whether DM affects TB drug concentrations and also in other populations, considering that pharmacokinetics can differ between different ethnic groups as a result of genetic variation in metabolic and transporter enzymes.24 It is particularly important to study this subject in African TB patients because the African continent has the highest TB incidence and mortality rates per 100 000 persons per year25 and a crisis of DM is evolving in this part of the world.26
Patients and methods
Objectives and study design
The objective of this study was to assess whether pharmacokinetic parameters of first-line TB drugs were different between adult TB patients with and without DM. To this end, we performed a prospective two-arm pharmacokinetic study among adult Tanzanian TB patients with and without DM, using intensive pharmacokinetic sampling performed at ‘steady-state’ during the intensive phase of treatment of drug-susceptible TB.
Study subjects
Based on available data on the pharmacokinetics of TB drugs,27 it was estimated that at least 16 participants were required in each group to be able to demonstrate a difference of at least 25% in the total exposure to the TB drug rifampicin while using a 5% significance level and 80% statistical power. Fewer patients would be needed to demonstrate the same difference in total exposure to pyrazinamide and ethambutol. At the time of the design of the study we did not have pharmacokinetic data on isoniazid gathered with our own bioanalytical assays.
Thus 40 adult (age ≥18 years) TB patients were included in this study; 20 TB patients without DM were recruited at Mawenzi Hospital, an outpatient TB treatment clinic in Moshi in northern Tanzania, and 20 TB patients with DM were recruited from this same hospital as well as other institutions across the region. The diagnosis of TB was based on clinical symptoms and signs, chest X-ray examination and sputum smear microscopy performed in all patients. Diabetic patients were included if they had a previously established diagnosis of DM and were attending a diabetes clinic. In addition, DM was tested for at the time of pharmacokinetic sampling using WHO criteria28 where a fasting blood glucose concentration >7 mmol/L (126 mg/dL) was considered to indicate diabetes.
Ethics
Participants gave written informed consent and the study was approved by the local institutional research board at the Kilimanjaro Christian Medical Center (KCMC), Moshi, and by the Tanzanian National Institute of Medical Research.
Drug treatment
The patients were using TB treatment for drug-susceptible Mycobacterium tuberculosis according to the Tanzanian National Tuberculosis Guidelines. They were treated with fixed-dose combination (FDC) tablets manufactured by Sandoz (a division of Novartis), Mumbai, India and donated by Novartis through the WHO Global Drug Facility (GDF) which only uses TB drugs checked according to stringent WHO standards. Patients with a body weight >50 kg received four FDC tablets daily (i.e. 300 mg of isoniazid, 600 mg of rifampicin, 1600 mg of pyrazinamide and 1100 mg of ethambutol) and those below 50 kg received three FDC tablets daily (i.e. 225 mg of isoniazid, 450 mg of rifampicin, 1200 mg of pyrazinamide and 825 mg of ethambutol). Patients were all under community-based directly observed treatment (DOT). Diabetic patients were either on dietary management alone or were treated with oral hypoglycaemic agents and/or injectable insulin.
Pharmacokinetic sampling
Pharmacokinetic sampling was performed when patients were on TB treatment for at least 2 weeks, given the expected steady-state (stable pharmacokinetics) at that point. Patients were admitted on the sampling day and serial venous blood samples were collected just before and at 1, 2, 3, 4, 6, 8, 10 and 24 h after observed TB drug intake. Plasma was separated immediately and kept frozen at −80°C until transport on dry ice to the Netherlands for bioanalysis.
Patients fasted at least 8 h (from the preceding evening’s dinner to the next morning dose) before drug intake and took a standardized breakfast within 30 min after drug intake, which reflected the usual drug intake procedures in the study population. The standardized breakfast consisted of a cup (125 mL) of tea with milk and sugar together with either a small bowl of porridge or maandazi, a typical east African doughnut-like pastry.
Bioanalysis and pharmacokinetic data analysis
The total (protein-bound plus unbound) plasma concentrations of isoniazid, acetyl-isoniazid, rifampicin, desacetyl-rifampicin (the main metabolite of rifampicin), pyrazinamide and ethambutol were assessed by validated HPLC methods as described before.27
Pharmacokinetic evaluations were performed using standard non-compartmental methods in WinNonLin Version 4.1 (Pharsight Corp., Mountain View, CA, USA) as described before,19,27 to assess the total exposure (AUC0–24), Cmax with the corresponding Tmax, the apparent clearance (CL/F; in which F is bioavailability), the apparent volume of distribution (Vz/F) and the elimination half-life (t½).
Reference ranges for Cmax values were 3–6 mg/L for isoniazid, 8–24 mg/L for rifampicin, 20–50 mg/L for pyrazinamide and 2–6 mg/L for ethambutol.29
The acetylator status for isoniazid was determined phenotypically, either by assessing the elimination half-life of isoniazid (with participants with a t½ of >130 min being classified as slow acetylators and those with t½ <130 min being classified as fast/intermediate acetylators) or by calculation of the ratio of acetyl-isoniazid to isoniazid at 3 h after the dose (using this approach, patients with a ratio <1.5 were considered slow acetylators and those with a ratio >1.5 were fast/intermediate metabolizers30).
Statistical analysis
Patient characteristics were presented descriptively. For pharmacokinetic parameters AUC0–24, Cmax, CL/F, V/F and t½, analyses were performed on logarithmically transformed data, and geometric means were presented.
Differences in pharmacokinetic parameters between diabetic and non-diabetic TB patients (primary objective) were calculated with an independent-sample t-test on the log-transformed data. Tmax values were not transformed and were compared with the Wilcoxon rank-sum test. Pearson χ2 test was used to determine the difference in proportions of patients who reached reference peak plasma concentrations of the TB drugs.
Next to the effect of DM, the effects of age, gender, body weight, HIV status and acetylator status (based on isoniazid half-life) on the log-transformed AUC0–24 and Cmax values of the first-line TB drugs were assessed (secondary objective), using univariate linear regression analyses. Only if DM was associated with a pharmacokinetic measure was multivariate linear regression analysis performed to correct this association for potential confounding by one of the other determinants. Due to the relatively low patient numbers in this study, a full multivariate analysis was not feasible.
In an additional exploratory analysis, the association between the AUC0–24, Cmax of the TB drugs and the fasting blood glucose and glycated haemoglobin (HbA1C) levels was assessed among diabetic TB patients only, using rank correlation (Spearman’s rho).
All statistical analyses were performed using SPSS version 20 (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
We enrolled 40 subjects comprising 20 diabetic and 20 non-diabetic TB patients. For the pharmacokinetic data analysis, the data of one subject (with DM) were excluded because the patient had high pre-dose concentrations for all TB drugs, indicating that he had incorrectly taken the drugs at home prior to pharmacokinetic sampling. Patient characteristics of the remaining 39 patients are summarized in Table 1. Of these, 23% were female, their median age was 42 years and 33% were HIV infected. One patient had extrapulmonary TB (TB of the spine); all others had pulmonary TB.
Table 1.
Characteristics of Tanzanian TB patients with (n=20) and without (n=19) DMa
| All patients (n=39) | TB patients with DM (n=19) | TB patients without DM (n=20) | P b | |
|---|---|---|---|---|
| Gender (female) | 9 (23) | 4 (21) | 5 (25) | 1.00 |
| Age (years) | 42 (19–81) | 50 (30–81) | 38 (19–64) | 0.001 |
| Body weight (kg) | 56 (33–75) | 58 (33–75) | 55 (45–65) | 0.258 |
| BMI (kg/m2) | 19.1 (12.9–28.2) | 21.3 (12.9–28.2) | 19.0 (15.9–24.8) | 0.380 |
| Dose (mg/kg) | ||||
| rifampicin | 10.3 (8.0–13.6) | 10.0 (8.0-13.6) | 10.3 (9.2–12.0) | 0.101 |
| isoniazid | 5.2 (4.0–6.8) | 5.0 (4.0–6.8) | 5.2 (4.6–6.0) | 0.101 |
| pyrazinamide | 27.6 (21.3–36.4) | 26.7 (21.3–36.4) | 27.6 (24.6–32.0) | 0.101 |
| ethambutol | 19.0 (14.7–25.0) | 18.3 (14.7–25.0) | 19.0 (16.9–22.0) | 0.101 |
| HIV status (positive) | 13 (33) | 6 (32) | 7 (35) | 0.821 |
| FBG (mmol/L)c | 7.8 (5–32) | 15.9 (6.9–31.5) | 6.9 (5.0–8.0) | <0.001 |
| HBA1c (mmol/mol) | 55 (27–147) | 111 (65–147) | 39 (27–45) | <0.001 |
| Acetylator status based on isoniazid elimination half-life (slow acetylators)d | 22/39 (56) | 10/19 (53) | 12/20 (60) | 0.643 |
| Acetylator status based on acetyl-isoniazid/isoniazid concentration ratio at 3 h (slow acetylators)e,f | 18/37 (49) | 8/18 (44) | 10/19 (53) | 0.619 |
| Diabetes treatment | ||||
| dietary only | 3 (16) | – | ||
| metformin | 10 (53) | – | ||
| chlorpropamide | 9 (47) | – | ||
| glibenclamide | 6 (32) | – | ||
| Presenting symptoms | ||||
| cough | 29 (74) | 14 (74) | 15 (75) | |
| night sweats | 30 (77) | 13 (68) | 17 (85) | |
| fever | 10 (26) | 6 (32) | 4 (20) | |
| weight loss | 37 (95) | 17 (89) | 20 (100) | |
| anorexia | 6 (15) | 2 (11) | 4 (20) | |
| chest pain | 4 (10) | 2 (11) | 2 (10) | |
| shortness of breath | 2 (5) | 2 (11) | – | |
| haemoptysis | 4 (10) | 3 (16) | 1 (5) | |
| fatigue | 1 (3) | – | 1 (5) |
Data are presented as n (%), median (minimum–maximum) or n/N (%).
P values are derived from χ2 tests (categorical variables) or Wilcoxon rank-sum tests (continuous variables).
FBG was assessed in the morning of pharmacokinetic sampling.
Acetylator status based on isoniazid elimination half-life; participants with an elimination half-life >130 min were classified as slow metabolizers and those with a shorter elimination half-life were classified as fast/intermediate metabolizers.
Acetylator status based on the acetyl-isoniazid/isoniazid concentration ratio at time 3 h after the dose; participants with a ratio <1.5 were classified as slow metabolizers and those with a ratio >1.5 were classified as fast/intermediate metabolizers.
In samples of 2/39 patients, the measurement of acetyl-isoniazid concentrations at timepoint 3 h was not possible.
There was no difference in gender, body weight, BMI or dose per kilogram of the TB drugs between diabetic and non-diabetic TB patients. Diabetic TB patients were older than non-diabetic TB patients (median age 50 versus 38 years, P=0.001). As expected, the median fasting blood glucose (FBG) was higher for diabetic TB patients than non-diabetic TB patients (15.9 versus 6.9 mmol/L, P<0.001). All diabetic patients had HbA1c (range 65–147 mmol/mol) above the target limit of 53 mmol/mol for good control. The proportions of fast and slow acetylators for isoniazid were roughly similar (Table 1). Three out of 37 evaluable patients had discordant results when assessing the acetylator status based on the elimination half-life of isoniazid or on the acetyl-isoniazid/isoniazid concentration ratio at 3 h after the dose.
All but one of the diabetic patients already knew they had DM and were enrolled at a diabetic clinic. Three of the diabetic patients were not on antidiabetic medications; all others were on antidiabetic drugs. These antidiabetic and other co-administered drugs (data not shown) are not known to affect the exposure to TB drugs.
Pharmacokinetic parameters: effect of DM
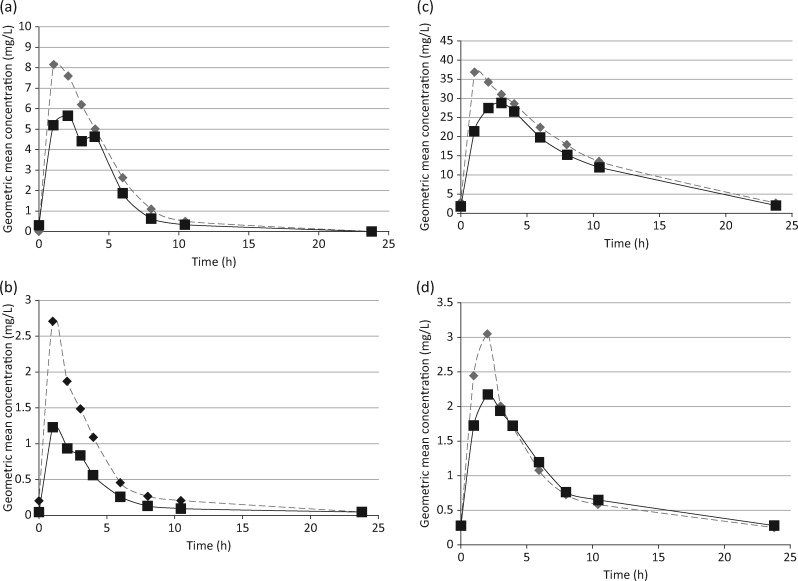
Table 2 shows the average pharmacokinetic parameters in diabetic and non-diabetic TB patients, and Figure 1 compares the plasma concentration–time curves of TB drugs in these patients.
Table 2.
Pharmacokinetic parameters of first-line TB drugs in Tanzanian diabetic and non-diabetic TB patientsa
| Drug/pharmacokinetic parameter | TB patients with DM (n=19) | TB patients without DM (n=20) | Ratio of value for TB patients with DM versus TB patients without DM (95% CI) | P |
|---|---|---|---|---|
| Rifampicin | ||||
| AUC0–24 (mg·h/L) | 29.9 (6.4–69.7) | 39.9 (27.4–68.3) | 0.75 (0.56–1.03) | 0.052 |
| Cmax (mg/L) | 7.9 (1.9–20.7) | 8.9 (5.9–14.8) | 0.89 (0.67–1.17) | 0.384 |
| Cmax below reference range, n (%)b | 9 (47) | 7 (35) | – | 0.433 |
| Tmax (h), median (range)c | 2.1 (0.9–4.2) | 1.1 (0.9–3.0) | 0.027 | |
| t½ (h) | 1.4 (1.0–2.7) | 1.8 (1.1–3.8) | 0.80 (0.66–0.97) | 0.026 |
| Vz (L) | 38.7 (14.3–143) | 37.3 (22.7–56.5) | 1.04 (0.77–1.40) | 0.798 |
| CL (L/h) | 18.6 (8.6–70.3) | 14.4 (8.8–21.9) | 1.29 (0.98–1.71) | 0.072 |
| desacetyl-rifampicin/rifampicin ratiod | ||||
| AUC0–24 | 0.125 (0.057) | 0.133 (0.035) | – | 0.672 |
| Cmax | 0.100 (0.039) | 0.107 (0.029) | – | 0.576 |
| Isoniazid | ||||
| AUC0–24 (mg·h/L) | 5.4 (0.7–26.9) | 10.6 (3.7–22.7) | 0.51 (0.30–0.87) | 0.015 |
| Cmax (mg/L) | 1.6 (0.4–5.8) | 2.8 (1.0–4.6) | 0.60 (0.40–0.89) | 0.013 |
| Cmax below reference range, n (%)b | 14 (74) | 11 (55) | – | 0.224 |
| Tmax (h), median (range)c | 1.0 (0.9–4.1) | 1.1 (0.7–2.9) | – | 0.855 |
| t½ (h) | 2.6 (1.1–5.0) | 2.5 (1.0–4.2) | 1.03 (0.75–1.34) | 0.985 |
| Vz (L) | 189 (42.0–637) | 99 (70.2–174) | 1.90 (1.27–2.85) | 0.003 |
| CL (L/h) | 51 (8.4–410) | 27 (13.0–60.7) | 1.91 (1.09–3.32) | 0.024 |
| Pyrazinamide | ||||
| AUC0–24 (mg·h/L) | 290 (123–420) | 344 (209–609) | 0.84 (0.69–1.02) | 0.083 |
| Cmax (mg/L) | 34.5 (21.4–46.2) | 38.2 (29.0–50.8) | 0.90 (0.80–1.02) | 0.090 |
| Cmax below reference range, n (%)b | 0 | 0 | – | – |
| Tmax (h), median (range)c | 1.1 (1.0–4.0) | 1.1 (0.7–3.0) | – | 0.252 |
| t½ (h) | 5.4 (2.9–9.6) | 6.3 (4.2–15.7) | 0.85 (0.68–1.07) | 0.154 |
| Vz (L) | 39.5 (19.9–57.5) | 40.4 (29.5–98.3) | 0.98 (0.81–1.19) | 0.832 |
| CL (L/h) | 5.1 (2.9–13.0) | 4.5 (2.6–6.5) | 1.15 (0.94–1.41) | 0.170 |
| Ethambutol | ||||
| AUC0–24 (mg·h/L) | 19.6 (7.5–40.4) | 20.2 (13.4–32.0) | 0.97 (0.77–1.22) | 0.789 |
| Cmax (mg/L) | 3.1 (1.3–6.3) | 3.3 (2.2–5.8) | 0.95 (0.75–1.21) | 0.672 |
| Cmax below reference range, n (%)b | 3 (16) | 0 (0) | −e | |
| Tmax (h), median (range)c | 2.0 (1.0–4.0) | 2.0 (0.9–2.2) | – | 0.317 |
| t½ (h) | 8.6 (2.8–18.2) | 9.6 (6.9–13.5) | 0.90 (0.70–1.16) | 0.384 |
| Vz (L) | 644 (324–2533) | 719 (491–965) | 0.90 (0.69–1.17) | 0.394 |
| CL (L/h) | 51.9 (20.5–126) | 52.0 (34.3–71.3) | 1.00 (0.78–1.27) | 0.985 |
Data are presented as geometric mean (minimum–maximum) unless stated otherwise.
By Pearson’s χ2 test.
By Wilcoxon rank-sum test.
Data are presented as mean (SD). An independent-sample t-test was used for testing.
The requirements for the Pearson’s χ2 test were not fulfilled as the frequency of one of the two cells was not ≥1.
Figure 1.
Geometric mean steady-state plasma concentration–time profiles of rifampicin (a), isoniazid (b), pyrazinamide (c) and ethambutol (d) in diabetic (squares and continuous line; n=19) and non-diabetic (diamonds and broken line, n=20) Tanzanian TB patients.
The average AUC0–24 for rifampicin was 25% lower in diabetic TB patients, which was a trend (P=0.052). No clinically relevant or statistically significant difference in rifampicin Cmax values was shown. Time to the rifampicin maximum concentration (Tmax) was longer in diabetic (2.1 h) than in non-diabetic TB patients (1.08 h, P=0.027; Table 2). There was no statistically significant difference in the desacetyl-rifampicin/rifampicin ratios for AUC0–24 or Cmax between diabetics and non-diabetics.
The geometric mean AUC0–24 for isoniazid was 49% lower in diabetic TB patients (P=0.015). Compared with non-diabetics, diabetic TB patients also had 40% lower values of isoniazid Cmax (P=0.013).
AUC0–24 and Cmax of pyrazinamide showed a trend to lower exposure in diabetic versus non-diabetic TB patients, but these differences did not reach statistical significance. Other pharmacokinetic parameters for pyrazinamide as well as those for ethambutol were also not significantly different (Table 2).
No statistically significant differences were found in proportions of diabetic versus non-diabetic patients with Cmax values of the TB drugs below reference ranges.
Pharmacokinetic parameters: effect of other characteristics and exploratory analyses
The results from the linear regression analyses are shown in Table 3. According to these analyses, TB patients with diabetes had lower rifampicin AUC0–24 values, reaching statistical significance. Diabetes was not associated with rifampicin Cmax, and male patients had a lower rifampicin Cmax than female TB patients.
Table 3.
Linear regression analysis for potential determinants for exposure to first-line TB drugs among Tanzanian diabetic and non-diabetic TB patients (n=39)a
| Univariate linear regression analysis |
Multivariate linear regression analysis |
|||
|---|---|---|---|---|
| unstandardized regression coefficient (95% CI) | P | unstandardized regression coefficient (95% CI) | P | |
| Rifampicin log10 AUC0–24 | ||||
| DM | −0.125 (−0.247 to −0.004) | 0.044 | – | |
| age (in years) | −0.004 (−0.008 to 0.001) | 0.142 | – | |
| gender (male versus female) | −0.090 (−0.240 to −0.059) | 0.228 | – | |
| body weight (in kg) | 0.003 (−0.004 to 0.009) | 0.389 | – | |
| HIV status | 0.008 (−0.046 to 0.221) | 0.191 | – | |
| Rifampicin log10Cmax | ||||
| DM | −0.052 (−0.169 to 0.065) | 0.373 | – | |
| age (in years) | −0.004 (−0.008 to 0.001) | 0.112 | – | |
| gender (male versus female) | −0.134 (−0.267 to −0.001) | 0.048 | – | |
| body weight (in kg) | 0.003 (−0.003 to 0.009) | 0.929 | – | |
| HIV status | 0.116 (−0.003 to 0.235) | 0.055 | – | |
| Isoniazid log10 AUC0–24 | ||||
| DM | −0.292 (−0.524 to −0.061) | 0.015 | −0.254 (−0.409 to −0.098) | 0.002 |
| age (in years) | −0.006 (−0.015 to −0.004) | 0.249 | – | |
| gender (male versus female) | 0.057 (−0.240 to 0.355) | 0.389 | – | |
| body weight (in kg) | −0.010 (−0.023 to 0.003) | 0.115 | – | |
| HIV status | 0.068 (−0.197 to 0.333) | 0.607 | – | |
| acetylator status (fast versus slow)b | −0.547 (−0.723 to −0.372) | <0.001 | −0.528 (−0.685 to −0.372) | <0.001 |
| Isoniazid log10Cmax | ||||
| DM | −0.225 (−0.394 to −0.056) | 0.010 | −0.197 (−0.381 to −0.012) | 0.037 |
| age (in years) | −0.007 (−0.014 to 0.000) | 0.050 | −0.004 (−0.011 to −0.003) | 0.256 |
| gender (male versus female) | −0.065 (−0.283 to 0.153) | 0.547 | – | |
| body weight (in kg) | −0.006 (−0.016 to 0.003) | 0.167 | – | |
| HIV status | 0.105 (−0.880 to 0.297) | 0.277 | – | |
| acetylator status (fast versus slow)b | −0.252 (−0.418 to −0.086) | 0.004 | −0.546 (−0.705 to −0.387) | <0.001 |
| Pyrazinamide log10 AUC0–24 | ||||
| DM | −0.075 (−0.160 to 0.010) | 0.083 | – | |
| age (in years) | 0.000 (−0.004 to 0.003) | 0.810 | – | |
| gender (male versus female) | −0.001 (−0.106 to 0.104) | 0.986 | – | |
| body weight (in kg) | −0.001 (−0.006 to 0.004) | 0.653 | – | |
| HIV status | 0.025 (−0.069 to 0.118) | 0.533 | – | |
| Pyrazinamide log10Cmax | ||||
| DM | −0.044 (−0.096 to 0.007) | 0.090 | – | |
| age (in years) | −0.001 (−0.003 to 0.001) | 0.307 | – | |
| gender (male versus female) | −0.065 (−0.125 to −0.004) | 0.036 | – | |
| body weight (in kg) | 0.000 (−0.003 to 0.003) | 0.912 | – | |
| HIV status | 0.059 (0.005 to 0.112) | 0.033 | – | |
| Ethambutol log10 AUC0–24 | ||||
| DM | −0.013 (−0.110 to 0.084) | 0.785 | – | |
| age (in years) | 0.001 (−0.003 to 0.005) | 0.624 | – | |
| gender (male versus female) | −0.068 (−0.181 to 0.045) | 0.230 | – | |
| body weight (in kg) | −0.002 (−0.007 to 0.003) | 0.430 | – | |
| HIV status | 0.027 (−0.075 to 0.130) | 0.593 | – | |
| Ethambutol log10Cmax | ||||
| DM | −0.022 (−0.125 to 0.082) | 0.672 | – | |
| age (in years) | −0.003 (−0.006 to 0.001) | 0.201 | – | |
| gender (male versus female) | −0.087 (−0.206 to 0.033) | 0.150 | – | |
| body weight (in kg) | 0.000 (−0.005 to 0.006) | 0.875 | – | |
| HIV status | 0.077 (−0.030 to 0.184) | 0.154 | – | |
The multivariate analysis was primarily used to correct for potential confounding for the association between DM and the exposure to TB drugs. Therefore, this multivariate analysis has only been performed for isoniazid log10 AUC0–24 and isoniazid log10Cmax. P values ≤0.05 are shown in bold.
In this analysis, acetylator status based on isoniazid elimination half-life was used, as this is considered the gold standard for phenotypic assessment of acetylator status.
The linear regression analysis showed that TB patients with DM or a fast acetylator status had a lower isoniazid AUC0–24 and lower isoniazid Cmax. These associations remained statistically significant in the multivariate linear regression analysis.
Neither diabetes nor any other parameters was associated with pyrazinamide AUC0–24, ethambutol AUC0–24 or ethambutol Cmax. However, female and HIV-positive TB patients had a higher pyrazinamide Cmax compared with either male patients or HIV-negative TB patients.
Within the group of TB patients with diabetes, none of the AUC0–24 and Cmax values was associated with either fasting blood glucose or HbA1c (data not shown).
Discussion
This is the first study that evaluates the exposure to all four first-line TB drugs in diabetic and non-diabetic TB patients as assessed by intensive pharmacokinetic sampling in an African population. We found a trend (P=0.052) for a lower AUC0–24 of rifampicin in diabetic versus non-diabetic TB patients when using a t-test to compare groups and a significantly lower rifampicin AUC0–24 when using linear regression analysis. Similarly, AUC0–24 and Cmax of isoniazid were decreased in diabetic TB patients, also when corrected for acetylator status which also predicted the AUC0–24 and Cmax of isoniazid, as expected.
Similar studies in Indonesia, Korea, Turkey, India and Peru have shown contradictory results,18–23 with some studies focusing on only one TB drug and some being limited by measurement of just a single or a few samples. Our study was carried out in a distinctly different population of Tanzanians (which admittedly cannot be regarded as one homogenous ethnic group), involved all four standard TB drugs and used intensive pharmacokinetic sampling (nine sampling points over a 24 h dosing interval). In one of the Indonesian studies, patients were matched for body weight to disentangle the effects of weight and DM.19 Although we did not match our patients for body weight, the distribution of weight (and therefore drug dose per kg) was the same in diabetic and non-diabetic TB patients, and weight was not a predictor of exposure to TB drugs in our multiple linear regression analyses. Since there is no evidence that antidiabetic drugs lower the concentration of TB drugs, we believe the observed differences in exposure are due to DM.
It is unknown how DM would affect the exposure to rifampicin and isoniazid. DM influences the pharmacokinetics of various other drugs by affecting: (i) absorption, due to changes in subcutaneous and muscle blood flow and delayed gastric emptying; (ii) distribution, due to non-enzymatic glycation of albumin; (iii) biotransformation, due to differential regulation of enzymes involved in drug metabolism and transport; and (iv) excretion, due to nephropathy.8,9 As to rifampicin, the current study suggests that the effect of DM is not mediated by a change in the biotransformation of rifampicin into its main metabolite desacetyl-rifampicin (Table 2). Furthermore, it is not known whether it is DM per se or the suboptimal control of the disease that affects exposure to TB drugs. Most of the diabetic TB patients in this study had suboptimally controlled diabetes, which is not surprising as rifampicin may induce hyperglycaemia31,32 and it lowers the exposure to many oral antidiabetic drugs.3 Fewer such interactions are expected when using metformin33 and insulin.
The lower exposure to TB drugs as shown in this and other studies18,20–22 may explain the poorer response to TB drugs in diabetic TB patients.10–17 In order to attain population average drug exposures that are associated with good treatment outcome in the majority of patients, it seems advisable to measure plasma TB drug concentrations and individualize doses in diabetic TB patients (therapeutic drug monitoring29,34), but this tool is not available in many resource-poor settings. An alternative is to increase the dose of rifampicin and isoniazid in the whole population of diabetic TB patients. Higher doses of rifampicin (up to 35 mg/kg daily) have been shown to be safe and tolerable in patients with pulmonary TB,35 and high-dose isoniazid is already being used for MDR TB.36 Clearly such intensified treatment of TB in diabetic patients needs to be evaluated in clinical trials first.
Our study findings may be limited by an unequal age distribution between the groups, the diabetic TB group having on average older patients than the non-diabetic TB group, as expected. However, age has not been found to be associated with pharmacokinetics of TB drugs in many studies including the current study.18,19,27 Similarly we did not match the groups on gender and weight, but the distribution of these parameters was the same in the two groups and they were also not significant predictors of TB drug exposure in multiple regression analyses. Furthermore, the number of patients (n=39) may be high for a pharmacokinetic study with intensive pharmacokinetic sampling, yet this number is relatively low to perform multiple regression analyses with several possible explanatory variables. Finally, proportions of fast and slow acetylators for isoniazid were similar among diabetics and non-diabetics (Table 1), but we realize that we used phenotyping methods (i.e. based on exposure to acetyl-isoniazid and isoniazid) to assess this acetylator status, whereas we evaluated the effect of DM on the pharmacokinetics of isoniazid at the same time. No funding for genotypic assessment of acetylator status was available.
In summary, we have shown lower isoniazid plasma concentrations and a trend towards decreased exposure to rifampicin in Tanzanian diabetic TB patients. We conclude that diabetes and not body weight differences are likely to be responsible for these differences in exposure. More studies in Africa are warranted to confirm our findings. There is a need for prospective evaluation of individualized dosing of isoniazid and rifampicin based on plasma concentration measurements (therapeutic drug monitoring) and for clinical trials on fixed, higher doses of these TB drugs in patients with TB and DM.
Acknowledgements
We wish to thank: all patients for participating in this study; the staff of the TB clinic at Mawenzi Hospital in Moshi and the research nurses at KCMC for their cooperation and effort; and the laboratory technicians at KCMC and at the Department of Pharmacy of Radboud university medical center, The Netherlands, for their technical support.
Funding
This study was supported by the African Poverty Related Infection Oriented Research Initiative (APRIORI), a research network granted by the Netherlands–African partnership for Capacity development and Clinical interventions Against Poverty-related diseases (NACCAP/EDCTP), and by funding from a UNESCO/L’Oreal For Young Women in Science Fellowship (awarded to A. Tostmann).
Transparency declarations
None to declare.
References
- 1. Boucot KR. Diabetes mellitus and pulmonary tuberculosis. J Chronic Dis 1957; 6: 256–79. [DOI] [PubMed] [Google Scholar]
- 2. Restrepo BI. Convergence of the tuberculosis and diabetes epidemics: renewal of old acquaintances. Clin Infect Dis 2007; 45: 436–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ruslami R, Aarnoutse RE, Alisjahbana B. et al. Implications of the global increase of diabetes for tuberculosis control and patient care. Trop Med Int Health 2010; 15: 1289–99. [DOI] [PubMed] [Google Scholar]
- 4. Pizzol D, Di Gennaro F, Chhaganlal KD. et al. Tuberculosis and diabetes: current state and future perspectives. Trop Med Int Health 2016; 21: 694–702. [DOI] [PubMed] [Google Scholar]
- 5. Jeon CY, Murray MB.. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med 2008; 5: e152.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ronacher K, Joosten SA, van Crevel R. et al. Acquired immunodeficiencies and tuberculosis: focus on HIV/AIDS and diabetes mellitus. Immunol Rev 2015; 264: 121–37. [DOI] [PubMed] [Google Scholar]
- 7. Baker MA, Harries AD, Jeon CY. et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med 2011; 9: 81.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gwilt PR, Nahhas RR, Tracewell WG.. The effects of diabetes mellitus on pharmacokinetics and pharmacodynamics in humans. Clin Pharmacokinet 1991; 20: 477–90. [DOI] [PubMed] [Google Scholar]
- 9. Dostalek M, Akhlaghi F, Puzanovova M.. Effect of diabetes mellitus on pharmacokinetic and pharmacodynamic properties of drugs. Clin Pharmacokinet 2012; 51: 481–99. [DOI] [PubMed] [Google Scholar]
- 10. Kimerling ME, Phillips P, Patterson P. et al. Low serum antimycobacterial drug levels in non-HIV-infected tuberculosis patients. Chest 1998; 113: 1178–83. [DOI] [PubMed] [Google Scholar]
- 11. Mehta JB, Shantaveerapa H, Byrd RP Jr. et al. Utility of rifampin blood levels in the treatment and follow-up of active pulmonary tuberculosis in patients who were slow to respond to routine directly observed therapy. Chest 2001; 120: 1520–4. [DOI] [PubMed] [Google Scholar]
- 12. Weiner M, Burman W, Vernon A. et al. Low isoniazid concentrations and outcome of tuberculosis treatment with once-weekly isoniazid and rifapentine. Am J Respir Crit Care Med 2003; 167: 1341–7. [DOI] [PubMed] [Google Scholar]
- 13. Weiner M, Benator D, Burman W. et al. Association between acquired rifamycin resistance and the pharmacokinetics of rifabutin and isoniazid among patients with HIV and tuberculosis. Clin Infect Dis 2005; 40: 1481–91. [DOI] [PubMed] [Google Scholar]
- 14. Chideya S, Winston CA, Peloquin CA. et al. Isoniazid, rifampin, ethambutol, and pyrazinamide pharmacokinetics and treatment outcomes among a predominantly HIV-infected cohort of adults with tuberculosis from Botswana. Clin Infect Dis 2009; 48: 1685–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pasipanodya JG, Srivastava S, Gumbo T.. Meta-analysis of clinical studies supports the pharmacokinetic variability hypothesis for acquired drug resistance and failure of antituberculosis therapy. Clin Infect Dis 2012; 55: 169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pasipanodya JG, McIlleron H, Burger A. et al. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J Infect Dis 2013; 208: 1464–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Svensson EM, Svensson RJ, te Brake LHM. et al. The potential for treatment shortening with higher rifampicin doses: relating drug exposure to treatment response in patients with pulmonary tuberculosis. Clin Infect Dis 2018; 67: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nijland HM, Ruslami R, Stalenhoef JE. et al. Exposure to rifampicin is strongly reduced in patients with tuberculosis and type 2 diabetes. Clin Infect Dis 2006; 43: 848–54. [DOI] [PubMed] [Google Scholar]
- 19. Ruslami R, Nijland HM, Adhiarta IG. et al. Pharmacokinetics of antituberculosis drugs in pulmonary tuberculosis patients with type 2 diabetes. Antimicrob Agents Chemother 2010; 54: 1068–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Babalik A, Ulus IH, Bakirci N. et al. Plasma concentrations of isoniazid and rifampin are decreased in adult pulmonary tuberculosis patients with diabetes mellitus. Antimicrob Agents Chemother 2013; 57: 5740–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chang MJ, Chae JW, Yun HY. et al. Effects of type 2 diabetes mellitus on the population pharmacokinetics of rifampin in tuberculosis patients. Tuberculosis (Edinb) 2015; 95: 54–9. [DOI] [PubMed] [Google Scholar]
- 22. Alfarisi O, Mave V, Gaikwad S. et al. Effect of diabetes mellitus on the pharmacokinetics and pharmacodynamics of tuberculosis treatment. Antimicrob Agents Chemother 2018; 62: e01383-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Requena-Mendez A, Davies G, Ardrey A. et al. Pharmacokinetics of rifampin in Peruvian tuberculosis patients with and without comorbid diabetes or HIV. Antimicrob Agents Chemother 2012; 56: 2357–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wilkinson GR. Drug metabolism and variability among patients in drug response. N Engl J Med 2005; 352: 2211.. [DOI] [PubMed] [Google Scholar]
- 25.WHO. Global Tuberculosis Report 2018. Geneva, Switzerland: WHO, 2018. http://www.who.int/tb/publications/global_report/en/. [Google Scholar]
- 26. Atun R, Davies JI, Gale EAM. et al. Diabetes in sub-Saharan Africa: from clinical care to health policy. Lancet Diabetes Endocrinol 2017; 5: 622–67. [DOI] [PubMed] [Google Scholar]
- 27. Ruslami R, Nijland HM, Alisjahbana B. et al. Pharmacokinetics and tolerability of a higher rifampin dose versus the standard dose in pulmonary tuberculosis patients. Antimicrob Agents Chemother 2007; 51: 2546–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO. Definition, Diagnosis, and Classification of Diabetes Mellitus and Its Complications. Geneva, Switzerland: WHO, 1999. https://apps.who.int/iris/handle/10665/66040. [Google Scholar]
- 29. Peloquin CA. Therapeutic drug monitoring in the treatment of tuberculosis. Drugs 2002; 62: 2169–83. [DOI] [PubMed] [Google Scholar]
- 30. Hutchings A, Routledge PA.. A simple method for determining acetylator phenotype using isoniazid. Br J Clin Pharmacol 1986; 22: 343–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takasu N, Yamada T, Miura H. et al. Rifampicin-induced early phase hyperglycemia in humans. Am Rev Respir Dis 1982; 125: 23–7. [DOI] [PubMed] [Google Scholar]
- 32. Waterhouse M, Wilson C, White VL. et al. Resolution of insulin-requiring diabetes after cessation of chemotherapy for tuberculosis. J R Soc Med 2005; 98: 270–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Te Brake LHM, Yunivita V, Livia R. et al. Rifampicin alters metformin plasma exposure but not blood glucose levels in diabetic tuberculosis patients. Clin Pharmacol Ther 2019; 105: 730–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Magis-Escurra C, van den Boogaard J, Ijdema D. et al. Therapeutic drug monitoring in the treatment of tuberculosis patients. Pulm Pharmacol Ther 2012; 25: 83–6. [DOI] [PubMed] [Google Scholar]
- 35. Boeree MJ, Heinrich N, Aarnoutse R. et al. High-dose rifampicin, moxifloxacin, and SQ109 for treating tuberculosis: a multi-arm, multi-stage randomised controlled trial. Lancet Infect Dis 2017; 17: 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.WHO. WHO Treatment Guidelines for Drug-Resistant Tuberculosis, 2016 Update. Geneva, Switzerland: WHO, 2016. https://apps.who.int/iris/bitstream/handle/10665/250125/9789241549639-eng.pdf?ua=1?sequence=1. [PubMed] [Google Scholar]