Abstract
Purpose
Laryngeal cancer is the most prevalent tumor type in head and neck cancers. Early diagnosis is considered as an important strategy for improving prognosis. The lncRNA DLX6-AS1 has been shown to modulate tumor phenotypes in several types of cancer, but the role of DLX6-AS1 in laryngeal cancer and its concrete mechanisms are not clear.
Methods
Tissue samples from laryngeal cancer patients and corresponding clinical data were used for detailed analysis. The laryngeal cancer cell lines HEp-2 and Tu-177 were studied. Cell proliferation, ROS production, mitochondrial respiratory function, intracellular and mitochondrial calcium influx were assessed. Western blotting, quantitative RT-PCR and luciferase assays were used to analyze the interactions. A xenografted tumor model was established to analyze the effects of DLX6-AS1 on tumor growth in vivo.
Results
lncRNA DLX6-AS1 had increased expression in tumor tissues compared with adjacent normal tissues and in higher clinical stages compared with lower stages, which was associated with poor prognosis. In detail, DLX6-AS1 knockdown decreased cell proliferation and affected key mitochondrial metabolic parameters in both HEp-2 and Tu-177 cells. Moreover, DLX6-AS1 knockdown suppressed TRPC3-mediated mitochondrial calcium uptake and ROS production. Furthermore, miR-26a functioned as a link between these two molecules, as it could be absorbed by DLX6-AS1 and thus regulated the levels of TRPC3. Finally, the DLX6-AS1/miR-26a/TRPC3 axis modulated laryngeal cancer proliferation both in vitro and in vivo.
Conclusion
This study provides new evidence that a novel lncRNA, DLX6-AS1, regulates mitochondrial calcium homeostasis, respiration and tumor proliferation via modulating the miR-26a/TRPC3 axis in laryngeal cancer.
Keywords: laryngeal cancer, DLX6-AS1, miR-26a, mitochondria, TRPC3
Introduction
According to cancer epidemiology statistics, laryngeal carcinoma is the most prevalent head and neck cancer and accounts for 25% to 30% of these cases and 1% to 2.5% of all malignancies.1,2 With advances in early diagnosis and treatment strategies, the prognosis of these patients has been quickly improved and early stage laryngeal carcinomas (T1 and T2) can have up to an 80% to 90% cure rate, whereas patients with advanced clinical stages have only a 60% chance of cure.3,4 In addition, the pathogenesis of laryngeal cancer is a complex, multistep-process disease that includes genetic dysregulation of protooncogenes and tumor suppressor genes.5 Therefore, it is urgent to investigate novel diagnostic markers involved in the initiation and progression of laryngeal cancer for early diagnosis and treatment.
Long noncoding RNAs (lncRNAs) are defined as noncoding RNAs with transcripts longer than 200 nucleotides in length. In recent years, lncRNAs have been shown to drive many important cancer phenotypes through their interactions with other cellular macromolecules including DNA, protein, and RNA.6 In laryngeal carcinomas, the role of lncRNAs has been reported7 and several lncRNAs were identified to play important roles in regulating the inflammation,8 proliferation and migration,9,10 cancer stemness,11 etc. Additionally, lncRNA DLX6-AS1 has been shown to promote tumor growth by modulating HSP90 expression in bladder cancer,12 regulating cell invasion in cervical cancer13 and epithelial-mesenchymal transition in gastric cancer,14 and promoting tumorigenesis through the STAT3 signaling pathway in liver cancer stem cells.15 However, the role of DLX6-AS1 in laryngeal cancer has not been defined.
Altered cellular metabolism is the hallmark of cancer cells and is the fundamental mechanism of tumor cells to adapt to the high demand of nutrients.16 In the metabolism of cancer cells, increased ROS production accounts for increased proliferation, and mitochondria are the main source of ROS in cells.17,18 Calcium is one of the most important second messengers in the cytoplasm and is also the key catalyst for a variety of mitochondrial enzymes located in the respiratory chain, such as pyruvate dehydrogenase and isocitrate dehydrogenase.19 Previous studies also indicated that calcium signaling participates in the tumorigenesis of multiple cancer types.20 Among the various calcium channels, the transient receptor potential channel, type C, member 3 (TRPC3) has been shown to be located both in the plasma membrane and mitochondrial inner membrane.21 TRPC3 has been shown to regulate both cellular and mitochondrial calcium homeostasis, but its role, expression and regulation in laryngeal cancer have not been defined in previous reports. In this study, the laryngeal cancer cell lines HEp-2 and Tu-177, clinical samples and grafted tumor models were used to analyze the effects and mechanism of lncRNA DLX6-AS1 on cellular proliferation while identifying the role of the DLX6-AS1/miR-26a/TRPC3 axis on mitochondrial function and ROS production.
Materials and Methods
Patients and Samples
The study protocols for using tumor samples and patient data were approved by the ethical committees of the Second Weihai Municipal Hospital affiliated to Qingdao University. The patients with laryngeal cancer were diagnosed by tissue biopsies and treated at this hospital. A written informed consent form was signed by each enrolled patient. Tumor and adjacent tissues were frozen in liquid nitrogen after extraction and then stored at −80 °C. In the process of data analysis, all enrolled patients were registered and numbered in a database with no personal information provided.
Cell Culture and Cell Proliferation Assay
The human laryngeal cancer cell lines HEp-2 and Tu-177 were purchased from the Cell Bank of Chinese Academy of Sciences (Shanghai, China). The two cell lines were cultured according to the ATCC (American Type Culture Collection) instructions. Cell proliferation assay was conducted using the Cell Counting Kit-8 (Dojindo Molecular Technologies, Kumamoto, Japan). The experiments were conducted according to the protocols and OD450 values were measured using a microplate reader.
Quantitative RT-PCR
The Quantitative reverse transcription-PCR was performed as previously described.22 Total RNAs and miRNA were extracted using TRIzol and a miRNA isolation kit (Invitrogen, Waltham, MA, USA). The primers used were as follows: DLX6-AS1: F: 5′-AGTTTCTCTCTAGATTGCCTT-3′ and R: 5′-ATTGACATGTTAGTGCCCTT-3′; miR-26a: F: 5′-GGATCCGCAGAAACTCCAGAGAGAAGGA-3′ and R: 3′-AAGCTTGCCTTTAGCAGAAAGGAGGTT-5′; U6 primer: F: 5′-ATCCGCAAAGACCTGT-3′ and R: 5′-GGGTGTAACACTAAG-3′; and GAPDH: F: 5′-GGGAAATTCAACGGCACAGT-3′ and R: 5′-AGATGGTGATGGGCTTCCC-3′.
Mitochondrial Function Assays
Mitochondrial respiratory functions were measured using the XF96 metabolic flux analyzer (Seahorse Biosciences, Billerica, MA, USA) to test the mitochondrial respiration (OCR) of cultured cells according to the manufacturer’s instructions. The results were analyzed using XFe Wave software (Seahorse Biosciences).
Cellular and mitochondrial ROS production was measured in cultured laryngeal cancer cells using DHE (dihydroethidium) and MitoSOX dyes (ThermoFisher, Waltham, MA, USA) according to the protocols. Mitochondrial ATP synthesis and H2O2 levels were detected using commercial experimental kits (Beyotime, Shanghai, China).
Western Blotting
Western blotting was conducted as previously described.23 Briefly, total proteins were extracted from cells or tissues and 30 to 60 μg proteins were loaded on gels and separated by electrophoresis. Then, the proteins were transferred to PVDF membranes, blocked and incubated with the corresponding primary and secondary antibodies. The final detection was performed using an electrochemiluminescence substrate (Merck-Millipore, Darmstadt, Germany). The primary antibodies used were anti-TRPC3 (Alomone Labs, Jerusalem, Israel) and anti-GAPDH (Santa Cruz Biotechnology, Dallas, TX, USA).
Intracellular and Mitochondrial Calcium Measurements
The protocols for measuring intracellular calcium uptake were described in previous studies.21 Briefly, the laryngeal cancer cells HEp-2 and Tu-177 were digested and incubated with 1 μmol/L Fura-2 dye for 30 mins in the dark after the corresponding treatments. Then, calcium-free Hank’s balanced salt solution (HBSS) was used for cell washing and fluorescence detection, with thapsigargin as the agonist. Fluorescence was measured at baseline and after treatment at an emission of 510 nm using excitation wavelengths of 340 and 380 nm. For mitochondrial calcium measurements, laryngeal cancer cells were subjected to specific treatments, and then the cells were incubated with 5 μmol/L Rhod-2 AM for 30 mins. Then, the cells were permeabilized with 10 μmol/L digitonin for five minutes in HBSS. ATP was used as an agonist for mitochondrial calcium detection at an emission wavelength of 581 nm and excitation wavelength of 552 nm.
Luciferase Reporter Assay
Dual luciferase reporter assays were performed to verify the binding between lncRNA DLX6-AS1, miR-26a and the 3ʹ-untranslated region (UTR) of TRPC3. In these experiments, pmirGLO-WT-DLX or pmirGLO-Mut-DLX and miR-26a mimics or miR-NC were co-transfected into laryngeal cancer cells. The pmirGLO-WT-TRPC3 or pmirGLO-Mut-TRPC3 were co-transfected with miR-26a mimics or miR-NC in a similar way. The pRL-TK plasmid was also co-transfected as the internal control. After 48 hrs, cells were lysed, and luciferase measurements were performed using a luciferase assay kit (Promega, WI, USA) and a Promega GloMax 20/20 machine.
Animal Experiments
Five-week-old male BALB/c nude mice were purchased from the animal center of Qingdao University. All experiments were conducted in accordance with the animal experimental protocols released by Qingdao University and approved by the Animal Care Committee of Qingdao University. The mice were randomly divided into four groups (HEp-2-siNC, HEp-2-siDLX, Tu-177-siNC and Tu-177-siDLX, each with six mice) and 2×106 cells were subcutaneously injected into the right flanks of the nude mice. Before injections, the cells were counted using Coulter Counter (Beckman Coulter Multisizer, Fulton, MO, USA). The size of the tumors was measured and recorded every five days. After 30 days, all mice were sacrificed and tumors were isolated for further analysis. The volume of each xenografted tumor was measured, and mitochondrial function analysis and immunoblotting were performed.
Statistical Analysis
The data in this study are presented as the mean ± SEM. The comparisons between two groups were performed by Student’s t-test, while the comparisons among three or more groups were conducted using one-way ANOVA. Two-tailed P values less than 0.05 were considered as statistically significant.
Results
DLX6-AS1 Expression Is Correlated with the Prognosis of Laryngeal Cancer Patients
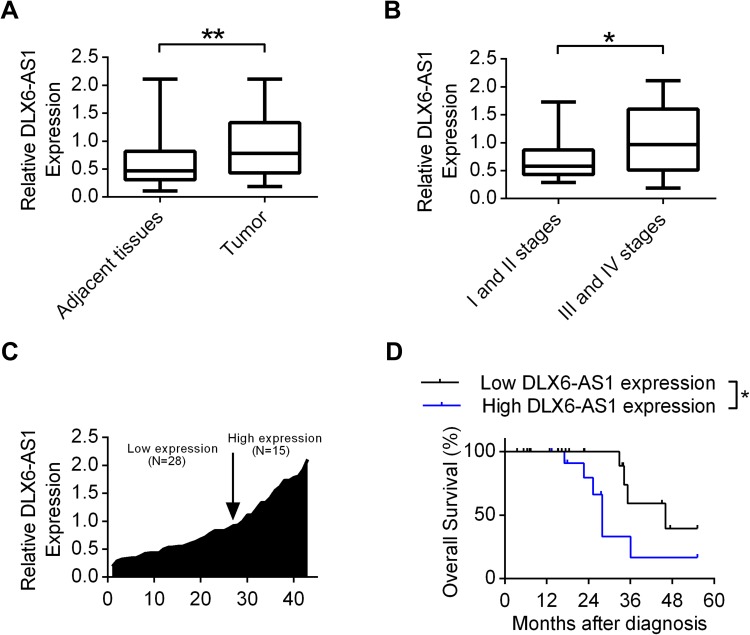
First, the expression profiles of DLX6-AS1 in tumor tissues and adjacent normal tissues of laryngeal cancer patients were analyzed by quantitative RT-PCR. The expression levels of DLX6-AS1 in tumors were increased compared with adjacent tissues (Figure 1A). Additionally, the patients enrolled were divided according to the clinical stages (stage I: 7 patients, stage II: 12 patients, stage III: 13 patients, and stage IV: 11 patients). It was shown that the tumor tissues from clinical stage III and IV patients had augmented DLX6-AS1 expression compared with those from stages I and II patients (Figure 1B). Furthermore, a total of 43 patients were classified into low and high expression levels according to the relative DLX6-AS1 levels of patient No.1 (Figure 1C). Table 1 shows that the expression levels of DLX6-AS1 correlated with primary tumor size, tumor clinical stage and metastasis, whereas no correlation was observed between DLX6-AS1 expression and gender/age. The Kaplan-Meier survival analysis in Figure 1D shows that patients with relatively higher expression of DLX6-AS1 had decreased overall survival compared with those with lower expression of DLX6-AS1. These results indicated that DLX6-AS1 influenced the prognosis of laryngeal cancer patients and might play important roles in the cell phenotypes of laryngeal tumor cells.
Figure 1.
The expression levels of DLX6-AS1 correlated with the prognosis of laryngeal cancer patients. (A) The expression levels of lncRNA DLX6-AS1 was investigated in tumor and adjacent tissues in laryngeal cancer patients using Quantitative RT-PCR. **P<0.01. (B) The expression levels of DLX6-AS1 was measured in laryngeal cancer patients with stages I and II verses stages III and IV by qRT-PCR. *P<0.05. (C) The relative expression levels of DLX6-AS1 were measured via qRT-PCR and rated relative to patient No.1. (D) The effects of DLX6-AS1 on overall survivals were plotted using Kaplan-Meier survival analysis. *P<0.05. Date were presented as Mean ± SD.
Table 1.
Correlation Between lncRNA DLX6-AS1 Expression and Clinicopathological Parameters of Patients with Laryngeal Cancer Enrolled
| Characteristics | Low DLX6-AS1 (N=28) | High DLX6-AS1 (N=15) | P values |
|---|---|---|---|
| Gender | 0.446 | ||
| Male | 20 | 9 | |
| Female | 8 | 6 | |
| Age (years) | 0.711 | ||
| ≥50 | 21 | 12 | |
| <50 | 7 | 3 | |
| Primary tumor | 0.005** | ||
| T1+T2 | 23 | 6 | |
| T3+T4 | 5 | 9 | |
| Tumor stage (TNM) | 0.019* | ||
| I, II | 16 | 3 | |
| III, IV | 12 | 12 | |
| Lymphatic metastasis (Yes) | 12 | 12 | 0.019* |
| Distant metastasis (Yes) | 3 | 4 | 0.177* |
Notes: *P<0.05, **P<0.01.
lncRNA DLX6-AS1 Increased Cell Proliferation by Promoting Mitochondrial ROS Production
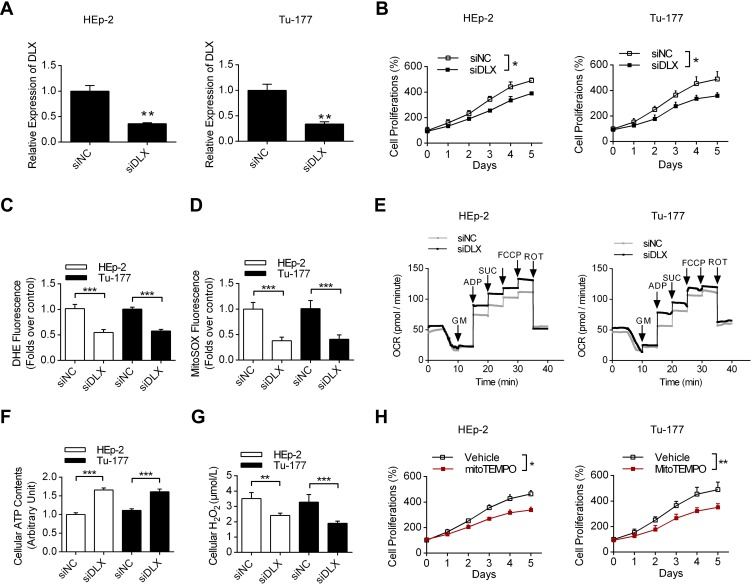
Then, using the laryngeal cancer cell lines HEp-2 and Tu-177, we first analyzed the efficacy of DLX6-AS1specific siRNA, and the results in Figure 2A show that the siRNA could effectively decrease the expression levels of DLX6-AS1. Moreover, DLX6-AS1 knockdown decreased the proliferation rates of both HEp-2 and Tu-177 cells compared with siNC treatment (Figure 2B). More importantly, the ROS levels of these two cells were detected using two fluorescence dyes, DHE and MitoSOX. The data showed that DLX6-AS1 knockdown decreased the production of both cellular and mitochondrial ROS in HEp-2 and Tu-177 cells (Figure 2C and D). Additionally, DLX6-AS1 knockdown upregulated key mitochondrial respiratory parameters, such as complex I OXPHOS, complex I+II OXPHOS and complex I+II ETS, in both HEp-2 and Tu-177 cells (Figure 2E). Figure 2F and G show that siDLX6-AS1 promoted ATP synthesis and decreased H2O2 production in laryngeal cancer cells, which is consistent with the effects on mitochondrial respiratory functions. Furthermore, the addition of the mitochondrial ROS scavenger mitoTEMPO also partially inhibited cell proliferation (Figure 2H), indicating that DLX6-AS1 is correlated with excessive ROS production and promotes cancer cell growth.
Figure 2.
The effects of DLX6-AS1 on cell proliferation and mitochondrial functions. (A) The effects of DLX6-AS1 specific siRNA on the levels of DLX6-AS1 using qRT-PCR in both HEp-2 and Tu-177 cells. **P<0.01. (B) The effects of DLX6-AS1 specific siRNA on cell proliferations. *P<0.05. (C, D) The effects of siDLX6-AS1 on cellular and mitochondrial ROS production in these two cells, using DHE and MitoSOX dyes, respectively. ***P<0.001. (E) The effects of siDLX6-AS1 on mitochondrial oxidative respiration using Seahorse respirometry. (F, G) The effects of DLX6-AS1 specific siRNA on ATP synthesis and H2O2 production in these two cells. **P<0.01, ***P<0.001. (H) The effects of mitochondrial specific ROS scavenger mitoTEMPO on cell proliferation in laryngeal cancer cells. *P<0.05, **P<0.01.
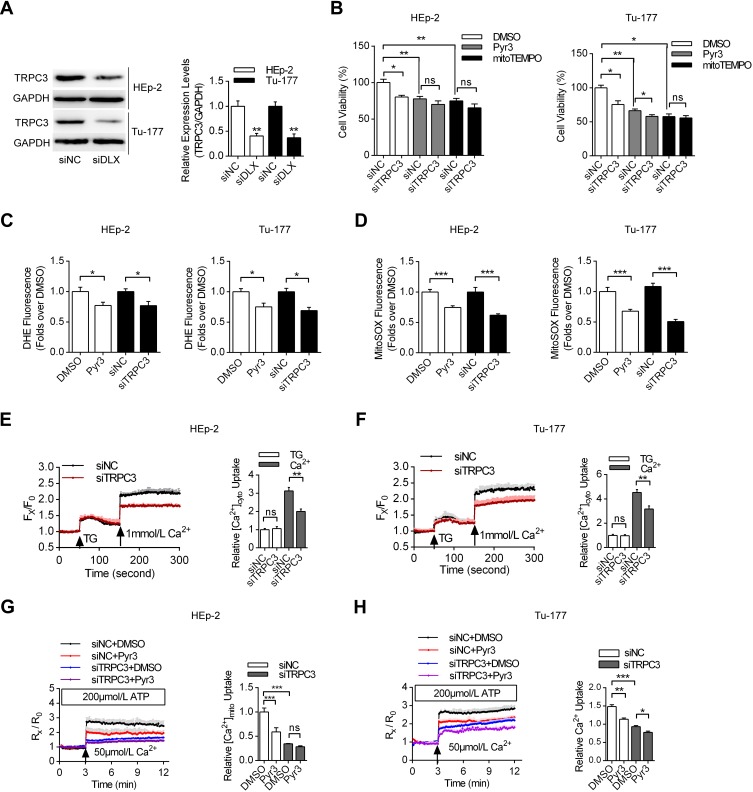
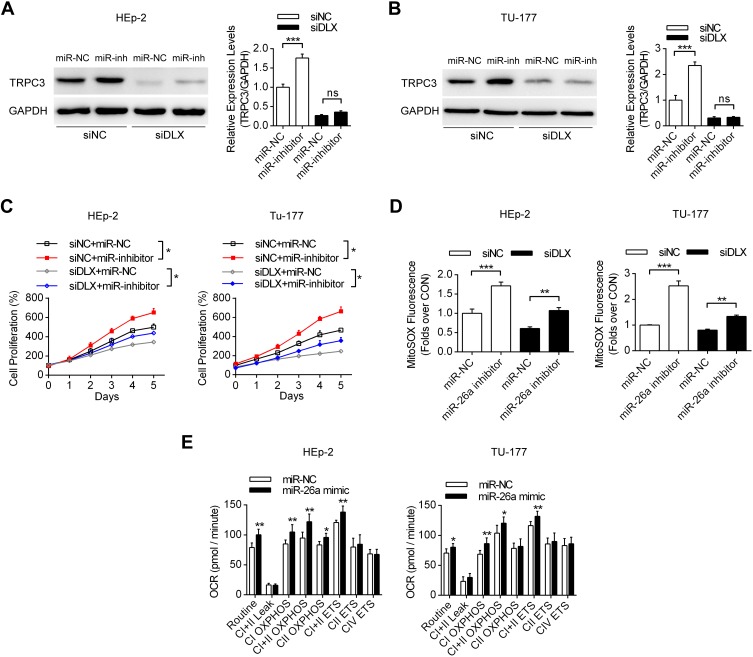
TRPC3 Upregulation Induced by lncRNA DLX6-AS1 Promoted Cell Proliferation and Mitochondrial Calcium Overload
Next, we analyzed the expression patterns of TRPC3, which is one of the key ion channels located in both the plasma membrane and the mitochondrial inner membrane.21 TRPC3 has been shown to regulate mitochondrial calcium uptake and ROS production. In Figure 3A, we detected whether DLX6-AS1 knockdown could affect the expression levels of TRPC3. It was shown that siDLX6-AS1 significantly decreased the TRPC3 expression levels in both HEp-2 and Tu-177 cells. Moreover, TRPC3 knockdown suppressed the proliferation of laryngeal cancer cells, while inhibition of TRPC3 by Pyr3 and depletion of mitochondrial ROS by mitoTEMPO partially decreased the differences between siNC and siTRPC3 (Figure 3B). Additionally, Figure 3C and D indicate that Pyr3 and TRPC3 knockdown decreased both the cellular and mitochondrial ROS production in HEp-2 and Tu-177 cells. Furthermore, we investigated the calcium homeostasis of these cancer cells. Figure 3E–H indicate that TRPC3 knockdown by specific siRNA decreased the cellular calcium uptake, induced by thapsigargin, and mitochondrial calcium uptake, induced by ATP in both HEp-2 and Tu-177 cells. These results indicated that TRPC3 plays vital roles in the regulation of cellular calcium homeostasis and cell proliferation.
Figure 3.
The effects of lncRNA DLX6-AS1-mediated TRPC3 upregulation on cell proliferation and calcium homeostasis. (A) The effects of DLX6-AS1 specific siRNA on the expression levels of TRPC3 using Western blotting. **P<0.01. (B) The effects of Pyr3 and mitoTEMPO on the laryngeal cancer cell proliferation treated with siNC and siTRPC3, respectively. *P<0.05, **P<0.01. (C, D) The effects of Pyr3 and TRPC3 specific siRNA on cellular and mitochondrial ROS production, compared with DMSO or siNC, respectively. *P<0.05, ***P<0.001. (E, F) The effects of siTRPC3 on cellular calcium uptake, induced by thapsigargin, compared with siNC. The laryngeal cancer cells were preincubated with Fura-2, with Fx/F0 indicated that the fluorescence values of certain dots versus the baseline of the measurements. **P<0.01. (G, H) The effects of TRPC3 inhibition on mitochondrial calcium uptake, induced by ATP in permeabilized cells. *P<0.05, **P<0.01, ***P<0.001.
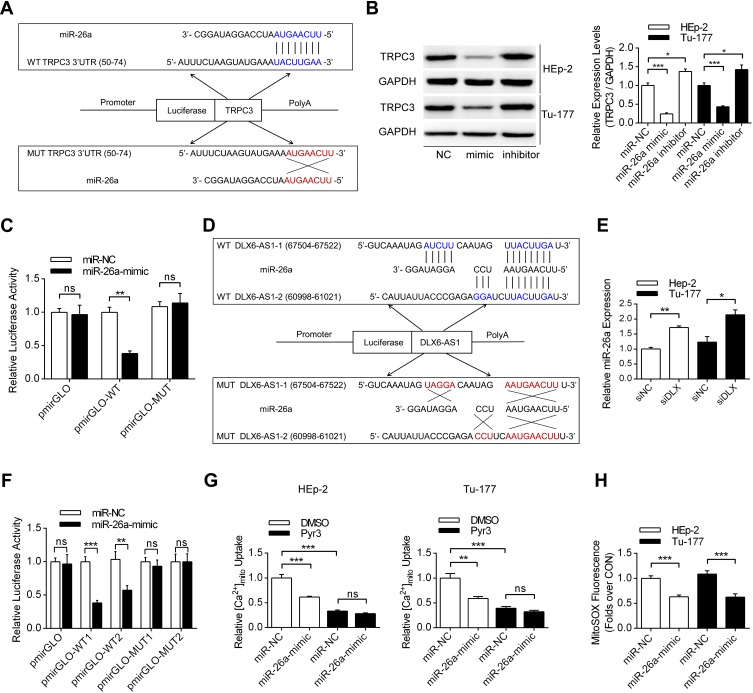
miR-26a Functioned as a Link Between lncRNA DLX6-AS1 and TRPC3
The lncRNA DLX6-AS1 could function as a decoy by absorbing microRNA and thus regulating the expression levels of key proteins. Among the several microRNAs binding to TRPC3 (miR-26a, miR-26b, miR-98), miR-26a could also be sponged by DLX6-AS1 using LncBase V2 database predictions. It was demonstrated that miR-26a could bind to the 3ʹ-UTR of TRPC3 mRNA and decrease the expression levels of TRPC3 in both HEp-2 and Tu-177 cells (Figure 4A and B). Moreover, in laryngeal cancer cells transfected with the wild-type pmirGLO plasmid, co-transfection with the miR-26a mimic decreased the relative luciferase activities in HEp-2 cells, while no significant difference was observed in transfections with the mutant plasmid (Figure 4C). Figure 4D and E present the binding sites between DLX6-AS1 and miR-26a, showing that siDLX6-AS1 significantly increased the expression levels of miR-26a in these two cells. Additionally, co-transfection of the wild-type pmirGLO plasmid with the miR-26a mimic decreased the luciferase activities compared with the miR-NC group, whereas in the subgroups transfected with the mutant plasmid, the addition of miR-26a did not obviously affect the luciferase activity (Figure 4F). Functional experiments demonstrated that miR-26a mimic transfection decreased mitochondrial calcium uptake and mitochondrial-specific ROS production (Figure 4G and H).
Figure 4.
MiR-26a functioned as a link between DLX6-AS1 and TRPC3. (A) The detailed binding sequence of miR-26a and wild-type TRPC3 3ʹ-UTR was presented, with the mutation sequence also presented. (B) The effects of miR-26a mimic and inhibitor on the expression levels of TRPC3 was demonstrated using Western blotting. *P<0.05, ***P<0.001. (C) The luciferase assay was conducted in HEp-2 cells co-transfected pmir-GLO-WT/MUT (TRPC3 3ʹ-UTR) plasmid and miR-NC/miR-26a-mimic. **P<0.01. (D) The binding sequence of both wild-type and mutant forms of DLX6-AS1 with miR-26a was presented. (E) The effects of DLX6-AS1 knockdown on the expression levels of miR-26a were demonstrated using qRT-PCR. *P<0.05, **P<0.01. (F) The luciferase assay was conducted in HEp-2 cells co-transfected pmir-GLO-WT/MUT (DLX6-AS1) plasmid and miR-NC/miR-26a-mimic. **P<0.01, ***P<0.001. (G) The effects of miR-26a-mimic and Pyr3 on mitochondrial calcium uptake in both HEp-2 and Tu-177 cells. **P<0.01, ***P<0.001. (H) The effects of miR-26a mimic on mitochondrial ROS production using MitoSOX dye. ***P<0.001.
DLX6-AS1/miR-26a/TRPC3 Axis Regulated Laryngeal Cancer Metabolism and Proliferation
We then analyzed the effects of the DLX6-AS1/miR-26a axis on the expression levels of TRPC3, cell proliferation and mitochondrial function. Figure 5A and B show that in both HEp-2 and Tu-177 cells, transfection with DLX6-AS1 siRNA decreased TRPC3 expression levels, while co-transfection with the miR-26a inhibitor partially recovered the TRPC3 levels compare with miR-NC. Moreover, the cell proliferation assay results in Figure 5C demonstrate that transfection with DLX6-AS1 siRNA decreased cell proliferation compared with siNC, while co-transfection with miR-26a inhibitor partially recovered cell proliferation compared with miR-NC. In detail, it was shown that siDLX6-AS1 in laryngeal cancer cells suppressed ROS synthesis, whereas miR-26a inhibitor co-transfection partially increased the ROS levels, consistent with the results of cell proliferation (Figure 5D). Seahorse Bioscience detections showed that miR-26a mimic increased the mitochondrial oxidative metabolism in both HEp-2 and Tu-177 cells (Figure 5E). Therefore, these results suggested that the DLX6-AS1/miR-26a/TRPC3 axis play a role in the proliferation of laryngeal cancer cells by modulating mitochondrial metabolism and ROS production.
Figure 5.
The effects of the DLX6-AS1/miR-26a/TRPC3 axis on mitochondrial functions and cell proliferation. (A, B) The effects of DLX6-AS1 siRNA and miR-26a on the expression levels of TRPC3 using Western blotting in laryngeal cancer cells. ***P<0.001. (C) The effects of DLX6-AS1 siRNA and miR-26a on cell proliferations in both HEp-2 and Tu-177 cells. *P<0.05. (D) The effects of DLX6-AS1 siRNA and miR-26a on mitochondrial ROS productions in laryngeal cancer cells. **P<0.01, ***P<0.001. (E) The effects of miR-26a on mitochondrial oxidative respiration. *P<0.05, **P<0.01, compare with corresponding miR-NC group. *P<0.05, **P<0.01.
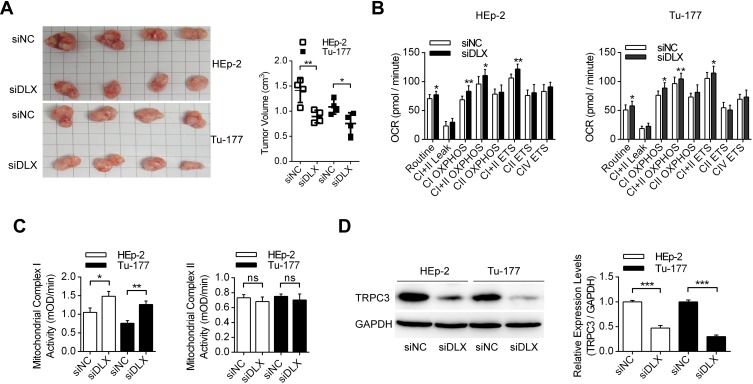
lncRNA DLX6-AS1 Promoted Laryngeal Tumor Growth in vivo
More specifically, the effects of lncRNA DLX6-AS1 in vivo were investigated using tumor-bearing nude mice. Figure 6A presents the morphologic characteristics of the excised tumors from nude mice generated by both HEp-2 and Tu-177 cells transfected with siNC and siDLX6-AS1. It was shown that DLX6-AS1 knockdown decreased the growth of tumors generated by both HEp-2 and Tu-177 cells. Moreover, mitochondrial oxidative metabolism was measured in laryngeal cancer cells from the siNC and siDLX6-AS1 groups, and the results demonstrated that DLX6-AS1 knockdown in vivo increased mitochondrial metabolism, mainly in complex I OXPHOS and complex I+II ETS (Figure 6B). More importantly, mitochondrial complex enzymatic activities were measured in tumor tissues, and the data indicated that DLX6-AS1 knockdown increased complex I activities and had no effect on mitochondrial complex II activities (Figure 6C). Using immunoblotting, it was shown that DLX6-AS1 knockdown also decreased the expression levels of TRPC3 in tumor tissues in both the HEp-2 and Tu-177 groups (Figure 6D). These results suggested that DLX6-AS1 promotes laryngeal tumor growth partially by upregulating TRPC3 expression levels.
Figure 6.
The effects of DLX6-AS1 siRNA on the tumor proliferation in vivo. (A) The effects of DLX6-AS1 siRNA on the tumor growth using nude mice models, with HEp-2 and Tu-177 cells pre-transfected with DLX6-AS1 specific siRNAs. The volume of each tumor was measured and analyzed. *P<0.05, **P<0.01. (B) The mitochondrial respiratory functions of grafted tumors were analyzed. *P<0.05, **P<0.01. (C) The enzymatic activities of mitochondrial complex I and complex II were measured using the tissues of grafted tumors. *P<0.05, **P<0.01. (D) The expression levels of TRPC3 in tumor tissues were detected by Western blotting. ***P<0.001.
Discussion
Investigating new diagnostic markers for laryngeal cancer is one of the most important strategies to improve the prognosis of patients. In particular, targeting the reprogrammed metabolism of cancer cells is a new emerging method for early prevention and effective therapy for several types of cancer. In this study, lncRNA DLX6-AS1 had different expression profiles in tumor tissues compared with adjacent normal tissues, which was correlated with poor prognosis. In detail, DLX6-AS1 knockdown decreased cell proliferation and changed the key mitochondrial metabolic parameters in both HEp-2 and Tu-177 cells. Moreover, DLX6-AS1 knockdown suppressed TRPC3-mediated mitochondrial calcium uptake and ROS production. Furthermore, miR-26a functioned as the link between these two molecules, as it could be absorbed by DLX6-AS1 which then regulated the levels of TRPC3. Finally, the DLX6-AS1/miR-26a/TRPC3 axis modulated cellular proliferation in vitro and in vivo. Our study provides new evidence that a novel lncRNA, DLX6-AS1, participates in tumorigenesis by regulating mitochondrial calcium homeostasis and respiration.
DLX6-AS1 is the antisense sequence of gene DLX6 (distal-less homeobox 6), which encodes a member of the homeobox transcription factor gene family similar to the Drosophila distal-less gene. Currently, DLX6-AS1 is identified as an oncogene in several types of cancer, such as colorectal cancer,24 bladder cancer,12 gastric cancer,14,25 pancreatic cancer.26 However, the role of DLX6-AS1 in laryngeal cancer has not been reported. More importantly, DLX6-AS1 is involved in the promotion of proliferation, migration, invasion, stemness and tumorigenesis, but little is known about the role of DLX6-AS1 in modulating intracellular functions, such as mitochondrial metabolism. In this study, we showed that DLX6-AS1 promoted proliferation by modulating TRPC3-mediated mitochondrial calcium uptake and respiratory functions.
Previous reports have shown that the regulatory mechanisms between lncRNAs and miRNAs are complicated. The most commonly accepted mechanism is that lncRNAs serve as competing endogenous RNAs (ceRNAs) to compete with miRNAs for binding to target mRNAs, thus forming a complex lncRNA/miRNA/mRNA network.27 Additionally, other mechanisms include translational inhibition, mRNA degradation, RNA decoys, recruitment of chromatin modifiers, regulation of protein activity, and regulation of the availability of miRNAs by sponging mechanisms.28 In this study, it was demonstrated that lncRNA DLX6-AS1 could function as an RNA decoy and sponge miR-26a, thus increasing the expression levels of the miR-26a target TRPC3, as demonstrated by luciferase assay.
Reprogrammed metabolism has been shown to participate in the tumorigenesis of all types of tumors. Although glycolysis has been considered as the major metabolic process for energy production in cancer cells, mitochondria play important roles. Porporato et al reviewed that in addition to exerting central bioenergetic functions, mitochondria could provide the building blocks for tumor anabolism, control redox and calcium homeostasis and govern cell death. Moreover, mitochondria are promising targets for the development of novel anticancer drugs.29 In our study, DLX6-AS1 knockdown suppressed mitochondrial ROS production and enhanced mitochondrial oxidative respiration, thus affecting cell proliferation. More specifically, NADH-ubiquinone oxidoreductase (complex I) is the largest complex of the mitochondrial electron transport chain and accounts for approximately 40% of the proton motive force required for mitochondrial ATP synthesis.30 In addition, complex I plays an essential role in biosynthesis and redox control during proliferation, which is consistent with the data in this study. Using a mitochondrial respiratory assay, it was shown that the oxidative respiration of complex I was regulated by TRPC3-mediated calcium influx and that the enzymatic activities of complex I increased in xenografted tumors pretreated with DLX6-AS1 siRNA.
TRPC3 belongs to the family of TRPCs, which is located both in the plasma membrane and mitochondria and regulates mitochondrial redox homeostasis.21 Previous studies revealed that TRPC3 regulated tumor phenotypes via calcium-signaling and its downstream pathways. Wang et al reported that TRPC3 modulated proliferation in breast cancer cells through the RASA4/MAPK pathway.31 Additionally, TRPC3 was reported to be regulated by another lncRNA, SNHG5, thus influencing growth and invasion in melanoma, but the concrete mechanism is not quite clear.32 In this study, we demonstrated that TRPC3 could be regulated by lncRNA DLX6-AS1, thus modulating mitochondrial calcium influx and ROS production. Inhibition of TRPC3 suppressed the proliferation of laryngeal cancer cells.
Conclusion
Collectively, this study shows that lncRNA DLX6-AS1 has aberrant expression in laryngeal cancer tissues compared with adjacent normal tissues and is correlated with the prognosis of these patients. Moreover, TRPC3 is one of the key targets of DLX6-AS1 via regulation of mitochondrial calcium homeostasis and respiratory functions. MiR-26a functions as the connection between these two elements, as it could be sponged by DLX6-AS1 and binds to the 3ʹ-UTR of TRPC3. lncRNA DLX6-AS1 promotes the proliferation of laryngeal cancer cells by modulating the miR-26a/TRPC3 pathway in vitro and in vivo. Targeting the DLX6-AS1/miR-26a/TRPC3 axis might become a promising treatment strategy in future therapeutics.
Disclosure
The authors declare no conflicts of interest in this study.
References
- 1.Peller M, Katalinic A, Wollenberg B, Teudt IU, Meyer JE. Epidemiology of laryngeal carcinoma in Germany, 1998–2011. Eur Arch Otorhinolaryngol. 2016;273(6):1481–1487. doi: 10.1007/s00405-016-3922-8 [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 3.Marioni G, Marchese-Ragona R, Cartei G, Marchese F, Staffieri A. Current opinion in diagnosis and treatment of laryngeal carcinoma. Cancer Treat Rev. 2006;32(7):504–515. doi: 10.1016/j.ctrv.2006.07.002 [DOI] [PubMed] [Google Scholar]
- 4.Hoffman HT, Porter K, Karnell LH, et al. Laryngeal cancer in the United States: changes in demographics, patterns of care, and survival. Laryngoscope. 2006;116(9Pt 2 Suppl 111):1–13. doi: 10.1097/01.mlg.0000236095.97947.26 [DOI] [PubMed] [Google Scholar]
- 5.He HJ, Bing H, Liu G. TSR2 induces laryngeal cancer cell apoptosis through inhibiting NF-kappaB signaling pathway. Laryngoscope. 2018;128(4):E130–E134. doi: 10.1002/lary.27035 [DOI] [PubMed] [Google Scholar]
- 6.Schmitt AM, Chang HY. Long noncoding RNAs in cancer pathways. Cancer Cell. 2016;29(4):452–463. doi: 10.1016/j.ccell.2016.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cossu AM, Mosca L, Zappavigna S, et al. Long non-coding RNAs as important biomarkers in laryngeal cancer and other head and neck tumours. Int J Mol Sci. 2019;20(14):3444. doi: 10.3390/ijms20143444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu Z, Xi K. LncRNA RGMB-AS1 promotes laryngeal squamous cell carcinoma cells progression via sponging miR-22/NLRP3 axis. Biomed Pharmacother. 2019;118:109222. doi: 10.1016/j.biopha.2019.109222 [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Wu N, Wang J, Li Z. LncRNA MEG3 inhibits cell proliferation and induces apoptosis in laryngeal cancer via miR-23a/APAF-1 axis. J Cell Mol Med. 2019;23(10):6708–6719. doi: 10.1111/jcmm.v23.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Su K, Wu H, Li J, Song D. LncRNA SNHG3 regulates laryngeal carcinoma proliferation and migration by modulating the miR-384/WEE1 axis. Life Sci. 2019;232:116597. doi: 10.1016/j.lfs.2019.116597 [DOI] [PubMed] [Google Scholar]
- 11.Yuan Z, Xiu C, Liu D, et al. Long noncoding RNA LINC-PINT regulates laryngeal carcinoma cell stemness and chemoresistance through miR-425-5p/PTCH1/SHH axis. J Cell Physiol. 2019;234(12):23111–23122. doi: 10.1002/jcp.v234.12 [DOI] [PubMed] [Google Scholar]
- 12.Fang C, Xu L, He W, Dai J, Sun F. Long noncoding RNA DLX6-AS1 promotes cell growth and invasiveness in bladder cancer via modulating the miR-223-HSP90B1 axis. Cell Cycle. 2019;18(23):1–12. doi: 10.1080/15384101.2019.1673633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tian Y, Wang YR, Jia SH. Knockdown of long noncoding RNA DLX6-AS1 inhibits cell proliferation and invasion of cervical cancer cells by downregulating FUS. Eur Rev Med Pharmacol Sci. 2019;23(17):7307–7313. doi: 10.26355/eurrev_201909_18836 [DOI] [PubMed] [Google Scholar]
- 14.Liang Y, Zhang CD, Zhang C, Dai DQ. DLX6-AS1/miR-204-5p/OCT1 positive feedback loop promotes tumor progression and epithelial-mesenchymal transition in gastric cancer. Gastric Cancer. 2020;23(2):212–227. doi: 10.1007/s10120-019-01002-1 [DOI] [PubMed] [Google Scholar]
- 15.Wu DM, Zheng ZH, Zhang YB, et al. Down-regulated lncRNA DLX6-AS1 inhibits tumorigenesis through STAT3 signaling pathway by suppressing CADM1 promoter methylation in liver cancer stem cells. J Exp Clin Cancer Res. 2019;38(1):237. doi: 10.1186/s13046-019-1239-3 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Ratnikov BI, Scott DA, Osterman AL, Smith JW, Ronai ZA. Metabolic rewiring in melanoma. Oncogene. 2017;36(2):147–157. doi: 10.1038/onc.2016.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Y, Karakhanova S, Hartwig W, et al. Mitochondria and mitochondrial ROS in cancer: novel targets for anticancer therapy. J Cell Physiol. 2016;231(12):2570–2581. doi: 10.1002/jcp.25349 [DOI] [PubMed] [Google Scholar]
- 18.Chiu HY, Tay EXY, Ong DST, Taneja R. Mitochondrial dysfunction at the centre of cancer therapy. Antioxid Redox Signal. 2020;10;32(5):309–330. doi: 10.1089/ars.2019.7898 [DOI] [PubMed] [Google Scholar]
- 19.Bravo-Sagua R, Parra V, Lopez-Crisosto C, Diaz P, Quest AF, Lavandero S. Calcium transport and signaling in mitochondria. Compr Physiol. 2017;7(2):623–634. doi: 10.1002/cphy.c160013 [DOI] [PubMed] [Google Scholar]
- 20.Sterea AM, El Hiani Y. The role of mitochondrial calcium signaling in the pathophysiology of cancer cells. Adv Exp Med Biol. 2020;1131:747–770. doi: 10.1007/978-3-030-12457-1_30 [DOI] [PubMed] [Google Scholar]
- 21.Feng S, Li H, Tai Y, et al. Canonical transient receptor potential 3 channels regulate mitochondrial calcium uptake. Proc Natl Acad Sci U S A. 2013;110(27):11011–11016. doi: 10.1073/pnas.1309531110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z, Guo J, Han X, et al. Metformin represses the pathophysiology of AAA by suppressing the activation of PI3K/AKT/mTOR/autophagy pathway in ApoE(-/-) mice. Cell Biosci. 2019;9:68. doi: 10.1186/s13578-019-0332-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao L, Zhang Z, Li Y, Zhao P, Chen Y. LncRNA H19/miR-let-7 axis participates in the regulation of ox-LDL-induced endothelial cell injury via targeting periostin. Int Immunopharmacol. 2019;72:496–503. doi: 10.1016/j.intimp.2019.04.042 [DOI] [PubMed] [Google Scholar]
- 24.Zhang JJ, Xu WR, Chen B, et al. The up-regulated lncRNA DLX6-AS1 in colorectal cancer promotes cell proliferation, invasion and migration via modulating PI3K/AKT/mTOR pathway. Eur Rev Med Pharmacol Sci. 2019;23(19):8321–8331. doi: 10.26355/eurrev_201910_19143 [DOI] [PubMed] [Google Scholar]
- 25.Wu Q, Ma J, Meng W, Hui P. DLX6-AS1 promotes cell proliferation, migration and EMT of gastric cancer through FUS-regulated MAP4K1. Cancer Biol Ther. 2020;21(1):17–25. doi: 10.1080/15384047.2019.1647050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang J, Ye Z, Mei D, Gu H, Zhang J. Long noncoding RNA DLX6-AS1 promotes tumorigenesis by modulating miR-497-5p/FZD4/FZD6/Wnt/beta-catenin pathway in pancreatic cancer. Cancer Manag Res. 2019;11:4209–4221. doi: 10.2147/CMAR.S194453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505(7483):344–352. doi: 10.1038/nature12986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akhade VS, Pal D, Kanduri C. Long noncoding RNA: genome organization and mechanism of action. Adv Exp Med Biol. 2017;1008:47–74. doi: 10.1007/978-981-10-5203-3_2 [DOI] [PubMed] [Google Scholar]
- 29.Porporato PE, Filigheddu N, Pedro JMB, Kroemer G, Galluzzi L. Mitochondrial metabolism and cancer. Cell Res. 2018;28(3):265–280. doi: 10.1038/cr.2017.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Urra FA, Munoz F, Lovy A, Cardenas C. The mitochondrial Complex(I)ty of cancer. Front Oncol. 2017;7:118. doi: 10.3389/fonc.2017.00118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Qi YX, Qi Z, Tsang SY. TRPC3 regulates the proliferation and apoptosis resistance of triple negative breast cancer cells through the TRPC3/RASA4/MAPK Pathway. Cancers (Basel). 2019;11(4):E558. doi: 10.3390/cancers11040558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao J, Zeng K, Liu Y, Gao L, Liu L. LncRNA SNHG5 promotes growth and invasion in melanoma by regulating the miR-26a-5p/TRPC3 pathway. Onco Targets Ther. 2019;12:169–179. doi: 10.2147/OTT.S184078 [DOI] [PMC free article] [PubMed] [Google Scholar]